Abstract
An outcome of low-grade B cell non-Hodgkins's lymphomas is the transformation to high-grade diffuse large B cell lymphomas (DLBL). To investigate the mechanisms of clonal evolution in the transformation to DLBL, we performed longitudinal molecular analyses of immunoglobulin (Ig), VHDJH gene sequences expressed in cases of chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma (SLL), and follicular lymphoma (FL) that transformed to DLBL. Among the neoplastic CLL and SLL cells and their respective high-grade transformants, there was no evidence for a clonotypic shift or acquired mutations in the expressed Ig VHDJH gene segments, as further confirmed by a specific and sensitive PCR-single strand polymorphism analysis. In contrast, among the FL cells there was a high degree of intraclonal diversification with highly divergent VHDJH gene sequences. Despite this intraclonal heterogeneity, the related DLBL expressed a collinear but unique VHDJH gene sequence. The intraclonal genealogical tree for the FL case demonstrated that the DLBL emerged in association with unique VHDJH gene mutational events. Among the intraclonal FL and related DLBL transformants, the nature and distribution of the Ig VHDJH gene mutations were consistent with antigenic selection. Thus, clonal evolution in the transformation from low- to high-grade B cell lymphoma may involve distinct pathways which vary according to the cellular origin and the type of the progenitor B cell tumor.
Keywords: B lymphocyte, Clonal expansion, Ig gene, Somatic hypermuation
1 Introduction
In the natural history of low-grade non-Hodgkin's lymphoma (NHL), a prolonged indolent phase may be followed by clinical progression toward intermediate or high-grade disease [1, 2]. Transformation to high-grade diffuse large B cell lymphoma (DLBL) occurs in up to 5 % of chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL), and in 30 % of follicular lymphoma (FL) cases [3–6]. In most cases, histological transformation is asociated with clinical acceleration of the disease and shortened survival [7, 8].
The mechanisms of morphological transformation, clonal evolution and clinical progression in transformed DLBL are poorly understood. Some investigators have indicated the high-grade DLBL cells share the same clonal root with the pre-existing low-grade NHL cells, based on observations that the original and transformed neoplastic B cell populations bear identical Ig VHDJH gene rearrangements [9–12]. Others have suggested that the morphological transformation from low- to high-grade B cell lymphoma is often associated with alteration of the expressed Ig genes indicating that clonal diversification is an important event in the emergence of DLBL [13–15].
To identify the pathways of clonal selection and outgrowth in the transformation of low-grade lymphomas, we performed longitudinal molecular analyses of the Ig VHDJH gene sequences expressed by CLL, SLL and FL B cells in cases that transformed to DLBL. In each case, we found that the morphological high-grade transformants were clonally related to the original tumor, and in each transformant there was an absolute lack of intraclonal diversification. While in CLL and SLL the transformants arose without any change from the original low-grade B cell population, in FL the high-grade transformant arose as a further and distinct mutant from an initial, highly diversified low-grade lymphoma B cell clonotype.
2 Results
2.1 Histology, cytology and oncogene configuration of low-grade B lymphomas and corresponding high-grade DLBL
The pathological findings for the three cases are summarized in Table 1. The original tumor was CLL in case 3557, SLL in case 1186 and FL (provisional cytological grade I) in case 7473. In all three cases, the histology of the second biopsy was classified as DLBL, and the transformed cells expressed the same antigens as the respective low-grade NHL cells prior to transformation. Whereas DNA isolated from the CLL and SLL cells and their respective transformed DLBL cells did not show evidence for bcl-1 or bcl-2 oncogene translocation by PCR analysis, DNA from both the FL and the corresponding transformed DLBL revealed a translocation of the bcl-2 gene resulting in fusion with the JH gene (not shown).
Table 1.
Summary of pathological data for three cases of low-grade NHL (CLL, SLL and FL) that transformed to DLBL
| Case 3557 | Case 1186 | Case 7473 | ||||
|---|---|---|---|---|---|---|
| Clinical data | ||||||
| Time interval | 1 month | 4 months | 18 months | |||
| Sample | PBa) | Ln | LN | LN | LN | LN |
| Cytology and histology | CLL | DLBL | SLL | DLBL | FL (Grade I) | DLBL |
| Immunophenotype | ||||||
| IgM | + | + | + | + | – | – |
| IgD | – | – | + | + | – | – |
| IgG | – | – | – | – | + | + |
| IgA | – | – | – | – | – | – |
| χ | – | – | + | + | + | + |
| λ | + | + | – | – | – | – |
| Surface antigens | ||||||
| HLA-DR | + | + | + | + | + | + |
| CD10 | – | – | – | – | – | – |
| CD19 | + | + | + | + | + | + |
| CD20 | + | + | + | + | + | + |
| CD23 | + | + | + | + | – | – |
| CD3 | – | – | – | – | – | – |
| CD5 | + | + | – | – | – | – |
| Oncogene translocation | ||||||
| bcl-1 | – | – | – | – | – | – |
| bcl-2 (major breakpoint region) | – | – | – | – | + | + |
| bcl-2 (minor cluster region) | – | – | – | – | – | – |
PB, peripheral blood.
2.2 Ig VHDJH genes expressed by CLL, SLL, FL and corresponding DLBL cells
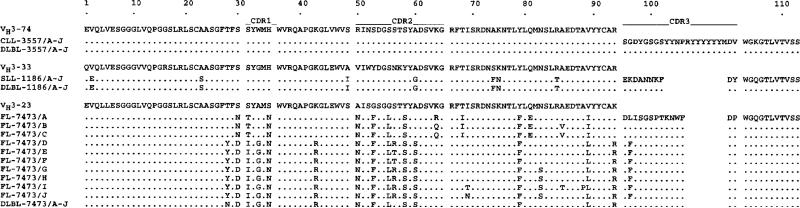
Ig VHDJH sequences from the CLL, SLL, FL and the respective DLBL cells were cloned and sequenced after PCR DNA amplification in separate reactions using the different Ig VH gene family-specific leader primers, each in conjunction with a consensus JH antisense primer. In each CLL, SLL, FL and corresponding DLBL cell sample, cDNA was amplified using the VH3 gene family-specific primer, and ten sequences from independent bacterial isolates were analyzed after cloning the amplified cDNA. The nucleotide and deduced amino acid sequences of the VHDJH gene segments, and those of the closest germ-line gene sequences, are depicted in Figs. 1 and 2, and are summarized in Table 2. All nucleic acid sequences derived from the CLL and corresponding DLBL cells (case 3557) were found to be identical. This VHDJH gene segment consisted of an unmutated germ-line V3–74 gene juxtaposed with D3–9 and JH6 genes [16–18], and with some intervening N segment additions (unencoded nucleotides). Likewise, in the SLL B cells (case 1186), the ten VHDJH gene sequences were identical and matched those of the ten corresponding DLBL cells. The expressed VHDJH gene segment consisted of a V3–33 gene with 12 nucleotide changes, juxtaposed to D5–24 and JH4 genes, with some intervening N segment additions [18–21].
Figure 1.
Nucleotide sequences of the Ig VHDJH genes expressed by the CLL (case 3557), SLL (case 1186) and FL (case 7473) and corresponding DLBL cells. In each case, the top sequence is provided for comparison and represents that of the closest reported germ-line VH, D, or JH gene. Dots indicate sequence identity, solid lines mark CDR, and numbers indicate the amino acid residues.
Figure 2.
Deduced amino acid sequences of the Ig VHDJH gene segments expressed by the CLL, SLL, FL and corresponding DLBL cells. Dots indicate identity and solid lines mark the CDR.
Table 2.
Analysis of Ig VH genes expressed by CLL, SLL, FL and morphologically transformed DLBL cells
| Case No. | Sample (Clone) | Intraclonal diversity | Closest germ-line gene | Nucleotide identity (%) | CDR1 and CDR2 | FR1, FR2 and FR3 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ra | p b | Sc | R | p | S | |||||
| 3557 | CLL (A-J) | no | HV3-74 | 100.0 | 0(0.00) | 0.00 | 0 | 0 ( 0.00) | 0.00 | 0 |
| DLBL (A-J) | no | HV3-74 | 100.0 | 0 ( 0.00) | 0.00 | 0 | 0 ( 0.00) | 0.00 | 0 | |
| 1186 | SLL (A-J) | no | HV3-33 | 96.0 | 1 (2.10) | 0.25 | 1 | 6 ( 6.93) | 1.95 × 10−1 | 4 |
| DLBL (A-J) | no | HV3-33 | 96.0 | 1 (2.10) | 0.25 | 1 | 6 ( 6.93) | 1.95 × 10−1 | 4 | |
| 7473 | FL (A) | yes | HV3-23 | 92.2 | 12 (4.03) | 1.35 × 10−4*d | 3 | 5 (13.24) | 4.23 × 10−4* | 3 |
| FL (B) | yes | HV3-23 | 91.8 | 11 (4.20) | 9.71 × 10−4* | 3 | 6 (13.81) | 9.75 × 10−4* | 4 | |
| FL (C) | yes | HV3-23 | 91.8 | 11 (4.20) | 9.71 × 10−4* | 3 | 6 (13.81) | 9.75 × 10−4* | 4 | |
| FL (D) | yes | HV3-23 | 90.1 | 12 (5.08) | 1.64 × 10−3* | 5 | 7 (16.69) | 2.11 × 10−4* | 5 | |
| FL (E) | yes | HV3-23 | 88.4 | 13 (5.95) | 2.37 × 10−3* | 7 | 7 (19.57) | 1.00 × 10−5* | 7 | |
| FL (F) | yes | HV3-23 | 88.4 | 13 (5.95) | 2.37 × 19−3* | 7 | 7 (19.57) | 1.00 × 10−5* | 7 | |
| FL (G) | yes | HV3-23 | 89.1 | 12 (5.60) | 3.90 × 10−3* | 6 | 8 (18.42) | 1.47 × 10−4* | 6 | |
| FL (H) | yes | HV3-23 | 88.8 | 12 (5.78) | 5.18 × 10−3* | 6 | 8 (19.00) | 8.30 × 10−5* | 7 | |
| FL (I) | yes | HV3-23 | 87.4 | 13 (6.48) | 5.11 × 10−3* | 6 | 11 (21.30) | 4.12 × 10−4* | 7 | |
| FL (J) | yes | HV3-23 | 88.8 | 12 (5.78) | 5.18 × 10−3* | 6 | 9 (19.00) | 3.11 × 10−4* | 6 | |
| DLBL (A-J) | no | HV3-23 | 89.1 | 11 (5.60) | 1.08 × 10−2* | 7 | 7 (18.42) | 3.50 × 10−5* | 7 | |
R, number of detected and (expected) R mutations.
p, probability.
S, number of detected S mutations.
statistically significant.
statistically significant.
In contrast with the VHDJH sequences found in the CLL and SLL B cells, among the FL cells (case 7473), the VHDJH sequences from ten independent isolates were collinear but each unique (FL-7473/A–J). These sequences encompassed a V3–23 gene rearranged with D1–7 and JH5 genes, with multiple intervening N segment additions [18, 20–23]. The degree of the intraclonal VHDJH gene diversification was marked, such that we detected 39 distinct and putatively independent somatic point mutations among the FL cells of case 7473. The sequence variation among the FL subclones (up to 32 base changes were observed when comparing subclone B or C to subclone I) was far above the Taq polymerase error rate in our laboratory (less than 2 × 10−4 base), suggesting that Taq infidelity did not account for the observed intraclonal VHDJH sequence diversity. In addition, the VHDJH gene sequences of the ten independent isolates from the transformed DLBL cells of the FL case (DLBL-7473/A–J) were all identical. These were collinear with, but distinct from, any of the FL VHDJH sequences, with which they shared 25 of the total 55 distinct mutations at separate residues in the ten FL B cell isolates, as compared to the germ-line (V3–23) sequence (Fig. 1).
In an attempt to outline the relatedness and evolution of the FL and transformed DLBL cells in case 7473, we aligned the ten VHDJH gene sequences of the FL clone and that of the DLBL. From the pattern of shared and unique mutations, and the assumption that shared mutations occurred due to single events rather than independent mutations, a genealogical tree for case 7473 was constructed and a putative ancestor VHDJH gene sequence inferred (Fig. 3). There were multiple differences (up to 26) between the DLBL and the FL (subclone B or C) VHDJH gene segment sequences. Overall, the pattern of point mutations encompassed at least four generations, and suggested that a divergent FL subclone gave rise to the DLBL.
Figure 3.
Genealogical tree outlining the clonal evolution of the FL case and transformed DLBL. Ig VHDJH genes were sequenced using ten independent isolates from the FL tumor and from the associated DLBL. The ten sequences expressed in the FL tumor demonstrated considerable heterogeneity (FL-A, B, C, D, E, F, G, H, I, J), whereas the ten DLBL sequences were identical. By aligning these FL and DLBL VHDJH gene segment sequences, a putative ancestor Ig gene sequence was deduced, and a genealogical tree was constructed. The common ancestor and putative intermediate clones are depicted as gray circles, and the number of mutations in each clone as compared to the closest ancestor is indicated. The 13 differences between the putative common ancestor and the first malignant cell would account for the mutations observed within the FL-A, B, C, and D elements that emerged in the third and fourth generations.
2.3 Identification of the germ-line V3–33 and V3–33 genes in cases 1186 and 7473
The Ig VH gene sequences expressed by the SLL and FL B cells (cases 1186 and 7473) were 96.0 % and 88.4–92.2 % identical to those of the V3–33 and V3–23 germ-line genes, respectively [20, 21, 23]. To confirm the presence of V3–23 and V3–33 as putative VH gene templates for the respective rearranged VHDJH segments in these patients, we used a PCR-based method with primers designed to amplify only the germ-line, unrearranged forms of the V3–33 and V3–23 genes (see Sect. 4.2). Amplification of a PCR product from genomic DNA was feasible in each case due to the presence of a few non-malignant cells within each tumor sample, and/or the presence of unrearranged V3–33 or V3–23 genes in the non-expressed chromosomes in the tumor cells. The genomic VH sequence PCR products were cloned and sequenced using plasmid DNA from six independent bacterial isolates in each case. In case 1186, the nucleotide sequences from all six isolates were 100 % identical to that of the reported germ-line V3–33 gene. In case 7473, three cloned plasmids incorporated sequences that were identical to V3–23 germ-line gene, and three cloned plasmids contained inserts with sequences identical to that of the V3–7 germ-line gene [23]. These findings are consistent with our contention that the Ig VH genes rearranged in the neoplastic cells of cases 1186 and 7473 consisted of mutated V3–33 and V3–23 genes.
2.4 Further analysis of the intraclonal Ig VHDJH gene diversity in CLL, SLL, FL and related DLBL cells
PCR-single strand chain polymorphism (SSCP) analysis is a highly specific and sensitive method to detect mutations in single-strand DNA, so much so that a single mutated sequence can be detected among approximately 1000 wild-type single-strand DNA chains. To confirm and extend the findings provided by the sequence analyses, we performed PCR-SSCP analyses after radioactively labeling the Ig VHDJH cDNA from each tumor. In each case, we used the V3 gene family-specific leader sense primer and the consensus antisense JH gene primer. In the CLL and SLL (cases 3557 and 1186) B cells, SSCP analysis of the PCR-amplified cDNA revealed two distinct bands, corresponding to the denatured strands of DNA, and a third band representing a minor population of non-denatured, double-strand DNA fragments (Fig. 4). This cDNA electrophoretic mobility was absolutely reproduced by the cDNA from the respective DLBL cells, indicating that intraclonal diversity, if present in the CLL, SLL, or the associated transformed DLBL cells, was less than that which could be detected by SSCP, i.e. less than one mutant per 1000 identical, wild-type cells.
Figure 4.

PCR-SSCP analysis of the expressed Ig VH genes in the original CLL, SLL, and FL tumor and corresponding DLBL cells. cDNA corresponding to leader-JH region of the expressed Ig VHDJH gene segment were amplified in the presence of [α-32P]dCTP, denatured, and separated by PAGE prior to autoradiography. In each case, non-denatured PCR products (ND) were used as controls, as indicated.
Consistent with the intraclonal heterogeneity of the VHDJH gene segment cDNA sequences, the PCR-SSCP analysis of the Ig VHDJH in the FL cells of case 7473 yielded a smear-like pattern. However, as in the other two DLBL cases, PCR-SSCP analysis of the FL-associated DLBL cells revealed only two electrophoretic bands, indicating that these cells expressed a single Ig VHDJH gene sequence. Thus, the PCR-SSCP analysis further indicated that FL cell clonotype was highly diversified in the Ig VHDJH gene sequence, but the corresponding high-grade NHL transformant consisted of a homogenous B cell population without intraclonal divergence.
2.5 Search for the DLBL clonotype in the corresponding FL cell population
The sequence and PCR-SSCP analyses of Ig VHDJH genes expressed by the DLBL cells in case 7473 showed that the cells consisted of a monoclonal cell population without intraclonal diversification. Comparable analyses of the FL cells from the same case revealed at striking degree of intraclonal divergence. To determine whether the DLBL cells emerged by ongoing somatic hypermutation from one of the intraclonal FL mutants, or resulted from the “fixation” of a pre-existing FL mutant, we developed a PCR specific for the DLBL VHDJH sequence using a DLBL clone-specific g22 sense primer in conjunction with the consensus JH antisense primer, capable of discriminating and amplifying the DLBL VHDJH cDNA even when significantly outnumbered by other irrelevant sequences. We performed a dose-dependent analysis to measure the sensitivity of this ad hoc PCR, as described in Sect. 4.3, and determined that it could detect the 7473 DLBL-specific VHDJH sequence in mixtures of DLBL and HL-60 cells at ratios up to 1:104 to 1:105 (Fig. 5). When we used these primer pairs and the same experimental conditions to attempt to amplify DNA from the 7473 FL cells, we did not succeed in multiple attempts to amplify an appropriate 310-bp cDNA fragment (Fig. 5). These experiments indicated that the FL tumor cells did not comprise the DLBL mutant, or that if such a mutant were present, its frequency of representation was less than 1:104 to 1:105 cells.
Figure 5.

Detection of the the clone-specific Ig VHDJH gene sequence in DLBL and FL cells of case 7473. DLBL cells of case 7473 were mixed with HL-60 cells in ratios of 1:10 × 1:106. cDNA was prepared, and clone-specific primers were used to PCR amplify the specific DLBL Ig VHDJH gene sequence. Electrophoretic fractionation of the PCR products in 2 % agarose containing 1 μ/ml ethidium bromide shows detection of the specific DLBL Ig VHDJH gene sequence in 7374 DLBL cell “diluted” up to 10−4 to 10−5 in “irrelevant” cells, but not in the corresponding (“undiluted”) FL cells.
2.6 Analysis of the somatic point mutations in the rearranged Ig VH genes
In the absence of negative or positive selective pressure on a gene product, nucleotide changes yielding amino acid replacement (R) mutations or silent (S) mutations are randomly distributed throughout the coding sequence. In the SLL and related DLBL cells (case 1186), the frequencies of R mutations in the VH gene segment complementarity-determining regions (CDR) and framework regions (FR) were not different from those expected by chance alone, suggesting the lack of a selective pressure applied to this Ig VH gene product (Table 2). In contrast, each of the ten collinear Ig VHDJH gene sequences expressed by the FL B cells (case 7473) displayed higher and lower numbers of R mutations in the CDR and FR, respectively, than theoretically expected. The probability that such an excess of R mutations in the CDR arose by chance alone was negligible. Likewise, the likelihood that the scarcity of R mutations in this VH gene FR was due to chance alone was exceedingly small (Table 2). Accordingly, the probability that the excess and scarcity of R mutations in the CDR and FR of the Ig V segment expressed by the DLBL cells of this tumor arose by chance only was also negligible. Thus, the number and distribution of R mutations in the Ig VHDJH gene segment of the SLL and related DLBL cells suggests a random pattern of hypermutation independent of any selective pressure. In contrast, in the FL and related DLBL cells, the pattern of R mutations is highly consistent with the application of a selective pressure to the Ig VHDJH gene product, and suggests that antigen played a role in the evolution of these FL cells and their transformation to high-grade lymphoma.
3 Discussion
We investigated the clonal evolution of three low-grade B cell lymphomas by performing longitudinal analyses of Ig VHDJH gene sequences expressed by CLL, SLL, FL and their clonally related DLBL high-grade transformants. The comparison of Ig VHDJH gene sequences expressed by lymphoma cells before and after transformation indicated that there exist distinct clonal evolution pathways which depend on the cellular origin of the low-grade NHL. In the lymphomas putatively originating from early germinal center B cells or centroblasts (CLL and SLL), the expressed Ig VHDJH genes were unmutated or minimally mutated. These sequences were strictly conserved when those tumors transformed to DLBL. In contrast, the FL B cells expressed in a high degree of intraclonal Ig VHDJH gene diversification, so much so that as many as 39 different and putatively independent somatic point mutations were observed among ten independent isolates. This tremendous intraclonal diversity was lost in the transformation to high-grade lymphoma, such that analysis of the Ig VHDJH sequences from ten independent plasmid clones, as well as SSCP analysis of cDNA prepared from the transformed tumor, indicated that the final B cell population in the DLBL expressed a single Ig VHDJH sequence. Finally, despite the absolute homogeneity and lack of intraclonal diversification among the high-grade DLBL cells, the pattern of somatic mutations was consistent with a process of antigenic selection, possibly begun at the low-grade lymphoma stage.
In our studies, the combination of sequencing and SSCP analyses demonstrated that intraclonal Ig VHDJH gene diversification did not occur among the CLL or SLL cells, and extends other reports that CLL B cells undergo no or minimal Ig somatic mutation [24–27]. Our present findings indicate that these tumors remain stable in their expressed Ig genes, even after transformation to DLBL, and support our recent demonstration that Ig VHDJH gene sequences in CLL can be absolutely conserved over a 2-year period [28]. Thus, intraclonal stability of CLL and SLL B cells seems to continue throughout transformation to DLBL, which is not associated with somatic intraclonal diversification.
In striking contrast to the CLL and SLL B cells, the FL and its associated transformant B cells showed evidence for ongoing somatic hypermutation and intraclonal diversification. SSCP and sequence analyses of the Ig VHDJH genes indicated a high degree of intraclonal divergence in the original FL tumor. However, the ten distinct isolates from the associated DLBL cells demonstrated identical Ig VHDJH sequences. The DLBL Ig VHDJH sequence was collinear (clonally related) with those of the FL, but could not be detected in the original tumor. Based on the Ig VHDJH sequence analyses in this case, we constructed a genealogical tree for the FL and its associated transformed DLBL. The pattern of somatic hypermutation supported the hypothesis that a single, somatically mutated FL subclone gave rise to the transformed DLBL. These results strengthen and extend those of Ottensmeier et al. [29], who examined Ig VHDJH sequences in a FL case which evolved over time. In that case, there was significant intraclonal Ig VHDJH gene variation among cells in an initial FL tumor, but at the time of clinical relapse and histological progression to DLBL, a single and discrete pattern of Ig gene expression emerged.
There are two possible models to explain the evolution of the DLBL clonotype in our FL case. First, the DLBL clonotype might have been present among highly divergent clones in the original FL. Eventually this clone predominated and became the single tumor clone. This model for lymphoma progression has been outlined by Friedman et al. [30], based on longitudinal analysis of Ig gene expression in an autoreactive lymphoma. Alternatively, as a result of ongoing Ig gene hypermutation, the FL tumor cell population might have become increasingly heterogeneous, such that a clonal variant emerged which gave rise to the DLBL clone. To distinguish between these two possible mechanisms in FL transformation, we used a PCR specific for the DLBL clonotype and determined that the DLBL sequence was absent in the FL sample, or was present in a copy number of less than 10−4 to 10−5 cells. The pattern of somatic mutations suggested that a new neoplastic clonotypic variant gave rise to the transformed DLBL cells clone. However, bot the SSCP and the sequence data support the absence of ongoing somatic mutation of the Ig VHDJH gene sequence in the FL-associated DLBL transformed clone, suggesting that the more aggressive, transformed clone lost the capacity to undergo mutation in the Ig gene. They also extend the suggestion by Stiernholm et al. [31] that hypermutation is absent in DLBL tumor cells in vivo and in propagated DLBL cells in vitro, and document in a single case the intraclonal evolution from heterogeneity to homogeneity.
Recent work from several groups has provided strong circumstantial evidence for a role of antigen in clonal selection and progression of B cell tumors including IgG isotype-switched CLL [32], FL [9, 33, 34], and Burkitt's lymphoma [35–37]. In our study, the high intraclonal Ig VHDJH gene sequence conservation observed in both CLL and SLL cells before and after DLBL transformation was consistent with the lack of a role of antigen in the clonal expansion of these tumors. In contrast, in the FL case there was tremendous intraclonal diversity in the original tumor, but only a single, related but unique clone in the corresponding DLBL. In both the low- and high-grade tumor cells, the pattern of somatic mutations was highly consistent with selection of R mutations by antigen, supporting the hypothesis that the outgrowth of the FL B cells and, perhaps, the subsequent transformation to high-grade DLBL, were associated with selective antigenic pressure.
Self antigens have been implicated in the development of some B cell tumors [30, 35, 38], but genetic or epigenetic events, such as mutations in the p53 [39, 40], bcl-2 [41], c-myc [42] proto-oncogenes, have also been called into question in the transformation of FL to DLBL cells. In addition, deletions of the p16 cell cycle regulatory gene have been identified in a high proportion of DLBL lymphomas that transformed from FL [43]. Thus, in the context of antigen-dependent clonal expansion and selection, other acquired mutations in cell regulatory elements might favor the outgrowth of a particular subclone, such that ultimately the tumor consists of a homogeneous population of B cells expressing a unique VHDJH sequence.
4 Materials and methods
4.1 Origin, phenotypic characterization and protooncogene configuration of the neoplastic cells
Peripheral blood and/or lymph node biopsy samples of three patients observed at The New York Presbyterian Hospital-Weill Medical College of Cornell University who had low-grade NHL that progressed to diffuse aggressive NHL were selected for this study based on the availability of material for molecular analyses. The samples were classified according to the Revised European-American Lymphoma Classification proposed by the International Lymphoma Study Group [2].
The phenotype of the lymphoma cells was determined by immunohistochemical staining of frozen and/or paraffin tissue sections and cytospin preparations using a three-step avidin-biotin immunoperoxidase method with the following polyclonal and monoclonal antibodies: IgM, IgD, IgA, IgG, CD3 (CD3), L26 (CD20 Dako Corp., Santa Barbara, CA), ϰ, λ (Organon Teknika-Cappel, West Chester; NY), HLA-DR, T1 (CD5, United Biomedical Corp., Hauppauge, NY), cALLa (CD10), Leu 20 (CD23; Becton Dickinson, San Jose, CA), and B4 (CD19; Coulter Immunology, Hialeah, FL). A PCR method was used to analyze the bcl-1 and bcl-2 protooncogene configurations. For the analysis of the bcl-1 rearrangement, chromosome 11-specific P2 and P3 sense primers were used in conjunction with an JH antisens primer [44, 45]. For the analysis of the bcl-2 rearrangement, major break point region (mbr)- and minor cluster region (mcr)-specific sense primers of chromosome 18 were used in conjunction with a JH antisense primer [45–47].
4.2 PCR amplification, cloning and sequencing of the expressed Ig VHDJH and the corresponding genomic Ig VH genes
Genomic DNA and total RNA were extracted from cryopreserved mononuclear cells suspensions or tissue blocks by salt extraction and guanidine isothiocyanate, respectively [48, 49]. RNA (5 μg) was reverse transcribed into cDNA using M-MLV reverse transcriptase (Superscript RNase H Reverse Transcriptase, Gibco-BRL Life Technologies, Grand Island, NY), in conjunction with a poly(dT)12–13 primer according to the manufacturer's instructions.
In each tumor, Ig VHDJH cDNA were amplified by PCR using sense Ig VH gene family-specific (V1, V2, V3, V4, V5 and V6) leader primers in conjunction with an antisense consensus JH primer in independent reactions, as described [23, 28, 50, 51]. The (unrearranged) germ-line V3–33 gene was amplified from case 1186 using the sense g27 primer (5'TTCACCTTCAGTAGCTATGGC-3') specific for the CDR1 sequence of the V3–33 [23] in conjunction with the degenerated antisense hept 3 primer [5'GGAATTC(AC)TG(AG)C(CT)TCCCCTC(AG)CT(CG)3'] specific for the VH3 gene heptamer recombination signal sequence [23]. Similarly, the V3–23 gene was amplified from genomic DNA using the sense g25 primer (5'TTTAGCAGCTATGCCATGAGC-3') specific for the CDR1 sequence of the V3–23 gene, in conjunction with the antisense hept 3 primer [23]. Thirty PCR cycles were performed with denaturation at 94 C for 1 min, annealing at 58 C for 1 min, and extension at 72 C for 2 min. The PCR products were cloned in pCR™ II vectors using the TA cloning system (Invitrogen Corporation, San Diego, CA) [28, 52]. For each cDNA preparation, at least ten independent plasmid isolates were sequenced using small-scale plasmid preparations and the Sequenase® system (United States Biochemical Corp., Cleveland, OH, version 2.0). The sequences were analyzed using MacVector software (Eastman Kodak Co., New Haven, CT, version 4.5) and the GeneBank data base.
4.3 PCR-SSCP analysis of Ig VHDJH gene segment DNA
PCR-SSCP analysis of the expressed Ig VHDJH gene sequences performed by adaptation of a reported method [53]. cDNA were amplified by PCR with the sense Ig VH3 family-specific leader and the consensus JH antisense primers in independent reactions, as described above, using 100 ng cDNA, 10 pM of each primer, 2.5 l dNTP, 1 μCi [α–32P] dCTP (NEN; specific activity, 3000 Ci/mM), 10 mM (pH 8.8), 50 mM KCl, 1.5 mM MgCl2, 0.5 U Taq polymerase, in a final volume of 10 μl. The reaction mixtures (2 μl) were diluted 1:25 in 0.1 % SDS, 10 mM EDTA, and then mixed 1:1 with a sequencing stop solution. Samples were heated at 95 °C for 5 min, chilled on ice, and immediately subjected to electrophoresis in 6 % acrylamide-TBE (1.0 M Tris, 0.9 M boric acid, 0.01 M EDTA, pH 8.4) with 10 % glycerol. After electrophoresis at room temperature for 14–16 h (4–8 W), the gels were fixed in 10 % acetic acid, air dried, and analyzed by autoradiography.
4.4 Clonotype-specific amplification of Ig VHDJH gene segment DNA in DLBL cells of case 7473 and determination of the PCR sensitivity
A PCR-based approach was developed to verify whether the DLBL clonotype of case 7473 was represented among the corresponding FL cells. The case-specific g22 gene primer (5'-TGTGCAGCCTCTGGATTCAAT-3') for the DLBL Ig VH gene and the consensus antisense JH primer were used in PCR for 30 cycles with denaturation at 94 °C for 1 min, annealing at 55 °C for 1 min and extension at 72 °C for 2 min. The sensitivity of this PCR-based approach was evaluated using mixtures of case 7473 DLBL cells and HL-60 cells at ratios of 1:10, 1:102, 1:103, 1:104, 1:105, and 1:106 prior to mRNA extraction, cDNA synthesis, PCR amplification of the clone-specific VHDJH sequence, and analysis by electrophoresis in 2 % agarose with ethidium bromide.
4.5 Analysis of somatic point mutations
The number of expected R mutations in the CDR and FR of the Ig VH gene sequences was calculated using the formula RCDR or RFR = n × (CDR Rf or FR Rf) × (CDRrel or FRrel), where n is the total number of observed mutations, and Rf is the replacement frequency inherent to each Ig VH gene [51, 54], and CDRrel and FRrel are the relative size of the CDR and FR, respectively. A binomial probability model was used to evaluate whether the excess or scarcity of R mutations in CDR and FR was due to chance alone: p = {n!/[k!(n−k)!]} × qk × (1−q)n−k, where q = the probability that an R mutation will localize to CDR or FR (q = CDRrel × CDR Rf or FRrel × FR Rf), and k = the number of observed R mutations in the CDR or FR [51, 54].
Acknowledgments
This work was supported by U.S. Public Health Service grants AR 40908 and CA 68541 to P.C., and the Hungarian Ministry of Culture and Education grant FKFP 0931/97 to A.M.
Abbreviations
- CLL
Chronic lymphocytic leukemia
- D
Diversity (region)
- DLBL
Diffuse large B cell lymphoma
- FL
Follicular lymphoma
- JH
Joining heavy chain (region)
- PCR-SSCP
PCR-single strand chain polymorphism (analysis)
- R
Replacement (mutation)
- S
Silent (mutation)
- SLL
Small lymphocytic leukemia
- VH
Variable heavy chain (region)
- CDR
Complementarity-determining region
- FR
Framework region
References
- 1.Ersboll J, Schultz HB, Pedersen-Bjergaard J, Nissen NI. Follicular low-grade non-Hodgkins's lymphoma: long-term outcome with or without tumor progression. Eur. J. Haematol. 1989;42:155–163. doi: 10.1111/j.1600-0609.1989.tb01205.x. [DOI] [PubMed] [Google Scholar]
- 2.Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, Delsol G, De Wolf-Peeters C, Falini B, Gatter KC. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84:1361–1392. [PubMed] [Google Scholar]
- 3.Cullen MH, Lister TA, Brearley RI, Shand WS, Stansfeld AG. Histological transformation of non-Hodgkin's lymphoma: a prospective study. Cancer. 1979;44:645–651. doi: 10.1002/1097-0142(197908)44:2<645::aid-cncr2820440234>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 4.Garvin AJ, Simon RM, Osborne CK, Merrill J, Young RC, Berard CW. An autopsy study of histologic progression in non-Hodgkins's lymphomas. 192 cases from the National Cancer Institute. Cancer. 1983;52:393–398. doi: 10.1002/1097-0142(19830801)52:3<393::aid-cncr2820520302>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 5.Harris NL, Ferry JA. Follicular lymphoma and related disorders (germinal center lymphomas). In: Knowles DM, editor. Neoplastic Hematopathology. Williams & Wilkins; Baltimore: 1992. pp. 645–674. [Google Scholar]
- 6.Foucar K. B cell chronic lymphocytic and prolymphocytic leukemia. In: Knowles DM, editor. Neoplastic Hematopathology. Williams & Wilkins; Baltimore: 1992. pp. 1181–1208. [Google Scholar]
- 7.Hubbard SM, Chabner BA, De Vita VT, Jr., Simon R, Berard CW, Jones RB, Garvin AJ, Canellos GP, Osborne CK, Young RC. Histologic progression in non-Hodgkins's lymphoma. Blood. 1982;59:258–264. [PubMed] [Google Scholar]
- 8.Oviatt DL, Cousar JB, Collins RD, Flexner JM, Stein RS. Malignant lymphomas of follicular center cell origin in humans. V. Incidence, clinical features, and prognostic implications of transformation of small cleaved cell nodular lymphoma. Cancer. 1984;53:1109–1114. doi: 10.1002/1097-0142(19840301)53:5<1109::aid-cncr2820530516>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 9.Zelenetz AD, Chen TT, Levy R. Histologic transformation of follicular lymphoma to diffuse lymphoma represents tumor progression by a single malignant B cell. J. Exp. Med. 1991;173:197–207. doi: 10.1084/jem.173.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foon KA, Thiruvengadam R, Saven A, Bernstein ZP, Gale RP. Genetic relatednesses of lymphoid malignancies. Transformation of chronic lymphocytic leukemia as a model. Ann. Int. Med. 1993;119:63–73. doi: 10.7326/0003-4819-119-1-199307010-00011. [DOI] [PubMed] [Google Scholar]
- 11.Cherepakhin V, Baird SM, Meisenholder GW, Kipps TJ. Common clonal origin of chronic lymphocytic leukemia and high-grade lymphoma of Richter's syndrome. Blood. 1993;82:3141–3147. [PubMed] [Google Scholar]
- 12.Matolcsy A, Inghirami G, Knowles DM. Molecular genetic demonstration of the diverse evolution of Richter's syndrome (chronic lymphocytic leukemia and subsequent large cell lymphoma). Blood. 1994;83:1363–1372. [PubMed] [Google Scholar]
- 13.Miyamura K, Osada H, Yamauchi T, Itoh M, Kodera Y, Suchi T, Takahashi T, Ueda R. Single clonal origin of neoplastic B-cells with different immunoglobulin light chains in patient with Richter's syndrome. Cancer. 1990;66:140–144. doi: 10.1002/1097-0142(19900701)66:1<140::aid-cncr2820660125>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 14.Schots R, Dehou MF, Jochmans K, Heirman C, de Waele M, van Camp B, Thielemans K. Southern blot analysis in a case of Richter's syndrome. Evidence for a postrearrangement heavy chain gene deletion associated with the altered phenotype. Am. J. Clin. Pathol. 1991;95:571–577. doi: 10.1093/ajcp/95.4.571. [DOI] [PubMed] [Google Scholar]
- 15.Nakamine H, Masih AS, Sanger WG, Wickert RS, Mitchell DW, Armitage JO, Weisenburger DD. Richter's syndrome with different immunoglobulin ligh chain types. Molecular and cytogenetic features indicate a common clonal origin. Am. J. Clin. Pathol. 1992;97:656–663. doi: 10.1093/ajcp/97.5.656. [DOI] [PubMed] [Google Scholar]
- 16.Ichihara Y, Matsuoka H, Kurosawa Y. Organization of human immunoglobulin heavy chain diversity gene loci. EMBO J. 1988;7:4141–4150. doi: 10.1002/j.1460-2075.1988.tb03309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rechavi G, Bienz B, Ram D, Ben-Neriah Y, Cohen JB, Zakut R, Givol D. Organization and evolution of immunoglobulin VH gene subgroups. Proc. Natl. Acad. Sci. USA. 1982;79:4405–4409. doi: 10.1073/pnas.79.14.4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ravetch JV, Siebenlist U, Korsmeyer S, Wald-mann T, Leder P. Structure of the human immunoglobulin mu locus: characterization of embryonic and rearranged J and D genes. Cell. 1981;27:583–591. doi: 10.1016/0092-8674(81)90400-1. [DOI] [PubMed] [Google Scholar]
- 19.Olee T, Yang PM, Siminovitch KA, Olsen NJ, Hillson J, Wu J, Kozin F, Carson DA, Chen PP. Molecular basis of an autoantibody-associated restriction fragment length polymorphism that confers susceptibility to autoimmune diseases. J. Clin. Invest. 1991;88:193–203. doi: 10.1172/JCI115277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuda F, Shin EK, Nagaoka H, et al. Structure and physical map of 64 variable segments in the 3′0.8-megabase region of the human immunoglobulin heavy-chain locus. Nat. Genet. 1993;3:88–94. doi: 10.1038/ng0193-88. [DOI] [PubMed] [Google Scholar]
- 21.Matsuda F, Ishii K, Bourvagnet P, Kuma K, Hayashida H, Miyata T, Honjo T. The complete nucleotide sequence of the human immunoglobulin heavy chain variable region locus. J. Exp. Med. 1998;188:2151–2162. doi: 10.1084/jem.188.11.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen PP, Liu MF, Sinha S, Carson DA. A 16/6 idiotype-positive anti-DNA antibody is encoded by a conserved VH gene with no somatic mutation. Arthritis Rheum. 1988;31:1429–1431. doi: 10.1002/art.1780311113. [DOI] [PubMed] [Google Scholar]
- 23.Tomlinson IM, Walter G, Marks JD, Llewelyn MB, Winter G. The repertoire of human germline VH sequences reveals about fifty groups of VH segments with different hypervariable loops. J. Mol. Biol. 1992;227:776–798. doi: 10.1016/0022-2836(92)90223-7. [DOI] [PubMed] [Google Scholar]
- 24.Schroeder HW, Jr., Dighiero G. The pathogenesis of chronic lymphocytic leukemia: analysis of the antibody repertoire [see comments]. Immunol. Today. 1994;15:288–294. doi: 10.1016/0167-5699(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 25.Rassenti LZ, Kipps TJ. Lack of extensive mutations in the VH5 genes used in common B cell chronic lymphocytic leukemia. J. Exp. Med. 1993;177:1039–1046. doi: 10.1084/jem.177.4.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kipps TJ. Immunoglobulin genes in chronic lymphocytic leukemia. Blood Cells. 1993;19:615–625. discussion 631–632. [PubMed] [Google Scholar]
- 27.Maloum K, Davi F, Magnac C, Pritsch O, McIntyre E, Valensi F, Binet JL, Merle-Beral H, Dighiero G. Analysis of VH gene expression in CD5+ and CD5− B-cell chronic lymphocytic leukemia. Blood. 1995;86:3883–3890. [PubMed] [Google Scholar]
- 28.Schettino EW, Cerutti A, Chiorazzi N, Casali P. Lack of intraclonal diversification in Ig heavy and light chain V region genes expressed by CD5+IgM+ chronic lymphocytic leukemia B cells: a multiple time point analysis. J. Immunol. 1998;160:820–830. [PMC free article] [PubMed] [Google Scholar]
- 29.Ottensmeier CH, Thompsett AR, Zhu D, Wilkins BS, Sweetenham JW, Stevenson FK. Analysis of VH genes in follicular and diffuse lymphoma shows ongoing somatic mutation and multiple isotype transcripts in early disease with changes during disease progression. Blood. 1998;91:4292–4299. [PubMed] [Google Scholar]
- 30.Friedman DF, Cho EA, Goldman J, Carmack CE, Besa EC, Hardy RR, Silberstein LE. The role of clonal selection in the pathogenesis of an autoreactive human B cell lymphoma. J. Exp. Med. 1991;174:525–537. doi: 10.1084/jem.174.3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stiernholm N, Kuzniar B, Berinstein NL. Absence of immunoglobulin variable region hypermutation in a large cell lymphoma after in vivo and in vitro propagation. Blood. 1992;80:738–743. [PubMed] [Google Scholar]
- 32.Hashimoto S, Dono M, Wakai M, Allen SL, Lichtman SM, Schulman P, Vinciguerra VP, Ferrarini M, Silver J, Chiorazzi N. Somatic diversification and selection of immunoglobulin heavy and light chain variable region genes. in IgG+ CD5+ chronic lymphocytic leukemia B cells. J. Exp. Med. 1995;181:1507–1517. doi: 10.1084/jem.181.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zelenetz AD, Chen TT, Levy R. Clonal expansion in follicular lymphoma occurs subsequent to antigenic selection. J. Exp. Med. 1992;176:1137–1148. doi: 10.1084/jem.176.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bahler DW, Levy R. Clonal evolution of a follicular lymphoma: evidence for antigen selection. Proc. Natl. Acad. Sci. USA. 1992;89:6770–6774. doi: 10.1073/pnas.89.15.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riboldi P, Gaidano G, Schettino EW, Steger TG, Knowles DM, Dalla-Favera R, Casali P. Two acquired immunodeficiency syndrome-associated Burkitt's lymphomas produce specific anti-i IgM cold agglutinins using somatically mutated VH4–21 segments. Blood. 1994;83:2952–2961. [PMC free article] [PubMed] [Google Scholar]
- 36.Jain R, Roncella S, Hashimoto S, Carbone A, Francia di Celle P, Foa R, Ferrarini M, Chiorazzi N. A potential role for antigen selection in the clonal evolution of Burkitt's lymphoma. J. Immunol. 1994;153:45–52. [PubMed] [Google Scholar]
- 37.Tamaru J, Hummel M, Marafioti T, Kalvelage B, Leoncini L, Minacci C, Tosi P, Wright D, Stein H. Burkitt's lymphomas express VH genes with a moderate number of antigen-selected somatic mutations. Am. J. Pathol. 1995;147:1398–1407. [PMC free article] [PubMed] [Google Scholar]
- 38.Roncella S, Cutrona G, Favre A, Ulivi M, Fais F, Signorini A, Grossi CE, Chiorazzi N, Ferrarini M. Apoptosis of Burkitt's lymphoma cells induced by specific interaction of surface IgM with a self-antigen: implications for lymphomagenesis in acquired immuno-deficiency syndrome. Blood. 1996;88:599–608. [PubMed] [Google Scholar]
- 39.Sander CA, Yano T, Clark HM, Harris C, Londo DL, Jaffe ES, Raffeld M. p53 mutation is associated with progression in follicular lymphomas. Blood. 1993;82:1994–2004. [PubMed] [Google Scholar]
- 40.Lo Coco F, Gaidano G, Louie DC, Offit K, Chaganti RS, Dalla-Favera R. p53 mutations are associated with histologic transformation of follicular lymphoma. Blood. 1993;82:2289–2295. [PubMed] [Google Scholar]
- 41.Matolcsy A, Casali P, Warnke RA, Knowles DM. Morphologic transformation of follicular lymphoma is associated with somatic mutation of the trans-located Bcl-2 gene. Blood. 1996;88:3937–3944. [PubMed] [Google Scholar]
- 42.Yano T, Jaffe ES, Longo DL, Raffeld M. MYC rearrangements in histologically progressed follicular lymphomas. Blood. 1992;80:758–767. [PubMed] [Google Scholar]
- 43.Elenitoba-Johnson KSJ, Gascoyne RD, Lim MS, Chhanabai M, Jaffe ES, Raffeld M. Homo-zygous deletions at chromosome 9p21 involving p16 and p15 are associated with histologic progression in follicle center lymphoma. Blood. 1998;91:4677–4685. [PubMed] [Google Scholar]
- 44.Rimokh R, Berger F, Delsol G, Digonnet I, Rouault JP, Tigaud JD, Gadoux M, Coiffier B, Bryon PA, Magaud JP. Detection of the chromosomal translocation t(11; 14) by polymerase chain reaction in mantle cell lymphomas. Blood. 1994;83:1871–1875. [PubMed] [Google Scholar]
- 45.Matolcsy A, Casali P, Nador RG, Liu YF, Knowles DM. Molecular characterization of IgA- and/ or IgG-switched chronic lymphocytic leukemia B cells. Blood. 1997;89:1732–1739. [PMC free article] [PubMed] [Google Scholar]
- 46.Ngan BY, Nourse J, Cleary ML. Detection of chromosomal translocation t(14; 18) within the minor cluster region of bcl-2 by polymerase chain reaction and direct genomic sequencing of the enzymatically amplified DNA in follicular lymphomas. Blood. 1989;73:1759–1762. [PubMed] [Google Scholar]
- 47.Crescenzi M, Seto M, Herzig GP, Weiss PD, Griffith RC, Korsmeyer SJ. Thermostable DNA polymerase chain amplification of t(14; 18) chromosome breakpoints and detection of minimal residual disease. Proc. Natl. Acad. Sci. USA. 1988;85:4869–4873. doi: 10.1073/pnas.85.13.4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chirgwin JM, Przybyla AE, MacDonald RJ, Rutter WJ. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- 50.Deane M, Amlot P, Pappas H, Norton JD. Independent clonal origin of T- and B-cell clones in a composite lymphoma. Leuk. Res. 1991;15:811–817. doi: 10.1016/0145-2126(91)90465-6. [DOI] [PubMed] [Google Scholar]
- 51.Chang B, Casali P. The CDR1 sequences of a major proportion of human germline Ig VH genes are inherently susceptible to amino acid replacement. Immunol. Today. 1994;15:367–373. doi: 10.1016/0167-5699(94)90175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schettino EW, Chai S, Kasaian MT, Schroeder HW, Casali P. VHDJH gene sequence and antigen-reactivity of mAbs produced by human B-1a, B-1b, and B-2 cells. J. Immunol. 1997;158:2477–2489. [PMC free article] [PubMed] [Google Scholar]
- 53.Gaidano G, Ballerini P, Gong JZ, Inghirami G, Neri A, Newcomb EW, Magrath IT, Knowles DM, Dalla-Favera R. p53 mutations in human lymphoid malignancies: association with Burkitt lymphoma and chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA. 1991;88:5413–5417. doi: 10.1073/pnas.88.12.5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chang B, Casali P. A sequence analysis of human germline Ig VH and VL genes. The CDR1 sequences of a major proportion of hu-man germline VH but not VL genes display a high inherent susceptibility to amino acid re-placement. Ann. N.Y. Acad. Sci. 1995;764:171–179. [PubMed] [Google Scholar]