Abstract
Objective
To investigate the in vitro ability of antiphospholipid antibodies (aPL) to bind human tro-phoblast cells and to affect gonadotropin secretion and invasiveness.
Methods
Antiphospholipid antibody IgG from women with recurrent miscarriages, β2-glycoprotein I (β2GPI)-independent IgG aPL human monoclonal antibody (mAb) (519), and IgM anti-β2GPI human mAb (TM1G2) were investigated for their binding to trophoblasts cultured for various amounts of time, their ability to affect invasiveness of Matrigel-coated filters, and their release of human chorionic gonadotropin (hCG).
Results
Polyclonal IgG aPL, as well as mAb 519 and TM1G2, bound to trophoblasts, the highest binding being found when cells displayed the greatest amount of syncytium formation. TM1G2 binding was found to be β2GPI dependent. Both polyclonal and monoclonal aPL, but not the controls, significantly reduced hCG release and Matrigel invasiveness.
Conclusion
These findings suggest that aPL recognition of both anionic PL and adhered β2GPI on trophoblast cell structures might represent a potential pathogenetic mechanism for defective placentation in women with the antiphospholipid syndrome.
Antiphospholipid antibodies (aPL) are associated with poor obstetric outcome, such as recurrent abortions, fetal death, growth retardation, and early preeclampsia (1). Passive transfer of whole immunoglobulin fractions from aPL-positive sera has been found to induce fetal loss and growth retardation in pregnant naive mice, suggesting a direct pathogenetic role (2–4).
Although it has been assumed that aPL are directed against anionic PL, current advances in the field suggest that antibodies to PL-binding plasma proteins, such as β2-glycoprotein I (β2GPI), can be detected in standard aPL assays (5). Antibodies specific for β2GPI have been identified and found to be associated with the clinical manifestations of the antiphospholipid syndrome (APS) (6–22). The in vivo immunohistologic demonstration of β2GPI on trophoblast surfaces (23,24) and the induction of fetal loss by anti-β2GPI antibodies in experimental animal models (25,26) suggested a role of anti-β2GPI antibodies in fetal loss. Moreover, even murine and human aPL monoclonal antibodies (mAb) specifically reacting with anionic PL in the absence of any plasma cofactor have been shown to produce fetal loss, growth retardation, placental deposition, and necrosis in experimental animal models (3,27,28).
Although experimental models have emphasized the role of thrombotic phenomena in placental tissue (4,27), studies in humans have shown that thrombotic events cannot account for all of the histopathologic findings in placentae from women with the APS (29,30). The possibility of direct villous and extravillous trophoblastic damage by aPL through the recognition of phosphatidylserine (PS) exposed during syncytium formation has been suggested (31). Reported direct effects of aPL on trophoblasts have included inhibition of the intercytotrophoblast fusion process (31), of human chorionic gonadotropin (hCG) or placental lactogen secretion (31,32), and/or of trophoblast invasiveness (31). Furthermore, whole IgG fractions from APS patient sera or xenogenic murine anti-PS mAb have been shown to displace annexin V from trophoblasts (and endothelial cell surfaces in the case of human IgG), thus creating conditions favorable to procoagulant state in vitro (31,33).
The purpose of the present study was to investigate the in vitro ability of IgG from sera containing high levels of aPL to bind human trophoblast cells and to affect hCG secretion and invasiveness. Furthermore, to identify whether specific effects were related to individual antibody subpopulations, human mAb reacting with β2GPI or with anionic PL in the absence of any plasma cofactor were investigated for their ability to reproduce the binding to trophoblast cell membranes and the modulation of hormone secretion as well as invasiveness. From our results, it appears that trophoblast cells might represent one target for circulating aPL reacting with β2GPI and/or with “pure” anionic PL (whose binding is independent of any plasma cofactor) and that such antibodies affect trophoblast differentiation–related activities.
PATIENTS AND METHODS
Patients
Two patients with primary APS (34) were studied. Patient 1 had persistently strong positivity for IgG anticardiolipin antibodies (aCL) (>100 GPL), anti-β2GPI antibodies, and lupus anticoagulant (LAC), and had a history of deep venous thrombosis and 2 pregnancies, both of which ended in spontaneous abortion (one in the first trimester and one in the second trimester). Patient 2 had persistently moderate positivity for IgG aCL (>60 GPL) and anti-β2GPI antibodies and had had 3 pregnancies, all of which ended in spontaneous abortion (one abortion during the first trimester, one during the second trimester, and one during the third trimester). Two aPL-positive women, each of whom had had 2 uncomplicated pregnancies, were studied as controls. IgG fractions were purified from sera on protein G–Sepharose (Mab Trap-GII; Pharmacia-Biotech, Uppsala, Sweden) as previously described (35).
Anticardiolipin and anti-PS antibody assay
Anticardiolipin antibodies were detected by solid-phase enzyme-linked immunosorbent assay (ELISA) as previously described (35,36). Briefly, plates were coated with CL (50 µg/ml in ethanol; Sigma-Aldrich, Milan, Italy) by evaporating overnight at 4°C. Plates were then blocked with 10% fetal calf serum (FCS; Sigma-Aldrich)−0.15M phosphate buffered saline (PBS), pH 7.4, for 2 hours, washed 3 times with FCS-PBS, and then incubated with samples for 2 hours. After further washes, 100 µl of alkaline phosphatase-conjugated affinity-purified goat anti-human IgG or IgM (Sigma-Aldrich) was added to the plates and incubated for 1 hour. After washing with FCS–PBS, p-nitrophenylphosphate substrate was used to reveal IgG or IgM binding. Optical density (OD) values of the enzymatic reactions were read at 405 nm with a microplate photometer (Platereader; Bio-Rad, Milan, Italy). Values were expressed as GPL or MPL units, and sera were considered positive when levels were >10 units. Anti-PS activity was evaluated using an ELISA comparable with that described above, with plates coated with PS (50 µg/ml in methanol/chloroform, 3/1 [volume/volume]; Sigma-Aldrich). To evaluate the β2GPI dependence of both whole sera and purified IgG fractions, aPL assays were also performed in the absence of FCS, using gelatin (0.5%; Sigma-Aldrich) in the blocking buffer as previously described (37).
Purification of human β2GPI and anti-β2GPI antibody assay
Human β2GPI was purified from normal human serum (NHS), and anti-β2GPI antibodies were detected by solid-phase assay as previously described (10,24,35,38). Sera were considered positive if OD values were >0.262 for IgG and >0.282 for IgM (3 SD above the mean in 100 healthy controls).
Lupus anticoagulant
The presence of LAC was evaluated using different assays, i.e., standard activated partial thromboplastin time, kaolin clotting time (39), tissue thromboplastin inhibition test (40), and dilute Russell’s viper venom time. These tests were performed according to the guidelines of the Scientific and Standardization Committee of the International Society on Thrombosis and Hemostasis (41).
Human monoclonal antibodies
Two human IgM mAb obtained from hybridized Epstein-Barr virus (EBV)-induced B cell lines from an APS patient were investigated: TM1G2 is an IgM that has been shown to recognize β2GPI both complexed with anionic PL (in CL-coated plates) and alone in γ-irradiated β2GPI-coated plates. TM1B9 is an IgM that does not display any anti-β2GPI reactivity, used as a negative control (42–44).
The human aCL IgG mAb (519) was generated from a hybridized EBV-induced B cell line from a healthy subject; it has been previously characterized as an IgG that binds anionic PL in a β2GPI-independent way (27). Human mAb 57, used as an isotype-matched mAb control, is a previously characterized high-affinity rabies virus-neutralizing IgG1 (45).
Cell cultures
Placentae were obtained from healthy women immediately after uncomplicated vaginal delivery at 36 weeks of gestation. Cytotrophoblast cells were isolated as detailed elsewhere (46). Briefly, placental tissues were rinsed 3 times in cold Dulbecco’s modified Eagle’s medium (DMEM)-10% FCS (Gibco BRL, Grand Island, NY). After mincing, the tissues were submitted to repeated enzymatic digestions in Ringer-bicarbonate buffer containing 0.25 % trypsin (Gibco BRL) and DNase I (Sigma-Aldrich) at 37°C in a shaking water bath. The supernatants were filtered through a 42-µm mesh filter and centrifuged (200g at room temperature for 7 minutes); then the cell suspension was layered over a preformed Percoll (Pharmacia-Biotech) gradient in Hanks’ balanced salt solution (HBSS; Gibco BRL) (46). The gradient was made from 5–70% Percoll (v/v) by dilutions of 90% Percoll (9 parts Percoll, HBSS 10×, 1 part) and layered in a 50-ml conical polystyrene centrifuge tube. After centrifugation (200g at room temperature for 20 minutes), the middle layer was removed, washed, and then resuspended in DMEM. Cell viability was >90% by trypan blue dye exclusion.
The purity of the cell preparation was evaluated by immunohistochemical staining for markers of 1) macrophages (3%, determined using a polyclonal anti–α1-chymotrypsin antibody) (Dako, Santa Barbara, CA), 2) fibroblasts (2%, determined using a polyclonal antivimentin antibody) (Labsystems, Helsinki, Finland), and 3) syncytiotrophoblast (1%, determined using an mAb against low molecular weight cytokeratins) (Labsystem, Chicago, IL). The enriched (95%) cytotrophoblast cells (5 × 105/ml) were cultured in DMEM-10% FCS in 96-well plates at 37°C in 5% CO2/95% air.
Cell cultures were performed for 24, 48, or 72 hours in standard medium. In some experiments, cells grown in standard medium were washed (at 24, 48, or 72 hours) and cultured in serum-free medium for a further 12 hours. Cell number per well was evaluated before and after washing and serum-free medium culture to rule out the possibility of artifacts due to cell detachment. An OD cell-counting method previously described (47) was used; no significant cell loss was found under the different experimental conditions (data not shown).
Cytotrophoblasts at different times of culture were further assayed for the cytoplasmic presence of hCG as a marker for syncytial trophoblasts (46). Briefly, cells were fixed with Bouin’s solution and washed twice with PBS at room temperature, and endogenous peroxidase activity was quenched by 30-minute incubation with 0.3% H2O2. Nonspecific Ig binding was blocked by 20-minute incubation with 5% goat serum in PBS (Dako) and, after further washes with PBS, an anti-hCG murine mAb (Dako) was applied; binding was revealed using the AEC Substrate System (Dako). After washing, the cells were counterstained with hematoxylin.
Binding assays
On day 1 (24 hours), day 2 (48 hours), and day 3 (72 hours) of culture, the medium was removed and the cells incubated at room temperature for 1 hour with serial protein concentrations of the different antibody preparations. Polyclonal IgG (ranging from 1.5 µg/ml to 125 µg/ml) and anti-β2GPI IgM mAb (TM1G2; ranging from 0.6 µg/ml to 50 µg/ml) binding was measured in complete medium at a final volume of 100 µl. Since the binding of the β2GPI-independent aCL IgG mAb (519) to CL-coated plates has been found to be inhibited by the addition of β2GPI (or FCS) (27), serial concentrations of mAb 519 (ranging from 0.6 µg/ml to 50 µg/ml) were incubated with trophoblast cells that had been washed extensively and then cultured in serum-free medium. IgG or IgM binding was evaluated as stated above.
In vitro invasion assay
In preliminary experiments, we found that cultured primary cytotrophoblasts from 36-week placentae displayed a higher invasion index in comparison with cells from term-delivery (40 weeks) placentae. Therefore, for the in vitro invasion assays, we obtained cytotrophoblasts from women in spontaneous labor undergoing vaginal delivery at 36 weeks of gestation. Trophoblast cells were plated on Matrigel invasion chamber inserts containing polyethylene terephthalate membranes with 8 µm-diameter pores in 24-well tissue culture plates. The cells were then labeled for 36 hours with 10 µCi/ml 3H-thymidine (Amersham, Milan, Italy) in DMEM–10% FCS, after which they were trypsinized, washed, and resuspended in complete medium at a concentration of 106/ml. Five hundred-microliter samples of the cell suspensions were added in duplicate to the upper well of transwell chambers, and 750 µl of 3T3 cell medium was added to the lower wells as a chemoattractant (48). In the experiments performed in serum-free medium, fibronectin (5 µg/ml; Sigma-Aldrich) was added to the lower wells. After a 72-hour incubation, media from the upper and lower wells were removed and placed in separate tubes. PBS washes of the upper wells were pooled with the media. Five hundred microliters of a trypsin solution (0.01% in PBS) was added to each lower well, with the transwell in place in order to remove adhered cells.
Trypsin solutions were pooled with the incubation media from the lower wells. The membranes were carefully removed with a small scalpel and placed in separate tubes for determination of radioactivity. Radioactivity of the fractions from each compartment as well as of the Matrigel-coated filters was evaluated with a scintillation counter (Beckman, Fullerton, CA). The invasion index was calculated from the amount of radioactivity in the lower wells, expressed as a percentage of the sum of the radioactivity in all of the compartments plus the membranes. Each experiment was carried out in duplicate with primary human trophoblasts in the presence of 1) medium alone, 2) polyclonal normal or APS IgG (3, 30, 60, or 125 µg/ml), 3) human anti-β2GPI mAb (TM1G2) or control mAb TM1B9 (3, 12, or 50 µg/ml), or 4) human β2GPI-independent aCL IgG mAb 519 or control mAb 57 (3, 12, or 50 µg/ml).
Hormone secretion
Primary trophoblast cells were cultured in complete medium in the presence of polyclonal IgG (ranging from 3 µg/ml to 125 µg/ml), anti-β2GPI (TM1G2), and its negative control (TM1B9; 25 µg/ml). Cultures (25 µg/ml) performed in serum-free medium with washing were used for the human aCL mAb (mAb 519) or the negative control mAb (mAb 57). The medium and the Ig were changed daily from the first day of incubation. After 72-hour culture, the cells were treated for 24 hours with 10−7M gonadotropin-releasing hormone (GnRH) (Lutrepulse; Ferring Arzneimittel, GmbH, Kiel, Germany). At the end of the incubation period, the media were removed and stored at −20°C for hCG determination. The assay was performed with a commercial radioimmunoassay kit (Radim, Rome, Italy). The intra- and interassay coefficients of variation were <12% and <8%, respectively.
Statistical analysis
Statistical analyses were performed using two-way analysis of variance for multiple comparisons.
RESULTS
Anti-PL activity of the IgG fractions
Table 1 shows aCL and anti-β2GPI binding of the patients’ IgG fractions. Assays performed with PS-coated plates showed higher OD values for IgG fractions from patients with the APS in comparison with those displayed by IgG from NHS (OD 2.876 ± 0.231 and 1.321 ± 0.156 for IgG from patient sample 1 at 125 µg/ml and 30 µg/ml, respectively, and 2.242 ± 0.123 and 1.654 ± 0.147 for IgG from patient sample 2 at 125 µg/ml and 30 µg/ml, respectively, versus 0.127 ± 0.031 and 0.167 ± 0.021 for NHS IgG sample 1 and sample 2, respectively, both at 125 µg/ml [mean ± SD of triplicate experiments]). When gelatin-blocked plates were used, both aCL (Table 1) and anti-PS activity (data not shown) decreased to background values.
Table 1.
Levels of aCL in standard and gelatin-modified assays and anti-β2GPI activity of sera and IgG fractions*
| IgG |
|||||
|---|---|---|---|---|---|
| Serum | 0 | 3 µg/ml | 30 µg/ml | 125 µg/ml | |
| APS patient 1 | |||||
| aCL | 108 | 20 | 874 | 2,015 | 1,797 |
| Anti-β2GPI | 1,988 | 14 | 64 | 1,152 | 2,391 |
| aCL modified | 28 | 16 | 25 | 21 | 28 |
| APS patient 2 | |||||
| aCL | 74 | 30 | 547 | 1,455 | 1,980 |
| Anti-β2GPI | 987 | 30 | 180 | 255 | 516 |
| aCL modified | 14 | 16 | 25 | 21 | 28 |
| NHS 1 | |||||
| aCL | 7 | 1 | 4 | 18 | 61 |
| Anti-β2GPI | 184 | 21 | 27 | 90 | 173 |
| aCL modified | 4 | 16 | 18 | 21 | 19 |
| NHS 2 | |||||
| aCL | 5 | 8 | 4 | 18 | 26 |
| Anti-β2GPI | 98 | 10 | 21 | 29 | 41 |
| aCL modified | 6 | 16 | 25 | 21 | 28 |
Anticardiolipin (aCL) values are expressed as GPL units. Anti–β2-glycoprotein I (anti-β2GPI) values are expressed as optical density values (× 10−3) (mean of triplicate experiments; SD was no more than 10% of the mean values). APS = antiphospholipid syndrome; NHS = normal human serum (from women negative for antiphospholipid antibody and with no history of fetal loss).
Primary cytotrophoblast cells
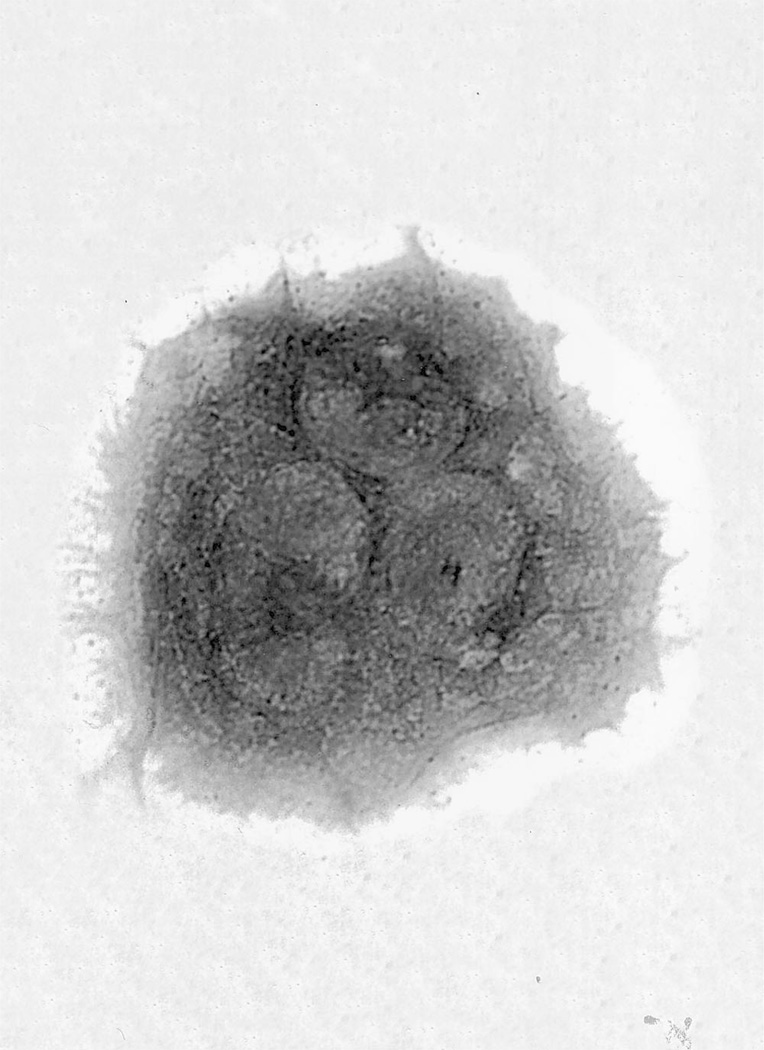
At the beginning of the cell culture, enriched cytotrophoblasts were single mononuclear cells with 2 or 3 aggregates (Figure 1A), as in previous studies (32). After 48 hours (Figure 1B), increasing numbers of syncytia were detectable, and they became the dominant form at 72 hours (Figure 1C). Immunohistologic studies performed with an anti-hCG antiserum revealed cytoplasmic reactivity in syncytial cells (Figure 2).
Figure 1.
Percoll gradient-purified human cytotrophoblasts. Cells were stained after 24 hours (A), 48 hours (B), and 72 hours (C) of culture. (Hematoxylin and eosin stained; original magnification × 400.)
Figure 2.
Immunohistochemical demonstration of human chorionic gonadotropin in Percoll gradient-purified human cytotrophoblasts at 72 hours of culture (original magnification × 600).
Binding of aPL antibodies to trophoblast cells
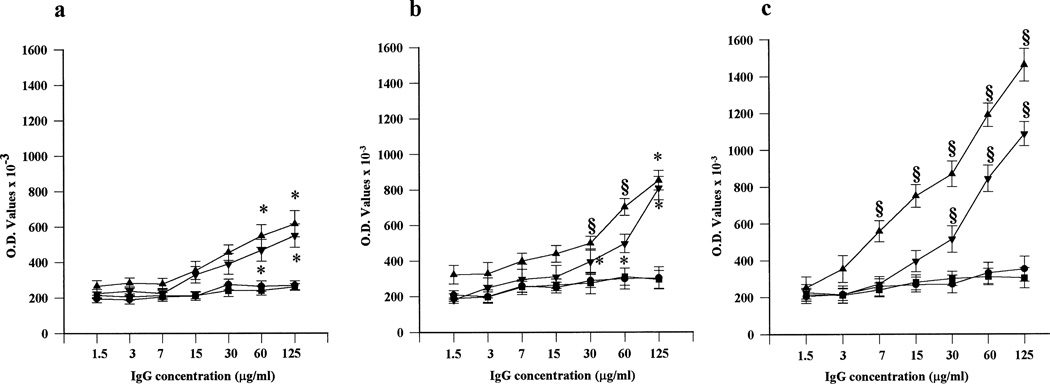
As shown in Figure 3, IgG preparations from APS sera displayed time-dependent trophoblast binding. The highest binding was seen at 72 hours of culture, when the largest differentiation of cytotrophoblasts into syncytiotrophoblasts was found. While APS IgG retained their trophoblast-binding activity with protein concentrations as low as 7–15 µg/ml, control IgG did not display any significant binding at any of the tested concentrations.
Figure 3.
IgG binding to primary cytotrophoblast cells. Serial protein concentrations of IgG from patients with the antiphospholipid syndrome (APS) (▲,▼) or from normal human serum (NHS) (●, ▪) were evaluated after 24 hours (a), 48 hours (b), and 72 hours (c) of culture. Dose- and time-dependent binding was found with APS IgG, but not with NHS IgG. Values are the mean ± SD. * = P < 0.05; § = P < 0.01, APS IgG versus NHS IgG. O.D. = optical density.
To formally demonstrate the relationship between the formation of multinucleated cells and antibody binding, cytotrophoblast cells were incubated for 24, 48, or 72 hours, removed from the plates (by gentle trypsinization and scraping), and counted in a hematocytometer. Parallel cultures were carried out for APS and NHS IgG binding. Table 2 shows that the highest antibody binding was detected in cultures in which the largest numbers of multinucleated cells were found.
Table 2.
Morphologic changes and polyclonal IgG binding of cultured cytotrophoblasts*
| Time of culture |
|||
|---|---|---|---|
| 24 hours | 48 hours | 72 hours | |
| Single cells, %† | 81 ± 2 | 30 ± 2‡ | 10 ± 2‡ |
| Aggregates, %† | 15 ± 3 | 39 ± 5‡ | 9 ± 1 |
| Syncytia, %† | 4 ± 1 | 31 ± 3‡ | 81 ± 2‡ |
| APS patient 1 | |||
| IgG, OD§ | 0.534 ± 15‡ | 0.798 ± 93‡ | 1.205 ± 98‡ |
| APS patient 2 | |||
| IgG, OD§ | 0.081 ± 2 | 0.605 ± 51‡ | 0.858 ± 101‡ |
| NHS IgG, OD§ | 0.101 ± 45 | 0.123 ± 37 | 0.111 ± 67 |
All experiments were performed 3 times on different placentae. Values are the mean ± SD. OD = optical density (see Table 1 for other definitions).
Percentage of 200 counted cells.
P< 0.01 versus 24-hour cultures.
All IgG fractions were tested at a final protein concentration of 60 µg/ml.
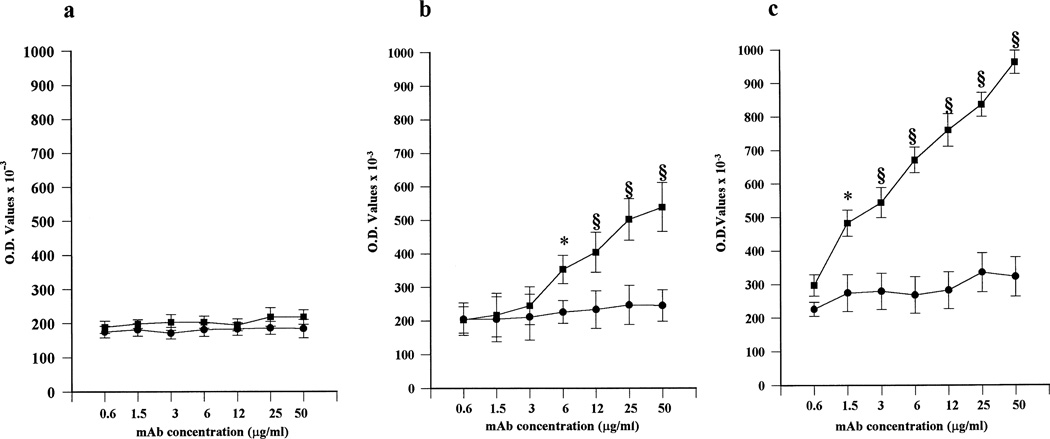
In order to demonstrate a role of β2GPI in aPL trophoblast binding, comparable cultures were performed in the presence of serial concentrations of human anti-β2GPI mAb TM1G2. TM1G2 displayed dose-dependent binding that reached the highest values with cells cultured for 72 hours (Figure 4). In contrast, after 72 hours, trophoblast cells that had been extensively washed and then cultured in serum-free medium to remove adherent β2GPI showed binding values comparable with those found with the irrelevant control TM1B9 (data not shown). Addition of purified human β2GPI (5 µg/ml) restored TM1G2 binding but did not affect TM1B9 binding (OD 0.784 ± 0.117 and 0.201 ± 0.097, respectively, mean ± SD of triplicate experiments). These findings suggest that β2GPI adhering to trophoblast membranes might represent a target antigen for aPL.
Figure 4.
Binding of human anti–β2-glycoprotein I (anti-β2GPI) monoclonal antibody (mAb) to primary cytotrophoblast cells. Serial protein concentrations of anti-β2GPI mAb TM1G2 (▪) or control mAb TM1B9 (●) were evaluated after 24 hours (a), 48 hours (b), and 72 hours (c) of culture. Dose- and time-dependent binding was seen with TM1G2, but not with TM1B9. Values are the mean ± SD. * = P < 0.05; § = P < 0.01, TM1G2 versus TM1B9. O.D. = optical density.
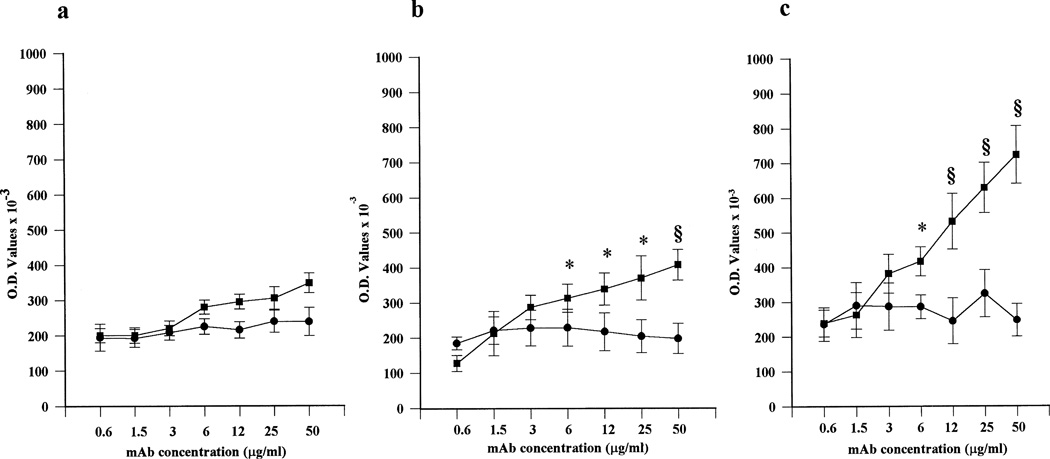
It has been reported that a murine anti-PS IgG mAb is able to recognize anionic PL exposed on the surface of trophoblast cells during syncytiotrophoblast formation (28,31). To evaluate whether comparable reactivity could be displayed by a human IgG mAb that is able to react with anionic PL in a β2GPI-independent manner (27), we incubated trophoblast cells with serial concentrations of mAb 519. Since binding of mAb 519 to CL-coated plates has been shown to be inhibited by the presence of human β2GPI or FCS (27), the experiments were performed with trophoblast cells in serum-free medium in order to avoid any possible interference by bovine serum cofactors. Dose- and time-dependent binding to human trophoblast cells was seen with mAb 519, but not with the irrelevant control (mAb 57). The binding was evident after 48 hours of culture and reached highest levels with cells cultured for 72 hours (Figure 5). Thus, the exposure of anionic PL on trophoblast cell surface can be also recognized by aPL in a β2GPI-independent manner.
Figure 5.
Binding of human IgG anticardiolipin (aCL) mAb to primary cytotrophoblast cells in serum-free medium cultures. Serial protein concentrations of mAb 519 (▪) or control mAb 57 (●) were evaluated after 24 hours (a), 48 hours (b), and 72 hours (c) of culture. Dose- and time-dependent binding was seen with 519, but not with 57. Values are the mean ± SD. * = P < 0.05; § = P < 0.01, 519 versus 57. See Figure 4 for other definitions.
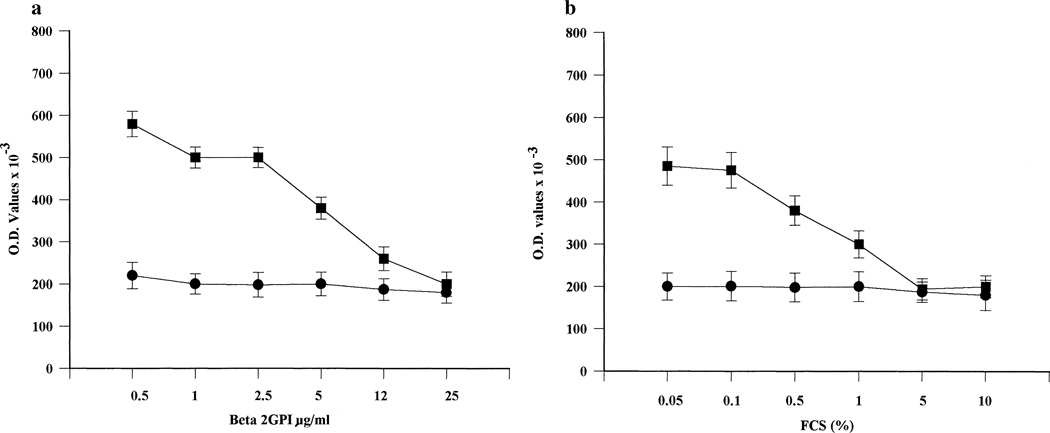
Interestingly, human β2GPI (or other serum cofactors present in whole FCS) was able to make anionic trophoblast PL less accessible for mAb 519 binding. As previously found with CL-coated plates (27), the addition of serial concentrations of human β2GPI or whole FCS inhibited mAb 519 binding to trophoblasts in a dose-dependent manner (Figure 6). In trophoblast cell cultures performed with complete medium, binding of mAb 519 could be detected only after 72 hours and at the highest mAb protein concentrations (mean ± SD OD 0.286 ± 0.012 with 25-µg/ml concentrations and 0.387 ± 0.011 with 50-µg/ml concentrations in complete medium, versus 0.490 ± 0.020 with 25-µg/ml concentrations and 0.714 ± 0.095 with 50-µg/ml concentrations in serum-free medium).
Figure 6.
Binding of human IgG anticardiolipin mAb 519 (▪) or control mAb 57 (●), in the presence of serial concentrations of human β2-glycoprotein I (Beta 2GPI) (a) or fetal calf serum (FCS) (b) to trophoblast cells cultured for 72 hours. Values are the mean ± SD. See Figure 4 for other definitions.
Control experiments performed with human skin fibroblast monolayers (kindly provided by Dr. F. Malavasi, University of Turin, Turin, Italy) cultured in flat-bottomed microtiter plates (2 × 104 cells/well) did not show any binding activity with any of the polyclonal or monoclonal Ig preparations (data not shown).
Effect of aPL on in vitro trophoblast invasivness
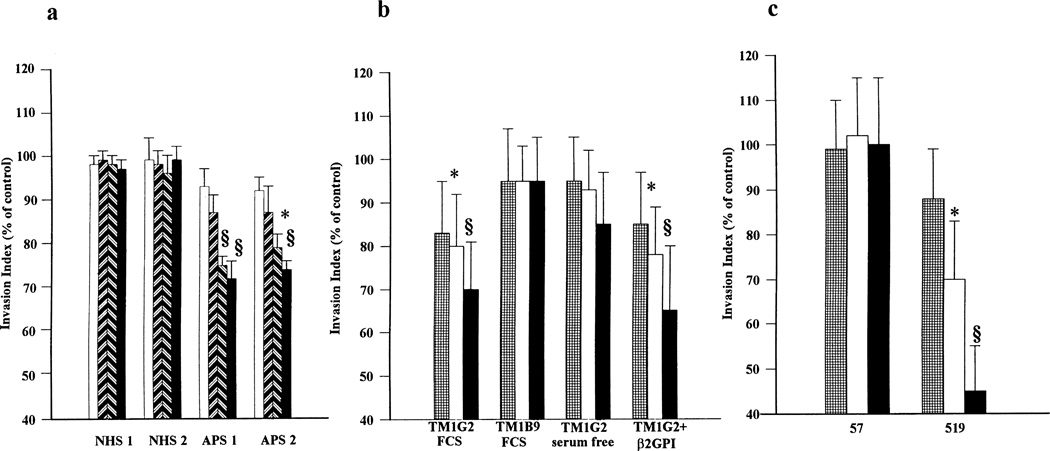
Trophoblast differentiation is characterized by the development of extravillous trophoblasts that migrate into maternal myometrium. Matrigel cultures have been reported to be useful in in vitro assays to evaluate trophoblast invasiveness (48). Incubation with polyclonal IgG from patients with the APS but not with IgG from NHS (60–125 µg/ml) significantly reduced trophoblast cell invasiveness. Figure 7 shows the results of a 3-day in vitro Matrigel invasion assay. Further experiments were performed to evaluate the potential role of an IgM anti-β2GPI mAb on trophoblast invasion. As seen in Figure 7, TM1G2, in contrast to the irrelevant control (TM1B9), significantly reduced trophoblast invasiveness at the highest protein concentrations (12 µg/ml and 50 µg/ml). Comparable experiments carried out in serum-free medium did not demonstrate any effect; however, the addition of exogenous purified human β2GPI (5 µg/ml) restored the mAb-mediated inhibition (Figure 7).
Figure 7.
Matrigel invasion assay in the presence of a, IgG fractions from normal human serum (NHS) or from patients with the antiphospholipid syndrome (APS) at serial protein concentrations ( = 3 µg/ml;
= 3 µg/ml;  = 30 µg/ml;
= 30 µg/ml;  = 60 µg/ml;
= 60 µg/ml;  = 125 µg/ml), b, human anti–β2-glycoprotein I (anti-β2GPI) monoclonal antibody (mAb) at serial protein concentrations (
= 125 µg/ml), b, human anti–β2-glycoprotein I (anti-β2GPI) monoclonal antibody (mAb) at serial protein concentrations ( = 3 µg/ml;
= 3 µg/ml;  = 12 µg/ml;
= 12 µg/ml;  = 50 µg/ml) (the effect of mAb TM1G2 was investigated in cultures performed in medium with and without fetal calf serum [FCS] or in serum-free medium with 5 µg/ml of β2GPI added), and c, human anticardiolipin mAb 519 and control mAb 57 at serial protein concentrations (
= 50 µg/ml) (the effect of mAb TM1G2 was investigated in cultures performed in medium with and without fetal calf serum [FCS] or in serum-free medium with 5 µg/ml of β2GPI added), and c, human anticardiolipin mAb 519 and control mAb 57 at serial protein concentrations ( = 3 µg/ml;
= 3 µg/ml;  = 12 µg/ml;
= 12 µg/ml;  = 50 µg/ml). Values are the mean and SD of triplicate experiments. * = P < 0.05; § = P < 0.01, APS IgG versus NHS IgG, TM1G2 versus TM1B9, and 519 versus 57.
= 50 µg/ml). Values are the mean and SD of triplicate experiments. * = P < 0.05; § = P < 0.01, APS IgG versus NHS IgG, TM1G2 versus TM1B9, and 519 versus 57.
Finally, we investigated aCL mAb 519 in the same in vitro model of trophoblast invasiveness. As shown in Figure 7, under serum-free conditions, the IgG aCL mAb, but not its irrelevant control, was able to significantly impair Matrigel invasion in a dose-dependent manner. In cultures performed in serum containing medium, the effect was detectable to a lesser degree, and only with mAb concentrations of 50 µg/ml (data not shown).
Effect of aPL antibodies on trophoblast hCG secretion
In accordance with our previous data (32), addition of GnRH (10−7M) to trophoblast cells significantly increased hCG secretion. The presence of normal polyclonal IgG (3–125 µg/ml) did not modify basal or GnRH-induced hCG production (Table 3). In contrast, APS IgG (at 30 µg/ml and 125 µg/ml) significantly reduced GnRH-induced hCG stimulation.
Table 3.
Effect of APS and control IgG on basal and GnRH-induced hCG*
| Untreated cells |
GnRH |
|||||
|---|---|---|---|---|---|---|
| + 3 µg/ ml IgG |
+ 30 µg/ ml IgG |
+ 125 µg/ ml IgG |
+ 3 µg/ ml IgG |
+ 30 µg/ ml IgG |
+ 125 µg/ ml IgG |
|
| NHS 1 IgG | 14 ± 3 | 15 ± 3 | 14 ± 3 | 24 ± 2† | 24 ± 4† | 21 ± 5‡ |
| NHS 2 IgG | 12 ± 2 | 14 ± 3 | 14 ± 3 | 22 ± 6† | 23 ± 6† | 23 ± 6‡ |
| APS patient 1 IgG | 14 ± 2 | 13 ± 3 | 15 ± 2 | 20 ± 7† | 16 ± 4‡ | 14 ± 6§ |
| APS patient 2 IgG | 13 ± 4 | 12 ± 3 | 12 ± 4 | 20 ± 3† | 16 ± 4 | 15 ± 3§ |
Values are mIU/ml human chorionic gonadotropin (hCG) (mean ± SD of 5 experiments). Values in untreated cells without IgG and gonadotropin-releasing hormone (GnRH; 107M)-treated cells without IgG were 13 ± 6 mIU/ml and 23 ± 9 mIU/ml, respectively. NHS = normal human serum.
P < 0.02 versus cultures with IgG alone.
Antiphospholipid syndrome (APS) patient IgG (30–125 µg/ml) significantly inhibited GnRH-induced hCG secretion (P < 0.05).
P < 0.02 versus the mean value in GnRH-treated cells without IgG.
Comparable experiments were performed in the presence of the different mAb. Anti-β2GPI mAb TM1G2 reduced GnRH-induced hCG secretion without changing basal hCG levels (Table 4). Anticardiolipin mAb 519 significantly inhibited both basal and GnRH-induced hCG secretion under serum-free conditions (Table 4). In contrast, the respective irrelevant controls (mAb TM1B9 and 57) did not modify hCG secretion. These experiments were performed using different trophoblast tissues obtained from 5 women, with comparable results.
Table 4.
Effects of anti-β2GPI and aCL human monoclonal antibodies (mAb, 25 µg/ml) on basal and GnRH-induced hCG production*
| Anti-β2GPI |
aCL |
|||||
|---|---|---|---|---|---|---|
| Control | mAb TM1G2 |
mAb TM1B9 |
Control (serum-free) |
mAb 519 |
mAb 57 |
|
| Basal | 10 ± 2 | 8.5 ± 2 | 11 ± 3 | 14 ± 3 | 10 ± 2 | 15 ± 5 |
| GnRH, 10−7M | 18 ± 7‡ | 10 ± 2.9† | 23 ± 4 | 25 ± 4‡ | 11 ± 4† | 27 ± 3 |
Values are mIU/ml hCG (mean ± SD of triplicate experiments). Anti-β2GPI = anti–β2-glycoprotein I; aCL = anticardiolipin (see Table 3 for other definitions).
P < 0.05 versus trophoblast cells in control medium in the presence of the irrelevant mAb.
P < 0.02 versus untreated cells.
DISCUSSION
Most of the clinical manifestations of the APS can be related to thrombotic events (1); however, placental thrombosis cannot explain all of the pregnancy complications that occur in women with this syndrome (29,30). In this regard, it has been hypothesized that aPL can directly attack trophoblasts (31,32), but it is still unclear what pathogenetic mechanisms play a role and which aPL subpopulations are involved.
In the present study, we found that IgG fractions from women with APS bind to trophoblasts in vitro. The highest degree of binding was found after 72 hours of culture, when the cells displayed the largest syncytial groups. Since the APS IgG fractions used in the present study displayed clear binding to CL-coated plates (and to β2GPI-coated plates) only in the presence of serum, we can assume that trophoblast binding might be largely related to antibodies reacting with the cofactor present on the trophoblast cell membranes. Our results are consistent with the demonstration that PS is exposed on the external cell surface during intertrophoblastic fusion, with this exposure being maximal after 72 hours of culture (31). Exposure of anionic PS can in turn offer a substrate for cationic β2GPI. The complex of β2GPI and membrane PS might expose new cryptic epitopes or an increased antigen density suitable for anti-β2GPI antibodies (5).
Because of the presence of FCS in the cell culture medium, it is reasonable to assume that bovine β2GPI was available for binding to the cell surfaces, as we previously found in human in vitro endothelial cell cultures (49). The wide cross-reactivity of aPL with β2GPI from different species (35) might explain the binding of anti-β2GPI present in our IgG fractions. In accordance with this, we found that a human IgM anti-β2GPI mAb bound to human trophoblast monolayers only in the presence of exogenous β2GPI. The immunohistochemical demonstration of β2GPI binding to trophoblast cell surfaces in both normal and abnormal placentae (23,24) suggests that aPL reactivity with trophoblasts might occur in vivo through cell membrane-adhered β2GPI.
The notion that anionic PL exposure has a role in trophoblast differentiation was further supported by the binding of a human IgG aCL mAb that recognizes negatively charged PL in the absence of any plasma cofactor. Interestingly, even mAb 519 displayed the highest binding when cells were cultured for periods of time known to allow the maximal exposure of PS. This finding suggests that even β2GPI-independent aPL can react with trophoblasts and supports the idea of the pathogenicity of mAb 519 in passive transfer animal models of aPL-induced fetal loss (27). It is useful to point out that externally exposed anionic PL should be bound in vivo by circulating or tissue PL-binding proteins, such as β2GPI, annexin V, or placental anticoagulant protein I. This binding is supposed to “neutralize” the potentially procoagulant anionic PL and to make it less accessible for anti-PL antibodies (31,50). An in vitro equivalent of such an in vivo phenomenon is mirrored by the partial inhibition of 519 binding to trophoblasts by exogenous β2GPI or FCS. However, we found that with the use of high concentrations of 519, potentially achievable in vivo, the binding could be recovered, suggesting that high levels of “pure” aCL can, at least in part, also overcome the β2GPI-dependent hindrance of anionic PL in vivo (27).
Taken together, these data strongly suggest that trophoblast cell membranes behave as targets for both β2GPI-dependent and β2GPI-independent aPL. Trophoblast differentiation is physiologically characterized by the development of invasive extravillous structures and by hCG secretion. Recently, Rote et al showed that murine anti-PS mAb bound to trophoblast cells and prevented their in vitro invasiveness and hCG secretion (31). We obtained comparable results with spontaneously occurring polyclonal IgG from patients with the APS. The heterogeneity of antigen specificity of such whole IgG fractions makes it difficult to precisely identify the antibody population responsible for modulation of trophoblast function. The fact that human “pure” aCL and anti-β2GPI mAb inhibit trophoblast invasiveness provides new information on the role of these autoantibody subpopulations in APS-associated fetal loss. Interestingly, in studies of the binding of aCL mAb 519 and anti-β2GPI mAb TM1G2, we were also able to reproduce the inhibitory effect on hCG secretion found with the polyclonal IgG fractions from APS patients.
We have confirmed in this study that antigenic structures suitable for β2GPI-independent aPL are present on trophoblasts. In addition, we demonstrated for the first time that trophoblast cells express epitopes that can be recognized by β2GPI-dependent or anti-β2GPI antibodies.
The autoantibody binding and consequent functional modulation might represent key mechanisms by which aPL can affect embryo implantation and pregnancy outcome. These findings are in accordance with the pathogenetic activity of both anti-β2GPI and human mAb 519 in murine passive transfer models of fetal loss in the APS (25,27) and with the reported association between the presence of anti-β2GPI antibodies and fetal loss (8–11,19–22). In addition, defective placentation might be responsible for some of the early fetal losses reported to frequently occur in women with the APS (51). The recent demonstration that Ig aCL and anti-β2GPI mAb affect human choriocarcinoma cell line proliferation in vitro (52) is consistent with our findings. The mechanism(s) by which aPL can affect both trophoblast invasiveness and hCG secretion is not clear, but antibody binding to anionic PL or to adhered β2GPI on trophoblast cell membranes seems to be a necessary prerequisite. In conclusion, our data suggest that both β2GPI-independent aPL and anti-β2GPI-dependent antibodies can bind to human trophoblast cells in vitro and that, once bound, they can affect normal trophoblast implantation and development.
Acknowledgments
Supported in part by Ministero Pubblica Istruzione, Progetti 40% and 60%, 1995 (Dr. Meroni) and NIH grant no. AR-40908 (Dr. Casali).
REFERENCES
- 1.McNeil HP, Chesterman CN, Krilis SA. Immunology and clinical importance of antiphospholipid antibodies. Adv Immunol. 1991;49:193–280. doi: 10.1016/s0065-2776(08)60777-4. [DOI] [PubMed] [Google Scholar]
- 2.Branch D, Dudley DJ, Mitchell MD, Creighton KA, Abbott TM, Dayanes RA. Immunoglobulin G fraction from patients with antiphospholipid antibodies caused fetal death in BALB/c mice: a model for autoimmune fetal loss. Am J Obstet Gynecol. 1990;63:210–216. doi: 10.1016/s0002-9378(11)90700-5. [DOI] [PubMed] [Google Scholar]
- 3.Blank M, Cohen J, Toder V, Shoenfeld Y. Induction of antiphospholipid syndrome in naive mice with mouse lupus monoclonal and human polyclonal anti-cardiolipin antibodies. Proc Natl Acad Sci USA. 1991;88:3069–3073. doi: 10.1073/pnas.88.8.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piona A, La Rosa L, Tincani A, Faden D, Magro G, Grasso S, et al. Placental thrombosis and fetal loss after passive transfer of mouse lupus monoclonal or human polyclonal anti-cardiolipin antibodies in pregnant naive BALB/C mice. Scand J Immunol. 1995;41:427–432. doi: 10.1111/j.1365-3083.1995.tb03588.x. [DOI] [PubMed] [Google Scholar]
- 5.Roubey RA. Autoantibodies to phospholipid-binding plasma proteins: a new view of lupus anticoagulants and other phospholipid antibodies. Blood. 1994;89:2854–2867. [PubMed] [Google Scholar]
- 6.Viard JP, Amoura Z, Bach JF. Association of anti-β2 glycoprotein I antibodies with lupus-type circulating anticoagulant and thrombosis in systemic lupus erythematosus. Am J Med. 1992;93:181–186. doi: 10.1016/0002-9343(92)90049-h. [DOI] [PubMed] [Google Scholar]
- 7.Matsuda J, Saitoh N, Gohchi K, Gotoh M, Tsukamoto M. Detection of β-2-glycoprotein-I-dependent antiphospholipid antibodies and anti-beta-2-glycoprotein-I antibody in patients with systemic lupus erythematosus and in patients with syphilis. Int Arch Allergy Immunol. 1994;103:239–244. doi: 10.1159/000236634. [DOI] [PubMed] [Google Scholar]
- 8.El-Kadi HS, Keil LB, DeBari VA. Analytical and clinical relationships between human IgG autoantibodies to β2 glycoprotein I and anticardiolipin antibodies. J Rheumatol. 1995;22:2233–2237. [PubMed] [Google Scholar]
- 9.Kaburaki J, Kuwana M, Yamamoto M, Matsuura E, Ikeda Y. Clinical significance of phospholipid-dependent anti-β2 glycoprotein I (beta 2-GPI) antibodies in systemic lupus erythematosus. Lupus. 1995;4:472–476. doi: 10.1177/096120339500400609. [DOI] [PubMed] [Google Scholar]
- 10.Balestrieri G, Tincani A, Spatola L, Allegri F, Prati E, Cattaneo R, et al. Anti-β2 glycoprotein I antibodies: a marker of antiphospholipid syndrome? Lupus. 1995;4:122–130. doi: 10.1177/096120339500400208. [DOI] [PubMed] [Google Scholar]
- 11.McNally T, Mackie IJ, Machin SJ, Isenberg DA. Increased levels of β2 glycoprotein-I antigen and beta 2 glycoprotein-I antibodies are associated with a history of thromboembolic complications in patients with SLE and primary antiphospholipid syndrome. Br J Haematol. 1995;34:1031–1036. doi: 10.1093/rheumatology/34.11.1031. [DOI] [PubMed] [Google Scholar]
- 12.Cabiedes J, Cabral AR, Alarcon-Segovia D. Clinical manifestations of the antiphospholipid syndrome in patients with systemic lupus erythematosus associate more strongly with anti-β-2-glycoprotein-I than with antiphospholipid antibodies. J Rheumatol. 1995;22:1899–1906. [PubMed] [Google Scholar]
- 13.Martinuzzo ME, Forastiero RR, Carreras LO. Anti β2 glycoprotein I antibodies: detection and association with thrombosis. Br J Haematol. 1995;89:397–402. doi: 10.1111/j.1365-2141.1995.tb03317.x. [DOI] [PubMed] [Google Scholar]
- 14.Koike T, Matsuura E. Anti-β2-glycoprotein I antibody: specificity and clinical significance. Lupus. 1996;5:378–380. doi: 10.1177/096120339600500508. [DOI] [PubMed] [Google Scholar]
- 15.Amengual O, Atsumi T, Khamashta MA, Koike T, Hughes GR. Specificity of ELISA for antibody to β2-glycoprotein I in patients with antiphospholipid syndrome. Br J Rheumatol. 1997;35:1239–1243. doi: 10.1093/rheumatology/35.12.1239. [DOI] [PubMed] [Google Scholar]
- 16.Sanmarco M, Soler C, Christides C, Raoult D, Weiller PJ, Gerolami V, et al. Prevalence and clinical significance of IgG isotype anti-β2 glycoprotein I antibodies on antiphospholipid syndrome: a comparative study with anticardiolipin antibodies. J Lab Clin Med. 1997;129:499–506. doi: 10.1016/s0022-2143(97)90004-4. [DOI] [PubMed] [Google Scholar]
- 17.Teixido M, Font J, Reverter JC, Cervera R, Tassies D, Ingelmo M, et al. Anti-β2 glycoprotein I antibodies: a useful marker for the antiphospholipid syndrome. Br J Rheumatol. 1997;36:113–116. doi: 10.1093/rheumatology/36.1.113. [DOI] [PubMed] [Google Scholar]
- 18.Swadzba J, De Clerck LS, Stevens WJ, Bridts CH, van Cotthem KA, Musial J, et al. Anticardiolipin, anti-β(2)-glycoprotein I, antiprothrombin antibodies, and lupus anticoagulant in patients with systemic lupus erythematosus with a history of thrombosis. J Rheumatol. 1997;24:1710–1715. [PubMed] [Google Scholar]
- 19.Falcon CR, Martinuzzo ME, Forastiero RR, Cerrato GS, Carreras LO. Pregnancy loss and autoantibodies against phospholipid-binding proteins. Obstet Gynecol. 1997;89:975–980. doi: 10.1016/s0029-7844(97)00115-4. [DOI] [PubMed] [Google Scholar]
- 20.La Rosa L, Covini G, Galperin C, Catelli L, Del Papa N, Reina G, et al. Anti-mitochondrial M5 type antibody represents one of the serological markers for anti-phospholipid syndrome distinct from anti-cardiolipin and anti-β2-glycoprotein I antibodies. Clin Exp Immunol. 1998;112:144–151. doi: 10.1046/j.1365-2249.1998.00562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Day HM, Thiagarajan P, Ahn C, Reveille JD, Tinker KF, Arnett FC. Autoantibodies to β2-glycoprotein I in systemic lupus erythematosus and primary antiphospholipid antibody syndrome: clinical correlations in comparison with other antiphospholipid antibody tests. J Rheumatol. 1998;25:667–674. [PubMed] [Google Scholar]
- 22.Cucurull E, Espinoza LR, Mendez E, Molina JF, Molina J, Ordi-Ros J, et al. Anticardiolipin and anti-β2glycoprotein I antibodies in patients with systemic lupus erythematosus: comparison between Colombians and Spaniards. Lupus. 1999;8:134–141. doi: 10.1191/096120399678847533. [DOI] [PubMed] [Google Scholar]
- 23.McIntyre JA. Immune recognition at the maternal-fetal interface: overview. Am J Reprod Immunol. 1992;28:127–131. doi: 10.1111/j.1600-0897.1992.tb00773.x. [DOI] [PubMed] [Google Scholar]
- 24.La Rosa L, Meroni PL, Tincani A, Balestrieri G, Faden D, Lojacono A, et al. β2 glycoprotein I and placental anticoagulant protein I in placentae from patients with antiphospholipid syndrome. J Rheumatol. 1994;21:1684–1693. [PubMed] [Google Scholar]
- 25.George J, Blank M, Levy Y, Meroni PL, Damianovich M, Tincani A, et al. Differential effects of anti-β2-glycoprotein I antibodies on endothelial cells and on the manifestations of experimental antiphospholipid syndrome. Circulation. 1998;97:900–906. doi: 10.1161/01.cir.97.9.900. [DOI] [PubMed] [Google Scholar]
- 26.Blank M, Faden D, Tincani A, Kopolovic J, Goldberg I, Gilburd B, et al. Immunization with anticardiolipin cofactor (β-2-glycoprotein I) induces experimental antiphospholipid syndrome in naive mice. J Autoimmun. 1994;7:441–455. doi: 10.1006/jaut.1994.1032. [DOI] [PubMed] [Google Scholar]
- 27.Ikematsu W, Luan F-L, La Rosa L, Beltrami B, Nicoletti F, Buyon JP, et al. Human anticardiolipin monoclonal autoantibodies cause placental necrosis and fetal loss in BALB/c mice. Arthritis Rheum. 1998;41:1026–1039. doi: 10.1002/1529-0131(199806)41:6<1026::AID-ART9>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 28.Vogt E, Ng AK, Rote NS. A model for the antiphospholipid antibody syndrome: monoclonal antiphosphatidylserine antibody induces intrauterine growth restriction in mice. Am J Obstet Gynecol. 1996;174:700–707. doi: 10.1016/s0002-9378(96)70453-2. [DOI] [PubMed] [Google Scholar]
- 29.Out HJ, Kooijman CD, Bruinse HW, Derksen RH. Histopatho-logical findings in placentae from patients with intra-uterine fetal death and anti-phospholipid antibodies. Eur J Obstet Gynecol Reprod Biol. 1991;41:179–186. doi: 10.1016/0028-2243(91)90021-c. [DOI] [PubMed] [Google Scholar]
- 30.Salafia CM, Starzyk K, Lopez-Zeno J, Parke A. Fetal losses and other obstetrical manifestations in the antiphospholipid syndrome. In: Asherson RA, Cervera R, JCPiette JC, Shoenfeld Y, editors. The antiphospholipid syndrome. Boca Raton (FL): CRC Press; 1996. pp. 117–131. [Google Scholar]
- 31.Rote NS, Vogt E, DeVere G, Obringer AR, Ng AK. The role of placental trophoblast in the pathophysiology of the antiphospholipid antibody syndrome. Am J Reprod Immunol. 1998;39:125–136. doi: 10.1111/j.1600-0897.1998.tb00344.x. [DOI] [PubMed] [Google Scholar]
- 32.Di Simone N, De Carolis S, Lanzone A, Ronsisvalle E, Giannice R, Caruso A. In vitro effect of antiphospholipid antibody-containing sera on basal and gonadotropin releasing hormone-dependent human chorionic gonadotropin release by cultured trophoblast cells. Placenta. 1995;16:75–83. doi: 10.1016/0143-4004(95)90083-7. [DOI] [PubMed] [Google Scholar]
- 33.Rand JH, Wu X, Andree HAM, Lockwood CJ, Guller S, Scher J, et al. Pregnancy loss in the antiphospholipid-antibody syndrome: a possible thrombogenic mechanism. N Engl J Med. 1997;337:154–160. doi: 10.1056/NEJM199707173370303. [DOI] [PubMed] [Google Scholar]
- 34.Alarcon-Segovia D, Sanchez-Guerrero J. Primary antiphospholipid syndrome. J Rheumatol. 1989;16:482–488. [PubMed] [Google Scholar]
- 35.Tincani A, Spatola L, Prati E, Allegri F, Ferremi P, Cattaneo R, et al. The anti-β2-glycoprotein I activity in human antiphospholipid syndrome sera is due to monoreactive low-affinity autoantibodies directed to epitopes located on native β2-glycoprotein I and preserved during species evolution. J Immunol. 1996;157:5732–5738. [PubMed] [Google Scholar]
- 36.Harris EN. The second international anti-cardiolipin standardization workshop: the Kingston Anti-Phospholipid Antibody Study Group. Am J Clin Pathol. 1990;94:476–484. doi: 10.1093/ajcp/94.4.476. [DOI] [PubMed] [Google Scholar]
- 37.Allegri F, Balestrieri G, Cattaneo R, Martinelli M, Tincani A, Barcellini W, et al. The plasma cofactor and anticardiolipin antibodies. Clin Exp Rheumatol. 1990;8:613–615. [PubMed] [Google Scholar]
- 38.Poltz E, Wurm H, Kostner GM. Investigation on β2-glycoprotein I in the rat: isolation from serum and demonstration in lipoprotein density fraction. J Biochem. 1994;21:1684–1693. doi: 10.1016/0020-711x(80)90229-3. [DOI] [PubMed] [Google Scholar]
- 39.Exner T, Richard KA, Kronenberg HA. A sensitive test demonstrating lupus anticoagulant and its behavioural patterns. Br J Haematol. 1978;40:143–151. doi: 10.1111/j.1365-2141.1978.tb03648.x. [DOI] [PubMed] [Google Scholar]
- 40.Schleider MA, Nachman RL, Jaffe EA, Coleman M. A clinical study of the lupus anticoagulant. Blood. 1976;48:499–509. [PubMed] [Google Scholar]
- 41.Brandt JT, Triplett DA, Alving B, Scharrer I. Criteria for diagnosis of lupus anticoagulants: an update. Thromb Haemost. 1995;74:1185–1190. [PubMed] [Google Scholar]
- 42.Ichikawa K, Khamashta MA, Koike T, Matsuura E, Hughes GRV. β2-glycoprotein I reactivity of monoclonal anticardiolipin antibodies from patients with the antiphospholipid syndrome. Arthritis Rheum. 1994;37:1453–1461. doi: 10.1002/art.1780371008. [DOI] [PubMed] [Google Scholar]
- 43.Roubey RAS, Eisenberg RA, Harper MF, Winfield JB. “Anticardiolipin” autoantibodies recognize β2-glycoprotein I in the absence of phospholipid: importance of antigen density and bivalent binding. J Immunol. 1995;154:954–960. [PubMed] [Google Scholar]
- 44.Del Papa N, Sheng YH, Raschi E, Kandiah DA, Tincani A, Khamashta MA, et al. Human β2-glycoprotein I binds to endothelial cells through a cluster of lysine residues that are critical for anionic phospholipid binding and offers epitopes for anti-β2-glycoprotein I antibodies. J Immunol. 1998;160:5572–5578. [PubMed] [Google Scholar]
- 45.Dietzschold B, Gore M, Casali P, Ueki Y, Rupprecht CE, Notkins AL, et al. Biological characterization of human monoclonal antibodies to rabies virus. J Virol. 1990;64:3087–3090. doi: 10.1128/jvi.64.6.3087-3090.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss JF., III Purification, characterization and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 1986;118:1567–1582. doi: 10.1210/endo-118-4-1567. [DOI] [PubMed] [Google Scholar]
- 47.Ferraro G, Meroni PL, Del Papa N, Barcellini W, Borghi MO, Lazzarin A, et al. In vitro modulation of human endothelial cell growth by Kaposi’s sarcoma sera. J AIDS. 1990;3:677–682. [PubMed] [Google Scholar]
- 48.Graham CH, Connelly I, MacDougall JR, Kerbel RS, Stetler-Stevenson WG, Lala PK. Resistance of malignant trophoblast cells to both the antiproliferative and antiinvasive effects of transforming growth factor-β. Exp Cell Res. 1994;214:93–99. doi: 10.1006/excr.1994.1237. [DOI] [PubMed] [Google Scholar]
- 49.Del Papa N, Guidali L, Sala A, Buccellati C, Khamashta MA, Ichikawa K, et al. Endothelial cells as target for antiphospholipid antibodies: human polyclonal and monoclonal anti-β2-glycoprotein I antibodies react in vitro with endothelial cells through adherent β2-glycoprotein I and induce endothelial activation. Arthritis Rheum. 1997;40:551–561. doi: 10.1002/art.1780400322. [DOI] [PubMed] [Google Scholar]
- 50.Roubey RAS. Immunology of the antiphospholipid antibody syndrome. Arthritis Rheum. 1996;39:1444–1454. doi: 10.1002/art.1780390903. [DOI] [PubMed] [Google Scholar]
- 51.Lakasing L, Poston L. Adverse pregnancy outcome in the antiphospholipid syndrome: focus for future research. Lupus. 1997;6:681–684. doi: 10.1177/096120339700600901. [DOI] [PubMed] [Google Scholar]
- 52.Chamley LM, Duncalf AM, Mitchell MD, Johnson PM. Action of anticardiolipin and antibodies to β2-glycoprotein-I on trophoblast proliferation as a mechanism for fetal death. Lancet. 1998;352:1037–1038. doi: 10.1016/s0140-6736(05)60080-3. [DOI] [PubMed] [Google Scholar]