Abstract
Retinol binding protein 4 (RBP4), is synthesized in liver where it binds vitamin A, retinol, and transports it to tissues throughout the body. It has been shown in some studies that the level of circulating RBP4 increases with body mass, and the protein has been implicated as a mediator in development of insulin resistance and the metabolic disease. Adipose tissue serves as another site of RBP4 synthesis, accounting for its designation as an adipokine. In addition to its function as a transport protein, RBP4 serves as a signaling molecule which, by binding to the membrane receptor STRA6, triggers downstream activation of pro-oncogenic pathways including JAK2/STAT3/5. Taken together, available information suggests the possibility that RBP4 may be a link between obesity and cancer.
Introduction
Vitamin A, retinol, is an essential nutrient obtained from food sources. In the enterocytes, retinol is esterified to retinylesters which are incorporated into chylomicrons and secreted to the circulation to be taken up by the liver. The liver stores the vitamin and provides it to the body in times of dietary vitamin A deficiency. As retinol is a hydrophobic compound, it cannot exit from tissues on its own. Instead, it is secreted from the liver into blood bound to retinol-binding protein 4 (RBP4, encoded for by the RBP4 gene), a soluble 21 kDa polypeptide which contains one binding site for retinol [1]. Thus, Retinol circulates in one of two forms: it is incorporated in chylomicrons, or it is bound to RBP4. While RBP4 can bind other retinoids in vitro, this is not considered physiologically relevant. RBP4 allows retinol to remain in the circulation and provides it to extrahepatic tissues. Indeed, in RBP4-null mice, vitamin A is sequestered in the liver and animals rapidly become deficient if it is not provided in the food [2]. Hence, while chylomicrons-associated retinol can comprise a major fraction of the vitamin in blood following a meal, peripheral tissues solely rely on the RBP4-retinol complex for obtaining retinol during vitamin A deficiency. Notably, RBP4 is secreted from the liver only when complexed with retinol and its blood levels are reduced upon depletion of the vitamin. The nature of the signal that triggers secretion of RBP4-retinol from the liver or whether such a signal exists is currently unknown.
Although the liver comprises the main storage site for retinol and the main site of RBP4 synthesis, the protein can be both synthesized in and secreted from adipose tissue and is consequently referred to as an adipokine. In addition to hepatic and adipose tissue, some other tissues including lung, kidney, testis, brain, and retinal pigment epithelium in the eye also synthesize and presumably secrete RBP4. Overall, while liver is expected to contribute most circulating RBP4, as much as 20% has been estimated to derive from adipose tissue [3]. Further demonstrating the importance of the liver as the major source of circulating RBP4, blood levels of the protein significantly decrease with progressive hepatic fibrosis and cirrhosis [4]. In plasma, retinol-bound RBP4 (holo-RBP4) is associated with another protein, transthyretin (TTR), a 56-kDa protein which, in addition to binding RBP4, functions as a carrier for thyroid hormone [5]. Binding of RBP4 to TTR serves to prevent the loss of the smaller protein from the circulation by filtration in the glomeruli. Thus, as unbound RBP4 does not bind TTR and is therefore filtered by kidney, the predominant fraction of circulating RBP4 is retinol bound. The holo-RBP4:TTR complex circulates at a molar stoichiometry approximating 1:1 but this ratio can change under different physiological conditions. For example, while it has been reported that serum levels of RBP4 are elevated in obese mice [6, 7], plasma levels of TTR do not change under these circumstances [8]. It has been suggested that elevation of serum RBP4 levels in obese animals originates from upregulation of its expression in adipose tissue [7].
RBP4 was discovered to affect insulin sensitivity in a seminal paper published in 2005 by Barbara Kahn and coauthors [7]. This paper was the first to link the retinol carrier with reduced expression of the insulin-responsive glucose transporter, GLUT4, and the first to introduce the notion that adipose tissue-derived RBP4 possesses signaling properties that affects systemic insulin resistance. These authors showed also that injection of recombinant RBP4 into C57BL mice resulted in insulin resistance and glucose intolerance [7]. This investigative team further showed in a follow-up human study that serum levels of RBP4 correlated strongly with not only insulin resistance, obesity, and impaired glucose tolerance, but also with indicators of metabolic syndrome such as waist-to-hip ratio (WHR), body mass index (BMI), systolic blood pressure, and decreased HDL cholesterol [6]. These studies strongly suggest that RBP4 may play a potentially causative role in insulin resistance and its sequela such as metabolic syndrome and type 2 diabetes mellitus. Recent observations also strikingly showed that RBP4 is closely involved in cancer development [9]. RBP4 may thus constitute a potentially important link between obesity, diabetes and cancer and the protein and its associated signaling appear to be promising candidates for clinical intervention
RBP4 in obesity, insulin resistance, and the metabolic syndrome: an epidemiological review
The work by Kahn, et al [7] has generated tremendous interest in the relationship of RBP4 with insulin resistance and metabolic syndrome, a constellation of metabolic abnormalities believed to be driven by insulin resistance. However, data from subsequent epidemiological studies on the association of RBP4 with insulin resistance have not been entirely consistent. While a large number of studies have reported a positive association of circulating levels of RBP4 with various parameters of insulin resistance [10–23], others have not [24–29]. In a cohort of 3,289 Chinese patients aged 50–70 years, the largest of these studies, the authors found a strong and independent association of RBP4 with metabolic syndrome and decreased insulin sensitivity in both men and women [14]. An intervention study in Chinese patients showed that oral Rosiglitazone, a peroxisome proliferator receptor γ (PPARγ) agonist that serves as an insulin sensitizer, markedly decreased the circulating levels of RBP4, corroborating the initial discovery made in rodents [7, 13]. These two studies conducted in a Chinese population, the largest epidemiological investigations reported to date, together with a number of other smaller studies showing a positive association for RBP4, provide supporting evidence for an etiological role of RBP4 in insulin resistance.
Among the studies reporting null results, the largest included 365 male patients with either type 2 diabetes mellitus (DM) or coronary artery disease [25]. Another study reported that administration of pioglitazone, another PPARγ agonist and insulin sensitizer, had no effect on plasma levels of RBP4 although RBP4 gene expression in adipose and muscle tissues were increased [27]. It is speculated that part of the discrepancies among studies may be due to differences in the ELISA method to determine RBP4 levels across studies [30]. The original assays that linked RBP4 to insulin resistance were designed to detect very small amounts of RBP4, but the reagents have changed throughout the course of the following years [31]. In addition, variations in age of the sample populations may have contributed to discrepancies as one study noted marked differences in the association of RBP4 with insulin resistance between younger and older patients [26].
For various parameters of metabolic syndrome, RBP4 has been consistently and positively associated with low density lipoproteins (LDL) [14, 25, 26, 32], triglycerides [13, 14, 26, 33, 34], total cholesterol [14, 25, 33, 35], and blood pressure [14, 33, 34]. Similarly, RBP4 was also observed to have a consistent and inverse association with high density lipoproteins (HDL) [14, 32–34]. Only one study observed contrary results with no relationship between RBP4 and blood pressure, cholesterol, or triglycerides [29].
Similarly to the relationship of RBP4 with insulin resistance, the epidemiological assessments of the correlation of serum and plasma levels of the protein with obesity varied. While the first investigation of RBP4 and obesity in human subjects noted a strong correlation with BMI and WHR, subsequent findings were less consistent depending on the specific measures of adiposity used [31]. Among the findings that blood levels of RBP4 were related with obesity, strong correlations were observed separately with BMI and waist circumference, visceral fat opposed to subcutaneous fat, liver fat, and various inflammatory markers related to obesity [10, 13, 14, 16, 22, 27, 31, 32, 36], although not in all studies [12, 24, 37, 38]. Again, the largest of these studies was performed in a Chinese cohort and identified independent associations with BMI and waist circumference [14].
A few studies were able to evaluate levels of RBP4 before and after weight loss interventions, such as gastric bypass surgery, physical activity intervention, and dietary intervention [10, 16, 33, 36, 37, 39–42]. All except one study evaluating physical activity intervention in a sample of Korean women noted marked decreases in levels of RBP4 after a variety of obesity interventions [39]. Of the several measures of obesity, one of the more consistent associations was between circulating RBP4 and visceral adipose tissue [32, 36, 42]. A few of these investigations were able to evaluate RBP4 gene expression in patient’s visceral versus subcutaneous fat yet there was no clear consensus as to preferential expression [24, 43].
Similar to epidemiological studies relating RBP4 with insulin resistance and metabolic syndrome, the majority of evidence is convincing for an association between RBP4 and obesity. Given the consistent evidence, circulating RBP4 appears to be related to central adiposity, particularly visceral adipose tissue. Though the two may be correlated, results of preferential RBP4 gene expression in adipose tissue were inconclusive and the mechanism through which excess weight leads to elevation of circulating RBP4 levels remain to be elucidated.
Circulating RBP4 and neoplasia
Energy imbalance and, in particular, insulin resistance and obesity have been identified as major risk factors for several cancers [44–46]. The potential role of RBP4 in obesity and insulin resistance raise the intriguing possibility that a positive relationship between serum levels of RBP4 and risk of cancer exists. To-date, only a few studies have investigated such a possibility [47–51]. Of these, three have been reports of large scale proteomic analysis [49–51] and two have evaluated its potential as a cancer biomarker [47, 48]. Results showed that increased sera levels of RBP4 were found in pancreatic cancer patients, compared to controls [48].
We have recently shown, in a cohort of subjects with average-risk, scheduled for screening colonoscopy, that high circulating levels of RBP4 are associated with increased risk of colon adenomas, a precursor lesion of colorectal cancer. Furthermore, we found that the positive association was limited to ‘lean’ subjects (with BMI lower than the cohort median < 27 kg/m2) with over 2-fold increase of risk among those in the top tertile of RBP4 as compared to those in the lowest tertile of RBP4 [47]. In sharp contrast, no association was found for those with higher BMI (≥ cohort median 27 kg/m2). As shown in Table 1, including or excluding participants with DM did not alter the results. To our knowledge, this is the first epidemiological study to show an association of RBP4 with risk of neoplasia. The underlying mechanisms by which RBP4 may promote carcinogenesis is a priority area of investigation given the well-established etiologic role of obesity and insulin resistance in various cancers, including colorectal and breast cancers [52, 53], and the emerging role of RBP4 in the development of insulin resistance.
Table 1.
Odds ratio for colon adenoma by RBP4 tertiles
| BMI < 27.8 | BMI ≥ 27.8 | |||||||
|---|---|---|---|---|---|---|---|---|
| 1st Tertile | 2nd Tertile | 3rd Tertile | p(trend) | 1st Tertile | 2nd Tertile | 3rd Tertile | p(trend) | |
| Including DM* | 1 | 1.84 | 2.14 | 0.03 | 1 | 1.16 | 1.01 | 0.99 |
| OR (95% CI) | (ref) | (0.91 3.72) | (1.04 4.11) | (ref) | (0.64–2.21) | (0.53–1.97) | ||
| Excluding DM* | 1 | 2.16 | 2.47 | 0.03 | 1 | 1.01 | 0.96 | 0.91 |
| OR (95% CI) | (ref) | (0.97–4.81) | (1.12–5.29) | (ref) | (0.50–2.07) | (0.46–2.02) | ||
Covariates included: age, race, gender, BMI, family history of colorectal cancer, packyears, non-steroidal anti-inflammatory drug (NSAID) use
RBP4 initiates cell signaling by activating STRA6, a cognate cytokine receptor
Retinol can enter cells from circulating holo-RBP4 by two distinct mechanisms. Due to its lipophilic nature, it can readily diffuse through the plasma membrane [54–57]. At some tissues, retinol is also internalized by a plasma membrane protein termed STRA6 which binds extracellular holo-RBP4, and mediates uptake of the vitamin into cells while leaving RBP4 in the circulation [58]. In the adult, STRA6 is expressed in the blood-brain barrier, retinal pigment epithelium (RPE) in the eye, brain, spleen, kidney, testis, female genital tract and adipose tissue [59, 60]. Interestingly, it has been reported that STRA6 is upregulated in multiple human cancers including Wilm’s kidney tumors, melanomas, and breast, colorectal, ovarian, and endometrial cancers [59]. The functional significance of upregulation of STRA6 in carcinoma cells is unknown. Interestingly, although vitamin A is critical for embryonic development, vision, reproduction and post-natal life [61, 62], characterization of STRA6-null mice showed that, with the exception of RPE cells, ablation of Stra6 has little effect on the retinoid content of tissues and does not disrupt physiological functions that critically depend on the vitamin A metabolite retinoic acid either in the embryo or in the adult, even under conditions of vitamin A deficiency [63–65]. Hence, the major fraction of vitamin enters cells by free diffusion through the plasma membranes and, although STRA6 partially contributes to retinol uptake by cells, it does not appear to be mandatory for vitamin A availability in tissues other than the eye. These observations suggest that STRA6 may have biological function(s) other than serving as a retinol transporter.
Our recent studies revealed that STRA6 functions as surface signaling receptor [8, 64, 66–69]. The cytosolic tail of STRA6 contains the amino acid sequence tyr-thr-leu-leu, recognizable as a phosphotyrosine motif. Such a motif is often used by cytokine signaling receptors that recruit and activate the tyrosine kinases Janus kinases (JAKs), and the transcription factors STATs. Binding of extracellular cognate ligands to cytokine receptors results in activation of JAKs which, in turn, phosphorylate STATs. Activated STATs translocate to the nucleus where they induce expression of specific target genes. JAK/STAT pathways thus regulate gene expression in response to more than 30 cytokines, hormones, and growth factors [70]. Our studies showed that transport of retinol from holo-RBP4 across STRA6 results in recruitment and activation of JAK2. The exact mechanism by which STRA6-mediated retinol transport activates JAK2 is unknown at present, but, by analogy to gp130 activation, may involve JAK phosphorylation [71]. Following its activation, JAK2 catalyzes the phosphorylation the STRA6, leading, in a cell-specific manner, to recruitment and activation of STAT3 or STAT5.
These observations establish that RBP4 functions as a classical cytokine to initiate cell signaling transduced by its cognate receptor STRA6. The two functions of STRA6 are critically interdependent, i. e. receptor activation requires retinol transport, and the transport does not occur if STRA6 phosphorylation is impaired [68]. Both retinol transport and cell signaling by STRA6 also rely on the presence of two intracellular proteins: cellular retinol-binding protein 1 (CRBP1), and the retinol metabolizing enzyme lecithin:retinol acyl transferase (LRAT) [68, 69]. CRBP1 associates with an intracellular region of STRA6 where it serves as a direct acceptor for retinol. Upon its ligation, CRBP1 dissociates from the receptor and delivers retinol to LRAT which catalyzes its esterification to the storage form retinylesters. LRAT thus maintains an inward-directed retinol concentration gradient, enabling continuing transport and signaling. In addition to STRA6-associated CRBP1, a major fraction of this binding protein exists in a cytosolic pool which binds retinol that enters cells by diffusion through the plasma membrane. However, retinol that enters the cell by the later mechanism neither activates STRA6 nor triggers cell signaling [66].
STRA6 thus couples ‘sensing’ serum levels of holo-RBP and intracellular retinol transport and metabolism to cell signaling (Fig. 1). Importantly, we found that TTR, the binding partner of holo-RBP4 in blood, effectively competes with STRA6 for RBP4 and thus blocks both retinol uptake and cell signaling mediated by the receptor [8]. Consequently, STRA6 functions only under conditions where serum level of RBP4 exceeds that of TTR, e.g. in obese animals [8], or in tissues that express very high levels of STRA6, such as the eye [63, 64].
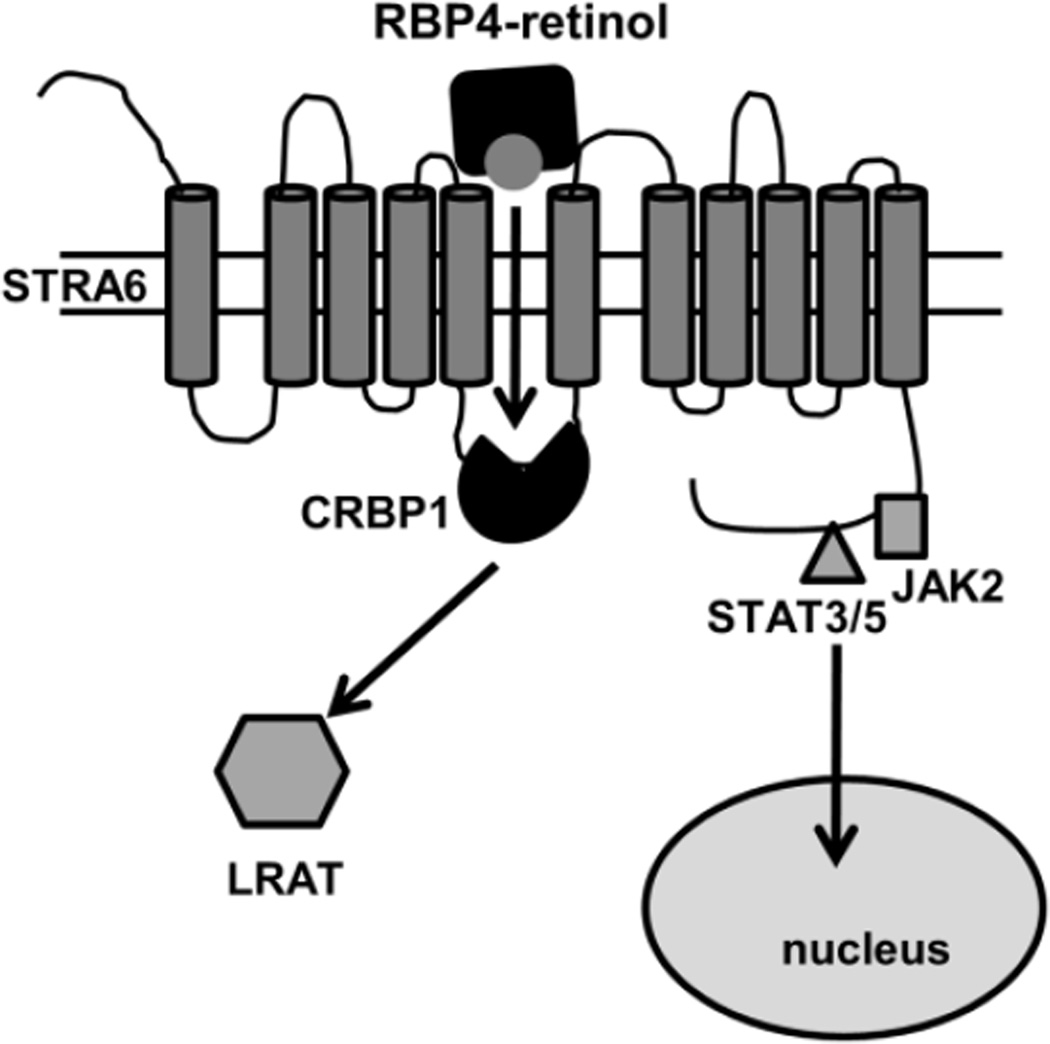
Figure 1. The RBP4/STRA6 pathway.
STRA6 binds extracellular holo-RBP4 and mediates transport of retinol to receptor-associated cellular retinol-binding protein 1 (CRBP1). Retinol transfer triggers phosphorylation of STRA6, resulting in recruitment and activation of JAK2 and its associated transcription factors STAT3/STAT5. Activated STAT translocates to the nucleus where it induces target gene expression. Upon binding retinol, CRBP1 dissociates from STRA6 and delivers retinol to lecithin:retinol acyl transferase (LRAT) which catalyzes the formation of retinylesters. STRA6 (GeneID 64220RBP) was drawn in accordance with prediction of a model generated by the software http://bp.nuap.nagoya-u.ac.jp/sosui.
RBP4/STRA6 signaling in regulation of insulin responses and lipid homeostasis
The discovery that activation of STRA6 by holo-RBP triggers JAK2/STAT3/5 signaling provides a clear rationale for understanding how elevated serum levels of RBP4 induce insulin resistance. A well-established STAT target gene in adipose tissue and muscle is Socs3, a member of the Suppressors of Cytokine Signaling (Socs) group of genes which encode for negative regulators of cytokine receptors [72, 73]. SOCS3 efficiently inhibits the activity of several cytokine receptors and is a potent suppressor of insulin receptor (IR) signaling [74, 75]. It has been demonstrated that holo-RBP4 inhibits IR activation and blocks insulin-triggered mobilization of the insulin responsive glucose transporter Glut4 to the plasma membranes and that this activity is mediated by SOCS3 whose expression is upregulated following activation of the holo-RBP4/STRA6 pathway [64, 66]. Further attesting to the central role of the RBP4/STRA6 pathway in suppressing insulin signaling, STRA6-null mice were found to be protected from insulin resistance brought about by ectopic administration of RBP4, as well as from insulin resistance brought about by feeding a high fat diet [64]. Moreover, even partial reduction of STRA6 expression restricted to adipocytes led to improved insulin sensitivity in obese mice [76].
Another STAT target gene in adipocytes is PPARγ, a key regulator of adipogenesis and of lipid homeostasis in adipocytes [77]. In accordance with upregulation of this nuclear receptor, holo-RBP4 treatment increases triglyceride accumulation, an activity that critically depended on STRA6 and its associated signaling [66].
RBP4/STRA6 signaling in cancer
Expression of STRA6 as well as RBP4 is upregulated in various human cancers, including colorectal and breast cancers [9, 59]. The discovery that STRA6 signaling triggered by holo-RBP4 activates a JAK2/STAT3/5 cascade provides a clue to the functional significance of the increased expression of these proteins in tumors. These STATs, considered to be oncogenes, are associated with inflammation, oncogenic transformation, survival, proliferation, invasion, and angiogenesis [78–81]. Tumor-promoting STAT target genes include the cell cycle regulators cyclin D1 and cyclin D3, the oncogene c-Myc, the growth factor VEGF, genes involved in migration and invasion such as MMP-9, and anti-apoptotic genes including survivin, Mcl-1, and Bcl-XL [79, 82]. Hence, an intriguing possibility is that STRA6 and its associated machinery are involved in oncogenic activities. In support of this notion, it has been shown that RBP4 and STRA6 promote oncogenic properties in cultured mammary and colon carcinoma cells where they induce various oncogenic hallmarks including cell proliferation, migration, invasion, and ability to form colonies in soft agar. STRA6 and RBP4 were also found to induce oncogenic transformation of fibroblasts [9] and to potently promote tumorigenesis by colon cancer cells in xenograft mouse models [9].
As discussed above, a large body of epidemiological studies indicates that obesity is a risk factor for multiple types of cancers [80–82]. The association is particularly strong with colorectal cancer where convincing evidence identified obesity to be a cause for the disease [52]. Breast and colorectal cancer are also associated with insulin resistance. For example, high levels of fasting insulin have been associated with a 2–3-fold increase in risk of mortality from breast cancer [83]. The observations that the RBP4/STRA6 pathway is highly oncogenic and that serum RBP4 levels are elevated in obese animals suggest that this path comprises an important mediator through which excess weight promotes cancer development, and may serve as a useful target for disrupting the obesity-cancer linkage.
Acknowledgement
This work was supported by NIH Grants RO1DK088969 to NN; RO1CA136726, UO1CA181770 to LL; and Case Center for Transdisciplinary Research on Energetics and Cancer, UA54CA116867, and Case GI SPORE P50CA50964 to LL and NAB.
Contributor Information
Noa Noy, Email: nxn51@case.edu/noyn@ccf.org, Department of Cellular and Molecular Medicine, Lerner Research Institute, Cleveland Clinic Foundation, Case Comprehensive Cancer Center, 9500 Euclid Avenue/NC10, Cleveland OH 44195, Tel: 216-444-8423.
Li Li, Email: lxl62@case.edu, Department of Family Medicine and Community Health, Case Western Reserve University, Case Comprehensive Cancer Center, 11000 Cedar Avenue, Suite 402, Cleveland OH 44106-7136, Tel: 216-368-5437.
Matthew V. Abola, Email: mva9@case.edu, Department of Family Medicine and Community Health, Research Division, Case Western Reserve University, 11000 Cedar Avenue, Suite 200, Cleveland OH 44106-7136, Tel: 216-286-4923.
Nathan A. Berger, Email: nab@case.edu, Departments of Medicine, Biochemistry and Genetics, Center for Science Health and Society, Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, 10900 Euclid Avenue, Cleveland OH 44106-4971, Tel: 216-368-4084.
References
- 1.Noy N. Retinoid-binding proteins: mediators of retinoid action. Biochem J. 2000;348(Pt 3):481–495. [PMC free article] [PubMed] [Google Scholar]
- 2.Quadro L, Blaner WS, Salchow DJ, Vogel S, Piantedosi R, Gouras P, Freeman S, Cosma MP, Colantuoni V, Gottesman ME. Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein. Embo J. 1999;18(17):4633–4644. doi: 10.1093/emboj/18.17.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsutsumi C, Okuno M, Tannous L, Piantedosi R, Allan M, Goodman DS, Blaner WS. Retinoids and retinoid-binding protein expression in rat adipocytes. J Biol Chem. 1992;267(3):1805–1810. [PubMed] [Google Scholar]
- 4.Yagmur E, Trautwein C, Leers MP, Gressner AM, Tacke F. Elevated apoptosis-associated cytokeratin 18 fragments (CK18Asp386) in serum of patients with chronic liver diseases indicate hepatic and biliary inflammation. Clin Biochem. 2007;40(9–10):651–655. doi: 10.1016/j.clinbiochem.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 5.Raz A, Goodman DS. The interaction of thyroxine with human plasma prealbumin and with the prealbumin-retinol-binding protein complex. J Biol Chem. 1969;244(12):3230–3237. [PubMed] [Google Scholar]
- 6.Graham TE, Yang Q, Bluher M, Hammarstedt A, Ciaraldi TP, Henry RR, Wason CJ, Oberbach A, Jansson PA, Smith U, Kahn BB. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med. 2006;354(24):2552–2563. doi: 10.1056/NEJMoa054862. [DOI] [PubMed] [Google Scholar]
- 7.Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436(7049):356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 8.Berry DC, Croniger CM, Ghyselinck NB, Noy N. Transthyretin blocks retinol uptake and cell signalling by the holo-retinol-binding protein receptor STRA6. Mol Cell Biol. 2012;32(19):3851–3859. doi: 10.1128/MCB.00775-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berry DC, Levi L, Noy N. Holo-retinol-binding protein and its receptor STRA6 drive oncogenic transformation. Cancer Res. 2014;74(21):6341–6351. doi: 10.1158/0008-5472.CAN-14-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balagopal P, Graham TE, Kahn BB, Altomare A, Funanage V, George D. Reduction of Elevated Serum Retinol Binding Protein in Obese Children by Lifestyle Intervention: Association with Subclinical Inflammation. The Journal of Clinical Endocrinology & Metabolism. 2007;92(5):1971–1974. doi: 10.1210/jc.2006-2712. [DOI] [PubMed] [Google Scholar]
- 11.Cho YM, Youn B-S, Lee H, Lee N, Min S-S, Kwak SH, Lee HK, Park KS. Plasma retinol-binding protein-4 concentrations are elevated in human subjects with impaired glucose tolerance and type 2 diabetes. Diabetes Care. 2006;29(11):2457–2461. doi: 10.2337/dc06-0360. [DOI] [PubMed] [Google Scholar]
- 12.Gavi S, Stuart LM, Kelly P, Melendez MM, Mynarcik DC, Gelato MC, McNurlan MA. Retinol-binding protein 4 is associated with insulin resistance and body fat distribution in nonobese subjects without type 2 diabetes. The Journal of Clinical Endocrinology and Metabolism. 2007;92(5):1886–1890. doi: 10.1210/jc.2006-1815. [DOI] [PubMed] [Google Scholar]
- 13.Jia W, Wu H, Bao Y, Wang C, Lu J, Zhu J, Xiang K. Association of serum retinol-binding protein 4 and visceral adiposity in Chinese subjects with and without type 2 diabetes. The Journal of Clinical Endocrinology and Metabolism. 2007;92(8):3224–3229. doi: 10.1210/jc.2007-0209. [DOI] [PubMed] [Google Scholar]
- 14.Qi Q, Yu Z, Ye X, Zhao F, Huang P, Hu FB, Franco OH, Wang J, Li H, Liu Y, Lin X. Elevated Retinol-Binding Protein 4 Levels Are Associated with Metabolic Syndrome in Chinese People. The Journal of Clinical Endocrinology & Metabolism. 2007;92(12):4827–4834. doi: 10.1210/jc.2007-1219. [DOI] [PubMed] [Google Scholar]
- 15.Abetew DF, Qiu C, Fida NG, Dishi M, Hevner K, Williams MA, Enquobahrie DA. Association of retinol binding protein 4 with risk of gestational diabetes. Diabetes Research and Clinical Practice. 2013;99(1):48–53. doi: 10.1016/j.diabres.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haider DG, Schindler K, Prager G, Bohdjalian A, Luger A, Wolzt M, Ludvik B. Serum retinol-binding protein 4 is reduced after weight loss in morbidly obese subjects. The Journal of Clinical Endocrinology and Metabolism. 2007;92(3):1168–1171. doi: 10.1210/jc.2006-1839. [DOI] [PubMed] [Google Scholar]
- 17.Kowalska I, Strączkowski M, Adamska A, Nikolajuk A, Karczewska-Kupczewska M, Otziomek E, Górska M. Serum Retinol Binding Protein 4 Is Related to Insulin Resistance and Nonoxidative Glucose Metabolism in Lean and Obese Women with Normal Glucose Tolerance. The Journal of Clinical Endocrinology & Metabolism. 2008;93(7):2786–2789. doi: 10.1210/jc.2008-0077. [DOI] [PubMed] [Google Scholar]
- 18.Li L, Wang C, Bao Y, Wu H, Lu J, Xiang K, Jia W. Serum retinol-binding protein 4 is associated with insulin secretion in Chinese people with normal glucose tolerance. Journal of Diabetes. 2009;1(2):125–130. doi: 10.1111/j.1753-0407.2009.00024.x. [DOI] [PubMed] [Google Scholar]
- 19.Mills JP, Furr HC, Tanumihardjo SA. Retinol to retinol-binding protein (RBP) is low in obese adults due to elevated apo-RBP. Experimental Biology and Medicine (Maywood, N.J.) 2008;233(10):1255–1261. doi: 10.3181/0803-RM-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reinehr T, Stoffel-Wagner B, Roth CL. Retinol-Binding Protein 4 and Its Relation to Insulin Resistance in Obese Children before and after Weight Loss. The Journal of Clinical Endocrinology & Metabolism. 2008;93(6):2287–2293. doi: 10.1210/jc.2007-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suh J-B, Kim S-M, Cho G-J, Choi K-M, Han J-H, Taek Geun H. Elevated serum retinol-binding protein 4 is associated with insulin resistance in older women. Metabolism: Clinical and Experimental. 2010;59(1):118–122. doi: 10.1016/j.metabol.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 22.Stefan N, Hennige AM, Staiger H, Machann J, Schick F, Schleicher E, Fritsche A, Häring H-U. High circulating retinol-binding protein 4 is associated with elevated liver fat but not with total, subcutaneous, visceral, or intramyocellular fat in humans. Diabetes Care. 2007;30(5):1173–1178. doi: 10.2337/dc06-2342. [DOI] [PubMed] [Google Scholar]
- 23.Sun L, Qi Q, Zong G, Ye X, Li H, Liu X, Zheng H, Hu FB, Liu Y, Lin X. Elevated plasma retinol-binding protein 4 is associated with increased risk of type 2 diabetes in middle-aged and elderly Chinese adults. The Journal of Nutrition. 2014;144(5):722–728. doi: 10.3945/jn.113.189860. [DOI] [PubMed] [Google Scholar]
- 24.Bajzová M, Kováciková M, Vítková M, Klimcáková E, Polák J, Kovácová Z, Viguerie N, Vedral T, Mikulásek L, Srámková P, Srp A, Hejnová J, Langin D, Stich V. Retinol-binding protein 4 expression in visceral and subcutaneous fat in human obesity. Physiological Research/Academia Scientiarum Bohemoslovaca. 2008;57(6):927–934. doi: 10.33549/physiolres.931379. [DOI] [PubMed] [Google Scholar]
- 25.Eynatten Mv, Lepper PM, Liu D, Lang K, Baumann M, Nawroth PP, Bierhaus A, Dugi KA, Heemann U, Allolio B, Humpert PM. Retinol-binding protein 4 is associated with components of the metabolic syndrome, but not with insulin resistance, in men with type 2 diabetes or coronary artery disease. Diabetologia. 2007;50(9):1930–1937. doi: 10.1007/s00125-007-0743-8. [DOI] [PubMed] [Google Scholar]
- 26.Gavi S, Qurashi S, Stuart LM, Lau R, Melendez MM, Mynarcik DC, McNurlan MA, Gelato MC. Influence of age on the association of retinol-binding protein 4 with metabolic syndrome. Obesity (Silver Spring, Md.) 2008;16(4):893–895. doi: 10.1038/oby.2007.138. [DOI] [PubMed] [Google Scholar]
- 27.Yao-Borengasser A, Varma V, Bodles AM, Rasouli N, Phanavanh B, Lee M-J, Starks T, Kern LM, Spencer HJ, Rashidi AA, McGehee RE, Fried SK, Kern PA. Retinol Binding Protein 4 Expression in Humans: Relationship to Insulin Resistance, Inflammation, and Response to Pioglitazone. The Journal of Clinical Endocrinology & Metabolism. 2007;92(7):2590–2597. doi: 10.1210/jc.2006-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henze A, Frey SK, Raila J, Tepel M, Scholze A, Pfeiffer AFH, Weickert MO, Spranger J, Schweigert FJ. Evidence That Kidney Function but Not Type 2 Diabetes Determines Retinol-Binding Protein 4 Serum Levels. Diabetes. 2008;57(12):3323–3326. doi: 10.2337/db08-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ülgen F, Herder C, Kühn MC, Willenberg HS, Schott M, Scherbaum WA, Schinner S. Association of serum levels of retinol-binding protein 4 with male sex but not with insulin resistance in obese patients. Archives of Physiology and Biochemistry. 2010;116(2):57–62. doi: 10.3109/13813451003631421. [DOI] [PubMed] [Google Scholar]
- 30.Graham TE, Wason CJ, Bluher M, Kahn BB. Shortcomings in methodology complicate measurements of serum retinol binding protein (RBP4) in insulin-resistant human subjects. Diabetologia. 2007;50(4):814–823. doi: 10.1007/s00125-006-0557-0. [DOI] [PubMed] [Google Scholar]
- 31.Graham TE, Smith U, Kahn BB. Retinol-Binding Protein 4 and Insulin Resistance Correspondence. New England Journal of Medicine. 2006;355(13):1392–1395. doi: 10.1056/NEJMc061863. [DOI] [PubMed] [Google Scholar]
- 32.Lee J-W, Im J-A, Lee H-R, Shim J-Y, Youn B-S, Lee D-C. Visceral adiposity is associated with serum retinol binding protein-4 levels in healthy women. Obesity (Silver Spring, Md.) 2007;15(9):2225–2232. doi: 10.1038/oby.2007.264. [DOI] [PubMed] [Google Scholar]
- 33.Broch M, Gómez JM, Auguet MT, Vilarrasa N, Pastor R, Elio I, Olona M, García-España A, Richart C. Association of Retinol-Binding Protein-4 (RBP4) with Lipid Parameters in Obese Women. Obesity Surgery. 2010;20(9):1258–1264. doi: 10.1007/s11695-010-0200-5. [DOI] [PubMed] [Google Scholar]
- 34.Lin C-C, Lai M-M, Li T-C, Li C-I, Liu C-S, Chen C-C, Wu M-T. Relationship between serum retinol-binding protein 4 and visfatin and the metabolic syndrome. Diabetes Research and Clinical Practice. 2009;85(1):24–29. doi: 10.1016/j.diabres.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 35.Lee K, Villena JA, Moon YS, Kim KH, Lee S, Kang C, Sul HS. Inhibition of adipogenesis and development of glucose intolerance by soluble preadipocyte factor-1 (Pref-1) J Clin Invest. 2003;111(4):453–461. doi: 10.1172/JCI15924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee J-W, Lee H-R, Shim J-Y, Im J-A, Lee D-C. Abdominal visceral fat reduction is associated with favorable changes of serum retinol binding protein-4 in nondiabetic subjects. Endocrine Journal. 2008;55(5):811–818. doi: 10.1507/endocrj.k08e-030. [DOI] [PubMed] [Google Scholar]
- 37.Gómez-Ambrosi J, Rodríguez A, Catalán V, Ramírez B, Silva C, Rotellar F, Gil MJ, Salvador J, Frühbeck G. Serum retinol-binding protein 4 is not increased in obesity or obesity-associated type 2 diabetes mellitus, but is reduced after relevant reductions in body fat following gastric bypass. Clinical Endocrinology. 2008;69(2):208–215. doi: 10.1111/j.1365-2265.2007.03156.x. [DOI] [PubMed] [Google Scholar]
- 38.Janke J, Engeli S, Boschmann M, Adams F, Böhnke J, Luft FC, Sharma AM, Jordan J. Retinol-Binding Protein 4 in Human Obesity. Diabetes. 2006;55(10):2805–2810. doi: 10.2337/db06-0616. [DOI] [PubMed] [Google Scholar]
- 39.Choi KM, Kim TN, Yoo HJ, Lee KW, Cho GJ, Hwang TG, Baik SH, Choi DS, Kim SM. Effect of exercise training on A-FABP, lipocalin-2 and RBP4 levels in obese women. Clinical Endocrinology. 2009;70(4):569–574. doi: 10.1111/j.1365-2265.2008.03374.x. [DOI] [PubMed] [Google Scholar]
- 40.Hermsdorff HHM, Zulet MÁ, Abete I, Martínez JA. Discriminated benefits of a Mediterranean dietary pattern within a hypocaloric diet program on plasma RBP4 concentrations and other inflammatory markers in obese subjects. Endocrine. 2009;36(3):445–451. doi: 10.1007/s12020-009-9248-1. [DOI] [PubMed] [Google Scholar]
- 41.Jüllig M, Yip S, Xu A, Smith G, Middleditch M, Booth M, Babor R, Beban G, Murphy R. Lower Fetuin-A, Retinol Binding Protein 4 and Several Metabolites after Gastric Bypass Compared to Sleeve Gastrectomy in Patients with Type 2 Diabetes. PLoS ONE. 2014;9(5) doi: 10.1371/journal.pone.0096489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tschoner A, Sturm W, Engl J, Kaser S, Laimer M, Laimer E, Weiss H, Patsch JR, Ebenbichler CF. Retinol-binding protein 4, visceral fat, and the metabolic syndrome: effects of weight loss. Obesity (Silver Spring, Md.) 2008;16(11):2439–2444. doi: 10.1038/oby.2008.391. [DOI] [PubMed] [Google Scholar]
- 43.Klöting N, Graham TE, Berndt J, Kralisch S, Kovacs P, Wason CJ, Fasshauer M, Schön MR, Stumvoll M, Blüher M, Kahn BB. Serum retinol-binding protein is more highly expressed in visceral than in subcutaneous adipose tissue and is a marker of intra-abdominal fat mass. Cell Metabolism. 2007;6(1):79–87. doi: 10.1016/j.cmet.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 44.Hursting SD, Berger NA. Energy balance, host-related factors, and cancer progression. J Clin Oncol. 2010;28(26):4058–4065. doi: 10.1200/JCO.2010.27.9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 46.LeRoith D, Novosyadlyy R, Gallagher EJ, Lann D, Vijayakumar A, Yakar S. Obesity and type 2 diabetes are associated with an increased risk of developing cancer and a worse prognosis; epidemiological and mechanistic evidence. Exp Clin Endocrinol Diabetes. 2008;116(Suppl 1):S4–S6. doi: 10.1055/s-2008-1081488. [DOI] [PubMed] [Google Scholar]
- 47.Abola MV, Thompson CL, Chen Z, Chak A, Berger NA, Kirwan JP, Li L. Serum levels of retinol-binding protein 4 and risk of colon adenoma. Endocrine-Related Cancer. 2015;22(2):L1–L4. doi: 10.1530/ERC-14-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.El-Mesallamy HO, Hamdy NM, Zaghloul AS, Sallam AM. Clinical value of circulating lipocalins and insulin-like growth factor axis in pancreatic cancer diagnosis. Pancreas. 2013;42(1):149–154. doi: 10.1097/MPA.0b013e3182550d9d. [DOI] [PubMed] [Google Scholar]
- 49.Gray IC, Phillips SMA, Lee SJ, Neoptolemos JP, Weissenbach J, Spurr NK. Loss of the Chromosomal Region 10q23–25 in Prostate Cancer. Cancer Research. 1995;55(21):4800–4803. [PubMed] [Google Scholar]
- 50.Lorkova L, Pospisilova J, Lacheta J, Leahomschi S, Zivny J, Cibula D, Zivny J, Petrak J. Decreased concentrations of retinol-binding protein 4 in sera of epithelial ovarian cancer patients: a potential biomarker identified by proteomics. Oncology Reports. 2012;27(2):318–324. doi: 10.3892/or.2011.1513. [DOI] [PubMed] [Google Scholar]
- 51.Tsunoda S, Smith E, De Young NJ, Wang X, Tian Z-Q, Liu J-F, Jamieson GG, Drew PA. Methylation of CLDN6, FBN2, RBP1, RBP4, TFPI2, and TMEFF2 in esophageal squamous cell carcinoma. Oncology Reports. 2009;21(4):1067–1073. doi: 10.3892/or_00000325. [DOI] [PubMed] [Google Scholar]
- 52.W.I.A.f.R.o. Weight Control and Physical Activity. IARC Handbooks of Cancer Prevention. In: Vainio H, Bianchini F, editors. Cancer. Vol. 6. Lyon, France: IARCPress; 2002. [PubMed] [Google Scholar]
- 53.Gilbert CA, Slingerland JM. Cytokines, obesity, and cancer: new insights on mechanisms linking obesity to cancer risk and progression. Annu Rev Med. 2013;64:45–57. doi: 10.1146/annurev-med-121211-091527. [DOI] [PubMed] [Google Scholar]
- 54.Fex G, Johannesson G. Transfer of retinol from retinol-binding protein complex to liposomes and across liposomal membranes. Methods Enzymol. 1990;189:394–402. doi: 10.1016/0076-6879(90)89313-7. [DOI] [PubMed] [Google Scholar]
- 55.Noy N, Xu ZJ. Interactions of retinol with binding proteins: implications for the mechanism of uptake by cells. Biochemistry. 1990;29(16):3878–3883. doi: 10.1021/bi00468a012. [DOI] [PubMed] [Google Scholar]
- 56.Noy N, Blaner WS. Interactions of retinol with binding proteins: studies with rat cellular retinol-binding protein and with rat retinol-binding protein. Biochemistry. 1991;30(26):6380–6386. doi: 10.1021/bi00240a005. [DOI] [PubMed] [Google Scholar]
- 57.Noy N, Xu ZJ. Kinetic parameters of the interactions of retinol with lipid bilayers. Biochemistry. 1990;29(16):3883–3888. doi: 10.1021/bi00468a013. [DOI] [PubMed] [Google Scholar]
- 58.Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, Wiita P, Bok D, Sun H. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science. 2007;315(5813):820–825. doi: 10.1126/science.1136244. [DOI] [PubMed] [Google Scholar]
- 59.Szeto W, Jiang W, Tice DA, Rubinfeld B, Hollingshead PG, Fong SE, Dugger DL, Pham T, Yansura DG, Wong TA, Grimaldi JC, Corpuz RT, Singh JS, Frantz GD, Devaux B, Crowley CW, Schwall RH, Eberhard DA, Rastelli L, Polakis P, Pennica D. Overexpression of the retinoic acid-responsive gene Stra6 in human cancers and its synergistic induction by Wnt-1 and retinoic acid. Cancer Res. 2001;61(10):4197–4205. [PubMed] [Google Scholar]
- 60.Pasutto F, Sticht H, Hammersen G, Gillessen-Kaesbach G, Fitzpatrick DR, Nurnberg G, Brasch F, Schirmer-Zimmermann H, Tolmie JL, Chitayat D, Houge G, Fernandez-Martinez L, Keating S, Mortier G, Hennekam RC, von der Wense A, Slavotinek A, Meinecke P, Bitoun P, Becker C, Nurnberg P, Reis A, Rauch A. Mutations in STRA6 cause a broad spectrum of malformations including anophthalmia, congenital heart defects, diaphragmatic hernia, alveolar capillary dysplasia, lung hypoplasia, and mental retardation. Am J Hum Genet. 2007;80(3):550–560. doi: 10.1086/512203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilson JG, Roth CB, Warkany J. An analysis of the syndrome of malformations induced by maternal vitamin A deficiency. Effects of restoration of vitamin A at various times during gestation. Am J Anat. 1953;92(2):189–217. doi: 10.1002/aja.1000920202. [DOI] [PubMed] [Google Scholar]
- 62.Wald G. The molecular basis of visual excitation. Nature. 1968;5156;219:800–807. doi: 10.1038/219800a0. [DOI] [PubMed] [Google Scholar]
- 63.Ruiz A, Mark M, Jacobs H, Klopfenstein M, Hu J, Lloyd M, Habib S, Tosha C, Radu RA, Ghyselinck NB, Nusinowitz S, Bok D. Retinoid content, visual responses and ocular morphology are compromised in the retinas of mice lacking the retinol-binding protein receptor, STRA6. Invest Ophthalmol Vis Sci. 2012 doi: 10.1167/iovs.11-8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Berry DC, Jacobs H, Marwarha G, Gely-Pernot A, O'Byrne SM, DeSantis D, Klopfenstein M, Feret B, Dennefeld C, Blaner WS, Croniger CM, Mark M, Noy N, Ghyselinck NB. The STRA6 receptor is essential for retinol-binding protein-induced insulin resistance but not for maintaining vitamin A homeostasis in tissues other than the eye. J Biol Chem. 2013;288(34):24528–24539. doi: 10.1074/jbc.M113.484014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Terra R, Wang X, Hu Y, Charpentier T, Lamarre A, Zhong M, Sun H, Mao J, Qi S, Luo H, Wu J. To Investigate the Necessity of STRA6 Upregulation in T Cells during T Cell Immune Responses. PLoS One. 2013;8(12):e82808. doi: 10.1371/journal.pone.0082808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Berry DC, Jin H, Majumdar A, Noy N. Signaling by vitamin A and retinol-binding protein regulates gene expression to inhibit insulin responses. Proc Natl Acad Sci U S A. 2011;108(11):4340–4345. doi: 10.1073/pnas.1011115108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berry DC, Noy N. Signaling by vitamin A and retinol-binding protein in regulation of insulin responses and lipid homeostasis. Biochim Biophys Acta. 2012;1821(1):168–176. doi: 10.1016/j.bbalip.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berry DC, O'Byrne SM, Vreeland AC, Blaner WS, Noy N. Cross Talk between Signaling and Vitamin A Transport by the Retinol-Binding Protein Receptor STRA6. Mol Cell Biol. 2012;32(15):3164–3175. doi: 10.1128/MCB.00505-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marwarha G, Berry DC, Croniger CM, Noy N. The retinol esterifying enzyme LRAT supports cell signaling by retinol-binding protein and its receptor STRA6. FASEB J. 2014;28(1):26–34. doi: 10.1096/fj.13-234310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264(5164):1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 71.Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334(Pt 2):297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Starr R, Willson TA, Viney EM, Murray LJ, Rayner JR, Jenkins BJ, Gonda TJ, Alexander WS, Metcalf D, Nicola NA, Hilton DJ. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387(6636):917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- 73.Croker BA, Kiu H, Nicholson SE. SOCS regulation of the JAK/STAT signalling pathway. Semin Cell Dev Biol. 2008;19(4):414–422. doi: 10.1016/j.semcdb.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Krebs DL, Hilton DJ. A new role for SOCS in insulin action. Suppressor of cytokine signaling. Sci STKE. 2003;2003(169):PE6. doi: 10.1126/stke.2003.169.pe6. [DOI] [PubMed] [Google Scholar]
- 75.Pirola L, Johnston AM, Van Obberghen E. Modulation of insulin action. Diabetologia. 2004;47(2):170–184. doi: 10.1007/s00125-003-1313-3. [DOI] [PubMed] [Google Scholar]
- 76.Zemany L, Kraus BJ, Norseen J, Saito T, Peroni OD, Johnson RL, Kahn BB. Downregulation of STRA6 in adipocytes and adipose stromovascular fraction in obesity and effects of adipocyte-specific STRA6 knockdown in vivo. Mol Cell Biol. 2014;34(6):1170–1186. doi: 10.1128/MCB.01106-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lehrke M, Lazar MA. The many faces of PPARgamma. Cell. 2005;123(6):993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 78.Koptyra M, Gupta S, Talati P, Nevalainen MT. Signal transducer and activator of transcription 5a/b: biomarker and therapeutic target in prostate and breast cancer. Int J Biochem Cell Biol. 2011;43(10):1417–1421. doi: 10.1016/j.biocel.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aggarwal BB, Kunnumakkara AB, Harikumar KB, Gupta SR, Tharakan ST, Koca C, Dey S, Sung B. Signal transducer and activator of transcription-3, inflammation, and cancer: how intimate is the relationship? Ann N Y Acad Sci. 2009;1171:59–76. doi: 10.1111/j.1749-6632.2009.04911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ferbeyre G, Moriggl R. The role of Stat5 transcription factors as tumor suppressors or oncogenes. Biochim Biophys Acta. 2011;1815(1):104–114. doi: 10.1016/j.bbcan.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 81.Quesnelle KM, Boehm AL, Grandis JR. STAT-mediated EGFR signaling in cancer. J Cell Biochem. 2007;102(2):311–319. doi: 10.1002/jcb.21475. [DOI] [PubMed] [Google Scholar]
- 82.Leeman RJ, Lui VW, Grandis JR. STAT3 as a therapeutic target in head and neck cancer. Expert Opin Biol Ther. 2006;6(3):231–241. doi: 10.1517/14712598.6.3.231. [DOI] [PubMed] [Google Scholar]
- 83.Goodwin PJ, Ennis M, Pritchard KI, Trudeau ME, Koo J, Madarnas Y, Hartwick W, Hoffman B, Hood N. Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study. J Clin Oncol. 2002;20(1):42–51. doi: 10.1200/JCO.2002.20.1.42. [DOI] [PubMed] [Google Scholar]