Abstract
To analyze the structure and formally ascertain the B-1a cellular origin of IgM rheumatoid factor (RF) autoantibodies, we generated 4 IgM RF mAb-producing cell lines using sorted (surface CD5+) B-1a cells from a patient with active rheumatoid arthritis. The RF mAb111, mAb112, mAb113, and mAb114 were monoreactive and displayed a relatively high affinity for human IgG Fc fragment (Kd, 3.1 × 10−7 to 6.8 × 10−7 M). The B-1a origin of the lymphocytes that gave rise to the IgM RF was confirmed by the expression of surface CD5 and specific CD5 mRNA by all mAb-producing cell lines. Analysis of the genes encoding the RF mAb VH and VL regions revealed that members of the VHI and VHIII families were utilized in conjunction with VκIIIa, VκIIIb, or VλI genes. JH3 and JH4 genes were each utilized twice. The H chain CDR3 sequences were divergent and variable in length. The RF mAb VH genes were identical or closely related to those expressed in the “restricted” fetal B cell repertoire and/or were JH-proximal. For instance, mAb111 VH gene likely constituted a mutated variant of the expressed fetal 20P3 which is the second most JH-proximal gene (125 kb from JH). In addition, the expressed VH and VL genes were among those that have been found to encode other RF, different autoantibodies, high affinity antibodies induced by exogenous Ag, and natural autoantibodies in the adult and neonatal B cell repertoires. When compared with those of known germline genes, the expressed V gene sequences displayed a number of differences. By cloning and sequencing DNA from PMN of the same patient whose B lymphocytes were used for the mAb generation, we showed that such differences resulted from somatic hypermutation in the RF mAb112 VH gene. The germline gene (112GL) that presumably gave rise to the RF mAb112 VH segment was identical to the expressed fetal 51P1 gene. The distribution and the high replacement to silent mutation ratio of the nucleotide mutations in RF mAb112 VH segment were highly consistent with their selection by Ag. RF mAb113 was clonally related to RF mAb112, as shown by the utilization of the same sets of VHI-D-JH4 and VκIIIb-Jκ4 genes, displaying identical junctional sequences, and the presence of two identical replacement and one silent mutations. The presence of a high number of unique amino acid changes, a total of 14 and 9 in the two RF mAb VH and VL segments, respectively, suggested that RF mAb112-and mAb113-producing cells diverged relatively early in their expansion from the common progenitor clone. These studies show that high affinity RF can arise through a hypermutation process from antibodies expressed by B-1a cells. They also suggest that the affinity maturation of a human autoimmune response can apply to IgM and is independent of Ig class switch.
Rheumatoid factors are IgM, IgG, IgA, or IgE antibodies that bind to the Fc portion of autologous IgG (1). They are characteristic of but not exclusive to patients with rheumatoid arthritis. Structural analysis of RF3 from normal humans and patients with monoclonal gammopathies have suggested that these autoantibodies display a low affinity for IgG Fc fragment (Kd, 10−3 to 10−5 M), and are, in general, encoded in a restricted set of V region genes in virtually unmutated (germline) configuration (2, 3). Such RF are part of the larger population of Ig that bind a variety of self and exogenous Ag, including those on pathogenic microorganisms, collectively termed natural antibodies or autoantibodies (4, 5). In healthy subjects, most natural antibodies, including those binding IgG Fc fragment, are polyreactive, i.e., they bind multiple Ag, and are produced by CD5+ B lymphocytes (6–10), now referred to as B-1a cells (11–13).
Rheumatoid patients, like healthy subjects, display circulating natural RF autoantibodies that are polyreactive and have a low affinity for IgG Fc fragment (14). Rheumatoid patients also display monoreactive, high affinity RF (Kd, ~ 10−7 M), that can form large immune aggregates, possibly triggering the inflammatory pathways that lead to tissue damage (14). In these patients, most of both the low and high affinity RF autoantibodies are produced by B-1a cells, which are greatly increased in number and can account for more than 50% of the circulating B lymphocytes (7, 9, 10, 14). Recent studies have suggested that high affinity RF can utilize VH and VL segments similar to those of their low affinity (polyreactive) counterparts, but in a somatically mutated configuration (15–20). However, the somatically mutated status of RF V genes has been formally verified in only few cases (21–24), and the cellular origin of the putatively mutated RF has never been unequivocally established. Lack of somatic mutations would be consistent with the IgM class of most RF and with their B-1a cell origin. IgM autoantibodies are, in general, in germline configuration (4, 5, 9, 12, 19, 25–27), and B-1a lymphocytes have long been thought to be primordial cellular elements lacking the machinery necessary to hypermutate the expressed Ig V genes (9–12).
Using sorted B-1a cells from a patient with rheumatoid arthritis, we generated four cell clones producing monoreactive high affinity IgM RF mAb. In each clone, the B-1a cell origin was confirmed by the analysis of surface CDS and that of specific CD5 mRNA expression. When compared with those of germline Ig V genes, the sequences of the RF displayed a number of differences. In at least one RF mAb, these differences were shown to result from a process of somatic mutation. Thus, IgM RF autoantibodies can undergo an Ag-directed affinity maturation process involving a hypermutation mechanism that operates on Ig V genes expressed by B-1a cells.
Materials and Methods
Generation of RF mAb-secreting cell lines
B lymphocytes were enriched from PBMC obtained from a 58-year-old female (V.M.H.) with active rheumatoid arthritis. B-1a cells were purified by sorting using specific mouse mAb to CD20 and CD5 (Coulter, Hialeah, FL) and an EPICS Elite (Coulter) fluorescence-activated cell sorter (6, 13, 14, 28–30). Purified B-1a cells were infected with EBV for 1 h and then immediately plated at different numbers/well in 96-well plates in presence of irradiated feeders (14,28–31). After a 2-wk culture, EBV-transformed B cells producing IgM RF were selected using a specific ELISA and cloned (14, 28–31). To yield maximal mAb production rate, these EBV-transformed B cells were fused with the F3B6 cell line to yield EBV-transformed B cell hybrids (32). Both cloned EBV-transformed cells and EBV-transformed B cell hybrids were used for Ig V gene analysis, whereas only cloned EBV-transformed cells were used for analysis of surface CD5 and CD5 mRNA expression.
RF mAb binding activities and Kd
The mAb were analyzed for Ag-binding using specific ELISA (28–31). The mAb Kd for IgG Fc fragment were measured using a competitive inhibition ELISA as originally proposed by Friguet et al. (32), and as we reported in previous studies (including those in references 5, 8, 10, 14, 17, 28, 30, and 31). Briefly, samples of PBS-Tween 20 (0.05 ml) containing 1% BSA and 0.2 µg of a given mAb (4.2 × 10−9 M) were mixed with samples of PBS-Tween 20 (0.05 ml) containing 1% BSA and 2 to 200 µg (i.e., 1.6 × 10−6 to 1.6 × 10−4 M) of soluble human polyclonal IgG Fc fragment (28–31). After an 18-h incubation at room temperature, the mixtures were transferred into ELISA plates precoated with 0.1 ml/well of bicarbonate buffer containing 5 µg/ml of IgG fragment. After a 1-h incubation and subsequent washing with PBS-Tween 20, the amount of mAb bound to solid-phase IgG Fc fragment was measured using a peroxidase-conjugated affinity-purified goat antibody to human µ Ig H chain (28–31). For each mAb, the binding activity measured in the presence of soluble IgG Fc fragment was expressed as percentage of that measured under identical conditions but in absence of soluble IgG Fc fragment. The latter yielded an absorbance of 0.5 to 0.6 at 492 nm, which fell within the linear portion of the dose-saturable binding curve and corresponded to ~50% of the maximum binding, as shown in Figure 1 (2 µg/ml of mAb). The Kd was calculated from the slope of the line determined by plotting the ratio of total to unbound mAb vs the reciprocal concentration of soluble Ag expressed as molarity (33). Even at the lowest Ag concentration, the IgG Fc fragment molarity exceeded by at least 100-fold that of the mAb. Under these conditions, the Kd values derived by the use of the competitive inhibition ELISA have been found to be comparable with those calculated by the means of conventional methods in various Ag-antibody systems involving multivalent linkages (33).
FIGURE 1.
Binding of the IgM RF mAb to solid-phase Ag, and Kd for IgC Fc fragment. The binding of each mAb to IgG Fc fragment, β-galactosidase from Escherichia coli, tetanus toxoid, ssDNA, human recombinant insulin, actin, and phosphorylcholine is expressed as optical absorbance at 492 nm.
Analysis of CD5-specific mRNA expression
Levels of CD5 mRNA expressed by mAb-producing B cell clones and T cells were analyzed using the PCR method we recently devised (33). Human-mouse F3B6 heterohybridoma cells were used as negative controls (M. T. Kasaian and P. Casali, unpublished observations). Briefly, poly(A)+ RNA was purified from EBV-transformed B cell clones, T cells, or F3B6 cells using the Micro Fast-Track mRNA isolation kit (Invitrogen, La Jolla, CA). mRNA from each B cell line (250 ng), T cells (1–250 ng), and F3B6 cells (250 ng) was reverse-transcribed using MMLV reverse transcriptase (Superscript RNase H-Reverse Transcriptase, GIBCO-BRL, Life Technologies, Gaithersburg, MD). cDNA were PCR-amplified using the β-actin-specific sense [5′ GTACCACTGGCAT CGTGATGGACT 3′] and antisense [5′ATCCACACGGAGTACTTGCGCTCA 3′] oligonucleotide primers or the CD5-specific sense [5′ AG-GACGGATGGCACATGGTTT 3′] and antisense [5′ TTGTCCTGGGCC TCATAGCT 3′] oligonucleotide primers, designed on the basis of the human genomic β-actin DNA (34) and CD5 cDNA (35) sequences, respectively (30). Each PCR reaction was performed in a 50-µl volume for a total of 25 cycles of amplification consisting of: denaturation, 94°C (1 min); annealing, 60°C (1 min); and extension, 72°C (2 min). Amplified DNA was applied to a 1.2% agarose gel containing 1 µg/ml of ethidium bromide, fractionated and transferred onto a nylon membrane (Gene Screen, Du Pont Co., NEN Research Products, Boston, MA) for hybridization with the internal CD5-specific oligonucleotide [5′ CCAGAAGACAACACCTCCAA 3′] probe labeled with [32P]-γ-ATP (Du Pont Co., NEN Research Products). After hybridization, filters were washed twice with 2X SSC/0.1% SDS at room temperature for 30 min and twice with 1X SSC/0.1% SDS at 52°C for 30 min. Autoradiography was carried out using Kodak XAR-5 film (Eastman Kodak Co., Rochester, NY).
Cloning and sequencing of expressed Ig VH and VL genes
mRNA was isolated from the established hybrid cell lines and transcribed into cDNA as described above. Second-strand cDNA synthesis and amplification were performed by PCR using 100 ng of first strand cDNA template in a 50 µl reaction volume containing 200 µM of each dNTP, 2.5 U of Taq polymerase (Perkin-Elmer Cetus, Emeryville, CA) and 10 pmol of each oligonucleotide primer. The degenerate sense oligonucleotide primers consisted of a sequence encompassing an area of the leader region of different VH gene families plus an EcoRI site. Primers were as follows: ALT 1 (VHI) [5′ GGGAATTCATGGACTGGACCTGGAGG(AG)TC(CT) TCT(GT)C 3′]; ALT 2 (VHIII) [5′ GGGAATTCATGGAG(CT)TTGGG-CTGA-(CG)CTGG(CG)TTT (CT)T 3′]; and ALT 4 (VHIV) [5′ GGGAATTCATGAA(AG)CA(TC)CTG-TGGTTCTT(CT)(AC)T(CT) CT(CG)C 3′]. The antisense oligonucleotide primer consisted of the reverse complement [5′ CCGAATTC AGACGAGGGGG AAAAGGGTTT 3′] of a 21-nucleotide Cµ sequence plus an EcoRI site. The oligonucleotide sense primers homologous to the leader regions of the Vκ and VλL chain gene subgroups were as follows: VκI-II (VκI and VκII L chains) [5′ AGCTCCTGGGGCT-(GC)-CT(AG)(AC)TGCTCT 3′]; VκIII [5′ TCTCTTCCTCCTGCTACTCTGGCT 3′]; VκIV [5′ ATGGTGTTGCAGACCCAGGTCTTC 3′]; VλI [5′ ATG(GA) CC-(TG) GCT(CT)CCCTCTCCTCCT 3′]; Vλ II-VI (VλII, VλIII, VλIV, and VλVI L chains) [5′ ATG(AG)C(CT)-TGGACCC(CT)(AT)CTC(CT)(TG)-(TG)TT 3′]. The Ig Cκ chain antisense oligonucleotide primer was [5′ CTGCTCATCAGATGGCGG-GAAGA 3′] and the Cλ chain antisense oligonucleotide primer was [5′ TTGGCTTGAAGCTCCTCAG-AGGA 3′]. For PCR amplification of VH and Vλ genes, 30 cycles consisting of the following steps were used: denaturation, 94°C (1 min); annealing, 52°C (1 min); and extension, 72°C (2 min). For Vκ gene amplification, an annealing temperature of 58°C was utilized. PCR products were purified with low melt agarose and ligated into pCR1000 plasmid vector (Invitrogen). The legation mixture was used to transform INVαF′ competent cells according to the manufacturer’s protocol (Invitrogen). Recombinant clones were selected according to the length of the insert and sequenced by the dideoxy chain termination method using the Taq Sequencing Kit (Promega, Madison, WI). Each VH or VL sequence was derived from the analysis of at least four independent recombinant clones. Differences in nucleotide sequences among different recombinant clones were observed in few cases (<2 × 10−3/base, consistent with the error rate of the Taq polymerase) and such variants were excluded from the sequence analysis.
Analysis of genomic VH segments from PMN DNA and hybridoma DNA
Because of the outbreeding of the human population and its high degree of V gene polymorphism, assessment of the somatically mutated status of an expressed VH segment requires identification of the autologous germline gene from which it arises (36). Genomic DNA was extracted from the mAb112-producing B cell line and from the peripheral blood PMN isolated from the patient whose B cells were used to generate mAb112. B cell or PMN DNA (200 ng) was supplemented with the appropriate sense and antisense oligonucleotide primers (10 pmol each). For PCR amplification, 30 cycles consisting of the following steps were used: denaturation, 94°C (1 min); annealing, 58°C (1 min); and extension, 72°C (2 min). The oligonucleotides used were as follows: sense HI-6 primer, encompassing a sequence [5′ CTGGAGGTTCCTCTTTGTGGT 3′] (residues −47 to −27 of cDNA) (31) shared by the mAb112 VH and the closest germline (hv1263) gene leaders; antisense HI-7 primer, consisting of the reverse complement [5′ GCCGTGTCATCAGATCTCAGG 3′] of a FR3 sequence (residues 255 to 275) (31) shared by mAb112 and the germline hv1263 VH genes, except for one base difference (A instead of C) at position 268; and sense 112CDR1 primer, consisting of the sequence [5′ GCAGCCATACTCTCAGTTG 3′] encompassing the 3′ end of framework region (FR)1, the whole complementarity determining region (CDR)1, and the 5′ portion of FR2 (residues 89 to 107) of the expressed mAb112 VH gene. This sequence differed in four bases from that of hv1263. PCR-amplified VH DNA was analyzed by fractionation on a 1.2% agarose gel, blotting and Southern hybridization with the 32P-labeled oligonucleotide probe 112CDR1. The PMN DNA PCR-amplified product was used to clone and sequence the germline VH gene segment that putatively gave rise to the expressed mAb112 VH gene.
Analysis of DNA sequence data
DNA sequencing data were analyzed using a Model 6000-410 VAX computer (Digital Equipment Corp., Marlboro, MA) and the software package of Genetics Computer Group of the University of Wisconsin, Release 6, the FASTA program (37), and the GenBank Database.
Results
RF mAb-producing cell lines and RF mAb
Using sorted B-1a cells from a patient with rheumatoid arthritis, we generated four RF mAb (mAb111, mAb112, mAb113, and mAb114)-producing cell lines. Three RF mAb utilized κ and one λ L chains; all were monoreactive and all bound in a dose-saturable fashion to IgG Fc fragment but to none of the other self or exogenous Ag tested (Fig. 1). The RF IgM mAb Kd values for IgG Fc fragment, as measured by competitive inhibition ELISA (see details in Materials and Methods), were relatively low (high affinity), ranging from 3.1 × 10−7 to 6.8 × 10−7 M (Fig. 1).
CD5 expression
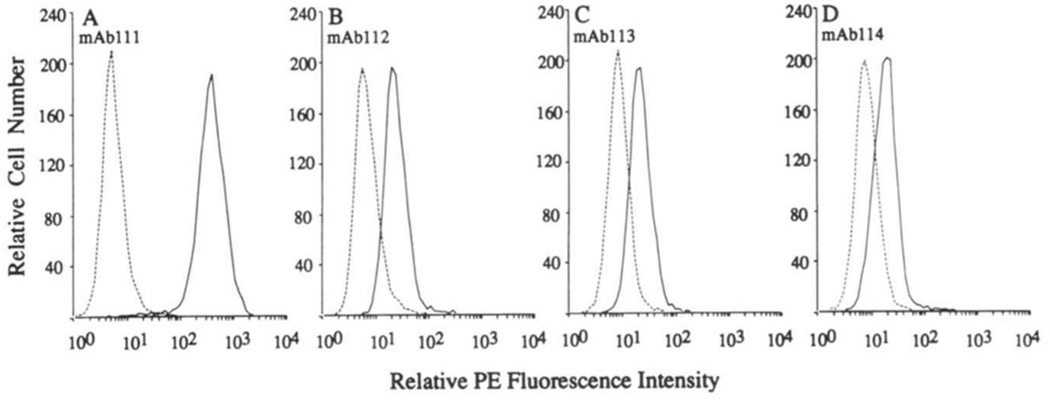
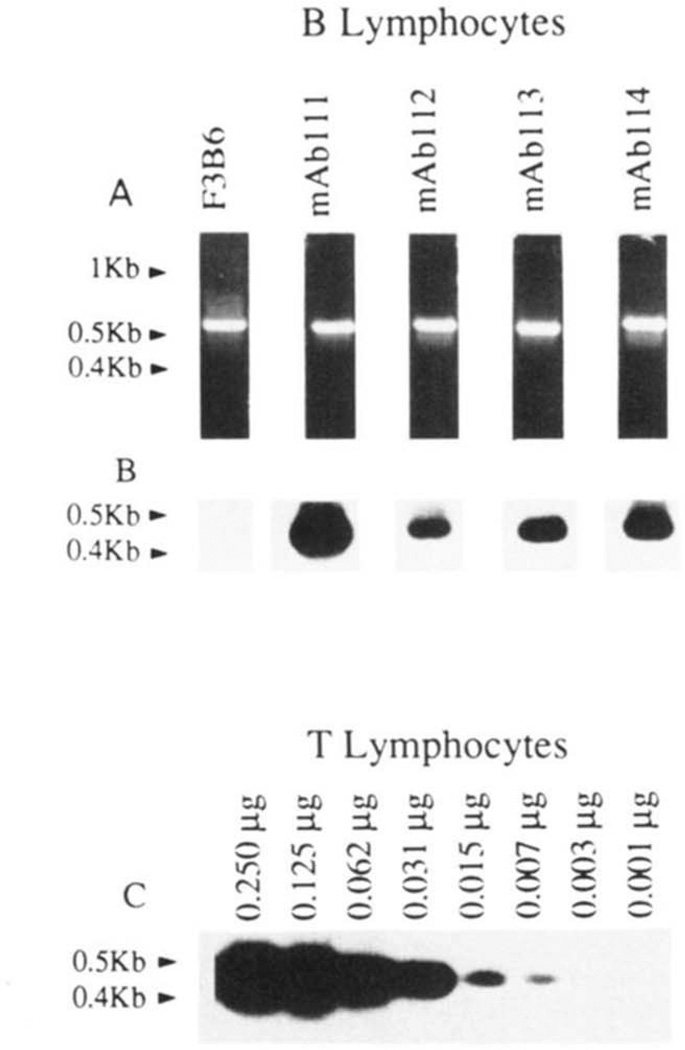
The B-1a cell origin of the four RF mAb-producing cell lines was confirmed by their expression of surface CD5 protein and CD5 mRNA. Fluorescence flow cytometry analysis using mouse mAb to CD5 showed that all RF mAb-producing EBV-transformed B cell lines expressed surface CD5, although at different densities (Fig. 2). CD5 mRNA expression by these cell lines was demonstrated by PCR (Fig. 3, A and B). The specificity of the PCR method has been detailed in our original report (30) and was further supported by: 1) the proportionality between amount of input mRNA and amount of CD5 DNA amplified (Fig. 3C); 2) the large amount of CD5 DNA amplified from the mAb111-producing B cell line, which expressed surface CD5 at the highest density; and 3) the failure to amplify CD5 DNA from the F3B6 B hybridoma (Fig. 3, A and B), which consistently does not express CD5 (M. T. Kasaian and P. Casali, unpublished observations).
FIGURE 2.
Expression of surface CD5 by the RF mAb-producing B cells. EBV-transformed cells were reacted with biotinylated mouse IgG2a mAb to CD5 (solid lines) or with biotinylated mouse IgG2a mAb of irrelevant specificity (dotted lines), and then with PE-labeled streptavidin. Cells were analyzed by fluorescence flow cytometry.
FIGURE 3.
Expression of CD5 mRNA by the RF mAb-producing B cells. mRNA from the EBV-transformed B cell lines (0.250 µg), T lymphocytes (0.250 to 0.001 µg), or the fusion partner F3B6 hybrid cells were reverse-transcribed. cDNA were amplified by PCR using β-actin or CD5-specific primers and fractionated on 1.2% agarose gel (see Materials and Methods). A, ethidium bromide-stained gel containing amplified β-actin DNA (~0.6 kb); B, hybridization of the 32P-labeled “internal” CD5-specific oligonucleotide probe with fractionated CD5 DNA amplified from cDNA reverse-transcribed from the mAb-producing cell lines or the fusion partner F3B6 cells; C, hybridization of 32P-labeled CD5-specific oligonucleotide probe to fractionated DNA amplified from cDNA independently reverse-transcribed from different amounts (0.250 to 0.001 µg) of purified T cell mRNA.
IgM RF mAb VH segments
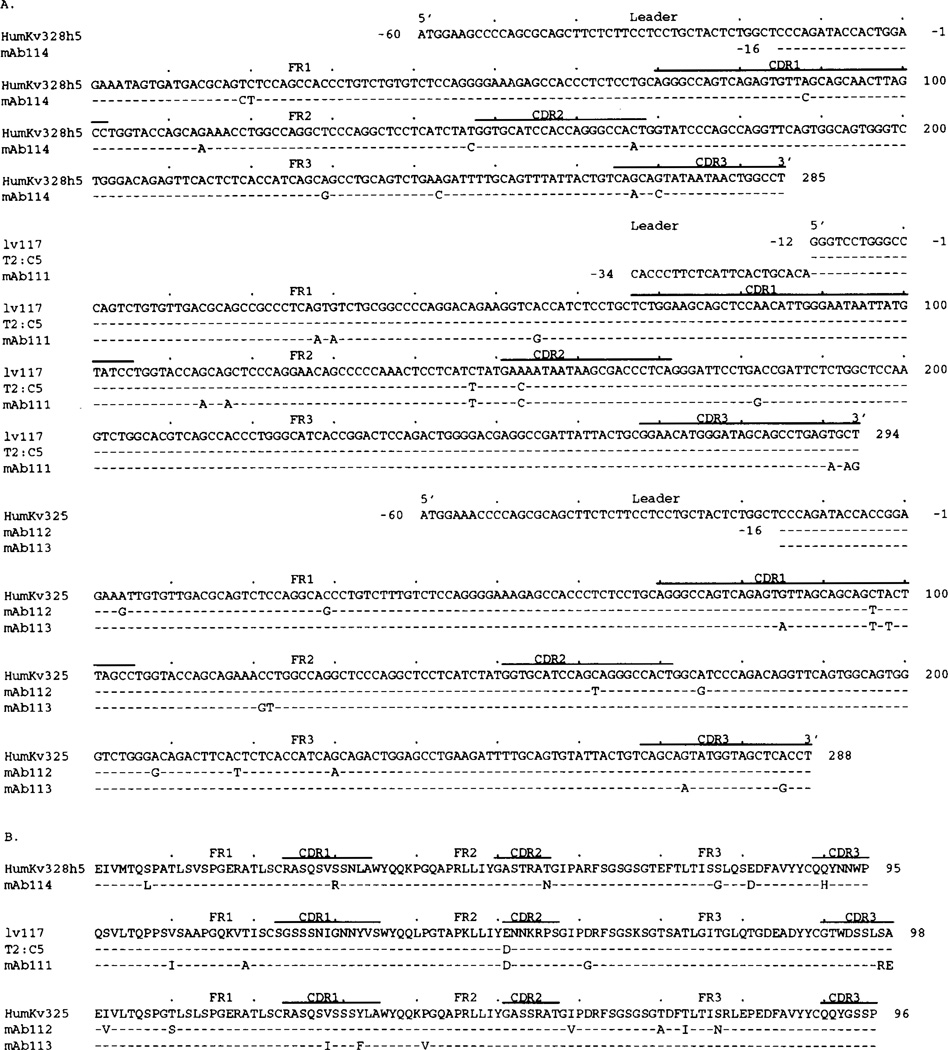
Figure 4 shows the nucleotide (A) and deduced amino acid (B) sequences of the four RF mAb VH regions. Table I summarizes the differences in nucleotide and predicted amino acid changes when compared with the closest known germline genes. The mAb114 VH gene sequence displayed the highest degree of identity with that of the germline VHIII HHG19 gene (Kueppers, unpublished observations). The 25 nucleotide differences were scattered throughout FR and CDR and yielded R/S ratios of 1.1 and 2.3, respectively. The mAb111 VH gene sequence was highly similar to the germline (VHI) VI-2 sequence (38), which is identical with that of the expressed fetal 20p3 gene (39). The nucleotide differences resulted in putative replacement changes and a high R/S ratio in the CDR. The mAb112 and mAb113 VH gene sequences were similar to that of the germline hv1263 gene (40), which is more than 98% identical with the expressed fetal 51p1 gene (39), and shared seven nucleotide differences throughout the coding region. In mAb112, only 6 of the 11 FR differences resulted in replacement changes, whereas all but one did in the CDR. In mAb113 VH gene, only two of the seven nucleotide FR differences resulted in replacement changes, and 8 of 12 did in the CDR.
FIGURE 4.
Nucleotide (A) and deduced amino acid (B) sequences of the VH genes utilized by the IgM RF mAb. In each cluster, the top sequence is given for comparison and represents the germline VH gene displaying the highest identity to the expressed VH genes. The HHG19 VH gene belongs to the VHIII family. VI-2 and hv1263 are members of the VHI gene family. Dashes indicate identities. Solid lines above each cluster encompass CDR sequences. Small letters (in the case of hv1263 and 112GL) denote intron sequences. 112GL is the germline sequence we amplified from PMN DNA of the patient whose B cells were used for the generation of mAb112. Sequences encompassed by the oligonucleotide primers are underlined. The present sequences are available from EMBL/GenBank/DDBJ under accession numbers L14453, L14454, L14455, and L14456.
Table I.
VH and VL segments of monoreactive high affinity IgM RF mAb
| VH Gene |
Db Gene |
VL Gene |
JL Gene |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clone | Chain |
Closet VH Member [Family] |
% Nucleotide Identity (Amino Acid)a |
Number of Nucleotide Differences (Number of Replacement Changes) |
JH Gene |
Closest VL Member [Subgroup] |
% Nucleotide Identity (Amino Acid)a |
Number of Nucleotide Differences (Number of Replacement Changes) |
|||||||||||||||
| H, L | FR1 | CDR1 | FR2 | CDR2 | FR3 | Total in |
FR1 | CDR1 | FR2 | CDR2 | FR3 | CDR3 | Total in |
||||||||||
| FR (R/S) |
CDR (R/S) |
FR (R/S) |
CDR (R/S) |
||||||||||||||||||||
| mAb114 | µ, κ | HHg19c | 91.4 | 7 | 4 | 0 | 6 | 8 | 15 | 10 | D21/9 | JH3 | humKv328h5d | 96.5 | 2 | 1 | 2 | 1 | 2 | 2 | 6 | 4 | Jκ1 |
| [VHIII] | (87.7) | (3) | (3) | (0) | (4) | (5) | (1.1) | (2.3) | [VκIIIa] | (93.7) | (2) | (1) | (0) | (1) | (2) | (1) | (2.0) | (3.0) | |||||
| mAb111 | µ, κ | VI–2c | 94.9 | 3 | 1 | 2 | 4 | 5 | 10 | 5 | DLR4 | JH3 | 1v117d | 96.9 | 3 | 0 | 3 | 1 | 1 | 3 | 6 | 3 | Jλ2/Jλ3 |
| [VHI] | (89.8) | (2) | (1) | (1) | (3) | (4) | (2.3) | (4.0) | [VλI] | (94.9) | (2) | (0) | (0) | (1) | (1) | (2) | (0.6) | (0.3) | |||||
| mAb112 | µ, κ | hv1263c | 92.8 | 3 | 4 | 2 | 6 | 6 | 11 | 10 | DA1 | JH4 | humKv325d | 97.2 | 2 | 1 | 0 | 1 | 4 | 0 | 6 | 2 | Jκ4 |
| [VHI] | (84.7) | (1) | (3) | (1) | (6) | (4) | (1.2) | (9.0) | DA4 DN4 (inv) |
[VκIIIb] | (93.8) | (2) | (0) | (0) | (0) | (4) | (0) | (6:0)f | (0.0) | ||||
| 112GL | 94.5e | 2 | 4 | 2 | 3 | 5 | 9 | 7 | |||||||||||||||
| [VHI] | (88.8)e | (1) | (3) | (1) | (3) | (3) | (1.2) | (6.0) | |||||||||||||||
| mAb113 | µ, κ | hv1263c | 93.5 | 1 | 1 | 1 | 11 | 5 | 7 | 12 | DA1 | JH4 | humKv325d | (97.6) | 0 | 2 | 2 | 0 | 0 | 2 | 2 | 5 | Jκ4 |
| [VHI] | (89.8) | (0) | (1) | (0) | (7) | (2) | (0.4) | (2.0) | DA4 DN4 (inv) |
[VκIIIb] | (96.9) | (0) | (2) | (2) | (0) | (0) | (0) | (2:0)f | (0.7) | ||||
| 112GL | 94.8e | 0 | 1 | 1 | 9 | 4 | 5 | 10 | |||||||||||||||
| [VHI] | (92.8)e | (0) | (1) | (0) | (5) | (1) | (0.2) | (1.5) | |||||||||||||||
Compared with the genomic germline sequences.
The D21/9 gene has been reported by L. Buluwela et al., EMBO J. 7:2003 (1988); all other D genes have been reported by Y. Ichiara et al., EMBO J. 7:4141 (1988).
The complete sequences of the genomic germline VH genes are as follows: HHg19 was reported by R. Kueppers (GenBank accession number X62128); VI-2 by E. K. Shin et al., EMBO J. 10:3641 (1991); hv1263 by P. P. Chen et al., Arthritis Rheum. 32:72 (1989).
The complete sequences of the genomic VL genes are as follows: humKv328h5, by M. Liu et al., J. Immunol. 142:688 (1989), 1v117 by K. A. Siminovitch et al., J. Clin. Invest. 84:1675 (1989); humKv325 by T J. et al., J. Exp. Med. 167:840 (1988).
% identity calculated assuming that the 3′ portion of the germline 112GL sequence is identical with that of hv1263, as suggested by the mAb112 and mAb113 VH sequences.
R/S ratios expressed as quotients are underlined.
IgM RF mAb D and JH genes; H chain CDR3 and FR4 structure
Comparison of the expressed D segment sequences with those of the known germline D and DIR segments (41–45) revealed identities between the expressed and germline D genes in all RF mAb (Fig. 5). All expressed D genes were flanked by some N segment additions. The mAb114 D gene contained a stretch of 34 bases similar to the D21/9 gene sequence. The mAb111 D gene consisted of 37 bases with the highest degree of identity with the DLR4 sequence (43). mAb112 and mAb113 displayed virtually identical 30 base D segments, which likely arose from a process of fusion of an inverted DN4 gene with a DA1/DA4 gene. The only nucleotide difference was silent.
FIGURE 5.
Nucleotide (A) and deduced amino acid (B) sequences of the D and JH segments of the RF mAb. Germline D genes are given for comparison. Dashes indicate identities. Inverted DN4 sequence is the reverse complement of the germline DN4 gene sequence. The deduced amino acid sequences are divided in CDR3 and FR4. The present sequences are available from EMBL/GenBank/DDBJ under accession numbers L14453, L14454, L14455, and L14456.
Comparison of the expressed JH gene sequences with those of the known germline JH genes (Fig. 5A) (41) showed that both mAb114 and mAb111 utilized complete and mutated forms of the JH3 gene, whereas mAb112 and mAb113 utilized identical and truncated unmutated JH4 genes, both displaying an A instead of G variation which is present in other expressed JH genes (36, 39, 46, 47) and in the prototypic JH4 sequence proposed by Yamada et al. (46).
Figure 5B shows the deduced amino acid sequences of the D-JH genes of the four mAb, in which each sequence is divided into CDR3 and FR4 stretches according to Kabat et al. (48). The CDR3 sequences were divergent and ranged in length from 13 to 17 amino acids; the four FR sequences displayed minimal diversity.
IgM RF mAb VL-JL segments
Figure 6 shows the nucleotide (A) and deduced amino acid (B) sequences of the four RF mAb VL genes and Table I summarizes the nucleotide differences and the predicted replacement changes. mAb114 utilized a (humKv328h5) VκIIIa gene (49) with minimal changes; mAb111 utilized a VλI gene highly similar to the germline lv117 (50) and the expressed T2:C5 (51) genes. In both the mAb114 VκIIIa and the mAb111 VλI segments, the nucleotide differences resulted in higher putative R/S mutation ratios in the CDR than in the FR. Both mAb112 and mAb113 utilized a (humKv325) VκIIIb gene (25) with few changes. In mAb112 VκIIIb segment, the nucleotide differences were mainly concentrated in the FR and all but two resulted in amino acid changes. In the mAb113 VκIIIb segment, the nucleotide differences were mainly in the CDR and about half were silent.
FIGURE 6.
Nucleotide (A) and deduced amino acid (B) sequences of the VL genes utilized by IgM RF mAb. The top sequence in each cluster is used for comparison. Identities are indicated by dashes. Solid lines above each cluster encompass CDR sequences. Sequences encompassed by the oligonucleotide primers are underlined. The humKv328h5 and humKv325 genes belong to the VκIIIa and VκIIIb subgroups, respectively. The Iv117 and T2:C5 genes belong to the VλI subgroup. The present sequences are available from EMBL/GenBank/DDBJ under accession numbers L14451, L14452, L14457, and L14458.
Figure 7 shows the nucleotide (A) and deduced amino acid (B) sequences of the RF mAb JL segments. The mAb114 Jκ1 segment differed in three nucleotides and amino acids from the published germline sequence (52) and was 5′ flanked by an unencoded CCG sequence yielding an additional proline at the amino acid level. Similar N additions have been reported recently at the VκIIIb-Jκ junctions of other RF (53). The mAb111 Jλ segment was encoded in a truncated Jλ2/Jλ3 (54) gene with a single silent mutation. mAb112 and the mAb113 utilized identical Jκ4 segments (52).
FIGURE 7.
Nucleotide (A) and deduced amino acid (B) sequences of the JL segments used by RF mAb. The top germline sequence in each cluster is used for comparison. Identities are indicated by dashes. Solid lines above each cluster encompass CDR3 sequences. The present sequences are available from EMBL/GenBank/DDBJ under accession numbers L14451, L14452, L14457, and L14458.
Somatic mutations in the RF mAb112 VH segment
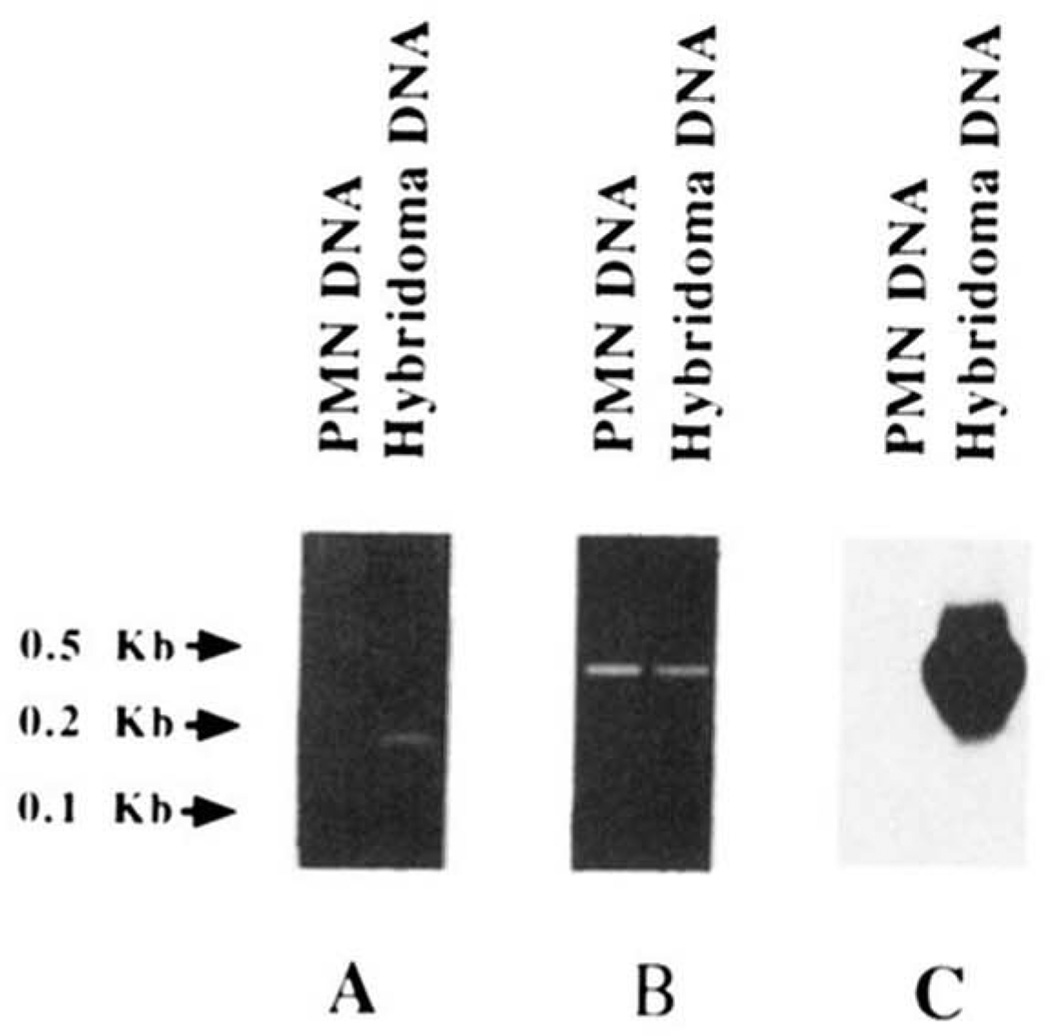
Because the distribution of the nucleotide differences when compared with the hv1263 sequence suggested that mAb112 VH gene consisted of a mutated form of the hv1263 or a hv1263-like gene, we performed PCR amplifications using ad hoc oligonucleotide primers and genomic DNA from autologous PMN or DNA from the RF mAb112-producing hybridoma. The sense 112CDR1 primer, encompassing the entire mAb112 CDR1 sequence and flanking regions, and differing in four nucleotides from the corresponding hv1263 gene sequence, was used in conjunction with the antisense HI-7 primer, encompassing a FR3 sequence identical in the expressed mAb112 and hv1263 VH gene except for a T instead of G in position 267 (Fig. 4A). The two combined primers yielded an amplification product when utilized in PCR involving DNA from the hybridoma but not from autologous PMN (Fig. 8A). The size of the amplified product (~180 bp) was consistent with that of the sequence spanning residues 89 to 275 of the mAb112 VH gene. Utilization of the same antisense (HI-7) primer in conjunction with the sense HI-6 primer encompassing a leader sequence stretch shared by the mAb112 and hv1263 genes (Fig. 4A) resulted in the amplification of a ~400-bp product from both hybridoma and PMN DNA (Fig. 8B). Southern blot analysis showed that the ~400-bp DNA amplified from hybridoma, but not from PMN, hybridized with the 32P-labeled 112CDR1 oligonucleotide probe (Fig. 8C). These experiments suggested that the expressed mAb112 VH gene constituted a somatically mutated form of a germline hv1263 or hv1263-like gene. To identify the autologous germline gene that putatively gave rise to the expressed mAb112 VH gene, the DNA amplified from PMN DNA was cloned and sequenced. Throughout the coding area, the sequenced gene (112GL) displayed 94.5% and 98.3% identity with the sequences of the mAb112 VH gene and the germline hv1263 gene, respectively (Fig. 4 and Table I), and was identical to the expressed fetal 51p1 sequence (39). The 112GL sequence shared 6 of the 23 nucleotide differences displayed by mAb112 VH and hv1263 gene sequences, strongly supporting the hypothesis that 112GL is the germline gene that gave rise to the mutated mAb112 VH gene. In this segment, consistent with their selection by Ag, the nucleotide changes were clustered mainly in CDR and resulted in a high R/S ratio (Fig. 4 and Table I).
FIGURE 8.
Evidence for somatic mutations in the mAb112 VH gene. A, ethidium bromide staining of amplified DNA after fractionation in agarose gel electrophoresis. Using the CDR1 (112CDR1) and the FR3 (HI-7) oligonucleotide primers (see Materials and Methods), a PCR amplification product of appropriate size was obtained by priming DNA from the mAb112-producing cell line (hybridoma DNA) but not from autologous PMN (PMN DNA). B, ethidium bromide staining of amplified DNA after fractionation in agarose gel electrophoresis. Using the leader (HI-6) and FR3 (HI-7) sequence oligonucleotide primers, amplification products of identical size were obtained by priming DNA from both mAb112 hybridoma (hybridoma DNA) and autologous PMN (PMN DNA). C, Southern blot hybridization of the PCR products shown in B with the 32P-labeled oligonucleotide 112CDR1 probe encompassing the entire CDR1 of the mAb112 VH gene. A strong, positive hybridization signal was detected only with DNA amplified from the mAb112-producing B cell line.
RF mAb112 and mAb113 are clonally related
Both the utilization of the same sets of VH, D, JH and VL, JL genes and the identity of the junctional VH and VL sequences, including identical N segment additions at both flanks of the D genes, supported the contention that mAb112 and mAb113 were clonally related. The mAb112- and mAb113-producing cell lines were generated from two different 96-well plate microcultures that had been independently seeded with freshly isolated B cells after a mere 1-h infection with EBV (28–32), strongly suggesting that the mAb112- and mAb113-producing cells existed as two related but discrete lymphocytes in vivo before their in vitro immortalization with EBV. In addition to two identical replacement mutations (amino acid residues 34 and 79), mAb112 and mAb113 VH segments accumulated nine and five unique amino acid changes, respectively (Table II). As a result of these changes, the two RF mAb VH segments displayed five conserved (residues 27, 52, 54, 68, and 85) and six nonconserved (residues 32, 33, 42, 57, 59, and 63) amino acid differences. The VκIIIb genes displayed a single identical (silent) change and 13 unique changes (Table II). These yielded six and three putative amino acid replacements in mAb112 and mAb113, respectively. As a result of these changes, the two RF mAb VκIII segments displayed three nonconserved (residues 32, 70, 73) and six conserved (residues 2, 10, 28, 41, 59, and 77) amino acid differences. The relatively high number of unique mutations in RF mAb112 and mAb113 V segments suggested that the mAb diverged early in their expansion from a common progenitor clone.
Table II.
Differences in RF mAb112 and mAb113 VH and VκIIIb segment deduced amino acid sequences
| Region | Residue | Germline Segment | RF Segments |
|
|---|---|---|---|---|
| mAb112 | mAb113 | |||
| VH (112GL) | ||||
| FR1 | 27 | G, polar | S, polar | G, polar |
| CDR1 | 32 | Y, polar | H,a positiveb | Y, polar |
| 33 | A | T, polar | A | |
| 34 | I | L | L | |
| FR2 | 42 | G, polar | R, positive | G, polar |
| CDR2 | 52 | I | T, polar | N, polar |
| 54 | I | V | F | |
| 57 | T, polar | T, polar | R, positive | |
| 59 | N, polar | K, positive | S, polar | |
| 63 | K, positive | K, positive | N, polar | |
| FR3 | 68 | V | L | V |
| 79 | A | T, polar | T, polar | |
| 85 | S, polar | G, polar | S, polar | |
| VκIIIb (Humky 325) | ||||
| FR1 | 02 | I | V | I |
| 10 | T, polar | S, polar | T, polar | |
| CDR1 | 28 | V | V | I |
| 32 | Y, polar | Y, polar | F | |
| FR2 | 41 | P | P | V |
| CDR2 | 59 | I | V | I |
| FR3 | 70 | T, polar | A | T, polar |
| 73 | T, polar | I | T, polar | |
| 77 | S, polar | N, polar | S, polar | |
Nonconserved amino acid differences between mAb112 and mAb113 V segments are underlined.
Positively changed.
Discussion
In this report, we analyzed the structure of human high affinity RF of the IgM class. In addition, we tested the hypothesis that high affinity IgM RF can be somatically mutated and are produced by B-1a cells. We generated four monoreactive high affinity IgM RF mAb using sorted B-1a cells from a patient with rheumatoid arthritis. The B-1a cellular origin of the mAb RF-producing cell lines was confirmed by their expression of surface CD5 and of CD5 mRNA. Comparison of the RF mAb V genes with the closest germline genes revealed the presence of a number of nucleotide differences that resulted from a process of somatic mutation, possible driven by Ag, as formally demonstrated in at least one RF mAb. Thus, affinity maturation of a human autoimmune response can apply to IgM, is independent of Ig class switch and can involve a hyper-mutation process in Ig expressed by B-1a cells.
The high affinity RF mAb utilized two genes of the VHI family in association with either VλI or VκIIIb genes (mAb111, mAb112, and mAb113) and a gene of the VHIII family in association with VκIIIa gene (mAb114). Preferential association of VHIII and VHI with VκIIIa and VκIIIb genes, respectively, has been reported in a number of RF (1–3, 15, 16, 19). In addition, VH and/or VL genes, similar and/or identical to those of the present RF mAb (including the only Vλ gene, VλI, found) encode a variety of other RF, autoantibodies, natural antibodies, and Ig of neoplastic origin (9, 15, 16, 19, 25, 55–58). Many of these antibodies bear cross-reactive idiotypes (CRI), including 17.109, 6B6.6, and G6 (1, 2, 9), that may play an important regulatory role in the development and homeostasis of the B cell repertoire (59), and are predominant at early ontogenetic stages, i.e., in the fetus and in neonatal cord blood (60). Accordingly, the VHI genes utilized by our RF mAb, VI-2 (mAb111) and 112GL (mAb112 and 113) are identical to the 20P3 and 51P1 genes, respectively, which are expressed in the “restricted” fetal antibody repertoire (39). It has been suggested that VH genes expressed early in ontogeny are JH-proximal and frequently encode natural autoantibodies in the neonate and the adult (3, 39, 47, 50). According to the physical map of the human VH locus recently proposed by Matsuda et al. (61), the germline VI-2/20P3 (mAb111) and HHg19 (mAb114) VH genes are among the most JH-proximal genes, located only 125 and 190 kb, respectively, 5′ of the JH locus. However, the germline 51P1 gene (112GL, mAb112 and mAb113) has been mapped 800 to 900 kb 5′ of the JH locus (62). The physical genomic location of the hv1263 gene remains to be determined.
The somatically mutated status of the RF mAb112 VH segment was ascertained and the autologous germline gene that putatively gave rise to it and its clonally related RF mAb113 was identified. Consistent with an Ag-driven selection, mAb112 VH gene CDR and FR displayed R/S ratios (6 and 1.25, respectively) comparable with those of the V genes of some high affinity antibodies and autoantibodies (22, 36, 56, 63–70). Similarly, it seems likely that, the gene encoding the VH region of RF mAb111 constitutes a somatically mutated form of the VI-2 germline gene. Likewise, the RF mAb114 VH gene could represent a mutated form of the HHg19 germline gene or, alternatively, the expression of a still uncharacterized VHIII gene. A number of putative silent mutations were accumulated in mAb113 and mAb114 VH genes. They yielded a relatively low R/S ratio in the CDR. A similar feature was displayed by a human IgM RF reported by Randen et al. (21), and, to a lesser extent, by mAb isolated during the secondary BALB/c response to influenza virus hemagglutinin (HA) (64,65). The occurrence of a “mutational wastage” possibly inherent to an anti-phosphocholine B cell affinity maturation process has been mimicked in vitro by Chen et al. (71).
When compared with the corresponding germline genes, both mAb111 and mAb114 VL segments displayed putative replacement mutations at higher frequencies in the CDR than in the FR. In mAb114, such mutations extended throughout the Jκ segment. Both mAb112 and mAb113 VκIIIb segments displayed high R/S mutation ratios throughout the FR but low R/S mutation ratios in the respective CDR. Thus, the structure of the VκIIIb segments of these RF may be consistent with a role for the FR in Ag-binding. This is further supported by the demonstration that VκIIIb FR residues are directly involved in the binding of IgG Fc fragment (72). The involvement of FR residues in Ag binding would not be limited to VL segments but it may be also a property of VH segments. A high number of FR amino acid substitutions has been observed in VH segments of an IgM RF (21) and a human anti-idiotypic mAb (73). Finally, the participation of both VH and VL FR residues in making contact with Ag and in contributing to affinity maturation has been shown for murine antibodies to phOx (74).
The VH genes utilized by the four mAb RF appeared to have accumulated mutations at a higher rate than did the expressed VL genes. A total of 40 amino acid mutations, of which 18 were verified, were present in the VH segments of the four RF mAb, as compared with 21 accumulated in the VL segments. A higher number of mutations in the VH than in the VL regions has been observed in human and mouse IgG RF. Eleven and three amino acid changes were displayed by the VH and VL regions, respectively, of a human RF IgG reported by Olee et al. (22). Seven amino acid differences were present in the VH region of the murine RF mAb AM 5 (when compared with the related mAb AM 2), but none in the VL region (66). A higher number of mutations in the VH than the VL region is not unique to the autoimmune response to IgG Fc fragment. In six different specific mAb arising in the murine secondary antibody response to influenza virus HA, a total of 41 amino acid changes accumulated in the VH regions compared with only two changes in the VL segments (75). It is not clear why mutations accumulate in some V genes less frequently than in others (66, 68, 76, 77). Different mutational rates may be exerted on different genes. Also, changes in some V regions might lead to Ag-binding loss mutants more frequently than in others.
RF mAb113 is clonally related to mAb112. The two mAb utilize the same sets of VHI-D-JH4 and VκIIIb-Jκ4 genes and display identical junctional sequences, including N segment additions, among which a GAG encoding an identical glutamic acid residue that is thought to be important in IgG Fc fragment binding (15). Because the D and N region sequences are generated by multiple independent events (i.e., sequence of the rearranged D gene, localization of the V-D and V-J splice sites, and random nucleotide addition), it is highly improbable that clones with identical junctional sequences would arise independently. Despite their relatedness, the two RF mAb differed by a number of unique amino acids, a total of 14 and nine in the VH and VκIIIb segments, respectively. Differences in somatic point mutations have been observed in other clonally related antibodies to self and exogenous Ag. Two clonally related human IgM RF reported by Randen et al. (21) displayed a total of nine and eight unique mutations in the VH and VL segments, respectively; a specific murine IgG, mAb H37–88, isolated during a secondary response to influenza virus HA displayed six and two unique amino acid mutations in the VH and VL segments, respectively (75).
The structure of RF mAb112 and mAb113 suggests that their respective producing cells diverged early in clonal expansion and were preceded by a number of intermediate elements. Although they differed in a number of somatic mutations, mAb112 and mAb113 displayed very similar Kd for IgG Fc fragment. This apparent discrepancy may be explained in at least two ways: 1) The two RF mAb are equally efficient in binding Ag. The two identical (residues 34 and 79) and two conserved (residues 52 and 54) amino acid replacements shared by mAb112 and mAb113 are crucial for binding IgG Fc fragment and the additional replacements (a total of 13 in the two VH and six in the two VκIIIb segments) (Table II) do not significantly affect binding to Ag. Consistent with this hypothesis is the suggestion that only 25 to 50% of randomly acquired mutations impair the ability of a VH segment to bind Ag (65, 71, 77); 2) The two RF mAb display similar affinities (Kd) for Ag but differ in their overall Ag-binding efficiencies. Different antibodies with similar affinities can display significant differences in binding the same Ag. For instance, recent studies by Foote and Milstein (78) suggest that association rate (kon) rather than affinity distinguishes the antibodies of the mature from those of the early responses. While most of the specific antibodies arising in the secondary and/or tertiary immune response to phOx display Kd comparable to those of the antibodies that emerged in the late primary response, their kon are at least 10-fold higher. Thus, the antibody-producing cell clones of the secondary and tertiary responses are positively selected because of the higher kon of their surface receptors for Ag. Similarly, the higher number of both VH and VL segment mutations in mAb112, when compared with mAb113, could have resulted in a higher kon in the absence of a significant change in Kd. This hypothesis remains to be tested.
Our data demonstrate that IgM RF can undergo an Ag-directed affinity maturation process mediated by a hyper-mutation mechanism independent of Ig class switch. Evidence that Ig V gene hypermutation can be independent and precedes H chain switch has been provided by analysis of murine IgM arising in the late primary antibody responses to phOx (61) and to β(1–6)-galactan (79), and by the relatively high frequency of mutations in V genes expressed by IgM-bearing circulating B lymphocytes expressing VHV genes in adult humans (80). The notable overlap of the V genes utilized by these and other RF with those of the early antibody repertoire and natural antibodies suggest that high affinity RF arise from physiological natural antibodies through a process of affinity maturation. Application of other antigenic pressures to similar VH genes, e.g., 51P1 utilized by RF mAb112 and mAb113, can drive affinity maturation of responses, involving Ig class switch, to other self Ag and foreign Ag. Mutated variants of the 51P1/hv1263 VH genes are utilized by an anti-cardiolipin/ssDNA IgG mAb (56), by a rabies virus-neutralizing IgG mAb (36), and by an IgG mAb to cytomegalovirus (81).
Based on data in the human (30, 82) and the mouse (12, 83), it has been proposed that the human B cell repertoire includes at least three distinct subsets: (surface CD5+) B-1a cells, which emerge from progenitors in the fetal splanchnic district and persist throughout life by virtue of their self-replenishing nature; (surface CD5−, but CD5 mRNA+) B-1b cells, the progenitors of which can be found in the splanchnic district and, possibly, in adult bone marrow; and (surface CD5− and CD5 mRNA−) B-2 cells (“conventional” B lymphocytes), which arise in the fetal liver and are continuously replenished in adult life by progenitors in the bone marrow. B-2 cells would provide the precursors for specific Ag-induced antibody-producing (mainly IgG) cells and include memory B lymphocytes. B-1a and B-1b cells constitute the source of natural autoantibodies in the fetus, neonate and adult (9–13). In addition, B-1a cells are major contributors to production of autoantibodies in patients with rheumatoid arthritis and Sjogren’s syndrome, and in autoimmune NZB mice (7, 9–14), while B-1b cells contribute significantly to the production of anti-DNA autoantibodies in SLE patients (M. T. Kasaian et al., manuscript in preparation). The ability to induce in vitro CD5 on conventional B cells by use of certain activating agents, such as phorbol ester in humans (84) or anti-IgM in mice (85), has raised the possibility that in some cases CD5 expression occurs on cells not generated by the “fetal” pathway and that such cells differ in other respects from “true” B-1a cells. As yet, this possibility cannot be excluded and remains to be investigated. The present demonstration that somatically mutated IgM RF are produced by B-1a cells suggests that, under different conditions, B-1a and, possibly, B-1b cells, previously committed to natural antibody production, can be driven by Ag to produce different high affinity autoantibodies. Collectively, our findings questions the established dogma that B-1a lymphocytes are primordial cellular elements incapable of undergoing somatic hypermutation, but rather indicate that these lymphocytes can participate in the affinity maturation of a response to self Ag. The factors imposing a selective pressure on B-1a cells and the mechanism leading to the generation of somatically mutated high affinity autoantibodies remain to be determined.
Acknowledgments
We thank Dr. M. T. Kasaian and Dr. J. Hirst for help with fluorescence-activated cell sorting. We are grateful to Dr. H. Ikematsu for help with the gene cloning studies.
Footnotes
This work was supported by U. S. Public Health Service Grant AR-40908. This is publication 18 from The Jeanette Greenspan Laboratory for Cancer Research. P.C. is a Kaplan Cancer Scholar. L. M. was recipient of a fellowship from ITALFARMACO S.p.A., Milan, Italy.
Abbreviations used in this paper: RF, rheumatiod factor(s); CDR, complementarity determining region; FR, framework region; HA, hemagglutinin; Kd, dissociation constant; kon, on rate; PCR, polymerase chain reaction; phOx, 2-phenyl-5-oxazolone; R/S, replacement to silent mutation ratio.
References
- 1.Carson DA, Chen PP, Fox RI, Kipps TJ, Jirik F, Goldfien RD, Silverman G, Radoux V, Fong S. Rheumatoid factors and immune networks. Annu. Rev. Immunol. 1987;5:109. doi: 10.1146/annurev.iy.05.040187.000545. [DOI] [PubMed] [Google Scholar]
- 2.Chen PP, Silverman GP, Liu M-F, Carson DA. Idiotypic and molecular characterization of human rheumatoid factors. In: Carson DA, Chen PP, Kipps TJ, editors. Idiotypes in Biology and Medicine. Vol. 48. Basel: Chem. Immunol., S. Karger; 1990. p. 63. [PubMed] [Google Scholar]
- 3.Chen PP, Olsen NJ, Yang P-M, Soto-Gil RW, Olee T, Siminovitch KA, Carson DA. From human autoantibodies to fetal antibody repertoire to B cell malignancy: it’s a small world after all. Int. Rev. Immunol. 1990;5:239. doi: 10.3109/08830189009056732. [DOI] [PubMed] [Google Scholar]
- 4.Avrameas S. Natural autoantibodies: from “horror autotoxicus” to “gnothi seauton.”. Immunol. Today. 1991;12:154. doi: 10.1016/S0167-5699(05)80045-3. [DOI] [PubMed] [Google Scholar]
- 5.Riboldi P, Kasaian MT, Mantovani L, Ikematsu H, Casali P. Natural antibodies. In: Bona CA, Siminovitch K, Zanetti M, Theofilopoulos AN, editors. The Molecular Pathology of Autoimmune Diseases. Philadelphia, PA: Gordon and Breach Science Publishers; 1993. pp. 45–64. [Google Scholar]
- 6.Casali P, Burastero SE, Nakamura M, Inghirami G, Notkins AL. Human lymphocytes making rheumatoid factor and antibody to ssDNA belong to the Leu-1+ B-cell subset. Science. 1987;236:77. doi: 10.1126/science.3105056. [DOI] [PubMed] [Google Scholar]
- 7.Hardy RR, Hayakawa K, Shimizu M, Yamasaki K, Kishimoto T. Rheumatoid factor secretion from human Leu-1+ B cells. Science. 1987;236:81. doi: 10.1126/science.3105057. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura M, Burastero SE, Notkins AL, Casali P. Human monoclonal rheumatoid factor-like antibodies from CD5 (Leu-1)+ B cells are polyreactive. J. Immunol. 1988;140:4180. [PubMed] [Google Scholar]
- 9.Kipps TJ. The CD5+ B cell. Adv. Immunol. 1989;47:117. doi: 10.1016/s0065-2776(08)60663-x. [DOI] [PubMed] [Google Scholar]
- 10.Kasaian MT, Ikematsu H, Casali P. CD5+ B lymphocytes. Proc. Soc. Exp. Biol. Med. 1991;197:226. doi: 10.3181/00379727-197-43250. [DOI] [PubMed] [Google Scholar]
- 11.Kantor AB. The development and repertoire of B-1 cells (CD5 B cells) Immunol. Today. 1991;12:384. doi: 10.1016/0167-5699(91)90136-H. [DOI] [PubMed] [Google Scholar]
- 12.Hardy RR. Variable gene usage, physiology and development of Ly-1+ (CD5+) B cells. Curr. Opin. Immunol. 1992;4:181. doi: 10.1016/0952-7915(92)90010-c. [DOI] [PubMed] [Google Scholar]
- 13.Casali P, Kasaian MT, Haughton G. B-1 (CD5 B) cells. In: Coutinho A, Kazatchkine MD, editors. Autoimmunity. New York: John Wiley; 1993. In press. [Google Scholar]
- 14.Burastero SE, Casali P. Characterization of human CD5 (Leu-1, OKT1)+ B lymphocytes and the antibodies they produce. Contrib. Microbiol. Immunol. 1989;11:231. [PubMed] [Google Scholar]
- 15.Pascual V, Randen I, Thompson K, Sioud M, Ferre O, Natvig J, Capra JD. The complete nucleotide sequences of the heavy chain variable regions of six monospecific rheumatoid factors derived from Epstein-Barr virus-transformed B cells isolated from the synovial tissue of patients with rheumatoid arthritis. J. Clin. Invest. 1990;86:1320. doi: 10.1172/JCI114841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Victor KD, Randen I, Thompson K, Forre O, Natvig JB, Fu SM, Capra JD. Rheumatoid factors isolated from patients with autoimmune disorders are derived from germline genes distinct from those encoding the Wa, Po, and B1a cross-reacting idiotypes. J. Clin. Invest. 1990;87:1603. doi: 10.1172/JCI115174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harindranath N, Goldfarb IS, Ikematsu H, Burastero SE, Wilder RL, Notkins AL, Casali P. Complete sequence of the genes encoding the VH and VL regions of low and high affinity monoclonal IgM and IgA1 rheumatoid factors produced by CD5+ B cells from a rheumatoid arthritis patient. Int. Immunol. 1991;3:865. doi: 10.1093/intimm/3.9.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams RC, Jr, Malone CC, Casali P. Heteroclitic polyclonal and monoclonal anti-Gm(a) and anti-Gm(g) human rheumatoid factors react with epitopes induced in Gm (a-), Gm(g-) IgG by interaction with antigen or by non-specific aggregation. A possible mechanism for the in vivo generation of rheumatoid factors. J. Immunol. 1992;149:1817. [PMC free article] [PubMed] [Google Scholar]
- 19.Pascual V, Victor K, Randen I, Thomson K, Steinitz M, Forre O, Fu S-M, Natvig JB, Capra JD. Nucleotide sequence analysis of rheumatoid factors and polyreactive antibodies derived from patients with rheumatoid arthritis reveals diverse use of VH and VL gene segments and extensive variability in CDR3. Scand. J. Immunol. 1992;36:349. doi: 10.1111/j.1365-3083.1992.tb03108.x. [DOI] [PubMed] [Google Scholar]
- 20.Lee KL, Bridges SL, Jr, Koopman WJ, Schroeder HW., Jr The immunoglobulin kappa light chain repertoire expressed in the synovium of a patient with rheumatoid arthritis. Arthritis Rheum. 1992;35:905. doi: 10.1002/art.1780350809. [DOI] [PubMed] [Google Scholar]
- 21.Randen I, Brown D, Thompson KM, Hughens-Jones N, Pascual V, Victor K, Capra JD, Forre O, Natvig JB. Clonally related IgM rheumatoid factors, undergo affinity maturation in the rheumatoid synovial tissue. J. Immunol. 1992;148:3296. [PubMed] [Google Scholar]
- 22.Olee T, Liu EW, Huang D-F, Soto-Gil RW, Deftos M, Kozin F, Carson DA, Chen PP. Genetic analysis of self-associating immunoglobulin G rheumatoid factors from two rheumatoid synovia implicates an antigen driven response. J. Exp. Med. 1992;172:831. doi: 10.1084/jem.175.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soto-Gil RW, Olee T, Klink BK, Kenny TP, Robbins DL, Carson DA, Chen PP. A systematic approach to defining the germline gene counterparts of a mutated autoantibody from a patient with rheumatoid arthritis. Arthritis Rheum. 1992;35:356. doi: 10.1002/art.1780350316. [DOI] [PubMed] [Google Scholar]
- 24.Gause A, Kuppers R, Mierau R. A somatically mutated VκIV gene encoding a human rheumatoid factor light chain. Clin. Exp. Immunol. 1992;88:430. doi: 10.1111/j.1365-2249.1992.tb06467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kipps TJ, Tomhave E, Chen PP, Carson DA. Autoantibody-associated kappa light chain variable region gene expressed in chronic lymphocytic leukemia with little or not somatic mutation. Implications for etiology and immunotherapy. J. Exp. Med. 1988;167:840. doi: 10.1084/jem.167.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanz I, Casali P, Thomas JW, Notkins AL, Capra JD. Nucleotide sequences of eight human natural autoantibodies VH region reveals apparent restricted use of VH families. J. Immunol. 1989;142:4054. [PubMed] [Google Scholar]
- 27.Casali P. Immunoglobulin M. In: Roitt IM, Delves PJ, editors. Encyclopaedia of Immunology. II. Harcourt Brace Jovanovich, London: Academic Press Ltd.; 1992. pp. 743–747. [Google Scholar]
- 28.Ueki Y, Goldfarb IS, Harindranath N, Gore M, Koprowski H, Notkins AL, Casali P. Clonal analysis of a human antibody response. Quantitation of precursors of antibody-producing cells and generation and characterization of monoclonal IgM, IgG and IgA to rabies virus. J. Exp. Med. 1990;171:19. doi: 10.1084/jem.171.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kasaian MT, Casali P. Analysis of the human CD5−CD45RAlow B cell subset. Ann. NY Acad. Sci. 1992;651:59. doi: 10.1111/j.1749-6632.1992.tb24593.x. [DOI] [PubMed] [Google Scholar]
- 30.Kasaian MT, Ikematsu H, Casali P. Identification and analysis of a novel CD5− B lymphocyte subset producing natural antibodies. J. Immunol. 1992;148:2690. [PMC free article] [PubMed] [Google Scholar]
- 31.Nakamura M, Burastero SE, Ueki Y, Larrick JW, Notkins AL, Casali P. Probing the normal and autoimmune B cell repertoire with EBV. Frequency of B cells producing monoreactive high affinity autoantibodies in patients with Hashimoto’s disease and SLE. J. Immunol. 1988;141:4165. [PubMed] [Google Scholar]
- 32.Larrick JW, Chiang YL, Sheng-Dong R, Senyk G, Casali P. Generation of specific monoclonal antibodies by in vitro expansion of human B cells. A novel recombinant DNA approach. In: Borrebaek CAK, editor. In Vitro Immunization in Hybridoma Technology. Amsterdam: Elsevier; 1988. pp. 231–246. [Google Scholar]
- 33.Friguet B, Chaffotte AF, Djavadi,-Ohaniance L, Goldberg ME. Measurement of the true affinity constant in solution of antigen-antibody complexes by enzyme-linked immunosorbent assay. J. Immunol. Methods. 1985;77:305. doi: 10.1016/0022-1759(85)90044-4. [DOI] [PubMed] [Google Scholar]
- 34.Nakajima-Iijima S, Hamada H, Reddy P, Kakunaga T. Molecular structure of the human cytoplasmic β-actin gene: interspecies homology of sequences in the introns. Proc. Natl. Acad. Sci. USA. 1985;82:6133. doi: 10.1073/pnas.82.18.6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones NH, Clabby ML, Dialynas DP, Huang H-JS, Herzenberg LA, Strominger JL. Isolation of complementary DNA clones encoding the human lymphocyte glycoprotein T1/Leu-1. Nature. 1986;323:346. doi: 10.1038/323346a0. [DOI] [PubMed] [Google Scholar]
- 36.Ikematsu H, Harindranath N, Ueki Y, Notkins AL, Casali P. Clonal analysis of a human antibody response. II. Sequences of the VH genes of human monoclonal IgM, IgG and IgA to rabies virus reveal preferential utilization of the VHIII family members and somatic hypermutation. J. Immunol. 1993;150:1325. [PMC free article] [PubMed] [Google Scholar]
- 37.Parson WR. Rapid and sensitive sequence comparison with FASTP and FASTA. Methods Enzymol. 1990;183:63. doi: 10.1016/0076-6879(90)83007-v. [DOI] [PubMed] [Google Scholar]
- 38.Shin EK, Matsuda F, Nagaoka H, Fukita Y, Imai T, Yokoyama K, Soeda E, Honjo T. Physical map of the 3′ region of the human immunoglobulin heavy chain locus: clustering of autoantibody-related variable segments in one haplotype. EMBO J. 1991;10:361. doi: 10.1002/j.1460-2075.1991.tb04930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schroeder HW, Jr, Hillson JL, Perlmutter RM. Early restriction of the human antibody repertoire. Science. 1987;238:791. doi: 10.1126/science.3118465. [DOI] [PubMed] [Google Scholar]
- 40.Chen PP, Liu M-F, Glass CA, Sinha S, Kipps TJ, Carson DA. Characterization of two immunoglobulin VH genes that are homologous to human rheumatoid factors. Arthritis Rheum. 1989;32:72. doi: 10.1002/anr.1780320112. [DOI] [PubMed] [Google Scholar]
- 41.Ravetch JV, Siebenlist U, Korsmeyer S, Waldmann T, Leder P. Structure of the human immunoglobulin µ locus: characterization of embryonic and rearranged J and D genes. Cell. 1981;27:583. doi: 10.1016/0092-8674(81)90400-1. [DOI] [PubMed] [Google Scholar]
- 42.Siebenlist V, Ravetch JV, Korsmeyer S, Waldmann T, Leder P. Human immunoglobulin D segments encoded in tandem multigene families. Nature. 1981;294:631. doi: 10.1038/294631a0. [DOI] [PubMed] [Google Scholar]
- 43.Ichihara Y, Matsuoka H, Kurosawa Y. Organization of human immunoglobulin heavy chain diversity gene loci. EMBO J. 1988;7:4141. doi: 10.1002/j.1460-2075.1988.tb03309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buluwela L, Albertson DG, Sherrington P, Rabbitts PH, Spurr N, Rabbitts TH. The use of chromosomal translocations to study human immunoglobulin gene organization: mapping DH segments within 35 kb of the Cµ gene and identification of a new DH locus. EMBO J. 1988;7:2003. doi: 10.1002/j.1460-2075.1988.tb03039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsuda R, Kee KH, Nakai S, Sato T, Kodaira M, Zong SQ, Ohno H, Fukuhara S, Honjo T. Dispersed localization of D segments in the human immunoglobulin heavy-chain locus. EMBO J. 1988;7:1047. doi: 10.1002/j.1460-2075.1988.tb02912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamada M, Wasserman R, Reichard BA, Shane S, Caton AJ, Rovera G. Preferential utilization of specific immunoglobulin heavy chain diversity and joining segments in adult human peripheral blood B lymphocytes. J. Exp. Med. 1991;173:395. doi: 10.1084/jem.173.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schroeder HW, Jr, Wang JY. Preferential utilization of conserved immunoglobulin heavy chain variable gene segments during human fetal life. Proc. Natl. Acad. Sci. USA. 1990;87:6146. doi: 10.1073/pnas.87.16.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kabat EA, Wu TT, Perry HM, Gottesman KS, Foeller C. Sequences of Proteins of Immunological Interest. 5th Ed. Washington, DC: U. S. Department of Health and Human Services; 1991. [Google Scholar]
- 49.Liu MR, Robbins DL, Crowley JJ, Sinha S, Kozin R, Kipps TJ, Carson DA, Chen PP. Characterization of four homologous L chain variable region genes that are related to 6B6.6 idiotype positive human rheumatoid factor L chain. J. Immunol. 1989;142:688. [PubMed] [Google Scholar]
- 50.Siminovitch KA, Misener V, Kwong PC, Song Q-L, Chen PP. A natural antibody is encoded by germline heavy and lambda light chain variable region genes without somatic mutation. J. Clin. Invest. 1989;84:1675. doi: 10.1172/JCI114347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beristein N, Levy S, Levy R. Activation of an excluded immunoglobulin allele in a human B lymphoma cell line. Science. 1989;244:337. doi: 10.1126/science.2496466. [DOI] [PubMed] [Google Scholar]
- 52.Hieter PA, Maizel JV, Jr, Leder P. Evolution of human immunoglobulin κ J region gene. J. Biol. Chem. 1982;257:1516. [PubMed] [Google Scholar]
- 53.Martin T, Blaison G, Levallois H, Pasquali JL. Molecular analysis of the VκIII-Jκ junctional diversity of polyclonal rheumatoid factors during rheumatoid arthritis frequently reveals N addition. Eur. J. Immunol. 1992;22:1773. doi: 10.1002/eji.1830220716. [DOI] [PubMed] [Google Scholar]
- 54.Udey JA, Blomberg B. Human λ light chain locus: organization and DNA sequences of three genomic J regions. Immunogenetics. 1987;25:63. doi: 10.1007/BF00768834. [DOI] [PubMed] [Google Scholar]
- 55.Kipps TJ, Robbins BA, Carson DA. Uniform high frequency expression of autoantibody-associated cross-reactive idiotypes in the primary B cell follicles of human fetal spleen. J. Exp. Med. 1990;171:189. doi: 10.1084/jem.171.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Es JH, Aanstoot H, Gmelig-Meyling FHJ, Derksen RHWM, Logtenberg T. A human systemic lupus erythematosus-related anti-cardiolipin/single-strand DNA autoantibody is encoded by somatically mutated variant of the developmentally restricted 51P1 VH gene. J. Immunol. 1992;149:2234. [PubMed] [Google Scholar]
- 57.Pascual V, Capra JD. Human immunoglobulin heavy-chain variable region genes: organization, polymorphism, and expression. Adv. Immunol. 1991;49:1. doi: 10.1016/s0065-2776(08)60774-9. [DOI] [PubMed] [Google Scholar]
- 58.Newkirk MM, Mageed RA, Jefferis R, Chen PP, Capra JD. Complete amino acid sequences of variable regions of two human IgM rheumatoid factors, Bor and Kas of the Wa idiotypic family, reveal restricted use of heavy and light chain variable and joining region gene segments. J. Exp. Med. 1987;166:550. doi: 10.1084/jem.166.2.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Siminovitch KA, Chen PP. The biological significance of human natural autoimmune responses: relationship to the germline, early immune and malignant B cell variable gene repertoire. Int. Rev. Immunol. 1990;5:265. doi: 10.3109/08830189009056734. [DOI] [PubMed] [Google Scholar]
- 60.Lydyard PM, Quarter-Papafino RP, Bröker BM, Mackenzie L, Hay FC, Youinou PY, Jefferis R, Mageed RA. The antibody repertoire of early human B cells. III. Expression of cross-reactive idiotypes characteristic of certain rheumatoid factor and identifying VκIII, VHI and VHIII gene family products. Scand. J. Immunol. 1990;32:709. doi: 10.1111/j.1365-3083.1990.tb03214.x. [DOI] [PubMed] [Google Scholar]
- 61.Matsuda F, Shin EK, Nagaoka H, Matsumura R, Haino M, Fukita Y, Taka-ishi S, Imai T, Riley JH, Anand R, Soeda E, Honjo T. Structure and physical map of 64 variable segments in the 3′ 0.8-megabase region of the human immunoglobulin heavy-chain locus. Nature Genetics. 1993;3:88. doi: 10.1038/ng0193-88. [DOI] [PubMed] [Google Scholar]
- 62.Willems van Dijk K, Milner LA, Sasso EH, Milner ECB. Chromosomal organization of the heavy chain variable region gene segments comprising the human fetal antibody repertoire. Proc. Natl. Acad. Sci. USA. 1992;89:10430. doi: 10.1073/pnas.89.21.10430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rudikoff S, Pawlita M, Pumphrey J, Heller M. Somatic diversification of immunoglobulins. Proc. Natl. Acad. Sci. USA. 1984;81:2162. doi: 10.1073/pnas.81.7.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McKean D, Huppi K, Bell M, Staud L, Gerhard W, Weigert MG. Generation of antibody diversity in the immune response of BALB/c mice to influenza virus hemagglutinin. Proc. Natl. Acad. Sci. USA. 1984;81:3180. doi: 10.1073/pnas.81.10.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Clarke HS, Huppi K, Ruezinsky D, Staudt L, Gerhard W, Weigert MG. Inter- and intraclonal diversity in the antibody response to influenza hemagglutinin. J. Exp. Med. 1985;161:687. doi: 10.1084/jem.161.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shlomchik MJ, Marshak-Rothstein A, Wolfowicz CB, Rothstein TL, Weigert MG. The role of clonal selection and somatic mutation in autoimmunity. Nature. 1987;328:805. doi: 10.1038/328805a0. [DOI] [PubMed] [Google Scholar]
- 67.Berek C, Milstein C. Mutation drift and repertoire shift in the maturation of the immune response. Immunol. Rev. 1987;96:23. doi: 10.1111/j.1600-065x.1987.tb00507.x. [DOI] [PubMed] [Google Scholar]
- 68.Levy NS, Malipiero UV, Lebecque SG, Gearhart PJ. Early onset of somatic mutation in immunoglobulin VH genes during the primary immune response. J. Exp. Med. 1989;169:2007. doi: 10.1084/jem.169.6.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shlomchik M, Mascelli M, Shan H, Radic MZ, Pisetsky D, Marshak-Rothstein A, Weigert MG. Anti-DNA antibodies from autoimmune mice arise by clonal expansion and somatic mutation. J. Exp. Med. 1990;171:265. doi: 10.1084/jem.171.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Manheimer-Lory A, Katz JB, Pillinger M, Ghossein C, Smith A, Diamond B. Molecular characteristics of antibodies bearing an anti-DNA-associated idiotype. J. Exp. Med. 1991;174:1639. doi: 10.1084/jem.174.6.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen C, Roberts VA, Rittenberg MB. Generation and analysis of random point mutations in an antibody CDR2 sequence: many mutated antibodies lose their affinity to bind antigen. J. Exp. Med. 1992;176:855. doi: 10.1084/jem.176.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hay FC, Soltys AJ, Tribbik G, Geyem HM. Framework peptides from κIIIb rheumatoid factor light chains with binding activity for aggregated IgG. Eur. J. Immunol. 1991;21:1837. doi: 10.1002/eji.1830210809. [DOI] [PubMed] [Google Scholar]
- 73.van der Heijden RW, Bunschoten H, Pascual V, Uytdehaag FGCM, Osterhaus ADME, Capra JD. Nucleotide sequencing of a human monoclonal anti-idiotypic antibody specific for a rabies virus neutralizing monoclonal idiotypic antibody reveals extensive somatic variability suggestive of an Ag-driven immune response. J. Immunol. 1990;144:2835. [PubMed] [Google Scholar]
- 74.Alzari PM, Spinelli S, Mariuzza RA, Boulot G, Poljak RJ, Jarvis JM, Milstein C. Three-dimensional structure determination of an anti-phenyloxazolone antibody: the role of somatic mutation and heavy/light chain pairing in the maturation of an immune response. EMBO J. 1990;9:3807. doi: 10.1002/j.1460-2075.1990.tb07598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Clarke S, Rickert R, Wloch MK, Staudt L, Gerhard W, Weigert MG. The BALB/c secondary response to the Sb site of influenza virus hemagglutinin. Non-random mutation and unequal number of VH and Vκ mutations. J. Immunol. 1990;145:2286. [PubMed] [Google Scholar]
- 76.Cumano A, Rajewsky K. Clonal recruitment and somatic mutation in the generation of immunological memory to the hapten NP. EMBO J. 1986;5:2459. doi: 10.1002/j.1460-2075.1986.tb04522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Caton AJ, Browle GG, Staudt LM, Gerhard W. Structural and functional implications of a restricted antibody response to a defined antigenic region on the influenza virus hemagglutinin. EMBO J. 1986;5:1577. doi: 10.1002/j.1460-2075.1986.tb04399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Foote J, Milstein C. Kinetic maturation of an immune response. Nature. 1991;352:530. doi: 10.1038/352530a0. [DOI] [PubMed] [Google Scholar]
- 79.Hartman AB, Rudikoff S. VH genes encoding the immune response to β-(1, 6)-galactan: somatic mutation in IgM molecules. EMBO J. 1984;3:3023. doi: 10.1002/j.1460-2075.1984.tb02249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van Es JH, Gmelig-Meyling FHJ, Logtenberg T. High frequency of somatically mutated IgM molecules in the human blood B cell repertoire. Eur. J. Immunol. 1992;22:2761. doi: 10.1002/eji.1830221046. [DOI] [PubMed] [Google Scholar]
- 81.Newkirk MM, Gram H, Heinrich F, Östberg L, Capra JD, Wasserman RL. Complete protein sequences of the variable regions of the cloned heavy chain of a human anti-cytomegalovirus antibody reveal a striking similarity to human monoclonal rheumatoid factors of the wa idiotypic family. J. Clin. Invest. 1988;81:1511. doi: 10.1172/JCI113483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sovalson N, Kearney JR. The human fetal omentum: a site of B cell generation. J. Exp. Med. 1992;175:397. doi: 10.1084/jem.175.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kantor AB, Stall AM, Adams S, Herzenberg LA, Herzenberg LA. Differential development of progenitor activity for three B cell lineages. Proc. Natl. Acad. Sci. USA. 1992;89:3320. doi: 10.1073/pnas.89.8.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Werner-Favre C, Vischer TL, Wohlwend D, Zubler RH. Cell surface CD5 is a marker for activated human B cells. Eur. J. Immunol. 1989;19:1209. doi: 10.1002/eji.1830190709. [DOI] [PubMed] [Google Scholar]
- 85.Ying-zi C, Rabin E, Wortis HH. Treatment of murine CD5− B cells with anti-Ig, but not LPS, induces surface CD5: two B-cell activation pathways. Int. Immunol. 1991;3:467. doi: 10.1093/intimm/3.5.467. [DOI] [PubMed] [Google Scholar]