Abstract
Background: Fetal alcohol spectrum disorders (FASDs) are conditions characterized by physical anomalies, neurodevelopmental abnormalities, and neurocognitive deficits, including intellectual, executive, and memory deficits. There are no specific biological treatments for FASDs, but rodent models have shown that prenatal or postnatal choline supplementation reduces cognitive and behavioral deficits. Potential mechanisms include phospholipid production for axonal growth and myelination, acetylcholine enhancement, and epigenetic effects.
Objective: Our primary goal was to determine whether postnatal choline supplementation has the potential to improve neurocognitive functioning, particularly hippocampal-dependent memory, in children with FASDs.
Design: The study was a double-blind, randomized, placebo-controlled pilot trial in children (aged 2.5–5 y at enrollment) with FASDs (n = 60) who received 500 mg choline or a placebo daily for 9 mo. Outcome measures were Mullen Scales of Early Learning (primary) and the elicited imitation (EI) memory paradigm (secondary).
Results: The administration proved feasible, and choline was well tolerated. Participants received a dose on 88% of enrolled days. The only adverse event linked to choline was a fishy body odor. Choline supplementation improved the secondary outcome (EI) only after immediate recall performance was controlled for, and the outcome was moderated by age. The treatment effect on EI items recalled was significant in the younger participants (2.5- to ≤4.0-y-olds); the young choline group showed an increase of 12–14 percentage points greater than that of the young placebo group on delayed recall measures during treatment. However, there was a marginal baseline difference in delayed item recall between the young choline and placebo groups as well as a potential ceiling effect for item recall, both of which likely contributed to the observed treatment effect. We also observed a trend toward a negative effect of choline supplementation on the immediate EI recall of ordered pairs; the young placebo group showed an increase of 8–17 percentage points greater than that of the choline group during treatment. There was an inverse relation between choline dose (in mg/kg) and memory improvement (P = 0.041); the data suggest that weight-adjusted doses may be a better alternative to a fixed dose in future studies. Limitations included trend-level baseline differences in performance, the post-hoc determination of age moderation, and potential ceiling effects for the memory measure.
Conclusions: This pilot study suggests that an additional evaluation of choline supplementation as an intervention for memory functioning in children with FASDs is warranted. The observed interaction between age and choline’s effect on EI suggests that potential sensitive periods should be considered in future work. This trial was registered at clinicaltrials.gov as NCT01149538.
Keywords: children, fetal alcohol spectrum disorder, fetal alcohol syndrome, memory, randomized double-blind placebo-controlled trial
INTRODUCTION
Fetal alcohol spectrum disorders (FASDs)9 represent a profound public health crisis with prevalence estimates as high as 2–5% in the United States and Western Europe (1). Individuals with fetal alcohol syndrome (FAS), which is the most severe form of FASD, have high rates of intellectual impairment (2, 3). Individuals with other FASDs, including partial fetal alcohol syndrome (pFAS) and alcohol-related neurodevelopmental disorder, are seriously affected by deficits in attention, executive functioning, and memory among other skills (4–6). Currently, there have been very few cognitive and behavioral interventions for FASDs (7–9), and there are no biological treatments.
Preclinical models of FASDs have consistently identified the hippocampus as particularly vulnerable to insult, and numerous experiments revealed memory deficits in animals exposed to alcohol prenatally (10–12). Studies of memory in children with prenatal alcohol exposure also have shown deficits in hippocampus-mediated encoding processes (13, 14). In normally developing animals, choline supplementation during gestation and the early postnatal period enhances performances on measures of cognition including memory (15–17). In animals exposed prenatally to alcohol, there has been strong evidence that dietary choline supplementation prenatally during hippocampal neurogenesis and postnatally, as late as days 21–30 during rapid hippocampal differentiation in the rodent (equivalent to human early childhood), attenuates memory and behavioral deficits that are normally observed (18, 19). Improvements have been seen in cognitive functions and behaviors that rely on the hippocampus including visual-spatial learning, spatial reversal learning, and fear conditioning (20).
At the time of the writing of this article, there were no published human trials of choline supplementation in FASDs to our knowledge. We previously reported on a pilot study that examined the safety and tolerability of choline in children with FASDs (n = 20) (21), and in the current study, we report on the results of the completed trial that included the participants from the previous pilot study. The trial enrolled 60 participants with FASDs who were aged 2.5–5 y. The goals of the overall study were to establish the feasibility of long-term choline supplementation in a large sample of children with FASDs and, because of compelling basic science findings, to examine the efficacy of choline as a neurocognitive treatment by specifically targeting behaviors dependent on hippocampal integrity. Thus, we hypothesized that choline would improve performances on a hippocampus-dependent memory task [elicited imitation (EI)] and, more generally, on a test of global cognitive functioning.
METHODS
The study was a randomized, double-blind, placebo-controlled trial conducted at the University of Minnesota from June 2010 to May 2014. Participants underwent an informed consent process, and all procedures were approved by the University’s Institutional Review Board. Additional oversight was provided by the University’s clinical trial monitoring program as well as an independent Data Safety Monitoring Board. Choline was administered under the Federal Drug Administration Investigational New Drug application 107085. The trial was registered at clinicaltrials.gov as NCT01149538 on 21 June 2010 before the first participant’s enrollment. A complete description of methods and procedures was reported in Wozniak et al. (21).
Subjects
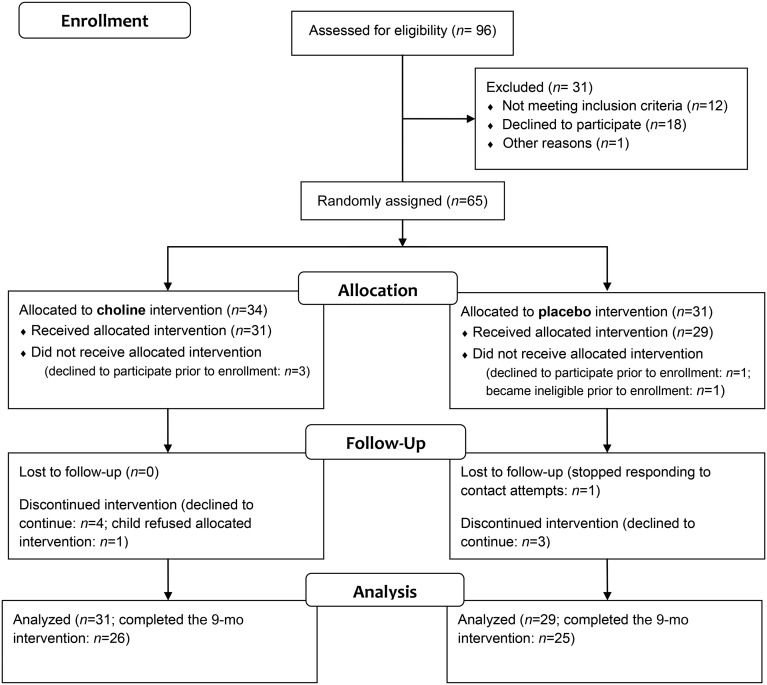
Children with FASDs (aged 2.5–5.0 y at enrollment) were recruited from the University’s FASD Clinic and International Adoption Clinic. Sixty children received the allocated intervention (Table 1) of whom 85% (n = 51) completed the 9-mo study (Figure 1).
TABLE 1.
Baseline characteristics of participants who received the allocated intervention1
| Choline (n = 31) | Placebo (n = 29) | |
| Age, y | 3.79 ± 0.802 | 3.92 ± 0.76 |
| Sex, n (%) | ||
| M | 12 (39) | 10 (35) |
| F | 19 (61) | 19 (65) |
| Racial categories, n (%) | ||
| White | 16 (52) | 9 (31) |
| Black or African American | 5 (16) | 9 (31) |
| American Indian/Alaska Native | 4 (13) | 7 (24) |
| Asian | 1 (3) | 1 (4) |
| More than one race | 5 (16) | 3 (10) |
| Ethnic category, n (%) | ||
| Hispanic or Latino | 1 (3) | 1 (3) |
| Not Hispanic or Latino | 29 (94) | 27 (93) |
| Unknown | 1 (3) | 1 (4) |
| Dysmorphic facial features, n (%) | ||
| Lip (score of 4 or 5) | 16 (52) | 10 (35) |
| Philtrum (score of 4 or 5) | 13 (42) | 9 (31) |
| Palpebral fissure (≤10th percentile)3 | 21 (68) | 20 (69) |
| ≥2 facial features present | 20 (65) | 17 (59) |
| Growth deficiency (≤10th percentile), n (%) | ||
| Height3 | 4 (13) | 0 (0) |
| Weight | 5 (16) | 1 (3) |
| Deficient brain growth (≤10th percentile) OFC,3 n (%) | 3 (10) | 0 (0) |
| Alcohol exposure, n (%) | ||
| Alcohol confirmed | 24 (77) | 26 (90) |
| Alcohol suspected | 7 (23) | 3 (10) |
| Drug exposure, n (%) | ||
| Other drug exposure suspected | 21 (68) | 21 (72) |
| IOM diagnostic category, n (%) | ||
| FAS | 5 (16) | 5 (17) |
| Partial FAS | 15 (48) | 11 (38) |
| ARND | 11 (36) | 13 (45) |
| Baseline cognitive-functioning scores4 | ||
| Mullen Visual Reception | 41 ± 12 | 44 ± 16 |
| Mullen Fine Motor | 42 ± 9 | 40 ± 15 |
| Mullen Receptive Language | 41 ± 9 | 40 ± 12 |
| Mullen Expressive Language | 40 ± 8 | 41 ± 11 |
| Mullen Early Learning Composite | 83 ± 14 | 84 ± 21 |
ARND, alcohol-related neurodevelopmental disorder; FAS, fetal alcohol syndrome; IOM, Institute of Medicine; OFC, occipital-frontal circumference.
Mean ± SD (all such values).
Data from the study baseline were missing from participants who were uncooperative and from whom an accurate measure could not be obtained for palpebral fissure length (choline: n = 3), height (choline: n = 1), and OFC (choline: n = 1). Data acquired from previous clinical evaluations are included for these participants.
Data were missing for 2 participants (choline: n = 2) who were unable to finish testing.
FIGURE 1.
Flowchart of the randomized clinical trial of postnatal choline supplementation.
Exclusion criteria were the presence of another developmental disorder (e.g., autism, Down syndrome), neurologic disorder, traumatic brain injury, or other medical condition that affects the brain. Psychiatric comorbidity, such as attention-deficit hyperactivity disorder or learning disorder, was not exclusionary because comorbidity is common with FASDs (22). All but one participant (a twin born at 36 wk who weighed1360 g) had a birth weight >1500 g.
To characterize the sample diagnostically, we applied modified Institute of Medicine (IOM) criteria (23) to the growth, facial dysmorphology, and alcohol-exposure data collected in the clinic and during the baseline visit. Of 60 participants, 10 individuals (17%) met the criteria for FAS; 24 individuals (40%) met criteria for pFAS; and 24 individuals (40%) met criteria for alcohol-related neurodevelopmental disorder (Table 1).
Because IOM criteria do not specifically characterize cognitive functioning, we further applied CDC central nervous system (CNS) criteria for FASDs (24) [see our previous article for details (21)]. Nineteen participants (32%) met the CNS criteria for an FASD diagnosis on the basis of deficient brain growth, whereby 15 subjects (25%) had global cognitive impairment (>2 SDs below the average), and 57 subjects (95%) had deficits of >1 SD in ≥3 domains (e.g., intellectual, language, motor, visual-perceptual, adaptive functioning, and behavioral domains). One participant (2%) was deficient in only one domain. Twenty-six participants (43%) met ≥2 CNS criteria.
Eighty-three percent of subjects (n = 50) had confirmed prenatal alcohol exposure, including a self-report by the biological mother or social service records that indicated heavy maternal use during pregnancy. Participants with maternal alcohol use at rank 3 or 4 in the University of Washington diagnostic system (25) were included. Ten participants had unconfirmed alcohol exposure, but alcohol use was suspected, and all 10 subjects had dysmorphic faces and cognitive deficits as previously defined. The 10 subjects met the modified IOM criteria for FAS (n = 1) or pFAS (n = 9). In 42 cases, other prenatal drug use was suspected. There was no difference in suspected drug use between the 2 treatment arms (Table 1). In all cases, alcohol was the predominant substance of abuse, and alcohol use was extensive.
Procedures
Participants received the supplement daily for 9 mo. The length of the intervention was selected to measure the potential developmental change in response to the treatment and to maintain a feasible daily administration of the experimental agent in preschool-age children, who are a group for whom supplementation can be challenging.
The study was designed to be completed in 2 phases. The primary outcome assessed in the first phase was side effects or adverse events, the results of which have been reported previously (21). The second phase was designed to further evaluate safety and tolerability and to measure the effect of choline on neurocognitive functioning. The primary outcome was an assessment of global cognitive functioning with the use of the Mullen Scales of Early Learning. The secondary outcome was an assessment of hippocampal-dependent function with the use of the EI memory task. Event-related potential data were also collected as an electrophysiological measure of brain functioning; however, event-related potential results are not reported in the current article because they were beyond the scope of the current report.
In-person assessments took place at the University of Minnesota at baseline (before receiving the allocated intervention) and at 6 and 9 mo. Phone visits occurred 2 wk after baseline and then monthly to monitor compliance and adverse events. The Mullen Scales of Early Learning were administered at baseline and at 9 mo. EI was administered at all 3 visits (at baseline and at 6 and 9 mo).
Allocated intervention
Participants were randomly assigned in a one-to-one allocation ratio with the use of preprepared computerized block-randomization schedules by the University’s Investigational Drug Services unit to receive 500 mg choline (1.25 g choline bitartrate) or a placebo daily for 9 mo. A concealed allocation was implemented, and the research team and participants were blinded to group assignments. The allocated intervention was supplied in coded light-blocking foil packets that contained a powdered, fruit-flavored drink mix that was developed for the study. Packet dosages and stability were evaluated with the use of HPLC by an outside laboratory. The dosage was within 0.13% of the target, on average, and stable (within 5.2% of target dosage over the study duration). Parents were instructed to administer 1 dose/d by mixing it with 4 fl oz (118.3 mL) H2O.
Measures
Feasibility of choline supplementation
Compliance, fidelity, and adverse events were monitored via calendar log sheets, dietary recalls, and serum choline concentrations. Parents used calendar log sheets to document the proportion of the allocated intervention the children consumed each day. If <100% of the drink was consumed, the amount and reason were recorded.
Detailed 24-h food recalls were administered at baseline and at 6 and 9 mo with the use of the automated self-administered 24-h recall system (26) to evaluate the potential confounding influence of changes in dietary choline intake. Dietary data were included only if the parent recalled all meals and snacks from the 24-h period (e.g., data were not included if the parent had no information about the child’s intake at school on that day). Numbers of subjects are reported for all dietary intake data. Families were instructed to refrain from adding dietary supplements during the study, which was reinforced at all study visits including phone visits.
Serum choline and betaine concentrations were measured at baseline and at 6 and 9 mo. Parents were asked to administer the allocated intervention 3 h before the scheduled blood draw (venipuncture). Choline and betaine were assayed with the use of liquid chromatography/electrospray ionization–isotope dilution mass spectrometry (27).
A physician completed a physical examination at each in-person visit, which included a review of major organ systems, to assess adverse events. Adverse events were also monitored during monthly phone visits. Changes in body or urine odor were monitored because high serum choline concentrations have been associated with fishy odor that is due to trimethylamine formation (28). For a subsample, plasma was assayed for trimethylamine N-oxide (TMAO) with the use of liquid chromatography/electronspray ionization–isotope dilution mass spectrometry (29). The TMAO assay was added midway through the study and was only done on a random subset because of the cost. Compliance problems with the administration of the allocated intervention were also monitored monthly.
Mullen Scales of Early Learning (primary outcome)
The Mullen Scales of Early Learning (30) is a measure of global cognitive development with the use of normative data from birth to 68 mo of age. The measure assesses visual reception, fine motor, receptive language, and expressive language abilities, and yields t scores for each of these subtests with a mean ± SD of 50 ± 10 points. Subtest scores are summed and converted to an Early Learning Composite, which is an age-scaled intelligence quotient–like score with a mean ± SD of 100 ± 15 points.
EI (secondary outcome)
The EI paradigm assesses explicit memory ability in preverbal children via the behavioral imitation of action sequences (31, 32). This paradigm is a nonverbal analog to verbal memory report (33) and requires support from the hippocampus (34). The paradigm reflects normal developmental changes in memory ability within the age range of the current study (35), is sensitive to neurodevelopmental disruption (36), and is predictive of later memory abilities in school-age children (37). The task involves sets of toys that are used in event sequences. Each sequence has a theme (e.g., going camping) and incorporates multiple toys that are used in a prescribed sequence of 9 individual actions (e.g., baiting a hook, catching a fish, and setting up a tent).
At each assessment, participants were shown 2 different 9-item sequences. The procedure included a free play period and a recall measure for each sequence. During free play, children were given the toys to manipulate for 2 min. The free play provided a control for spontaneous occurrences of target actions. An experimenter modeled the event sequence twice with narration. The child was directed to recall the event sequence in one of 2 conditions (immediate or delayed). For immediate recall, the child was asked to imitate the sequence after modeling. For delayed recall, the toys were removed for 15 min after which the child was asked to reproduce the sequence. Immediate recall provided a measure of attention to the task and the encoding of items and sequences. Delayed recall provided a measure of hippocampal-dependent long-term memory. For each child, the 2 sequences were drawn from a larger set of available sequences that were, in turn, counterbalanced across conditions (immediate and delayed) and visits.
The production of individual items (e.g., baiting a hook, catching a fish, and setting up a tent) and the correctly ordered pairs of items (i.e., baiting the hook before catching the fish and then setting up the tent) was assessed for both free play and the recall for each condition (immediate and delayed). Sessions were video recorded and scored offline by trained raters. Twenty percent of the videos were coded by multiple raters to ensure reliability (93%). The variables analyzed were the percentage of correct individual items produced (maximum: 9) and the percentage of correctly ordered pairs produced (maximum: 8) for the following 3 conditions: free play, immediate recall, and delayed recall. Only immediate and delayed recall measures were used in the current study. There were no group differences in EI free -play performance.
Statistical analyses
Feasibility and tolerability analyses
The distributions of the compliance log-sheet variables were nonnormal and leptokurtic, and therefore, medians and IQRs are reported. Between-group comparisons of these variables were conducted with the use of Mann-Whitney U tests. The distribution of serum TMAO was also nonnormal at the 6- and 9-mo visits, and medians and IQRs for TMAO are reported in addition to the mean value for each visit. For between-group comparisons of categorical variables, Fisher’s exact test was used because of small cell sizes. The Freeman-Halton extension of the Fisher’s exact test (38) was used for contingency tables that were larger than 2 × 2. t tests were used for between -group comparisons of continuous variables.
Mixed-model specification
Growth curve analyses with the use of linear mixed models were performed to test for treatment differences in growth trajectories (intercepts, which represented baseline differences, and slopes, which represented the differential change over time) for the feasibility and neurocognitive data. The analyses, which modeled fixed effects and random child-specific intercepts, were conducted with the SAS (version 9.4) Proc Mixed procedure (SAS Institute Inc.) with the use of a restricted maximum likelihood estimation (REML). The REML with an estimated df procedure (39) was used because data were not available for all participants at each time point, and the REML yields valid variable estimates with incomplete data without imputing missing data or using list-wise deletion. The variables are estimated under the assumption that the missing data could be ignored (40), which was assumed in the current study. The following 2 types of general linear mixed models (random-effects models) were initially considered: 1) models with child-specific intercepts only and 2) models with both child-specific intercepts and child-specific slopes. Models were compared with the use of the Akaike information criterion, which takes into account both the degree of model fit to the data and the model complexity. Intercept-only models were shown to have consistently better Akaike information criterion values than the intercept and slope models did. Longitudinal analyses were conducted as intention-to-treat analyses with all available data from participants who received the allocated treatment (choline: n = 31; placebo: n = 29) included in the analyses independent of their completion of the study and compliance during the study (Figure 1).
For data collected at 3 visits (e.g., EI memory data), the linear slope term was specified to estimate the growth trajectory across 3 time points (time 0: baseline; time 1: 6-mo visit; time 1.5: 9-mo visit). The intercept represents the estimated value at baseline (time 0) in the growth curves, and the linear slope represents the change in outcome over the time during treatment, with one unit of time along the x-axis representing 6 mo of treatment. For data collected at 2 visits (e.g., Mullen Scales), the linear slope term was specified across 2 time points (time 0: baseline; time 1: 9-mo visit).
Curve fit
Before the main effects of treatment were tested, unconditional growth-curve analyses were performed to determine whether the general growth trajectory for each longitudinal outcome was linear or nonlinear (quadratic) regardless of the treatment group (Supplemental Equation 1). Unconditional analyses (which did not include the treatment group in the model) gave growth-curve estimates for the entire sample. The appropriate slope (linear or quadratic) was tested in the conditional analyses (with the treatment group added to the analysis) to examine treatment-group differences (Supplemental Equations 2–6). All reported longitudinal analyses tested for the linear change unless otherwise specified.
Tests for demographic moderation of main treatment effects
For the neurocognitive data (Mullen Scales and EI), 2 sets of conditional longitudinal analyses were performed to test the treatment effect. First, the main effect of treatment was examined without demographic covariates or moderators (Supplemental Equations 2 and 4). Second, 3-way interactions between the treatment group and age, race, or FASD diagnosis were examined to test for moderation (i.e., whether the association between treatment and neurocognitive outcomes differed as a function of these subject variables) (Supplemental Equations 3 and 5). For each significant 3-way interaction, simple slope analyses were completed to evaluate the change in performance over time for each value of the moderator (41).
Assessment of main treatment effects with and without demographic covariates
Variables were included as covariates if there was a difference in the group distribution even after random assignment and if there was a potential association with the outcome measures. Covariates included age, race, and FASD diagnosis. Because dysmorphic facial features, growth deficiency, deficient brain growth, and alcohol exposure form the basis of an FASD diagnosis, these variables were not included again as independent covariates. The remaining demographic characteristics were not included as covariates because they were very closely matched or had no known association with the outcome measures. Analyses are presented with and without the covariates included in the model (Supplemental Equations 5 and 6). Effect sizes are presented for both the unadjusted (no covariates) and adjusted (with covariates) models.
Immediate EI performance as a covariate
For EI, the hypothesis was that choline would improve the performance on the delayed recall condition because it depends on hippocampal integrity. Choline’s effects in preclinical models have been predominantly on the developing hippocampus (18–20). Growth-curve analyses were completed for a delayed performance with the corresponding immediate condition performance included as a covariate in the model (Supplemental Equations 4–6 and Supplemental Equation 7). The child’s immediate performance represents the ability to attend to the stimuli and encode a sequence and was included in the models to control for the variability introduced by these nondelay–related characteristics of the child’s performance.
Effect size
For significant linear slope results, effect sizes (Cohen’s d values) were determined by first standardizing the change scores (the difference between the estimated mean baseline and 9-mo follow-up scores) by the SD of the raw baseline scores for each treatment group (42). The difference between the standardized change scores for the 2 treatment groups was calculated. Effect sizes for significant moderators were determined by calculating the difference between the effect for the placebo and treatment groups for each value of the moderator.
RESULTS
Feasibility of choline supplementation: compliance, fidelity, and adverse events
Completed log sheets were returned by 95% of participants. Participants reportedly received a partial dose (at least one-quarter) or a full dose of the allocated intervention on 88% (IQR: 69–96%) of the days in the study. Reported days that participants received a dose did not differ between the 2 arms [choline: median: 87% (IQR: 72–93%); placebo: median: 90% (IQR: 69–96%); P = 0.306]. The 24-h dietary recall data revealed no differences in dietary choline intake across the 2 arms at any point (Table 2). Significant increases in serum choline (102%) and betaine (106%) concentrations occurred with choline supplementation (Table 2).
TABLE 2.
Results of growth-curve analyses that examined treatment-group differences in dietary choline intake, serum choline, physical examination results, and Mullen Early Learning Scales by treatment arm1
| Unadjusted values |
Growth-curve analyses, γ ± SE; P |
|||||
| Choline (n = 31) |
Placebo (n = 29) |
|||||
| n | Mean ± SD | n | Mean ± SD | Estimated intercept, choline vs. placebo | Estimated linear slope, choline vs. placebo | |
| Dietary choline, mg/d | −11.29 ± 23.87; 0.637 | −6.87 ± 21.58; 0.751 | ||||
| Baseline | 30 | 177 ± 88 | 23 | 195 ± 60 | ||
| 6 mo | 18 | 231 ± 99 | 21 | 221 ± 104 | ||
| 9 mo | 23 | 194 ± 86 | 18 | 223 ± 86 | ||
| Serum choline, μM | −0.25 ± 0.82; 0.766 | 4.77 ± 0.75; <0.0001* | ||||
| Baseline | 22 | 6.53 ± 1.62 | 23 | 7.20 ± 1.51 | ||
| 6 mo | 21 | 13.18 ± 4.15 | 17 | 7.38 ± 1.68 | ||
| 9 mo | 20 | 13.23 ± 4.19 | 18 | 7.16 ± 1.68 | ||
| Serum betaine, μM | −0.78 ± 8.60; 0.928 | 28.70 ± 5.69; <0.0001* | ||||
| Baseline | 22 | 42.96 ± 16.64 | 23 | 48.91 ± 9.79 | ||
| 6 mo | 21 | 85.27 ± 39.07 | 17 | 50.92 ± 17.45 | ||
| 9 mo | 20 | 88.83 ± 50.85 | 18 | 50.38 ± 17.26 | ||
| TMAO,2 μM | 1.89 ± 17.81; 0.916 | −98.34 ± 42.84; 0.033* | ||||
| Baseline | 7 | 2.87 ± 1.94 | 6 | 0.98 ± 0.66 | ||
| 6 mo | 7 | 70.73 ± 68.94 | 6 | 3.22 ± 1.51 | ||
| 9 mo | 5 | 29.34 ± 21.57 | 5 | 4.94 ± 7.39 | ||
| Height z score | −0.49 ± 0.23; 0.038* | −0.06 ± 0.06; 0.322 | ||||
| Baseline | 30 | −0.35 ± 0.89 | 29 | 0.12 ± 0.86 | ||
| 6 mo | 20 | −0.20 ± 0.85 | 18 | 0.29 ± 0.76 | ||
| 9 mo | 25 | −0.19 ± 0.86 | 22 | 0.35 ± 0.92 | ||
| Weight z score | −0.43 ± 0.27; 0.114 | 0.04 ± 0.04; 0.319 | ||||
| Baseline | 31 | −0.14 ± 1.18 | 29 | 0.29 ± 0.81 | ||
| 6 mo | 26 | 0.11 ± 1.21 | 24 | 0.32 ± 0.80 | ||
| 9 mo | 26 | 0.12 ± 1.25 | 25 | 0.31 ± 0.80 | ||
| Blood pressure, mm Hg | 2.46 ± 2.48; 0.323 | −1.60 ± 2.46; 0.515 | ||||
| Systolic | ||||||
| Baseline | 27 | 103 ± 11 | 28 | 101 ± 9 | ||
| 6 mo | 23 | 102 ± 8 | 24 | 100 ± 12 | ||
| 9 mo | 23 | 100 ± 8 | 24 | 100 ± 7 | ||
| Diastolic | 2.44 ± 2.71; 0.368 | −3.83 ± 2.56; 0.137 | ||||
| Baseline | 27 | 63 ± 14 | 28 | 61 ± 10 | ||
| 6 mo | 23 | 61 ± 12 | 24 | 62 ± 7 | ||
| 9 mo | 23 | 60 ± 8 | 24 | 64 ± 9 | ||
| Mullen Early Learning Scales score | ||||||
| Early Learning Composite | −1.82 ± 4.73; 0.701 | −0.36 ± 3.09; 0.908 | ||||
| Baseline | 29 | 83.2 ± 13.7 | 29 | 84.3 ± 21.4 | ||
| 9 mo | 26 | 87.1 ± 16.4 | 25 | 89.6 ± 21.6 | ||
| Visual Reception | −3.26 ± 3.69; 0.381 | −0.30 ± 2.98; 0.920 | ||||
| Baseline | 29 | 41.2 ± 11.8 | 29 | 44.4 ± 16.2 | ||
| 9 mo | 26 | 43.5 ± 10.8 | 25 | 47.0 ± 13.9 | ||
| Fine Motor | 1.34 ± 3.30; 0.69 | −0.67 ± 2.69; 0.805 | ||||
| Baseline | 29 | 42.0 ± 9.3 | 29 | 40.1 ± 15.0 | ||
| 9 mo | 26 | 44.4 ± 10.1 | 25 | 43.3 ± 15.8 | ||
| Receptive Language | 0.44 ± 2.88; 0.879 | −0.04 ± 2.19; 0.984 | ||||
| Baseline | 29 | 40.6 ± 9.4 | 29 | 39.8 ± 12.4 | ||
| 9 mo | 26 | 42.2 ± 10.8 | 25 | 41.8 ± 12.3 | ||
| Expressive Language | −1.31 ± 2.57; 0.614 | 0.08 ± 2.72; 0.978 | ||||
| Baseline | 29 | 40.3 ± 8.5 | 29 | 41.2 ± 10.8 | ||
| 9 mo | 26 | 42.6 ± 10.3 | 25 | 44.2 ± 10.3 | ||
Longitudinal analyses with linear mixed models with the use of restricted maximum likelihood estimations were used to compare differences in growth curves between treatment groups (Supplemental Equation 2). For the growth-curve analyses, the estimated intercept column contains comparisons of baseline values (time 0), and the estimated linear slope column contains comparisons of change over time including baseline (time 0), 6 mo (time 1), and 9 mo (time 1.5). For Mullen Scales, the time included baseline (time 0) and 9 mo (time 1). *P < 0.05.
TMAO, trimethylamine N-oxide. The slope term reported for TMAO was quadratic and represented a significant nonlinear change. Median (IQR) values for TMAO (μM) were as follows—at baseline: choline group, 2.90 μM (0.60–4.60 μM); placebo group, 1.00 μM (0.28–1.60 μM); at 6 mo: choline group, 38.3 μM (13.50–150.70 μM); placebo group, 3.30 μM (2.08–4.05 μM); and at 9 mo: choline group, 40.40 μM (6.25–46.90 μM); placebo group, 1.50 μM (1.15–10.45 μM).
A fishy odor was the only adverse event that occurred differentially in the choline arm during treatment (Table 3). This odor was due to trimethylamine formation by gut bacteria; trimethylamine is oxidized in the liver to form TMAO. During treatment, serum TMAO concentrations reached 22-times higher in the choline arm than in the placebo arm (Table 2; 6 mo). A fishy odor was episodically noticeable to parents (mostly when changing clothes, bathing, or toileting) but was not noticeable to the research assistant who administered the outcome measures. Physical examination results, including height, weight, and blood pressure, remained consistent for both groups throughout the duration of the study (Table 2).
TABLE 3.
Number of participants reporting symptoms at baseline and number reporting new symptoms (adverse events that were not present at baseline) at least once during the course of treatment1
| Choline (n = 31) | Placebo (n = 29) | P (Fisher’s exact test) | |
| Administration problems with the supplement, n (%) | |||
| Baseline | — | — | — |
| New symptoms during treatment | 13 (42) | 12 (41) | 1.000 |
| General health, n (%) | |||
| Baseline | 7 (23) | 3 (10) | −0.302 |
| New symptoms during treatment | 8 (28) | 5 (18) | 0.530 |
| Skin, n (%) | |||
| Baseline | 9 (29) | 15 (52) | 0.113 |
| New symptoms during treatment | 4 (14) | 4 (14) | 1.000 |
| Ear, nose, and throat, n (%) | |||
| Baseline | 1 (3) | 3 (10) | 0.346 |
| New symptoms during treatment | 1 (4) | 0 (0) | 1.000 |
| Cardiovascular, n (%) | |||
| Baseline | 2 (7) | 2 (7) | 1.000 |
| New symptoms during treatment | 1 (3) | 0 (0) | 1.000 |
| Respiratory, n (%) | |||
| Baseline | 5 (16) | 10 (35) | 0.139 |
| New symptoms during treatment | 5 (17) | 4 (14) | 1.000 |
| Gastrointestinal, n (%) | |||
| Baseline | 14 (45) | 12 (41) | 0.800 |
| New symptoms during treatment | 9 (31) | 7 (25) | 0.770 |
| Fishy body odor, n (%) | |||
| Baseline | 0 (0) | 0 (0) | 1.000 |
| New symptoms during treatment | 15 (52) | 1 (4) | <0.001* |
| Genitourinary, n (%) | |||
| Baseline | 6 (19) | 2 (7) | 0.257 |
| New symptoms during treatment | 3 (10) | 8 (29) | 0.103 |
| Musculoskeletal, n (%) | |||
| Baseline | 3 (10) | 7 (24) | 0.175 |
| New symptoms during treatment | 0 (0) | 0 (0) | 1.000 |
| Neurologic, n (%) | |||
| Baseline | 15 (48) | 11(38) | 0.446 |
| New symptoms during treatment | 6 (21) | 4 (14) | 0.730 |
| Allergy, n (%) | |||
| Baseline | 7 (23) | 10 (35) | 0.394 |
| New symptoms during treatment | 0 (0) | 2 (7) | 0.237 |
| Other, n (%) | |||
| Baseline | 13 (42) | 9 (31) | 0.431 |
| New symptoms during treatment | 8 (28) | 13 (46) | 0.175 |
During treatment, sample sizes were as follows: choline group: n = 29; placebo group: n = 28. *P < 0.05.
Mullen Scales of Early Learning (primary outcome measure)
Intercept (baseline difference) and linear slope (change over time) results were examined to test the main effect of treatment on the Mullen Scales (Supplemental Equation 2). None of the intercept or linear slope results reached significance, which indicated that there were no differences between the 2 treatment arms before treatment or during treatment of the Mullen Early Learning Composite or of any of the subscales (Table 2). None of the 3-way interactions used to test moderation for the main effect of treatment on the linear slope reached significance for the Mullen Scales (Supplemental Equation 3).
The Mullen Early Learning Composite was correlated (with age controlled for) with EI delayed performance for items (partial r = 0.56, P < 0.001) and ordered pairs (partial r = 0.47, P < 0.001) at baseline but not at the 9-mo visit (P > 0.17 for all). In other words, the Mullen scales and EI measures similarly reflected the participant’s level of functioning at baseline, but the 2 measures were differentially responsive to the intervention.
EI (secondary outcome measure)
Intercept and linear slope results were examined to test the main effect of treatment on the growth trajectory of EI (items and ordered pairs; Supplemental Equation 4). For the whole sample, none of the intercept or linear slope results reached significance for delayed recall (items or ordered pairs), which indicated that there were no differences between the 2 arms (choline compared with placebo) before treatment or during treatment of EI items (estimated means for choline at baseline: 74%; at 6 mo: 81%; and at 9 mo: 84%; estimated means for the placebo at baseline: 79%; at 6 mo: 83%; and at 9 mo: 85%). There were no differences for ordered pairs (estimated means for choline at baseline: 41%; at 6 mo: 52%; and at 9 mo: 58%; estimated means for the placebo at baseline: 44%; at 6 mo: 52%; and at 9 mo: 57%). Thus, for the whole sample, there was not a significant effect of choline on EI delayed memory performance).
Race and FASD diagnosis as potential moderators of choline’s effects on EI
To examine whether the treatment effect on EI delayed recall was moderated by race or FASD diagnosis, 3-way interactions for the linear slope were examined (Supplemental Equation 5). None of the analyses reached significance, which showed that the treatment effect on delayed recall did not differ between children of different races or with different FASD diagnoses.
Age as a moderator of choline’s effects on EI
Because preclinical data showed that early choline supplementation in prenatally exposed animals is associated with greater cognitive improvements than later supplementation is (43), we hypothesized that age would be an important factor in the human response to choline supplementation [the potential interaction with age was discussed in our earlier article on the first pilot study (21)]. To examine whether the treatment effect on delayed EI performance was moderated by age, 3-way interactions between the treatment arm, age at baseline (as a continuous variable), and either the intercept or linear slope were examined on EI delayed performance (items and ordered pairs). Immediate (nondelayed) performance was included as a covariate (Supplemental Equation 5). The intercept was significant for items [γ = 11.60 (95% CI: 0.23, 22.98), t(114) = 2.02, P = 0.046] but not for ordered pairs [γ = 3.91 (95% CI: −6.74, 14.56), t(118) = 0.73, P = 0.469]. The linear slope was also significant for items [γ = −10.76 (95% CI: −20.41, −1.10), t(83.1) = −2.21, P = 0.030] but not for ordered pairs [γ = −4.28 (95% CI: −13.59, 5.03), t(84.5) = −0.91, P = 0.363]. In summary, the group difference (choline compared with placebo) in the rate of EI improvement over time differed depending on the child’s age.
Age as a moderator of choline’s effects on EI with the use of split-age groups
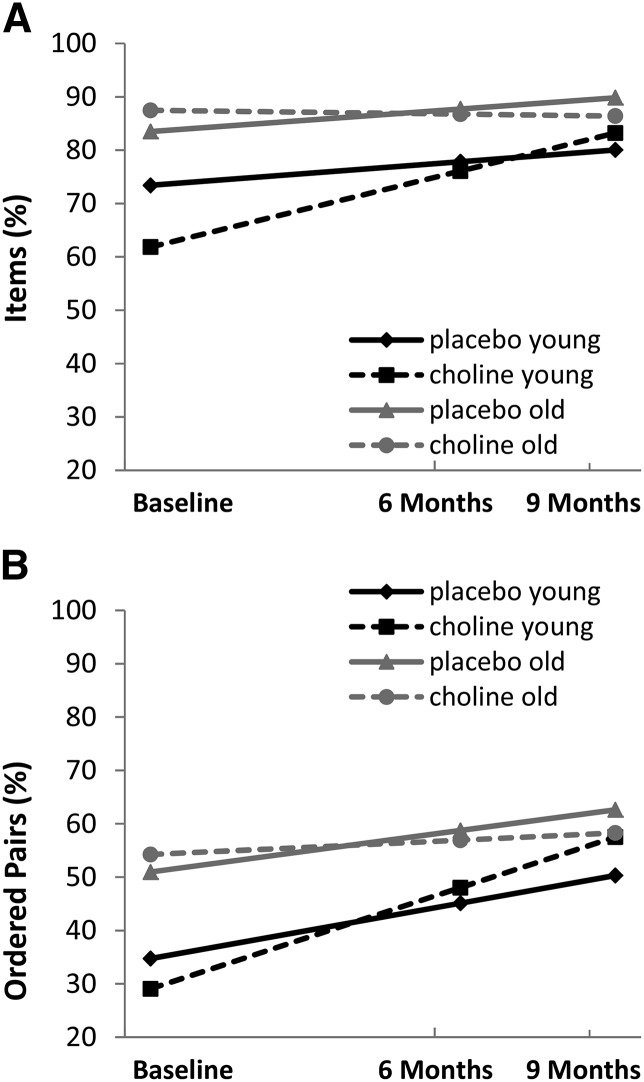
To more easily illustrate the moderating role of age on the treatment effect and to gain an understanding of effect sizes, the sample was split into 2 groups on the basis of the median age as follows: a younger group (n = 30; placebo: n = 13; choline: n = 17) consisting of 2.5- to ≤4.0-y-olds and an older group consisting of >4.0–5.0-y-olds (n = 30; placebo: n = 16; choline: n = 14). Three-way interactions were examined between the treatment arm, age group, and either the intercept or linear slope for delayed EI performance (items and ordered pairs; Supplemental Table 1 and Supplemental Equation 5). In the model for items, there was a trend-level 3-way interaction between the treatment group, age group, and linear slope [γ = −14.75 (95% CI: −30.38, 0.88), t(84.9) = −1.88, P = 0.064] but not for the intercept [ γ = 15.52 (95% CI: −3.20, 34.25), t(109) =1.64, P = 0.103]. For ordered pairs, there was a trend-level 3-way interaction between the treatment group, age group, and linear slope [γ = −13.68 (95% CI: −28.89, 1.52), t(89.1) = −1.79, P = 0.077] but not for the intercept [γ = 8.95 (95% CI: −8.43, 26.34), t(117) = 1.02, P = 0.310]. As in the previous evaluation, age appeared to play a role in the response to choline in terms of the rate of improvement in EI delayed performance.
Figure 2 shows the estimated growth curves for the treatment arms by age group for EI delayed recall (items and ordered pairs) while controlling for immediate performance. There was a marginal intercept (baseline) difference between treatment arms and age groups for individual items during the delayed EI condition whereby the young age group, particularly the young choline group, performed lower by chance. This difference in intercepts was not likely due to dietary choline because there was no difference in dietary choline intake at baseline between the young choline group (35% of whom met adequate intake) and the young placebo group (40% of whom met adequate intake) (P = 0.807).
FIGURE 2.
Treatment effect on elicited imitation delayed performance was moderated by age for items [slope: γ = −14.75 (95% CI: −30.38, 0.88), t(84.9) = −1.88, P = 0.064] and ordered pairs [slope: γ = −13.68 (95% CI: −28.89), 1.52, t(89.1) = −1.79, P = 0.077]. The largest improvement in elicited imitation delayed performance occurred in the young choline group (2.5- to ≤4.0-y-olds). (A) Percentages of individual items recalled. (B) Percentages of ordered pairs recalled. Choline: n = 30 (young: n = 16; old: n = 14); placebo: n = 29 (young: n = 13; old: n = 16).
During treatment, the largest improvement in delayed EI performance was in the young choline group. Post hoc analyses revealed significant differences in the simple slopes for both delayed items and ordered pairs between treatment groups (choline compared with placebo) for the young-age group but not for the old-age group (Table 4). The simple slope values estimated the change over 6 mo (from times 0 to 1). The change over 9 mo of treatment was computed as 1.5 times the simple slope. For delayed items, the young choline group showed an increase of 21% over 9 mo of treatment compared with 7% in the young placebo group (Table 4). For delayed ordered pairs, the change was 28% in the young choline group compared with 16% in the young placebo group (Table 4). Effect sizes were large for both outcome measures in the young group.
TABLE 4.
Simple slope results by age group for performance on the elicited imitation delayed condition1
| Choline (n = 30), γ ± SE | Placebo (n = 29), γ ± SE | t test [t(28), P] | Effect size, d | |
| Delayed condition | ||||
| Items | ||||
| Young | 14.24 ± 3.84 | 4.43 ± 4.04 | −2.41, 0.023 | 0.54 |
| Old | −0.71 ± 3.98 | 4.23 ± 3.90 | 1.21, 0.235 | — |
| Ordered pairs | ||||
| Young | 18.97 ± 3.65 | 10.39 ± 4.00 | −2.18, 0.038 | 0.50 |
| Old | 2.69 ± 3.79 | 7.79 ± 3.73 | 1.31, 0.201 | — |
| Immediate condition | ||||
| Items | ||||
| Young | 8.48 ± 4.23 | 13.72 ± 4.41 | 1.18, 0.125 | — |
| Old | 2.59 ± 4.36 | 3.89 ± 4.24 | 0.29, 0.386 | — |
| Ordered pairs | ||||
| Young | 8.75 ± 4.45 | 20.01 ± 4.80 | 2.36, 0.013 | −0.46 |
| Old | 11.46 ± 4.61 | 5.50 ± 4.46 | −1.27, 0.107 | — |
The simple slope was used to estimate the change in delayed performance (%) per 6-mo unit of treatment. The change over 9 mo of treatment can be computed as 1.5 times the change per 6 mo of treatment.
Evaluation of demographic covariates
Race and FASD diagnosis were examined as potential confounding variables in the model with age as a moderator to determine whether the association between the treatment group and linear slope in the young group was altered by these variables (Supplemental Equation 6). With race and FASD diagnosis included as covariates, the adjusted effect size for the linear slope in the young group for delayed EI items was d = 0.58 (unadjusted effect size: d = 0.54) and, for delayed EI ordered pairs, was d = 0.42 (unadjusted effect size: d = 0.50).
Alternate analysis without controlling for immediate EI performance
The 3-way interaction between the age group, treatment group, and growth curve for delayed EI performance was also examined without controlling for immediate recall performance (Supplemental Equation 3). Results did not reach significance for any of the growth-curve variables for the delayed performance of items [intercept: γ = 10.51 (95% CI: −10.98, 32.01), t(105) = 0.97, P = 0.334; slope: γ = −12.46 (95% CI: −28.56, 3.63), t(98.3) = −1.54, P = 0.128] or ordered pairs [intercept: γ = 0.86 (95% CI: −19.11, 20.84), t(115) = 0.09, P = 0.931; slope: γ = −7.50 (95% CI: −23.73, 8.37), t(98.8) = −0.94, P = 0.351]. In other words, choline’s effect of improving delayed EI performance was evident only when nondelay characteristics of the child’s performance were controlled for.
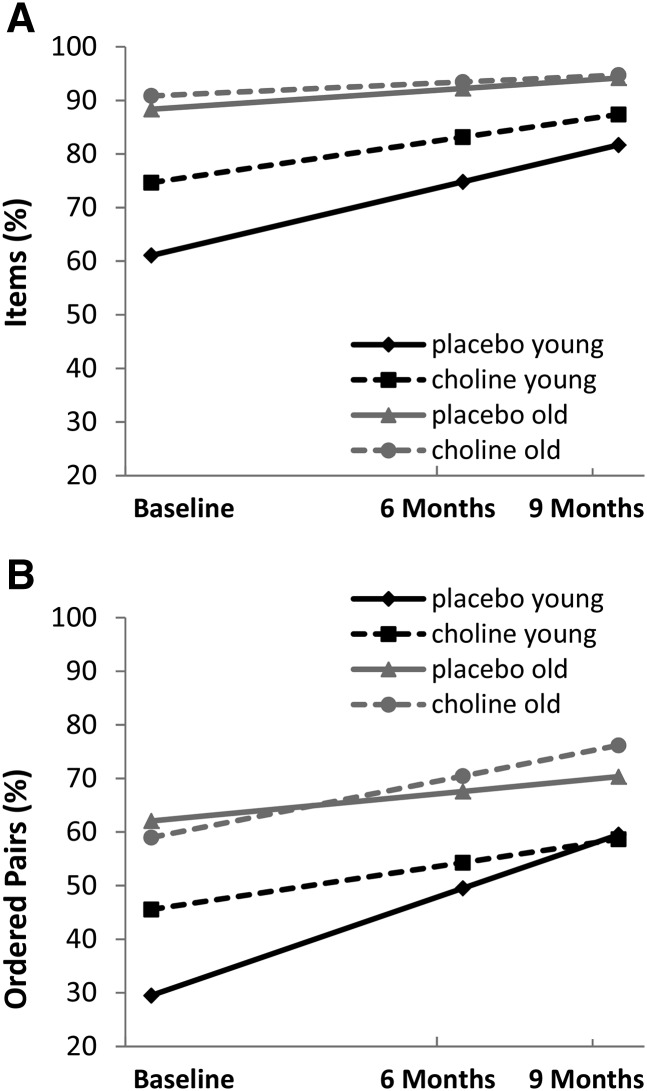
Additional analyses of immediate EI performance
Growth trajectories for immediate EI performance are presented by age group and treatment arm in Figure 3 (Supplemental Equation 3). Choline was not associated with improvement in the immediate condition for items [intercept: γ = −11.06 (95% CI: −32.31, 10.19), t(105) = −1.03, P = 0.304; slope: γ = 3.94 (95% CI: −13.19, 21.08), t(86.6) = 0.46, P = 0.649]. There was a trend toward significance in the 3-way interaction for ordered pairs in the immediate condition (intercept: γ = −19.14 (95% CI: −40.43, 2.15), t(119) = −1.78, P = 0.078; slope: γ = 17.23 (95% CI: −0.97, 35.42), t(93.2) = 1.88, P = 0.063]. Post hoc analyses revealed a significant difference in the simple slopes for immediate ordered pairs between treatment groups (choline compared with placebo) for the young-age group but not for the old-age group (Table 4). The young choline group showed less improvement for ordered pairs, with an increase of 13% over 9 mo compared with 30% in the young placebo group.
FIGURE 3.
Treatment effect on elicited imitation immediate performance. Choline was not associated with the immediate score for items [intercept: γ = −11.06 (95% CI: −32.31, 10.19), t(105) = −1.03, P = 0.304; slope: γ = 3.94 (95% CI: −13.19, 21.08), t(86.6) = 0.46, P = 0.649]. A trend was seen for immediate ordered pairs [intercept: γ = −19.14 (95% CI: −40.43, 2.15), t(119) = −1.78, P = 0.078; slope: γ = 17.23 (95% CI: −0.97, 35.42), t(93.2) = 1.88, P = 0.063]. (A) Percentages of individual items recalled. (B) Percentages of ordered pairs recalled. Choline: n = 30 (young: n = 16; old: n = 14); placebo: n = 29 (young: n = 13; old: n = 16).
Last, to put these results in context, immediate and delayed performances were correlated at the baseline visit (items: partial r = 0.59, P < 0.001; ordered pairs: partial r = 0.60, P < 0.001) and at 6 mo (items: partial r = 0.61, P < 0.001; ordered pairs: partial r = 0.55, P = 0.001) but not at 9 mo (items: partial r = 0.15, P = 0.322; ordered pairs: partial r = 0.26, P = 0.074) while controlling for age. EI delayed and immediate performances were correlated at baseline and 6 mo, but the 2 measures were differentially responsive to the intervention and were no longer correlated at 9 mo.
Average daily choline dose
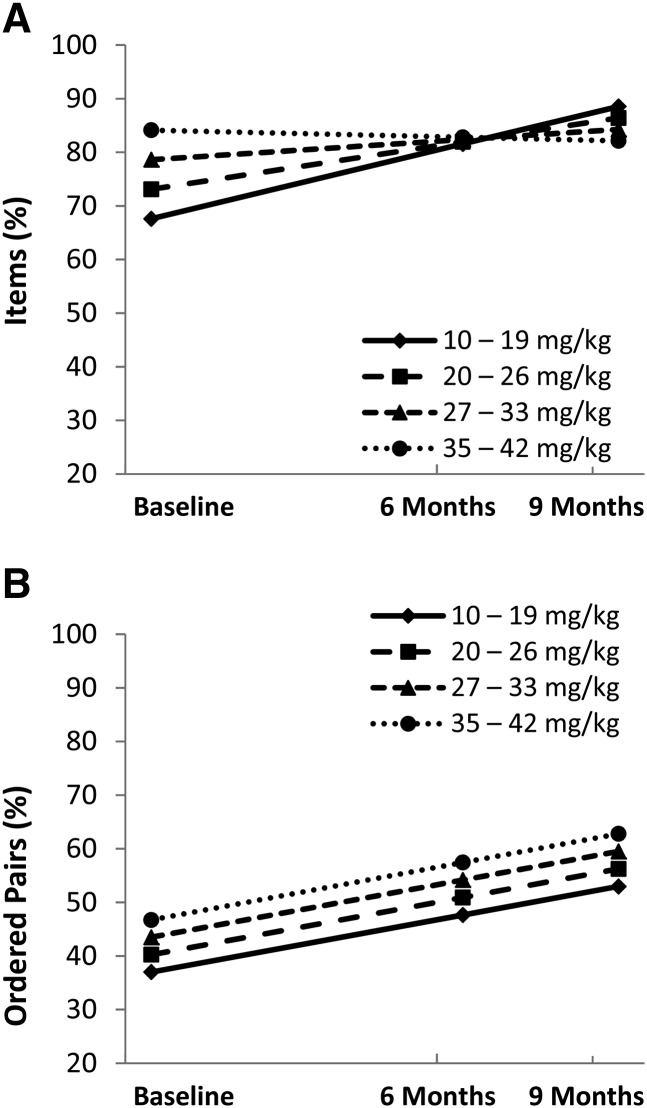
The association between the average daily choline dose and EI delayed recall was examined in the choline arm. To estimate the mean choline dose received throughout the study, the full dose (500 mg) was corrected for the percentage of days participants received any supplement on the basis of parent-report log sheets. The corrected dose was divided by the child’s weight at baseline to yield the average daily choline dose received per kilogram of body weight (mg/kg).
Because of the interaction between the treatment arm and age on EI performance, age was included as a covariate in the dosage analyses. EI immediate recall performance was also included as a covariate (Supplemental Equation 7). Linear slope results were significant for the choline dose (mg/kg) on EI delayed recall for items [γ = −0.82 (95% CI: −1.60, −0.04), t(34.9) = −2.13, P = 0.041] but not for ordered pairs [γ = −0.30 (95% CI: −1.10, 0.49), t(36.6) = −0.77, P = 0.446]. Intercept results for the choline dose (mg/kg) did not reach significance for EI items (P = 0.152) or for ordered pairs (P = 0.343). Figure 4 depicts this linear association between the dose (divided into quartiles) and EI delayed performance. Subjects in the lowest quartile (i.e., the lowest mg · kg−1 · d−1) showed greater improvement of delayed recall for items (21%) than subjects in the highest quartile did (−2%).
FIGURE 4.
Lower daily dose of choline was associated with a greater treatment effect of choline for elicited imitation delayed items [slope: γ = −0.82 (95% CI: −1.60, −0.04), t(34.9) = −2.13, P = 0.041) (A) but not for ordered pairs [slope: γ = −0.30 (95% CI: −1.10, 0.49), t(36.6) = −0.77, P = 0.446] (B). The daily dose was not associated with the intercept for elicited imitation delayed items (P = 0.152) or for ordered pairs (P = 0.343). Choline group (n = 25)—10–19 mg/kg: n = 6; 20–26 mg/kg: n = 6; 27–33 mg/kg: n = 7; and 35–42 mg/kg: n = 6.
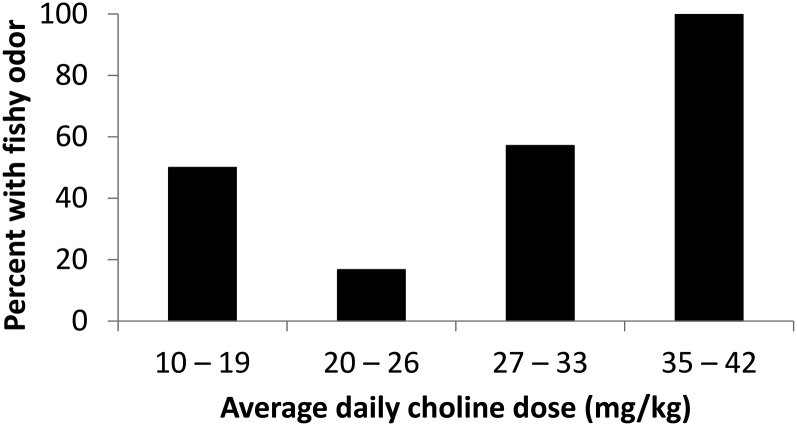
Finally, the association between the choline dose and presence of fishy odor was examined. The prevalence of fishy odor was greater in the highest quartile for choline dose (100% of subjects reported a fishy odor at some point during the 9 mo) than in the lower 3 quartiles for choline dose (42% of subjects reported a fishy odor) (P = 0.020). There were no differences in the presence of a fishy odor between the lowest 3 quartiles for choline dose (Figure 5).
FIGURE 5.
Prevalence of fishy body odor was greater in the highest quartile for the choline dose than in the lower 3 quartiles for the choline dose (P = 0.020; Fisher’s exact test). There were no differences in the presence of a fishy odor between the lowest 3 quartiles for the choline dose. Choline group (n = 25)—10–19 mg/kg: n = 6; 20–26 mg/kg: n = 6; 27–33 mg/kg: n = 7; and 35–42 mg/kg: n = 6.
DISCUSSION
This pilot study represents an initial evaluation of the potential efficacy of choline as a cognitive intervention in FASDs. As in our previous study (21), choline had high tolerability and was associated with no serious adverse events at 500 mg/d in 2–5-y-olds with FASDs. The study evaluated choline’s effects on cognition in a randomized, double-blind, placebo-controlled trial. Age-dependent improvements were seen on a hippocampus-dependent memory task. In this small pilot sample, younger participants (2.5- to ≤4.0-y-olds) showed greater improvement than older participants (>4.0–5.0-y-olds) did, although there are caveats to interpreting these findings as will be discussed. Young participants in the choline arm showed an increase of 12–14 percentage points greater than in the placebo arm in long-delay memory after 9 mo of supplementation, which was a potentially meaningful improvement. Although we could not characterize the clinical significance of this memory improvement (there are no normative standards for this measure), we do know that an EI performance change is expected in this age range (35), and an EI improvement has implications for future cognitive ability. For example, in one longitudinal study, EI performance at 20 mo of age predicted ≤37% of the variance in explicit memory skill at 6 y of age (37).
In the current study, the effect of choline was seen in the secondary outcome measure (EI delayed memory performance) but not in the primary outcome measure (global cognitive ability). Note that, although EI was the secondary outcome measure, it was considered from the start to be a critical outcome measure because of choline’s presumed effects on hippocampal development [see Wozniak et al. (21)]. Cognitive deficits are common in FASDs even when a patient’s intelligence quotient is average (44), which suggests that individual neural systems (e.g., hippocampus) may be the most-appropriate targets for an intervention rather than global cognition. The hippocampus is heavily interconnected with other systems (45, 46), and its integrity is critical to the development, functioning, and organization of other domains. Future studies may examine downstream effects of choline on other domains affected in FASDs (e.g., attention, executive function, and behavior regulation).
The observed interaction between choline’s effect on memory and younger age was consistent with the potential underlying mechanisms of choline and with existing preclinical data. Prenatally, choline affects neurogenesis, thereby contributing to increased cell proliferation and decreased apoptosis in the hippocampus (47, 48). Postnatal choline may affect synaptogenesis as well as continued hippocampal growth (49), which is rapid during the first 2 y of life and slower thereafter (50). In humans, the hippocampus continues to develop into the fourth year of life (51). In preclinical models, both early supplementation (postnatal days 11–20) and late supplementation (postnatal days 21–30) attenuate cognitive deficits from prenatal alcohol with an advantage for early supplementation (43). The current human results suggest that the specific benefits for delayed sequential memory in FASDs are evident in the first 2–3 y of life, but our results were less conclusive for older children; we observed a potential ceiling effect in the EI data from the 4–5-y-olds (this was also evident in the younger group) that may have masked treatment effects in the older group. Also, we reiterate that the initial random assignment was not stratified by age; instead, the sample was evaluated for age interactions and was eventually split by age after data collection. For these reasons, an additional examination of choline’s potential in 4–5-y-olds may be warranted in future studies.
There are other important caveats to consider. Despite random assignment, there was a significant interaction between age and treatment group (P = 0.046), with the young choline group having slightly lower delayed EI performance than the other groups did for items at baseline. The regression to the mean could have contributed to the young choline group showing the largest improvement as opposed to the effect being purely a treatment effect. Another caveat to consider is that item scores were in the 80–90% correct range at the final study visit, which suggested a potential ceiling effect. Together, these factors likely contributed to the observed treatment effect. In a future choline study, the task difficulty could be increased to better evaluate the full potential range of choline’s effects.
In the current data, the effect of choline on EI delayed performance depended on controlling for the child’s concurrent EI immediate performance, which we believed to be largely a function of the child’s attention and initial encoding. There is some evidence in the literature that prenatal choline supplementation has the potential to improve certain aspects of attention (52). The relation between postnatal choline supplementation and attentional capacity in the context of prenatal alcohol exposure is less clear. Choline was expected to improve long-term memory storage and retrieval processes that were measured in the delayed EI condition because choline’s mechanisms of action are in the hippocampus and choline typically improves long-delay memory. This was the observed pattern for the recall of individual items. Choline’s effects on immediate memory were harder to predict on the basis of the existing literature, but there was some reason to believe that choline could also improve it. In fact, choline did not affect EI immediate performance for items but did improve delayed EI performance for items, which suggested that the effect of choline supplementation is specific to long-delay memory and that controlling for immediate performance may be warranted. However, for EI delayed ordered pairs, the results were more complex. Post hoc analyses suggested that choline may have a negative effect on immediate memory for ordered pairs. Although both treatment arms showed an improvement in immediate performance for ordered pairs during the 9 mo of the study, choline supplementation slightly attenuated this growth for ordered pairs. Thus, choline’s effects may be multifaceted, and future studies will need to carefully assess for unexpected changes of this type in nondelay aspects of memory performance.
Data from the current study may influence future choline dosing in studies of children with FASDs. The adequate intake is 200 mg for ages 1–3 y and 250 mg for ages 4–8 y (53). On average, children with FASDs consume insufficient amounts of choline (54). In the current study, the 500-mg dose was selected to bring all participants to sufficiency, to provide supplementation, and to keep amounts in the tolerable range of 1000 mg/d (53). The 500-mg dose substantially raised free serum choline and betaine concentrations. Many subjects in the choline arm (52%) experienced an adverse event of a fishy odor. This event occurred across the dosage range for body weight (mg/kg) but was universal (100%) at the highest dosage. The dosage data showed an inverse relation with memory performance, but there are caveats to consider. Differential doses were not assigned. Rather, because the allocated dose was universal, the individual dosage varied as a function of body weight and compliance (the majority of variance was due to body weight). The measure with which dosage was most associated (EI delayed items) approached a ceiling at baseline and at 9 mo. This effect may have exaggerated the inverse relation between the dose and treatment response. There was no relation between the dose and delayed EI ordered pairs score (for which there was no ceiling effect). For these reasons, the observed relation between the dosage and outcomes needs to be considered tentative and replicated accordingly. Nonetheless, these data, together with the side-effect data, suggest that supplementation with choline beyond the recommended adequate intake for treatment purposes should take into account the child’s weight.
The only adverse event that occurred differentially in the choline arm was a fishy odor. The odor was from trimethylamine, which is formed when choline becomes available to gut bacteria (28). Trimethylamine is converted to TMAO in the liver. We observed significant elevations in serum TMAO concentrations in the choline arm. In rodent models, increased plasma concentrations of TMAO have been suggested as a potential contributor to atherosclerosis (55). Atherosclerosis-prone (apolipoprotein E–negative) mice fed choline or TMAO showed greater aortic root atherosclerotic plaque (56). TMAO concentrations are also associated with acute cardiovascular events in cardiac patients (56, 57). However, it is not known whether TMAO “has a direct effect on pathogenesis, is an epiphenomenal biomarker, or is a precursor to a more direct effector” (58). One study in hamsters showed an inverse relation between plasma TMAO and atherosclerosis (59). Furthermore, fish is a rich source of trimethylamine (60), but dietary fish intake is associated with decreased risk of cardiovascular disease (61). Practically speaking, smaller choline doses given multiple times per day (instead of a single bolus) will reduce or eliminate trimethylamine formation, thereby allowing for the potential management of this adverse effect in future studies.
Additional studies are needed to determine the optimal dosage that improves memory performance and minimizes the fishy odor and TMAO increase. Longitudinal studies will determine the permanency of choline’s effects. Furthermore, the minimum adequate length of treatment has yet to be established because the current study tested only the 9-mo duration as a starting point in this line of research.
In conclusion, this pilot study suggests that an additional evaluation of choline as a potential intervention for memory functioning may be warranted in children with FASDs. The results of the trial are encouraging because, to our knowledge, there have been no other intervention studies that have shown similar effects in FASDs nor are there other promising biological interventions ready for human clinical trials.
Acknowledgments
We thank Xueqing Zhao from the University of North Carolina at Chapel Hill Nutrition Research Institute, for assistance with the measurements of choline, betaine, and TMAO. We also thank Jennifer D Thomas, Darlette G Luke, Kristine V Lukasik, Gary Schneider, Martin A Erickson, James D Neaton, Iris W Borowsky, Nimi P Singh, Megan Finsaas, Kristin E Sandness, and Carrie J Moore.
The authors’ responsibilities were as follows—JRW, JPR, MGK, SHZ, and MKG: designed the research; JRW, CJB, JKE, BAF, HLH, JPR, MGK, NCM, and SHZ: conducted the research; JRW, AJF, and AMB: analyzed the data; JRW and AJF: wrote the manuscript; JRW: had primary responsibility for the final content of the manuscript; and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: CNS, central nervous system; EI, elicited imitation; FAS, fetal alcohol syndrome; FASD, fetal alcohol spectrum disorder; IOM, Institute of Medicine; pFAS, partial fetal alcohol syndrome; REML, restricted maximum likelihood estimation; TMAO, trimethylamine N-oxide.
REFERENCES
- 1.May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, Hoyme HE. Prevalence and epidemiologic characteristics of FASD form various research methods with an emphasis on recent in-school studies. Dev Disabil Res Rev 2009;15:176–92. [DOI] [PubMed] [Google Scholar]
- 2.Abel EL. An update on incidence of FAS: FAS is not an equal opportunity birth defect. Neurotoxicol Teratol 1995;17:437–43. [DOI] [PubMed] [Google Scholar]
- 3.Pulsifer MB. The neuropsychology of mental retardation. J Int Neuropsychol Soc 1996;2:159–76. [DOI] [PubMed] [Google Scholar]
- 4.Coles CD, Brown RT, Smith IE, Platzman KA, Erickson S, Falek A. Effects of prenatal alcohol exposure at school age. I. Physical and cognitive development. Neurotoxicol Teratol 1991;13:357–67. [DOI] [PubMed] [Google Scholar]
- 5.Kodituwakku PW. Defining the behavioral phenotype in children with fetal alcohol spectrum disorders: a review. Neurosci Biobehav Rev 2007;31:192–201. [DOI] [PubMed] [Google Scholar]
- 6.Mattson SN, Riley EP. A review of the neurobehavioral deficits in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcohol Clin Exp Res 1998;22:279–94. [DOI] [PubMed] [Google Scholar]
- 7.Kable JA, Coles CD, Taddeo E. Socio-cognitive habilitation using the math interactive learning experience program for alcohol-affected children. Alcohol Clin Exp Res 2007;31:1425–34. [DOI] [PubMed] [Google Scholar]
- 8.Bertrand J. Interventions for children with fetal alcohol spectrum disorders (FASDs): overview of findings for five innovative research projects. Res Dev Disabil 2009;30:986–1006. [DOI] [PubMed] [Google Scholar]
- 9.Nash K, Stevens S, Greenbaum R, Weiner J, Koren G, Rovet J. Improving executive functioning in children with fetal alcohol spectrum disorders. Child Neuropsychol 2015;21:191–209. [DOI] [PubMed] [Google Scholar]
- 10.Berman RF, Hannigan JH. Effects of prenatal alcohol exposure on the hippocampus: spatial behavior, electrophysiology, and neuroanatomy. Hippocampus 2000;10:94–110. [DOI] [PubMed] [Google Scholar]
- 11.Livy DJ, Miller EK, Maier SE, West JR. Fetal alcohol exposure and temporal vulnerability: effects of binge-like alcohol exposure on the developing rat hippocampus. Neurotoxicol Teratol 2003;25:447–58. [DOI] [PubMed] [Google Scholar]
- 12.Miller MW. Generation of neurons in the rat dentate gyrus and hippocampus: effects of prenatal and postnatal treatment with ethanol. Alcohol Clin Exp Res 1995;19:1500–9. [DOI] [PubMed] [Google Scholar]
- 13.Pei JR, Rinaldi CM, Rasmussen C, Massey V, Massey D. Memory patterns of acquisition and retention of verbal and nonverbal information in children with fetal alcohol spectrum disorders. Can J Clin Pharmacol 2008;15:e44–56. [PubMed] [Google Scholar]
- 14.Willoughby KA, Sheard ED, Nash K, Rovet J. Effects of prenatal alcohol exposure on hippocampal volume, verbal learning, and verbal and spatial recall in late childhood. J Int Neuropsychol Soc 2008;14:1022–33. [DOI] [PubMed] [Google Scholar]
- 15.Meck WH, Smith RA, Williams CL. Pre- and postnatal choline supplementation produces long-term facilitation of spatial memory. Dev Psychobiol 1988;21:339–53. [DOI] [PubMed] [Google Scholar]
- 16.Meck WH, Williams CL. Characterization of the facilitative effects of perinatal choline supplementation on timing and temporal memory. Neuroreport 1997;8:2831–5. [DOI] [PubMed] [Google Scholar]
- 17.Cheng RK, MacDonald CJ, Williams CL, Meck WH. Prenatal choline supplementation alters the timing, emotion, and memory performance (TEMP) of adult male and female rats as indexed by differential reinforcement of low-rate schedule behavior. Learn Mem 2008;15:153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas JD, Biane JS, O’Bryan KA, O’Neill TM, Dominguez HD. Choline supplementation following third-trimester-equivalent alcohol exposure attenuates behavioral alterations in rats. Behav Neurosci 2007;121:120–30. [DOI] [PubMed] [Google Scholar]
- 19.Thomas JD, La Fiette MH, Quinn VR, Riley EP. Neonatal choline supplementation ameliorates the effects of prenatal alcohol exposure on a discrimination learning task in rats. Neurotoxicol Teratol 2000;22:703–11. [DOI] [PubMed] [Google Scholar]
- 20.Wagner AF, Hunt PS. Impaired trace fear conditioning following neonatal ethanol: reversal by choline. Behav Neurosci 2006;120:482–7. [DOI] [PubMed] [Google Scholar]
- 21.Wozniak JR, Fuglestad AJ, Eckerle JK, Kroupina MG, Miller NC, Boys CJ, Brearley AM, Fink BA, Hoecker HL, Zeisel SH, et al. . Choline supplementation in children with fetal alcohol spectrum disorders has high feasibility and tolerability. Nutr Res 2013;33:897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Connor MJ, Shah B, Whaley S, Cronin P, Gunderson B, Graham J. Psychiatric illness in a clinical sample of children with prenatal alcohol exposure. Am J Drug Alcohol Abuse 2002;28:743–54. [DOI] [PubMed] [Google Scholar]
- 23.Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller JH, Aragon AS, Khaole N, et al. . A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 Institute of Medicine criteria. Pediatrics 2005;115:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bertrand J, Floyd LL, Weber MK, Fetal Alcohol Syndrome Prevention Team, Division of Birth Defects and Developmental Disabilities, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention (CDC). Guidelines for identifying and referring persons with Fetal Alcohol Syndrome. MMWR Recomm Rep 2005;54:1–14. [PubMed] [Google Scholar]
- 25.Astley SJ. Diagnostic guide for fetal alcohol spectrum disorders: the 4-digit diagnostic code, 3rd ed. Seattle (WA): University of Washington; 2004. [Google Scholar]
- 26.National Cancer Institute. Automated self-administered 24-hour recall (ASA24)-beta. Bethesda (MD): National Cancer Institute; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koc H, Mar MH, Ranasinghe A, Swenberg JA, Zeisel SH. Quantitation of choline and its metabolites in tissues and foods by liquid chromatography/electrospray ionization-isotope dilution mass spectrometry. Anal Chem 2002;74:4734–40. [DOI] [PubMed] [Google Scholar]
- 28.Zeisel SH, daCosta KA, Youssef M, Hensey S. Conversion of dietary choline to trimethylamine and dimethylamine in rats: dose-response relationship. J Nutr 1989;119:800–4. [DOI] [PubMed] [Google Scholar]
- 29.Miller CA, Corbin KD, da Costa K-A, Zhang S, Zhao X, Galanko JA, Blevins T, Bennett BJ, O’Connor A, Zeisel SH. Effect of egg ingestion on trimethylamine-N-oxide production in humans: a randomized, controlled, dose-response study. Am J Clin Nutr 2014;100:778–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mullen EM. Mullen Scales of Early Learning. Circle Pines (MN): American Guidance Service Inc.; 1995. [Google Scholar]
- 31.Bauer PJ. Development of memory in early childhood. Hove (United Kindom): Psychology Press; 1997. [Google Scholar]
- 32.Bauer PJ. Recalling past events: from infancy to early childhood. Ann Child Dev 1995;11:25–71. [Google Scholar]
- 33.Bauer PJ. Building toward a past: construction of a reliable long-term memory system. Mahwah (NJ): Erlbaum; 2002. [Google Scholar]
- 34.McDonough L, Mandler JM, McKee RD, Squire LR. The deferred imitation task as a nonverbal measure of declarative memory. Proc Natl Acad Sci USA 1995;92:7580–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riggins T, Miller NC, Bauer PJ, Georgieff MK, Nelson CA. Electrophysiological indices of memory for temporal order in early childhood: implications for the development of recollection. Dev Sci 2009;12:209–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riggins T, Miller NC, Bauer PJ, Georgieff MK, Nelson CA. Consequences of low neonatal iron status due to maternal diabetes mellitus on explicit memory performance in childhood. Dev Neuropsychol 2009;34:762–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riggins T, Cheatham CL, Stark E, Bauer PJ. Elicited imitation performance at 20 months predicts memory abilities in school-aged children. J Cogn Dev 2013;14:593–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Freeman GH, Halton JH. Note on an exact treatment of contingency, goodness of fit and other problems of significance. Biometrika 1951;38:141–9. [PubMed] [Google Scholar]
- 39.Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 1997;53:983–97. [PubMed] [Google Scholar]
- 40.Diggle PJ, Heagerty P, Liang KY, Zeger SL. Analysis of longitudinal data. 2nd ed. New York: Oxford University Press; 2002. [Google Scholar]
- 41.Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. J Educ Behav Stat 2006;31:437–48. [Google Scholar]
- 42.Feingold A. Effect sizes for growth-modeling analysis for controlled clinical trials in the same metric as for classical analysis. Psychol Methods 2009;14:43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ryan SH, Williams JK, Thomas JD. Choline supplementation attenuates learning deficits associated with neonatal alcohol exposure in the rat: effects of varying the timing of choline administration. Brain Res 2008;1237:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eme R, Millard E. Fetal alcohol spectrum disorders: a literature review with screening recommendations. Sch Psychol 2012;66:12–20. [Google Scholar]
- 45.Kim JJ, Baxter MG. Multiple brain-memory systems: the whole does not equal the sum of its parts. Trends Neurosci 2001;24:324–30. [DOI] [PubMed] [Google Scholar]
- 46.White NM, McDonald RJ. Multiple parallel memory systems in the brain of the rat. Neurobiol Learn Mem 2002;77:125–84. [DOI] [PubMed] [Google Scholar]
- 47.Albright CD, Friedrich CB, Brown EC, Mar MH, Zeisel SH. Maternal dietary choline availability alters mitosis, apoptosis and the localization of TOAD-64 protein in the developing fetal rat septum. Brain Res Dev Brain Res 1999;115:123–9. [DOI] [PubMed] [Google Scholar]
- 48.Albright CD, Tsai AY, Friedrich CB, Mar MH, Zeisel SH. Choline availability alters embryonic development of the hippocampus and septum in the rat. Brain Res Dev Brain Res 1999;113:13–20. [DOI] [PubMed] [Google Scholar]
- 49.Zeisel SH. The fetal origins of memory: the role of dietary choline in optimal brain development. J Pediatr 2006;149(5 Suppl):S131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Utsunomiya H, Takano K, Okazaki M, Mitsudome A. Development of the temporal lobe in infants and children: analysis by MR-based volumetry. AJNR Am J Neuroradiol 1999;20:717–23. [PMC free article] [PubMed] [Google Scholar]
- 51.Dani SU, Hori A, Walter GF. Principals of neural aging. New York: Elsevier; 1997. [Google Scholar]
- 52.Mohler EG, Meck WH, Williams CL. Sustained attention in adult mice is modulated by prenatal choline availability. Int J Comp Psychol 2001;14:136–50. [Google Scholar]
- 53.Food and Nutrition Board - Institute of Medicine. Dietary Reference Intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, panthotenic acid, biotin, and choline. Washington (DC): National Academy Press; 1998. [PubMed] [Google Scholar]
- 54.Fuglestad AJ, Fink BA, Eckerle JK, Boys CJ, Hoecker HL, Kroupina MG, Zeisel SH, Georgieff MK, Wozniak JR. Inadequate intake of nutrients essential for neurodevelopment in children with fetal alcohol spectrum disorders (FASD). Neurotoxicol Teratol 2013;39:128–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, et al. . Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011;472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, et al. . Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 2013;19:576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 2013;368:1575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Loscalzo J. Gut microbiota, the genome, and diet in atherogenesis. N Engl J Med 2013;368:1647–9. [DOI] [PubMed] [Google Scholar]
- 59.Martin JC, Canlet C, Delplanque B, Agnani G, Lairon D, Gottardi G, Bencharif K, Gripois D, Thaminy A, Paris A. 1H NMR metabonomics can differentiate the early atherogenic effect of dairy products in hyperlipidemic hamsters. Atherosclerosis 2009;206:127–33. [DOI] [PubMed] [Google Scholar]
- 60.Zeisel SH, DaCosta KA. Increase in human exposure to methylamine precursors of N-nitrosamines after eating fish. Cancer Res 1986;46:6136–8. [PubMed] [Google Scholar]
- 61.He K, Song Y, Daviglus ML, Liu K, Van Horn L, Dyer AR, Greenland P. Accumulated evidence on fish consumption and coronary heart disease mortality: a meta-analysis of cohort studies. Circulation 2004;109:2705–11. [DOI] [PubMed] [Google Scholar]