Abstract
Background: The Canadian Health Measures Survey (CHMS) is an ongoing cross-sectional national survey that includes a measure of 25-hydroxyvitamin D [25(OH)D] by immunoassay. For cycles 1 and 2, the collection period occurred approximately every 2 y, with a new sample of ∼5600 individuals.
Objective: The goal was to standardize the original 25(OH)D CHMS values in cycles 1 and 2 to the internationally recognized reference measurement procedures (RMPs) developed by the US National Institute for Standards and Technology (NIST) and Ghent University, Belgium.
Design: Standardization was accomplished by using a 2-step procedure. First, serum samples corresponding to the original plasma samples were remeasured by using the currently available immunoassay method. Second, 50 serum samples with known 25(OH)D values assigned by the NIST and Ghent reference method laboratories were measured by using the currently available immunoassay method. The mathematical models for each step—i.e., 1) YCurrent = XOriginal and 2) YNIST-Ghent = XCurrent —were estimated by using Deming regression, and the 2 models were solved to obtain a single equation for converting the “original” values to NIST-Ghent RMP values.
Results: After standardization (cycles 1 and 2 combined), the percentage of Canadians with 25(OH)D values <40 nmol/L increased from 16.4% (original) to 19.4% (standardized), and values <50 nmol/L increased from 29.0% (original) to 36.8% (standardized). The 25(OH)D standardized distributions (cycles 1 and 2 analyzed separately) were similar across age and sex groups; slightly higher values were associated with cycle 2 in the young and old. This finding contrasts with the original data, which indicated that cycle 2 values were lower for all age groups.
Conclusion: The shifts in 25(OH)D distribution brought about by standardization indicate its importance in drawing correct conclusions about potential population deficiencies and insufficiencies and in permitting the comparison of distributions between national surveys.
Keywords: standardization, vitamin D, immunoassay, population survey, adequacy, CHMS
INTRODUCTION
The Canadian Health Measures Survey (CHMS)11 is an ongoing cross-sectional national survey that includes a measure of plasma total 25-hydroxyvitamin D [25(OH)D] by immunoassay. During the first 2 cycles, the survey repeated approximately every 2 y (2007–2009 and 2009–2011), with a new sample of ∼5600 individuals monitored for each cycle (1). The distribution of 25(OH)D values within the population (2, 3) is used by Health Canada to assess vitamin D status (4), which affects food-fortification policies and identifies potential needs within the food supply. Because Canada is a northern country with a more limited productive sunlight period (5–7), vitamin D status is of greater concern in general and to individuals of nonwhite ethnic origin who may be at higher risk of insufficiency because of darker skin pigmentation (7, 8). Evaluating factors that may influence 25(OH)D concentrations in these individuals is difficult because of their lower prevalence within the Canadian population, necessitating combining CHMS cycles to obtain sufficient data to enable analysis of age, sex, BMI, dietary group, and sun exposure.
Combining data from different collection cycles can be problematic. Several factors can influence 25(OH)D concentrations over time, including direct effects on intake [public health messaging, abundance in the food supply, or changes in the doses found in commonly consumed supplements (9)], problems with assay drift and/or shift despite the use of external control programs (10), and changes in instrumentation or assay composition (11). For the CHMS, instrument performance issues necessitated a change in the autoimmunoanalyzer between cycles 1 and 2. In addition, a change in the Diasorin method at the end of cycle 2 restricted the analyses to serum 25(OH)D; all previous analyses had been performed with the use of plasma. A calibration study was therefore required to link the values from CHMS cycles 1 and 2 to the current method. In recognition of these issues, and of the difficulties of comparing across the different assay platforms used by national surveys (11, 12), the US Office of Dietary Supplements (NIH) in collaboration with the National Institute of Standards and Technology (NIST), CDC, Ghent University, and 8 national surveys (including the CHMS) organized the Vitamin D Standardization Program (VDSP) (13, 14). The VDSP reference measurement system includes internationally recognized reference measurement procedures (RMPs) developed by the NIST (15) and the University of Ghent (16) and Standard Reference Materials, which can be purchased from the NIST (17, 18).
Here we describe the procedure used to standardize the 25(OH)D values from CHMS cycles 1 and 2 to the RMPs and present equations for converting the originally released data to the VDSP RMP standard. This procedure minimizes changes in assay performance for each cycle and greatly increases the usefulness of the data by allowing comparison across cycles.
METHODS
Subjects
The CHMS (1, 19) is a cross-sectional survey of roughly 5600 Canadians per cycle that repeated approximately every 2 y for cycle 1 (2007–2009) and cycle 2 (2009–2011). Each cycle included samples from 15 sites across Canada chosen at random over the calendar year (20). The survey excluded residents of Indian reserves, Crown lands, certain remote regions, and institutions and full-time members of the Canadian Forces. Cycle 1 consisted of 5604 subjects (48% males) aged 6–79 y (median age: 33 y) who reported to a mobile examination center for physical measurements. This represents 51.7% of the individuals originally contacted. Of these individuals, plasma 25(OH)D data were obtained for 5306 (95%) subjects. The second cycle consisted of 6395 subjects (48.1% males) aged 3–79 y (median age: 32 y; overall response rate: 55.5%); plasma 25(OH)D data were obtained for 6030 (94%) subjects. The combined sample size for cycles 1 and 2 was 11,336. Each cycle was weighted to be representative of >96% of the Canadian population.
Sampling, analysis, and quality control
Plasma samples were collected and analyzed as previously described (21, 22). 25(OH)D was measured by using a LIAISON autoimmunoanalyzer (Diasorin Inc.). LIAISON 25OH Vitamin D TOTAL Assay integrals, LIAISON system liquid, starter reagents, and reaction modules were also obtained from Diasorin Inc. The assay was performed as indicated in the manufacturer’s product insert. The autoimmunoanalyzer was changed between cycles 1 and 2 as a result of problems with the instrument.
Quality controls were as previously described (22). In addition, the laboratory was in proficient standing in the Vitamin D External Quality Assessment Scheme (23). Assay drift was monitored starting with cycle 2 by using in-house pooled material at 3 concentrations of 25(OH)D. CVs for data from cycle 1 (10.8%) and cycle 2 (10.2%) were calculated by using the 3 concentrations of Bio-Rad external control samples previously reported (22). CVs were calculated according to guidelines (EP5-A2) approved by the Clinical Laboratory and Standards Institute (24).
Standardization
The VDSP reference measurement system and associated RMPs from the NIST and Ghent laboratories were described in detail previously (13, 14). The CV for the measurement of vitamin D in these laboratories was 1.86%. The protocol for standardizing CHMS plasma 25(OH)D values from cycles 1 and 2 was complicated by a change in the assay formulation, which occurred at the end of CHMS cycle 2 (December 2012). This necessitated the adoption of a 2-step procedure, similar to that reported by Cashman et al. [(25; Supplemental Figure 1 and Supplemental Table 1]. First, the number of samples to be remeasured in each cycle was calculated by using each cycle’s 25(OH)D range of concentrations, quartile cutoffs, and CVs for the original assay and new assay. The calculated sample size was 90 for each cycle. Next, serum samples corresponding to plasma samples originally analyzed in CHMS cycles 1 and 2 [original 25(OH)D ≥10 nmol/L] were selected by dividing the range of interest into intervals by using the quantiles of the observed samples and uniformly sampling equal number of Xi’s from each of the subintervals (26). All samples with values >100 nmol/L were also selected, totaling 107 (cycle 1) and 109 (cycle 2) samples. However, a single outlier (>4 SD) was removed from the cycle 1 data, and 3 outliers were removed from the cycle 2 data (>4 SD), which resulted in a total sample of 106 for both cycles 1 and 2.
The selected CHMS serum samples were measured in singlet with the current version of the LIAISON assay (code 310600) by using 3 different lot numbers (on 3 different days) for cycle 1 and 4 different lot numbers (on 4 different days) for cycle 2. A weighted Deming regression analysis was then conducted to establish the relation between the originally measured plasma 25(OH)D values (old assay method) and serum 25(OH)D values measured by using the current assay method, assuming that the ratio of the CVs for the different methods was constant. For cycle 1, the variance ratio used for the Deming regression (plasma/serum) was 1.141 and for cycle 2 was 0.926.
The second step involved standardizing the current immunoassay to the NIST and Ghent RMPs. To do this, 50 serum samples from individual donors (with target values assigned as the mean values from the NIST and Ghent University RMPs) were measured by using the current LIAISON procedure. The serum samples were collected by Solomon Park using Clinical Laboratory and Standards Institute guidelines (27) and were originally sent to the CHMS laboratory in November 2012 as part of an interlaboratory comparison study sponsored by the VDSP. The 50 serum samples were analyzed once on 13 July 2012 along with samples from cycle 1 and once on each of 4 d (31 October 2012, 1 November 2012, 12 December 2012, and 1 January 2013) along with samples from cycle 2. For cycle 1, the variance ratio used for the Deming regression (plasma/serum) was 56.07 and for cycle 2 was 47.74.
Statistical analysis
Weighted Deming Regression analyses were run by using CBStat5 (K. Linnet Charlottenlund) and verified by using the Method Comparison Regression program in R. The proportion of the Canadian population with 25(OH)D values <30, <40, and <50 nmol/L were calculated by using the combined data from cycles 1 and 2. Representational weights for individuals, released with data for each CHMS cycle, were used to obtain nationally representative distributions of 25(OH)D status. The value of 40 nmol/L has been defined as the equivalent of the estimated average requirement (EAR) for vitamin D (28). The proportion of the population with values <40 nmol/L is therefore an estimate of the percentage of the population with inadequate vitamin D status (29). Population distributions were calculated by using SAS version 9.2 with CIs calculated by using SUDAAN 10.1 as described in the CHMS data release by Statistics Canada (30). Comparisons between means were calculated by using the z test.
RESULTS
Standardizing the originally measured 25(OH)D values
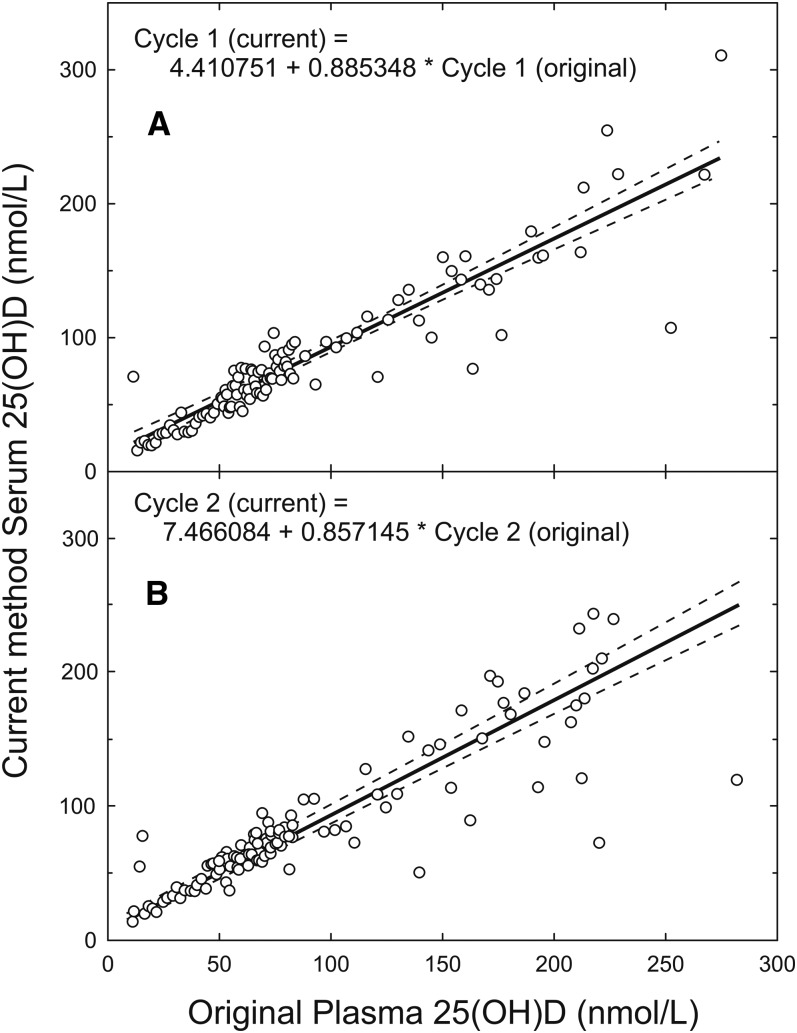
The standardization procedure (Supplemental Figure 1) involved 2 separate measurements to convert the originally released CHMS 25(OH)D values (2, 3). First, linear relations between the originally reported CHMS plasma 25(OH)D values (cycles 1 and 2) and serum 25(OH)D values were obtained by remeasuring the original 25(OH)D samples by using the current version of the immunoassay (Figure 1). After this step, overall cycle 1 values decreased from 67.7 ± 1.2 nmol/L (original) to 64.4 ± 1.1 nmol/L (mean ± SE, n = 5306; P = 0.05) and cycle 2 values decreased slightly from 63.8 ± 1.9 nmol/L (original) to 62.1 ± 1.6 nmol/L (mean ± SE, n = 6030; P = 0.6).
FIGURE 1.
Relation between 25(OH)D in plasma CHMS samples originally reported and values remeasured in serum via the current immunoassay for CHMS cycles 1 (A) and 2 (B). n = 106 for each cycle. The solid line represents the weighted Deming regression fit. The dashed lines represent the 95% CI. CHMS, Canadian Health Measures Survey; 25(OH)D, 25-hydroxyvitamin D.
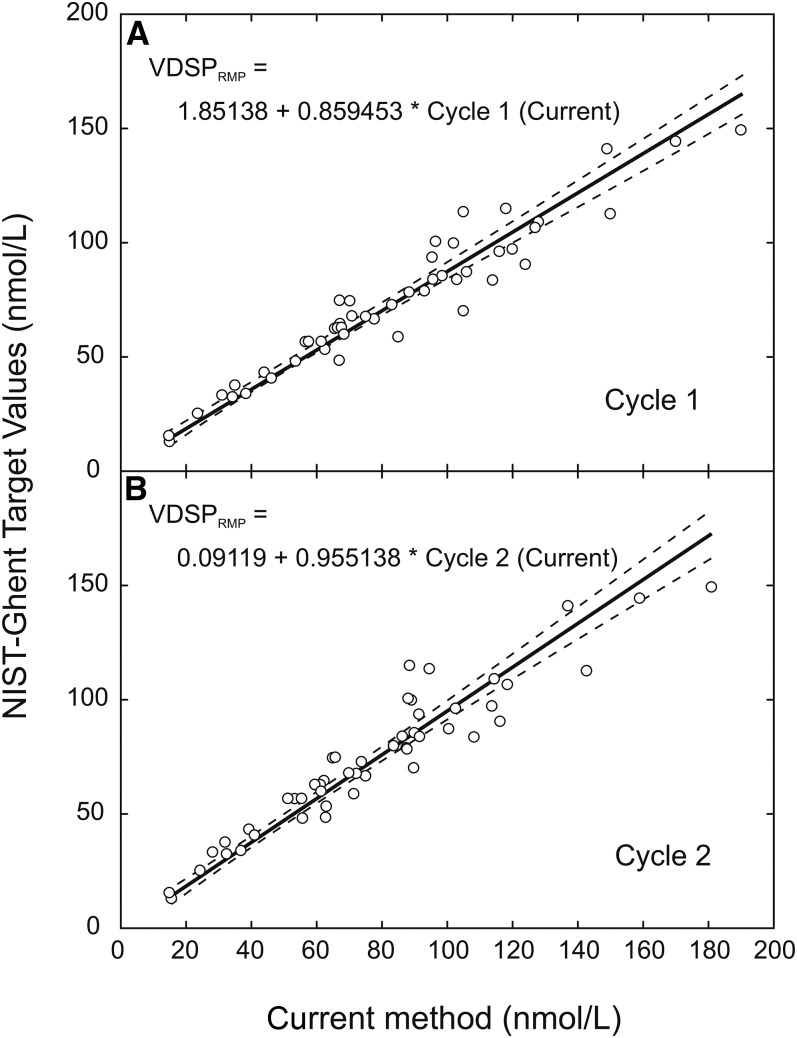
Second, relations between the current immunoassay method and RMP values from Ghent and the NIST were derived by measuring 50 serum samples with values assigned by the NIST and Ghent RMP [(25); Figure 2]. The NIST- and Ghent-assigned values represent primary standards because their values have been measured by using the 25(OH)D RMP. Thus, the second measurement calibrated the ”current method” to the RMP. Substituting the regression result from the first step for the “current” variable in the equations from the second step and simplifying resulted in equations for standardizing the originally measured values in cycles 1 and 2:
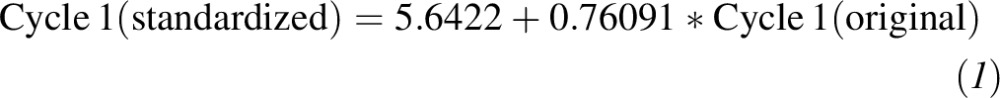 |
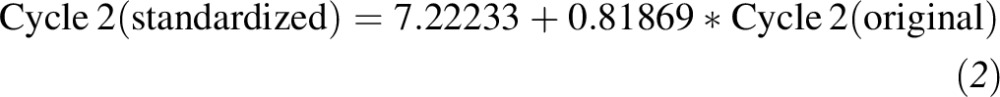 |
FIGURE 2.
Relation between NIST-Ghent–assigned VDSP values (VDSPRMP) and values measured by immunoassay via the current immunoassay method for cycles 1 (A) and 2 (B). The solid line represents the weighted Deming regression fit to the data (n = 50 samples). The dashed lines represent the 95% CI. NIST, National Institute of Standards and Technology; RMP, reference measurement procedure; VDSP, Vitamin D Standardization Program.
The final combined equations reduced the overall population mean 25(OH)D values to 57.2 ± 0.9 nmol/L (mean ± SE, P < 0.0001 compared with original) for cycle 1 and to 59.4 ± 1.6 (mean ± SE, P = 0.084 compared with original) for cycle 2.
Distribution of 25(OH)D values in Canada over cycles 1 and 2 (2007–2011)
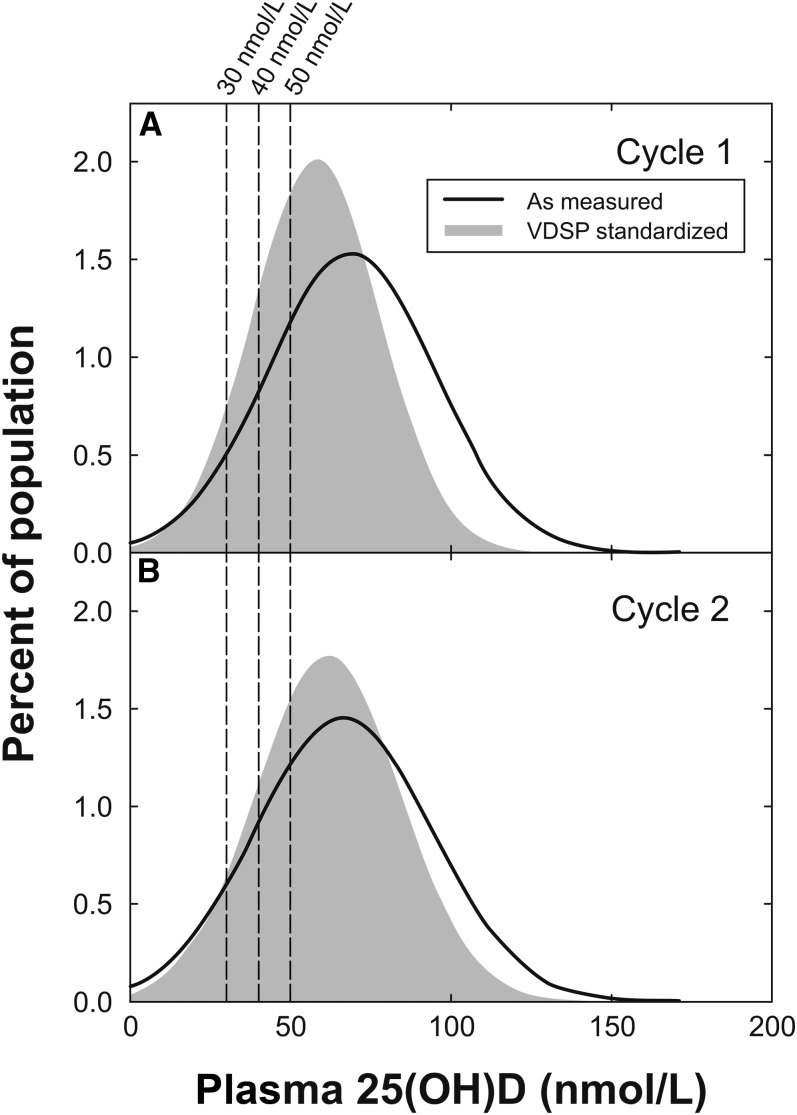
Standardization shifted the 25(OH)D distribution to the left for both cycles 1 and 2, but the effect was larger for cycle 1 (Figure 3). The standardization procedure also tended to bring in the tails of the original distribution so that the standardized data more closely reflected a normal distribution. As a result of standardization, the mean concentration of 25(OH)D decreased from 65.7 ± 1.1 to 58.3 ± 0.9 nmol/L after standardization (P < 0.00001; Table 1). The downward shift in the distribution also led to an increase in the proportion of the overall population falling below the physiologically equivalent vitamin D EAR value of 40 nmol/L when compared against the originally reported CHMS data (Table 1 and Figure 3). Further analysis showed some notable age and sex differences. For example, 16.6% of males aged 19–30 y had 25(OH)D values <30 nmol/L, whereas only 5.5% of age-matched females had 25(OH)D values <30 nmol/L (P < 0.001). In addition, of subjects aged 31–50 y, 27.9% of males compared with 18.1% of females had 25(OH)D values <40 nmol/L (P = 0.02) and 49.8% of males compared with 36.1% of females had 25(OH)D values <50 nmol/L (P < 0.009; Table 2). Other differences were not significant.
FIGURE 3.
Distribution of Canadian Health Measures Survey values (weighted for population representation) before (solid line) and after (filled gray) standardization by using Equation 1 for cycles 1 (A) and 2 (B). Vertical dashed lines show cutoff values of 30, 40, and 50 nmol/L. n = 5306 for cycle 1 and 6030 for cycle 2. VDSP, Vitamin D Standardization Program; 25(OH)D, 25-hydroxyvitamin D.
TABLE 1.
25(OH)D distribution in the Canadian population (cycles 1 and 2 combined) before and after standardization: ages and sexes combined1
| Original (n = 11,336) | Standardized (n = 11,336) | P value2 | |
| Mean 25(OH)D, nmol/L | 65.7 ± 1.1 (63.3, 68.0) | 58.3 ± 0.9 (56.4, 60.2) | <0.00001 |
| Median 25(OH)D, nmol/L | 63.8 (61.3, 66.3) | 56.7 (54.6, 58.8) | |
| Percentage of population <30 nmol/L | 7.7 ± 1.4 (5.6, 10.5) | 7.4 ± 1.2 (5.5, 10.0) | NS |
| Percentage of population <40 nmol/L | 16.4 ± 1.8 (13.2, 20.1) | 19.4 ± 1.9 (16.0, 23.4) | NS |
| Percentage of population <50 nmol/L | 29.0 ± 2.1 (25.0, 33.3) | 36.8 ± 2.2 (32.4, 41.5) | 0.02 |
All values are ± SEs; 95% CIs in parentheses. 25(OH)D, 25-hydroxyvitamin D.
Comparison between original values and standardized values by Bonferroni-corrected z test.
TABLE 2.
Effect of standardization on the distribution of 25(OH)D values in the Canadian population by age and sex groups1
| Percentage of population below indicated value |
||||
| Sex and age | Subjects, n | <30 nmol/L | <40 nmol/L | <50 nmol/L |
| Male | ||||
| All ages | ||||
| Original | 5484 | 9.4 ± 1.7 (6.7, 12.9) | 19.2 ± 2.3 (15.2, 23.8) | 32.1 ± 2.4 (27.6, 37.1) |
| Standardized | 5484 | 9.1 ± 1.6 (6.6, 12.5) | 22.6 ± 2.4 (18.3, 27.5) | 40.8 ± 2.6 (35.5, 46.2) |
| 9–13 y | ||||
| Original | 800 | NA | 9.7 ± 2.9 (5.9, 15.6)2 | 21.8 ± 3.6 (15.8, 29.3) |
| Standardized | 800 | NA | 12.1 ± 3.1 (7.6, 18.8)2 | 29.4 ± 3.5 (23.1, 36.7) |
| 14–18 y | ||||
| Original | 630 | NA | 16.3 ± 5.0 (9.4, 26.6)2 | 28.5 ± 5.2 (19.8, 39.2) |
| Standardized | 630 | NA | 21.2 ± 5.0 (13.6, 31.5)2 | 35.7 ± 5.4 (25.9, 46.8) |
| 19–30 y | ||||
| Original | 570 | 17.1 ± 3.9 (11.2, 25.1)2 | 27.2 ± 4.3 (19.9, 36.0) | 39.9 ± 4.3 (31.5, 48.8) |
| Standardized | 570 | 16.6 ± 3.9 (10.8, 24.8)2 | 31.5 ± 4.4 (23.7, 40.6) | 49.6 ± 4.3 (40.9, 58.4) |
| 31–50 y | ||||
| Original | 1387 | 10.3 ± 1.9 (7.4, 14.3) | 24.0 ± 3.5 (18.1, 31.2) | 40.5 ± 3.5 (33.6, 47.7) |
| Standardized | 1387 | 10.2 ± 1.7 (7.5, 13.8) | 27.9 ± 3.7 (21.3, 35.6) | 49.8 ± 3.7 (42.3, 57.4) |
| 51–70 y | ||||
| Original | 1116 | 6.6 ± 1.5 (4.4, 9.6)2 | 14.7 ± 1.7 (10.5, 20.3) | 25.5 ± 2.5 (20.9, 30.6) |
| Standardized | 1116 | 6.1 ± 1.2 (4.3, 8.6) | 17.2 ± 2.6 (12.9, 22.7) | 34.1 ± 2.8 (28.7, 40.0) |
| 71–79 y | ||||
| Original | 317 | 3.2 ± 1.3 (1.8, 5.8)2 | 7.8 ± 1.6(5.4, 11.0)2 | 16.3 ± 1.8 (13.2, 19.9) |
| Standardized | 317 | NA | 9.8 ± 1.5 (7.5, 12.9) | 23.4 ± 3.2 (17.9, 30.0) |
| Female | ||||
| All ages | ||||
| Original | 5852 | 6.0 ± 1.3 (4.1, 8.6)2 | 13.5 ± 1.6 (10.8, 16.9) | 25.8 ± 2.0 (22.1, 29.8) |
| Standardized | 5852 | 5.7 ± 1.2 (4.0, 8.2)2 | 16.3 ± 1.7 (13.3, 19.8) | 32.8 ± 2.1 (28.8, 37.1) |
| 9–13 y | ||||
| Original | 779 | 7.2 ± 3.0 (3.8, 13.5)2 | 14.2 ± 3.9 (8.7, 22.3)2 | 26.7 ± 3.4 (20.7, 33.6) |
| Standardized | 779 | NA | 15.9 ± 3.9 (10.1, 24.0)2 | 33.7 ± 3.5 (27.2, 40.9) |
| 14–18 y | ||||
| Original | 581 | 8.5 ± 2.3 (5.3, 13.3)2 | 14.6 ± 3.0 (10.0, 20.8)2 | 24.7 ± 3.8 (18.2, 32.6) |
| Standardized | 581 | 8.6 ± 2.8 (5.0, 14.4)2 | 16.6 ± 3.0 (11.8, 22.9) | 33.0 ± 4.1 (25.5, 41.5) |
| 19–30 y | ||||
| Original | 668 | 5.7 ± 1.6 (3.6, 9.1)2 | 18.1 ± 4.5 (11.4, 27.4)2 | 32.6 ± 4.6 (24.3, 42.1) |
| Standardized | 668 | 5.5 ± 1.8 (3.2, 9.2)2 | 21.3 ± 4.4 (14.3, 30.5)2 | 39.9 ± 4.6 (31.1, 49.5) |
| 31–50 y | ||||
| Original | 1647 | 6.4 ± 1.8 (4.0, 10.1)2 | 14.7 ± 2.0 (11.4, 18.8) | 28.0 ± 2.8 (22.8, 33.8) |
| Standardized | 1647 | 6.2 ± 1.8 (3.8, 9.9)2 | 18.1 ± 2.2 (14.4, 22.6) | 36.1 ± 3.1 (30.1, 42.5) |
| 51–70 y | ||||
| Original | 1204 | 5.7 ± 1.6 (3.5, 9.0)2 | 10.6 ± 1.8 (7.7, 14.4) | 21.9 ± (18.0, 26.3) |
| Standardized | 1204 | 5.5 ± 1.7 (3.5, 8.5)2 | 13.2 ± 2.0 (9.9, 17.4) | 28.1 ± 2.1 (24.7, 31.7) |
| 71–79 y | ||||
| Original | 354 | NA | 9.0 ± 2.9 (5.3, 15.0)2 | 17.6 ± 3.6 (12.1, 25.0)2 |
| Standardized | 354 | NA | 10.3 ± 2.7 (6.5, 16.0)2 | 22.3 ± 3.5 (16.4, 29.5) |
All values are cumulative population percentages ± SEs; 95% CIs in parentheses. NA, not available (the number of respondents was so low that values were too unreliable to be published); 25(OH)D, 25-hydroxyvitamin D.
Use with caution.
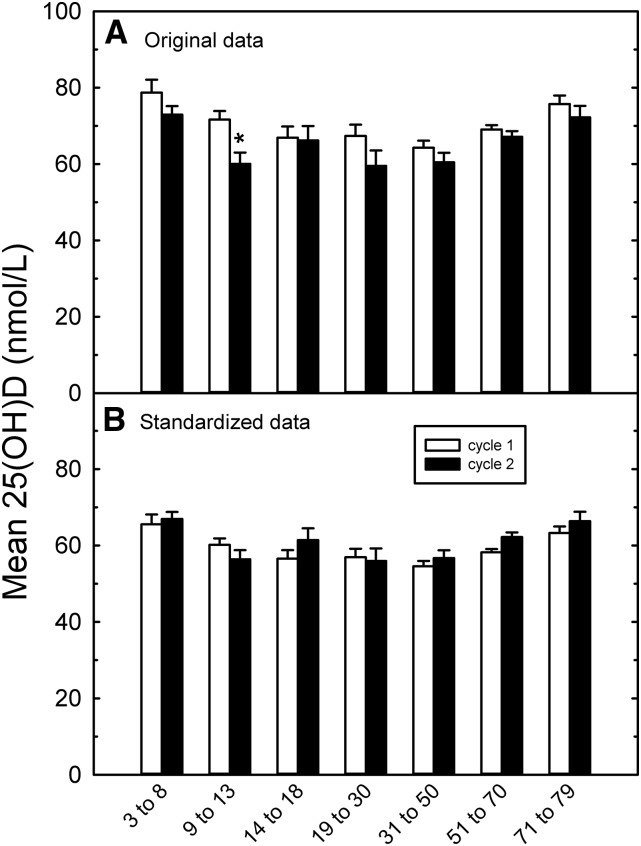
Standardization also allowed comparison of the data from cycle 2 (collected during 2009–2011) with that of cycle 1 (collected during 2007–2009) to analyze for time-associated changes in 25(OH)D status. The original data showed decreased 25(OH)D concentrations in cycle 2 for most of the age groups (Figure 4A); the mean value across all ages and sexes decreased from 67.7 ± 1.2 nmol/L (mean ± SE, n = 5306; cycle 1) to 63.8 ± 1.8 nmol/L (mean ± SE, n = 6030; cycle 2). This trend disappeared or was reversed after standardization (Figure 4B). Comparison of the standardized data by age and sex groups showed a significant increase in 25(OH)D in females aged 51–70 y: 58.1 ± 0.9 nmol/L (n = 550 for cycle 1 compared with 65.4 nmol/L for cycle 2, n = 566; P = 0.012, Bonferroni corrected; Supplemental Table 2). However, this difference disappeared when the sexes were combined (Figure 4B).
FIGURE 4.
Mean ± SEM 25(OH)D values pooled by Dietary Reference Intake age categories (male + female) in cycles 1 and 2 for the original reported data (A) and the standardized data (B). See Supplemental Table 1 for the sample numbers per group and individual values. Significant differences between cycles were determined by z test and are indicated by an asterisk. 25(OH)D, 25-hydroxyvitamin D.
DISCUSSION
The ability to compare survey results over the long term is a critical step in the development of government policies, requiring an understanding of time-dependent changes in status and differences in racial-ethnic groups that make up populations. The VDSP procedure offers a mechanism for this comparison by providing a procedure for standardizing past results. Standardization is important for all methods because immunoassays can be subject to matrix effects (31, 32), and the newer liquid chromatography–tandem mass spectrometry methods have been shown to be variable among laboratories (33).
The CHMS survey exemplified the need for standardization. The relatively lower proportion of the Canadians who are nonwhite (∼20%) makes it difficult to analyze for dietary, seasonal, and latitude effects within a single cycle because this group is not oversampled in the current design. Only by combining data from multiple cycles will it be possible to use CHMS data to analyze for factors that affect 25(OH)D. There were also methodologic concerns: samples were measured over several years, and the method/instrumentation changed significantly over the 3 cycles since its beginning. The immunoanalyzer was changed after cycle 1, and the assay was modified by the manufacturer at the completion of cycle 2 (switch from plasma to serum). Whereas crossover data (collected throughout) and quality-control data indicated no obvious issues, assay drift was monitored only starting with CHMS cycle 2. The greater reduction in CHMS cycle 1 values (compared with cycle 2 values) showed that undetected assay drift was an issue. This may have been the result of many factors, including the intended use of clinical assay systems to compare values to acceptable ranges or population distributions (33) compared with the constant requirement for accuracy in a research setting. The potential effect of different operators (11, 12, 33) did not apply to cycles 1 and 2 of the CHMS, for which a single principal operator performed all of the measurements; however, this did not rule out subtle procedural changes that may have occurred. External controls used during this period are unlikely to have helped correct for assay drift (22).
The VDSP standardization procedure used here was validated by using data collected in Ireland (25). This method is not tied to any predefined mathematical model. For example, the Irish national survey data were best fit by a piecewise linear model, and the predicted values were well matched to samples re-analyzed by liquid chromatography–tandem mass spectrometry (25). In the current study, the data were best fit by a linear model, although some points fell below the line at high (>100 nmol/L) values of 25(OH)D as a result of issues of repeatability in this region (22). A potential drawback of this procedure is that the standardization of 25(OH)D concentrations at the high end of the distribution may be problematic because of the apparent breakdown of some assays in this range. This may arise, in part, because the antibodies used in many immunoassays are highly cross-reactive with 24,25-dihydroxyvitamin D3 (34). This was not a major factor in assessing the CHMS distribution for adequacy at the EAR value, which fell well below the median of the curve.
The other major concern with a 2-step procedure is the greater potential for introducing measurement error, because the best estimates from each derived relation have their own uncertainty and potential bias. This means that the variability associated with the coefficients in our final (combined) equations is higher than that of the 2 original regressions. Note that, when equations are used to estimate population risk at a defined cutoff value, it is common to ignore the variance associated with standardization in the final reported values. Thus, the values reported in the current study were calculated assuming that the standardization introduced no extra uncertainty. In addition to increased uncertainty, there is also the potential for lot-to-lot bias and for assay shift between measures when more than one step is performed. In the current study, this was minimized by performing measures within a short time period with reagents with the same lot numbers. Although this was possible for cycle 2, instrument issues arose during the remeasure of cycle 1, which made it necessary to analyze this cycle over different times.
Standardization had an effect on the survey results and reversed the originally observed decreasing trend in 25(OH)D over time. From a population perspective, a decreasing trend appeared counterintuitive because of increased awareness of a link between vitamin D and health. Part of the decrease in 25(OH)D over time could have been attributable to changes in the methodologic bias itself, which have been documented (33, 35). This could have accounted for a greater correction required for cycle 1, especially because the overall LIAISON method bias among laboratories decreased during the time frame of cycle 2 (33, 35).
Relative to the original data, a higher percentage (∼20%) of the overall population had 25(OH)D values <40 nmol/L—a value consistent with a daily vitamin D intake equivalent to the EAR value of 400 IU/d (10 μg/d), which potentially indicated an inadequate intakes and/or inadequate sun exposure to meet daily needs. This suggests that vitamin D supplementation may be required for some Canadians to meet the Institute of Medicine recommendations, given their current dietary habits and sun exposure and the current vitamin D availability in the food supply. This would be in addition to the current mandatory fortification of milk and margarine that exists in Canada (36). Dietary sources of vitamin D can significantly contribute to 25(OH)D status. For example, a previous report (not standardized) in Canada showed that regular milk drinkers (≥1 time/d) had, on average, 9-nmol/L higher 25(OH)D values than those consuming milk <1 time/d (2). That analysis also showed that ∼33% of Canadians from cycle 2 consumed a vitamin D–containing supplement, and these individuals, on average, had 16-nmol/L higher plasma 25(OH)D values during the winter and 10-nmol/L higher values during the summer (2). Correspondingly, a lower percentage of supplement users fell below the 40-nmol/L cutoff value (15.6%) than did nonsupplement users (6.4%; note that these values were not standardized). Higher rates of insufficiency associated with winter in a northern country such as Canada are not unexpected, where very little productive sunlight exposure occurs during the winter months, even on sunny days (37), when individuals are most likely to be indoors or covered up.
In summary, CHMS 25(OH)D values have been standardized to the NIST and Ghent University laboratory RMPs through the VDSP. Standardization allowed values from cycles 1 and 2 to be combined, which permitted a more thorough examination of factors that affect 25(OH)D status in Canadians by functionally increasing the total number of survey participants. The standardized values were lower overall, with a concomitant increase in the percentage of individuals with a concentration <40 nmol/L compared with nonstandardized values. Standardization also reversed an apparent time-dependent decrease in 25(OH)D status, which indicated its usefulness for analyzing data from ongoing, long-term surveys. Note that the standardization results presented here cannot be generalized to other laboratories using the same method or to any other method; standardization is a procedure for transforming data collected with a single method by a single laboratory to an RMP.
Acknowledgments
We thank Shirley Bryan and Scott McLean at Statistics Canada for organizing and coordinating the CHMS collection, Penny Jee and Lois Fernandez at Health Canada for quality control, Rosemary Schleicher (NHANES) for extremely helpful discussions and advice, the laboratory of Linda M Thienpont for helping to assign values to the standardization samples, and all individuals who voluntary participated in the CHMS.
The authors’ responsibilities were as follows—KS, CTS, RD-A, LT, and SPJB: designed the research; KS, CTS, RD-A, and LT: conducted the research and provided essential analyses; SPJB and CTS: wrote the manuscript and had primary responsibility for the final content of the manuscript; and all authors: contributed to the revision and final approval of the manuscript and analyzed and interpreted the data. The sponsors had no influence on the study design, data collection, data analysis, data interpretation, or content and submission of the manuscript. None of the authors had a conflict of interest.
Footnotes
Abbreviations used: CHMS, Canadian Health Measures Survey; EAR, estimated average requirement; NIST, National Institute for Standards and Technology; RMP, reference measurement procedure; VDSP, Vitamin D Standardization Program; 25(OH)D, 25-hydroxyvitamin D.
REFERENCES
- 1.Tremblay MS, Connor Gorber S. Canadian health measures survey: brief overview. Can J Public Health 2007;98:453–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whiting SJ, Langlois KA, Vatanparast H, Greene-Finestone LS. The vitamin D status of Canadians relative to the 2011 Dietary Reference Intakes: an examination in children and adults with and without supplement use. Am J Clin Nutr 2011;94:128–35. [DOI] [PubMed] [Google Scholar]
- 3.Janz T, Pearson C. Vitamin D blood levels of Canadians. Ottawa (Canada): Statistics Canada; 2013. [Google Scholar]
- 4.Hollis BW. The determination of circulating 25-hydroxyvitamin D: no easy task. J Clin Endocrinol Metab 2004;89:3149–51. [DOI] [PubMed] [Google Scholar]
- 5.Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr 2005;135:317–22. [DOI] [PubMed] [Google Scholar]
- 6.Vieth R, Cole D, Hawker G, Trang H, Rubin L. Wintertime vitamin D insufficiency is common in young Canadian women, and their vitamin D intake does not prevent it. Eur J Clin Nutr 2001;55:1091–7. [DOI] [PubMed] [Google Scholar]
- 7.Hanley DA, Davison KS. Vitamin D insufficiency in North America. J Nutr 2005;135:332–7. [DOI] [PubMed] [Google Scholar]
- 8.Durazo-Arvizu RA, Camacho P, Bovet P, Forrester T, Lambert EV, Plange-Rhule J, Hoofnagle AN, Aloia J, Tayo B, Dugas LR, et al. . 25-Hydroxyvitamin D in African-origin populations at varying latitudes challenges the construct of a physiologic norm. Am J Clin Nutr 2014;100:908–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yetley EA. Assessing the vitamin D status of the US population. Am J Clin Nutr 2008;88:558S–64S. [DOI] [PubMed] [Google Scholar]
- 10.Yetley EA, Pfeiffer CM, Schleicher RL, Phinney KW, Lacher DA, Christakos S, Eckfeldt JH, Fleet JC, Howard G, Hoofnagle AN. NHANES monitoring of serum 25-hydroxyvitamin D: a roundtable summary. J Nutr 2010;140:2030S–45S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carter GD. 25-Hydroxyvitamin D: a difficult analyte. Clin Chem 2012;58:486–8. [DOI] [PubMed] [Google Scholar]
- 12.Carter GD, Carter R, Jones J, Berry J. How accurate are assays for 25-hydroxyvitamin D? Data from the international vitamin D external quality assessment scheme. Clin Chem 2004;50:2195–7. [DOI] [PubMed] [Google Scholar]
- 13.Sempos CT, Vesper HW, Phinney KW, Thienpont LM, Coates PM. Vitamin D status as an international issue: national surveys and the problem of standardization. Scand J Clin Lab Invest Suppl 2012;243:32–40. [DOI] [PubMed] [Google Scholar]
- 14.Binkley N, Sempos CT, Vitamin DSP. Standardizing vitamin d assays: the way forward. J Bone Miner Res 2014;29:1709–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tai SS, Bedner M, Phinney KW. Development of a candidate reference measurement procedure for the determination of 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 in human serum using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal Chem 2010;82:1942–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stepman HC, Vanderroost A, Van Uytfanghe K, Thienpont LM. Candidate reference measurement procedures for serum 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 by using isotope-dilution liquid chromatography-tandem mass spectrometry. Clin Chem 2011;57:441–8. [DOI] [PubMed] [Google Scholar]
- 17.Phinney KW. Development of a standard reference material for vitamin D in serum. Am J Clin Nutr 2008;88(Suppl):511S–2S. [DOI] [PubMed] [Google Scholar]
- 18.Phinney KW, Bedner M, Tai SS, Vamathevan VV, Sander LC, Sharpless KE, Wise SA, Yen JH, Schleicher RL, Chaudhary-Webb M, et al. . Development and certification of a standard reference material for vitamin D metabolites in human serum. Anal Chem 2012;84:956–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Day B, Langlois R, Tremblay M, Knoppers B-M. Canadian Health Measures Survey: ethical, legal and social issues. Health Rep 2007;18:37–51. [PubMed] [Google Scholar]
- 20.Giroux S. Canadian Health Measures Survey: sampling strategy overview. Health Rep 2007;18(Suppl):31–6. [PubMed] [Google Scholar]
- 21.Langlois K, Greene-Finestone L, Little J, Hidiroglou N, Whiting S. Vitamin D status of Canadians as measured in the 2007 to 2009 Canadian Health Measures Survey. Health Rep 2010;21:47–55. [PubMed] [Google Scholar]
- 22.Sarafin K, Hidiroglou N, Brooks SPJ. A comparison of two immunoassays for analysing plasma 25-hydroxyvitamin D. Open Clin Chem J 2011;45:45–9. [Google Scholar]
- 23.Carter GD, Carter CR, Gunter E, Jones J, Jones G, Makin HL, Sufi S. Measurement of vitamin D metabolites: an international perspective on methodology and clinical interpretation. J Steroid Biochem Mol Biol 2004;89-90:467–71. [DOI] [PubMed] [Google Scholar]
- 24.Tholen DW, Kallner A, Kennedy JW, Krouwer JS, Meier K. Evaluation of precision performance of quantitative measurement methods; approved guideline—second edition. Evaluation 2004;24(25). [Google Scholar]
- 25.Cashman KD, Kiely M, Kinsella M, Durazo-Arvizu RA, Tian L, Zhang Y, Lucey A, Flynn A, Gibney MJ, Vesper HW, et al. . Evaluation of Vitamin D Standardization Program protocols for standardizing serum 25-hydroxyvitamin D data: a case study of the program’s potential for national nutrition and health surveys. Am J Clin Nutr 2013;97:1235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian L, Durazo-Arvizu RA, Myers G, Brooks S, Sarafin K, Sempos CT. The estimation of calibration equations for variables with heteroscedastic measurement errors. Stat Med 2014;33:4420–36. [DOI] [PubMed] [Google Scholar]
- 27.Clinical Laboratory and Standards Institute. Preparation and validation of commutable frozen human serum pools as secondary reference materials for cholesterol measurement procedures; approved guideline. Wayne (PA): CLSI; 2004. [Google Scholar]
- 28.Institute of Medicine. Dietary Reference Intakes for calcium and vitamin D. Washington (DC): National Academy Press; 2011. [Google Scholar]
- 29.Taylor CL, Carriquiry AL, Bailey RL, Sempos CT, Yetley EA. Appropriateness of the probability approach with a nutrient status biomarker to assess population inadequacy: a study using vitamin D. Am J Clin Nutr 2013;97:72–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nadkarni MA, Caldon CE, Chhour KL, Fisher IP, Martin FE, Jacques NA, Hunter N. Carious dentine provides a habitat for a complex array of novel Prevotella-like bacteria. J Clin Microbiol 2004;42:5238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heijboer AC, Blankenstein MA, Kema IP, Buijs MM. Accuracy of 6 routine 25-hydroxyvitamin D assays: influence of vitamin D binding protein concentration. Clin Chem 2012;58:543–8. [DOI] [PubMed] [Google Scholar]
- 32.Farrell C, Soldo J, Williams P, Herrmann M. 25-Hydroxyvitamin D testing: challenging the performance of current automated immunoassays. Clin Chem Lab Med 2012;50:1953–63. [DOI] [PubMed] [Google Scholar]
- 33.Carter GD. Accuracy of 25-hydroxyvitamin D assays: confronting the issues. Curr Drug Targets 2011;12:19–28. [DOI] [PubMed] [Google Scholar]
- 34.Cashman KD, Hayes A, Galvin K, Merkel J, Jones G, Kaufmann M, Hoofnagle AN, Carter GD, Durazo-Arvizu RA, Sempos CT. Significance of serum 24,25-dihydroxyvitamin D in the assessment of vitamin D status: a double-edged sword? Clin Chem 2015;61:636–45. [DOI] [PubMed] [Google Scholar]
- 35.Carter GD, Berry JL, Gunter E, Jones G, Jones JC, Makin HL, Sufi S, Wheeler MJ. Proficiency testing of 25-hydroxyvitamin D (25-OHD) assays. J Steroid Biochem Mol Biol 2010;121:176–9. [DOI] [PubMed] [Google Scholar]
- 36. Health Canada. Food and Drug Regulations (CRC, c. 870). Food and Drugs Act Division 3. Addition of vitamins, mineral, nutrients or amino acids to foods. D.03.002. 2014.
- 37.Engelsen O, Brustad M, Aksnes L, Lund E. Daily duration of vitamin D synthesis in human skin with relation to latitude, total ozone, altitude, ground cover, aerosols and cloud thickness. Photochem Photobiol 2005;81:1287–90. [DOI] [PubMed] [Google Scholar]