Abstract
Background: Adherence to a Mediterranean-type diet is linked to a lower risk of mortality and chronic disease, but the association with the progression of age-related macular degeneration (AMD) and genetic susceptibility is unknown.
Objective: We examined the association of adherence to the Mediterranean diet and genetic susceptibility with progression to advanced AMD.
Design: Among 2525 subjects in the AREDS (Age-Related Eye Disease Study), 1028 eyes progressed to advanced AMD over 13 y. Baseline data for demographic and behavioral covariates were collected by using questionnaires. Dietary data were collected from food-frequency questionnaires. The alternate Mediterranean diet (aMeDi) score (range: 0–9) was constructed from individual intakes of vegetables, fruit, legumes, whole grains, nuts, fish, red and processed meats, alcohol, and the ratio of monounsaturated to saturated fats. Ten genetic loci in 7 genes [complement factor H (CFH), age-related maculopathy susceptibility 2/high-temperature requirement A serine peptidase 1 (ARMS2/HTRA1), complement component 2 (C2), complement factor B (CFB), complement component 3 (C3), collagen type VIII α 1 (COL8A1), and RAD51 paralog B (RAD51B)] were examined. Survival analysis was used to assess individual eyes for associations between incident AMD and aMeDi score, as well as interaction effects between aMeDi score and genetic variation on risk of AMD.
Results: A high aMeDi score (score of 6–9) was significantly associated with a reduced risk of progression to advanced AMD after adjustment for demographic, behavioral, ocular, and genetic covariates (HR: 0.74; 95% CI: 0.61, 0.91; P-trend = 0.007). The aMeDi score was significantly associated with a lower risk of incident advanced AMD among subjects carrying the CFH Y402H nonrisk (T) allele (P-trend = 0.0004, P-interaction = 0.04). The aMeDi score was not associated with AMD among subjects who were homozygous for the risk (C) allele.
Conclusion: Higher adherence to a Mediterranean diet was associated with reduced risk of progression to advanced AMD, which may be modified by genetic susceptibility. This trial was registered at clinicaltrials.gov as NCT00594672.
Keywords: AMD progression, Mediterranean diet, genetics, macular degeneration, nutrition
INTRODUCTION
Age-related macular degeneration (AMD)8 is a chronic and degenerative disease of the central part of the retina. AMD is the most common cause of blindness in industrialized countries (1, 2) and can significantly reduce quality of life (3). Visual impairment due to advanced AMD is expected to increase as the elderly population grows and the prevalence of AMD rises, with an estimated 50% increase by 2020 (2). Advanced forms of the disease include neovascular disease and geographic atrophy (GA), which are generally preceded by asymptomatic early and intermediate stages and can lead to irreversible blindness. Although treatments that inhibit vascular endothelial growth factor are available for neovascular disease, they are not curative (4). Major genetic factors related to AMD are now well known (5), and important behavioral components have been identified, including diet and smoking (1, 5, 6). Addressing modifiable risk factors could be invaluable for reducing the medical and social burden due to AMD.
Epidemiologic studies have shown that single nutrients, foods, or food groups are associated with both onset and progression of AMD. A high consumption of fish (7–11), nuts (8), lutein and zeaxanthin (12–18), and EPA and DHA (7, 8, 10, 11, 19–24) was associated with a reduced risk of AMD, whereas the consumption of red meat (25) and trans fats (8) was associated with an increased risk. These studies used either a single-nutrient or a single-food approach. Foods and nutrients are consumed in combination, however, and they may have synergistic effects.
The traditional Mediterranean diet is characterized by high consumption of plant foods, moderate consumption of fish and wine, low consumption of dairy and meat, and intake of MUFAs as the primary fat source (26). A higher adherence to a Mediterranean-type diet is linked to lower rates of mortality (27), chronic disease, and stroke (28), as well as healthy aging (29), but the association with AMD has not been fully explored. In the CAREDS (Carotenoids Age-Related Eye Disease Study), a high adherence to the Mediterranean diet was associated with a lower prevalence of early AMD (30). Other studies recently evaluated associations between dietary patterns and prevalence of early and advanced AMD with the use of principal components analysis (PCA) (31, 32) and the Healthy Eating Index (HEI) (30, 33).
We hypothesized that adhering to a Mediterranean-type diet could help reduce progression to advanced AMD and that the benefit of this dietary pattern could be modified by genetic risk. We therefore investigated the association between adherence to the Mediterranean-type diet and progression to advanced stages of AMD, controlling for 10 major genetic variants, and explored gene-diet interactions.
METHODS
Age-Related Eye Disease Study population
Details of the AREDS (Age-Related Eye Disease Study) of the National Eye Institute of the NIH have been reported (34). The AREDS included a multicenter randomized clinical trial to assess the effect of antioxidant and mineral supplements on the risk of AMD and cataracts as well as a longitudinal study of progression to advanced AMD. The protocol was approved by a data and safety monitoring committee and by each institutional review board for the 11 participating ophthalmic centers before initiation of the study. Participants were aged 55–80 y at baseline and were required to have at least one eye with a visual acuity no worse than 20/32. In addition, at least one eye of each participant had to be free from eye disease that could complicate the assessment of AMD, and that eye could not have had previous ocular surgery (except for cataract surgery and unilateral photocoagulation for AMD). Potential participants were excluded for illness or disorders that would have made long-term follow-up or compliance with the study protocol unlikely or difficult. Informed consent was obtained from participants before enrollment. Research followed the tenets of the Declaration of Helsinki. This study enrolled 4757 participants from 1992 to 1998. This trial was registered at clinicaltrials.gov as NCT00594672.
Procedures
Data on demographic factors, environmental exposures, medical history, drug use, habitual diet, and ocular status were obtained through general questionnaires and ophthalmic examinations. Trained graders, masked to clinical and phenotypic information from previous years, ascertained signs of AMD from annual stereoscopic color images by using a standardized and validated protocol at a single reading center. Retinal photographs were taken according to a standardized protocol by AREDS-certified photographers with the use of AREDS-certified cameras (35). Photographs were scheduled at baseline, at the 2-y visit, and annually thereafter during follow-up.
Study subjects
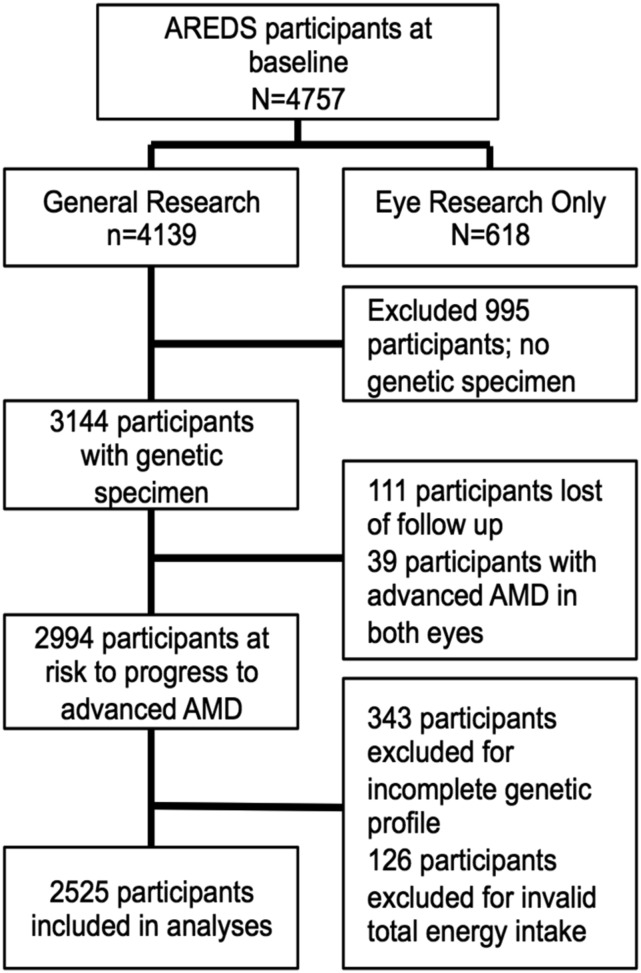
Data were accessed from the NIH Database of Genotypes and Phenotypes. Figure 1 shows the selection procedures for subjects included in the present study. Among the 4757 participants at baseline, we excluded 618 subjects who consented only to “eye research.” For these subjects, phenotype and genetic data could not be linked and therefore could not be included in these analyses. Among the remaining 4139 subjects who consented to “general research,” we excluded 995 subjects for lack of a genetic specimen. Of the 3144 subjects with a genetic specimen, 111 were removed from the data set due to lack of follow-up information. Thirty-nine participants with advanced AMD in both eyes at baseline were also removed. Furthermore, an additional 469 subjects were excluded from the data set: 343 as a result of incomplete genotyping information [genotyping rate of <100% across the 10 single nucleotide polymorphisms (SNPs) evaluated] and 126 due to an inappropriate total energy intake (TEI; valid TEI range: 600–3200 kcal for women and 600–4200 kcal for men). Complete data, including AMD grade at baseline and at least one follow-up visit, dietary data, demographic, behavioral, and genetic covariates, were available for 2525 subjects (4663 eyes) at risk of progression to advanced AMD.
FIGURE 1.
Flowchart showing the selection of subjects for this study who are at risk of progression to advanced AMD from the AREDS cohort. AMD, age-related macular degeneration; AREDS, Age-Related Eye Disease Study.
Definition of progression
Eyes were classified by using the Clinical Age-Related Maculopathy Staging (CARMS) system (36). Conversion from the AREDS to the CARMS grading system was based on all available phenotype data for all follow-up visits, as described in Yu et al. (37). The AREDS system uses the Wisconsin grading classification, and for the purpose of the trial, combined intermediate AMD with noncentral GA into one grade (grade 3) and combined central atrophy and neovascular disease along with visual loss due to AMD into another grade (grade 4). When conducting genetic analyses, the classification of specific subphenotypes can be informative. We therefore used the CARMS system to reclassify subjects into a separate GA category (central or noncentral), because there is no evidence that these 2 types are different in their etiology, and retained neovascular disease as a separate category. Visual loss due to AMD is not part of the CARMS system, and advanced cases were classified on the basis of their phenotype only, which is useful for the purpose of evaluating progression over time for various phenotypes. CARMS grades were defined as follows: no drusen or only a few small drusen (<63 μm) were assigned to grade 1 (no AMD); intermediate drusen (63–124 μm) were assigned to grade 2 (early AMD); large drusen (≥125 μm) were assigned to grade 3 (intermediate AMD) as long there were no signs of advanced AMD; GA (both central and noncentral) was classified as grade 4, if there was GA in the center grid or anywhere within the grid and there was no record of hemorrhage; and neovascular disease, or grade 5, if there were any definitive signs of neovascular AMD such as hemorrhagic retinal detachment, hemorrhage under the retina or retinal pigment epithelium, or subretinal fibrosis, regardless of visual acuity.
Progression was defined as either eye progressing from no, early, or intermediate AMD at baseline to advanced disease (either GA or neovascular disease) at any point during the 13-y study period. Follow-up ended when an eye progressed to either GA or neovascular disease. Eyes with advanced AMD at baseline were excluded from the analysis.
Dietary assessment and Mediterranean diet score
Data on food consumption were collected at enrollment with a validated, self-administered, 90-item, semiquantitative food-frequency questionnaire based on the National Cancer Institute Health Habits and History Questionnaire (version 2.1). Subjects were asked to report how often, on average, they consumed each food or beverage item during the past year. Consumption was classified into 9 categories from “never or less than one per month” to “6 or more per day.” For each item, average serving size was recorded as “small,” “medium,” or “large,” with respect to standard examples. The University of Minnesota Nutrition Coordinating Center Food Composition Database (version 31) was used with the estimated quantity of nutrient intake and DietSys software (version 3.0; Block Dietary Data Systems) to derive individual nutrient values for each questionnaire item. The instrument was validated through a telephone-administered 24-h dietary recall 3 and 6 mo postenrollment in a sample of 197 randomly selected participants.
The alternate Mediterranean diet (aMeDi) score is a validated score widely used to estimate adherence to the Mediterranean diet in the US population (38). This score is a slightly modified version of the original Mediterranean diet score published by Trichopoulou et al. (39) and is more representative of the dietary patterns observed in the US population. The original Mediterranean diet, which also includes 9 components, does not distinguish between whole and refined grains and includes dairy products rather than nuts.
The aMeDi score includes 9 components: vegetables (excluding potatoes), fruit, legumes, whole grains, nuts, fish, red and processed meats alcohol, and the MUFA-to-SFA ratio. The daily intake of each food or beverage group was calculated as the sum of the number of medium servings consumed per day. For each component hypothesized to benefit health, 1 point was given if intake was above the sex-specific median and zero otherwise. For alcohol, 1 point was given to men if their consumption was between 2.8 and 12.6 g/d and women if their consumption was between 1 and 7.3 g/d. These cutoffs, corresponding to the third quartile of distribution of total alcohol consumption in this population, were chosen to represent mild to moderate consumption. For components presumed to be detrimental to health, 1 point was given if intake was below the sex-specific median and zero otherwise (Supplemental Table 1 presents details with regard to the aMeDi scoring system). The total aMeDi score was calculated by adding the scores (0 or 1 point) for each food category for each participant. Scores ranged from 0 (nonadherence) to 9 (perfect adherence).
Demographic and behavioral covariates
Baseline age, sex, education, smoking, BMI (in kg/m2), AREDS treatment group, supplement use, and ocular characteristics were evaluated. Pack-years was defined as the product of smoking duration (years) by average packs of cigarettes smoked per day. The median value among ever-smokers was 20 pack-years. AREDS treatment was defined as “any AREDS treatment” for subjects in the antioxidant-alone or zinc-alone or the antioxidant-plus-zinc groups and as “placebo” for subjects in the placebo group. AREDS treatment groups were randomly assigned in the AREDS clinical trial. Supplement use was defined as “never” for subjects who reported never having taken a multivitamin supplement and who were not taking Centrum (Pfizer) during the study and as “ever” for subjects who declared that they had taken a multivitamin supplement in the past or at present or who were taking Centrum during the study.
Genotype data
DNA samples were obtained from the AREDS repository. Genotypes for 10 SNPs associated with AMD in 7 different genes were determined by using previously reported methods (40, 41). The following SNPs were evaluated as covariates: complement factor H (CFH) Y402H (rs1061170), CFH (rs1410996), CFH R1210C (rs121913059), age-related maculopathy susceptibility 2/high-temperature requirement A serine peptidase 1 (ARMS2/HTRA1) (rs10490924), complement component 2 (C2) E318D (rs9332739), complement factor B (CFB) R32Q (rs641154), complement component 3 (C3) R102G (rs2230199), C3 K155Q (rs147859257), collagen type VIII α 1 (COL8A1) (rs13095226), and RAD51 paralog B (RAD51B) (rs8017304).
Statistical analyses
Subjects were classified according to categories of the aMeDi score. The aMeDi score categories—low (range: 0–3), medium (range: 4–5), or high (range: 6–9)—were defined to be nutritionally relevant and close to each tertile of the distribution of the aMeDi score in this sample.
Baseline characteristics of progressors and nonprogressors were compared by using logistic regression to estimate ORs and 95% CIs for progression to advanced AMD by using the subject as the unit of analysis. These comparisons were adjusted for age and sex. Associations between baseline characteristics, daily nutrient intake, food components, and aMeDi score were assessed by using ordinal logistic regression adjusted for age, sex, and TEI.
Relation between aMeDi score and progression to advanced AMD
For analysis of incident outcomes, we assessed progression to advanced AMD over 13 y using survival analysis methodology. The associations of aMeDi score with progression to advanced AMD were analyzed by using Cox proportional hazards models with the individual eye as the unit of analysis [by using PROC PHREG with the covariate aggregate option in SAS 9.3 (SAS Institute), which allows for the use of correlated data in eye-specific analyses] (42). Model 1 was adjusted for age (≤64, 65–74, or >74 y), sex, AREDS treatment (placebo or any AREDS treatment), AMD grade at baseline for both the study and the fellow eye (CARMS grade for each eye), and TEI (continuous variable). Model 2 was adjusted for all variables in model 1 plus education (high school or less or more than high school), smoking history (never smoker, <20 pack-years, or ≥20 pack-years), BMI (<25, 25–29, or ≥30), supplement use (never or ever), and the 10 AMD SNPs reported above. Low aMeDi score was designated as the reference group. P-trend was calculated for multivariate models by using the median value of the aMeDi score for each category.
Secondary analyses
Interactions between all AMD genes and aMeDi score were also analyzed. Interaction terms for the number of risk alleles and aMeDi score were assessed separately for each genetic variant with the use of multiplicative models. Each interaction term was introduced independently into a demographic, behavioral, ocular, and genetic-adjusted Cox proportional hazards model (model 2 described above). For the significant interaction term including the CFH Y402H variant, we evaluated HRs and 95% CIs for the association between aMeDi score and progression using a Cox proportional hazards model adjusted for demographic, behavioral, ocular, and genetic factors (model 2 excluding CFH Y402H) for each CFH Y402H genotype group [at least 1 nonrisk allele (CT/TT) compared with a homozygous risk allele (CC)]. P-trend was calculated by using the median value for each aMeDi score category. To assess the combined effect of the 10 genetic variants, we calculated a composite genetic risk score using the sum of the regression coefficients multiplied by the corresponding number of risk alleles summed over the 10 genetic variants used in our comprehensive prediction model (41). The interaction between this genetic risk score (classified as below the median or greater than or equal to the median) and the aMeDi score was analyzed by using the same model as for single SNPs described above.
We also assessed whether associations of aMeDi score may be due to individual dietary components by examining associations between the individual components of the aMeDi score and advanced AMD. Each component was first introduced independently into a demographic, behavioral, ocular, and genetic-adjusted Cox proportional hazards model (model 2). The 9 components of the aMeDi score were then introduced simultaneously in the same model.
We evaluated whether associations between aMeDi score and progression to advanced AMD may differ between participants who had received the AREDS treatment and participants who had not. These associations were separately evaluated for each AREDS treatment group (any AREDS treatment and placebo) by using a Cox proportional hazards model adjusted for demographic, behavioral, ocular, and genetic factors (model 2 excluding AREDS treatment). P-trend was calculated by using the median value for each aMeDi score category.
We calculated a composite risk score using regression coefficients for the following covariates: age, sex, AMD grade at baseline for both eyes, AREDS treatment, TEI, smoking, education, BMI, supplement use, 10 genetic loci, and aMeDi score (41). Progression to advanced AMD was assessed according to the age-specific quartile of the risk score. The low-risk category, corresponding to the lowest quartile of distribution, was used as the reference group.
All statistical analyses were performed with the use of SAS software, version 9.3. P values <0.05 were considered significant in all analyses.
RESULTS
Baseline demographic, behavioral, ocular, and genetic characteristics among progressors and nonprogressors adjusted for age and sex are shown in Supplemental Table 2. Among 2525 subjects, 744 (29.5%) progressed to advanced AMD over 13 y. The mean follow-up time was 8.7 y (range: 1–13 y). Progressors to advanced AMD tended to be older (P-trend < 0.0001), have a lower level of education (P-trend < 0.0001), have a history of smoking (P-trend < 0.0001), and have a higher BMI (P-trend = 0.0006) and were more likely to have taken a supplement (P = 0.001) than were nonprogressors. Sex did not differ between progressors and nonprogressors (P = 0.26). Subjects with advanced AMD in one eye were at high risk of progression to advanced AMD (P-trend < 0.0001). The risk alleles for CFH variants, ARMS2/HTRA1 (T), C3 variants (G), and COL8A1 (C) were significantly associated with an increased risk of progression to advanced AMD. The protective alleles of C2 (C), CFB (T), and RAD51B (G) were significantly associated with a decreased risk of progression to advanced AMD.
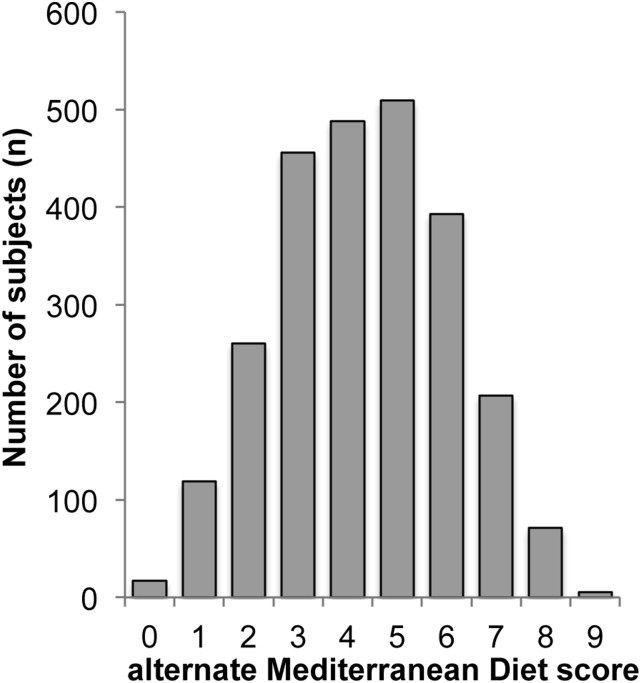
Figure 2 shows the distribution of the aMeDi score in our study population. We divided aMeDi score into 3 categories close to the tertile of the distribution: low aMeDi score (0–3; n = 852), medium aMeDi score (4–5; n = 997), and high aMeDi score (6–9; n = 676).
FIGURE 2.
Distribution of the alternate Mediterranean diet score. AREDS (Age-Related Eye Disease Study) cohort, n = 2525.
Table 1 shows baseline demographic, behavioral, ocular, and genetic characteristics according to the aMeDi score categories adjusted for age, sex, and TEI. Subjects with a higher aMeDi score tended to be male (P = 0.01) and have a higher educational level (P < 0.0001), no history of smoking (P-trend < 0.0001), a lower BMI (P < 0.0001), and a history of supplement use (P = 0.003). Subjects with intermediate AMD in the worst eye or advanced AMD in one eye and nonadvanced AMD fellow eye at baseline had a lower aMeDi score (P < 0.0001). Age and AREDS treatment were not associated with the aMeDi score. The CFH rs1410996 variant was associated with the aMeDi score (P-trend = 0.04). CFH Y402H, CFH R1210C, and the variants in ARMS2/HTRA1, C2, CFB, C3, COL8A1, and RAD51B were not significantly associated with the aMeDi score. As expected, a higher aMeDi score was characterized by a higher intake of fruit, vegetables, legumes, whole grains, nuts, and fish and by a lower intake of red and processed meats as well as higher mild to moderate alcohol consumption and a higher MUFA-to-SFA ratio (Supplemental Table 3).
TABLE 1.
Baseline demographic, behavioral, ocular, and genetic characteristics by categories of aMeDi score1
| aMeDi score, n (%) |
||||
| Low: 0–3 (n = 852) | Medium: 4–5 (n = 997) | High: 6–9 (n = 676) | P-trend2 | |
| Age, y | 0.86 | |||
| ≤64 | 147 (17.3) | 186 (18.7) | 117 (17.3) | |
| 65–74 | 555 (65.1) | 646 (64.8) | 455 (67.3) | |
| >74 | 150 (17.6) | 165 (16.5) | 104 (15.4) | |
| Sex | 0.01 | |||
| Male | 367 (43.1) | 444 (44.5) | 313 (46.3) | |
| Female | 485 (56.9) | 553 (55.5) | 363 (53.7) | |
| Educational level | <0.0001 | |||
| High school or less | 385 (45.2) | 293 (29.4) | 160 (23.7) | |
| More than high school | 467 (54.8) | 704 (70.6) | 516 (76.3) | |
| Smoking, pack-years | <0.0001 | |||
| Never | 384 (45.1) | 449 (45.0) | 350 (51.8) | |
| <20 | 176 (20.6) | 250 (25.1) | 164 (24.2) | |
| ≥20 | 292 (34.3) | 298 (29.9) | 162 (24.0) | |
| BMI, kg/m2 | <0.0001 | |||
| <25 | 245 (28.8) | 333 (33.4) | 250 (37.0) | |
| 25–29 | 358 (42.0) | 425 (42.6) | 306 (45.3) | |
| ≥30 | 249 (29.2) | 239 (24.0) | 120 (17.7) | |
| AREDS treatment | 0.53 | |||
| Placebo | 269 (31.6) | 297 (29.8) | 207 (30.6) | |
| Any AREDS treatment | 583 (68.4) | 700 (70.2) | 469 (69.4) | |
| Supplement use | 0.003 | |||
| Never | 305 (35.8) | 297 (29.8) | 200 (29.6) | |
| Ever | 547 (64.2) | 700 (70.2) | 476 (70.4) | |
| Baseline grade in each eye3 | <0.0001 | |||
| 1,1/1,2/2,2 | 380 (44.6) | 478 (47.9) | 360 (53.2) | |
| 1,3/2,3/3,3 | 312 (36.6) | 376 (37.7) | 234 (34.6) | |
| 1,4/2,4/3,4 | 19 (2.2) | 26 (2.6) | 14 (2.1) | |
| 1,5/2,5/3,5 | 141 (16.6) | 117 (11.8) | 68 (10.1) | |
| CFH rs1061170 (Y402H) | 0.69 | |||
| TT | 255 (29.9) | 301 (30.2) | 198 (29.3) | |
| CT | 369 (43.3) | 465 (46.6) | 311 (46.0) | |
| CC | 228 (26.8) | 231 (23.2) | 167 (24.7) | |
| CFH rs1410996 | 0.04 | |||
| TT | 98 (11.5) | 133 (13.4) | 86 (12.7) | |
| CT | 329 (38.6) | 398 (39.9) | 292 (43.2) | |
| CC | 425 (49.9) | 466 (46.7) | 298 (44.1) | |
| CFH rs121913059 (R1210C) | 0.89 | |||
| CC | 847 (99.4) | 994 (99.7) | 672 (99.4) | |
| CT | 5 (0.6) | 3 (0.3) | 4 (0.6) | |
| ARMS2/HTRA1 rs10490924 | 0.07 | |||
| GG | 418 (49.1) | 516 (51.8) | 339 (50.1) | |
| GT | 330 (38.7) | 379 (38.0) | 279 (41.3) | |
| TT | 104 (12.2) | 102 (10.2) | 58 (8.6) | |
| C2 rs9332739 (E318D) | 0.80 | |||
| GG | 790 (92.7) | 935 (93.8) | 623 (92.2) | |
| CC/CG | 62 (7.3) | 62 (6.2) | 53 (7.8) | |
| CFB rs641153 (R32Q) | 0.14 | |||
| CC | 725 (85.1) | 869 (87.2) | 589 (87.1) | |
| CT/TT | 127 (14.9) | 128 (12.8) | 87 (12.9) | |
| C3 rs2230199 (R102G) | 0.91 | |||
| CC | 495 (58.1) | 552 (55.4) | 400 (59.2) | |
| CG | 311 (36.5) | 374 (37.5) | 241 (35.6) | |
| GG | 46 (5.4) | 71 (7.1) | 35 (5.2) | |
| C3 rs147859257 (K155Q) | 0.46 | |||
| TT | 832 (97.6) | 977 (98.0) | 663 (98.1) | |
| GT | 20 (2.4) | 20 (2.0) | 13 (1.9) | |
| COL8A1 rs13095226 | 0.64 | |||
| TT | 692 (81.2) | 789 (79.1) | 544 (80.5) | |
| CT/TT | 160 (18.8) | 208 (20.9) | 132 (19.5) | |
| RAD51B rs8017304 | 0.13 | |||
| AA | 371 (43.5) | 402 (40.3) | 274 (40.5) | |
| AG | 377 (44.3) | 463 (46.5) | 318 (47.1) | |
| GG | 104 (12.2) | 132 (13.2) | 84 (12.4) | |
AREDS cohort, n = 2525. AMD, age-related macular degeneration; aMeDi, alternate Mediterranean diet; AREDS, Age-Related Eye Disease Study; ARMS2, age-related maculopathy susceptibility 2; CFB, complement factor B; CFH, complement factor H; COL8A1, collagen type VIII α 1; C2, complement component 2; C3, complement component 3; HTRA1, high-temperature requirement A serine peptidase 1; RAD51B, RAD51 paralog B.
P-trend for logistic ordinal regression adjusted for age, sex, and total energy intake.
Represents the CARMS (Clinical Age-Related Maculopathy Staging system) grade in each eye, as follows: 1,1 (no AMD, no AMD)/1,2 (no AMD, early AMD)/2,2 (early AMD, early AMD); 1,3 (no AMD, intermediate AMD)/2,3 (early AMD, intermediate AMD)/3,3 (intermediate AMD, intermediate AMD); 1,4 (no AMD, geographic atrophy)/2,4 (early AMD, geographic atrophy)/3,4 (intermediate AMD, geographic atrophy); 1,5 (no AMD, neovascular AMD)/2,5 (early AMD, neovascular AMD)/3,5 (intermediate AMD, neovascular AMD).
Age- and sex-adjusted daily nutrient intake among aMeDi score categories adjusted for age, sex, and TEI are shown in Supplemental Table 4. A higher aMeDi score was significantly associated with a lower daily intake of total fat, SFAs, and MUFAs and with a higher daily intake of PUFAs and EPA+DHA (P < 0.0001 for all fats). A greater aMeDi score was also significantly associated with a higher TEI and with a higher daily intake of β-carotene, α-carotene, cryptoxanthin, lutein+zeaxanthin, lycopene, folate, vitamins B-1 (thiamin), B-3 (niacin), B-6 (pyridoxamine), C, and E (P < 0.0001 for all nutrients) and with a lower retinol daily intake (P = 0.0001). Vitamin B-2 (riboflavin), vitamin B-12, and vitamin D were not associated with the aMeDi score.
Table 2 shows multivariate associations between the aMeDi score and progression to advanced AMD. Among 4663 eyes, 1028 (22.0%) progressed to advanced AMD over 13 y. After adjustment for age, sex, AMD grade at baseline for both eyes, AREDS treatment, and TEI (model 1), a higher aMeDi score was significantly associated with a reduced risk of progression to advanced AMD (P-trend = 0.005). This association remained significant after adjustment for other behavioral and genetic covariates (P-trend = 0.007). For each statistical model, subjects with a high aMeDi score (6–9) had a significantly reduced risk of progression to advanced AMD compared with subjects with a low aMeDi score (0–3) (HR: 0.74; 95% CI: 0.61, 0.91; model 2). For subjects with a medium aMeDi score (4–5), this association showed similar trends but did not reach significance (HR: 0.92; 95% CI: 0.78, 1.07; model 2). To assess whether the benefit of high adherence to the aMeDi score was due to specific nutrients related to AMD, we included dietary intake of lutein and zeaxanthin and EPA+DHA to the covariates evaluated in model 2. Associations between aMeDi score and progression to AMD remained significant for subjects with a high aMeDi score (HR: 0.76; 95% CI: 0.61, 0.95; P-trend = 0.028).
TABLE 2.
Associations between aMeDi score and progression to advanced AMD over 13 y1
| aMeDi score |
||||
| Low: 0–3 | Medium: 4–5 | High: 6–9 | P-trend2 | |
| Eye progressors/eyes at risk, n/n | 376/1542 | 413/1851 | 239/1270 | |
| Progressors, % | 24.4 | 22.3 | 18.8 | |
| HR (95% CI) | ||||
| Model 13 | 1.0 | 0.91 (0.77, 1.07) | 0.74 (0.61, 0.90) | 0.005 |
| Model 24 | 1.0 | 0.92 (0.78, 1.07) | 0.74 (0.61, 0.91) | 0.007 |
AREDS cohort, n = 4663 eyes. AMD, age-related macular degeneration; aMeDi, alternate Mediterranean diet; AREDS, Age-Related Eye Disease Study; ARMS2, age-related maculopathy susceptibility 2; CFB, complement factor B; CFH, complement factor H; COL8A1, collagen type VIII α 1; C2, complement component 2; C3, complement component 3; HTRA1, high-temperature requirement A serine peptidase 1; RAD51B, RAD51 paralog B.
Calculated by using median values within each category.
Adjusted for age, sex, AREDS treatment, AMD grade at baseline for both eyes, and total energy intake.
Adjusted for age, sex, AREDS treatment, AMD grade at baseline for both eyes, total energy intake, educational level, smoking, BMI, supplement use, and 10 genetic variants [CFH rs1061170 (Y402H), CFH rs1410996, CFH rs121913059 (R1210C), ARMS2/HTRA1 rs10490924, C2 rs9332739 (E318D), CFB rs641153 (R32Q), C3 rs2230199 (R102G), C3 rs147859257 (K155Q), COL8A1 rs13095226, and RAD51B rs8017304].
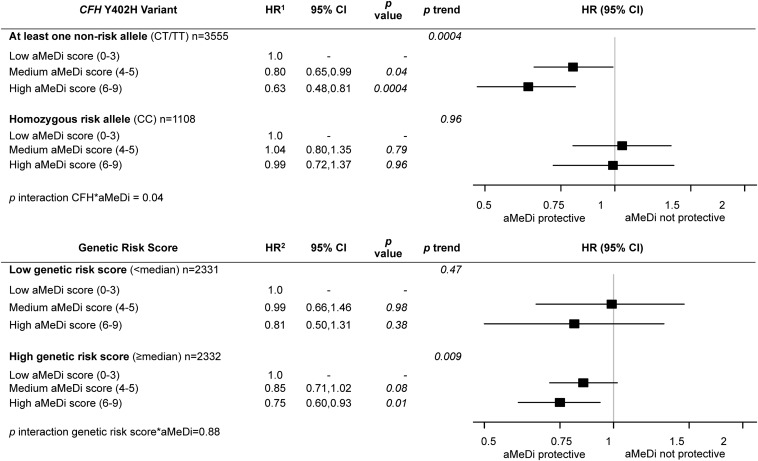
Figure 3 shows multivariate associations between the aMeDi score and progression to advanced AMD according to the CFH Y402H genotype adjusted for age, sex, AMD grade at baseline for both eyes, AREDS treatment, TEI, smoking, educational level, BMI, supplement use, and 9 genetic loci (excluding CFH Y402H). The interaction between CFH Y402H and aMeDi score was significant (P-interaction = 0.04; CC compared with CT/TT). There was a significant protective effect of greater adherence to an aMeDi among subjects carrying the CFH Y402H nonrisk allele (CT/TT) (HR: 0.63; 95% CI: 0.48, 0.81) but no significant beneficial effect of greater adherence to an aMeDi among those who were homozygous for the risk allele (CC) (HR: 0.99; 95% CI: 0.72, 1.37). Interactions between aMeDi score and other SNPs were not significant. Figure 3 also shows the associations between the aMeDi score and progression to advanced AMD according to our composite genetic risk score. The interaction between aMeDi score and the genetic risk score was not significant (P = 0.88). Subjects with a higher genetic risk score (above the median) had a significant protective effect of a greater adherence to an aMeDi (HR: 0.75; 95% CI: 0.60, 0.93), but this risk was not different from that observed for subjects with a lower genetic risk score (below the median) (HR: 0.81; 95% CI: 0.50, 1.31).
FIGURE 3.
Effect of adherence to the aMeDi on progression to advanced age-related macular degeneration according to CFH Y402H genotypes and composite genetic risk score. AREDS cohort, n = 2525 subjects/n = 4663 eyes. 1HRs (95% CIs) were estimated by using Cox proportional hazards models with individual eye as the unit of analysis, adjusted for age, sex, AMD grade at baseline for both eyes, AREDS treatment, total energy intake, educational level, smoking, BMI, supplement use, and the other 9 genetic variants [CFH rs1410996, CFH rs121913059 (R1210C), ARMS2/HTRA1 rs10490924, C2 rs9332739 (E318D), CFB rs641153 (R32Q), C3 rs2230199 (R102G), C3 rs147859257 (K155Q), COL8A1 rs13095226, and RAD51B rs8017304]. 2HRs (95% CIs) were estimated by using univariate Cox proportional hazards models with individual eye as the unit of analysis, adjusted for age, sex, AMD grade at baseline for both eyes, AREDS treatment, total energy intake, educational level, smoking, BMI, and supplement use. P-trend was calculated by using median values within each category. Low aMeDi score (0–3) was the referent. The genetic risk score includes the 10 genetic variants [CFH rs1061170 (Y402H), CFH rs1410996, CFH rs121913059 (R1210C), ARMS2/HTRA1 rs10490924, C2 rs9332739 (E318D), CFB rs641153 (R32Q), C3 rs2230199 (R102G), C3 rs147859257 (K155Q), COL8A1 rs13095226, and RAD51B rs8017304]. AMD, age-related macular degeneration; aMeDi, alternate Mediterranean diet; AREDS, Age-Related Eye Disease Study; ARMS2, age-related maculopathy susceptibility 2; CFB, complement factor B; CFH, complement factor H; COL8A1, collagen type VIII α 1; C2, complement component 2; C3, complement component 3; HTRA1, high-temperature requirement A serine peptidase 1; RAD51B, RAD51 paralog B.
Associations between aMeDi score components and progression to advanced AMD are presented in Table 3. After adjustment for demographic, behavioral, ocular, and genetic factors, vegetable and fish components were significantly associated with progression to advanced AMD. Subjects with vegetable and fish consumption above the median had a reduced risk of progression to advanced AMD compared with subjects with intakes below the median [HRs of 0.81 (95% CI: 0.71, 0.94) (P = 0.005) and 0.82 (95% CI: 0.71, 0.95) (P = 0.007), respectively]. These associations remained significant after adjustment for other aMeDi score components (P = 0.03 and P = 0.04, respectively).
TABLE 3.
Associations between aMeDi score components and progression to advanced AMD1
| aMeDi score components2 |
||||||
| Nonadjusted3 |
Adjusted4 |
|||||
| HR | 95% CI | P | HR | 95% CI | P | |
| Fruit | 0.92 | 0.80, 1.07 | 0.29 | 0.99 | 0.85, 1.15 | 0.89 |
| Vegetables | 0.81 | 0.71, 0.94 | 0.005 | 0.85 | 0.73, 0.99 | 0.03 |
| Legumes | 0.98 | 0.85, 1.13 | 0.80 | 1.02 | 0.88, 1.18 | 0.83 |
| Fish | 0.82 | 0.71, 0.95 | 0.007 | 0.86 | 0.74, 0.99 | 0.04 |
| Red meat | 0.86 | 0.73, 1.00 | 0.05 | 0.88 | 0.75, 1.04 | 0.12 |
| MUFA-to-SFA ratio | 1.13 | 0.98, 1.29 | 0.10 | 1.14 | 0.99, 1.31 | 0.07 |
| Alcohol | 0.87 | 0.73, 1.03 | 0.11 | 0.88 | 0.74, 1.04 | 0.13 |
| Whole grains | 0.89 | 0.77, 1.03 | 0.13 | 0.93 | 0.80, 1.07 | 0.30 |
| Nuts | 1.01 | 0.87, 1.17 | 0.90 | 1.00 | 0.87, 1.16 | 0.97 |
AREDS cohort, n = 4663 eyes. AMD, age-related macular degeneration; aMeDi, alternate Mediterranean diet; AREDS, Age-Related Eye Disease Study; ARMS2, age-related maculopathy susceptibility 2; CFB, complement factor B; CFH, complement factor H; COL8A1, collagen type VIII α 1; C2, complement component 2; C3, complement component 3; HTRA1, high-temperature requirement A serine peptidase 1; RAD51B, RAD51 paralog B.
aMeDi score components were defined as below or above the median.
Model adjusted for age, sex, AREDS treatment, AMD grade at baseline for both eyes, total energy intake, educational level, smoking, BMI, supplement use, and 10 genetic variants [CFH rs1061170 (Y402H), CFH rs1410996, CFH rs121913059 (R1210C), ARMS2/HTRA1 rs10490924, C2 rs9332739 (E318D), CFB rs641153 (R32Q), C3 rs2230199 (R102G), C3 rs147859257 (K155Q), COL8A1 rs13095226, and RAD51B rs8017304].
Model adjusted for age, sex, AREDS treatment, AMD grade at baseline for both eyes, total energy intake, educational level, smoking, BMI, supplement use, 10 genetic variants [CFH rs1061170 (Y402H), CFH rs1410996, CFH rs121913059 (R1210C), ARMS2/HTRA1 rs10490924, C2 rs9332739 (E318D), CFB rs641153 (R32Q), C3 rs2230199 (R102G), C3 rs147859257 (K155Q), COL8A1 rs13095226, and RAD51B rs8017304], and 9 aMeDi components.
Table 4 shows the multivariate associations between aMeDi score and progression to advanced AMD according to AREDS treatment adjusted for age, sex, AMD grade at baseline for both eyes, TEI, educational level, smoking, BMI, supplement use, and 10 genetic loci. A high aMeDi score was significantly associated with a reduced risk of progression to advanced AMD in both the AREDS treatment and placebo groups [HRs of 0.76 (95% CI: 0.61, 0.96) and 0.60 (95% CI: 0.40, 0.92), respectively]. Among subjects with a medium aMeDi score, trends were similar for both treatment groups but did not reach significance.
TABLE 4.
Associations between aMeDi score and progression to advanced AMD over 13 y according to AREDS treatment1
| AREDS treatment group |
||||||||
| AREDS treatment |
Placebo |
|||||||
| n/n | HR2 | 95% CI | P-trend3 | n/n | HR2 | 95% CI | P-trend3 | |
| Eye progressors/eyes at risk | 789/3201 | 239/1462 | ||||||
| aMeDi score | ||||||||
| Low (0–3) | Ref | — | 0.03 | Ref | — | 0.04 | ||
| Medium (4–5) | 0.91 | 0.75, 1.09 | 0.93 | 0.65, 1.33 | ||||
| High (6–9) | 0.76 | 0.61, 0.96 | 0.60 | 0.40, 0.92 | ||||
AREDS cohort, n = 4663 eyes. AMD, age-related macular degeneration; aMeDi, alternate Mediterranean diet; AREDS, Age-Related Eye Disease Study; ARMS2, age-related maculopathy susceptibility 2; CFB, complement factor B; CFH, complement factor H; COL8A1, collagen Type VIII α 1 ; C2, complement component 2; C3, complement component 3; HTRA1, high-temperature requirement A serine peptidase 1; RAD51B, RAD51 paralog B; Ref, reference.
Model adjusted for age, sex, AMD grade at baseline for both eyes, total energy intake, educational level, smoking, BMI, supplement use, and 10 genetic variants [CFH rs1061170 (Y402H), CFH rs1410996, CFH rs121913059 (R1210C), ARMS2/HTRA1 rs10490924, C2 rs9332739 (E318D), CFB rs641153 (R32Q), C3 rs2230199 (R102G), C3 rs147859257 (K155Q), COL8A1 rs13095226, and RAD51B rs8017304].
Calculated by using median values within each category.
Supplemental Table 5 shows associations between a composite risk score including age, sex, AMD grade at baseline for both eyes, TEI, educational level, smoking, BMI, supplement use, all genetic variants, and aMeDi score and progression to advanced AMD. A higher risk score was associated with an increased risk of progression to advanced AMD (P-trend < 0.0001). In the highest quartile, 65.2% of eyes progressed to advanced AMD compared with 1% in the lowest quartile. This composite risk score underscores the marked differences in risk of progression according to baseline genetic and nongenetic characteristics.
DISCUSSION
High adherence to the Mediterranean diet was associated with a 26% lower risk of progression to advanced AMD. The risk of progression was also significantly reduced among subjects with the CFH Y402H nonrisk allele (T) but not the homozygous risk genotype (CC). Fish and vegetable consumption was associated with a lower risk of progression. Taking an AREDS supplement rather than placebo did not alter the protective effect of the Mediterranean diet on the risk of progression. Assessing overall diet with respect to ocular outcomes is relatively new in ophthalmic epidemiology, and this study is the first, to our knowledge, to explore the association between aMeDi score and progression to advanced AMD. It is also the first, to our knowledge, to evaluate genetic factors and their interactions with overall diet in a large prospective cohort.
Previous studies of dietary patterns have been cross-sectional or case-control studies, focused on early AMD, or used small samples. In a cross-sectional study of the prevalence of early AMD, the CAREDS showed a protective effect of high adherence to the Mediterranean diet (OR: 0.34; 95% CI: 0.08, 0.98; P-trend = 0.046) (30). The HEI also assessed whole diet and health outcomes. A retrospective case-control study observed that subjects in the highest quartile of HEI had a lower prevalence of advanced AMD (OR: 0.54; 95% CI: 0.30, 0.99) (33). Moreover, the CAREDS reported a 46% decreased risk of early AMD in the highest quintile of the HEI (OR: 0.54; 95% CI: 0.33, 0.88; P-trend = 0.01) (30).
PCA methods for defining dietary patterns are based on empirical guidelines. Decisions about grouping foods and the number of factors are subjective. One study identified 6 factors with the use of PCA in a cross-sectional analysis of 108 cases of advanced AMD. Nuts were associated with a lower prevalence of advanced AMD and red meat was associated with a higher prevalence of advanced AMD (31). In our study, subjects with lower red and processed meat consumption had a decreased risk of progression to advanced AMD, but this association was not significant. An earlier prospective analysis, however, found that nut consumption reduced the risk of progression to advanced AMD (8). Another PCA of the AREDS cohort examined dietary patterns (32). Oriental dietary patterns were associated with a reduced risk of early and advanced AMD, and Western dietary patterns were associated with an increased risk of early and late AMD. Our findings are based on prospective analyses and also have the advantage of combining these 2 patterns into a single comprehensive and validated score that includes high consumption of beneficial foods and moderate consumption of deleterious foods.
No study has previously explored whether genetic susceptibility could modify associations between the entire diet (instead of specific nutrients) and AMD. Our results expand on a previous study that showed that beneficial associations between DHA and AMD could be affected by genotype (23). A significant protective effect of DHA was observed among subjects with the CFH Y402H homozygous nonrisk genotype (TT), and no significant effect of DHA was observed among those with the homozygous risk genotype (CC). AREDS supplementation with antioxidant plus zinc was also associated with a greater reduction in AMD progression in subjects with the low-risk genotype compared with those with the high-risk genotype for CFH Y402H (43). The population-based Alienor Study and the clinical trial Nutritional AMD Treatment 2 showed that subjects carrying the homozygous risk genotype had lower plasma concentrations of long-chain ω-3 PUFAs (22) and had a limited benefit of DHA supplementation (44). These studies suggest that subjects with the CC genotype experience limited benefits from both diet and supplementation. In our study, nonsignificant interactions between our genetic risk score, as well as other AMD SNPs, and aMeDi score may reflect the specific effect of the CFH Y402H variant on diet.
CFH is one of the main genes implicated in AMD and is a key regulator of complement through inhibiting the alternative pathway. The risk allele appears to impair this regulatory function of the CFH gene, leading to complement overactivation and thereby increasing AMD risk (45). Healthy diets are usually rich in lutein, zeaxanthin, and EPA and DHA and are associated with reduced serum concentrations of C-reactive protein (46, 47), a marker of systemic inflammation related to AMD. It is plausible that high adherence to the Mediterranean diet reduces the risk of AMD by modulating immune and inflammatory responses, resulting in a stronger effect among subjects with the nonrisk allele for CFH Y402H.
Another pooled study suggested a protective association between dietary lutein and zeaxanthin and AMD among subjects with the high-risk genotype for CFH Y402H and ARMS2 (48). Although we found a protective association between aMeDi score and progression to late AMD among subjects with a high genetic risk score, the interaction was not significant. These results highlight the complexity of interactions between genetic and environmental exposures.
It is important to highlight that our findings are consistent with associations between AMD risk and intakes of single nutrients (9–24, 34) or foods (7–10, 19) identified in other studies. Although these nutrients were not directly incorporated into our aMeDi score, high scores were associated with high dietary intakes of several individual nutrients related to low prevalence or progression of AMD in previous studies, including EPA and DHA (9–11, 19–24), vitamins C and E (12, 34), and lutein and zeaxanthin (12–18). In our study, the protective association of aMeDi score with AMD progression was driven by the fish and vegetable components, the main sources of ω-3 PUFAs, lutein, and zeaxanthin. We also reported stronger associations for the aMeDi score than for individual components.
The biological basis for the documented health benefits of the Mediterranean diet score involves a decrease in oxidative stress and inflammation (49–51). Both processes also participate in the pathophysiology of AMD (1, 5). Subjects with higher adherence to the Mediterranean diet typically have higher plasma concentrations of some beneficial biomarkers (50, 51). In sensitivity analyses, we showed the protective effect of consuming a Mediterranean diet beyond simply the use of antioxidant and zinc supplementation. Adopting healthy habits such as consuming a Mediterranean-type diet, and ultimately complementing individual dietary patterns with an appropriate supplement, may play an important role in the risk of progression to advanced stages of AMD.
Limitations of the study design include the assessment of dietary intake only at baseline: long-term intake was not taken into account. The aMeDi score uses cutoffs based on the study population and results may not be generalizable. To strengthen our analyses, we excluded subjects with unusually high or low TEI. We used a well-known and validated score to assess diet and probable synergistic effects between nutrients. Our aMeDi score was developed by using sex-specific thresholds to better account for differences between men and women. Other strengths include standardized data collection for a large and well-defined cohort, extensive follow-up time, and a high number of subjects who progressed to advanced AMD. Our analyses accounted for time to progression in each eye and included 10 genetic variants associated with AMD. Dietary assessment was conducted before the development of advanced AMD, reducing possible reverse causation. On the basis of this study, a Mediterranean-type diet that includes the consumption of vegetables, fruit, whole grains, legumes, nuts, and fish; moderate consumption of alcohol; and limited intake of red and processed meats may reduce the risk of progression to advanced AMD.
In conclusion, these results suggest that higher adherence to a Mediterranean-type diet is associated with a reduced risk of progression to advanced AMD. This association might be moderated by the CFH Y402H variant. Among subjects with 1 or 2 nonrisk alleles, those with a high aMeDi score had an approximately one-third lower risk of progression than did those with lower adherence to this healthy diet, although these gene-diet associations require confirmation by other studies. Modifiable factors such as dietary intake are therefore of utmost importance in preventing this disease.
Acknowledgments
The authors’ responsibilities were as follows—BMJM, BR, and JMS: designed and conducted the research; BMJM and BR: analyzed the data or performed the statistical analysis; and BMJM, RES, BR, and JMS: wrote the manuscript and had primary responsibility for the final content. None of the authors had a conflict of interest.
Footnotes
Abbreviations used: AMD, age-related macular degeneration; aMeDi, alternate Mediterranean diet; AREDS, Age-Related Eye Disease Study; ARMS2, age-related maculopathy susceptibility 2; CAREDS, Carotenoids Age-Related Eye Disease Study; CARMS, Clinical Age-Related Maculopathy Staging; CFB, complement factor B; CFH, complement factor H; COL8A1, collagen type VIII α 1; C2, complement component 2; C3, complement component 3; dbGaP, Database of Genotypes and Phenotypes; GA, geographic atrophy; HEI, Healthy Eating Index; HTRA1, high-temperature requirement A serine peptidase 1; PCA, principal components analysis; RAD51B, RAD51 paralog B; SNP, single-nucleotide polymorphism; TEI, total energy intake; VEGF, vascular endothelial growth factor.
REFERENCES
- 1.Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet 2012;379:1728–38. [DOI] [PubMed] [Google Scholar]
- 2.Friedman DS, O’Colmain BJ, Munoz B, Tomany SC, McCarty C, de Jong PT, Nemesure B, Mitchell P, Kempen J; Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol 2004;122:564–72. [DOI] [PubMed] [Google Scholar]
- 3.Mangione CM, Gutierrez PR, Lowe G, Orav EJ, Seddon JM. Influence of age-related maculopathy on visual functioning and health-related quality of life. Am J Ophthalmol 1999;128:45–53. [DOI] [PubMed] [Google Scholar]
- 4.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY, Marina Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355:1419–31. [DOI] [PubMed] [Google Scholar]
- 5.Sobrin L, Seddon JM. Nature and nurture- genes and environment- predict onset and progression of macular degeneration. Prog Retin Eye Res 2014;40:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zampatti S, Ricci F, Cusumano A, Marsella LT, Novelli G, Giardina E. Review of nutrient actions on age-related macular degeneration. Nutr Res 2014;34:95–105. [DOI] [PubMed] [Google Scholar]
- 7.Seddon JM, Rosner B, Sperduto RD, Yannuzzi L, Haller JA, Blair NP, Willett W. Dietary fat and risk for advanced age-related macular degeneration. Arch Ophthalmol 2001;119(8):1191–9. [DOI] [PubMed] [Google Scholar]
- 8.Seddon JM, Cote J, Rosner B. Progression of age-related macular degeneration: association with dietary fat, transunsaturated fat, nuts, and fish intake. Arch Ophthalmol 2003;121:1728–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seddon JM, George S, Rosner B. Cigarette smoking, fish consumption, omega-3 fatty acid intake, and associations with age-related macular degeneration: the US Twin Study of Age-Related Macular Degeneration. Arch Ophthalmol 2006;124:995–1001. [DOI] [PubMed] [Google Scholar]
- 10.Chong EW, Kreis AJ, Wong TY, Simpson JA, Guymer RH. Dietary omega-3 fatty acid and fish intake in the primary prevention of age-related macular degeneration: a systematic review and meta-analysis. Arch Ophthalmol 2008;126(6):826–33. [DOI] [PubMed] [Google Scholar]
- 11.Augood C, Chakravarthy U, Young I, Vioque J, de Jong PT, Bentham G, Rahu M, Seland J, Soubrane G, Tomazzoli L, et al. Oily fish consumption, dietary docosahexaenoic acid and eicosapentaenoic acid intakes, and associations with neovascular age-related macular degeneration. Am J Clin Nutr 2008;88(2):398–406. [DOI] [PubMed] [Google Scholar]
- 12.Seddon JM, Ajani UA, Sperduto RD, Hiller R, Blair N, Burton TC, Farber MD, Gragoudas ES, Haller J, Miller DT, et al. ; Eye Disease Case-Control Study Group. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. JAMA 1994;272:1413–20. [PubMed] [Google Scholar]
- 13.Mares-Perlman JA, Fisher AI, Klein R, Palta M, Block G, Millen AE, Wright JD. Lutein and zeaxanthin in the diet and serum and their relation to age-related maculopathy in the Third National Health and Nutrition Examination Survey. Am J Epidemiol 2001;153:424–32. [DOI] [PubMed] [Google Scholar]
- 14.Delcourt C, Carriere I, Delage M, Barberger-Gateau P, Schalch W, POLA Study Group. Plasma lutein and zeaxanthin and other carotenoids as modifiable risk factors for age-related maculopathy and cataract: the POLA Study. Invest Ophthalmol Vis Sci 2006;47:2329–35. [DOI] [PubMed] [Google Scholar]
- 15.Cho E, Hankinson SE, Rosner B, Willett WC, Colditz GA Prospective study of lutein/zeaxanthin intake and risk of age-related macular degeneration. Am J Clin Nutr 2008;87(6):1837–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seddon JM, Reynolds R, Rosner B. Associations of smoking, body mass index, dietary lutein, and the LIPC gene variant rs10468017 with advanced age-related macular degeneration. Mol Vis 2010;16:2412–24. [PMC free article] [PubMed] [Google Scholar]
- 17.Ma L, Dou HL, Wu YQ, Huang YM, Huang YB, Xu XR, Zou ZY, Lin XM. Lutein and zeaxanthin intake and the risk of age-related macular degeneration: a systematic review and meta-analysis. Br J Nutr 2012;107:350–9. [DOI] [PubMed] [Google Scholar]
- 18.Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 2013;309:2005–15. [DOI] [PubMed] [Google Scholar]
- 19.Sangiovanni JP, Agron E, Meleth AD, Reed GF, Sperduto RD, Clemons TE, Chew EY; Age-Related Eye Disease Study Research Group. ω-3 Long-chain polyunsaturated fatty acid intake and 12-y incidence of neovascular age-related macular degeneration and central geographic atrophy: AREDS report 30, a prospective cohort study from the Age-Related Eye Disease Study. Am J Clin Nutr 2009;90:1601–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan JS, Wang JJ, Flood V, Mitchell P. Dietary fatty acids and the 10-year incidence of age-related macular degeneration: the Blue Mountains Eye Study. Arch Ophthalmol 2009;127:656–65. [DOI] [PubMed] [Google Scholar]
- 21.Merle B, Delyfer MN, Korobelnik JF, Rougier MB, Colin J, Malet F, Feart C, Le Goff M, Dartigues JF, Barberger-Gateau P, et al. Dietary omega-3 fatty acids and the risk for age-related maculopathy: the Alienor Study. Invest Ophthalmol Vis Sci 2011;52:6004–11. [DOI] [PubMed] [Google Scholar]
- 22.Merle BM, Delyfer MN, Korobelnik JF, Rougier MB, Malet F, Feart C, Le Goff M, Peuchant E, Letenneur L, Dartigues JF, et al. High concentrations of plasma n-3 fatty acids are associated with decreased risk for late age-related macular degeneration. J Nutr 2013;143:505–11. [DOI] [PubMed] [Google Scholar]
- 23.Reynolds R, Rosner B, Seddon JM. Dietary omega-3 fatty acids, other fat intake, genetic susceptibility, and progression to incident geographic atrophy. Ophthalmology 2013;120:1020–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merle BM, Benlian P, Puche N, Bassols A, Delcourt C, Souied EH; Nutritional Age-Related Macular Degeneration Treatment Study Group. Circulating omega-3 fatty acids and neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci 2014;55:2010–9. [DOI] [PubMed] [Google Scholar]
- 25.Chong EW, Simpson JA, Robman LD, Hodge AM, Aung KZ, English DR, Giles GG, Guymer RH. Red meat and chicken consumption and its association with age-related macular degeneration. Am J Epidemiol 2009;169:867–76. [DOI] [PubMed] [Google Scholar]
- 26.Willett WC, Sacks F, Trichopoulou A, Drescher G, Ferro-Luzzi A, Helsing E, Trichopoulos D. Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr 1995;61(6 Suppl):1402S–6S. [DOI] [PubMed] [Google Scholar]
- 27.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med 2003;348:2599–608. [DOI] [PubMed] [Google Scholar]
- 28.Sofi F, Abbate R, Gensini GF, Casini A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis. Am J Clin Nutr 2010;92:1189–96. [DOI] [PubMed] [Google Scholar]
- 29.Samieri C, Sun Q, Townsend MK, Chiuve SE, Okereke OI, Willett WC, Stampfer M, Grodstein F. The association between dietary patterns at midlife and health in aging: an observational study. Ann Intern Med 2013;159:584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mares JA, Voland RP, Sondel SA, Millen AE, Larowe T, Moeller SM, Klein ML, Blodi BA, Chappell RJ, Tinker L, et al. Healthy lifestyles related to subsequent prevalence of age-related macular degeneration. Arch Ophthalmol 2011;129:470–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amirul Islam FM, Chong EW, Hodge AM, Guymer RH, Aung KZ, Makeyeva GA, Baird PN, Hopper JL, English DR, Giles GG, et al. Dietary patterns and their associations with age-related macular degeneration: the Melbourne Collaborative Cohort Study. Ophthalmology 2014;121(7):1428–34.e2. [DOI] [PubMed] [Google Scholar]
- 32.Chiu CJ, Chang ML, Zhang FF, Li T, Gensler G, Schleicher M, Taylor A. The relationship of major American dietary patterns to age-related macular degeneration. Am J Ophthalmol 2014;158(1):118–27.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montgomery MP, Kamel F, Pericak-Vance MA, Haines JL, Postel EA, Agarwal A, Richards M, Scott WK, Schmidt S. Overall diet quality and age-related macular degeneration. Ophthalmic Epidemiol 2010;17:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol 2001;119(10):1417–36. [DOI] [PMC free article] [PubMed]
- 35.Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study (AREDS): design implications. AREDS report no. 1. Control Clin Trials 1999;20(6):573–600. [DOI] [PMC free article] [PubMed]
- 36.Seddon JM, Sharma S, Adelman RA. Evaluation of the clinical age-related maculopathy staging system. Ophthalmology 2006;113:260–6. [DOI] [PubMed] [Google Scholar]
- 37.Yu Y, Reynolds R, Rosner B, Daly MJ, Seddon JM. Prospective assessment of genetic effects on progression to different stages of age-related macular degeneration using multistate Markov models. Invest Ophthalmol Vis Sci 2012;53:1548–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fung TT, Rexrode KM, Mantzoros CS, Manson JE, Willett WC, Hu FB. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation 2009;119:1093–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trichopoulou A, Kouris-Blazos A, Wahlqvist ML, Gnardellis C, Lagiou P, Polychronopoulos E, Vassilakou T, Lipworth L, Trichopoulos D. Diet and overall survival in elderly people. BMJ 1995;311:1457–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seddon JM, Reynolds R, Yu Y, Rosner B. Three new genetic loci (R1210C in CFH, variants in COL8A1 and RAD51B) are independently related to progression to advanced macular degeneration. PLoS One 2014;9:e87047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seddon JM, Silver RE, Kwong M, Rosner B. Risk prediction for progression of macular degeneration: 10 common and rare genetic variants, demographic, environmental, and macular covariates. Invest Ophthalmol Vis Sci 2015;56(4):2192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glynn RJ, Rosner B. Regression methods when the eye is the unit of analysis. Ophthalmic Epidemiol 2012;19:159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klein ML, Francis PJ, Rosner B, Reynolds R, Hamon SC, Schultz DW, Ott J, Seddon JM. CFH and LOC387715/ARMS2 genotypes and treatment with antioxidants and zinc for age-related macular degeneration. Ophthalmology 2008;115:1019–25. [DOI] [PubMed] [Google Scholar]
- 44.Merle BM, Richard F, Benlian P, Puche N, Delcourt C, Souied EH. CFH Y402H and ARMS2 A69S polymorphisms and oral supplementation with docosahexaenoic acid in neovascular age-related macular degeneration patients: the NAT2 study. PLoS One 2015;10:e0130816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schramm EC, Clark SJ, Triebwasser MP, Raychaudhuri S, Seddon JM, Atkinson JP. Genetic variants in the complement system predisposing to age-related macular degeneration: a review. Mol Immunol 2014;61(2):118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seddon JM, Gensler G, Klein ML, Milton RC. C-reactive protein and homocysteine are associated with dietary and behavioral risk factors for age-related macular degeneration. Nutrition 2006;22:441–3. [DOI] [PubMed] [Google Scholar]
- 47.Seddon JM, Gensler G, Rosner B. C-reactive protein and CFH, ARMS2/HTRA1 gene variants are independently associated with risk of macular degeneration. Ophthalmology 2010;117:1560–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang JJ, Buitendijk GH, Rochtchina E, Lee KE, Klein BE, van Duijn CM, Flood VM, Meuer SM, Attia J, Myers C, et al. Genetic susceptibility, dietary antioxidants, and long-term incidence of age-related macular degeneration in two populations. Ophthalmology 2014;121:667–75. [DOI] [PubMed] [Google Scholar]
- 49.Fung TT, McCullough ML, Newby PK, Manson JE, Meigs JB, Rifai N, Willett WC, Hu FB. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr 2005;82:163–73. [DOI] [PubMed] [Google Scholar]
- 50.Dai J, Jones DP, Goldberg J, Ziegler TR, Bostick RM, Wilson PW, Manatunga AK, Shallenberger L, Jones L, Vaccarino V. Association between adherence to the Mediterranean diet and oxidative stress. Am J Clin Nutr 2008;88:1364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bach-Faig A, Geleva D, Carrasco J, Ribas-Barba L, Serra-Majem L. Evaluating associations between Mediterranean diet adherence indexes and biomarkers of diet and disease. Public Health Nutr 2006;9(8A):1110–7. [DOI] [PubMed] [Google Scholar]