Abstract
Background: Folate status has been positively associated with cognitive function in many studies; however, some studies have observed associations of poor cognitive outcomes with high folate. In search of an explanation, we hypothesized that the association of folate with cognition would be modified by the interaction of high-folate status with a common 19-bp deletion polymorphism in the dihydrofolate reductase (DHFR) gene. To our knowledge, the cognitive effects of this gene have not been studied previously.
Objective: We examined the association between cognitive outcomes with the 19-bp deletion DHFR polymorphism, folate status, and their interaction with high or normal plasma folate.
Design: This was a pooled cross-sectional study of the following 2 Boston-based cohorts of community living adults: the Boston Puerto Rican Health Study and the Nutrition, Aging, and Memory in Elders study. Individuals were genotyped for the DHFR 19-bp deletion genotype, and plasma folate status was determined. Cognitive outcomes included the Mini-Mental State Examination, Center for Epidemiologic Studies Depression Scale, and factor scores for the domains of memory, executive function, and attention from a set of cognitive tests.
Results: The prevalence of the homozygous deletion (del/del) genotype was 23%. In a multivariable analysis, high folate status (>17.8 ng/mL) was associated with better memory scores than was normal-folate status (fourth–fifth quintiles compared with first–third quintiles: β ± SE = −0.22 ± 0.06, P < 0.01). Carriers of the DHFR del/del genotype had worse memory scores (β ± SE = −0.24 ± 0.10, P < 0.05) and worse executive scores (β = −0.19, P < 0.05) than did those with the del/ins and ins/ins genotypes. Finally, we observed an interaction such that carriers of the del/del genotype with high folate had significantly worse memory scores than those of both noncarriers with high-folate and del/del carriers with normal-folate (β-interaction = 0.26 ± 0.13, P < 0.05).
Conclusions: This study identifies a putative gene-nutrient interaction that, if confirmed, would predict that a sizable minority carrying the del/del genotype might not benefit from high-folate status and could see a worsening of memory. An understanding of how genetic variation affects responses to high-folate exposure will help weigh risks and benefits of folate supplementation for individuals and public health.
Keywords: cognition, dihydrofolate reductase, folate, gene-nutrient interaction, memory
INTRODUCTION
Folate status and metabolism have long been recognized as important nutritional determinants of cognitive function in older adults. Most studies in human populations have reported associations between low folate status and high plasma total homocysteine (tHcy)10 with poor cognitive function (1–8). However, some studies have shown an inverse association of high folate status with worse cognitive outcomes either as a function of poor vitamin B-12 status (9) or independent of vitamin B-12 status (10). Such inconsistencies may be explained partly by study designs that tended to focus on low rather than high folate status as the reference group, by differences between study populations with respect to the prevalence of low and high folic acid (FA) intake, by the genetic background of the study population, and by gene-nutrient interactions.
The enzyme dihydrofolate reductase (DHFR) is one potential nexus for such an interaction. The natural function of DHFR is to reduce endogenous dihydrofolate to form tetrahydrofolate, the derivatives of which are cofactors of one-carbon metabolism, and the synthesis of thymidylate. DHFR can also reduce FA (the synthetic form of folate used in dietary supplements and fortified foods) to dihydrofolate, thereby allowing its assimilation into the body’s folate pool. However, this latter reaction occurs in the following 2 steps: a slow first step where synthetic FA is reduced to the natural vitamer dihydrofolate and a subsequent second step that proceeds quickly in which dihydrofolate is reduced to tetrahydrofolate. Because the initial reduction of FA to dihydrofolate in humans is very slow, excess FA may compete for the enzyme with the endogenous substrate dihydrofolate, thereby disrupting the normal cycling of the folate pool through dihydrofolate to tetrahydrofolate (11). Theoretically, this effect could interfere with downstream brain processes that require normal folate and one-carbon metabolism.
Although severe congenital mutations in the DHFR gene can cause hematologic and neurologic symptoms of folate deficiency (12, 13), the functional significance of other polymorphisms is less certain. In 2004, Johnson et al. (14) described a 19-bp deletion polymorphism in the sequence of the DHFR gene intron 1. The polymorphism is highly prevalent, with the frequency of the del/del genotype reported between 10.5% and 48% in various populations (15–19). Although the deletion falls in a noncoding intronic region, the missing segment causes the loss of potential binding sites for transcription factors such as mothers against decapentaplegic homolog 4 (SMAD4) and specificity protein 1 transcription factor (SP1), which could alter its regulation (Supplemental Table 1). There have been conflicting reports on the association between the del/del genotype with homocysteine, neural tube defects, and early birth in populations from different ethnic backgrounds (18–22). However, the deletion has been reported to result in elevated messenger RNA expression of DHFR in lymphocytes (18, 22) and in higher plasma concentrations of unmetabolized FA (20). Theoretically, DHFR could also have indirect vascular effects because of its capacity to reduce dihydrobiopterin to tetrahydrobiopterin, which is a necessary cofactor in the synthesis of nitric oxide by endothelial nitric oxide synthase (23–25). Thus, it is possible that any cognitive effects of the DHFR del/del genotype may depend on gene-nutrient interactions between the enzyme and folate status and, particularly, on high FA intake.
To examine this hypothesis, we used a cross-sectional study of 2 pooled cohorts of adults who were living in Boston in whom folate status, DHFR 19-bp deletion genotype, and cognitive status were determined.
METHODS
Subjects and study design
The current analysis was performed in a pooled cross-sectional study of the following 2 Boston-based cohorts: the BPRHS (Boston Puerto Rican Health Study) and the NAME (Nutrition, Aging, and Memory in Elders) study, which were both initiated in 2004. A detailed description of the cohorts and methods are given elsewhere in our previous investigations (26–28). Briefly, the BPRHS consists of Boston-area residents of Puerto Rican origin aged 45–75 y. The NAME study is a cohort of community-based African American and non-Hispanic white elderly participants aged ≥60 y. Extensive data on nutritional and health outcomes were obtained from both cohorts, including identical neuropsychological tests and blood biochemical measurements. The DHFR genotype was obtained for this study from 1164 participants of the BPRHS and 872 participants of the NAME cohorts, which provided an initial sample size of n = 2036, 1402 subjects of whom had complete data for a full multivariable analysis. Neither cohort was powered a priori to study gene-nutrient interactions; however, the pooled data set allowed for the exploration of such interactions as described in our study of the C677T polymorphism in the methylenetetrahydrofolate reductase (MTHFR) gene, folate status, and cognition (27). Both study populations were exposed to FA fortification of flour and grain products. Ethical approval for the studies was granted by the Institutional Review Board of the Tufts Medical Center and Tufts University, and all participants gave informed consent for all tests including genotyping.
DNA isolation and genotyping
DNA was isolated with the use QIAamp DNA Blood Mini kits (Qiagen). The genotype was determined with the use of a 7300 Real-Time PCR system (Applied Biosystems). The following polymerase chain reaction primers were used: a forward primer (5′-TCGCTGTGTCCCAGAACATG-3′) and a reverse primer (5′-AGCGCAGACCGCAAGTCTG-3′). Two TaqMan TAMRA probes (Applied Biosystems) were used to target the 19-bp deletion. The assay identifies the DHFR alleles by measuring the change in fluorescence of the dyes associated with the probes. A FAM fluorescent probe was used to identify the insertion allele (ACC TGG GCG GGA CGC G TAMRA), and a VIC fluorescent probe was used for the deleted allele (TGG CCG ACT CCCGGCG TAMRA). The polymerase chain reaction consisted of a 3-ng template (genomic DNA), 950 nmol/L for forward and backward primers, 250 nmol/L of each probe and a TaqMan Universal PCR Master mix (Applied Biosystems) in a total reaction volume of 20 mL. Samples were denatured at 95°C for 10 min followed by 50 cycles of 92°C for 15 s and 60°C for 1 min. For all genotyping, blinded no-template controls and replicates of DNA samples were incorporated in each of the DNA sample plates, which were routinely checked. Apolipoprotein E was genotyped as described elsewhere (29).
Primary outcome: cognition
The Mini-Mental State Examination (MMSE) is a 30-point test designed to assess global cognition and screen for dementia; this examination was administered in both cohorts to determine eligibility (30). No participants in the current study had an MMSE score ≤10 because this was an exclusion criterion of the original cohorts to prevent floor effects. The cognitive testing battery for the NAME study (31) consisted of subtests from the Wechsler Memory Scale–III (Logical Memory and Word List Learning) and Wechsler Adult Intelligence Scale–III (Digit Span, Digit Symbol Coding, Block Design, and Matrix Reasoning) (32, 33), Trail Making Tests A and B (34), the Controlled Oral Word Association test (35), and the North American Adult Reading Test (36). The testing battery for the BPRHS cohort consisted of Word List Learning, Stroop Color-Word, Digit Span, and Letter Fluency subtests (37) as well as adaptations of the clock-drawing task (38) and figure-copying task (39). Both cohorts were administered the Center for Epidemiologic Studies Depression Scale (CESD) (32). The testing in both cohorts was supervised by the same research team and neuropsychologist.
A principal components analysis of cognitive testing scores was used to derive composite scores for each cohort separately (28–30). The analysis of cognitive factors was completed with the use of an orthogonal rotational procedure (Varimax). The adequacy of the correlation was assessed with the sue of Bartlett’s test and the Kaiser-Meyer-Olkin measure. Components with Eigen values >1.0 were interpreted in terms of the cognitive domain by items with component loading scores >│0.4│. The principal components analysis for both cohorts resulted in 3 components that were interpreted as measuring memory, attention, and executive function. By definition, the components had a mean ± SD of 0 ± 1. Component scores for each domain were pooled to combine the 2 cohorts and were used in subsequent data analyses to avoid issues associated with multiple testing.
Covariates
General demographic information including age, sex, ethnicity, education, and health history and behaviors was elicited by a questionnaire. Kidney function was evaluated by calculating the estimated glomerular filtration rate (eGFR) from serum creatinine with the use of the Modification of Diet in Renal Disease Study equation (40). The apolipoprotein E genotype was classified as carriers of the E4 allele (E3E4 and E4E4) and others (E3E3, E2E3, and E2E2). Thirty individuals with the E2E4 genotype were excluded from the analysis as per convention. Multivitamin use was classified as a dichotomous variable. For the BPRHS cohort only, the genetic admixture of the population was also examined as a covariate with the use of a first principal component derived from an EIGENSTRAT analysis of 100 ancestry informative markers (41). Ancestry informative markers were not available for the NAME cohort.
Biochemical measurements
Nutritional predictors included fasting plasma folate, vitamin B-12, vitamin B-6, and tHcy. tHcy was determined with the use of HPLC (42). Plasma creatinine was measured with the use of a modified Jaffe method (43). Plasma folate and vitamin B-12 were measured with an Immulite chemiluminescent assay (Diagnostic Products Corp./Siemens). Pyridoxal 5-phosphate (vitamin B-6) was determined with the use of the tyrosine decarboxylase apoenzyme method (44). Analyses in both cohorts were carried out by the same laboratory.
Statistical analysis
Descriptive statistics were performed for the individual and pooled cohorts with stratification for ethnicity and DHFR genotype. All variables were analyzed for normal distribution before and after transformation of any skewed variables that were log transformed as appropriate. Univariate analyses were performed for categorical variables with the use of the chi-square test, and continuous variables were analyzed with the use of an independent samples t test for comparison of homozygotes for the del/del genotype with all others (heterozygotes and homozygotes for the insertion variant; del/ins plus ins/ins). A 2-sided α = 0.05 was used to determine significance. A multivariable linear regression was performed to determine the association of cognitive outcomes with folate and DHFR status. Cognitive outcomes adjusted for covariates were compared across folate quintiles with the use of a 1-factor ANOVA with the Tukey post hoc test. General linear modeling (GLM) was used with the DHFR genotype and/or folate as fixed factors with and without an interaction term for folate × DHFR, where log-transformed folate was analyzed with the use of a cutoff for high folate (quintiles 4–5: >17.8 ng/mL) vs. normal folate (quintiles 1–3: ≤17.8 ng/mL). Outcome measures included the derived cognitive domains of memory, executive function, and attention as well as the MMSE and CESD scores. The following covariates were included in the models: self- identified ethnicity, age, sex, a reduced-category variable for education, eGFR, diabetes, hypertension, apolipoprotein E4 genotype, tHcy (log transformed), vitamin B-12 (log transformed), and vitamin B-6 (log transformed). Mean values are described as means ± SDs unless specified otherwise. All statistical analyses were performed with the use of SPSS 21 software (IBM SPSS Statistics for Windows, Version 21.0; IBM Corp.).
RESULTS
Table 1 shows unadjusted demographic and descriptive statistics for subjects of our pooled study population, who were genotyped for the DHFR gene, stratified by ethnicity and genotype. As described in our previous study (27), NAME participants were older and better educated and had a higher proportion of women than did the BPRHS cohort, and the individual cohorts had high burdens of chronic conditions including hypertension and diabetes. Stratified by ethnicity alone, the overall prevalence of diabetes and hypertension was highest in African Americans (42.2% and 90.2%, respectively). A high variability was shown in ethnic groups, including in education, the sex ratio, the prevalence of chronic conditions, concentrations of B vitamins, frequencies of genetic variants, and cognitive function. Cognitive impairment, as measured by the cutoff of 24 in the MMSE, was more-significantly more prevalent in African Americans (50.3%) and Puerto Ricans (58.1%) than in non-Hispanic whites (24.6%, P < 0.001). Elevated depressive symptoms (CESD score ≥16) were more prevalent in the Puerto Rican population (61.1%) than in the African American or non-Hispanic white population (37.3% and 32.2%, respectively, P < 0.001). Puerto Ricans had higher mean folate (19.3 ± 8.9 ng/mL) than did African Americans and non-Hispanic whites (14.5 ± 11.1 and 15.6 ± 9 ng/mL, respectively; P < 0.001). tHcy differed between all 3 ethnicities with lower means displayed by Puerto Ricans (9.2 ± 4.7 μmol/L) than by non-Hispanic whites and African Americans (11.6 ± 4.3 and 12.6 ± 6.3 μmol/L, respectively (P < 0.001). These data, which were further stratified by genotype, are given in Table 1.
TABLE 1.
Description of NAME and BPRHS populations1
| NAME |
||||||
| African Americans |
Non-Hispanic whites |
BPRHS, Puerto Ricans |
||||
| del/del | del/ins + ins/ins | del/del | del/ins + ins/ins | del/del | del/ins + ins/ins | |
| n (% within group) | 74 (32.9) | 151 (67.1) | 93 (22.0) | 329 (78.0) | 160 (22.1) | 565 (77.9) |
| Demographic | ||||||
| Age, y | 72.9 ± 8.82 | 72.7 ± 8.3 | 75.4 ± 8.2 | 76.0 ± 8.3 | 56.7 ± 7.4 | 57.3 ± 7.5 |
| Female, % | 82.4 | 82.1 | 67.7 | 72.9 | 71.3 | 71.8 |
| Education, % | ||||||
| Zero to fifth grade | 1.4 | 2.6 | 0.0 | 1.5 | 21.9 | 18.0 |
| Fifth to ninth grade | 21.6 | 15.9 | 6.5 | 10.0 | 25.0 | 26.9 |
| Ninth to 12th grade | 28.4 | 29.1 | 18.3 | 17.3 | 41.3 | 38.5 |
| College | 29.7 | 26.5 | 39.8 | 34.7 | 10.0 | 13.8 |
| Graduate school | 18.9 | 22.5 | 26.9 | 33.1 | 1.3 | 2.9 |
| Blood biochemistry | ||||||
| eGFR, mL · min−1 · 1.73 m−2 | 89.1 ± 35.7 | 85.6 ± 35.6 | 79.0 ± 30.8 | 81.2 ± 32.2 | 91.0 ± 32.2* | 86.8 ± 22.7* |
| Folate, ng/mL | 14.1 ± 10.6 | 14.0 ± 7.5 | 15.6 ± 7.7 | 15.5 ± 9.1 | 18.7 ± 8.1 | 19.6 ± 9.5 |
| Vitamin B-12, pg/mL | 657 ± 540 | 716 ± 1059 | 521 ± 238 | 530 ± 263 | 540 ± 311 | 552 ± 282 |
| Vitamin B-6,3 nmol/L | 62.0 ± 74.9 | 54.7 ± 48.6 | 78.4 ± 73.5 | 70.7 ± 73.2 | 61.1 ± 61.4 | 62.6 ± 68.4 |
| tHcy, μmol/L | 12.9 ± 9.2 | 12.0 ± 4.8 | 11.8 ± 4.4 | 11.6 ± 4.2 | 9.2 ± 5.0 | 9.2 ± 5.0 |
| Multivitamin use, % | 48.1 | 51.9 | 49.8 | 50.2 | 20.3** | 21.9** |
| Chronic condition | ||||||
| Diabetes, % | 32.4* | 47.0* | 34.4 | 28.6 | 40.6 | 37.3 |
| Hypertension, % | 93.2 | 88.7 | 88.2 | 83.3 | 61.3* | 70.3* |
| MMSE score | 24.5 ± 3.1 | 24.2 ± 3.6 | 26.0 ± 2.9 | 26.1 ± 3.0 | 23.6 ± 3.6 | 23.9 ± 3.1 |
| Cognitive impairment % | 47.3 | 51.7 | 26.9 | 24.0 | 60.0 | 57.6 |
| CESD score | 15.0 ± 10.9 | 13.7 ± 11.4 | 11.8 ± 9.5 | 12.6 ± 10.2 | 20.9 ± 13.2 | 20.6 ± 13.6 |
| Depressive symptoms % | 40.5 | 35.8 | 28.0 | 33.4 | 60.6 | 61.2 |
| Genetic background,% | ||||||
| apoE4 carriers | 29.7 | 27.2 | 23.7 | 17.9 | 21.3 | 24.5 |
Unadjusted demographic and descriptive statistics for subjects of the pooled study population who were genotyped for the DHFR gene are shown stratified by ethnicity and genotype. Univariate analyses were performed for categorical variables with the use of the chi-square test, and continuous variables were analyzed with the use of an independent samples t test. The cutoff for cognitive impairment was an MMSE score ≤24; the cutoff for depressive symptoms was a CESD score ≥16. *,**Significant pairwise comparisons between del/del and del/ins + ins/ins genotypes within separate ethnic subgroups: *P < 0.05, **P < 0.01. An independent samples t test and chi-square test were used to test significance in the 3 ethnicities. apoE4, apolipoprotein E4 allele; BPRHS, Boston Puerto Rican Health Study; CESD, Center for Epidemiologic Studies Depression Scale; DHFR, dihydrofolate reductase; eGFR, estimated glomerular filtration rate; MMSE, Mini-Mental State Examination; NAME, Nutrition, Aging, and Memory in Elders; tHcy, plasma total homocysteine.
Unadjusted mean ± SD (all such values).
Measured as pyridoxyl 5-phosphate.
The prevalence of the DHFR del/del genotype in the pooled cohort was 23.2%; however, it varied significantly between ethnic groups, ranging from 32.9% in African Americans to 22.1% and 20.0% in Hispanics and non-Hispanic whites, respectively (P = 0.003).
The population was stratified by both ethnicity and the DHFR genotype of homozygotes for the del/del variant and the group combining heterozygotes and homozygotes for the insertion variant (del/ins plus ins/ins) (Table 1). The prevalence of diabetes was lower in del/del carriers than in others only in African Americans (32.4% and 47.0%, respectively; P < 0.05), and the prevalence of hypertension was lower in del/del carriers than in the others only in Hispanics (61.3% and 70.3%, respectively; P < 0.01). The prevalence of cognitive impairment (MMSE score <24) or depressive symptoms (CESD score ≥16) did not differ significantly by genotype within any ethnic group.
Plasma folate and tHcy concentrations did not differ significantly in the groups. Folate deficiency was infrequent in both cohorts and all ethnic groups because this population was exposed to FA fortification of flour and grain products. The overall median and mean folate concentrations were 16.1 ng/mL and 17.6 ± 9.5 ng/mL, respectively. The prevalence of folate deficiency was low. Only 0.4% of subjects (n = 8) displayed severe folate deficiency (<3 ng/mL), and 7.3% of subjects (n = 147) showed mild folate deficiency (<7 ng/mL) (45).
Multivitamin use was more frequent in the NAME cohort than in the BPHRS cohort. Fifty percent of African Americans and 57% of non-Hispanic whites took multivitamins compared with only 22% of Puerto Ricans who did so. Overall, plasma folate in multivitamin users was higher than in nonusers (20.1 ± 10.0 vs. 16.0 ± 8. ng/mL, respectively; P < 0.01). However, neither the frequency of multivitamin use nor plasma folate differed by genotype (Table 1).
Neither log-transformed folate, as a continuous variable, nor dichotomous DHFR genotype was significantly correlated with any cognitive outcome in multivariable linear regression models (data not shown). As in the subsequent GLM analysis, cognitive outcomes were significantly associated with age, sex, ethnicity, and level of education. As previously reported for these cohorts, a lower vitamin B-12 status was associated with higher CESD scores, which indicated a greater frequency of depressive symptoms; vitamin B-6 status was positively associated with MMSE, attention, and executive function; and diabetes and hypertension were associated with lower MMSE scores. Kidney function (eGFR) and apolipoprotein E genotype were not significantly associated with cognition (Table 2) (27).
TABLE 2.
Variable estimates for the GLM model of cognitive outcomes by folate status and DHFR genotype in the pooled cohort1
| Variable | MMSE2 | CESD2 | FACattn2 | FACexec2 | FACmem2 | FACmem3 |
| DHFR del/del genotype | −0.13 ± 0.19 | 0.05 ± 0.75 | 0.01 ± 0.06 | 0.07 ± 0.05 | −0.08 ± 0.06 | −0.24 ± 0.1* |
| <17.8 ng folate/mL | −0.05 ± 0.18 | 1.02 ± 0.71 | 0.06 ± 0.06 | −0.09 ± 0.06 | −0.16 ± 0.06** | −0.22 ± 0.06** |
| DHFR del/del genotype × <17.8 ng folate/mL | 0.26 ± 0.13* | |||||
| Ethnicity | −0.21 ± 0.15 | 0.79 ± 0.59 | 0.22 ± 0.05† | −0.03 ± 0.04 | −0.19 ± 0.05† | −0.19 ± 0.05† |
| Age | −0.008 ± 0.009 | −0.33 ± 0.04† | 0.006 ± 0.003* | −0.02 ± 0.003† | −0.02 ± 0.003† | −0.02 ± 0.003† |
| Sex | −0.59 ± 0.18** | 3.73 ± 0.74† | −0.18 ± 0.06** | 0.03 ± 0.06 | 0.34 ± 0.06† | 0.34 ± 0.06† |
| Education | 1.02 ± 0.06† | −1.02 ± 0.25† | 0.19 ± 0.02† | 0.27 ± 0.02† | 0.12 ± 0.02† | 0.12 ± 0.02† |
| apoE4 genotype | −0.01 ± 0.19 | 0.33 ± 0.75 | −0.03 ± 0.06 | −0.005 ± 0.06 | −0.1 ± 0.06 | −0.1 ± 0.06 |
| eGFR | 0.005 ± 0.003 | −0.02 ± 0.01 | −0.001 ± 0.001 | 0.001 ± 0.001 | 0.001 ± 0.001 | 0.001 ± 0.001 |
| tHcy4 | 0.13 ± 0.26 | −0.78 ± 1.05 | −0.05 ± 0.09 | −0.18 ± 0.08* | 0.004 ± 0.09 | 0.01 ± 0.09 |
| Vitamin B-124 | 0.34 ± 0.18 | −1.51 ± 0.71* | 0.01 ± 0.06 | −0.08 ± 0.05 | 0.06 ± 0.06 | 0.07 ± 0.06 |
| Vitamin B-64 | 0.24 ± 0.12* | −0.73 ± 0.46 | 0.13 ± 0.04† | 0.1 ± 0.03** | −0.05 ± 0.04 | −0.05 ± 0.04 |
| Diabetes | −0.46 ± 0.17** | −0.37 ± 0.68 | −0.12 ± 0.06* | −0.12 ± 0.06 * | −0.18 ± 0.05† | −0.08 ± 0.06 |
| Hypertension | 0.42 ± 0.20* | 1.27 ± 0.80 | 0.09 ± 0.07 | −0.06 ± 0.06 | 0.06 ± 0.06 | 0.06 ± 0.07 |
All values are βs (variable estimates) ± SEs. The table shows variable estimates for the association of the listed variables with cognitive outcomes in the pooled cohort as analyzed with the use of GLM. Covariates included self-identified ethnicity, age, sex, education, eGFR, diabetes, hypertension, apoE4 genotype, tHcy, vitamin B-12, and vitamin B-6. *P < 0.05, **P < 0.01, †P < 0.001. apoE4, apolipoprotein E4; CESD, Center for Epidemiologic Studies Depression Scale; DHFR, dihydrofolate reductase; eGFR, estimated glomerular filtration rate; FACattn, factor score for attention; FACexec, factor score for executive function; FACmem, factor score for memory; GLM, general linear modeling; MMSE, Mini-Mental State Examination; tHcy, plasma total homocysteine.
Variable estimates for the main-effects model without the interaction term DHFR del/del genotype × <17.8 ng folate/mL.
Variable estimates for the full GLM model with an interaction term.
Plasma analytes were log transformed.
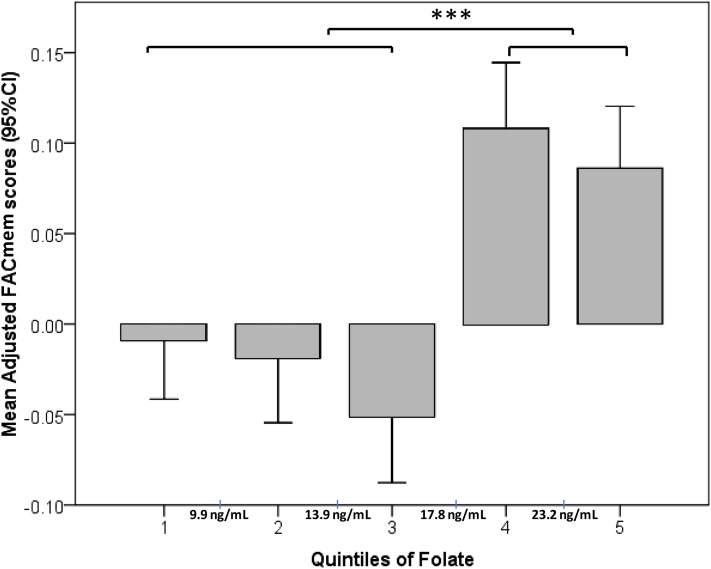
To test the hypothesis that the effects of folate and the DHFR del/del genotype would manifest under conditions of high folate status rather than in a linear fashion, we examined the effects of folate quintiles on the adjusted cognitive outcomes in the pooled cohort. Although the linear regression across quintiles was NS, an ANOVA analysis of the resulting adjusted memory scores showed significantly lower memory scores for the first 3 quintiles of folate than for the 2 upper quintiles (Figure 1, P < 0.01). The descriptive analysis showed that the 2 upper quintiles of the pooled population had relatively high plasma folate (>17.8 ng/mL; mean: 26 ± 9.1 ng/mL; range: 17.9–114.4 ng/mL) than did the lower 3 quintiles, which on average, had normal plasma folate (≤17.8 ng/mL; mean: 11.9 ± 3.8 ng/mL; range: 2–17.8 ng/mL). This value (17.8 ng/mL) was very close to the mean folate concentration of the study population (17.6 ng/mL). Therefore, we chose 17.8 ng/mL as a cutoff to explore the effect of high vs. normal folate status on cognitive function. To test the hypothesis that high vs. normal folate status and the DHFR genotype can modify cognitive outcomes through the interaction with each other, we used this cutoff and the DHFR genotype as fixed factors in a full GLM model as previously described.
FIGURE 1.
Mean (95% CI) adjusted memory scores by quintiles of folate in the pooled cohort. The graph shows results of an ANOVA with Tukey post hoc testing for differences between mean adjusted memory scores by quintiles of plasma folate concentrations. n = 280/quintile. Memory scores were adjusted with the use of a multivariate linear regression for ethnicity, age, sex, education, estimated glomerular filtration rate, diabetes, hypertension, apolipoprotein E4 genotype, plasma total homocysteine, vitamin B-12, and vitamin B-6. Cutoff values between quintiles are marked on the x axis. ***P < 0.001. FACmem, factor score for memory.
Table 3 shows the effect of genotype on cognitive outcomes for pooled and ethnicity-stratified results. The pooled analysis showed that carriers of the del/del genotype had significantly worse adjusted mean memory scores (−0.03 vs. 0.04; P < 0.0001) and executive function (−0.08 vs. 0.02; P = 0.001) than did noncarriers. Although these differences in memory in the pooled results appeared to have been driven largely by non-Hispanic whites, the trends for worse function in the del/del genotype were similar but NS in African Americans. Adjusted attention scores and indicators of clinical cognitive impairment or depression (MMSE and CESD scores) in the pooled results did not differ by genotype (Table 2). Because only the BPRHS had data on genetic admixture, we could only adjust for this variable in the stratified analysis of the BPRHS cohort. The addition of admixture as a covariate in the model enlarged the difference in memory between genotypes (Supplemental Table 2).
TABLE 3.
Cognitive outcomes by ethnicity and genotype in the NAME, BPRHS, and pooled cohorts1
| NAME |
||||||||
| African Americans |
Non-Hispanic whites |
BPRHS, Puerto Ricans |
Pooled cohort |
|||||
| del/del | del/ins + ins/ins | del/del | del/ins + ins/ins | del/del | del/ins + ins/ins | del/del | del/ins + ins/ins | |
| FACmem2 | 0.12 ± 0.27 | 0.18 ± 0.27 | −0.10 ± 0.23* | −0.03 ± 0.26* | −0.07 ± 0.30† | 0.04 ± 0.31† | −0.03 ± 0.29† | 0.04 ± 0.30† |
| FACmem3 | 0.09 ± 0.26** | 0.20 ± 0.27** | −0.11 ± 0.25** | −0.03 ± 0.26** | −0.05 ± 0.31† | 0.04 ± 0.30† | −0.04 ± 0.30† | 0.04 ± 0.30† |
| FACexec3 | −0.18 ± 0.42 | −0.07 ± 0.42 | −0.04 ± 0.38 | −0.002 ± 0.39 | −0.06 ± 0.47* | 0.04 ± 0.48* | −0.08 ± 0.44** | 0.02 ± 0.45** |
| FACattn3 | −0.27 ± 0.26 | −0.27 ± 0.26 | 0.15 ± 0.28 | 0.09 ± 0.27 | −0.06 ± 0.31 | −0.02 ± 0.30 | −0.05 ± 0.33 | −0.03 ± 0.31 |
| MMSE3 | 24.9 ± 1.3 | 25.1 ± 1.3 | 25.4 ± 1.3 | 25.7 ± 0.2 | 23.8 ± 1.6* | 24.1 ± 1.6* | 24.5 ± 1.6 | 24.6 ± 1.6 |
| CESD3 | 14.1 ± 3.5 | 13.7 ± 3.4 | 12.8 ± 3.4 | 13.0 ± 0.5 | 20.8 ± 3.4 | 20.1 ± 3.2 | 17.0 ± 5.1 | 17.2 ± 5.0 |
All values are adjusted estimated means ± SDs. The table shows comparisons of differences in cognitive function by genotype in African Americans, non-Hispanic whites, and Puerto Ricans in the NAME, BPRHS, and pooled cohort. *,**,†Significant pairwise comparisons between del/del and del/ins + ins/ins genotypes within separate ethnic subgroups: *P < 0.05, **P < 0.01, †P < 0.001. CESD, Center for Epidemiologic Studies Depression Scale; DHFR, dihydrofolate reductase; FACattn, factor score for attention; FACexec, factor score for executive function; FACmem, factor score for memory; MMSE, Mini-Mental State Examination.
Cognitive outcome values for the full general linear modeling model including an interaction term for the DHFR del/del genotype × <17.8 ng folate/mL.
Cognitive outcome values for the main model without the interaction term.
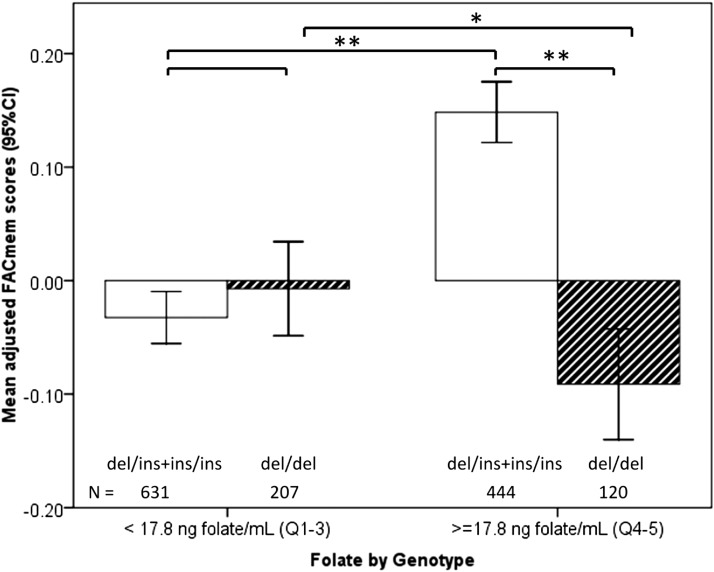
Variable estimates for the GLM models are given in Table 3, Both the del/del genotype and plasma folate concentrations less than the cutoff were significantly and negatively associated with memory (β = −0.24, P < 0.05; β = -0.22, P < 0.01, respectively). The interaction between the DHFR genotype and folate status was significant (β = 0.26, P < 0.05; Table 3). Carriers of the del/del genotype in the high-folate category had significantly lower memory scores than did others with high folate (P < 0.001; Figure 2) and also compared with those of carriers with normal folate (P < 0.05, Figure 2). After adjustment for folate and DHFR, tHcy (log transformed) showed a significant inverse association with executive function but not with memory (β = −0.18, P < 0.05; Table 3). Neither folate nor tHcy was significantly associated with the cognitive domain of attention. Multivitamin use was not associated with any cognitive outcome and did not alter the interaction between high folate and genotype and was, therefore, excluded from the final models to avoid overfitting.
FIGURE 2.
Interaction of dihydrofolate reductase genotype and plasma folate with respect to memory in the pooled cohort. The graph shows results of a comparison of mean (95% CI) adjusted memory scores by genotype below and above the plasma folate cutoff of 17.8 ng folate/mL. Memory scores were adjusted in the general linear model for ethnicity, age, sex, education, estimated glomerular filtration rate, diabetes, hypertension, apolipoprotein E4 genotype, plasma total homocysteine, vitamin B-12, and vitamin B-6. *P < 0.05, ** P < 0.01. FACmem, factor score for memory.
DISCUSSION
In this cross-sectional study, we tested the hypothesis that the genetic variation in the folate metabolic pathway, namely a 19-bp deletion in the DHFR gene, would be directly associated with cognitive function or would modify the association of folate status and cognition through the interaction with a high concentration of circulating folate. The significant interaction shown between folate and DHFR genotype and memory function is supportive of this hypothesis.
Although an increased folate status across the population should have a net benefit for memory in older adults, a causal interaction would predict that a sizable minority carrying the del/del genotype may not share the benefit and could see a worsening of memory. We observed that the 23% of our study population carrying the DHFR del/del variant had poorer adjusted memory scores than did those of the other genotypes (Tables 2 and 3). Moreover, in subjects in the high-folate category, approximately one of 5 individuals corresponding to those who carried the del/del genotype did not appear to benefit from high folate (Figure 2). Instead, these subjects had significantly worse memory. In other words, causality would predict that, in a population with a similar distribution of the genotype and folate, for every 4 individuals who stand to benefit from high folate, one individual might incur risk.
The functional cognitive effect of DHFR could help to explain the findings of adverse associations between high folate and poor cognitive outcomes in some studies. For example, in the Framingham cohort, the accelerated cognitive decline that was associated with the lower 2 quartiles of vitamin B-12 (<258 pmol/L) was significantly exacerbated by a higher folate status (>8.9 ng/mL), and in the Chicago Health and Aging Project, the rate of cognitive decline in individuals with folate intake in the top quintile of total intake (median: 742 μg/d) was more than double that of those in the lowest quintile of intake (median: 186 μg/d) even after adjustment for vitamin B-12 (9, 10). In our study, the association and interaction of folate and DHFR genotype with memory were independent of vitamin B-12. We were underpowered to examine a potential 3-way interaction with low vitamin B-12 status because relatively few subjects had sufficiently low vitamin B-12: Compared with the Framingham study, in which 40% of the population had vitamin B-12 concentrations <258 pmol/L, only 7.6% of the population in our study had concentrations below this value.
A gene-nutrient interaction between excessive FA with DHFR might also explain inconsistencies in the results of clinical trials that studied the efficacy of B-vitamin supplementation for lowering homocysteine and preventing cognitive decline, particularly when high doses of FA were used in the treatment arm (46). Note that, although many of the null trials used high doses of FA (1000–5000 μg/d) (47–49), trials that showed benefit used more-modest doses of FA (800 μg/d) (50, 51). If this association were causal, memory outcomes would tend to the null in populations with high FA intake and a high prevalence of the del/del genotype. The elucidation of the interplay of such genetic and nutritional heterogeneity is likely to prove more informative than would obscuring the complexity of the phenomena in meta-analyses (46, 52–54).
Notably, the nutritional and genetic associations in the current study differed with the cognitive domain. Although folate was specifically associated with memory, homocysteine was independently associated with executive function, and neither was associated with attention. These seemingly specific associations have been observed before (51, 55–58) and suggest that the possibility of different underlying mechanisms should be explored, and differences should be elucidated. These findings on the relation of homocysteine to cognition differed slightly from our previous analysis of this study population in which no significant association was shown between folate or tHcy status and cognitive outcomes. Several factors may explain these differences. Apart from analyzing the DHFR polymorphism rather than the MTHFR genotype, our previous analysis focused on low folate status, which is known to destabilize the MTHFR C677T variant. Consequently, we did not make a dichotomous comparison between normal folate status and high folate status. In the current study, adjustment for DHFR and high folate may have allowed the association of tHcy with executive function to reach significance. Moreover, the significant effect of tHcy could be explained by the fact that the current analysis was restricted to individuals genotyped for the DHFR 19-bp polymorphism with complete data.
Since 1996, the fortification of cereal grain products in the United States has led to significant increases in the intake of FA. As expected in a population exposed to FA fortification, the plasma folate concentration in our study was relatively high but with a median that was only 0.5 ng/mL higher than the National Health and Nutrition Examination Survey’s median for American adults >60 y of age measured at the time of our study. The prevalence of a high serum folate concentration (>20 ng/mL) in the US population rose in older persons from 7% before fortification to 38% after fortification and decreased to 31.8% during the period of our study (59). Although fortification has apparently reached its original goals (60), questions remain about its safety for untargeted subpopulations, especially cancer patients with malignant and premalignant tumors, and with respect to possible long-term effects of fetal exposure to high folate in utero (61–67). Some countries have revised and lowered their recommended daily intake FA (68). Despite concerns regarding possible negative effects of FA supplementation, an upper limit for plasma folate has yet to be determined. The WHO, e.g., set the upper limit at 20 ng/mL, relying on “the assay’s upper limit capabilities without dilutions, and not on biological implications for health” (69).
Evidence has suggested that humans have a limited capacity to metabolize high doses of FA efficiently (11, 70), which results in the appearance of unmetabolized FA in the circulation. Concern that this unmetabolized FA could have adverse effects on health has, thus far, only limited evidence (71–73). Our findings suggest that the appearance of FA in circulation may be determined in part by interactions between exposure and genetic variation. If FA intake alone cannot explain the variability in unmetabolized FA in the plasma, genetic variation that can influence the individual’s ability to metabolize the fortified FA should be examined. Although observational studies cannot establish cause and effect, our study suggests that the DHFR 19-bp polymorphism may interact with FA intake to entrain metabolic events in one-carbon metabolism than can alter cognition.
By virtue of its cross-sectional design, the study had several limitations. The observational findings did not indicate a cause and effect; findings were generated on a subset of the general population at a specific point in time; and although this was a multi-ethnic population, ethnic, genetic, and socioeconomic backgrounds could have influenced the results, despite our efforts to adjust the analysis for known confounders and for all relevant variables.
Future work to replicate these findings in prospective studies will need to account for ethnicity, nonlinear effects, cutoffs for high folate, and potentially specific associations with different cognitive domains and other outcomes. Studies that distinguish different folate forms in blood, such as unmetabolized FA, and possibly measure biopterin and nitrate could help elucidate a mechanism of action. Finally, additional research is needed to probe the functionality of the 19-bp polymorphism and what molecular mechanisms may allow it to interact with different metabolites to exert normal and pathological effects.
In conclusion, this study identifies a putative gene-nutrient interaction that, if confirmed, could help account for conflicting outcomes in observational and interventional studies of how folate affects cognition. The study underscores the need for a nuanced understanding of how genetic variation in the gene affects individual responses to folate status so that we can more-adequately weigh risks and benefits of folate supplementation for individuals and make necessary adjustments in public health policy.
Acknowledgments
The authors’ responsibilities were as follows—DP, AB, and DM: analyzed the data; DM: conducted the genotyping; TMS: oversaw the cognitive testing; AMT, IHR, KLT, JMO, C-QL, and LDP: conceived and designed the DHFR analysis; JS: oversaw the biochemical assays; IHR: was the principal investigator for the NAME study; KLT: was principal investigator for the BPRHS study; DP and AMT: wrote the manuscript with substantive input from all authors; and AMT: had primary responsibility for the final content of the manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: BPRHS, Boston Puerto Rican Health Study; CESD, Center for Epidemiologic Studies Depression Scale; DHFR, dihydrofolate reductase; eGFR, estimated glomerular filtration rate; FA, folic acid; GLM, general linear modeling; MMSE, Mini-Mental State Examination; MTHFR, methylenetetrahydrofolate reductase; NAME, Nutrition, Aging, and Memory in Elders; tHcy, plasma total homocysteine.
REFERENCES
- 1.Dimopoulos N, Piperi C, Salonicioti A, Psarra V, Flerri G, Nounopoulos C, Lea RW, Kalofoutis A. Association of cognitive impairment with plasma levels of folate, vitamin B12 and homocysteine in the elderly. In Vivo 2006;20:895–9. [PubMed] [Google Scholar]
- 2.Dimopoulos N, Piperi C, Salonicioti A, Psarra V, Gazi F, Papadimitriou A, Lea RW, Kalofoutis A. Correlation of folate, vitamin B12 and homocysteine plasma levels with depression in an elderly Greek population. Clin Biochem 2007;40:604–8. [DOI] [PubMed] [Google Scholar]
- 3.Kado DM, Karlamangla AS, Huang MH, Troen A, Rowe JW, Selhub J, Seeman TE. Homocysteine versus the vitamins folate, B6, and B12 as predictors of cognitive function and decline in older high-functioning adults: MacArthur Studies of Successful Aging. Am J Med 2005;118:161–7. [DOI] [PubMed] [Google Scholar]
- 4.Quadri P, Fragiacomo C, Pezzati R, Zanda E, Forloni G, Tettamanti M, Lucca U. Homocysteine, folate, and vitamin B-12 in mild cognitive impairment, Alzheimer disease, and vascular dementia. Am J Clin Nutr 2004;80:114–22. [DOI] [PubMed] [Google Scholar]
- 5.Ramos MI, Allen LH, Haan MN, Green R, Miller JW. Plasma folate concentrations are associated with depressive symptoms in elderly Latina women despite folic acid fortification. Am J Clin Nutr 2004;80:1024–8. [DOI] [PubMed] [Google Scholar]
- 6.Ramos MI, Allen LH, Mungas DM, Jagust WJ, Haan MN, Green R, Miller JW. Low folate status is associated with impaired cognitive function and dementia in the Sacramento Area Latino Study on Aging. Am J Clin Nutr 2005;82:1346–52. [DOI] [PubMed] [Google Scholar]
- 7.Selhub J, Troen A, Rosenberg IH. B vitamins and the aging brain. Nutr Rev 2010;68(Suppl 2):S112–8. [DOI] [PubMed] [Google Scholar]
- 8.Tucker KL, Qiao N, Scott T, Rosenberg I, Spiro A 3rd. High homocysteine and low B vitamins predict cognitive decline in aging men: the Veterans Affairs Normative Aging Study. Am J Clin Nutr 2005;82:627–35. [DOI] [PubMed] [Google Scholar]
- 9.Morris MS, Selhub J, Jacques PF. Vitamin B-12 and folate status in relation to decline in scores on the mini-mental state examination in the framingham heart study. J Am Geriatr Soc 2012;60:1457–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris MC, Evans DA, Bienias JL, Tangney CC, Hebert LE, Scherr PA, Schneider JA. Dietary folate and vitamin B12 intake and cognitive decline among community-dwelling older persons. Arch Neurol 2005;62:641–5. [DOI] [PubMed] [Google Scholar]
- 11.Bailey SW, Ayling JE. The extremely slow and variable activity of dihydrofolate reductase in human liver and its implications for high folic acid intake. Proc Natl Acad Sci USA 2009;106:15424–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banka S, Blom HJ, Walter J, Aziz M, Urquhart J, Clouthier CM, Rice GI, de Brouwer AP, Hilton E, Vassallo G, et al. . Identification and characterization of an inborn error of metabolism caused by dihydrofolate reductase deficiency. Am J Hum Genet 2011;88:216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cario H, Smith DE, Blom H, Blau N, Bode H, Holzmann K, Pannicke U, Hopfner KP, Rump EM, Ayric Z, et al. . Dihydrofolate reductase deficiency due to a homozygous DHFR mutation causes megaloblastic anemia and cerebral folate deficiency leading to severe neurologic disease. Am J Hum Genet 2011;88:226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson WG, Scholl TO, Spychala JR, Buyske S, Stenroos ES, Chen X. Common dihydrofolate reductase 19–base pair deletion allele: a novel risk factor for preterm delivery. Am J Clin Nutr 2005;81:664–8. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi H, Horino M, Morishita M, Tazoe Y, Tsuboi S, Matsuyama T, Kosuge K, Yamada H, Tsuji D, Inoue K, Itoh K. Dihydrofolate reductase gene intronic 19-bp deletion polymorphisms in a Japanese population. Drug Metab Pharmacokinet 2010;25:516–8. [DOI] [PubMed] [Google Scholar]
- 16.Eroglu A, Egin Y, Cam R, Akar N. The 19-bp deletion of dihydrofolate reductase (DHFR), methylenetetrahydrofolate reductase (MTHFR) C677T, Factor V Leiden, prothrombin G20210A polymorphisms in cancer patients with and without thrombosis. Ann Hematol 2009;88:73–6. [DOI] [PubMed] [Google Scholar]
- 17.Gemmati D, De Mattei M, Catozzi L, Della Porta M, Serino ML, Ambrosio C, Cuneo A, Friso S, Krampera M, Orioli E, et al. . DHFR 19-bp insertion/deletion polymorphism and MTHFR C677T in adult acute lymphoblastic leukaemia: is the risk reduction due to intracellular folate unbalancing? Am J Hematol 2009;84:526–9. [DOI] [PubMed] [Google Scholar]
- 18.Parle-McDermott A, Pangilinan F, Mills JL, Kirke PN, Gibney ER, Troendle J, O’Leary VB, Molloy AM, Conley M, Scott JM, Brody LC. The 19-bp deletion polymorphism in intron-1 of dihydrofolate reductase (DHFR) may decrease rather than increase risk for spina bifida in the Irish population. Am J Med Genet A 2007;143A:1174–80. [DOI] [PubMed] [Google Scholar]
- 19.van der Linden IJ, Nguyen U, Heil SG, Franke B, Vloet S, Gellekink H, den Heijer M, Blom HJ. Variation and expression of dihydrofolate reductase (DHFR) in relation to spina bifida. Mol Genet Metab 2007;91:98–103. [DOI] [PubMed] [Google Scholar]
- 20.Kalmbach RD, Choumenkovitch SF, Troen AP, Jacques PF, D’Agostino R, Selhub J. A 19-base pair deletion polymorphism in dihydrofolate reductase is associated with increased unmetabolized folic acid in plasma and decreased red blood cell folate. J Nutr 2008;138:2323–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stanisławska-Sachadyn A, Brown KS, Mitchell LE, Woodside JV, Young IS, Scott JM, Murray L, Boreham CA, McNulty H, Strain JJ, Whitehead AS. An insertion/deletion polymorphism of the dihydrofolate reductase (DHFR) gene is associated with serum and red blood cell folate concentrations in women. Hum Genet 2008;123:289–95. [DOI] [PubMed] [Google Scholar]
- 22.Xu X, Gammon MD, Wetmur JG, Rao M, Gaudet MM, Teitelbaum SL, Britton JA, Neugut AI, Santella RM, Chen J. A functional 19-base pair deletion polymorphism of dihydrofolate reductase (DHFR) and risk of breast cancer in multivitamin users. Am J Clin Nutr 2007;85:1098–102. [DOI] [PubMed] [Google Scholar]
- 23.Cabo R, Hernes S, Slettan A, Haugen M, Ye S, Blomhoff R, Mansoor MA. Effects of polymorphisms in endothelial nitric oxide synthase and folate metabolizing genes on the concentration of serum nitrate, folate, and plasma total homocysteine after folic acid supplementation: a double-blind crossover study. Nutrition 2015;31:337–44. [DOI] [PubMed] [Google Scholar]
- 24.Crabtree MJ, Tatham AL, Hale AB, Alp NJ, Channon KM. Critical role for tetrahydrobiopterin recycling by dihydrofolate reductase in regulation of endothelial nitric-oxide synthase coupling: relative importance of the de novo biopterin synthesis versus salvage pathways. J Biol Chem 2009;284:28128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitsett J, Rangel Filho A, Sethumadhavan S, Celinska J, Widlansky M, Vasquez-Vivar J. Human endothelial dihydrofolate reductase low activity limits vascular tetrahydrobiopterin recycling. Free Radic Biol Med 2013;63:143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tucker KL, Mattei J, Noel SE, Collado BM, Mendez J, Nelson J, Griffith J, Ordovas JM, Falcon LM. The Boston Puerto Rican Health Study, a longitudinal cohort study on health disparities in Puerto Rican adults: challenges and opportunities. BMC Public Health 2010;10:107. [DOI] [PMC free article] [PubMed]
- 27.Moorthy D, Peter I, Scott TM, Parnell LD, Lai C, Crott JW, Ordova JM, Selhub J, Griffith J, Rosenberg IH, Tucker KL, et al. . Status of vitamins B-12 and B-6 but not of folate, homocysteine, and the methylenetetrahydrofolate reductase C677T polymorphism are associated with impaired cognition and depression in adults. J Nutr 2012;142:1554–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott TM, Peter I, Tucker KL, Arsenault L, Bergethon P, Bhadelia R, Buell J, Collins L, Dashe JF, Griffith J, et al. The Nutrition, Aging, and Memory in Elders (NAME) Study: design and methods for a study of micronutrients and cognitive function in a homebound elderly population. Int J Geriatr Psychiatry 2006;21:519–28. [DOI] [PubMed]
- 29.Sun X, Chiu CC, Liebson E, Crivello NA, Wang L, Claunch J, Folstein M, Rosenberg I, Mwamburi DM, Peter I, et al. . Depression and plasma amyloid beta peptides in the elderly with and without the apolipoprotein E4 allele. Alzheimer Dis Assoc Disord 2009;23:238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Folstein MF, Robins LN, Helzer JE. The Mini-Mental State Examination. Arch Gen Psychiatry 1983;40:812. [DOI] [PubMed] [Google Scholar]
- 31.Scott TM, Peter I, Tucker KL, Arsenault L, Bergethon P, Bhadelia R, Buell J, Collins L, Dashe JF, Griffith J, et al. . The Nutrition, Aging, and Memory in Elders (NAME) study: design and methods for a study of micronutrients and cognitive function in a homebound elderly population. Int J Geriatr Psychiatry 2006;21:519–28. [DOI] [PubMed] [Google Scholar]
- 32.Wechsler D. Manual for the Wechsler Memory Scale. San Antonio (TX): Psychological Corporation/Harcourt Brace and Company; 1997. [Google Scholar]
- 33.Wechsler D. Wechsler Adult Intelligence Scale. San Antonio (TX): The Psychological Corporation/Harcourt Brace and Company; 1997. [Google Scholar]
- 34.Heaton RK, Grant I. Comprehensive norms for an expanded Halstead-Reitan battery. Odessa (FL_: Psychological Assessment Resources, Inc.; 1991. [Google Scholar]
- 35.Benton AL, Amsher KD. Multilingual aphasia examination. Iowa City (IA): AJA Associates; 1989. [Google Scholar]
- 36.Blair JR, Spreen O. Predicting premorbid IQ: a revision of the national adult reading test. Clin Neuropsychol 1989;3:129–36. [Google Scholar]
- 37.Artiola I, Fortuna L, Hermosillo Romo D, Heaton RK, Pardee III RE. Manual of Standards and Procedures for the Neuropsychological Battery in Spanish. Manual de normas y procedimientos para la bateria neuropsicologíca en Español. Tucson (AZ): M Press; 2000. (in Spanish). [Google Scholar]
- 38.Wolf-Klein GP, Silverstone FA, Levy AP, Brod MS. Screening for Alzheimer’s disease by clock drawing. J Am Geriatr Soc 1989;37:730–4. [DOI] [PubMed] [Google Scholar]
- 39.Beery KE. Adapted from the VMI: developmental test of visual-motor integration. 3rd rev. Cleveland (OH): Modern Curriculum Press; 1989. [Google Scholar]
- 40.Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van Lente F, Chronic Kidney Disease Epidemiology Collaboration.. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 2007;53:766–72. [DOI] [PubMed] [Google Scholar]
- 41.Lai CQ, Tucker KL, Choudhry S, Parnell LD, Mattei J, Garcia-Bailo B, Beckman K, Burchard EG, Ordovas JM. Population admixture associated with disease prevalence in the Boston Puerto Rican Health Study. Hum Genet 2009;125:199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Araki A, Sako Y. Determination of free and total homocysteine in human plasma by high-performance liquid chromatography with fluorescence detection. J Chromatogr 1987;422:43–52. [DOI] [PubMed] [Google Scholar]
- 43.Soldin SJ, Wan BS, Cherian AG. The measurement of creatinine: a comparison between the Beckman Creatinine Analyzer II and the Selective Analyzer GSA IID. Clin Biochem 1981;14:165–8. [DOI] [PubMed] [Google Scholar]
- 44.Shin YS, Rasshofer R, Friedrich B, Endres W. Pyridoxal-5′-phosphate determination by a sensitive micromethod in human blood, urine and tissues; its relation to cystathioninuria in neuroblastoma and biliary atresia. Clin Chim Acta 1983;127:77–85. [DOI] [PubMed] [Google Scholar]
- 45.Selhub J, Rosenberg IH. Assessment of vitamin depletion. In: Wright RA, Heymsfeld S, editors. Nutritional assessment. Boston (MA): Blackwell Scientific Publications Inc.; 1984. p. 209–14.
- 46.Nachum-Biala Y, Troen AM. B-vitamins for neuroprotection: narrowing the evidence gap. Biofactors 2012;38:145–50. [DOI] [PubMed] [Google Scholar]
- 47.Aisen PS, Schneider LS, Sano M, Diaz-Arrastia R, van Dyck CH, Weiner MF, Bottiglieri T, Jin S, Stokes KT, Thomas RG, et al. . High-dose B vitamin supplementation and cognitive decline in Alzheimer disease: a randomized controlled trial. JAMA 2008;300:1774–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brady CB, Gaziano JM, Cxypoliski RA, Guarino PD, Kaufman JS, Warren SR, Hartigan P, Goldfarb DS, Jamison RL. Homocysteine lowering and cognition in CKD: the Veterans Affairs homocysteine study. Am J Kidney Dis 2009;54:440–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McMahon JA, Green TJ, Skeaff CM, Knight RG, Mann JI, Williams SM. A controlled trial of homocysteine lowering and cognitive performance. N Engl J Med 2006;354:2764–72. [DOI] [PubMed] [Google Scholar]
- 50.de Jager CA, Oulhaj A, Jacoby R, Refsum H, Smith AD. Cognitive and clinical outcomes of homocysteine-lowering B-vitamin treatment in mild cognitive impairment: a randomized controlled trial. Int J Geriatr Psychiatry 2012;27:592–600. [DOI] [PubMed] [Google Scholar]
- 51.Durga J, van Boxtel MP, Schouten EG, Kok FJ, Jolles J, Katan MB, Verhoef P. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: a randomised, double blind, controlled trial. Lancet 2007;369:208–16. [DOI] [PubMed]
- 52.Clarke R, Bennett, Parish S, Lewington S, Skeaff M, Eussen SJ, Lewerin C, Stott DJ, Armitage J, Hankey GJ, Lonn E, et al. . Effects of homocysteine lowering with B vitamins on cognitive aging: meta-analysis of 11 trials with cognitive data on 22,000 individuals. Am J Clin Nutr 2014;100:657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garrard P, Jacoby R. B-vitamin trials meta-analysis: less than meets the eye. Am J Clin Nutr 2015;101:414–5. [DOI] [PubMed] [Google Scholar]
- 54.Smith AD, de Jager CA, Refsum H, Rosenberg IH. Homocysteine lowering, B vitamins, and cognitive aging. Am J Clin Nutr 2015;101:415–6. [DOI] [PubMed] [Google Scholar]
- 55.Bell IR, Edman JS, Miller J, Hebben N, Linn RT, Ray D, Kayne HL. Relationship of normal serum vitamin B12 and folate levels to cognitive test performance in subtypes of geriatric major depression. J Geriatr Psychiatry Neurol 1990;3:98–105. [DOI] [PubMed] [Google Scholar]
- 56.Miller JW, Green R, Ramos MI, Allen LH, Mungas DM, Jagust WJ, Haan MN. Homocysteine and cognitive function in the Sacramento Area Latino Study on Aging. Am J Clin Nutr 2003;78:441–7. [DOI] [PubMed] [Google Scholar]
- 57.Riggs KM, Spiro A 3rd, Tucker K, Rush D. Relations of vitamin B-12, vitamin B-6, folate, and homocysteine to cognitive performance in the Normative Aging Study. Am J Clin Nutr 1996;63:306–14. [DOI] [PubMed] [Google Scholar]
- 58.Scott TM, Tucker KL, Bhadelia A, Benjamin B, Patz S, Bhadelia R, Liebson E, Price LL, Griffith J, Rosenberg I, et al. . Homocysteine and B vitamins relate to brain volume and white-matter changes in geriatric patients with psychiatric disorders. Am J Geriatr Psychiatry 2004;12:631–8. [DOI] [PubMed] [Google Scholar]
- 59.Pfeiffer CM, Johnson CL, Jain RB, Yetley EA, Picciano MF, Rader JI, Fisher KD, Mulinare J, Osterloh JD. Trends in blood folate and vitamin B-12 concentrations in the United States, 1988–2004. Am J Clin Nutr 2007;86:718–27. [DOI] [PubMed] [Google Scholar]
- 60.Berry RJ, Bailey L, Mulinare J, Bower C, Folic Acid Working G. Fortification of flour with folic acid. Food Nutr Bull 2010;31(1 Suppl):S22–35. [DOI] [PubMed] [Google Scholar]
- 61.Bailey RLMJ, Yetley EA, Gahche JJ, Pfeiffer CM, Dwyer JT, Dodd KW, Sempos CT, Betz JM, Picciano MF. Serum unmetabolized folic acid in a nationally representative sample of adults ≥60 years in the United States, 2001-2002. Food Nutr Res 2012;56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barua SKS, Junaid MA. Folic acid supplementation in pregnancy and implications in health and disease. J Biomed Sci 2014;21:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Crider KS, Bailey LB, Berry RJ. Folic acid food fortification—its history, effect, concerns, and future directions. Nutrients 2011;3:370–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gylling B, Van Guelpen B, Schneede J, Hultdin J, Ueland PM, Hallmans G, Johansson I, Palmqvist R. Low folate levels are associated with reduced risk of colorectal cancer in a population with low folate status. Cancer Epidemiol Biomarkers Prev 2014;23:2136–44. [DOI] [PubMed] [Google Scholar]
- 65.Kim YI. Will mandatory folic acid fortification prevent or promote cancer? Am J Clin Nutr 2004;80:1123–8. [DOI] [PubMed] [Google Scholar]
- 66.Mason JB, Dickstein A, Jacques PF, Haggarty P, Selhub J, Dallal G, Rosenberg IH. A temporal association between folic acid fortification and an increase in colorectal cancer rates may be illuminating important biological principles: a hypothesis. Cancer Epidemiol Biomarkers Prev 2007;16:1325–9. [DOI] [PubMed] [Google Scholar]
- 67.Choi JH, Yates Z, Veysey M, Heo YR, Lucock M. Contemporary issues surrounding folic Acid fortification initiatives. Prev Nutr Food Sci 2014;19:247–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krawinkel M, Strohm D, Weissenborn A, Watzl B, Eichholzer M, Bärlocher K, Elmadfa I, Leschik-Bonnet E, Heseker H. Revised D-A-CH intake recommendations for folate: how much is needed? Eur J Clin Nutr 2014;68:719–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. WHO/NMH/NHD/EPG/12.1. Serum and red blood cell folate concentrations for assessing folate status in populations. 2012.
- 70.Patanwala I, King MJ, Barrett DA, Rose J, Jackson R, Hudson M, Philo M, Dainty JR, Wright AJ, Finglas PM, Jones DE. Folic acid handling by the human gut: implications for food fortification and supplementation. Am J Clin Nutr 2014;100:593–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morris MS, Jacques PF, Rosenberg IH, Selhub J. Circulating unmetabolized folic acid and 5-methyltetrahydrofolate in relation to anemia, macrocytosis, and cognitive test performance in American seniors. Am J Clin Nutr 2010;91:1733–44. [DOI] [PubMed] [Google Scholar]
- 72.Obeid R, Herrmann W. The emerging role of unmetabolized folic acid in human diseases: myth or reality? Curr Drug Metab 2012;13:1184–95. [DOI] [PubMed] [Google Scholar]
- 73.Troen AM, Mitchell B, Sorensen B, Wener MH, Johnston A, Wood B, Selhub J, McTiernan A, Yasui Y, Oral E, Potter JD, et al. . Unmetabolized folic acid in plasma is associated with reduced natural killer cell cytotoxicity among postmenopausal women. J Nutr 2006;136:189–94. [DOI] [PubMed] [Google Scholar]