Abstract
Background: Individual studies have suggested that circulating carotenoids, retinol, or tocopherols may be associated with prostate cancer risk, but the studies have not been large enough to provide precise estimates of associations, particularly by stage and grade of disease.
Objective: The objective of this study was to conduct a pooled analysis of the associations of the concentrations of 7 carotenoids, retinol, α-tocopherol, and γ-tocopherol with risk of prostate cancer and to describe whether any associations differ by stage or grade of the disease or other factors.
Design: Principal investigators of prospective studies provided individual participant data for prostate cancer cases and controls. Risk by study-specific fifths of each biomarker was estimated by using multivariable-adjusted conditional logistic regression in matched case-control sets.
Results: Data were available for up to 11,239 cases (including 1654 advanced stage and 1741 aggressive) and 18,541 controls from 15 studies. Lycopene was not associated with overall risk of prostate cancer, but there was statistically significant heterogeneity by stage of disease, and the OR for aggressive disease for the highest compared with the lowest fifth of lycopene was 0.65 (95% CI: 0.46, 0.91; P-trend = 0.032). No other carotenoid was significantly associated with overall risk of prostate cancer or with risk of advanced-stage or aggressive disease. For retinol, the OR for the highest compared with the lowest fifth was 1.13 (95% CI: 1.04, 1.22; P-trend = 0.015). For α-tocopherol, the OR for the highest compared with the lowest fifth was 0.86 (95% CI: 0.78, 0.94; P-trend < 0.001), with significant heterogeneity by stage of disease; the OR for aggressive prostate cancer was 0.74 (95% CI: 0.59, 0.92; P-trend = 0.001). γ-Tocopherol was not associated with risk.
Conclusions: Overall prostate cancer risk was positively associated with retinol and inversely associated with α-tocopherol, and risk of aggressive prostate cancer was inversely associated with lycopene and α-tocopherol. Whether these associations reflect causal relations is unclear.
Keywords: prostate cancer, carotenoids, retinol, tocopherols, vitamin E, vitamin A, pooled analysis, nested case-control study, biomarkers
INTRODUCTION
Previous studies have suggested that dietary and plasma carotenoids, retinol, and tocopherols might affect the development of prostate cancer, but overall this research has been inconclusive (1). The carotenoids that have been studied are α- and β-carotene, lycopene, lutein, zeaxanthin, β-cryptoxanthin, and canthaxanthin, together with retinol. Three of these carotenoids can be converted in the body into retinol, whereas others cannot but may have biological effects independent of vitamin A activity (2). α-Carotene, β-carotene, and β-cryptoxanthin can be converted into retinol; major sources are carrots, pumpkin, yellow and orange fruits and vegetables for α-carotene and β-carotene, and citrus fruits, especially oranges, for β-cryptoxanthin (3). Lycopene cannot be converted into retinol, and the major sources of lycopene in most diets are tomatoes and tomato products (3). Lutein and its stereoisomer, zeaxanthin, are not converted into retinol; major food sources are green vegetables and, to a lesser extent, eggs, because lutein is added to chicken feed to enhance the yellow color of egg yolks (3, 4). Canthaxanthin occurs naturally in some plants and mushrooms, but much of the canthaxanthin in diets is derived from its use as a feed additive to color foods such as eggs and farmed fish (5). Rich sources of retinol include fatty fish, dairy products, liver, cod liver oil, and vitamin supplements.
The tocopherols α- and γ-tocopherol have also been studied in relation to prostate cancer risk, and both have vitamin E activity. α-Tocopherol occurs in a wide range of foods, especially nuts, seeds, and vegetable oils such as sunflower seed oil, whereas the major sources of γ-tocopherol are specific vegetable oils such as soya and corn (maize) oil; α-tocopherol is also widely included in dietary supplements. α-Tocopherol is usually the predominant tocopherol in European diets, whereas γ-tocopherol is more predominant in the United States because of the greater use of soya and corn oils, but α-tocopherol is generally present in the plasma at higher concentrations than γ-tocopherol (6).
The Endogenous Hormones, Nutritional Biomarkers and Prostate Cancer Collaborative Group was established with the aim of pooling individual data from prospective studies of the associations of circulating concentrations of hormones and nutritional biomarkers with risk of prostate cancer (7–9). The objective of the current analysis was to examine the associations of the concentrations of 7 carotenoids, retinol, α-tocopherol, and γ-tocopherol with risk of prostate cancer and to describe whether any associations differ by stage or grade of the disease or other factors. Since the advent of prostate-specific antigen (PSA)44 testing for early detection, many men are diagnosed with very small prostate cancers, most of which progress slowly (10); therefore, the associations of biomarkers with aggressive prostate cancer were of particular interest.
We had several a priori hypotheses: that lycopene would be associated with a reduced risk of advanced-stage and aggressive prostate cancer (10), that retinol would be associated with an increased risk of prostate cancer (11), and that α-tocopherol would be associated with a reduced risk of prostate cancer in current smokers but not in nonsmokers (12). These 3 biomarkers are therefore discussed more fully than the other biomarkers.
METHODS
Identification of studies
The objective was to conduct a pooled analysis of individual participant data. Studies were eligible to join this collaborative group if they had data on circulating carotenoids, retinol, or tocopherols measured in blood samples collected before the diagnosis of prostate cancer for at least 75 cases (for at least one biomarker under investigation by the collaborative group). Studies were identified through searches using the terms carotenoid, retinol, vitamin A, tocopherol, vitamin E, and prostate cancer on computerized bibliographic systems, including PubMed, Web of Science, Cochrane Library, and CancerLit; through the reference lists of publications identified in this search; and through discussions with colleagues.
Collection of data
Individual participant data on circulating carotenoids, retinol, or tocopherols were available from 15 studies: the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study (ATBC) (11, 13, 14); the β-Carotene and Retinol Efficacy Trial (15); the CLUE I and CLUE II studies (named after the campaign slogan “Give us a Clue to Cancer”) (16, 17); the European Prospective Investigation into Cancer and Nutrition study (18); the Finnish Mobile Clinic Health Examination Survey (19); the Health Professionals Follow-Up Study (20); the Janus biobanks (21); the Melbourne Collaborative Cohort Study (22, 23); the Multiethnic Cohort (MEC) (24); the Prostate Cancer Prevention Trial (PCPT) (25); the Physicians’ Health Study (26); the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO) (27–29); the Prostate testing for cancer and Treatment (ProtecT) trial (30); and the SUpplémentation en VItamines et Minéraux Anti-oXydants trial (SU.VI.MAX) (31). Descriptions of the study designs are in Supplemental Table 1, and details of the assay methods are in Supplemental Table 2. Further details of the recruitment of participants, informed consent, ethical approval, and inclusion criteria are in the original publications (11–31); the procedures followed in the collaborating studies were in accordance with the ethical standards of the institution or regional committee on human experimentation, and approval was obtained from the relevant committees on human subjects. Most of the studies are traditional cohort studies in which blood samples were collected at recruitment and participants were followed for diagnosis of prostate cancer by their doctor; in these studies, there was no organized screening for prostate cancer, but PSA testing has been widely used since around 1990, with high frequency of use in some populations. Three studies (PCPT, PLCO, and ProtecT) were observational investigations based on trials that included organized screening for prostate cancer, and these 3 studies have unique design features. In PCPT, men were eligible to enter the trial if they had a normal digital rectal examination and had PSA ≤3 ng/mL; they then had annual digital rectal examinations and PSA determinations, and if not diagnosed with prostate cancer, they were asked to undergo a prostate biopsy at the end of the trial in year 7. In PLCO, men were eligible to enter the trial if they had a normal digital rectal examination and had PSA <4 ng/mL; they then had a digital rectal examination annually for 3 y and PSA screening annually for 5 y. ProtecT is a trial of different treatments for prostate cancer, in which men were screened with PSA, and those with PSA ≥3 ng/mL were offered diagnostic biopsy; men diagnosed at this time were included as cases for the observational study of biomarkers and prostate cancer. The data from ProtecT are reported here because on average the blood was collected several years before the cancer would have been diagnosed in an unscreened population (32), although the study could be considered cross-sectional rather than prospective. The number of cases varied between biomarkers; the largest numbers were for retinol, for which there were data for 11,239 cases and 18,541 controls; the smallest numbers were for canthaxanthin, for which there were data for 1737 cases and 2056 controls.
Data were not available for 5 of the studies identified: data could not be retrieved for the Japanese American study in Hawaii (with 142 cases) (33), investigators from the NHANES study in the United States (with 84 cases) (34) and the Wittenoom study in Western Australia (with 96 cases) (35) declined the invitation to collaborate, and the Kuopio Ischemic Heart Disease Risk Factor Study in Finland (with 68 cases) (36, 37) and the Basel study in Switzerland (with 30 cases) (38) were not invited because they had <75 eligible cases for any of the biomarkers under investigation by the collaborative group (including other nutritional biomarkers and hormones; this cutoff was agreed on by the collaborative group as a pragmatic criterion to include all studies with a reasonable sample size).
Where available, collaborators provided data for prostate cancer cases and controls on dates of birth and blood collection, time of blood collection, fasting status, marital status, ethnicity, educational attainment, family history of prostate cancer, height, weight, smoking status, alcohol intake, circulating total cholesterol, and circulating total PSA concentration at recruitment and diagnosis. Information on stage and grade for prostate cancer cases was also provided. To provide a common definition across studies, we defined a cancer as being localized if it was tumor/node/metastasis (TNM) stage ≤T2 with no reported lymph node involvement or metastases, stage ≤II, or the equivalent (i.e., a tumor that does not extend beyond the prostate capsule); advanced if it was TNM stage T3 or T4 and/or N1+ and/or M1, stage III–IV, or the equivalent (i.e., a tumor extending beyond the prostate capsule and/or lymph node involvement and/or distant metastases); or stage unknown. Aggressive disease was categorized as “no” for TNM stage ≤T3 with no reported lymph node involvement or metastases or the equivalent, “yes” for TNM stage T4 and/or N1+ and/or M1 and/or stage IV disease or death from prostate cancer, or unknown. Prostate cancer was defined as low grade if the Gleason sum was <8 or the equivalent (extent of differentiation good, moderate, or poor), high grade if the Gleason sum was at least 8 or the equivalent (undifferentiated), or grade unknown.
Statistical analysis
The methods of analysis were similar to those described previously by this collaborative group (7–9). All except 2 of the studies (ATBC and SU.VI.MAX) had used a matched case-control study design nested within either a prospective study or a randomized controlled trial, with matching on age at recruitment, date of blood collection (or follow-up time), and, where appropriate, other specific matching criteria (Supplemental Table 1); the original matching was retained in all analyses. Two studies (ATBC and SU.VI.MAX) had used a full cohort design, and for this collaborative analysis matched case-control sets were created for these studies by randomly matching up to 4 control subjects to each case patient by age at recruitment (within 1 y) and date of recruitment (within 6 mo). For the main analyses, men were categorized into fifths of concentration for each biomarker, with cutoffs defined by the study-specific quintiles of the distribution in control participants; in supplementary analyses, men were categorized into fifths with overall cutoffs set across all studies. The main method of analysis was logistic regression conditioned on the matching variables within each study. To provide a summary measure of the OR for subgroup analyses and to calculate the P value for trend, we replaced the categorical variable representing the fifths of the circulating biomarker with a continuous variable that was scored as 0, 0.25, 0.5, 0.75, or 1; because the midpoints of the lowest and highest fifths are the 10th and 90th percentiles of the study-specific biomarker concentrations, a unit increase in this variable can be taken to represent an 80 percentile increase in the study-specific biomarker. Age at blood collection (exact), BMI (in kg/m2; <25, 25–27.4, 27.5–29.9, ≥30, or not known), height (≤170, 171–175, 176–180, or >180 cm or not known), marital status (married or cohabiting, not married or cohabiting, or not known), educational status (did not graduate from high school/secondary school/college, high school/secondary school/college graduates, university graduates, or not known), and cigarette smoking (never smoker, past smoker, current, or not known) have been associated with prostate cancer risk in previous large studies (39–41) and were included in the conditional logistic regression models. For α-tocopherol, which is strongly correlated with total cholesterol, we also calculated the OR with further adjustment for total cholesterol (for the studies in which total cholesterol had been measured); we also examined the joint associations of α-tocopherol and γ-tocopherol with risk, with both classified by thirds of the distribution in controls. For each biomarker, heterogeneity in linear trends between studies was tested by comparing the χ2 values for models with and without a (study) × (linear trend) interaction term; we also examined whether there was heterogeneity by study design, comparing the prospective studies with the screening-based ProtecT trial. To test whether the linear-trend OR estimates for each biomarker varied according to certain case participant characteristics, we estimated ORs within subgroups for the following characteristics: age at diagnosis (<60, 60–69, or ≥70 y), years from blood collection to diagnosis (<3, 3–6, or ≥7 y), year of diagnosis (pre-1990, 1990–1994, 1995–1999, or 2000 onward), stage of disease (localized or advanced), aggressive disease (no or yes), and grade of disease (low or high). Controls in each matched set were assigned the value of their matched case for the case-defined factors (e.g., stage of disease); for multicase matched sets in which these case characteristics varied (e.g., some localized, some advanced stage), controls were randomly allocated to cases in the same proportions. Subgroup analyses were also conducted stratified according to the following participant characteristics: age at blood draw (<60 or ≥60 y), PSA at blood draw (<2 or ≥2 ng/mL), university or higher education (no or yes), BMI (<25 or ≥25), cigarette smoking (never, past, or current smoker), usual alcohol consumption (<10 or ≥10 g/d), and family history of prostate cancer (in father or brother: no or yes). These subgroups were defined a priori, as in previous publications from this collaborative group (7–9). Tests for heterogeneity for the case-defined factors were obtained by fitting separate models for each subgroup and assuming independence of the ORs by using a method analogous to a meta-analysis. Tests for heterogeneity for the non-case-defined factors were assessed with a χ2 test of interaction between subgroup and the continuous trend test variable. For aggressive disease, we also conducted further analyses restricted to these cases and their matched controls, calculating ORs in fifths of the distribution defined by the study-specific quintiles of the distribution in controls. The ORs shown in the figures are presented as squares with horizontal lines corresponding to the 95% CIs. The position of the square indicates the value of the OR, whereas the size of the square is inversely proportional to the variance of the logarithm of the OR and indicates the amount of statistical information available for that particular estimate. The open diamonds (the lateral points of which are the 95% CIs) represent the overall OR for an 80th percentile increase in the individual biomarkers.
The associations of the biomarkers with participant characteristics for control men were examined by using ANOVA to calculate geometric mean concentrations in categories of each characteristic, adjusted for study and age; geometric means were used because the log-transformed variables had distributions closer to normal than the untransformed variables.
All statistical analyses were carried out by using Stata 12 (StataCorp LP). All P values reported are 2-tailed, and P < 0.05 was considered statistically significant.
RESULTS
Baseline characteristics of prostate cancer cases and controls in each study are shown in Table 1. Mean age at blood collection varied from 47.7 y for cases and controls in Janus to 68.7 y for cases in MEC.
TABLE 1.
Participant characteristics by study and case-control status1
| Study/case-control status | Participants, n | Age at recruitment, y | Height, cm | BMI, kg/m2 | Married or cohabiting, % | Higher education, % | Current smokers, % | Alcohol intake, g/d |
| ATBC (11, 13, 14) | ||||||||
| Case | 2165 | 57.5 ± 5.12 | 174 ± 6 | 26.3 ± 3.6 | 83.1 | 5.4 | 100.0 | 16.8 ± 21.5 |
| Control | 6491 | 57.5 ± 5.1 | 174 ± 6 | 26.2 ± 3.6 | 83.2 | 4.7 | 100.0 | 16.7 ± 20.3 |
| CARET (15) | ||||||||
| Case | 693 | 60.1 ± 5.7 | 176 ± 7 | 28.3 ± 4.3 | 81.6 | 27.7 | 51.4 | 17.3 ± 24.9 |
| Control | 1387 | 59.8 ± 5.8 | 175 ± 7 | 28.1 ± 4.4 | 81.7 | 23.7 | 52.3 | 15.7 ± 26.1 |
| CLUE I (16) | ||||||||
| Case | 245 | 56.6 ± 10.2 | NA | NA | 90.6 | 13.9 | 26.1 | NA |
| Control | 427 | 55.5 ± 10.0 | NA | NA | 86.9 | 13.1 | 24.1 | NA |
| CLUE II (17) | ||||||||
| Case | 142 | 65.7 ± 8.0 | 176 ± 7 | 26.5 ± 3.8 | 85.9 | 16.9 | 9.9 | 6.4 ± 12.5 |
| Control | 284 | 65.6 ± 8.0 | 175 ± 6 | 26.7 ± 3.3 | 87.3 | 14.4 | 10.9 | 5.7 ± 12.6 |
| EPIC (18) | ||||||||
| Case | 1027 | 60.4 ± 6.0 | 173 ± 7 | 26.6 ± 3.3 | 89.2 | 26.1 | 23.5 | 21.2 ± 24.5 |
| Control | 1189 | 60.5 ± 6.2 | 173 ± 7 | 26.8 ± 3.6 | 89.6 | 20.9 | 27.3 | 20.4 ± 23.7 |
| FMC (19) | ||||||||
| Case | 37 | 64.7 ± 7.2 | 170 ± 6 | 25.9 ± 2.9 | 89.2 | NA | 36.1 | NA |
| Control | 66 | 65.0 ± 7.1 | 169 ± 6 | 25.3 ± 3.5 | 84.8 | NA | 25.0 | NA |
| HPFS (20) | ||||||||
| Case | 696 | 65.3 ± 7.4 | 177 ± 7 | 26.1 ± 3.4 | 93.5 | 100.0 | 5.0 | 11.4 ± 14.6 |
| Control | 696 | 65.2 ± 7.4 | 178 ± 6 | 26.1 ± 3.5 | 93.1 | 100.0 | 4.0 | 11.0 ± 14.9 |
| Janus (21) | ||||||||
| Case | 2106 | 47.7 ± 9.2 | 177 ± 7 | 25.5 ± 3.0 | NA | NA | 32.8 | NA |
| Control | 2106 | 47.7 ± 9.2 | 177 ± 7 | 25.6 ± 3.0 | NA | NA | 34.5 | NA |
| MCCS (22, 23) | ||||||||
| Case | 333 | 60.4 ± 6.7 | 172 ± 7 | 27.0 ± 3.5 | 77.8 | 25.8 | 7.8 | 19.4 ± 24.3 |
| Control | 635 | 57.9 ± 7.4 | 173 ± 7 | 27.1 ± 3.5 | 82.0 | 22.8 | 13.2 | 21.6 ± 26.4 |
| MEC (24) | ||||||||
| Case | 382 | 68.7 ± 7.0 | 174 ± 7 | 26.6 ± 4.1 | 77.9 | 33.3 | 14.0 | 22.7 ± 42.6 |
| Control | 761 | 68.6 ± 7.1 | 174 ± 8 | 26.9 ± 4.1 | 78.7 | 31.6 | 12.1 | 21.7 ± 38.0 |
| PCPT (25) | ||||||||
| Case | 963 | 63.4 ± 5.5 | 178 ± 7 | 27.4 ± 4.1 | 87.8 | 39.2 | 6.6 | 9.9 ± 16.3 |
| Control | 963 | 63.3 ± 5.6 | 177 ± 7 | 27.6 ± 4.0 | 87.6 | 37.1 | 7.7 | 8.8 ± 13.5 |
| PHS (26) | ||||||||
| Case | 968 | 58.0 ± 8.4 | 179 ± 6 | 24.7 ± 2.5 | NA | 100.0 | 9.4 | 6.9 ± 6.1 |
| Control | 1671 | 59.5 ± 8.3 | 178 ± 7 | 24.6 ± 2.5 | NA | 100.0 | 9.2 | 7.0 ± 6.1 |
| PLCO (27–29) | ||||||||
| Case | 691 | 65.0 ± 4.9 | 178 ± 6 | 27.1 ± 3.6 | 87.1 | 44.0 | 6.7 | 16.6 ± 30.7 |
| Control | 842 | 64.8 ± 4.7 | 178 ± 7 | 27.4 ± 3.9 | 86.7 | 42.6 | 9.0 | 16.7 ± 29.8 |
| ProtecT (30) | ||||||||
| Case | 1402 | 62.1 ± 5.0 | 176 ± 7 | 26.8 ± 3.5 | NA | NA | 14.6 | 24.2 ± 25.2 |
| Control | 1421 | 61.9 ± 5.0 | 176 ± 7 | 26.9 ± 3.8 | NA | NA | 12.9 | 24.1 ± 25.4 |
| SU.VI.MAX (31) | ||||||||
| Case | 85 | 55.2 ± 4.5 | 173 ± 7 | 25.8 ± 3.1 | 94.0 | 34.5 | 14.5 | 25.6 ± 21.2 |
| Control | 298 | 55.0 ± 4.5 | 174 ± 6 | 25.5 ± 2.8 | 87.0 | 37.0 | 12.8 | 27.1 ± 20.6 |
The cases and controls are nested in prospective studies, and the numbers of cases and controls are based on the number in complete matched sets for retinol plus HPFS participants in complete matched sets for β-carotene. ATBC, Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study; CARET, β-Carotene and Retinol Efficacy Trial; EPIC, European Prospective Investigation into Cancer and Nutrition; FMC, Finnish Mobile Clinic Health Examination Survey; HPFS, Health Professionals Follow-Up Study; MCCS, Melbourne Collaborative Cohort Study; MEC, Multiethnic Cohort; NA, data not available for this study; PCPT, Prostate Cancer Prevention Trial (placebo arm only); PHS, Physicians’ Health Study; PLCO, Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (screening arm only); ProtecT, Prostate testing for cancer and Treatment trial; SU.VI.MAX, SUpplémentation en VItamines et Minéraux Anti-oXydants trial.
Mean ± SD (all such values).
The characteristics of cases by study are shown in Table 2. The proportion of cases diagnosed before age 60 y varied from 1.6% in PCPT to 40.6% in Janus. The proportion of cases diagnosed from 1995 onward varied from 0% in the Finnish Mobile Clinic Health Examination Survey to 100% in MEC, PLCO, ProtecT, and SU.VI.MAX. The proportion of cases diagnosed at least 7 y after blood collection varied from 0% in CLUE II, Health Professionals Follow-Up Study, and ProtecT to 93.7% in Janus. The proportion of cases in studies with data on stage that were known to have advanced disease varied from 1.6% in PCPT to 25.4% in CLUE II, and the proportion of cases known to have aggressive disease varied from 0.8% in PCPT to 33.5% in CLUE I. The proportion of cases in studies with data on grade of disease that were known to have high-grade disease varied from 0.3% in MEC to 11.5% in the β-Carotene and Retinol Efficacy Trial. The geometric mean biomarker concentrations for cases and controls, by study, are shown in Table 3, and Supplemental Table 3 shows the correlations between the log-transformed biomarker concentrations in controls, standardized within each study and adjusted for age at blood collection.
TABLE 2.
Characteristics of the prostate cancer cases by study1
| Age at diagnosis, % |
Date of diagnosis, % |
Years from blood collection to diagnosis, % |
Stage of disease,2 % |
Aggressive disease,2 % |
Grade of disease,2 % |
|||||||||||||
| Study | <60 y | 60–69 y | ≥70 y | Pre-1990 | 1990–94 | 1995–onward | <3 y | 3–6 y | ≥7 y | Localized | Advanced | NK | No | Yes | NK | Low | High | NK |
| ATBC (11, 13, 14) | 2.6 | 36.5 | 60.9 | 4.1 | 12.4 | 83.6 | 3.9 | 9.0 | 87.2 | 44.6 | 21.4 | 34.0 | 43.7 | 26.6 | 29.7 | 59.5 | 5.8 | 34.7 |
| CARET (15) | 11.1 | 55.1 | 33.8 | 2.7 | 29.1 | 68.1 | 19.5 | 35.2 | 45.3 | 67.0 | 19.5 | 13.6 | 76.9 | 10.4 | 12.7 | 81.4 | 11.5 | 7.1 |
| CLUE I (16) | 10.2 | 36.3 | 53.5 | 46.5 | 42.4 | 11.0 | 8.6 | 9.4 | 82.0 | 54.7 | 20.8 | 24.5 | 53.9 | 33.5 | 12.7 | 82.0 | 7.3 | 10.6 |
| CLUE II (17) | 10.6 | 38.0 | 51.4 | 0.0 | 73.9 | 26.1 | 34.5 | 65.5 | 0.0 | 56.3 | 25.4 | 18.3 | 67.6 | 16.9 | 15.5 | 85.9 | 6.3 | 7.7 |
| EPIC (18) | 18.2 | 62.4 | 19.4 | 0.0 | 0.5 | 99.5 | 28.9 | 54.7 | 16.4 | 53.3 | 20.8 | 25.9 | 54.2 | 25.3 | 20.4 | 63.0 | 10.3 | 26.7 |
| FMC (19) | 13.5 | 43.2 | 43.2 | 100.0 | 0.0 | 0.0 | 29.7 | 64.9 | 5.4 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 |
| HPFS (20) | 13.1 | 38.8 | 48.1 | 0.0 | 11.4 | 88.6 | 45.0 | 55.0 | 0.0 | 88.1 | 4.7 | 7.2 | 83.8 | 10.6 | 5.6 | 80.6 | 7.8 | 11.6 |
| Janus (21) | 40.6 | 32.1 | 27.3 | 0.7 | 6.0 | 93.4 | 2.0 | 4.2 | 93.7 | 51.0 | 19.6 | 29.4 | 51.3 | 22.8 | 25.9 | 0.0 | 0.0 | 100.0 |
| MCCS (22, 23) | 15.0 | 49.5 | 35.4 | 0.0 | 14.7 | 85.3 | 23.1 | 29.7 | 47.1 | 88.6 | 10.2 | 1.2 | 96.7 | 2.1 | 1.2 | 86.5 | 11.4 | 2.1 |
| MEC (24) | 6.8 | 36.1 | 57.1 | 0.0 | 0.0 | 100.0 | 79.3 | 17.5 | 3.1 | 0.0 | 0.0 | 100.0 | 0.0 | 5.0 | 95.0 | 95.0 | 0.3 | 4.7 |
| PCPT (25) | 1.6 | 50.5 | 48.0 | 0.0 | 0.1 | 99.9 | 9.9 | 27.4 | 62.7 | 96.0 | 1.6 | 2.5 | 96.7 | 0.8 | 2.5 | 93.0 | 4.7 | 2.3 |
| PHS (26) | 12.0 | 44.4 | 43.6 | 22.3 | 40.5 | 37.2 | 6.5 | 15.2 | 78.3 | 82.4 | 12.7 | 4.9 | 77.9 | 17.8 | 4.3 | 87.1 | 9.9 | 3.0 |
| PLCO (27–29) | 5.8 | 56.2 | 38.1 | 0.0 | 0.0 | 100.0 | 59.9 | 39.7 | 0.4 | 87.3 | 12.7 | 0.0 | 93.1 | 6.9 | 0.0 | 93.6 | 5.8 | 0.6 |
| ProtecT (30) | 28.9 | 67.0 | 4.1 | 0.0 | 0.0 | 100.0 | 99.9 | 0.1 | 0.0 | 81.7 | 7.9 | 10.3 | 88.8 | 0.9 | 10.3 | 94.4 | 5.6 | 0.1 |
| SU.VI.MAX (31) | 34.1 | 65.9 | 0.0 | 0.0 | 0.0 | 100.0 | 15.3 | 38.8 | 45.9 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 85.9 | 8.2 | 5.9 |
ATBC, Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study; CARET, β-Carotene and Retinol Efficacy Trial; EPIC, European Prospective Investigation into Cancer and Nutrition; FMC, Finnish Mobile Clinic Health Examination Survey; HPFS, Health Professionals Follow-Up Study; MCCS, Melbourne Collaborative Cohort Study; MEC, Multiethnic Cohort; NK, not known; PCPT, Prostate Cancer Prevention Trial (placebo arm only); PHS, Physicians’ Health Study; PLCO, Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (screening arm only); ProtecT, Prostate testing for cancer and Treatment trial; SU.VI.MAX, SUpplémentation en VItamines et Minéraux Anti-oXydants trial; TNM, tumor/node/metastasis.
Stage of disease was defined as being advanced if it was TNM stage T3 or T4 and/or N1+ and/or M1, stage III–IV, or approximate equivalent (i.e., a tumor extending beyond the prostate capsule and/or lymph node involvement and/or distant metastases); localized if it was TNM stage ≤T2 with no reported lymph node involvement or metastases, stage 0–II, or approximate equivalent (i.e., a tumor that does not extend beyond the prostate capsule); or stage unknown. Aggressive disease was categorized as “yes” for TNM stage T4 and/or N1+ and/or M1 and/or stage IV disease or death from prostate cancer, “no” for TNM stage ≤T3 with no reported lymph node involvement or metastases or the equivalent, or unknown. Grade of disease defined as high grade if the Gleason sum was at least 8 or approximate equivalent (i.e., extent of differentiation of “none”), low grade if the Gleason sum was <8 or approximate equivalent (i.e., extent of differentiation of “poor,” “moderate,” or “good”), or grade unknown.
TABLE 3.
Prediagnostic geometric mean carotenoid, retinol, and tocopherol concentrations by study in cases and controls1
| Study and case-control status | α-carotene | β-carotene | Lycopene | Lutein | Zeaxanthin | β-cryptoxanthin | Canthaxanthin | Retinol | α-tocopherol | γ-tocopherol |
| ATBC (11, 13, 14) | ||||||||||
| Cases | NA | 336 (327, 346) | NA | NA | NA | NA | NA | 2045 (2027, 2064) | 26.6 (26.4, 26.9) | NA |
| Controls | NA | 335 (330, 341) | NA | NA | NA | NA | NA | 2016 (2006, 2027) | 27.1 (27.0, 27.3) | NA |
| CARET (15) | ||||||||||
| Cases | 70 (68, 73) | 301 (288, 315) | 574 (551, 597) | 225 (217, 233) | 41 (40, 43) | 115 (110, 120) | NA | 2306 (2265, 2348) | 29.7 (28.9, 30.6) | 5.55 (5.31, 5.80) |
| Controls | 70 (68, 72) | 292 (283, 302) | 572 (555, 589) | 228 (222, 234) | 41 (40, 42) | 116 (112, 119) | NA | 2257 (2228, 2287) | 29.9 (29.3, 30.5) | 5.61 (5.43, 5.79) |
| CLUE I 19902 (16) | ||||||||||
| Cases | NA | 461 (391, 543) | 603 (518, 701) | NA | NA | NA | NA | 2202 (2066, 2348) | NA | NA |
| Controls | NA | 479 (402, 571) | 639 (550, 743) | NA | NA | NA | NA | 2271 (2122, 2430) | NA | NA |
| CLUE I 20032 (16) | ||||||||||
| Cases | 42 (38, 47) | 149 (135, 166) | 598 (547, 653) | 242 (228, 258) | NA | 107 (94.7, 121) | NA | 2301 (2229, 2375) | 27.6 (26.5, 28.8) | 5.30 (4.91, 5.73) |
| Controls | 45 (42, 49) | 154 (142, 166) | 623 (583, 665) | 235 (225, 246) | NA | 104 (95.1, 113) | NA | 2316 (2261, 2373) | 28.3 (27.3, 29.3) | 5.67 (5.37, 5.99) |
| CLUE II (17) | ||||||||||
| Cases | 47 (41, 54) | 160 (142, 180) | 697 (632, 768) | 192 (178, 207) | NA | 113 (98.8, 129) | NA | 2381 (2288, 2478) | 31.0 (28.9, 33.2) | 5.21 (4.68, 5.79) |
| Controls | 48 (43, 53) | 151 (138, 164) | 719 (671, 771) | 200 (191, 210) | NA | 116 (106, 127) | NA | 2370 (2309, 2432) | 31.6 (30.3, 33.0) | 6.37 (5.93, 6.84) |
| EPIC-Europe (18) | ||||||||||
| Cases | 102 (97, 107) | 277 (265, 290) | 505 (484, 526) | 315 (305, 324) | 70 (68, 73) | 117 (111, 124) | 30.3 (29.0, 31.7) | 1915 (1888, 1943) | 31.4 (30.9, 31.9) | 2.34 (2.26, 2.43) |
| Controls | 100 (95, 105) | 283 (271, 295) | 504 (483, 525) | 311 (302, 320) | 67 (64, 69) | 114 (108, 120) | 28.9 (27.7, 30.1) | 1907 (1883, 1932) | 32.1 (31.6, 32.6) | 2.41 (2.33, 2.50) |
| EPIC-Norfolk (18) | ||||||||||
| Cases | 124 (102, 151) | 319 (274, 371) | 433 (361, 518) | 272 (246, 299) | 53 (47, 60) | 109 (89.8, 131) | 7.3 (6.2, 8.7) | 1776 (1666, 1894) | 25.2 (23.5, 27.0) | 1.59 (1.41, 1.78) |
| Controls | 118 (104, 134) | 301 (270, 335) | 411 (365, 464) | 264 (246, 284) | 52 (47, 57) | 101 (89.4, 115) | 6.7 (5.7, 7.8) | 1747 (1677, 1821) | 25.0 (23.8, 26.4) | 1.68 (1.53, 1.84) |
| FMC (19) | ||||||||||
| Cases | NA | 100 (74, 135) | NA | NA | NA | NA | NA | 2331 (2151, 2526) | 17.4 (15.9, 19.0) | NA |
| Controls | NA | 106 (87, 129) | NA | NA | NA | NA | NA | 2308 (2178, 2445) | 18.2 (16.9, 19.6) | NA |
| HPFS (20) | ||||||||||
| Cases | 110 (104, 116) | 368 (348, 389) | 723 (696, 750) | 235 (222, 249) | NA | 121 (115, 128) | NA | NA | NA | NA |
| Controls | 115 (110, 121) | 381 (361, 402) | 726 (700, 754) | 239 (226, 252) | NA | 124 (118, 131) | NA | NA | NA | NA |
| Janus (21) | ||||||||||
| Cases | NA | NA | NA | NA | NA | NA | NA | 2548 (2521, 2576) | NA | NA |
| Controls | NA | NA | NA | NA | NA | NA | NA | 2524 (2497, 2551) | NA | NA |
| MCCS (22, 23) | ||||||||||
| Cases | 81 (75, 88) | 501 (462, 544) | 400 (365, 439) | NA | NA | 154 (139, 171) | NA | 2434 (2358, 2513) | 34.0 (33.0, 35.1) | 1.53 (1.43, 1.64) |
| Controls | 77 (72, 83) | 470 (441, 502) | 438 (408, 469) | NA | NA | 159 (148, 171) | NA | 2402 (2339, 2466) | 34.7 (33.9, 35.6) | 1.60 (1.52, 1.67) |
| MEC (24) | ||||||||||
| Cases | 111 (103, 119) | 448 (412, 487) | 700 (666, 735) | 336 (322, 352) | 162 (155, 168) | 99.4 (93.8, 105) | NA | 4076 (3959, 4197) | 33.9 (32.4, 35.4) | 3.55 (3.29, 3.83) |
| Controls | 111 (105, 116) | 442 (417, 469) | 715 (690, 741) | 333 (323, 344) | 159 (154, 164) | 101 (97.2, 106) | NA | 4013 (3929, 4100) | 34.0 (32.9, 35.1) | 3.66 (3.48, 3.85) |
| PCPT (25) | ||||||||||
| Cases | 89 (85, 92) | 454 (435, 474) | 650 (632, 669) | 248 (235, 263) | 41 (39, 44) | 152 (147, 158) | NA | 2383 (2351, 2416) | 36.6 (35.7, 37.5) | 3.70 (3.54, 3.87) |
| Controls | 83 (80, 86) | 422 (403, 442) | 636 (618, 654) | 245 (230, 261) | 40 (37, 43) | 150 (145, 156) | NA | 2330 (2295, 2364) | 36.4 (35.5, 37.3) | 3.79 (3.63, 3.95) |
| PHS (26) | ||||||||||
| Cases | 107 (102, 111) | 147 (133, 161) | 536 (516, 557) | 259 (248, 270) | NA | 138 (132, 145) | NA | 2134 (2101, 2168) | 25.6 (24.9, 26.3) | 4.38 (4.25, 4.52) |
| Controls | 110 (106, 113) | 232 (217, 247) | 617 (600, 634) | 215 (208, 222) | NA | 131 (126, 135) | NA | 2023 (1998, 2048) | 26.0 (25.5, 26.4) | 4.37 (4.28, 4.47) |
| PLCO (27–29) | ||||||||||
| Cases | 135 (127, 144) | 311 (294, 329) | 1097 (1049, 1146) | 302 (292, 314) | 101 (98, 105) | 128 (121, 134) | 9.9 (9.4, 10.4) | 2359 (2306, 2414) | 40.0 (38.7, 41.3) | 6.27 (5.96, 6.60) |
| Controls | 127 (120, 134) | 285 (270, 300) | 1091 (1054, 1129) | 304 (294, 314) | 103 (100, 106) | 123 (118, 129) | 10.1 (9.6, 10.6) | 2393 (2345, 2442) | 40.2 (39.1, 41.4) | 6.34 (6.05, 6.64) |
| ProtecT (30) | ||||||||||
| Cases | NA | NA | NA | NA | NA | NA | NA | 1731 (1707, 1755) | NA | NA |
| Controls | NA | NA | NA | NA | NA | NA | NA | 1755 (1732, 1779) | NA | NA |
| SU.VI.MAX (31) | ||||||||||
| Cases | 179 (109, 292) | 375 (319, 442) | 441 (340, 573) | 272 (219, 338) | 86 (62, 120) | 213 (126, 361) | 33.8 (20.2, 56.7) | 2262 (2118, 2416) | 30.3 (28.6, 32.2) | NA |
| Controls | 169 (118, 243) | 380 (351, 410) | 475 (372, 607) | 258 (222, 300) | 71 (57, 89) | 175 (125, 245) | 25.1 (15.8, 39.9) | 2426 (2354, 2499) | 31.2 (30.3, 32.0) | NA |
Values are geometric means; 95% CI in parentheses. Units are nmol/L for each biomarker except for α-tocopherol and γ-tocopherol, which are measured in μmol/L. ATBC, Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study; CARET, β-Carotene and Retinol Efficacy Trial; EPIC, European Prospective Investigation into Cancer and Nutrition; FMC, Finnish Mobile Clinic Health Examination Survey; HPFS, Health Professionals Follow-Up Study; MCCS, Melbourne Collaborative Cohort Study; MEC, Multiethnic Cohort; NA, data not available for this study; PCPT, Prostate Cancer Prevention Trial (placebo arm only); PHS, Physicians’ Health Study; PLCO, Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (screening arm only); ProtecT, Prostate testing for cancer and Treatment trial; SU.VI.MAX, SUpplémentation en VItamines et Minéraux Anti-oXydants trial.
Dates that the assays were conducted.
Associations of circulating carotenoids, retinol, and tocopherols with overall prostate cancer risk
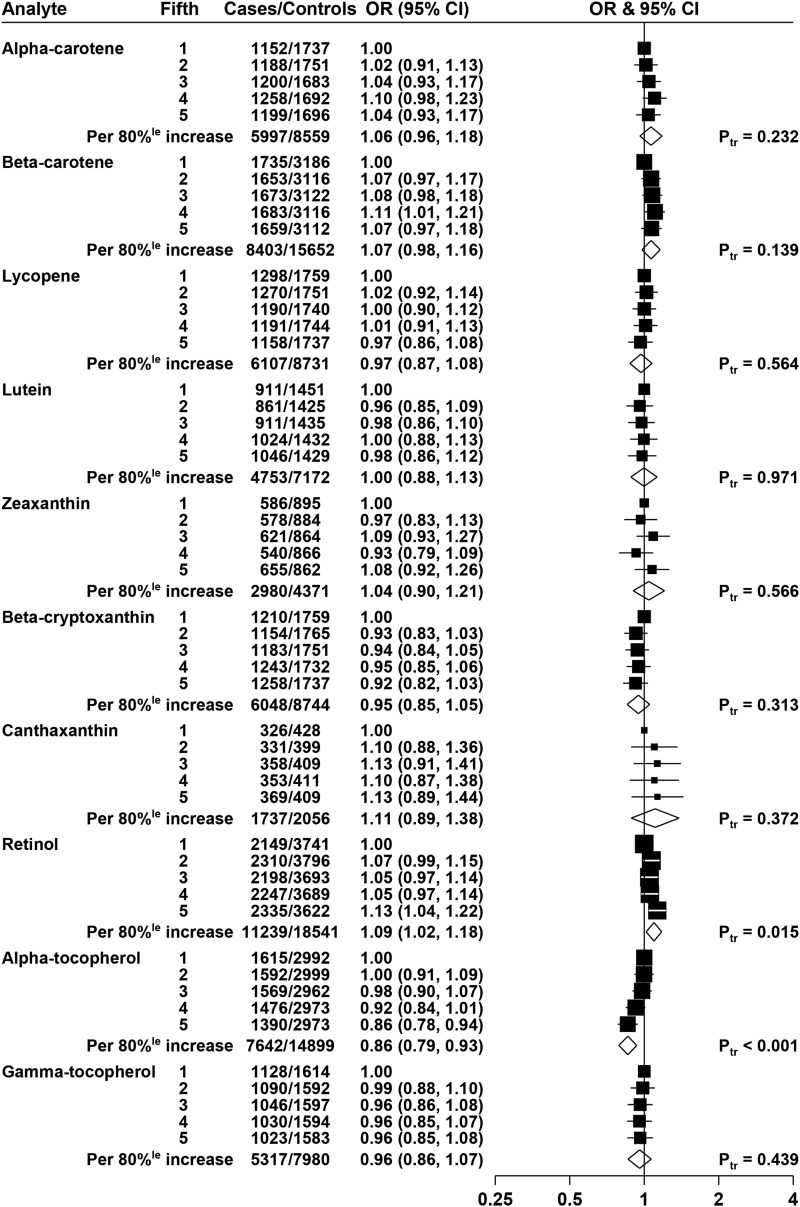
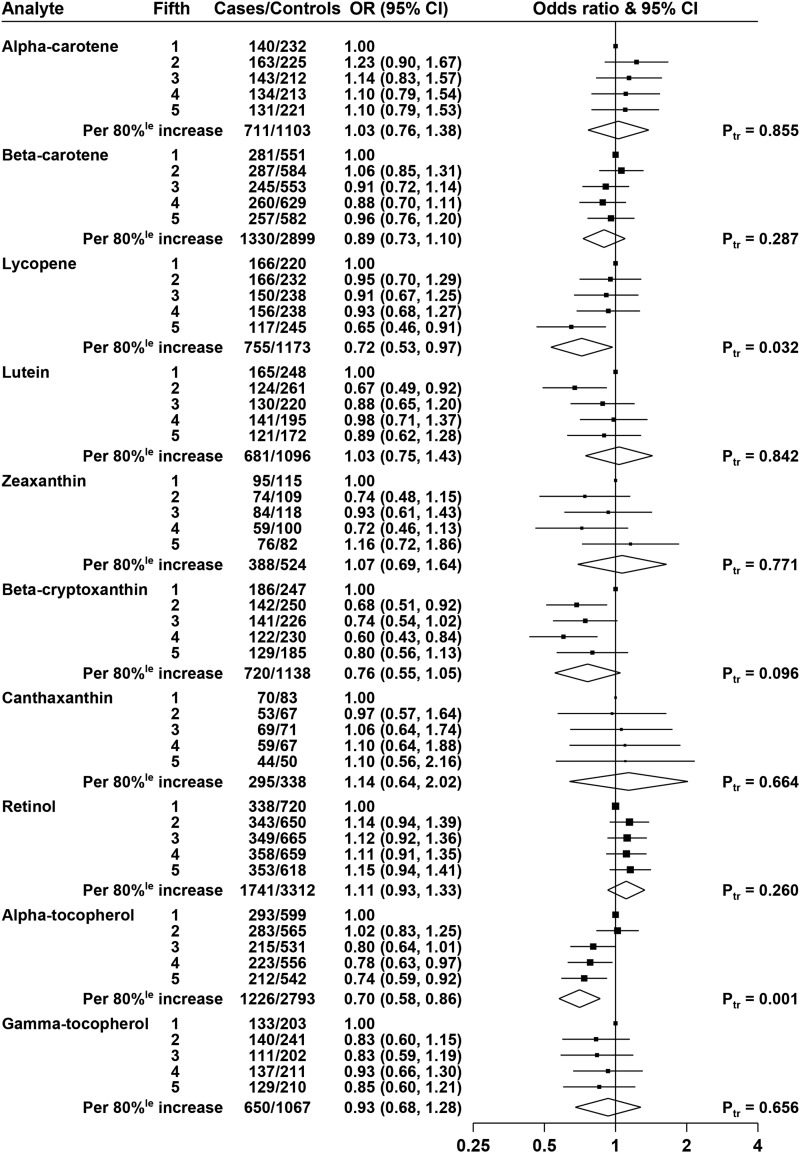
The overall ORs by quintiles of biomarkers, adjusted for age, height, BMI, educational attainment, marital status, and smoking, are shown in Figure 1. Lycopene was not statistically significantly associated with risk, with an OR of 0.97 (95% CI: 0.86, 1.08; P-trend = 0.56) for men in the highest fifth of lycopene compared with the lowest fifth, and none of the other carotenoids was statistically significantly associated with risk of prostate cancer.
FIGURE 1.
ORs for prostate cancer associated with carotenoids, retinol, and tocopherols. The black squares indicate the ORs in study-specific fifths, and the horizontal lines show the 95% CIs. The area of each square is proportional to the amount of statistical information (inverse of the variance of the logarithm of the OR). The diamonds show the OR for an increase in concentration from the 10th to the 90th percentile, and the widths of the diamonds show the 95% CIs. The χ2 tests for linear trend (Ptr) were calculated scoring the fifths as 0, 0.25, 0.5, 0.75, and 1. Estimates are from conditional logistic regression on case-control sets matched within each study and adjusted for age, marital status, educational attainment, smoking, height, and BMI.
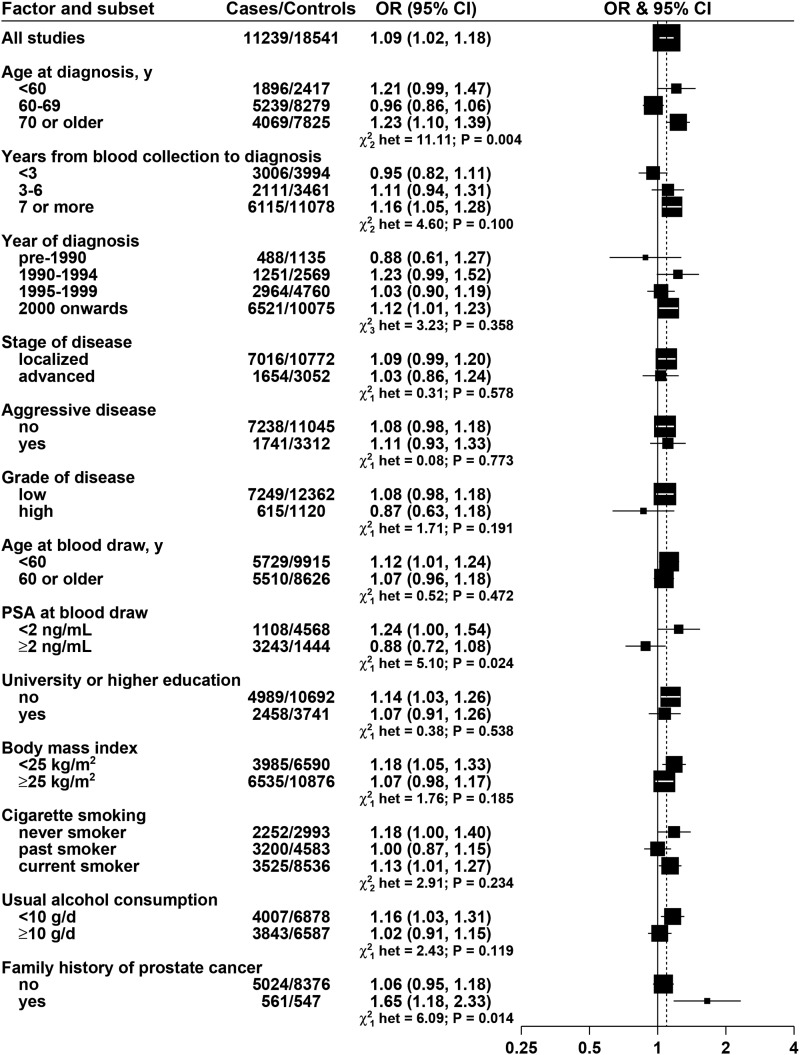
Retinol was positively associated with risk, with an OR of 1.13 (95% CI: 1.04, 1.22; P-trend = 0.015) for men in the highest fifth of retinol compared with the lowest fifth.
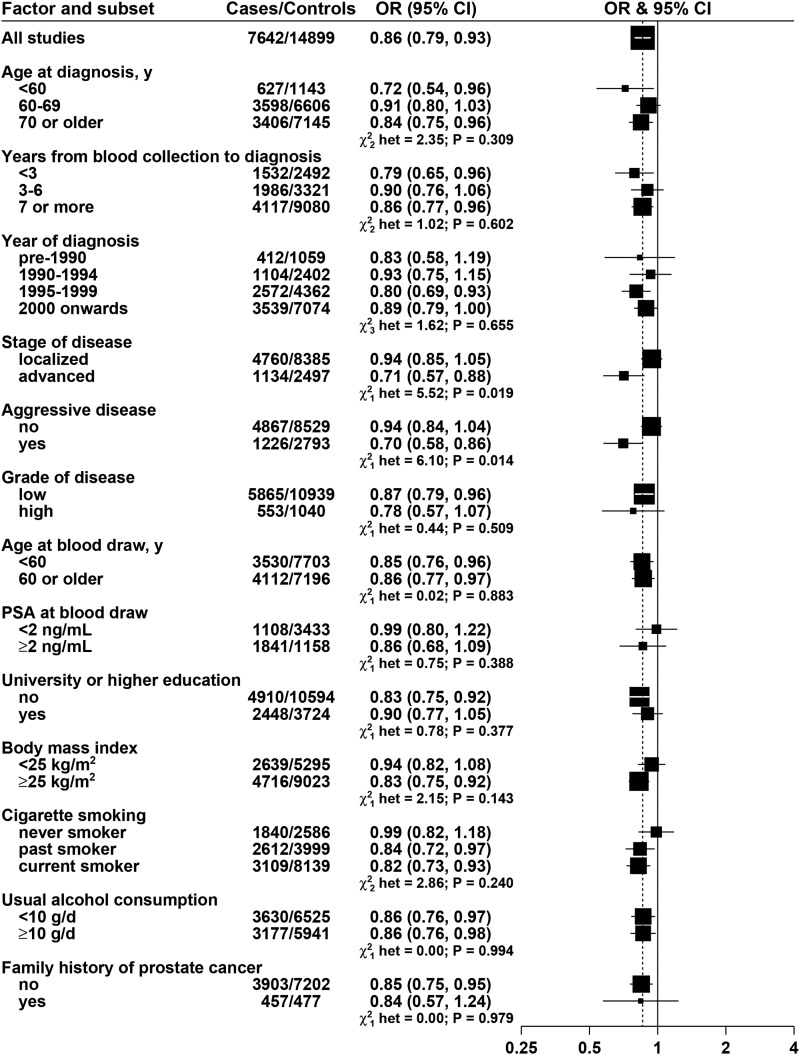
α-Tocopherol was inversely associated with risk, with an OR of 0.86 (95% CI: 0.78, 0.94, P-trend < 0.001) for men in the highest fifth of α-tocopherol; this association was essentially unchanged after further adjustment for total cholesterol (results not shown). γ-Tocopherol was not associated with risk, and analyses grouping men according to both α-tocopherol and γ-tocopherol did not show any interaction (results not shown; P-interaction = 0.27).
The results of analyses without adjustment for anthropometric and lifestyle factors (i.e., with allowance only for the matching variables) are shown in Supplemental Figure 1; the unadjusted (matched) results were broadly similar to the fully adjusted results for most biomarkers, with the exception that in the unadjusted results, both α-carotene and β-carotene were positively associated with prostate cancer risk [ORs in top fifth of 1.10 (95% CI: 0.98, 1.23), P-trend = 0.024 and 1.10 (95% CI: 1.00, 1.20), P-trend = 0.028, respectively).
Adjusted risk analyses were also performed with the fifths defined by using the quintile cutoffs determined for controls across all studies combined (shown in Supplemental Figure 2), with results qualitatively similar to those shown in Figure 1 that used study-specific quintiles. In these analyses, the ORs for men in the highest fifths of retinol and α-tocopherol were 1.16 (95% CI: 1.06, 1.27) and 0.88 (95% CI: 0.80, 0.97), respectively.
Supplemental Figures 3–12 show the relations of the biomarkers with prostate cancer risk for the individual studies, together with overall estimates and tests for heterogeneity between studies. It should be noted that because of the small number of cases in some studies and missing data on some covariates, these analyses are not adjusted for anthropometric and lifestyle factors; therefore, the all-study results shown in these figures differ slightly from those shown in Figure 1 (but are the same as the unadjusted results from prospective studies shown in Supplemental Figure 1). For retinol, there was evidence of heterogeneity between the prospective studies and the cross-sectional ProtecT study: the OR for an 80% increase in retinol was 1.13 (95% CI: 1.05, 1.22) in the prospective studies, 0.86 (95% CI: 0.69, 1.08) in ProtecT, and 1.10 (95% CI: 1.02, 1.18) overall, P-heterogeneity by study design = 0.027 (Supplemental Figure 10). There was no evidence of heterogeneity between studies in the association of any of the other biomarkers with prostate cancer risk.
Subgroup analyses by clinical and other characteristics
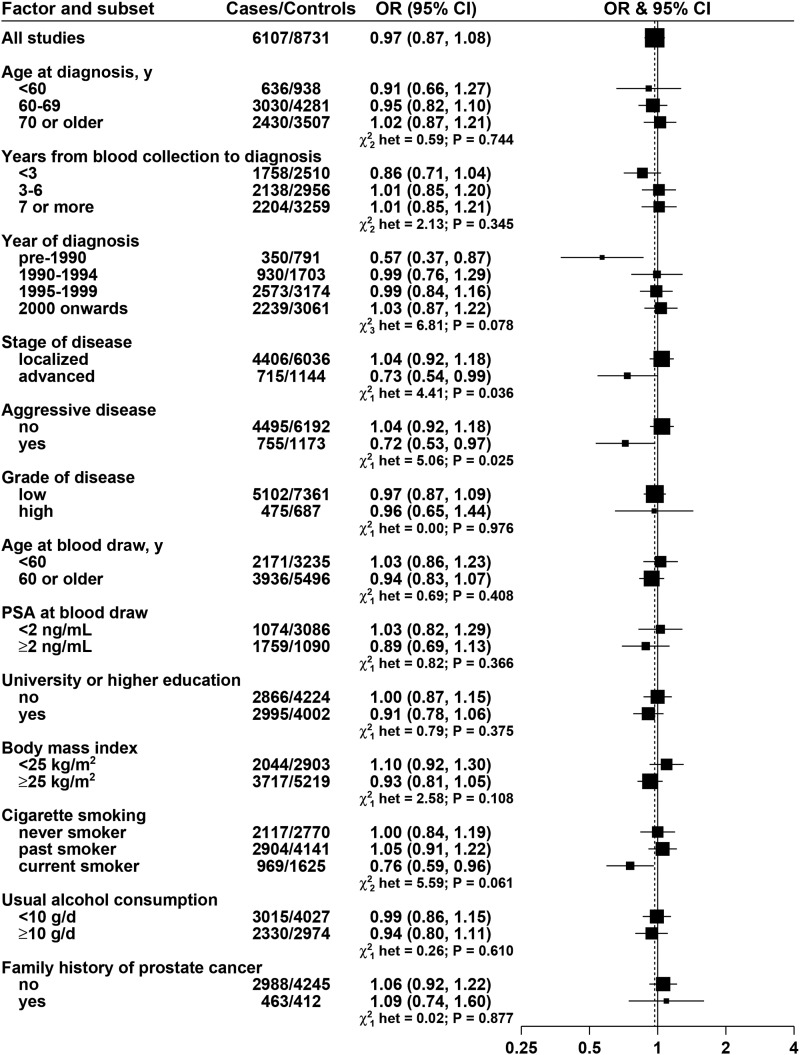
The relations of the biomarkers with risk subdivided by clinical and other characteristics are shown in Figures 2, 3, and 4 for lycopene, retinol, and α-tocopherol, respectively, and in Supplemental Figures 13–19 for all other biomarkers (these results are adjusted for age, height, BMI, educational attainment, marital status, and smoking). For lycopene (Figure 2), the association with prostate cancer risk varied significantly by stage and aggressiveness of the disease: OR for an 80% increase was 0.73 (95% CI: 0.54, 0.99), P-heterogeneity = 0.036, for advanced disease and 0.72 (95% CI: 0.53, 0.97), P-heterogeneity = 0.025, for aggressive disease. Lycopene was associated with a reduction in overall risk of prostate cancer for cases diagnosed before 1990 (OR: 0.57; 95% CI: 0.37, 0.87) but not in cases diagnosed later (P-heterogeneity between the 4 categories of year of diagnosis = 0.078).
FIGURE 2.
ORs for prostate cancer associated with lycopene concentration, according to characteristics of cases and controls. Each OR is the estimate of the linear trend obtained by replacing the categorical variables representing the fifths of lycopene concentration in controls by a continuous variable scored as 0, 0.25, 0.5, 0.75, and 1. Black squares indicate the OR, and the horizontal lines show the 95% CIs. The area of each square is proportional to the amount of statistical information (inverse of the variance of the logarithm of the OR). The vertical dotted line indicates the OR for all studies. Tests for heterogeneity are for the difference in the association of lycopene with prostate cancer risk between subgroups. Estimates are from conditional logistic regression on case-control sets matched within each study and adjusted for age, marital status, educational attainment, smoking, height and BMI. PSA, prostate-specific antigen.
FIGURE 3.
ORs for prostate cancer associated with retinol concentration, according to characteristics of cases and controls. Each OR is the estimate of the linear trend obtained by replacing the categorical variables representing the fifths of retinol concentration in controls by a continuous variable scored as 0, 0.25, 0.5, 0.75, and 1. Black squares indicate the OR, and the horizontal lines show the 95% CIs. The area of each square is proportional to the amount of statistical information (inverse of the variance of the logarithm of the OR). The vertical dotted line indicates the OR for all studies. Tests for heterogeneity are for the difference in the association of retinol with prostate cancer risk between subgroups. Estimates are from conditional logistic regression on case-control sets matched within each study and adjusted for age, marital status, educational attainment, smoking, height and BMI. PSA, prostate-specific antigen.
FIGURE 4.
ORs for prostate cancer associated with α-tocopherol concentration, according to characteristics of cases and controls. Each OR is the estimate of the linear trend obtained by replacing the categorical variables representing the fifths of α-tocopherol concentration in controls by a continuous variable scored as 0, 0.25, 0.5, 0.75, and 1. Black squares indicate the OR, and the horizontal lines show the 95% CIs. The area of each square is proportional to the amount of statistical information (inverse of the variance of the logarithm of the OR). The vertical dotted line indicates the OR for all studies. Tests for heterogeneity are for the difference in the association of α-tocopherol with prostate cancer risk between subgroups. Estimates are from conditional logistic regression on case-control sets matched within each study and adjusted for age, marital status, educational attainement, smoking, height and BMI. PSA, prostate-specific antigen.
For retinol, the association with prostate cancer risk did not vary by stage, aggressiveness, or grade of disease (Figure 3). Retinol was significantly positively associated with risk in men aged at least 70 y at diagnosis but not in men diagnosed at a younger age (P-heterogeneity = 0.004), positively associated with risk in men with PSA <2 ng/mL at blood collection but not in men with higher PSA (P-heterogeneity = 0.024), and positively associated with risk in men with a family history of prostate cancer but not in men without a family history (P-heterogeneity = 0.014).
α-Tocopherol was inversely associated with risk of advanced and aggressive prostate cancer [ORs for 80% increase: 0.71 (95% CI: 0.57, 0.88) and 0.70 (95% CI: 0.58, 0.86), respectively] but not with localized or nonaggressive disease (P-heterogeneity = 0.019 and 0.014, respectively; Figure 4). The inverse association of α-tocopherol with risk was significant in current smokers and past smokers but not in nonsmokers, but this was not a statistically significant difference (P-heterogeneity = 0.240, Figure 4); we also examined the association of α-tocopherol with risk subdivided by smoking in 2 categories: the ORs were 0.99 (95% CI: 0.82, 1.18) for never smokers and 0.83 (95% CI: 0.75, 0.91) for current and past smokers together (P-heterogeneity = 0.093).
There was also some evidence of heterogeneity between subgroups for α-carotene, lutein, and zeaxanthin. α-Carotene was positively associated with the risk of high-grade disease (OR for an 80% increase: 1.64; 95% CI: 1.12, 2.39) but not low-grade disease, P-heterogeneity = 0.012; with the risk of prostate cancer for men from whom blood was collected before age 60 y (OR for an 80% increase: 1.24; 95% CI: 1.05, 1.47) but not for men with blood collected at age ≥60 y, P-heterogeneity = 0.022; and with risk of prostate cancer in past smokers (OR for an 80% increase: 1.20; 95% CI: 1.04, 1.38) but not in never or current smokers, P-heterogeneity = 0.041 (Supplemental Figure 13). Lutein was inversely associated with the risk of prostate cancer diagnosed before age 60 y (OR for an 80% increase: 0.60; 95% CI: 0.41, 0.87) but not for cancers diagnosed at age ≥60 y, P-heterogeneity = 0.013 (Supplemental Figure 15). Zeaxanthin was positively associated with risk for men with a BMI <25 (OR for an 80% increase: 1.32; 95% CI: 1.02, 1.71) but not for overweight or obese men, P-heterogeneity = 0.047 (Supplemental Figure 16).
Analyses of the risk of aggressive prostate cancer in study-specific fifths of concentration for all biomarkers are shown in Figure 5; there were inverse associations with lycopene (OR in top fifth: 0.65; 95% CI: 0.46, 0.91) and α-tocopherol (OR in top fifth: 0.74; 95% CI: 0.59, 0.92), and no statistically significant associations with the other carotenoids, retinol, or γ-tocopherol. Similar analyses using overall cutoffs instead of study-specific cutoffs produced similar results: ORs in top fifth were 0.65 (95% CI: 0.46, 0.92) for lycopene and 0.70 (95% CI: 0.55, 0.90) for α-tocopherol (Supplemental Figure 20).
FIGURE 5.
ORs for aggressive prostate cancer associated with carotenoids, retinol, and tocopherols. The black squares indicate the ORs in study-specific fifths, and the horizontal lines show the 95% CIs. The area of each square is proportional to the amount of statistical information (inverse of the variance of the logarithm of the OR). The diamonds show the OR for an increase in concentration from the 10th to the 90th percentile, and the widths of the diamonds show the 95% CIs. The χ2 tests for linear trend (Ptr) were calculated by scoring the fifths as 0, 0.25, 0.5, 0.75, and 1. Estimates are from conditional logistic regression on case-control sets matched within each study and adjusted for age, marital status, educational attainment, smoking, height and BMI.
Cross-sectional analyses of the associations of the biomarkers with participant characteristics for control men are provided in Supplemental Figures 21–30. Geometric mean concentrations of lycopene were positively associated with higher educational achievement; were relatively low for men who were not married or cohabiting, men with a high BMI, and men who were current smokers; and were higher in moderate alcohol consumers than in men who consumed no alcohol or men who consumed a lot of alcohol (Supplemental Figure 23). Geometric mean concentrations of retinol were positively associated with educational attainment and alcohol intake and were relatively low for men with a low BMI and men who were current smokers (Supplemental Figure 28). Geometric mean concentrations of α-tocopherol were positively associated with educational attainment and were relatively low for men who were not married or cohabiting, men with a low BMI, and men who were current smokers (Supplemental Figure 29).
DISCUSSION
Lycopene was not associated with overall prostate cancer risk but was inversely associated with risk of advanced stage and aggressive disease. Lycopene was also significantly inversely associated with risk of prostate cancers diagnosed before 1990 (i.e., before the widespread use of PSA testing). Lycopene might reduce prostate cancer risk by various mechanisms such as by acting as an antioxidant or by inhibiting the cell cycle (1, 2), and further research on the relation of lycopene with prostate cancer is warranted (10).
For the other 6 carotenoids examined, there was no statistically significant association with overall risk of prostate cancer. α-Carotene and β-carotene were weakly positively associated with risk in the unadjusted analyses, but these associations were attenuated and no longer statistically significant after adjustment for anthropometric and lifestyle factors. There was some evidence of heterogeneity in associations for α-carotene (with grade of disease and age at blood collection), lutein (with age at diagnosis), and zeaxanthin (with BMI), but these differences may be due to chance.
Retinol was statistically significantly associated with prostate cancer overall, with a 13% higher risk in men with high retinol concentrations than in those with low concentrations. There was significant heterogeneity between the prospective studies and the cross-sectional ProtecT study, and when ProtecT was excluded, the overall association with retinol was increased to a 16% higher risk in men with high retinol concentrations than in those with low concentrations. This positive association of retinol with prostate cancer risk is difficult to interpret. Retinol concentrations are quite closely controlled by homeostasis and in well-nourished populations do not correlate strongly with vitamin A intake; for example, in ATBC, the correlation of dietary vitamin A intake with serum retinol was 0.05 (11). The association of retinol with prostate cancer risk may or may not reflect a causal relation; high concentrations of retinol might have adverse effects on the prostate, for example, through the insulin-like growth factor I receptor (42) or by antagonizing vitamin D (43), and it is also possible that retinol concentrations are associated with other unidentified metabolic risk factors for prostate cancer that should be explored further.
α-Tocopherol was statistically significantly inversely associated with prostate cancer risk, and this relation appeared to be restricted to advanced and aggressive prostate cancer, for which risk was about 25% lower in men in the highest compared with lowest fifth of the distribution of α-tocopherol. γ-Tocopherol was not associated with risk. The ATBC trial reported in 1998 that prostate cancer risk was lower for men who received a moderate, 50-IU supplemental dose/d of α-tocopherol than for those who did not (44), but subsequent randomized controlled trials found no reduction in prostate cancer risk for men given substantially higher doses of α-tocopherol (e.g., 400 IU/d) (45–48), and longer follow-up of the Selenium and Vitamin E Cancer Prevention Trial (SELECT) showed a significant 17% increase in risk of overall prostate cancer (most of which were early-stage, low-intermediate grade prostate cancer) for men given supplements of vitamin E alone (49). The ATBC trial reported that the reduction in risk was present for clinical cancer but not for latent cancers, which is consistent with our observation that the inverse association of α-tocopherol with risk was restricted to more advanced/aggressive disease (44), but the other trials did not report their results by stage of disease, and most of the cases in these trials were early stage (e.g., in the longer follow-up of the SELECT trial, <1% of cases had a T stage >2) (49). It should also be noted that all the men in ATBC were smokers, whereas the prevalence of smoking was low in the other trials, so it is possible that any effect of α-tocopherol may be influenced by smoking; in the current analyses, there was a significant inverse association of α-tocopherol with risk in current smokers and past smokers but not in nonsmokers, but this was not a significant difference. The recent trials indicate that high doses of supplemental α-tocopherol for a few years in middle-aged and elderly men do not reduce the risk of overall, largely PSA-detected prostate cancer, but it remains possible that relatively high intakes of dietary constituents rich in α-tocopherol might have some beneficial impact on the development of advanced prostate cancer. Alternatively, circulating α-tocopherol concentrations may be inversely associated with unidentified metabolic risk factors for prostate cancer.
The analyses described are based on a large amount of individual participant data, providing enough power to examine associations of the nutritional biomarkers with prostate cancer risk overall and for subgroups. Data were available for up to ∼11,000 cases, representing most of the worldwide data, since the 5 studies from which data were not obtained included a total of only 420 cases. Data were also available for several potential confounding factors, although we did not have enough nonwhite participants to provide reliable results for nonwhite ethnic groups. A caveat in the interpretation of the results is that we conducted many significance tests, especially for the subgroup analyses, and therefore some of the nominally significant results may be due to chance.
The main limitations of these analyses concern the differences between studies in design and in laboratory measures. PSA testing became widespread in the general population in many countries from the early 1990s onward, but use has varied between populations, whereas PSA screening was used systematically in 3 of the collaborating studies (PCPT, PLCO, and ProtecT). Most PSA-detected cancers are nonadvanced, and many are biologically indolent; therefore, the results reported for advanced-stage and aggressive cancers are of particular interest (10), although there might be some bias for these cases if men with a less health-conscious lifestyle are more likely to be diagnosed later in the course of the disease.
The biomarker measures for each study were all based on HPLC platforms but in different laboratories. Therefore, some of the differences in mean concentrations between studies may be due to between-laboratory variation, as well as differences in diet and other factors that affect the biomarkers. Our main analyses combined the data from different studies by using study-specific cutoffs, an approach that assumes that there are not large true differences in concentrations between studies and therefore that for any association with risk, the RRs across fifths of the distribution are similar in the different studies (50). As an alternative, we also report the main analyses by using cutoffs across all studies, an approach that makes the assumption that there is little variation between laboratories. The results from these 2 approaches were similar. In analyses of circulating carotenoids and breast cancer risk from 8 prospective studies, Eliassen et al. (51) reassayed 20 samples from each study and stated that their results were fairly consistent and robust regardless of whether they pooled study-specific RRs or calculated RRs from pooled recalibrated data.
Another limitation of the analyses is that they are based on only one blood sample for each participant. Several studies have investigated the reliability of these biomarkers over a few years. For example, the correlations between measures from 2 samples from the same individuals have been reported as 0.68 for retinol over 3 y (11), 0.44–0.46 for β-carotene and retinol over 4 y (19), and 0.35–0.52 for carotenoids, 0.58 for retinol, and 0.48–0.61 for tocopherols over 15 y (52). These reports suggest that single measures of these biomarkers provide moderately reliable information on usual concentrations over a few years but that any true associations with risk are likely to be underestimated.
In summary, the results from this large collaborative pooled analysis showed inverse associations of lycopene and α-tocopherol with risk of aggressive prostate cancer, as well as a positive association between retinol and overall prostate cancer risk. It is not clear whether these associations indicate any causal relations; therefore, to determine whether they are clinically relevant, more laboratory and clinical research is needed on the effects of these compounds on the behavior of prostate cancer cells and tissue.
Acknowledgments
WD Fraser undertook the assays for the ProtecT study.
The authors’ responsibilities were as follows—TJK, PNA, RCT, and NEA: conceived the idea, drafted the manuscript, had full access to all the data in the study, interpreted the results, and took responsibility for the integrity of the data and the accuracy of the data analysis; PNA: performed the statistical analyses; and all authors: provided data, critically revised the manuscript for intellectual content, and approved the final version of the manuscript. The authors reported no conflicts of interest relevant to this article.
Footnotes
Abbreviations used: ATBC, Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study; MEC, Multiethnic Cohort; PCPT, Prostate Cancer Prevention Trial; PLCO, Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial; ProtecT, Prostate testing for cancer and Treatment trial; PSA, prostate-specific antigen; SU.VI.MAX, SUpplémentation en VItamines et Minéraux Anti-oXydants trial; TNM, tumor/node/metastasis.
REFERENCES
- 1.Chan JM, Gann PH, Giovannucci EL. Role of diet in prostate cancer development and progression. J Clin Oncol 2005;23:8152–60. [DOI] [PubMed] [Google Scholar]
- 2.Krinsky NI, Johnson EJ. Carotenoid actions and their relation to health and disease. Mol Aspects Med 2005;26:459–516. [DOI] [PubMed] [Google Scholar]
- 3.O’Neill ME, Carroll Y, Corridan B, Olmedilla B, Granado F, Blanco I, Van den Berg H, Hininger I, Rousell AM, Chopra M, et al. A European carotenoid database to assess carotenoid intakes and its use in a five-country comparative study. Br J Nutr 2001;85:499–507. [DOI] [PubMed] [Google Scholar]
- 4.Schlatterer J, Breithaupt DE. Xanthophylls in commercial egg yolks: quantification and identification by HPLC and LC-(APCI)MS using a C30 phase. J Agric Food Chem 2006;54:2267–73. [DOI] [PubMed] [Google Scholar]
- 5.McKevith B. Is salmon salmon pink? The use of canthaxanthin in animal feeds. Nutr Bull 2003;28:243–5. [Google Scholar]
- 6.Ford ES, Schleicher RL, Mokdad AH, Ajani UA, Liu S. Distribution of serum concentrations of alpha-tocopherol and gamma-tocopherol in the US population. Am J Clin Nutr 2006;84:375–83. [DOI] [PubMed] [Google Scholar]
- 7.Roddam AW, Allen NE, Appleby P, Key TJ, Ferrucci L, Carter HB, Metter EJ, Chen C, Weiss NS, Fitzpatrick A, et al. Insulin-like growth factors, their binding proteins, and prostate cancer risk: analysis of individual patient data from 12 prospective studies. Ann Intern Med 2008;149:461–71, W83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roddam AW, Allen NE, Appleby P, Key TJ; Endogenous Hormones and Prostate Cancer Collaborative Group. Endogenous sex hormones and prostate cancer: a collaborative analysis of 18 prospective studies. J Natl Cancer Inst 2008;100:170–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crowe FL, Appleby PN, Travis RC, Barnett M, Brasky TM, Bueno-de-Mesquita HB, Chajes V, Chavarro JE, Chirlaque MD, English DR, et al. Circulating fatty acids and prostate cancer risk: individual participant meta-analysis of prospective studies. J Natl Cancer Inst 2014;106:dju240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giovannucci E. Commentary: Serum lycopene and prostate cancer progression: a re-consideration of findings from the prostate cancer prevention trial. Cancer Causes Control 2011;22:1055–9. [DOI] [PubMed] [Google Scholar]
- 11.Mondul AM, Watters JL, Männistö S, Weinstein SJ, Snyder K, Virtamo J, Albanes D. Serum retinol and risk of prostate cancer. Am J Epidemiol 2011;173:813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mondul AM, Rohrmann S, Menke A, Feinleib M, Nelson WG, Platz EA, Albanes D. Association of serum α-tocopherol with sex steroid hormones and interactions with smoking: implications for prostate cancer risk. Cancer Causes Control 2011;22:827–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartman TJ, Albanes D, Pietinen P, Hartman AM, Rautalahti M, Tangrea JA, Taylor PR. The association between baseline vitamin E, selenium, and prostate cancer in the alpha-tocopherol, beta-carotene cancer prevention study. Cancer Epidemiol Biomarkers Prev 1998;7:335–40. [PubMed] [Google Scholar]
- 14.Weinstein SJ, Wright ME, Lawson KA, Snyder K, Männistö S, Taylor PR, Virtamo J, Albanes D. Serum and dietary vitamin E in relation to prostate cancer risk. Cancer Epidemiol Biomarkers Prev 2007;16:1253–9. [DOI] [PubMed] [Google Scholar]
- 15.Goodman GE, Schaffer S, Omenn GS, Chen C, King I. The association between lung and prostate cancer risk, and serum micronutrients: results and lessons learned from beta-carotene and retinol efficacy trial. Cancer Epidemiol Biomarkers Prev 2003;12:518–26. [PubMed] [Google Scholar]
- 16.Hsing AW, Comstock GW, Abbey H, Polk BF. Serologic precursors of cancer: retinol, carotenoids, and tocopherol and risk of prostate cancer. J Natl Cancer Inst 1990;82:941–6. [DOI] [PubMed] [Google Scholar]
- 17.Huang HY, Alberg AJ, Norkus EP, Hoffman SC, Comstock GW, Helzlsouer KJ. Prospective study of antioxidant micronutrients in the blood and the risk of developing prostate cancer. Am J Epidemiol 2003;157:335–44. [DOI] [PubMed] [Google Scholar]
- 18.Key TJ, Appleby PN, Allen NE, Travis RC, Roddam AW, Jenab M, Egevad L, Tjønneland A, Johnsen NF, Overvad K, et al. Plasma carotenoids, retinol, and tocopherols and the risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition study. Am J Clin Nutr 2007;86:672–81. [DOI] [PubMed] [Google Scholar]
- 19.Knekt P, Aromaa A, Maatela J, Aaran RK, Nikkari T, Hakama M, Hakulinen T, Peto R, Teppo L. Serum vitamin A and subsequent risk of cancer: cancer incidence follow-up of the Finnish Mobile Clinic Health Examination Survey. Am J Epidemiol 1990;132:857–70. [DOI] [PubMed] [Google Scholar]
- 20.Wu K, Erdman JW Jr, Schwartz SJ, Platz EA, Leitzmann M, Clinton SK, DeGroff V, Willett WC, Giovannucci E. Plasma and dietary carotenoids, and the risk of prostate cancer: a nested case-control study. Cancer Epidemiol Biomarkers Prev 2004;13:260–9. [DOI] [PubMed] [Google Scholar]
- 21.Meyer HE, Robsahm TE, Bjørge T, Brustad M, Blomhoff R. Vitamin D, season and risk of prostate cancer: a nested case-control study within Norwegian health studies. Am J Clin Nutr 2013;97:147–54. [DOI] [PubMed] [Google Scholar]
- 22.Hodge AM, Simpson JA, Fridman M, Rowley K, English DR, Giles GG, Su Q, O’Dea K. Evaluation of an FFQ for assessment of antioxidant intake using plasma biomarkers in an ethnically diverse population. Public Health Nutr 2009;12:2438–47. [DOI] [PubMed] [Google Scholar]
- 23.Bassett JK, Severi G, Hodge AM, MacInnis RJ, Gibson RA, Hopper JL, English DR, Giles GG. Plasma phospholipid fatty acids, dietary fatty acids and prostate cancer risk. Int J Cancer 2013;133:1882–91. [DOI] [PubMed] [Google Scholar]
- 24.Gill JK, Franke AA, Steven Morris J, Cooney RV, Wilkens LR, Le Marchand L, Goodman MT, Henderson BE, Kolonel LN. Association of selenium, tocopherols, carotenoids, retinol, and 15-isoprostane F(2t) in serum or urine with prostate cancer risk: the multiethnic cohort. Cancer Causes Control 2009;20:1161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kristal AR, Till C, Platz EA, Song X, King IB, Neuhouser ML, Ambrosone CB, Thompson IM. Serum lycopene concentration and prostate cancer risk: results from the Prostate Cancer Prevention Trial. Cancer Epidemiol Biomarkers Prev 2011;20:638–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gann PH, Ma J, Giovannucci E, Willett W, Sacks FM, Hennekens CH, Stampfer MJ. Lower prostate cancer risk in men with elevated plasma lycopene levels: results of a prospective analysis. Cancer Res 1999;59:1225–30. [PubMed] [Google Scholar]
- 27.Schenk JM, Riboli E, Chatterjee N, Leitzmann MF, Ahn J, Albanes D, Reding DJ, Wang Y, Friesen MD, Hayes RB, et al. Serum retinol and prostate cancer risk: a nested case-control study in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Biomarkers Prev 2009;18:1227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peters U, Leitzmann MF, Chatterjee N, Wang Y, Albanes D, Gelmann EP, Friesen MD, Riboli E, Hayes RB. Serum lycopene, other carotenoids, and prostate cancer risk: a nested case-control study in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Biomarkers Prev 2007;16:962–8. [DOI] [PubMed] [Google Scholar]
- 29.Weinstein SJ, Peters U, Ahn J, Friesen MD, Riboli E, Hayes RB, Albanes D. Serum α-tocopherol and γ-tocopherol concentrations and prostate cancer risk in the PLCO Screening Trial: a nested case-control study. PLoS One 2012;7:e40204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilbert R, Metcalfe C, Fraser WD, Donovan J, Hamdy F, Neal DE, Lane JA, Martin RM. Associations of circulating retinol, vitamin E, and 1,25-dihydroxyvitamin D with prostate cancer diagnosis, stage, and grade. Cancer Causes Control 2012;23:1865–73. [DOI] [PubMed] [Google Scholar]
- 31.Hercberg S, Galan P, Preziosi P, Bertrais S, Mennen L, Malvy D, Roussel AM, Favier A, Briançon S. The SU.VI.MAX study: a randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch Intern Med 2004;164:2335–42. [DOI] [PubMed] [Google Scholar]
- 32.Finne P, Fallah M, Hakama M, Ciatto S, Hugosson J, de Koning H, Moss S, Nelen V, Auvinen A. Lead-time in the European Randomised Study of Screening for Prostate Cancer. Eur J Cancer 2010;46:3102–8. [DOI] [PubMed] [Google Scholar]
- 33.Nomura AM, Stemmermann GN, Lee J, Craft NE. Serum micronutrients and prostate cancer in Japanese Americans in Hawaii. Cancer Epidemiol Biomarkers Prev 1997;6:487–91. [PubMed] [Google Scholar]
- 34.Reichman ME, Hayes RB, Ziegler RG, Schatzkin A, Taylor PR, Kahle LL, Fraumeni JF Jr. Serum vitamin A and subsequent development of prostate cancer in the first National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Cancer Res 1990;50:2311–5. [PubMed] [Google Scholar]
- 35.Beilby J, Ambrosini GL, Rossi E, de Klerk NH, Musk AW. Serum levels of folate, lycopene, β-carotene, retinol and vitamin E and prostate cancer risk. Eur J Clin Nutr 2010;64:1235–8. [DOI] [PubMed] [Google Scholar]
- 36.Karppi J, Kurl S, Nurmi T, Rissanen TH, Pukkala E, Nyyssönen K. Serum lycopene and the risk of cancer: the Kuopio Ischaemic Heart Disease Risk Factor (KIHD) study. Ann Epidemiol 2009;19:512–8. [DOI] [PubMed] [Google Scholar]
- 37.Karppi J, Kurl S, Laukkanen JA, Kauhanen J. Serum β-carotene in relation to risk of prostate cancer: the Kuopio Ischaemic Heart Disease Risk Factor study. Nutr Cancer 2012;64:361–7. [DOI] [PubMed] [Google Scholar]
- 38.Eichholzer M, Stähelin HB, Lüdin E, Bernasconi F. Smoking, plasma vitamins C, E, retinol, and carotene, and fatal prostate cancer: seventeen-year follow-up of the prospective Basel study. Prostate 1999;38:189–98. [DOI] [PubMed] [Google Scholar]
- 39.Engeland A, Tretli S, Bjørge T. Height, body mass index, and prostate cancer: a follow-up of 950000 Norwegian men. Br J Cancer 2003;89:1237–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allen NE, Key TJ, Appleby PN, Travis RC, Roddam AW, Tjønneland A, Johnsen NF, Overvad K, Linseisen J, Rohrmann S, et al. Animal foods, protein, calcium and prostate cancer risk: the European Prospective Investigation into Cancer and Nutrition. Br J Cancer 2008;98:1574–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rohrmann S, Linseisen J, Allen N, Bueno-de-Mesquita HB, Johnsen NF, Tjønneland A, Overvad K, Kaaks R, Teucher B, Boeing H, et al. Smoking and the risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Br J Cancer 2013;108:708–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khillan JS. Vitamin a/retinol and maintenance of pluripotency of stem cells. Nutrients 2014;6:1209–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rohde CM, DeLuca HF. All-trans retinoic acid antagonizes the action of calciferol and its active metabolite, 1,25-dihydroxycholecalciferol, in rats. J Nutr 2005;135:1647–52. [DOI] [PubMed] [Google Scholar]
- 44.Heinonen OP, Albanes D, Virtamo J, Taylor PR, Huttunen JK, Hartman AM, Haapakoski J, Malila N, Rautalahti M, Ripatti S, et al. Prostate cancer and supplementation with alpha-tocopherol and beta-carotene: incidence and mortality in a controlled trial. J Natl Cancer Inst 1998;90:440–6. [DOI] [PubMed] [Google Scholar]
- 45.Gaziano JM, Glynn RJ, Christen WG, Kurth T, Belanger C, MacFadyen J, Bubes V, Manson JE, Sesso HD, Buring JE. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA 2009;301:52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360:23–33.12114037 [Google Scholar]
- 47.Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, Ross C, Arnold A, Sleight P, Probstfield J, et al. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA 2005;293:1338–47. [DOI] [PubMed] [Google Scholar]
- 48.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2009;301:39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klein EA, Thompson IM Jr, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, Minasian LM, Ford LG, Parnes HL, Gaziano JM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011;306:1549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Key TJ, Appleby PN, Allen NE, Reeves GK. Pooling biomarker data from different studies of disease risk, with a focus on endogenous hormones. Cancer Epidemiol Biomarkers Prev 2010;19:960–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eliassen AH, Hendrickson SJ, Brinton LA, Buring JE, Campos H, Dai Q, Dorgan JF, Franke AA, Gao YT, Goodman MT, et al. Circulating carotenoids and risk of breast cancer: pooled analysis of eight prospective studies. J Natl Cancer Inst 2012;104:1905–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Comstock GW, Burke AE, Hoffman SC, Norkus EP, Gross M, Helzlsouer KJ. The repeatability of serum carotenoid, retinoid, and tocopherol concentrations in specimens of blood collected 15 years apart. Cancer Epidemiol Biomarkers Prev 2001;10:65–8. [PubMed] [Google Scholar]