Abstract
Background: Vitamin D and iron deficiencies frequently co-exist. It is now appreciated that mechanistic interactions between iron and vitamin D metabolism may underlie these associations.
Objective: We examined interrelations between iron and vitamin D status and their regulatory hormones in pregnant adolescents, who are a group at risk of both suboptimal vitamin D and suboptimal iron status.
Design: The trial was a prospective longitudinal study of 158 pregnant adolescents (aged ≤18 y). Maternal circulating biomarkers of vitamin D and iron were determined at midgestation (∼25 wk) and delivery (∼40 wk). Linear regression was used to assess associations between vitamin D and iron status indicators. Bivariate and multivariate logistic regressions were used to generate the OR of anemia as a function of vitamin D status. A mediation analysis was performed to examine direct and indirect relations between vitamin D status, hemoglobin, and erythropoietin in maternal serum.
Results: Maternal 25-hydroxyvitamin D [25(OH)D] was positively associated with maternal hemoglobin at both midgestation and at delivery (P < 0.01 for both). After adjustment for age at enrollment and race, the odds of anemia at delivery was 8 times greater in adolescents with delivery 25(OH)D concentrations <50 nmol/L than in those with 25(OH)D concentrations ≥50 nmol/L (P <0.001). Maternal 25(OH)D was inversely associated with erythropoietin at both midgestation (P <0.05) and delivery (P <0.001). The significant relation observed between 25(OH)D and hemoglobin could be explained by a direct relation between 25(OH)D and hemoglobin and an indirect relation that was mediated by erythropoietin.
Conclusions: In this group of pregnant adolescents, suboptimal vitamin D status was associated with increased risk of iron insufficiency and vice versa. These findings emphasize the need for screening for multiple nutrient deficiencies during pregnancy and greater attention to overlapping metabolic pathways when selecting prenatal supplementation regimens.
Keywords: anemia, hepcidin, iron, pregnancy, vitamin D
INTRODUCTION
A recent report from the CDC showed that vitamin D and iron are 2 of the top 3 most prevalent nutrient deficiencies in the United States because ∼10% of women aged 12–49 y are iron deficient (defined as total body Fe <0 mg/kg), and 8% of Americans ≥1 y of age are vitamin D deficient (defined as serum 25-hydroxyvitamin D [25(OH)D]7 concentrations <30 nmol/L) (1). Risk of insufficiency of both of these nutrients is particularly high during pregnancy, and a deficiency of each nutrient has independently been associated with increased risk of adverse pregnancy outcomes. Iron deficiency and iron-deficiency anemia during pregnancy have been linked to increased risk of intrauterine growth restriction, premature delivery, low birth weight, postpartum depression, and maternal morbidity and mortality (2–4). Similarly, vitamin D deficiency during pregnancy has been associated with increased risk of preeclampsia, small for gestational age, neonatal hypocalcemia, impaired fetal bone development, and low neonatal vitamin D status (5–7).
For decades, vitamin D insufficiency and iron insufficiency have been noted to co-exist. Suboptimal vitamin D status has been shown to be associated with a greater prevalence of anemia across the life cycle from children to healthy adults to the elderly (8–11). This association is most often attributed to an overall poor-quality diet as noted as early as 1934: “Parsons (1934) says that anaemia is in no sense a symptom of rickets, although the degree of nutritional anaemia is not unusual, because a diet which is defective in one factor is likely to be defective in others” (12). However, diet quality is unlikely to be the primary factor responsible for these observed relations because the majority of vitamin D in healthy individuals is produced endogenously in the epidermis after exposure to UVB radiation (13). In addition, the degree to which biological mechanisms might underlie these associations has only begun to be highlighted, and emerging data suggest that vitamin D affects iron metabolism by modulating erythropoiesis and hepcidin production (14–18). In contrast, iron has been shown to modify the expression of fibroblast growth factor 23 (FGF23), which is a hormone that also regulates vitamin D metabolism (19–21).
With these observations in mind, the goal of this study was to explore possible associations between iron and vitamin D biomarkers in a cohort of pregnant adolescents with a high prevalence of both iron insufficiency and anemia (22) and suboptimal vitamin D status (23). In this study, we began to identify possible shared pathways by which iron insufficiency may affect vitamin D status and, conversely, by which suboptimal vitamin D status may alter iron homeostasis across gestation.
METHODS
Participants
This study was a substudy of a larger prospective longitudinal study (n = 171) conducted by our group that was designed to evaluate maternal and fetal bone health across gestation in pregnant adolescents. A priori calculations for sample-size estimates as well as recruitment strategies to allow for the loss to follow-up and missing data have been described previously (7). Of the 171 adolescents enrolled in the larger parent study, 158 subjects had vitamin D and iron status data available and, thus, were included in the current study.
For the parent study, a cohort of pregnant adolescents (aged ≤18 y) were recruited from the Rochester Adolescent Maternal Program in Rochester, New York, beginning in June 2007. All eligible pregnant adolescents who were attending the Rochester Adolescent Maternity Program clinic were approached and asked to participate in the study to ensure that the study population was representative of the patient population who attended the clinic. All procedures were approved by institutional review boards at Cornell University and the University of Rochester. Written informed consent was obtained from all study participants aged 15–18 y. If participants were ≤14 y old, both assent and parental consent were obtained. Teens who were carrying a single fetus were eligible if they were otherwise healthy and were at ≥12 wk of gestation at entry into the study. Subjects with HIV infection, eating disorders, malabsorption diseases, diabetes, pre-eclampsia, self-reported drug use, or any other diagnosed medical conditions that might affect mineral homeostasis were excluded. On entry to the study, maternal race, ethnicity, prepregnancy weight, and smoking history were self-reported. Participants attended up to 3 study visits across gestation, which were timed to coincide with early pregnancy, midpregnancy, and late pregnancy. During these visits, maternal anthropometric measures were recorded. Maternal blood (10 mL) was collected at midgestation (25.3 ± 3.4 wk) and at delivery (39.8 ± 1.2 wk). Serum was stored at −80°C until analysis. Results on calcitropic hormones (23, 24), fetal bone growth (7), bone turnover and osteoprotegerin (25), changes in maternal bone quality across gestation (26), maternal iron status, erythropoietin and hepcidin (22), pica behaviors (27), placental iron transporter expression (28–30), maternal diet and fetal adiposity (31), and placental vitamin D hydroxylase expression (24) in this cohort have been published.
Dietary assessment
To estimate habitual dietary intake during pregnancy, study personnel trained by research dietitians at the University of Rochester’s Clinical and Translational Science Center administered 24-h dietary recalls to study participants during their scheduled study visits. Dietary intake data were entered into Nutrient Data System for Research (versions 2006, 2008, and 2009; Nutrition Coordinating Center, University of Minnesota) and analyzed by a registered dietitian. As part of their routine prenatal care, all adolescents were prescribed a standard prenatal vitamin and mineral supplement that contained 27 mg elemental iron as ferrous fumarate. If adolescents were shown to be anemic, additional iron supplements (which contained 65 mg ferrous sulfate) were provided over the remainder of their pregnancies. In addition, midway through the study, if the teens were vitamin D insufficient [25(OH)D concentration <50 nmol/L), cholecalciferol (vitamin D3) supplements that contained 10 μg (400 IU) were prescribed to be taken until term (23). To assess the use of prenatal supplements, questionnaires were administered to teens at study visits, and supplement use was self-reported as described previously (22).
Biochemical assessment
Maternal and neonatal serum 25(OH)D concentrations were measured by Quest Laboratories, and calcitriol (1,25-dihydroxyvitamin D [1,25(OH)2D]) concentrations were measured with the use of a receptor-binding assay as previously reported (23). Intact parathyroid hormone was analyzed with the use of a commercially available ELISA (DSL Laboratories). Maternal and neonatal hemoglobin and hematocrit were analyzed by the Strong Memorial Hospital’s clinical laboratory with the use of the Cell Dyn 4000 system (Abbott Laboratories). Serum ferritin and soluble transferrin receptor (sTfR) were measured with the use of ELISAs from Ramco Laboratories (Ramco Laboratories Inc.), and serum iron was measured with the use of graphite furnace atomic absorption spectrophotometry (Perkin Elmer Analyst 800; Perkin Elmer). Maternal and neonatal serum hepcidin concentrations were measured by Intrinsic LifeSciences with the use of a competitive ELISA. Hepcidin concentrations <5.0 ng/mL (the limit of detection) were assigned a value of 2.5 ng/mL for analysis purposes. Serum erythropoietin was measured with the use of the IMMULITE 2000 Immunoassay System (Siemens).
To identify the presence of anemia across gestation, the following CDC thresholds of hemoglobin concentrations were used: <11 g/dL in the first trimester, <10.5 g/dL in the second trimester, and <11 g/dL in the third trimester (32). The following Institute of Medicine (IOM) hemoglobin thresholds were used to define anemia in African American adolescents in our study: 10.2 g/dL in the first and third trimesters and 9.7 g/dL in the second trimester (33). Adolescents were identified as having depleted iron stores if serum ferritin concentrations were <12 μg/L (32), whereas tissue iron deficiency was defined as sTfR concentrations >8.5 mg/L (34). Iron deficiency anemia was present if adolescents were anemic with serum ferritin concentrations <12 μg/L.
The following IOM cutoffs were used when vitamin D status was assessed: serum 25(OH)D concentrations <30 nmol/L (12 ng/mL) were suggestive of risk of vitamin D deficiency relative to bone health; serum 25(OH)D concentrations ≥40 nmol/L (16 ng/mL) reflected the desired concentration for a population median, and this value was used for the establishment of the estimated average requirement (EAR) for vitamin D; and it is thought that practically all persons are vitamin D sufficient at serum 25(OH)D concentrations ≥50 nmol/L (20 ng/mL) (35).
Statistical analysis
Statistical analyses were conducted with the use of JMP 10.0 software (SAS Institute Inc.) and Stata/MP 13.1 software (StataCorp LP). Some of the variables were transformed with the use of the ln to fit the assumptions of the model. A simple linear regression was used to evaluate associations between maternal and neonatal vitamin D status indicators and maternal and neonatal iron status indicators. Univariate and multivariate logistic regression analyses were used to calculate the OR of anemia as a function of vitamin D status. Potential differences in maternal and neonatal hemoglobin concentrations in adolescents as a function of vitamin D status were evaluated with the use of a 1-factor ANOVA. Multivariate regression models were constructed to determine the extent to which maternal vitamin D status and hemoglobin concentrations were associated with one another. A mediation analysis was performed to examine direct and indirect relations (as through erythropoietin) between vitamin D status and hemoglobin concentrations. These effects were estimated with the use of a structural equation model in Stata/MP 13.1 software. Age and race are known to affect both vitamin D status and iron status; therefore, we adjusted for these covariates in all multivariate analyses. Data are reported as means ± SDs unless otherwise noted. For all statistical analyses, differences with a P value <0.05 were considered significant.
RESULTS
Participant characteristics
Characteristics of the 158 pregnant adolescents with both iron and vitamin D data are shown in Table 1. Age at enrollment ranged from 13.6 to 18.7 y and averaged 17.1 ± 1.1 y. Sixty-four percent of adolescents were classified as either underweight or normal weight according to their prepregnancy BMI, whereas 36% of adolescents were considered overweight or obese. On average, adolescents entered prenatal care at 10.7 ± 5.3 wk of gestation and were enrolled into this study at 21.7 ± 5.5 wk of gestation. Teens delivered, on average, at 39.2 ± 3.0 wk, with 8.6% of teens delivering preterm (<37 wk of gestation), 19.1% of teens delivering early term (37–39 wk of gestation), and 17.8% of teens delivering late or postterm (≥41 wk of gestation).
TABLE 1.
Characteristics of pregnant adolescents1
| Maternal characteristic | Value |
| Total subjects, n | 158 |
| Maternal age at enrollment, y | 17.1 ± 1.1 [158]1 |
| Race | |
| African American, % | 64.6 |
| Caucasian, % | 35.4 |
| Ethnicity | |
| Hispanic, % | 24.7 |
| Non-Hispanic, % | 75.3 |
| Prepregnancy BMI, kg/m2 | 24.7 ± 5.4 [156] |
| Gestational weight gain, kg | 17.0 ± 7.9 [150] |
| Parity >0, n (%) | 158 (8.9) |
| Dietary vitamin D intake,2 IU/d | 203.6 ± 120.9 [156] |
| Dietary iron intake,2 mg/d | 18.6 ± 9.7 [156] |
| Neonatal birth weight, g | 3204 ± 582 [149] |
| Preterm birth, n (%) | 152 (8.6) |
Mean ± SD; n in brackets (all such values).
Intake was calculated from the mean of all 24-h dietary recalls administered (≤3 recalls/participant).
Maternal dietary intake and vitamin D and iron statuses
Daily dietary vitamin D intake ranged from 14 to 589 IU/d. The mean vitamin D intake (204 ± 121 IU/d) was approximately one-half of the IOM EAR of 400 IU for pregnant women aged ≤18 y (35). Dietary iron intake ranged from 5.5 to 104.4 mg/d, and mean intake was 18.6 ± 9.7 mg/d, which was 20% lower than the EAR of 23 mg/d for pregnant teens aged 14–18 y. Dietary intakes of iron and vitamin D were significantly positively associated with one another (R2 = 0.272, P <0.0001; n = 156).
Biochemical markers of vitamin D and iron status are shown in Table 2. Of the 158 adolescents enrolled in this study, 98% of subjects (n = 155 of 158) had a midgestation blood draw, and 83.5% of subjects (n = 132 of 158) had a blood draw at delivery. Seventeen percent of teens (n = 27 of 158) had blood collected at midgestation only, and 1.3% of teens (n = 2 of 158) had blood collected at delivery only. Variable sample sizes for the biochemical indicators were primarily due to missing data related to an insufficient serum volume for all biochemical analyses and delivery complications that hindered our ability to obtain a delivery blood collection. To determine whether participants with missing data were significantly different from participants with complete data, a comparison of means for continuous variables and chi-square tests for categorical variables were performed for the variables listed in Tables 1 and 2. Pregnant teens with missing data at delivery were shown to have significantly lower gestational age at delivery than did teens without missing data (mean gestational age at delivery: 36.9 ± 5.1 vs. 39.9 ± 1.3 wk of gestation, respectively; P < 0.0001). Serum iron at midgestation was significantly higher in participants with missing data at delivery than in those without missing data at delivery (P < 0.014). To account for these differences, we included the gestational age at delivery and midgestation serum iron as covariates in the multivariate models. However, results of these analyses showed that the gestational age at delivery and serum iron at midgestation were not significant predictors of any of the outcomes tested; therefore, we removed these variables from the final models.
TABLE 2.
Vitamin D and iron status at midgestation and at delivery in relation to maternal race1
| Whole cohort (n = 158)2 |
Pregnant Caucasian adolescents (n = 56)3 |
Pregnant African American adolescents (n = 102)4 |
||||
| Biochemical marker | Midgestation | Delivery | Midgestation | Delivery | Midgestation | Delivery |
| 25(OH)D, nmol/L | 56.0 ± 25.65 | 53.4 ± 29.1 | 64.6 ± 23.6 | 60.5 ± 33.6 | 51.2 ± 25.5 | 49.3 ± 25.4 |
| n | 155 | 133 | 55 | 49 | 100 | 84 |
| <50 nmol/L, % | 49.7 | 48.1 | 32.8 | 36.7 | 59.0 | 54.8 |
| <40 nmol/L, % | 22.6 | 31.6 | 5.5 | 24.5 | 32.0 | 35.7 |
| <30 nmol/L, % | 10.3 | 17.3 | 1.8 | 10.2 | 15.0 | 21.4 |
| 1,25(OH)2D, pmol/L | 280.5 ± 72.8 | 254.9 ± 73.4* | 275.5 ± 74.5 | 233.3 ± 70.0* | 283.5 ± 72.2 | 266.2 ± 73.2 |
| n | 100 | 96 | 37 | 33 | 63 | 63 |
| iPTH, pg/mL | 24.6 (17.4)6 | 39.3 (32.2)* | 21.9 (10.8) | 37.8 (21.6)* | 28.4 (18.3) | 41.6 (33.9)* |
| n | 91 | 80 | 35 | 29 | 56 | 51 |
| ≥46 pg/mL, % | 12.1 | 40.0 | 0 | 34.5 | 19.6 | 43.1 |
| Hemoglobin, g/dL | 11.2 ± 0.9 | 11.5 ± 1.3* | 11.6 ± 0.9 | 11.9 ± 1.2* | 11.0 ± 0.8 | 11.3 ± 1.4* |
| n | 120 | 141 | 40 | 50 | 80 | 91 |
| Anemia,7 % | 8.6 | 15.8 | 10.3 | 20.0 | 7.8 | 13.5 |
| Ferritin, μg/L | 17.9 (18.1) | 21.0 (19.7) | 17.3 (20.3) | 24.4 (18.8) | 18.2 (17.3) | 18.1 (18.8) |
| n | 147 | 130 | 51 | 48 | 96 | 82 |
| <12 μg/L, % | 26.5 | 22.3 | 29.4 | 16.7 | 25.0 | 25.6 |
| Serum iron, μg/L | 940 (675) | 1024 (796) | 970 (546) | 1063 (1021) | 934 (779) | 1022 (761) |
| n | 146 | 130 | 51 | 48 | 95 | 82 |
| sTfR, mg/L | 4.4 (2.3) | 4.8 (3.1)* | 4.5 (2.3) | 4.5 (3.1) | 4.3 (2.3) | 5.0 (3.4)* |
| n | 147 | 130 | 51 | 48 | 96 | 82 |
| >8.5 mg/L, % | 6.8 | 14.6 | 5.9 | 10.4 | 7.3 | 17.1 |
| Hepcidin, ng/mL | 22.05 (22.7) | 29 (35.2) | 22.6 (29.1) | 32.8 (50.1) | 22 (22.4) | 28 (27.0) |
| n | 138 | 119 | 49 | 43 | 89 | 76 |
| <5 ng/mL, % | 4.8 | 7.8 | 2.0 | 10.4 | 6.3 | 6.2 |
| Erythropoietin, mIU/mL | 27.3 (16.2) | 25.5 (19.1) | 26.7 (16.8) | 22.8 (19.3) | 28.3 (17.0) | 26.2 (18.5) |
| n | 144 | 125 | 50 | 45 | 94 | 80 |
*Significantly different from midgestation values with the use of paired t test, P <0.05. iPTH, intact parathyroid hormone; sTfR, soluble transferrin receptor; 1,25(OH)2D, 1,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D.
Midgestation: 26.0 ± 3.3 wk; delivery: 39.2 ± 3.0 wk.
Midgestation: 25.6 ± 3.4 wk; delivery: 39.2 ± 3.2 wk.
Midgestation: 26.2 ± 3.3 wk; delivery: 39.1 ± 2.9 wk.
Mean ± SD (all such values).
Median; IQR in parentheses (all such values).
Anemia prevalence was calculated with the use of race- and trimester-specific cutoffs as described in Methods.
At midgestation (26.0 ± 3.3 wk of pregnancy), 49.7% of teens had 25(OH)D concentrations <50 nmol/L (n = 77/155), and of these teens, 45% had 25(OH)D concentrations <40 nmol/L (n = 35), and 20.8% had 25(OH)D concentrations <30 nmol/L (n = 16). Neither these percentages nor individual 25(OH)D status changed from midgestation to delivery. Participants with 25(OH)D concentrations <50 nmol/L midway through the study received an additional 400 IU supplemental vitamin D/d. As described previously (23), only ∼26% of the adolescents who were given a vitamin D supplement reported taking it daily. Although the change in 25(OH)D concentration from midgestation to delivery was significantly greater in supplemented teens than in nonsupplemented teens (8.1 compared with −7.3 nmol/L, respectively; P = 0.003), the supplemented group still had a higher prevalence of 25(OH)D concentrations <50 nmol/L at delivery than that of the nonsupplemented group (57.8% compared with 43.2%, respectively; P = 0.11).
The prevalence of anemia (race and trimester adjusted) in this cohort increased across gestation and was 3.2% in the first trimester (9.3 ± 2.2 wk of gestation; n = 3 of 93), 5.4% in the second trimester (22.1 ± 3.9 wk of gestation; n = 5 of 92), 20.6% in the third trimester (32.9 ± 3.6 wk of gestation; n = 14 of 68), and 15.8% at delivery (39.4 ± 2.4 wk of gestation; n = 22 of 139). If we used hemoglobin cutoffs that were not adjusted for race, the prevalence of anemia would have been significantly greater during the second trimester (20.8% compared with 5.4%; P = 0.003), during the third trimester (37.7% compared with 20.6%; P = 0.03), and at delivery (32.6% compared with 15.8%; P = 0.001). In total, 114 adolescents (72% of the study cohort) were prescribed iron supplementation, and of these adolescents, 55% of subjects (n = 63) reported taking the iron supplement ≥2–5 times/wk. Teens who were prescribed an iron supplement exhibited a greater but nonsignificant increase in hemoglobin concentration from midgestation to delivery than did adolescents who were not prescribed an iron supplement (0.47 compared with 0.04 g/dL, respectively; P = 0.053). When the prevalence of anemia was examined, 12.8% of teens who were supplemented with additional iron were anemic at delivery (defined with the use of race- and trimester-specific cutoffs) compared with 24.3% of teens who were not provided additional iron supplements; however, this difference was NS (P = 0.098).
Relations between vitamin D and iron statuses
Maternal 25(OH)D was significantly and positively associated with the maternal hemoglobin concentration both at midgestation (R2 = 0.065, P = 0.005; n = 118) and at delivery (R2 = 0.084, P = 0.001; n = 125). When adolescents were stratified by vitamin D status, the subgroup analysis revealed that adolescents with 25(OH)D concentrations <50 nmol/L at midgestation had significantly lower midgestation hemoglobin concentrations than did those with 25(OH)D concentrations ≥50 nmol/L (P = 0.01) (Table 3). In contrast, midgestation hemoglobin concentrations did not differ significantly between adolescents with midgestation 25(OH)D concentrations <40 compared with ≥40 nmol/L (Table 3). Similarly, there were no significant differences in midgestation hemoglobin concentrations in adolescents with midgestation 25(OH)D concentrations <30 compared with ≥30 nmol/L (Table 3).
TABLE 3.
Differences in maternal hemoglobin concentrations at midgestation and at delivery as a function of maternal 25(OH)D status in pregnant adolescents1
| Serum 25(OH)D, nmol/L |
|||||||||
| <50 | ≥50 | P2 | <40 | ≥40 | P2 | <30 | ≥30 | P2 | |
| Midgestation | |||||||||
| n | 60 | 58 | 29 | 89 | 14 | 104 | |||
| Hemoglobin, g/dL | 11.0 ± 0.833 | 11.4 ± 0.92 | 0.01 | 10.9 ± 0.82 | 11.2 ± 0.91 | 0.11 | 10.9 ± 0.85 | 11.2 ± 0.90 | 0.31 |
| Anemic, % | 6.8 | 10.5 | 0.47 | 6.9 | 9.2 | 0.70 | 7.1 | 8.8 | 0.83 |
| Delivery | |||||||||
| n | 58 | 67 | 39 | 86 | 20 | 105 | |||
| Hemoglobin, g/dL | 11.1 ± 1.3 | 12.0 ± 1.1 | 0.0001 | 11.3 ± 1.2 | 11.7 ± 1.3 | 0.06 | 11.2 ± 1.2 | 11.7 ± 1.3 | 0.11 |
| Anemic, % | 22.8 | 6.1 | 0.006 | 18.4 | 11.8 | 0.33 | 10.0 | 14.6 | 0.58 |
Anemia was calculated with the use of race- and trimester-specific cutoffs as described in Methods. 25(OH)D, 25-hydroxyvitamin D.
Differences in hemoglobin concentration between subgroups of 25(OH)D status were assessed with the use of Student’s t test. Differences in anemia prevalence between subgroups of 25(OH)D status were calculated with the use of the chi-square test of independence.
Mean ± SD (all such values).
As shown in Table 3, pregnant adolescents with serum 25(OH)D concentrations <50 nmol/L had significantly lower hemoglobin concentrations at delivery than did teens with 25(OH)D concentrations ≥50 nmol/L (P = 0.0001). In addition, there was a trend toward significantly lower delivery hemoglobin concentrations in adolescents with delivery 25(OH)D concentrations <40 compared with ≥40 nmol/L (P = 0.06) (Table 3). However, delivery hemoglobin did not significantly differ between adolescents with delivery 25(OH)D concentrations <30 compared with ≥30 nmol/L (Table 3).
Race is known to be a determinant of both anemia and vitamin D insufficiency with African Americans being at greater risk of suboptimal vitamin D status and low hemoglobin concentrations than are other race and ethnic groups (36). Because the majority of the teens in this study were African American, we adjusted for race in all multivariate analyses. To assess significant predictors of hemoglobin concentrations at delivery, multivariate regression models were constructed (Table 4). With this approach, the delivery 25(OH)D concentration remained a significant determinant of the delivery hemoglobin concentration even after maternal age at enrollment and maternal race were controlled for.
TABLE 4.
Multiple regression analysis of the effect of maternal 25(OH)D at delivery on maternal hemoglobin at delivery1
| Variable | Coefficient ± SE (95% CI) | P |
| Intercept | 7.40 ± 1.76 (3.92, 10.88) | <0.0001 |
| Delivery 25(OH)D | 0.03 ± 0.01 (0.01, 0.06) | 0.0022 |
Model (R2 = 0.135, P = 0.0003; n = 125) was adjusted for maternal race and age at enrollment. 25(OH)D, 25-hydroxyvitamin D.
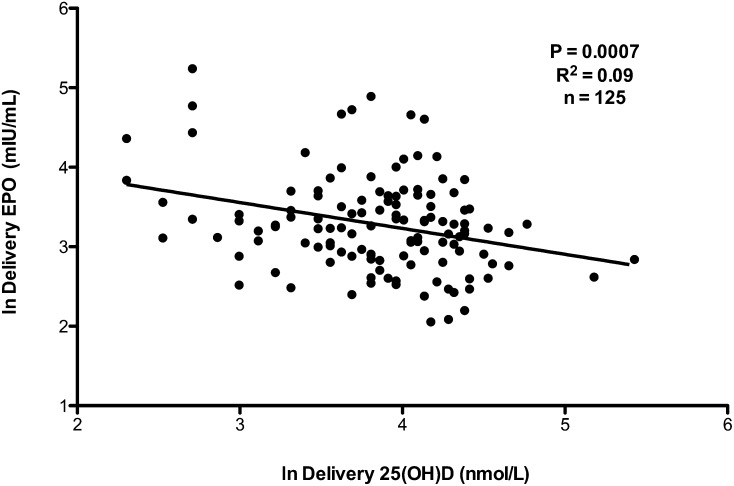
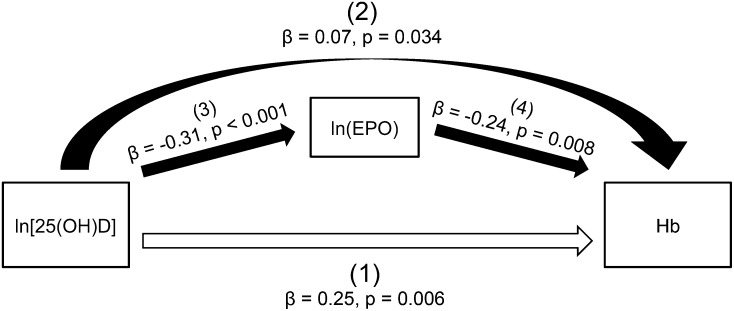
To determine whether maternal vitamin D status was associated with other biochemical markers of maternal iron status, simple linear regression analyses were performed. Results of these analyses showed that maternal 25(OH)D was negatively associated with maternal erythropoietin concentrations both at midgestation (R2 = 0.03, P = 0.04; n = 144) and at delivery (R2 = 0.09, P = 0.0007; n = 125) (Figure 1). Because of the growing literature on interrelations between vitamin D and hemoglobin and between vitamin D and erythropoietin, we developed a statistical model to isolate the direct effect of vitamin D on hemoglobin and the indirect effect of vitamin D on hemoglobin mediated by erythropoietin. As shown in Figure 2, after erythropoietin concentrations were controlled for, a significant and positive direct relation was shown between 25(OH)D and hemoglobin concentrations at delivery [depicted by (1) in Figure 2]. An indirect relation between 25(OH)D and hemoglobin was also shown [depicted by (2) in Figure 2], which was mediated by erythropoietin.
FIGURE 1.
At delivery, the ln(EPO) was significantly inversely associated with the ln[25(OH)D]. Data were analyzed with the use of a simple linear regression (P <0.05). EPO, erythropoietin; 25(OH)D, 25-hydroxyvitamin D.
FIGURE 2.
Mediating role of erythropoietin in the effect of 25(OH)D on Hb concentrations at delivery in 117 pregnant adolescents. The total effect of 25(OH)D on Hb was comprised of direct and indirect effects. The direct relation between 25(OH)D and Hb is shown by the straight open arrow at the bottom of the figure [(1); P = 0.006)]. The indirect effect of 25(OH)D on Hb, which is represented by the curved solid arrow [(2); P = 0.034], is mediated by EPO with the effect of 25(OH)D on EPO [(3); P < 0.001] and the effect of EPO on Hb [(4); P = 0.008] indicated by straight solid arrows in the middle of the figure. A mediation analysis was performed with the use of a structural equation model. EPO, erythropoietin; Hb, hemoglobin; 25(OH)D, 25-hydroxyvitamin D.
Potential relations between vitamin D status and circulating hepcidin concentrations were also explored. Results of this analysis revealed no significant associations between maternal 25(OH)D and circulating hepcidin concentrations at either midgestation (R2 = 0.002, P = 0.64; n = 145) or delivery (R2 = 0.01, P = 0.19; n = 128).
A significant positive association was shown between serum 25(OH)D concentration and serum iron at midgestation (R2 = 0.050, P = 0.006; n = 146) but not at delivery (R2 = 0.009, P = 0.27; n = 130). There were no significant correlations between vitamin D status and serum ferritin or between vitamin D status and sTfR in these adolescents at midgestation or at delivery. However, teens with delivery sTfR concentrations >8.5 mg/L had significantly lower delivery 25(OH)D concentrations than did those with sTfR concentrations ≤8.5 mg/L [mean 25(OH)D concentrations of 45.0 compared with 55.2 nmol/L, respectively; P = 0.0364].
There were no significant associations between maternal serum 1,25(OH)2D and any of the measured biomarkers of iron status. Specifically, at midgestation, serum 1,25(OH)2D was not associated with hemoglobin (R2 = 0.0005, P = 0.84; n = 89), erythropoietin (R2 = 0.0007, P = 0.96; n = 99), serum iron (R2 = 0.002, P = 0.65; n = 100), hepcidin (R2 = 0.017, P = 0.21; n = 100), ferritin (R2 = 0.003, P = 0.60; n = 100), or sTfR (R2 = 0.008, P = 0.38; n = 100). A similar lack of significant associations was evident between calcitriol and iron status biomarkers at delivery [i.e., hemoglobin (R2 = 3.5 × 10−6, P = 0.99; n = 91), erythropoietin (R2 = 0.031, P = 0.09; n = 94), hepcidin (R2 = 0.002, P = 0.70; n = 95), serum iron (R2 = 9.3 × 10−5, P = 0.92; n = 96); ferritin (R2 = 0.01, P = 0.29; n = 96), and sTfR (R2 = 0.0005, P = 0.83; n = 96)].
We evaluated whether circulating parathyroid hormone (PTH) concentrations were associated with circulating iron status markers. A linear regression analysis showed that delivery PTH concentrations were significantly positively correlated with delivery sTfR concentrations (R2 = 0.074, P = 0.015; n = 80) and delivery erythropoietin concentrations (R2 = 0.074, P = 0.017; n = 77). In addition, a significant inverse association between delivery PTH and delivery serum iron (R2 = 0.073, P = 0.015; n = 80) was also observed.
Anemia and vitamin D status
To determine whether vitamin D status at midgestation was associated with risk of anemia at delivery, a logistic regression analysis was performed. In unadjusted models, a serum 25(OH)D concentration <50 nmol/L at midgestation was associated with increased odds of anemia at delivery (OR: 3.7; 95% CI 1.3, 10.9; P = 0.0095). In fully adjusted models (which controlled for maternal race and age at enrollment), pregnant adolescents with 25(OH)D concentrations <50 nmol/L at midgestation had 4 times greater odds of being anemic at delivery than did adolescents with 25(OH)D concentrations ≥50 nmol/L (OR: 4.4; 95% CI 1.5, 14.2; P = 0.005).
As shown in Table 5, in both unadjusted and adjusted models, the odds of anemia at delivery were significantly greater in pregnant adolescents with 25(OH)D concentrations <50 compared with ≥50 nmol/L (P <0.05). The prevalence of anemia in teens with 25(OH)D concentrations <50 nmol/L was 22.8% (n = 13/57) compared with 6.1% in teens with 25(OH)D concentrations ≥50 nmol/L (n = 4/66). The odds of being anemic at delivery in adolescents with serum 25(OH)D concentrations <40 nmol/L were not greater than those shown in adolescents with serum 25(OH)D ≥40 nmol/L (Supplemental Table 1). Similarly, no difference in the odds of anemia at delivery was observed between teens with 25(OH)D concentrations <30 compared with ≥30 nmol/L (Supplemental Table 1).
TABLE 5.
ORs for anemia at delivery in pregnant adolescents with suboptimal vitamin D status
| Delivery 25(OH)D1 concentrations <50 vs. ≥50 nmol/L | OR (95% CI) | P |
| Unadjusted | 4.60 (1.51, 17.11) | 0.0064 |
| Adjusted2 | 7.97 (2.35, 33.67) | 0.0006 |
25(OH)D, 25-hydroxyvitamin D.
Logistic regression model was adjusted for maternal race and age at enrollment (n = 123).
DISCUSSION
To the best of our knowledge, this is the first study to show the existence of significant interrelations between vitamin D status and anemia during pregnancy. Pregnant teens with suboptimal serum 25(OH)D (<50 nmol/L) were significantly more likely to be anemic than were those with 25(OH)D concentrations ≥50 nmol/L. Maternal 25(OH)D was also significantly inversely associated with maternal erythropoietin concentrations at both midgestation and at delivery. The significant relation between vitamin D and hemoglobin could be explained by both a direct association between vitamin D and hemoglobin as well as by an indirect association that was mediated by erythropoietin.
In this study, pregnant adolescents with serum 25(OH)D concentrations <50 nmol/L (<20 ng/mL) were 4–8 times more likely to be anemic than were those with concentrations ≥50 nmol/L. Although, to our knowledge, this relation has not been previously reported during pregnancy, it is known to occur in other age and population groups. Healthy men and women (mean age: 65 y) with 25(OH)D concentrations <30 ng/mL (<75 nmol/L) were ∼1.9 times more likely to be anemic than were those with 25(OH)D concentrations ≥30 ng/mL (11). In a cohort of men and women aged ≥60 y, the prevalence of anemia was greater in participants with 25(OH)D concentrations <50 compared with ≥50 nmol/L (OR: 1.47; P = 0.02) (10). Similar relations have been observed in healthy children aged 1–21 y and in patients with chronic kidney disease (CKD) (8, 37). More recently, in a cohort of 638 healthy adult US men and women, subjects with 25(OH)D concentrations <50 nmol/L had significantly lower hemoglobin, hematocrit, and serum iron concentrations than those of subjects with 25(OH)D concentrations ≥50 nmol/L (38). These studies showed that poor vitamin D status and anemia coexist in several groups of various ages, races, and disease states.
In our study, circulating 25(OH)D was significantly inversely related to serum erythropoietin during pregnancy. Of note, a mediation analysis revealed that the significant indirect relation we observed between vitamin D and hemoglobin was at least partly mediated by erythropoietin. Clinically, this association has been recognized because vitamin D2 supplementation in hemodialysis patients (350,000 IU over a 4-mo period) significantly reduced the amount of erythropoietin needed to maintain hemoglobin concentrations >12.6 g/dL (16). Similarly, vitamin D2 supplementation in children with CKD (120,000 or 240,000 IU over a 3-mo period) significantly reduced the need for erythropoiesis-stimulating agents (17). These findings may have been mediated by a direct effect of calcitriol on erythroid precursors and the enhanced sensitivity of hematopoietic cells to erythropoietin (15) because calcitriol has been shown to not only induce the proliferation of erythroid-derived human stem cells but also augment mRNA and protein expressions of the erythropoietin receptor in hematopoietic tissues (14).
Another hypothesis to explain the relation between vitamin D and iron homeostasis may involve hepcidin, which is the hepatic hormone that increases during iron overload and inflammation to block the iron absorption and release from reticuloendothelial macrophages (39). In a recent study that involved healthy adult men and women, supplementation with 100,000 IU vitamin D2 significantly reduced circulating hepcidin within 24 h of supplementation (18). Similarly, lower serum calcitriol has been identified as a significant independent predictor of higher hepcidin in predialysis CKD patients (40). Potential underlying mechanisms that are responsible for this relation include the suppression of hepatic hepcidin transcription by vitamin D (18) and the vitamin D–mediated attenuation of inflammation, which, in turn, could reduce hepcidin production (41, 42).
In these adolescents, as expected, iron status worsened as pregnancy progressed, and significantly more participants were anemic at delivery than at midgestation. These results may partly explain why some of the strongest associations observed between 25(OH)D and erythropoietin and 25(OH)D and hemoglobin were evident at delivery. We hypothesize that we were unable to detect significant associations between 25(OH)D and other iron biomarkers that also function as acute-phase proteins at delivery because of delivery-associated inflammatory processes. In support of this hypothesis, recent data from our group showed that serum ferritin, hepcidin, and IL-6 were significantly elevated at delivery compared with midgestation, and thus, these indicators were not as reliable when we assessed iron status at this time (22). Our ability to detect significant associations between vitamin D and iron biomarkers were also likely improved by the fact that nearly two-thirds of our study population were African American. Teen pregnancy is known to disproportionately affect minorities (43), and non-Hispanic blacks have been shown to be at increased risk of suboptimal vitamin D status (36) and anemia (44) than are other racial or ethnic groups. These factors may have contributed to our ability to identify significant associations between vitamin D and iron status that may not be as apparent in adult pregnant women. Future studies are needed to explore whether these relations also exist in adult pregnant women.
Although the observed relations between biomarkers of vitamin D and iron were significant, vitamin D status explained only a small percentage of the variability in iron status. However, the R2 values we present (0.05) were similar to those reported (0.04) by Smith et al. (38). Because of the cross-sectional nature of our study, we could not infer causality. It is likely that iron status also affects vitamin D metabolism in part by augmenting the expression of FGF23 (20). FGF23 is a bone-derived hormone that regulates vitamin D metabolism by reducing 1α-hydroxylase expression and by increasing the activity of renal vitamin D 24-hydroxylase, which converts 25(OH)D and calcitriol into inactive metabolites (45). In humans, serum iron was significantly negatively correlated with circulating FGF23 in both healthy adults and adults with autosomal dominant hypophosphatemic rickets (21). In addition, plasma FGF23 concentrations were shown to be higher in Gambian children with anemia than in those without anemia, and hemoglobin was a significant independent predictor of circulating FGF23 concentrations (19). Unfortunately, we do not have any measures of FGF23 or serum phosphate in our pregnant adolescent population to explore this possibility.
In conclusion, to our knowledge, this is the first study to evaluate relations between vitamin D and iron during pregnancy. The cohort studied was sufficiently large and had high prevalences of both anemia and vitamin D insufficiency to explore these associations. Deficiencies of vitamin D or iron are known to be independently associated with negative pregnancy outcomes. Data from this study indicate that a suboptimal status of each of these nutrients may increase risk of the insufficiency of the other. These findings emphasize the need for screening for multiple nutrient deficiencies during pregnancy, and greater attention to overlapping metabolic pathways is warranted when selecting supplementation regimens. Furthermore, these data support the need for more-effective treatment strategies for improving vitamin D status during pregnancy because nearly 60% of teens supplemented with additional vitamin D had 25(OH)D concentrations <50 nmol/L at delivery. Additional studies are needed to identify the mechanism(s) by which vitamin D and iron may be affecting one another, and particular attention should be given to potential mediating factors involved such as erythropoietin. The elucidation of these underlying mechanisms is not only important for the treatment of chronic diseases such as CKD but may also have significant relevance to the maintenance of nutrient homeostasis in other physiologic states that increase nutrient demands including pregnancy.
Acknowledgments
The authors’ responsibilities were as follows—CET, TRK, and KOO: conducted the research; RG, RAQ, EMC, EKP, and KOO: designed the research; CET and FMV: analyzed the data; CET and KOO: wrote the manuscript; CET, RG, RAQ, EMC, EKP, MSR, and KOO: revised the manuscript; KOO: had primary responsibility for the final content of the manuscript; and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: CKD, chronic kidney disease; EAR, estimated average requirement; FGF23, fibroblast growth factor 23; IOM, Institute of Medicine; PTH, parathyroid hormone; sTfR, soluble transferrin receptor; 1,25(OH)2D, 1,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D.
REFERENCES
- 1.US Centers for Disease Control and Prevention. Second national report on biochemical indicators of diet and nutrition in the U.S. population 2012. Atlanta (GA): National Center for Environmental Health, April 2012. [Google Scholar]
- 2.Scholl TO, Hediger ML, Fischer RL, Shearer JW. Anemia vs iron deficiency: increased risk of preterm delivery in a prospective study. Am J Clin Nutr 1992;55:985–8. [DOI] [PubMed] [Google Scholar]
- 3.Beard JL, Hendricks MK, Perez EM, Murray-Kolb LE, Berg A, Vernon-Feagans L, Irlam J, Isaacs W, Sive A, Tomlinson M. Maternal iron deficiency anemia affects postpartum emotions and cognition. J Nutr 2005;135:267–72. [DOI] [PubMed] [Google Scholar]
- 4.Brabin BJ, Hakimi M, Pelletier D. An analysis of anemia and pregnancy-related maternal mortality. J Nutr 2001;131:604S–14S; discussion 614S–5S. [DOI] [PubMed] [Google Scholar]
- 5.Mulligan ML, Felton SK, Riek AE, Bernal-Mizrachi C. Implications of vitamin D deficiency in pregnancy and lactation. Am J Obstet Gynecol 2010;202:429 e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Javaid MK, Crozier SR, Harvey NC, Gale CR, Dennison EM, Boucher BJ, Arden NK, Godfrey KM, Cooper C, Princess Anne Hospital Study Group. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet 2006;367:36–43. [DOI] [PubMed] [Google Scholar]
- 7.Young BE, McNanley TJ, Cooper EM, McIntyre AW, Witter F, Harris ZL, O’Brien KO. Maternal vitamin D status and calcium intake interact to affect fetal skeletal growth in utero in pregnant adolescents. Am J Clin Nutr 2012;95:1103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atkinson MA, Melamed ML, Kumar J, Roy CN, Miller ER 3rd, Furth SL, Fadrowski JJ. Vitamin D, race, and risk for anemia in children. J Pediatr 2014;164:153–8.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blanco-Rojo R, Perez-Granados AM, Toxqui L, Zazo P, de la Piedra C, Vaquero MP. Relationship between vitamin D deficiency, bone remodelling and iron status in iron-deficient young women consuming an iron-fortified food. Eur J Nutr 2013;52:695–703. [DOI] [PubMed] [Google Scholar]
- 10.Perlstein TS, Pande R, Berliner N, Vanasse GJ. Prevalence of 25-hydroxyvitamin D deficiency in subgroups of elderly persons with anemia: association with anemia of inflammation. Blood 2011;117:2800–6. [DOI] [PubMed] [Google Scholar]
- 11.Sim JJ, Lac PT, Liu IL, Meguerditchian SO, Kumar VA, Kujubu DA, Rasgon SA. Vitamin D deficiency and anemia: a cross-sectional study. Ann Hematol 2010;89:447–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crowley N, Taylor S. Iron-resistant anaemia and latent rickets in schoolchildren. Arch Dis Child 1939;14:317–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holick MF. Skin: site of the synthesis of vitamin D and a target tissue for the active form, 1,25-dihydroxyvitamin D3. Ann N Y Acad Sci 1988;548:14–26. [DOI] [PubMed] [Google Scholar]
- 14.Alon DB, Chaimovitz C, Dvilansky A, Lugassy G, Douvdevani A, Shany S, Nathan I. Novel role of 1,25(OH)(2)D(3) in induction of erythroid progenitor cell proliferation. Exp Hematol 2002;30:403–9. [DOI] [PubMed] [Google Scholar]
- 15.Aucella F, Scalzulli RP, Gatta G, Vigilante M, Carella AM, Stallone C. Calcitriol increases burst-forming unit-erythroid proliferation in chronic renal failure. A synergistic effect with r-HuEpo. Nephron Clin Pract 2003;95:c121–7. [DOI] [PubMed] [Google Scholar]
- 16.Kumar VA, Kujubu DA, Sim JJ, Rasgon SA, Yang PS. Vitamin D supplementation and recombinant human erythropoietin utilization in vitamin D-deficient hemodialysis patients. J Nephrol 2011;24:98–105. [DOI] [PubMed] [Google Scholar]
- 17.Rianthavorn P, Boonyapapong P. Ergocalciferol decreases erythropoietin resistance in children with chronic kidney disease stage 5. Pediatr Nephrol 2013;28:1261–6. [DOI] [PubMed] [Google Scholar]
- 18.Bacchetta J, Zaritsky JJ, Sea JL, Chun RF, Lisse TS, Zavala K, Nayak A, Wesseling-Perry K, Westerman M, Hollis BW, et al. Suppression of iron-regulatory hepcidin by vitamin D. J Am Soc Nephrol 2014;25:564–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braithwaite V, Jarjou LM, Goldberg GR, Jones H, Pettifor JM, Prentice A. Follow-up study of Gambian children with rickets-like bone deformities and elevated plasma FGF23: possible aetiological factors. Bone 2012;50:218–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clinkenbeard EL, Farrow EG, Summers LJ, Cass TA, Roberts JL, Bayt CA, Lahm T, Albrecht M, Allen MR, Peacock M, et al. Neonatal iron deficiency causes abnormal phosphate metabolism by elevating FGF23 in normal and ADHR mice. J Bone Miner Res 2014;29:361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imel EA, Peacock M, Gray AK, Padgett LR, Hui SL, Econs MJ. Iron modifies plasma FGF23 differently in autosomal dominant hypophosphatemic rickets and healthy humans. J Clin Endocrinol Metab 2011;96:3541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee S, Guillet R, Cooper EM, Westerman M, Orlando M, Pressman E, O’Brien KO. Maternal inflammation at delivery affects assessment of maternal iron status. J Nutr 2014;144:1524–32. [DOI] [PubMed] [Google Scholar]
- 23.Young BE, McNanley TJ, Cooper EM, McIntyre AW, Witter F, Harris ZL, O’Brien KO. Vitamin D insufficiency is prevalent and vitamin D is inversely associated with parathyroid hormone and calcitriol in pregnant adolescents. J Bone Miner Res 2012;27:177–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Brien KO, Li S, Cao C, Kent T, Young BV, Queenan RA, Pressman EK, Cooper EM. Placental CYP27B1 and CYP24A1 expression in human placental tissue and their association with maternal and neonatal calcitropic hormones. J Clin Endocrinol Metab 2014;99:1348–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Easley B, McNanley T, Cooper E, McIntyre AW, Witter F, Harris ZL, O’Brien KO. Osteoprotegerin (OPG) differs by race and is related to infant birth weight Z-score in pregnant adolescents. J Dev. Orin Health Dis 2011;2:272–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whiner CM, Young BE, Witter FR, Harris ZL, Queenan RA, Cooper EM, O’Brien KO. Reductions in heel bone quality across gestation are attenuated in pregnant adolescents with higher prepregnancy weight and greater increases in PTH across gestation. J Bone Miner Res 2014;29:2109–17. [DOI] [PubMed] [Google Scholar]
- 27.Lumpish RA, Young SL, Lee S, Cooper E, Pressman E, Guillet R, O’Brien KO. Gestational iron deficiency is associated with pica behaviors in adolescents. J Nutr 2014;144:1533–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacks LM, Young MF, Easley BV, McNanley TJ, Cooper EM, Pressman EK, McIntyre AW, Orlando MS, Markowitz JL, Guillet R, et al. Placental expression of the home transporter, feline leukemia virus subgroup C receptor, is related to maternal iron status in pregnant adolescents. J Nutr 2011;141:1267–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao C, Pressman EK, Cooper EM, Guillet R, Westerman M, O’Brien KO. Placental home receptor LRP1 correlates with the home exporter FLVCR1 and neonatal iron status. Reproduction 2014;148:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young MF, Pressman E, Fehr ML, McNanley T, Cooper E, Guillet R, Orlando M, McIntyre AW, LA fond J, O’Brien KO. Impact of maternal and neonatal iron status on placental transferrin receptor expression in pregnant adolescents. Placenta 2010;31:1010–4. [DOI] [PubMed] [Google Scholar]
- 31.Whiner CM, Young BE, Pressman EK, Queenan RA, Cooper EM, O’Brien KO. Maternal diet but not gestational weight gain predicts central adiposity accretion in utero among pregnant adolescents. Int J Obese (Lund) 2015;39:565–70. [DOI] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention. Recommendations to prevent and control iron deficiency in the United States. MMWR Recommit Rep 1998;47:1–29. [PubMed] [Google Scholar]
- 33.National Research Council. Iron deficiency anemia: recommended guidelines for the prevention, detection, and management among U.S. children and women of childbearing age. Washington (DC): The National Academies Press; 1993. [PubMed] [Google Scholar]
- 34.Acheson A, Gjellerup P, Berglund M, Bremen K, Vaster M. Serum transferrin receptor: a specific marker of iron deficiency in pregnancy. Am J Clin Nutr 1998;68:1241–6. [DOI] [PubMed] [Google Scholar]
- 35.IOM (Institute of Medicine). Dietary reference intakes for calcium and vitamin D. Washington (DC): The National Academies Press; 2011. [PubMed] [Google Scholar]
- 36.Ganja V, Zhang X, Tangpricha V. Serum 25-hydroxyvitamin D concentrations and prevalence estimates of hypovitaminosis D in the U.S. population based on assay-adjusted data. J Nutr 2012;142:498–507. [DOI] [PubMed] [Google Scholar]
- 37.Patel NM, Gutierrez OM, Andress DL, Coyne DW, Levin A, Wolf M. Vitamin D deficiency and anemia in early chronic kidney disease. Kidney Int 2010;77:715–20. [DOI] [PubMed] [Google Scholar]
- 38.Smith EM, Alvarez JA, Martin GS, Zughaier SM, Ziegler TR, Tangpricha V. Vitamin D deficiency is associated with anaemia among African Americans in a US cohort. Br J Nutr 2015;113:1732–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004;306:2090–3. [DOI] [PubMed] [Google Scholar]
- 40.Carvalho C, Isakova T, Collerone G, Olbina G, Wolf M, Westerman M, Gutierrez OM. Hepcidin and disordered mineral metabolism in chronic kidney disease. Clin Nephrol 2011;76:90–8. [DOI] [PubMed] [Google Scholar]
- 41.Kendrick J, Targher G, Smits G, Chonchol M. 25-Hydroxyvitamin D deficiency and inflammation and their association with hemoglobin levels in chronic kidney disease. Am J Nephrol 2009;30:64–72. [DOI] [PubMed] [Google Scholar]
- 42.Panichi V, De Pietro S, Andreini B, Bianchi AM, Migliori M, Taccola D, Giovannini L, Tetta C, Palla R. Calcitriol modulates in vivo and in vitro cytokine production: a role for intracellular calcium. Kidney Int 1998;54:1463–9. [DOI] [PubMed] [Google Scholar]
- 43.Martin JA, Hamilton BE, Osterman MJ, Curtin SC, Matthews TJ. Births: final data for 2013. Natl Vital Stat Rep 2015;64:1–65. [PubMed] [Google Scholar]
- 44.Mei Z, Cogswell ME, Looker AC, Pfeiffer CM, Cusick SE, Lacher DA, Grummer-Strawn LM. Assessment of iron status in US pregnant women from the National Health and Nutrition Examination Survey (NHANES), 1999-2006. Am J Clin Nutr 2011;93:1312–20. [DOI] [PubMed] [Google Scholar]
- 45.Bergwitz C, Juppner H. Regulation of phosphate homeostasis by PTH, vitamin D, and FGF23. Annu Rev Med 2010;61:91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]