Abstract
Renal ischemia and reperfusion injury causes loss of renal epithelial cell polarity and perturbations in tubular solute and fluid transport. Na+,K+-ATPase, which is normally found at the basolateral plasma membrane of renal epithelial cells, is internalized and accumulates in intracellular compartments after renal ischemic injury. We previously reported that the subcellular distribution of Na+,K+-ATPase is modulated by direct binding to Akt substrate of 160 kD (AS160), a Rab GTPase-activating protein that regulates the trafficking of glucose transporter 4 in response to insulin and muscle contraction. Here, we investigated the effect of AS160 on Na+,K+-ATPase trafficking in response to energy depletion. We found that AS160 is required for the intracellular accumulation of Na+,K+-ATPase that occurs in response to energy depletion in cultured epithelial cells. Energy depletion led to dephosphorylation of AS160 at S588, which was required for the energy depletion–induced accumulation of Na,K-ATPase in intracellular compartments. In AS160-knockout mice, the effects of renal ischemia on the distribution of Na+,K+-ATPase were substantially reduced in the epithelial cells of distal segments of the renal tubules. These data demonstrate that AS160 has a direct role in linking the trafficking of Na+,K+-ATPase to the energy state of renal epithelial cells.
Keywords: cell physiology, transport physiology, cell biology, cell structure, cell signaling, epithelial, ischemia-reperfusion, MDCK
The Na,K-ATPase, or sodium pump, creates the driving force for solute and fluid transport in most tissues. The energy released through the hydrolysis of one molecule of ATP is used by the enzyme to drive export of three Na+ ions and the import of two K+ ions, and this transport is essential for the maintenance of cellular electrochemical gradients. The Na,K-ATPase is restricted in its distribution to the basolateral domain of the plasma membrane in most polarized epithelial cells.1 Many cell types appear to contain two functionally separable pools of Na,K-ATPase. In addition to the principal pool at the plasma membrane, a population of Na,K-ATPase is also frequently associated with the membranes of intracellular compartments.2–4 Physiologic stimuli can promote Na,K-ATPase endocytosis or translocation from the intracellular pool to the plasma membrane.2,5–7
The glucose transporter 4 (GLUT4) of muscle and fat cells is one of the best-studied examples of a transport protein whose activity is governed through stimulus-induced trafficking between the plasma membrane and intracellular compartments.8 GLUT4 is delivered to the cell surface from intracellular storage vesicles in response to stimuli that favor increased glucose uptake, including insulin and muscle contraction. AS160, or TBC1D4, is a Rab GTPase-activating protein (GAP) that participates in regulating the translocation of GLUT4 to the plasma membrane.9,10 AS160 is phosphorylated by Akt on at least six amino acid residues after insulin stimulation9 and this phosphorylation inhibits the Rab-GAP activity of AS160. Because the cell surface accumulation of GLUT4 is dependent upon the GTP-bound state of Rab 8, Rab 10, and Rab 14,11 inhibition of AS160’s Rab-GAP activity leads to a redistribution of GLUT4 from intracellular storage compartments to the plasma membrane. Consistent with this model, the small interfering RNA–induced knockdown (KD) of AS160 expression increases the basal levels of GLUT4 at the cell surface and concomitantly reduces the size of the intracellular pool.12,13 AS160 may also play a similar role in regulating the distribution and hence the activity of the epithelial sodium channel in response to aldosterone stimulation.14
Energy depletion in renal epithelial cells results in a redistribution of the cell surface Na,K-ATPase, resulting in its accumulation in intracellular compartments.15 Energy deprivation induced by renal ischemia can lead to AKI, which is a common condition associated with very significant morbidity and mortality.16–18 AKI is associated with detachment of the sodium pump from the plasma membrane’s subcortical cytoskeleton and with the loss of cell polarity and resultant impairment of renal function.7,19–21 Recovery from ischemic renal injury involves restitution of cellular polarity and return of the sodium pump from intracellular compartments to the plasma membrane.22
Recently, we reported that AS160 interacts directly with the Na,K-ATPase α-subunit and modulates the sodium pump’s subcellular distribution.23 In this study, we investigated the role of AS160 in mediating the accumulation of the Na,K-ATPase in intracellular compartments after energy depletion in cultured renal epithelial cells. Our data indicate that AS160 is essential for sodium pump accumulation in cytoplasmic compartments in response to ATP depletion and that the redistribution of the Na,K-ATPase in response to energy depletion is mediated by changes in the phosphorylation state of AS160. Our studies utilizing AS160 knockout (KO) mice further suggest that AS160 plays a role in governing the redistribution of the Na,K-ATPase in distal segments of the renal tubule that is induced by renal ischemia in vivo.
Results
AS160 Mediates the Intracellular Accumulation of Na,K-ATPase That Is Induced by Energy Depletion
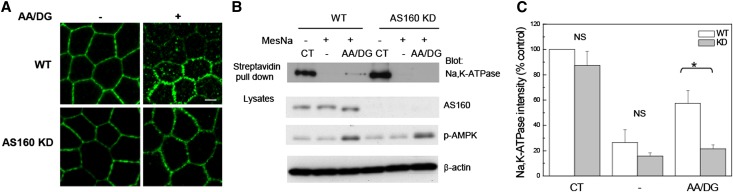
AS160 plays an important role in regulating the GLUT4 protein’s intracellular retention and its translocation to the plasma membrane in response to insulin or muscle contraction.24,25 Previously, we characterized the interaction between AS160 and Na,K-ATPase and found that AS160 interacts with the cytoplasmic NP domain of the α-subunit of the sodium pump.23 Na,K-ATPase is internalized and retained in intracellular compartments in response to energy depletion and renal ischemia.7,15 To investigate whether AS160 plays a role in this Na,K-ATPase redistribution after energy depletion, we examined the effects of energy depletion on the subcellular distribution of the Na,K-ATPase in a stable clonal cell line in which AS160 is robustly knocked down by small hairpin RNA (shRNA) (AS160 KD).23 Wild-type (WT) Madin-Darby Canine Kidney (MDCK) cells or AS160 KD MDCK cells were subjected or not to energy depletion. Energy depletion was achieved by incubating cells in a glucose-free medium containing antimycin A and 2-deoxy-glucose (AA/DG). A mAb directed against the Na,K-ATPase α-subunit was used to detect the distribution of the sodium pump. Figure 1 depicts the Na,K-ATPase localization in untreated WT and AS160 KD cells (−). After energy depletion treatment (AA/DG), the Na,K-ATPase was dramatically redistributed. Abundant Na,K-ATPase was detected in association with intracellular structures in the cytoplasm in the WT cells. The intracellular accumulation of the Na,K-ATPase in response to energy depletion was substantially reduced in the AS160 KD cell line, suggesting that AS160 plays an obligate role in sodium pump accumulation in intracellular compartments after energy depletion.
Figure 1.
shRNA-mediated KD of AS160 inhibits the intracellular accumulation of Na,K-ATPase that is induced by energy depletion. (A) Immunofluorescence analysis is performed to detect the distribution of the endogenous Na,K-ATPase in MDCK renal epithelial cells in culture. MDCK cells are stained with an antibody directed against the sodium pump α-subunit (α5). WT MDCK cells and MDCK cells knocked down for AS160 expression (AS160 KD) are treated with (+) or without (−) AA/DG. Typical results from one of five experiments are shown. (B) Cell surface biotinylation. WT or AS160 KD cells are biotinylated at the basolateral surface, then subjected or not to energy depletion. The biotin that remained exposed at the cell surface after the treatments is stripped by treating the cells with MesNa. Biotinylated proteins are recovered through incubation with streptavidin agarose beads. The biotinylated Na,K-ATPase α-subunit is detected with the mAb α5. Lysates are blotted with anti-AS160, anti–phospho-AMPK antibody, and with anti–β-actin to assess total protein loading. The extent of energy depletion is assessed by probing for phospho-AMPK. (C) Quantification of the biotinylated Na,K-ATPase band intensity normalized to the β-actin levels. The results indicate that the fraction of the pool of sodium pump that was initially biotinylated at the cell surface that accumulated within the cell following energy depletion is lower in the AS160 KD cells compared with the WT line. *P<0.01 (n=4). CT, total biotinylated Na,K-ATPase in untreated cells; −, biotinylated Na,K-ATPase in untreated cells subjected to MesNa strip; AA/DG, biotinylated Na,K-ATPase in energy-depleted cells treated with MesNa; p-AMPK, phospho-AMPK. Bar, 5 μm.
In order to confirm the results obtained by immunofluorescence and to quantify the extent of Na,K-ATPase accumulation in cytoplasmic compartments in response to energy depletion in the MDCK WT and AS160 KD cell lines, a surface biotinylation assay was performed. The basolateral surfaces of MDCK cells were labeled with Sulfo-NHS-SS biotin, after which cells were subjected to energy depletion (AA/DG). As a control, biotinylated cells were maintained in regular media during the treatment (−). To determine the total amount of plasma membrane Na,K-ATPase that was labeled, aliquots of cells were lysed immediately after the biotinylation process. After the energy depletion treatment or control incubation interval, the biotin that remained exposed at the plasma membrane was stripped through an incubation with the membrane-impermeable reducing agent 2-mercaptoethane sulfonate sodium (MesNa), allowing us to detect only the pool of internalized Na,K-ATPase that was protected from exposure to the MesNa reagent. Cell lysates were incubated with streptavidin beads and samples were subjected to SDS-PAGE and Western blotting. Figure 1B shows the resulting Western blot, and Figure 1C shows the quantification of the relative levels of biotinylated Na,K-ATPase detected under each condition. Similar levels of energy depletion were achieved in both the WT and AS160 KD cells, as evidenced by the comparable increase in the level of the phosphorylated form of the energy-sensing kinase adenosine monophosphate-stimulated protein kinase (AMPK) that is detected in both cell types. As expected, upon ATP depletion, the levels of intracellular Na,K-ATPase were significantly higher in the MDCK WT cells than in the AS160 KD cell line. These results are consistent with the results observed in the immunofluorescence experiments and suggest that AS160 is an essential component of the machinery that mediates the intracellular accumulation of Na,K-ATPase in response to energy depletion.
The AS160 and Na,K-ATPase Interaction Is Not Affected by Energy Depletion
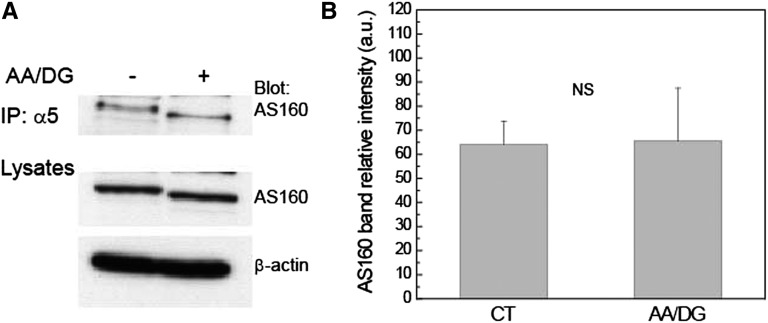
We previously showed that AS160 interacts directly with the Na,K-ATPase in MDCK cells.23 To analyze whether the interaction between these two proteins was affected by ATP depletion, a coimmunoprecipitation assay was performed. MDCK cells were treated or not with media lacking glucose in the presence of AA/DG. Total cell lysates were then incubated with protein G beads and α5 antibody to immunoprecipitate endogenous Na,K-ATPase α-subunit. The associated AS160 that coprecipitated with the sodium pump was detected by Western blotting with anti-AS160 antibody (Figure 2). The results indicated that the energy depletion treatment does not affect the extent of the interaction between AS160 and Na,K-ATPase. Interestingly, we observed a slight decrease in the apparent molecular weight of the coprecipitated AS160 as a result of the energy depletion treatment.
Figure 2.
The interaction between AS160 and Na,K-ATPase is not affected by energy depletion treatment. Endogenous Na,K-ATPase is immunoprecipitated using a mAb directed against the α-subunit (α5). Immunoprecipitates are probed with AS160 polyclonal antibody to detect endogenous AS160. MDCK cells are treated (+) or not (−) with energy depletion AA/DG, for 1 hour. (A) Immunoblot of the coimmunoprecipitation. Lysates are blotted with anti-AS160 to detect the endogenous levels of AS160 and with anti–β-actin as a control for the total protein loaded. (B) Coimmunoprecipitation quantification. The results indicate that the interaction between AS160 and sodium pump is not affected by energy depletion. n=3. IP, immunoprecipitation.
AS160 Phosphorylation at S588 Is Essential for Na,K-ATPase Intracellular Accumulation in Response to Energy Depletion
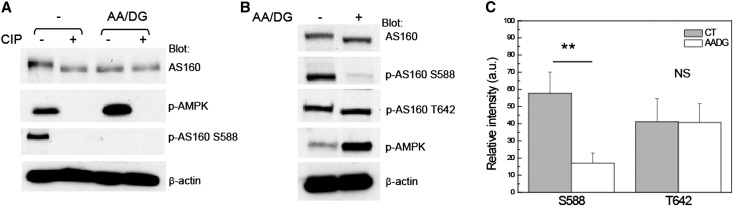
AS160 phosphorylation plays a critical role in determining whether GLUT4 is retained intracellularly or translocated to the plasma membrane.9 AS160 dephosphorylation is essential for GLUT4 intracellular storage under basal conditions.12,13 Because a decrease in the apparent molecular weight of AS160 was observed after ATP depletion, we explored whether this change was attributable to alterations in the phosphorylation state of AS160. We began this analysis by performing an alkaline phosphatase assay. Total cell lysates from MDCK cells subjected or not to energy depletion were incubated in the presence or absence of calf intestinal alkaline phosphatase, after which they were analyzed by Western blotting with anti-AS160 antibody. The results indicated that, in MDCK cell lysates treated with alkaline phosphatase, the apparent molecular weight of AS160 was reduced to a similar extent as is observed in material prepared from cells that were subjected to energy depletion alone (Figure 3A). This result supports the interpretation that the reduction of the apparent molecular weight of AS160 that follows energy depletion could be attributable to the effects of dephosphorylation.
Figure 3.
Energy depletion leads to AS160 dephosphorylation at S588. (A) The apparent molecular weight of AS160 decreases as a result of its dephosphorylation. MDCK cells are subjected (AA/DG) or not (−) to energy depletion for 1 hour. Total cell lysates are incubated in the presence (+) or absence (−) of 10 units of CIP for 30 minutes at 37°C. Lysates are blotted with anti-AS160 antibody to visualize the shift of the apparent molecular weight of AS160. Blots are also probed with anti–phospho-AMPK and p-AS160 S588 antibodies to document the efficacy of both the ATP depletion and the CIP treatments. Anti–β-actin is also used to assess the total amount of protein loaded. (B) Total MDCK cells lysates are blotted with rabbit anti-AS160 to detect the endogenous AS160 and with phospho-specific antibodies directed against the AS160 phosphorylation sites S588 and T642. Lysates are also probed with antibodies directed against p-AMPK. Blots are probed with anti–β-actin to assess the total amount of protein loaded. The data indicate that energy depletion is associated with a decrease in the apparent molecular weight of AS160 that is induced by the dephosphorylation of AS160 at S588. (C) Quantification of AS160 phosphorylation at S588 and T642 is based on four independent experiments. *P<0.01. Typical results of one of four experiments are shown. CIP, calf intestinal alkaline phosphatase; p-AMPK, phospho-AMPK.
To further explore the effects of energy depletion on AS160 phosphorylation, lysates were prepared from MDCK cells that had been subjected or not to the energy depletion protocol and analyzed by Western blotting (Figure 3, B and C). As observed previously, the apparent molecular weight of AS160 was reduced in lysates prepared from cells subjected to energy depletion. We tested the effects of energy depletion on the phosphorylation status of two specific AS160 phosphorylation sites: S588 and T642. The results show that the phosphorylation of AS160 T642 does not change in response to energy depletion. However, S588 phosphorylation was abolished after the treatment, suggesting that the change in the apparent molecular weight of AS160 induced by ATP depletion is due to the dephosphorylation of AS160 at S588. As noted previously, the dephosphorylated form of AS160 promotes the intracellular accumulation of GLUT4.9,12–14,26 It is tempting to suggest, by analogy, that the dephosphorylation of AS160 at S588 is responsible for the intracellular accumulation of Na,K-ATPase that accompanies energy depletion.
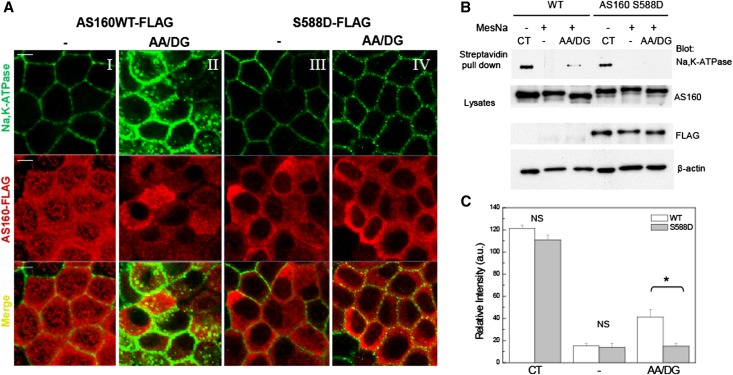
Finally, to test directly whether the intracellular accumulation of Na,K-ATPase in response to energy depletion is dependent upon dephosphorylation of AS160 at S588, we prepared constructs encoding wild-type human AS160 carrying a FLAG epitope tag (AS160 WT-FLAG) or a mutated version of human AS160 in which the serine at position 588 has been replaced with a phosphomimetic aspartic acid residue (AS160 S588D-FLAG). These constructs were expressed in MDCK cells in which expression of the endogenous AS160 had been reduced by shRNA KD (AS160 KD). The human sequence was used to generate the transfected constructs because this sequence is not susceptible to KD by the shRNA that was expressed in these cells. The resultant clonal cell lines do not express detectable levels of endogenous AS160 (Supplemental Figure 1) compared with control MDCK cells. Figure 4A depicts the immunofluorescence images obtained with these cell lines. Antibodies directed against the FLAG epitope and the α5 mAb were used to detect exogenous AS160 and endogenous Na,K-ATPase, respectively. AS160 WT-FLAG and AS160 S588D-FLAG cells were subjected (4A, II and IV) or not (4A, I and III) to energy depletion. As can be seen in Figure 4AII, Na,K-ATPase was detected in intracellular compartments after energy depletion in cells expressing AS160 WT-FLAG, demonstrating that the AS160 WT-FLAG construct can effectively substitute for the activity of the absent endogenous AS160 in the AS160 KD cells. By contrast, sodium pump was found predominantly at the plasma membrane after energy depletion in AS160 KD cells expressing the S588D mutant form of AS160 (Figure 4AIV). To measure the relative quantities of Na,K-ATPase present at the basolateral surface versus in intracellular compartments in response to energy depletion in cells expressing the S588D mutant form of AS160, a surface biotinylation/MesNa stripping assay was performed as described above. Figure 4B depicts the resultant Western blot. Consistent with the immunofluorescence results shown in Figure 4A, intracellular accumulation of Na,K-ATPase in MDCK WT cells treated with AA/DG was significantly higher than that measured in the AS160 S588D cell line. Quantification of the Na,K-ATPase band intensity detected under each condition is depicted in Figure 4C. Together, these findings strongly suggest that AS160 is necessary for the redistribution of Na,K-ATPase to intracellular structures in response to energy depletion and that the dephosphorylation of AS160 at S588 plays a crucial role in this process.
Figure 4.
Intracellular accumulation of Na,K-ATPase in response to energy depletion does not occur in cells expressing the S588D mutant of AS160. (A) Immunofluorescence analysis of the distribution of endogenous Na,K-ATPase. MDCK cells untreated (−) (I and III) or treated with energy depletion (AA/DG) (II and IV) are stained with an antibody directed against the Na,K-ATPase α-subunit (α5) and with anti-FLAG to detect exogenous AS160. Na,K-ATPase accumulates in intracellular compartments in response to AA/DG in cells stably transfected with AS160 WT-FLAG (II). However, Na,K-ATPase is not localized in intracellular structures in the AS160 S588D cell line treated with AA/DG (IV). (B) Cell surface biotinylation. MDCK WT or AS160 S588D cells are biotinylated at the basolateral surface, then exposed or not to energy depletion (AA/DG). The biotin that remained at the cell surface after the energy depletion is stripped by treating the cells with MesNa. The biotinylated sodium pump is detected with the antibody α5 directed against the Na,K-ATPase α-subunit. Lysates are blotted with anti-AS160, anti-FLAG, and with anti–β-actin antibodies to assess total protein loading. (C) Quantification of the biotinylated Na,K-ATPase band intensity normalized to the β-actin levels. The amount of sodium pump that was initially biotinylated at the cell surface and that accumulated within the cell after energy depletion is lower in the AS160 S588D cells compared with the MDCK WT cell line. *P<0.05 (n=3). CT, total biotinylated Na,K-ATPase in untreated cells; −, biotinylated Na,K-ATPase in untreated cells subjected to MesNa strip; AA/DG, biotinylated Na,K-ATPase in energy-depleted cells treated with MesNa. Bar, 5 μm.
Na,K-ATPase–Induced Endocytosis in Response to Energy Depletion Is a Unique Pathway That Is Not Regulated by Dynamin and Does Not Involve Caveolae
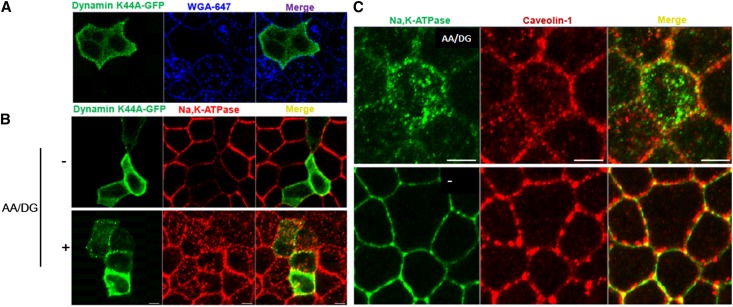
To investigate the mechanism that controls Na,K-ATPase endocytosis in response to energy depletion, MDCK cells were transfected with a dominant negative form of dynamin containing the mutation K44A. The cells were then treated or not with energy depletion for 30 minutes at 37°C according to our standard protocol, after which they were subjected to immunofluorescence analysis, the results of which are shown in Figure 5. To assess the effectiveness of dynamin K44A expression in blocking endocytosis in the transfected cells, MDCK cells were incubated with wheat germ agglutinin linked to Alexa-647 probe (WGA-647) for 30 minutes at 37°C (Figure 5A). In the absence of the dominant negative form of dynamin, the surface-bound WGA-647 was extensively internalized into the cells. As expected, in cells expressing dynamin K44A-green fluorescent protein (GFP), the endocytosis of WGA-647 was inhibited. MDCK cells transfected with dynamin K44A-GFP were treated (+AA/DG) or not with energy depletion (−) (Figure 5B). Interestingly, the Na,K-ATPase internalization induced by energy depletion continued to be detected in cells that express the dominant negative from of dynamin. These findings strongly suggest that the intracellular accumulation of Na,K-ATPase that follows energy depletion is not dependent upon the dynamin/clathrin pathway.
Figure 5.
Na,K-ATPase endocytosis after energy depletion does not involve dynamin or caveolin-1. (A) Immunofluorescence analysis of MDCK cells transfected with dynamin K44A-GFP and incubated with WGA-647. Cells positive for the expression of the dominant negative form of dynamin do not display WGA-647 endocytosis. (B) MDCK cells transfected with dynamin K44A-GFP are subjected or not (−) to energy depletion (AA/DG). Na,K-ATPase localization is determined with an antibody directed against the Na,K-ATPase α-subunit (α5). (C) Immunofluorescence analysis of endogenous Na,K-ATPase and caveolin-1 in MDCK cells untreated (−) or treated with energy depletion, (AA/DG). The results indicate that sodium pump endocytosis induced by energy depletion does not depend upon dynamin or involve transit through caveolin-1–containing compartments. Typical results of one of three experiments are presented. Bar, 5 μm.
It has been shown that caveolin-1 interacts with the Na,K-ATPase27 and is involved in the ouabain-induced endocytosis of the sodium pump.28 To assess whether the Na,K-ATPase becomes associated with caveolae in response to energy depletion, MDCK cells were subjected or not to energy depletion and examined by immunofluorescence analysis, which is depicted in Figure 5C. No colocalization between the intracellular pool of Na,K-ATPase and caveolin-1 was detected in cells subjected to energy depletion, suggesting that the redistribution of the Na,K-ATPase that follows energy depletion does not involve its transit through caveolae.
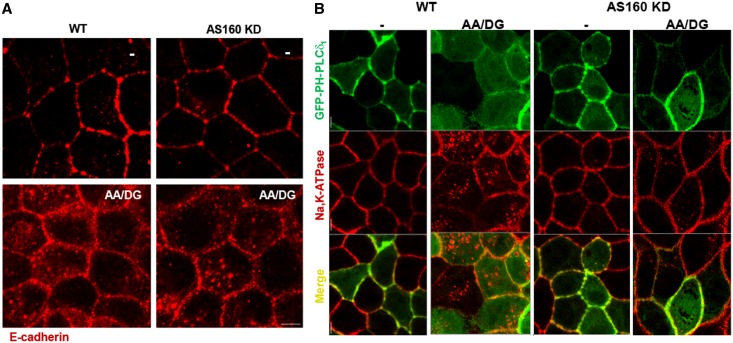
Like the Na,K-ATPase, E-cadherin is also endocytosed in response to energy depletion.15 To investigate the generality of the AS160-regulated mechanism that drives endocytosis and retention of the sodium pump, an immunofluorescence assay was performed to detect E-cadherin endocytosis after energy depletion. Figure 6A shows immunofluorescence images from MDCK (WT) and AS160 KD cells subjected or not to energy depletion. The results indicate that AS160 does not play an obligate role in the energy depletion–induced endocytosis and cytoplasmic retention of E-cadherin. Finally, we assessed whether the energy depletion–induced AS160-dependent internalization mechanism is specific for the Na,K-ATP or instead affects the entire basolateral plasma membrane. Toward this end, WT or AS160 KD MDCK cells were transfected to express the plasma membrane marker GFP-PH-PLCδ129 and then treated or not with energy depletion. The results indicated that GFP-PH-PLCδ1 is not internalized or retained in the cytoplasm (Figure 6B) in response to energy depletion. These observations strongly support the conclusion that AS160 regulates Na,K-ATPase trafficking in response to energy depletion via a unique pathway.
Figure 6.
The mechanism that regulates Na,K-ATPase trafficking after energy depletion is specific and AS160 dependent. (A) Immunofluorescence analysis of WT MDCK cells and AS160 KD MDCK cell lines stained with an antibody that detects endogenous levels of E-cadherin. Cells are treated (AA/DG) or not (−) with energy depletion for 30 minutes. The images indicate that E-cadherin endocytosis induced by energy depletion is AS160 independent. (B) Immunofluorescence images of WT and AS160 KD MDCK cells expressing a membrane marker GFP-PH-PLCδ1 that are treated (AA/DG) or not (−) with energy depletion. Endogenous Na,K-ATPase is detected with an antibody directed against the α-subunit (α5). The resultant immunofluorescence images show that energy depletion does not produce a generalized internalization of plasma membrane components, indicating that the AS160-dependent modulation of Na,K-ATPase distribution is specific. Bar, 5 μm.
AS160 Mediates the Intracellular Accumulation of the Na,K-ATPase in Epithelial Cells of Distal Segments after Renal Ischemia And Reperfusion Injury In Vivo
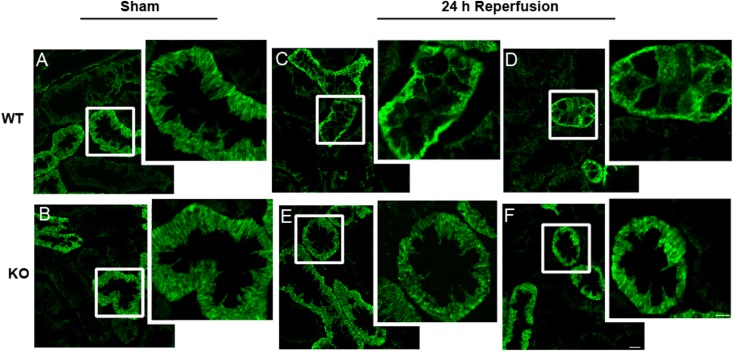
The Na,K-ATPase is internalized and mislocalized as a result of renal ischemia in vivo.7,30 To determine whether AS160 is required for the intracellular accumulation of the Na,K-ATPase as a consequence of renal ischemia, WT and AS160 null mice were subjected to renal ischemia and reperfusion. Both WT and AS160 KO animals underwent 30 minutes of bilateral renal ischemia that was imposed by clamping the renal pedicles, followed by 24 hours of reperfusion. Control animals of both genotypes were subjected to sham surgery, in which renal blood flow was not compromised. Immunofluorescence localization of the Na,K-ATPase in kidney sections of two different animals subjected to ischemia followed by 24 hours of reperfusion is depicted at high magnification in Figure 7 (lower-magnification images are presented in Supplemental Figure 2). In total, eight mice from both groups were analyzed. AS160 is expressed predominantly in the distal convoluted tubule (DCT).31 Kidney sections that exhibited calbindin-positive DCT profiles were selected for imaging of Na,K-ATPase distribution. In control and sham-treated animals, the Na,K-ATPase is localized to the basolateral infoldings of DCT epithelial cells (Figure 7, A and B). WT mice subjected to ischemia followed by 24 hours of reperfusion exhibited Na,K-ATPase mislocalization and accumulation of sodium pump in cytoplasmic structures in DCT cells (Figure 7, C and D). By contrast, the plasma membrane localization of the Na,K-ATPase was preserved and sodium pump accumulation in intracellular compartments was dramatically reduced in the DCT segments of the AS160 KO mice subjected to the ischemia and reperfusion protocol (Figure 7, E and F). The Na,K-ATPase distribution in each condition was scored as being predominantly basolateral or predominantly cytoplasmic by an observer who was blinded to the experimental conditions. Sections from three animals for each of the four experimental groups were analyzed, and >100 tubule cross-sections per animal were scored. As expected, in sections from WT and AS160 KO mice subjected to the sham procedure, a very small fraction of the tubules were scored as exhibiting extensive cytoplasmic labeling (4% and 5%, respectively). In animals subjected to ischemia followed by 24 hours of reperfusion, the fraction of tubules with predominantly cytoplasmic staining was significantly higher in the WT compared with the AS160 KO renal tissue (62% versus 43%, respectively; P<0.02). These results are consistent with the conclusion that AS160 plays an obligate role in the intracellular accumulation of Na,K-ATPase in DCT that accompanies renal ischemia and reperfusion.
Figure 7.
Renal ischemia–induced redistribution of Na,K-ATPase is reduced in the DCTs of AS160 KO mice. Immunofluorescence images of calbindin-positive tubules of kidney cortex stained with an antibody directed against the Na,K-ATPase α-subunit (α5). (A and B) WT (A) and AS160 KO (B) mice exposed to sham surgery. Tissue from two separate WT and AS160 KO animals exposed to 30 minutes of bilateral renal pedicle clamping followed by 24 hours of reperfusion. (C–F) Na,K-ATPase mislocalization after ischemia and reperfusion is less dramatic in the DCT cells of the AS160 KO mice (E and F) compared with those of the WT mice (C and D). Images are taken at a uniform contrast and brightness and are representative of at least eight animals per condition. Bar, 10 μm and 5 μm for low-magnification and enlarged images, respectively.
Because AS160 is not detectably expressed in proximal tubule (PT) epithelial cells,31 we anticipated that ischemia-induced internalization of the Na,K-ATPase in PT cells would occur to the same extent in WT and AS160 KO mice. Consistent with this hypothesis, we found that the extent of Na,K-ATPase internalization in response to ischemic injury was not appreciably different in PTs of WT versus AS160 KO animals (Supplemental Figure 3). No significant differences in the levels of serum creatinine measured after 24 hours of reperfusion were detected between AS160 null mice and WT mice, nor were substantial differences noted in the histologic appearance of renal tissue obtained from WT and AS160 KO mice subjected to the ischemia protocol (data not shown). To better quantify the extent of tissue damage, a terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling assay was performed (Supplemental Figure 4). The results indicate that the levels of cell death after injury are similar in the DCTs of WT and AS160 KO mice. Thus, as expected, the absence of AS160 does not alter the effects of ischemia and reperfusion on the distribution of the Na,K-ATPase in PT cells or on the severity of renal injury.
Discussion
ARF is a common and clinically important problem whose incidence and effect is increasing.32 Ischemic renal injury induces defects in tubular reabsorption of solute and fluid. The Na,K-ATPase creates the driving forces responsible for most ion transport in kidney tubules. Renal ischemia induces the loss of epithelial cell polarity, and the normally basolateral Na,K-ATPase relocalizes to intracellular compartments and to the apical surface.33,34 The mechanism that controls this Na,K-ATPase redistribution in response to energy deprivation is poorly understood. In this study, we characterized the role of AS160 in this process in vitro and in vivo and found that AS160 mediates the intracellular accumulation of the Na,K-ATPase after ischemic injury.
Intracellular compartments within renal epithelial cells can exchange Na,K-ATPase molecules with the pool of sodium pump present at the plasma membrane. Up to 30% of a renal epithelial cell’s complement of Na,K-ATPase may be contained in intracellular compartments that have the potential capacity to be translocated to the plasma membrane.5 The Na,K-ATPase can also be internalized by endocytosis and retained in intracellular structures in response to stimuli such as hormones and hypoxia. These trafficking processes appear to be controlled by a variety of protein kinases.5,35–38
We have found that AS160 mediates the intracellular accumulation of the Na,K-ATPase that is induced by energy depletion. We find that shRNA-mediated KD of AS160 expression is sufficient to prevent the relocalization of the Na,K-ATPase to intracellular structures in MDCK renal epithelial cells that have been subjected to ATP depletion. This result is consistent with the finding that small interfering RNA–mediated KD of AS160 increases the cell surface expression of both GLUT4 and the epithelial sodium channel in muscle and renal epithelial cells, respectively. AS160 appears to regulate the trafficking of both of these transport proteins between intracellular compartments and the cell surface.12–14 Recently, we described that AS160 interacts with the Na,K-ATPase through the cytosol-facing NP domain of the sodium pump’s α-subunit.23
Previous studies have shown that the reduction in the size of the pool of the Na,K-ATPase at the plasma membrane after hypoxia is specific and not a consequence of general membrane internalization, because levels of both the transferrin receptor and GLUT-1 remained unchanged.37 In this work, we find that sodium pump endocytosis induced by energy depletion is mediated by a pathway that is not dependent upon dynamin and does not involve passage through caveolae. The Na,K-ATPase endocytosis that occurs in response to energy depletion was not affected by the overexpression of a dominant negative form of dynamin and the Na,K-ATPase did not colocalize with caveolin-1 at any point during or after its internalization. It would appear, therefore, that the AS160-dependent mechanism that controls sodium pump endocytosis in response to energy depletion does not involve these two major endocytic pathways.
We find that the mechanism through which AS160 mediates Na,K-ATPase trafficking in response to energy depletion involves regulation of the phosphorylation of AS160 at S588. The phosphorylation state of AS160 determines its capacity to control GLUT4 translocation to the plasma membrane.39,40 Energy depletion treatment in MDCK cells results in a substantial decrease in the phosphorylation of AS160 at S588. We generated a stable cell line expressing a phosphomimetic form of AS160 by substituting S588 with an aspartic acid residue. When this construct is expressed in MDCK cells in which expression of the endogenous AS160 has been reduced by shRNA-mediated KD, the intracellular accumulation of the sodium pump that is induced by ATP depletion is substantially reduced compared with that observed in AS160 KD cells transfected to express WT AS160. Together, these results support the conclusion that the dephosphorylation of AS160 S588 is required to induce the intracellular accumulation of Na,K-ATPase. Furthermore, our data indicate that loss of phosphorylation at S588 is sufficient to induce the intracellular accumulation of the Na,K-ATPase and persistence of this phosphorylation is sufficient to prevent this relocalization. Further experiments will be required to determine whether and how the regulation of kinase and phosphatase activities contributes to determining the phosphorylation state of AS160 at S588.
Overexpression of a dominant active mutant form of AS160 (AS160-4P), which has four phosphorylation sites mutated to alanines, inhibits insulin-stimulated GLUT4 translocation to the plasma membrane in adipocytes, whereas the overexpression of the WT AS160 protein has no effect on this process.9 Addition of another mutation to AS160-4P, in the GAP domain at AS160 R973K, overcomes the inhibitory effect of AS160-4P. These findings indicate that the GAP activity of AS160 is required for it to manifest its capacity to induce the intracellular accumulation of GLUT4.9 Stöckli et al. suggested that S588 phosphorylation confers a stronger inactivation of the AS160 GAP activity than does phosphorylation of AS160 at T642 alone.26 Interpreted in light of these observations, our findings suggest that energy depletion results in the dephosphorylation of AS160 at S588, which triggers the activation of the AS160 GAP activity and results in the accumulation of the GDP-bound form of a critical Rab protein that participates in determining the subcellular distribution of the Na,K-ATPase. AS160 displays in vitro GAP activity toward several Rab proteins, including Rab 2A, Rab 8A, Rab 10, Rab 11, Rab 13, and Rab 14.11,13,41 Recently, Comellas et al. suggested that Rab 10 is implicated in sodium pump trafficking in response to insulin in pulmonary cells.42 It will be important to identify the Rab protein involved in the AS160-mediated Na,K-ATPase trafficking in response to energy depletion, as well as the role of the GAP domain of AS160 in this process.
Our studies utilizing AS160 KO mice indicate that at least some component of the intracellular accumulation of Na,K-ATPase that is induced by renal ischemia in distal segments of the nephron in vivo is dependent upon the participation of AS160. AS160 is expressed at high levels in the renal DCT.31 Our data indicate that, in the absence of AS160 expression, the relocalization of sodium pump that occurs in response to renal ischemia followed by 24 hours of reperfusion in cells of the DCT is substantially reduced. The absence of AS160 does not reduce the ischemia-induced internalization of the Na,K-ATPase in PTs, consistent with the lack of detectable AS160 expression in proximal segments.31 Clearly other mechanisms must be involved in driving the substantial internalization of Na,K-ATPase that occurs in response to ischemic injury in PTs.
A large body of literature documents that renal ischemia results in partial redistribution of the Na,K-ATPase from the basolateral domain of the plasma membrane to the apical surface in PT cells.20,43 Although no such loss of Na,K-ATPase polarity is observed in distal nephron segments, biochemical studies suggest that ischemic injury disrupts the attachment of the Na,K-ATPase to the subcortical cytoskeleton throughout the nephron, thus enhancing its susceptibility to endocytosic internalization.30,43 The ischemia-induced internalization of the Na,K-ATPase may be of both physiologic and pathophysiologic significance, because an analysis of cadaveric transplanted kidneys demonstrated that those kidneys that manifest delayed graft function exhibited significantly larger pools of intracellular Na,K-ATPase than did those that demonstrated prompt graft function.44 In addition, the redistribution of the sodium pump from the basolateral membrane to intracellular compartments could account at least in part for the decrease in tubular Na absorption that occurs as a consequence of renal ischemia. Our data suggest that, at least in the DCT, AS160 plays a central role in this clinically important process.
Concise Methods
Antibodies and Reagents
A polyclonal antibody directed against amino acids 1178–1189 of rat AS160 was obtained from EMD Millipore (Billerica, MA). Monoclonal anti–Na,K-ATPase antibody α5 is directed against the amino terminus of the rat Na,K-ATPase α1 subunit.45 Polyclonal phospho-specific antibodies recognizing AS160 phosphorylated at S588 and T642 were obtained from Symansis (New Zealand). Monoclonal anti–β-actin antibody and anti–calbindin polyclonal antibody were purchased from Abcam, Inc. (Cambridge, MA), and anti–caveolin-1 polyclonal and anti–E-cadherin mAbs were obtained from BD Transduction Laboratories (San Jose, CA). Rabbit anti–phospho-AMPK (T172) antibody was purchased from Cell Signaling Technology (Danvers, MA). The cell death quantification experiment was done using the terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling assay following the manufacturer’s protocol (Roche, Indianapolis, IN).
Cell Culture and Energy Depletion Protocol
MDCK cells were grown in a 5% CO2 and 95% air humidified incubator at 37°C in α-MEM (Invitrogen, Carlsbad, CA) supplemented with 10% FBS, 2 mM L-glutamine, 50 U/ml penicillin, and 50 g/ml streptomycin. The cells were subjected to energy depletion via incubation in prewarmed glucose-free DMEM containing 0.1 μM AA and 25 mM DG (Sigma-Aldrich, St. Louis, MO) to inhibit aerobic and substrate-dependent ATP generation, respectively. Cells were subjected to 30 minutes to 1 hour of energy depletion as previously described.46
Constructs and DNA Cloning
The sequence chosen for the shRNA construct targeting expression of canine AS160 (5′GCAAGGGAGCATGGTATTA3′) was subcloned into pSUPER plasmid (Oligoengine, Seattle, WA). The detailed description of the production and characterization of the stable KD AS160 MDCK cell line are presented elsewhere.23 The CMV-10 plasmid encoding the WT human AS160 was obtained from Thermo Scientific (Thermo Fisher Scientific, Rockford, IL) and the construct with a triple FLAG-tag inserted at the amino terminus (AS160 WT-FLAG) was generated according to previously described protocols.9,24 To create the pcDNA3.1-Hygro (+) plasmid encoding the human AS160 WT-FLAG, the AS160-FLAG sequence was inserted at NotI and BamHI restriction sites. The construct encoding the AS160-S588D mutation was generated in this plasmid with the QuikChange XL site-directed mutagenesis kit from Stratagene, and the mutations were verified by DNA sequencing. The AS160 WT-FLAG and S588D-FLAG constructs were transfected into the AS160 KD MDCK cell line with Lipofectamine 2000 (Invitrogen). The selection and maintenance of stable MDCK cell clones were performed in α-MEM containing 5 mg/ml G418 and Hygromycin B 0.4 mg/ml (Invitrogen). Clones were screened both for the reduced expression levels of endogenous AS160 and for the expression of the exogenous human AS160-FLAG by Western blotting. Constructs encoding a dominant negative form of dynamin (dynamin K44A-GFP) and a plasma membrane marker corresponding to a PI (4,5) P2 binding PH domain with a GFP tag (GFP-PH-PLCδ1) were kindly provided by Dr. Pietro De Camilli (Yale University, New Haven, CT).
Immunofluorescence
MDCK cells were plated on 12-mm Transwell filter inserts (Corning Life Sciences, Lowell, MA) and allowed to polarize for 3–4 days after reaching confluence. Cells were fixed with 4% paraformaldehyde and subsequently permeabilized with PBS containing 1 mg/ml BSA and 0.3% Triton X-100. Nonspecific binding was blocked using goat serum dilution buffer (33% goat serum, 40 mM NaPi, pH 7.4, 450 mM NaCl, and 0.6% Triton X-100). Primary and Alexa Fluor–conjugated secondary (Invitrogen) antibodies were diluted in goat serum dilution buffer. Cells were visualized with a confocal laser scanning microscope (model LSM-780; Carl Zeiss Microimaging, Thornwood, NY). Contrast and brightness settings were chosen so that all pixels were in the linear range. Images are the product of 8-fold line averaging.
Immunoprecipitation
MDCK cells treated or untreated with the energy depletion protocol were incubated with 1 ml of lysis buffer containing 150 mM NaCl, 50 mM Tris-HCl, pH 7.4, 1% Lubrol, 5 mM EDTA, protease inhibitors (Santa Cruz Biotechnology, Santa Cruz, CA) and phosphatase inhibitors (Sigma-Aldrich) for 30 minutes at 4°C. The insoluble fraction was removed through centrifugation at 10,000g for 30 minutes at 4°C. After the centrifugation, the supernatants were incubated with α5 antibody directed against the endogenous Na,K-ATPase and with protein G conjugated to Sepharose (Pierce, Rockford, IL) for 8 hours at 4°C. Beads were washed four times with lysis buffer. Proteins were eluted in SDS-PAGE sample buffer and separated by SDS-PAGE electrophoresis and analyzed by Western blotting. Blots were then probed with peroxidase-conjugated mouse secondary antibody and visualized with the enhanced chemiluminescence reagent (GE Healthcare, Little Chalfont, Buckinghamshire, UK).
Cell Surface Biotinylation
MDCK cells were plated on Transwell filter inserts (Corning, Corning, NY), and were allowed to polarize for 3–4 days after reaching confluence. Cells were first biotinylated at the basolateral surface with Sulfo-NHS-SS biotin (Thermo Fisher Scientific) as described previously,47 and then subjected to energy depletion. Biotin that remained exposed at the basolateral cell surface was stripped through incubation with 100 mM MesNa for 40 minutes at 4°C, and cells were washed with PBS++ (PBS supplemented with 10 mM MgCl2 and 1 mM CaCl2). Cells were then lysed in 1 ml of lysis buffer (1% Triton, 150 mM NaCl, 50 mM Tris, pH 7.4, and 1 mM EDTA, containing protease and phosphatase inhibitors), and incubated for 8 hours at 4°C with streptavidin-conjugated agarose beads (Pierce). Precipitated proteins were eluted from the beads through incubation in SDS-PAGE sample buffer supplemented with 100 mM dithiothreitol and analyzed by standard SDS-PAGE and Western immunoblotting. To assess the level of Na,K-ATPase expression, equal amounts of total lysates were subjected to SDS-PAGE and Western immunoblotting using a mAb (α5) directed against an epitope of the α-subunit of Na,K-ATPase. Band density was quantified using ImageJ software (National Institutes of Health).
Alkaline Phosphatase Treatment
MDCK cells were plated on six-well tissue culture plates and allowed to polarize for 3–4 days after reaching confluence. Cells were subjected to energy depletion as described above and then lysed in 1 ml of lysis buffer: 1% Triton X-100, 150 mM NaCl, 50 mM Tris, and 1 mM EDTA, containing protease inhibitors. Cells were incubated with lysis buffer for 30 minutes at 4°C, and the soluble fraction was recovered after a centrifugation at 10,000g for 30 minutes at 4°C. The lysates were incubated with or without 10 units of calf intestinal alkaline phosphatase (Sigma-Aldrich) for 30 minutes at 37°C. Samples that did not receive the calf intestinal alkaline phosphatase treatment were lysed in a buffer containing phosphatase inhibitors. Lysates were dissolved in SDS-PAGE sample buffer, separated by SDS-PAGE electrophoresis and examined by Western blotting.
Acute Renal Ischemia
Animal studies were conducted in accordance with the Yale School of Medicine Institutional Animal Care and Use Committee. The AS160 KO mice had been generated by targeted deletion of part of exon 1 of the AS160 gene as described by Lier et al.31 The 129/SvEv-C57BL/6 TBC1D4-deficient mice were crossed with C57/BL6 WT mice to generate a mixed 129/SvEv-C57BL/6 background. Eight-week-old male AS160 KO mice or WT littermates weighing 20–25 g were anesthetized with ketamine (100 mg/kg) by intraperitoneal injection and, using aseptic techniques, subjected to a laparotomy only (sham surgery) or a laparotomy with bilateral renal pedicle clamping (ischemia) for 30 minutes. Ischemia was confirmed by color change observed in kidneys after clamping. For analgesia, buprenorphine (0.05 mg/kg) was injected subcutaneously before surgical procedures and then postoperatively every 12 hours. Supportive fluids were given throughout the operative period, and hypothermia was prevented by use of an isothermal heating pad and warming lights. After 24 hours of reperfusion, the animals were once again anesthetized with ketamine (100 mg/kg) and subjected to laparotomy. Kidneys were collected after being snap frozen in situ. Frozen kidneys were stored at −80°C or immediately homogenized in 1 ml of cold homogenization buffer containing phosphatase and protease inhibitors (150 mM NaCl, 50 mM Tris-HCl, pH 7.4, 1% Triton X-100, and 5 mM EDTA). Homogenates were then prepared as previously published.48 Specimens were rotated for 1 hour at 4°C, centrifuged at 16,000g for 5 minutes at 4°C to separate nuclei and tissue debris, and then ultracentrifuged at 100,000g for 60 minutes at 4°C. Protein concentrations of supernatants were determined by the Bradford assay (Bio-Rad, Hercules, CA) and subjected to Western blot analysis.
Fixation and Preparation of Kidney Tissue
Kidney tissue was fixed according to our laboratory’s standard protocol.49 Briefly, mice were fixed via whole-body ventricular perfusion with PBS, pH 7.4, for 15 seconds followed with freshly prepared 4% formaldehyde in PBS for 2 minutes. Kidneys were removed, dissected into transverse slices 1 mm in thickness, and postfixed for 1 hour at room temperature, followed by three 15-minute washes in PBS. The tissue was then prepared for cryostat sectioning or further postfixed in PBS with 1% glutaraldehyde followed by osmication and embedding in EPOX-812 resin. Epoxy-embedded tissue was sectioned at 2 μm with a Reichert Ultracut-E ultramicrotome and stained with a mixture of 1% azure I and II in water and 0.5% sodium borate.
Analysis of Images of Renal Tissue
Random images of cortex sampling both proximal and distal tubules were obtained from three experiments and labeled only with image accession number, date, and time. Images were analyzed at a later time period in a randomly blinded fashion. All distal tubules in each image were counted. Images were scored based upon whether the subcellular distribution of the Na,K-ATPase was predominantly restricted to the basolateral plasma membrane or whether the Na,K-ATPase staining was predominantly distributed throughout the cytoplasm. Those with a predominantly cytoplasmic distribution were counted as a separate set and the ratio of tubules with a cytoplasmic distribution to the total number of distal tubules was obtained for each image. Over 100 tubule cross-sections were scored for each condition. The experimental groups were then correlated with date and time of accession and the results of all of the images of each group were combined to give totals of all tubules in each group and the ratio and percentage was calculated.
Wheat Germ Agglutinin Endocytosis Assay
MDCK cells were transfected or not with a plasmid encoding dynamin K44A-GFP, plated on Transwell filter inserts (Corning), and were allowed to polarize for 3–4 days. Cells were treated at the apical surface with 25 μg/ml WGA-647 conjugate (Invitrogen) for 30 minutes at 37°C. The apical WGA-647 that remained bound to the apical surface after this treatment was stripped with 0.1 M of N-acetyl-d-glucosamine (Sigma-Aldrich) for 10 minutes at 18°C with three different washes in a CO2 independent media (Invitrogen). After a wash step in in PBS++, cells were then fixed with 4% paraformaldehyde and analyzed by confocal microscopy, as described above.
Immunofluorescence Analyses of Mouse Kidneys and Measurement of Biochemical Parameters
Serum creatinine measurements were performed by the Mouse Metabolic Phenotyping Center at Yale University. Cryostat-prepared kidney sections were subjected to 15 minutes of antigen retrieval through incubation in 10 mM citrate buffer, pH 6.0. After multiple washes with PBS and quenching with 0.5 M NH4Cl, sections were washed with 1% SDS and then blocked with 0.1% BSA in 10% goat serum buffer. Primary antibody α5, directed against the sodium pump, was applied at a dilution of 1:400, followed by Alexa Fluor 488–conjugated goat anti-mouse secondary antibody at a 1:200 dilution (Molecular Probes, Carlsbad, CA).
Statistical Analyses
All experiments were carried out in at least three independent replicates. Values are expressed as means±SEM. Comparisons between experimental groups were made using the t test.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Vanathy Rajendran, SueAnn Mentone, Tom Ardito, and Deren Shao for their invaluable technical support. We also thank all of the members of the Caplan Laboratory for their helpful discussions and suggestions.
This work was supported by the Leducq Foundation Transatlantic Network for Hypertension and by the National Institutes of Health (Grants DK-072612 and DK-17433).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013101040/-/DCSupplemental.
References
- 1.Jørgensen PL: Sodium and potassium ion pump in kidney tubules. Physiol Rev 60: 864–917, 1980 [DOI] [PubMed] [Google Scholar]
- 2.Barlet-Bas C, Khadouri C, Marsy S, Doucet A: Enhanced intracellular sodium concentration in kidney cells recruits a latent pool of Na-K-ATPase whose size is modulated by corticosteroids. J Biol Chem 265: 7799–7803, 1990 [PubMed] [Google Scholar]
- 3.Horisberger JD, Rossier BC: Aldosterone regulation of gene transcription leading to control of ion transport. Hypertension 19: 221–227, 1992 [DOI] [PubMed] [Google Scholar]
- 4.Liang M, Tian J, Liu L, Pierre S, Liu J, Shapiro J, Xie ZJ: Identification of a pool of non-pumping Na/K-ATPase. J Biol Chem 282: 10585–10593, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Chibalin AV, Ogimoto G, Pedemonte CH, Pressley TA, Katz AI, Féraille E, Berggren PO, Bertorello AM: Dopamine-induced endocytosis of Na+,K+-ATPase is initiated by phosphorylation of Ser-18 in the rat alpha subunit and Is responsible for the decreased activity in epithelial cells. J Biol Chem 274: 1920–1927, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Sweeney G, Klip A: Regulation of the Na+/K+-ATPase by insulin: Why and how? Mol Cell Biochem 182: 121–133, 1998 [PubMed] [Google Scholar]
- 7.Molitoris BA, Geerdes A, McIntosh JR: Dissociation and redistribution of Na+,K(+)-ATPase from its surface membrane actin cytoskeletal complex during cellular ATP depletion. J Clin Invest 88: 462–469, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coster AC, Govers R, James DE: Insulin stimulates the entry of GLUT4 into the endosomal recycling pathway by a quantal mechanism. Traffic 5: 763–771, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Sano H, Kane S, Sano E, Mîinea CP, Asara JM, Lane WS, Garner CW, Lienhard GE: Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem 278: 14599–14602, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Bruss MD, Arias EB, Lienhard GE, Cartee GD: Increased phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle in response to insulin or contractile activity. Diabetes 54: 41–50, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Mîinea CP, Sano H, Kane S, Sano E, Fukuda M, Peränen J, Lane WS, Lienhard GE: AS160, the Akt substrate regulating GLUT4 translocation, has a functional Rab GTPase-activating protein domain. Biochem J 391: 87–93, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eguez L, Lee A, Chavez JA, Miinea CP, Kane S, Lienhard GE, McGraw TE: Full intracellular retention of GLUT4 requires AS160 Rab GTPase activating protein. Cell Metab 2: 263–272, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Larance M, Ramm G, Stöckli J, van Dam EM, Winata S, Wasinger V, Simpson F, Graham M, Junutula JR, Guilhaus M, James DE: Characterization of the role of the Rab GTPase-activating protein AS160 in insulin-regulated GLUT4 trafficking. J Biol Chem 280: 37803–37813, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Liang X, Butterworth MB, Peters KW, Frizzell RA: AS160 modulates aldosterone-stimulated epithelial sodium channel forward trafficking. Mol Biol Cell 21: 2024–2033, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mandel LJ, Doctor RB, Bacallao R: ATP depletion: A novel method to study junctional properties in epithelial tissues. II. Internalization of Na+,K(+)-ATPase and E-cadherin. J Cell Sci 107: 3315–3324, 1994 [DOI] [PubMed] [Google Scholar]
- 16.Brodie JC, Humes HD: Stem cell approaches for the treatment of renal failure. Pharmacol Rev 57: 299–313, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Thadhani R, Pascual M, Bonventre JV: Acute renal failure. N Engl J Med 334: 1448–1460, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Schrier RW, Wang W, Poole B, Mitra A: Acute renal failure: Definitions, diagnosis, pathogenesis, and therapy. J Clin Invest 114: 5–14, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaudio KM, Thulin G, Ardito T, Kashgarian M, Siegel NJ: Metabolic alterations in proximal tubule suspensions obtained from ischemic kidneys. Am J Physiol 257: F383–F389, 1989 [DOI] [PubMed] [Google Scholar]
- 20.Molitoris BA, Chan LK, Shapiro JI, Conger JD, Falk SA: Loss of epithelial polarity: A novel hypothesis for reduced proximal tubule Na+ transport following ischemic injury. J Membr Biol 107: 119–127, 1989 [DOI] [PubMed] [Google Scholar]
- 21.Riordan M, Sreedharan R, Wang S, Thulin G, Mann A, Stankewich M, Van Why S, Kashgarian M, Siegel NJ: HSP70 binding modulates detachment of Na-K-ATPase following energy deprivation in renal epithelial cells. Am J Physiol Renal Physiol 288: F1236–F1242, 2005 [DOI] [PubMed] [Google Scholar]
- 22.van Why SK, Kim S, Geibel J, Seebach FA, Kashgarian M, Siegel NJ: Thresholds for cellular disruption and activation of the stress response in renal epithelia. Am J Physiol 277: F227–F234, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Alves DS, Farr GA, Seo-Mayer P, Caplan MJ: AS160 associates with the Na+,K+-ATPase and mediates the adenosine monophosphate-stimulated protein kinase-dependent regulation of sodium pump surface expression. Mol Biol Cell 21: 4400–4408, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kane S, Sano H, Liu SC, Asara JM, Lane WS, Garner CC, Lienhard GE: A method to identify serine kinase substrates. Akt phosphorylates a novel adipocyte protein with a Rab GTPase-activating protein (GAP) domain. J Biol Chem 277: 22115–22118, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Cartee GD, Wojtaszewski JF: Role of Akt substrate of 160 kDa in insulin-stimulated and contraction-stimulated glucose transport. Appl Physiol Nutr Metab 32: 557–566, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Stöckli J, Davey JR, Hohnen-Behrens C, Xu A, James DE, Ramm G: Regulation of glucose transporter 4 translocation by the Rab guanosine triphosphatase-activating protein AS160/TBC1D4: Role of phosphorylation and membrane association. Mol Endocrinol 22: 2703–2715, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai T, Wang H, Chen Y, Liu L, Gunning WT, Quintas LE, Xie ZJ: Regulation of caveolin-1 membrane trafficking by the Na/K-ATPase. J Cell Biol 182: 1153–1169, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J, Liang M, Liu L, Malhotra D, Xie Z, Shapiro JI: Ouabain-induced endocytosis of the plasmalemmal Na/K-ATPase in LLC-PK1 cells requires caveolin-1. Kidney Int 67: 1844–1854, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Stauffer TP, Ahn S, Meyer T: Receptor-induced transient reduction in plasma membrane PtdIns(4,5)P2 concentration monitored in living cells. Curr Biol 8: 343–346, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Aufricht C, Bidmon B, Ruffingshofer D, Regele H, Herkner K, Siegel NJ, Kashgarian M, Van Why SK: Ischemic conditioning prevents Na,K-ATPase dissociation from the cytoskeletal cellular fraction after repeat renal ischemia in rats. Pediatr Res 51: 722–727, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Lier N, Gresko N, Di Chiara M, Loffing-Cueni D, Loffing J: Immunofluorescent localization of the Rab-GAP protein TBC1D4 (AS160) in mouse kidney. Histochem Cell Biol 138: 101–112, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Slocum JL, Heung M, Pennathur S: Marking renal injury: Can we move beyond serum creatinine? Transl Res 159: 277–289, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Molitoris BA, Falk SA, Dahl RH: Ischemia-induced loss of epithelial polarity. Role of the tight junction. J Clin Invest 84: 1334–1339, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Molitoris BA, Hoilien CA, Dahl R, Ahnen DJ, Wilson PD, Kim J: Characterization of ischemia-induced loss of epithelial polarity. J Membr Biol 106: 233–242, 1988 [DOI] [PubMed] [Google Scholar]
- 35.Tian J, Li X, Liang M, Liu L, Xie JX, Ye Q, Kometiani P, Tillekeratne M, Jin R, Xie Z: Changes in sodium pump expression dictate the effects of ouabain on cell growth. J Biol Chem 284: 14921–14929, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dada LA, Sznajder JI: Hypoxic inhibition of alveolar fluid reabsorption. Adv Exp Med Biol 618: 159–168, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Dada LA, Chandel NS, Ridge KM, Pedemonte C, Bertorello AM, Sznajder JI: Hypoxia-induced endocytosis of Na,K-ATPase in alveolar epithelial cells is mediated by mitochondrial reactive oxygen species and PKC-zeta. J Clin Invest 111: 1057–1064, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yudowski GA, Efendiev R, Pedemonte CH, Katz AI, Berggren PO, Bertorello AM: Phosphoinositide-3 kinase binds to a proline-rich motif in the Na+, K+-ATPase alpha subunit and regulates its trafficking. Proc Natl Acad Sci U S A 97: 6556–6561, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tzatsos A, Tsichlis PN: Energy depletion inhibits phosphatidylinositol 3-kinase/Akt signaling and induces apoptosis via AMP-activated protein kinase-dependent phosphorylation of IRS-1 at Ser-794. J Biol Chem 282: 18069–18082, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Towler MC, Hardie DG: AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res 100: 328–341, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Sun Y, Bilan PJ, Liu Z, Klip A: Rab8A and Rab13 are activated by insulin and regulate GLUT4 translocation in muscle cells. Proc Natl Acad Sci U S A 107: 19909–19914, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Comellas AP, Kelly AM, Trejo HE, Briva A, Lee J, Sznajder JI, Dada LA: Insulin regulates alveolar epithelial function by inducing Na+/K+-ATPase translocation to the plasma membrane in a process mediated by the action of Akt. J Cell Sci 123: 1343–1351, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Molitoris BA, Dahl R, Geerdes A: Cytoskeleton disruption and apical redistribution of proximal tubule Na(+)-K(+)-ATPase during ischemia. Am J Physiol 263: F488–F495, 1992 [DOI] [PubMed] [Google Scholar]
- 44.Alejandro VS, Nelson WJ, Huie P, Sibley RK, Dafoe D, Kuo P, Scandling JD, Jr, Myers BD: Postischemic injury, delayed function and Na+/K(+)-ATPase distribution in the transplanted kidney. Kidney Int 48: 1308–1315, 1995 [DOI] [PubMed] [Google Scholar]
- 45.Tamkun MM, Fambrough DM: The (Na+ + K+)-ATPase of chick sensory neurons. Studies on biosynthesis and intracellular transport. J Biol Chem 261: 1009–1019, 1986 [PubMed] [Google Scholar]
- 46.Seo-Mayer PW, Thulin G, Zhang L, Alves DS, Ardito T, Kashgarian M, Caplan MJ: Preactivation of AMPK by metformin may ameliorate the epithelial cell damage caused by renal ischemia. Am J Physiol Renal Physiol 301: F1346–F1357, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gottardi CJ, Dunbar LA, Caplan MJ: Biotinylation and assessment of membrane polarity: Caveats and methodological concerns. Am J Physiol 268: F285–F295, 1995 [DOI] [PubMed] [Google Scholar]
- 48.Mount PF, Hill RE, Fraser SA, Levidiotis V, Katsis F, Kemp BE, Power DA: Acute renal ischemia rapidly activates the energy sensor AMPK but does not increase phosphorylation of eNOS-Ser1177. Am J Physiol Renal Physiol 289: F1103–F1115, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Pluznick JL, Zou DJ, Zhang X, Yan Q, Rodriguez-Gil DJ, Eisner C, Wells E, Greer CA, Wang T, Firestein S, Schnermann J, Caplan MJ: Functional expression of the olfactory signaling system in the kidney. Proc Natl Acad Sci U S A 106: 2059–2064, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.