Abstract
Zinc-α2-glycoprotein (AZGP1) is a secreted protein synthesized by epithelial cells and adipocytes that has roles in lipid metabolism, cell cycling, and cancer progression. Our previous findings in AKI indicated a new role for AZGP1 in the regulation of fibrosis, which is a unifying feature of CKD. Using two models of chronic kidney injury, we now show that mice with genetic AZGP1 deletion develop significantly more kidney fibrosis. This destructive phenotype was rescued by injection of recombinant AZGP1. Exposure of AZGP1-deficient mice to cardiac stress by thoracic aortic constriction revealed that antifibrotic effects were not restricted to the kidney but were cardioprotective. In vitro, recombinant AZGP1 inhibited kidney epithelial dedifferentiation and antagonized fibroblast activation by negatively regulating TGF-β signaling. Patient sera with high levels of AZGP1 similarly attenuated TGF-β signaling in fibroblasts. Taken together, these findings indicate a novel role for AZGP1 as a negative regulator of fibrosis progression, suggesting that recombinant AZGP1 may have translational effect for treating fibrotic disease.
Keywords: fibrosis, epithelial, fibroblast, renal fibrosis, renal tubular epithelial cells, GF-β
Fibrosis of the kidney is present in almost all forms of chronic renal disease and has been identified as one of the most crucial determinants of progressive loss of renal function.1 Studying age-dependent differences in renal repair, we observed that mice treated with small interfering RNA (siRNA) against zinc-α2-glycoprotein (AZGP1) were unexpectedly susceptible to kidney fibrosis,2 suggesting a potential link between AZGP1 and the development of fibrosis.
AZGP1 is a secreted glycoprotein that is synthesized by adipocytes and epithelial cells of many organs. First isolated from human plasma, it was later found in other body fluids, such as saliva, sweat, milk, and urine.3,4 The biologic function of AZGP1 is still poorly understood, and although the molecular structure of AZGP1 resembles a secretory form of MHC-I, the protein seems to be devoid of immunologic functions.5 AZGP1 plays a role in lipid metabolism and potentially, cancer cachexia, where it has been described as a lipid-mobilizing factor.6 In agreement, AZGP1 mobilized fat in cultured adipocytes through stimulation of β3-adrenoceptors.7 Furthermore, rodents that were administered exogenous AZGP1 showed increased fat mobilization,8,9 whereas genetic deletion of AZGP1 was associated with increased body fat.5
With the molecular mass of 41 kD, AZGP1 is freely filtered in the glomerulus and subsequently cleared by kidney tubular cells through reabsorption and lysosomal degradation.10 Circulating AZGP1 significantly accumulates when renal clearance is disrupted in patients with acute and chronic renal failure.11–13 Stimulated by a uremic environment, adipocytes might secrete more AZGP1, further increasing protein levels.14 However, the functional effect of elevated AZGP1 levels in renal disease remains unclear. Because AZGP1 was inversely associated with proatherogenic factors and oxidative stress in patients undergoing hemodialysis, Leal et al.15 suggested that it might act as a cardiovascular protective factor in this patient population.
Similar to other organs, kidney fibrosis is characterized by a dedifferentiation of epithelial cells and an activation of interstitial fibroblasts. Like in other organs, the single most important mediator of kidney fibrosis is TGF-β.16 The key role of TGF-β in progressive renal disease was underlined by studies of TGF-β blockade and transgenic TGF-β overexpression.17,18 These studies showed that TGF-β can potently disrupt renal cell homeostasis by activating resting fibroblasts into myofibroblasts, which produce excessive amounts of collagen and other extracellular matrix components.1,16 In parallel, sustained TGF-β signaling promotes fibrosis progression by inducing the dedifferentiation of parenchymal epithelial cells.19 Interestingly, AZGP1 has been shown to promote epithelial cell differentiation and stabilization during carcinogenesis.20–23 Along these lines, AZGP1 was linked to inhibition of TGF-β–mediated dedifferentiation in pancreatic cancer cells.24 On the basis of these data, we tested the hypothesis that AZGP1 negatively regulates the development of organ fibrosis.
Results
AZGP1 Deficiency Exacerbates Experimental Kidney Fibrosis
On the basis of our previous data,2 we hypothesized that AZGP1 inhibits profibrotic processes in the kidney. To test this hypothesis, we induced experimental renal fibrosis in AZGP1-deficient mice. So far, the only phenotypical difference found in these mice was a moderately increased body weight combined with a reduced lipolytic activity.5 To minimize potentially confounding effects of this phenotypical difference, we used heterozygous littermates (AZGP1+/−) as controls instead of wild-type (AZGP1+/+) mice in most experiments. AZGP1+/− mice did not differ significantly from AZGP1−/− mice in body weight, kidney function, and triglyceride levels (Supplemental Figure 1, A–D). AZGP1+/− showed global AZGP1 expression at a level of approximately 50% of AZGP1+/+ mice (Supplemental Figure 1, E and F), and there were no discernable differences in renal expression levels of E-cadherin and vimentin, suggesting a similar tubulointerstitial composition between unstressed AZGP1−/− and AZGP1+/− kidneys (Supplemental Figure 1, G and H).
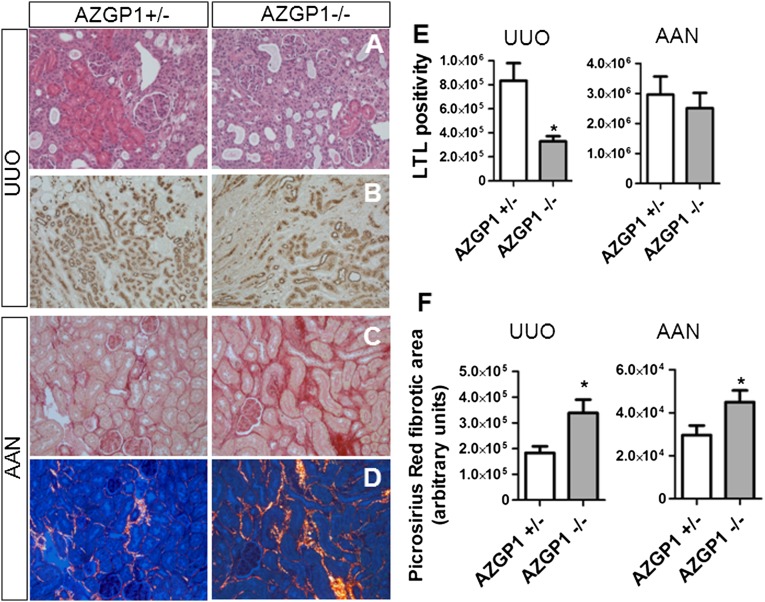
Mice were subjected to two well established experimental models of renal tubulointerstitial fibrosis: aristolochic acid nephropathy (AAN) and unilateral ureteral obstruction (UUO). In both models, kidneys of AZGP1−/− mice had more tubular damage and increased tubulointerstitial matrix expansion compared with AZGP1+/− kidneys (Figure 1, A and C). In UUO, AZGP1 deletion was associated with a more severe loss of intact tubular brush border membrane, a sign of epithelial damage and dedifferentiation, as shown by reduced Lotus Tetragonolobus Lectin (LTL) staining (Figure 1, B and E). In both models, AZGP1−/− kidneys had enhanced deposition of interstitial collagen as reflected by Picrosirius Red staining (Figure 1, C, D, and F).
Figure 1.
Genetic deficiency of AZGP1 exacerbates progression of renal fibrosis in UUO and AAN. (A) Representative images of hematoxylin/eosin staining of AZGP1+/− and AZGP1−/− kidney sections after 2 weeks of UUO. (B) Representative images showing LTL staining as a marker of intact proximal tubular brush border in UUO kidneys. Representative images of Picrosirius Red staining in (C) bright-field and (D) polarized light at 4 weeks of AAN. (E) Quantification of the LTL-positive area in the outer medulla region of UUO and AAN kidneys. (F) Quantification of birefringent collagen fibers in Picrosirius Red–stained kidney sections after UUO and AAN. UUO was evaluated for all data at 2 weeks, and AAN was evaluated for all data at 4 weeks. Values are given as means±SEMs (n=8 for E and F). Original magnifications, ×200 in A, C, and D; ×100 in B. *P<0.05.
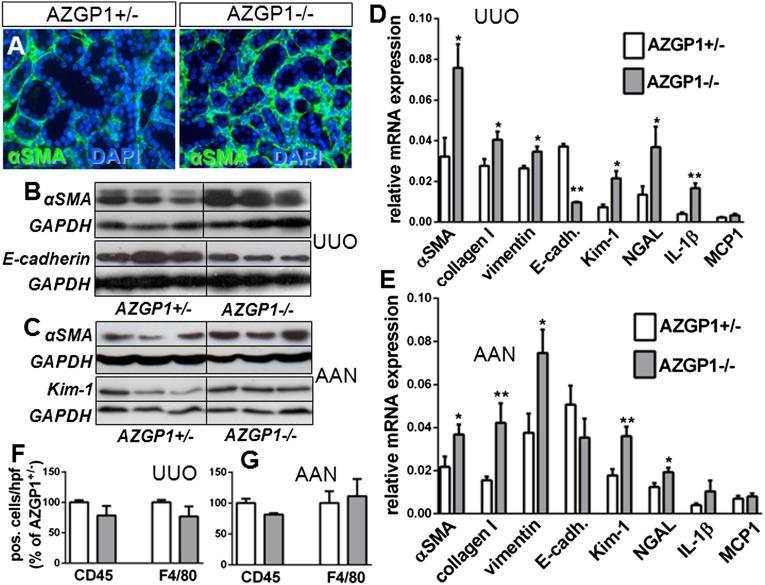
In addition, AZGP1−/− kidneys showed significantly increased interstitial α-Smooth Muscle Actin (αSMA), a marker of fibroblast activation, and enhanced expression of profibrotic Collagen I and vimentin in both models (Figure 2, A–E). No changes were found in the overall numbers of CD45-positive leukocytes and F4/80-positive macrophages. Renal expression of proinflammatory monocyte chemotactic protein-1 (MCP1) was not different, but mRNA levels of IL-1β were significantly higher in AZGP1−/− UUO kidneys (Figure 2, E and F). The expressions of tubular damage markers kidney injury molecule-1 (Kim-1) and neutrophil gelatinase–associated lipocalin (NGAL) were also markedly stronger in AZGP1−/− kidneys (Figure 2, C–E). Additionally, in UUO, there was a significant reduction of expression of the epithelial differentiation marker E-cadherin (Figure 2, B and D). For additional genotype-phenotype correlation, UUO was also performed in wild-type littermates. In the renal analysis, wild-type kidneys showed a mild trend for additional protection compared with kidneys from AZGP1+/− mice, but none of the differences reached statistical significance (Supplemental Figure 2).
Figure 2.
Genetic deficiency of AZGP1 is associated with enhanced epithelial dedifferentiation and extracellular matrix accumulation. (A) Representative images of immunofluorescence staining of αSMA in AZGP1+/− and AZGP1−/− kidney sections 4 weeks after AAN. (B) Representative Western blots for αSMA and E-cadherin at 2 weeks of UUO. (C) Representative Western blots for αSMA and Kim-1 at 4 weeks of AAN. (D and E) Quantitative RT-PCR for fibrosis genes, tubular damage markers, and inflammatory genes in UUO and AAN kidneys. (F and G) Quantification of cells positive for CD45 or F4/80 immunofluorescence in UUO and AAN kidney sections. Values are given as means±SEMs (n=8 for D–G). Original magnification, ×400 in A.*P<0.05; **P<0.005. DAPI, 4′,6-diamidino-2-phenylindole; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Deletion of AZGP1 Exacerbates Experimental Cardiac Fibrosis
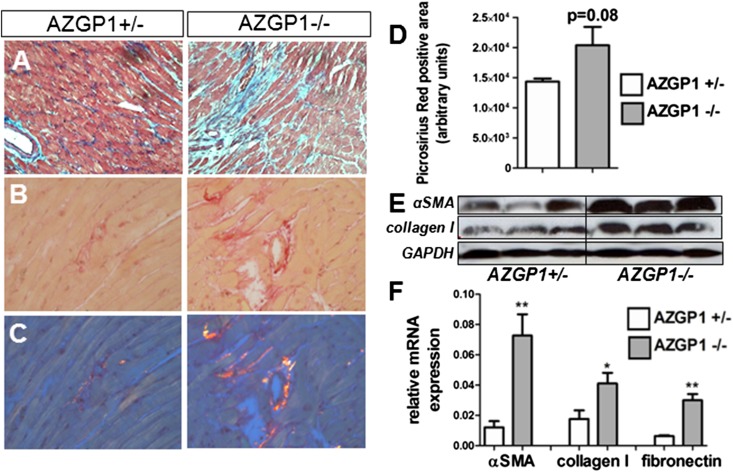
To determine whether the observed antifibrotic effects of AZGP1 were also present in other organs, we induced cardiac hypertrophy and fibrosis in AZGP1+/− and AZGP1−/− mice by a thoracic aortic constriction (TAC) model that leads to left ventricular pressure overload. After 4 weeks of TAC, AZGP1+/− and AZGP1−/− mice had significantly increased ventricular mass compared with sham-operated mice (139±9 and 142±5 mg versus 91±12 mg; each P<0.05), but there was no significant difference between the genotypes. Despite similar degrees of hypertrophy, AZGP1−/− hearts had more extracellular matrix deposition, which was indicated by Masson trichrome staining (Figure 3A), and an increased accumulation of collagen, which was evidenced by quantification of Picrosirius Red staining (Figure 3, B–D). This was accompanied by a significantly higher abundance of αSMA, Collagen I, and fibronectin in cardiac tissue of AZGP1−/− mice (Figure 3, E and F).
Figure 3.
Genetic deficiency of AZGP1 exacerbates progression of cardiac fibrosis in the TAC model. (A) Representative images of Masson trichrome–stained heart sections from AZGP1+/− and AZGP1−/− mice after 4 weeks of TAC. Representative images of Picrosirius Red staining in (B) bright-field and (C) polarized light microscopy. (D) Quantification of Picrosirius Red birefringent signal. (E) Representative Western blots of αSMA and Collagen I in cardiac tissue of AZGP1+/− and AZGP1−/− mice at 4 weeks of TAC. (F) Quantitative RT-PCR for fibrosis genes at 4 weeks of TAC. Values are given as means±SEMs (n=8 for each data point in D and F). Original magnifications, ×200 in A; ×400 in B and C. *P<0.05; **P<0.005. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Systemic Treatment with AZGP1 Rescues the Profibrotic Phenotype of AZGP1−/− Mice in UUO
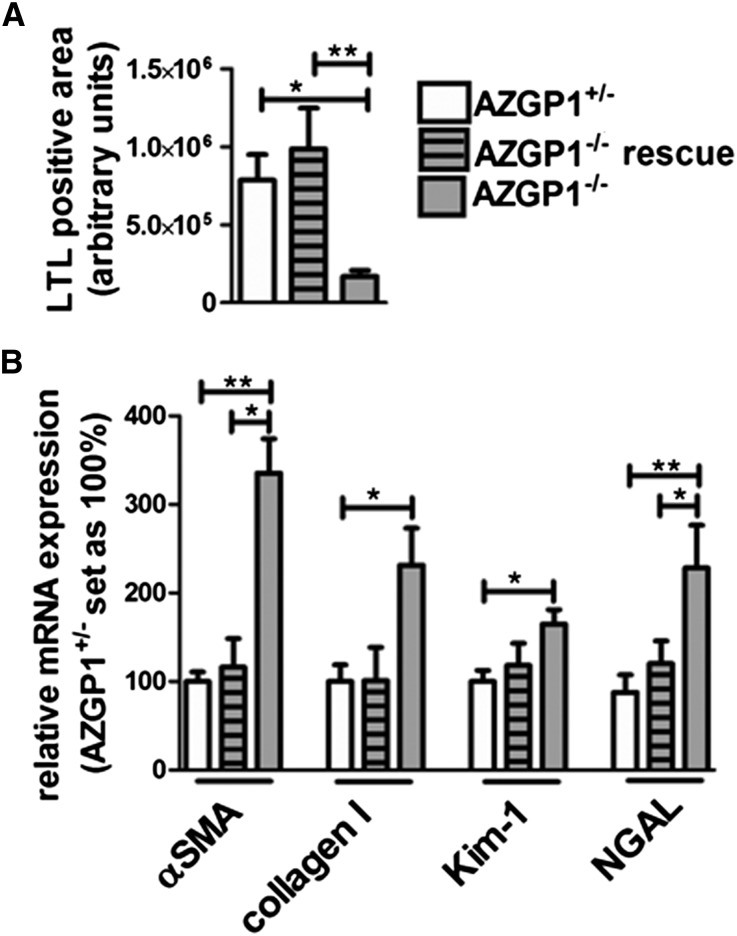
To test whether exogenous application of AZGP1 would rescue the profibrotic phenotype seen in AZGP1−/− mice, recombinant murine AZGP1 was injected into AZGP1−/− animals during UUO. Systemic treatment with 200 µg AZGP1 intravenously (approximately 8 mg/kg) on 5 consecutive days during UUO significantly attenuated profibrotic changes in AZGP1−/− kidneys, preserving the integrity of tubular brush border membranes (Figure 4A), suppressing the expression of profibrotic αSMA and Collagen I (Figure 4B), and reducing expression of tubular damage markers Kim-1 and NGAL to levels only slightly higher than in AZGP1+/− controls (Figure 4B).
Figure 4.
Administration of recombinant AZGP1 rescues profibrotic phenotype of AZGP1−/− kidneys. (A) Quantification of LTL-positive staining in 1-week UUO kidneys from AZGP1+/− and AZGP1−/− mice compared with AZGP1−/− mice after systemic injection of recombinant AZGP1 (200 µg intravenously on 5 consecutive days). (B) Quantitative RT-PCR for fibrosis and tubular damage markers. Values are given as means±SEMs (n=8 for each data point). *P<0.05; **P<0.005.
AZGP1 Attenuates TGF-β–Induced Effects in Renal Tubular Cells and Renal Fibroblasts
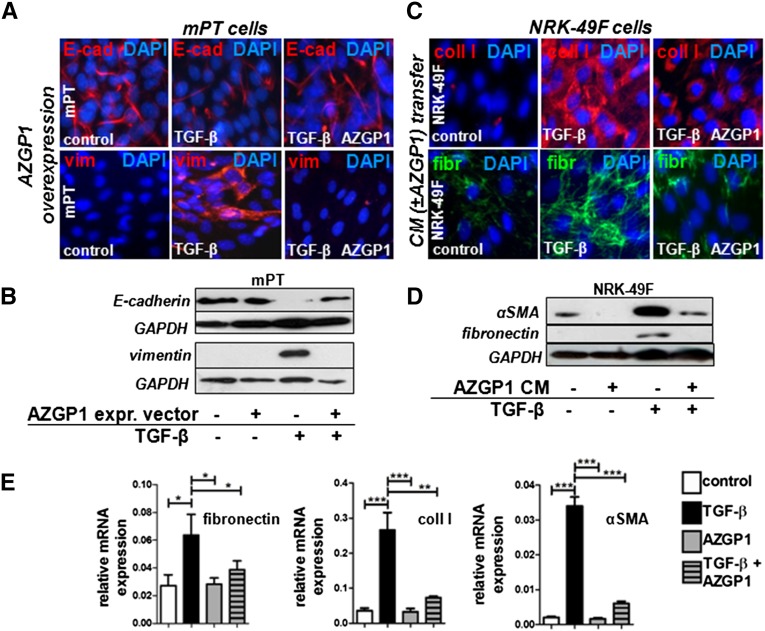
TGF-β is the single most important mediator of fibrosis in the kidney and other organs, exerting its detrimental effect by inducing epithelial dedifferentiation and activating local fibroblasts.16 To test whether the antifibrotic effects of AZGP1 might be explained through TGF-β antagonism, AZGP1 was overexpressed in cultured murine proximal tubular (mPT) cells that were challenged with TGF-β. On addition of TGF-β, AZGP1-overexpressing cells showed sustained E-cadherin expression and did not express vimentin, indicating an inhibition of TGF-β–induced epithelial dedifferentiation (Figure 5, A and B).
Figure 5.
AZGP1 negatively regulates the TGF-β–induced dedifferentiation of renal tubular cells and the activation of renal fibroblasts. (A) Representative immunocytochemistry for E-cadherin (E-cad) and vimentin (vim) showing the effect of TGF-β stimulation (2 ng/ml; 24 hours) on mPT cells transfected with AZGP1 overexpression vector or control vector. (B) Representative Western blots for E-cad and vim 24 hours after TGF-β stimulation of mPT cells transfected with AZGP1 overexpression vector or control vector. (C) Representative immunocytochemistry for Collagen I (coll I) and fibronectin (fibr) in normal rat kidney renal fibroblasts (NRK-49F) at 24 hours after TGF-β stimulation (2 ng/ml) in the presence of conditioned medium (CM) from mPT cells transfected with AZGP1 overexpression or control vector. (D) Representative Western blots of NRK-49F cells for αSMA and fibro after TGF-β stimulation in the presence of CM from mPT cells transfected with control or AZGP1 overexpression vector. (E) Quantitative RT-PCR for fibr, coll I, and αSMA of NRK-49F cells after 48 hours of TGF-β stimulation (2 ng/ml) with or without the presence of recombinant human AZGP1 (25 ng/ml). Values are given as means±SEMs (n=3 independent experiments for each data point in E). Original magnifications, ×400 in A and C. *P<0.05; **P<0.005; ***P<0.001. DAPI, 4′,6-diamidino-2-phenylindole; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Fibroblasts do not express AZGP1 but are exposed to it through local and systemic secretions.2 To mimic this situation, conditioned medium from AZGP1–overexpressing mPT cells was transferred to normal rat kidney fibroblasts (NRK-49Fs). When fibroblasts were subsequently stimulated with TGF-β, the typical profibrotic cell activation as mirrored by upregulation of Collagen I, fibronectin, and αSMA was significantly inhibited (Figure 5, C and D). The same effect was found when fibroblasts were exposed to purified recombinant human (Figure 5E) or recombinant murine AZGP1 (Supplemental Figure 3A).
AZGP1 Inhibits TGF-β Signaling by an Endocytosis-Dependent Mechanism
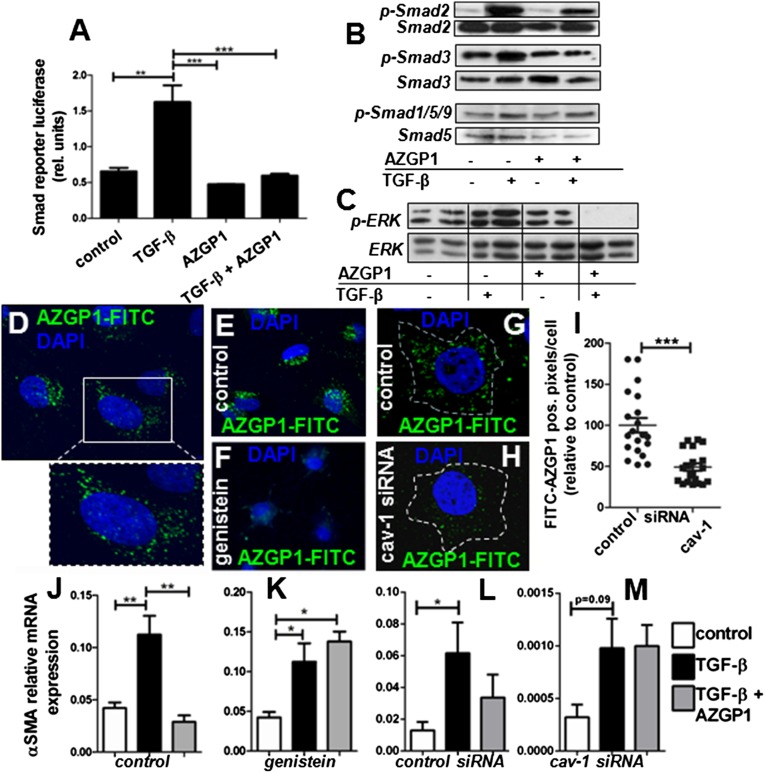
To elucidate the mechanism behind AZGP1 antagonism of the TGF-β pathway, we performed luciferase assays of the Smad promoter, revealing less TGF-β–induced transcription activity in the presence of AZGP1 (Figure 6A). Consistently, there was reduced TGF-β–induced phosphorylation of Smad2 and Smad3, whereas the mild increase in Smad1/Smad5/Smad9 phosphorylation was not significantly altered (Figure 6B). We also found that AZGP1 suppressed TGF-β–dependent extracellular signal–regulated kinase (ERK) phosphorylation, whereas it had no effect on basal ERK activation (Figure 6C). To test the possibility that AZGP1 antagonizes TGF-β by direct binding or competitive blocking of its receptor, coimmunoprecipitation studies were performed but failed to show any protein-protein interaction (Supplemental Figure 3, B and C). Similarly, propranolol blockade of the only described putative receptor of AZGP1, the β3-adrenergic receptor (β3-AR),25 had no significant effect on AZGP1-induced changes in fibroblasts (Supplemental Figure 3, D and E). Confocal microscopy revealed that fluochrome-labeled AZGP1 was readily internalized into renal fibroblasts (Figure 6D). Internalization was significantly diminished after preincubation with genistein, a tyrosine kinase inhibitor, which is often used to block caveolin-dependent endocytosis (Figure 6, E and F). More specific inhibition using caveolin-1 siRNA also resulted in a reduction in AZGP1 uptake (Figure 6, G–I). Diminished internalization of AZGP1 after genistein treatment and caveolin-1 knockdown was paralleled by an attenuated TGF-β inhibitory effect of AZGP1 (Figure 6, J–M). These results suggest a link between endocytosis and AZGP1-dependent antagonism of the TGF-β pathway.
Figure 6.
AZGP1 inhibits TGF-β–induced Smad and ERK phosphorylation, which is linked to its internalization by endocytosis. (A) Smad reporter luciferase assay in NRK-49F cells after 24 hours of TGF-β stimulation (2 ng/ml) in the presence or absence of recombinant AZGP1 (25 ng/ml). (B and C) Representative Western blots for phosphorylated Smad2, Smad3, Smad1/Smad5/Smad9, ERK, and their respective total protein after 24 hours of TGF-β stimulation (2 ng/ml) in the presence or absence of recombinant AZGP1 (25 ng/ml). (D) Representative confocal microscopy image showing NRK-49F cells at 2 hours after administration of FITC–labeled recombinant human AZGP1. Cellular uptake of labeled AZGP1 is significantly reduced by (E and F) pretreatment with genistein (200 µM) and (G and H) after siRNA-mediated knockdown of caveolin-1. (I) Quantification of cytoplasmic FITC–positive pixels after treatment with control or anticaveolin-1 siRNA. Quantitative RT-PCR shows change in AZGP1 effect on αSMA expression (J and K) after pretreatment with genistein (200 µM) and (L and M) after siRNA-mediated knockdown of caveolin-1. Values are given as means±SEMs (n=3 independent experiments for each data point in A, I, and J–M.) Original magnifications, ×63 in D–H. *P<0.05; **P<0.005; ***P<0.001.
AZGP1 in Patient Sera Exerts TGF-β Inhibitory Effects
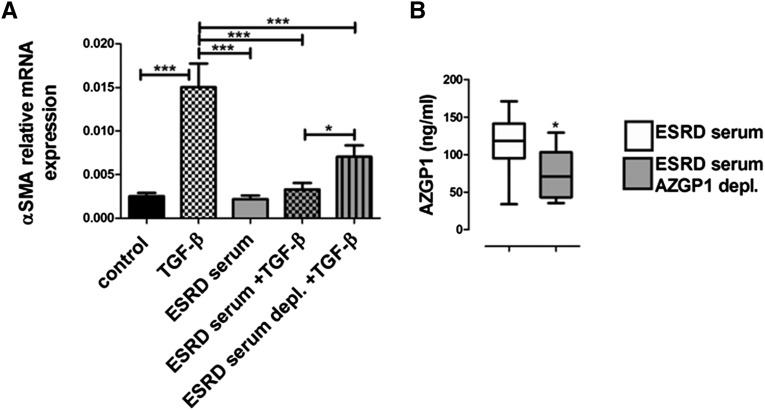
To test the hypothesis that serum AZGP1 might confer antifibrotic effects, we used sera from patients with ESRD, which have particularly high levels of circulating AZGP1.11–13 Fibroblasts that were exposed to medium supplemented with ESRD serum showed a markedly lower TGF-β response (Figure 7A). When AZGP1 levels were reduced by antibody-mediated depletion (Figure 7B), the inhibitory effect of ESRD serum was significantly attenuated (Figure 7A). These findings suggest that circulating AZGP1 in patient sera may act as a negative regulator of the TGF-β pathway, which suggests that individual AZGP1 levels could change the susceptibility to profibrotic disease processes.
Figure 7.
Sera from patients with ESRD negatively regulate TGF-β–mediated renal fibroblast activation in an AZGP1-dependent manner. (A) Quantitative RT-PCR for αSMA expression in NRK-49F cells with or without TGF-β stimulation (2 ng/ml) in the presence or absence of 5% ESRD patient sera. (B) AZGP1 levels in ESRD patient sera were reduced by antibody treatment as shown by ELISA quantification before and after depletion treatment. Values are given as means±SEMs (n=11 for each data point using sera from 11 patients with ESRD) in A and B. *P<0.05; ***P<0.001.
Discussion
In this study, we characterized AZGP1 as a negative regulator of TGF-β–induced effects in renal epithelial cells and renal fibroblasts. We found that genetic deletion of AZGP1 aggravated kidney and heart fibrosis in experimental injury models. This destructive phenotype was rescued by exogenous administration of recombinant AZGP1. Cell culture assays revealed that TGF-β antagonistic effects of AZGP1 were linked to endocytosis and also found in patient sera. Together, these findings introduce AZGP1 as a novel player in the balance of the epithelial-interstitial homeostasis.
One of the main observations of this study was that AZGP1 attenuated TGF-β–induced epithelial dedifferentiation and fibroblast activation, which are common features of organ fibrosis and cancer progression.26 Enhanced tumor progression has been shown to correlate with lower expression of epithelial AZGP1.20–23 Kong et al.24 found that AZGP1 expression can preserve epithelial integrity by promoting mesenchymal-to-epithelial transition in pancreatic cancer cells. Kong et al.24 attributed the prodifferentiation effects of AZGP1 to TGF-β antagonistic properties. We observed similar anti–TGF-β effects of AZGP1 in renal epithelial cells. Additionally, we found that AZGP1 attenuated TGF-β–induced activation of fibroblasts. These cell culture findings were congruent to our findings in knockout mice. Genetic AZGP1 deletion was associated with a more pronounced loss of epithelial differentiation and enhanced activation of fibroblasts in kidneys stressed by AAN or UUO. Both AAN and UUO importantly involve the TGF-β pathway.27,28 AZGP1-deficient mice were also more severely affected by TAC-induced cardiac fibrosis, which is also driven by TGF-β.29 Together with our cell culture results, these findings suggest that AZGP1 acquires its antifibrotic properties through inhibition of pathologic TGF-β pathway activation. In accordance with this, we found that physiologically low levels of TGF-β were associated with an inconspicuous baseline phenotype in unstressed AZGP1–deficient mice. Importantly, there was no significant difference between UUO kidneys from wild-type and AZGP1+/− mice, suggesting that a 50% reduction of normal AZGP1 is still compatible with an appropriate renal response to fibrotic stress.
A growing number of clinical studies suggesting that AZGP1 plays a role in many different cancers20–22 implies that our data might be relevant to the field of oncology. If it is correct that AZGP1 exerts antitumor effects through inhibition of TGF-β,24 AZGP1-deficient mice would be expected to have a higher susceptibility for tumor progression. The fact that we did not observe pathologic tumor formation argues against a pivotal role in cancer regulation. However, it is possible that antitumor effects of AZGP1 only become relevant in a tumor microenvironment containing increased TGF-β levels. In this context, it is important to keep in mind that TGF-β can also act as a tumor suppressor, possibly repressing carcinogenesis at early stages.30 Therefore, the TGF-β inhibitory effect of AZGP1 might be heterogeneous in cancer development, which should be explored in experimental studies.
AZGP1 is a glycoprotein secreted by most epithelial tissues, reaching a considerable serum concentration of 40–70 µg/ml in healthy individuals.11,13,31–33 This concentration changes with various diseases: decreasing in patients with HIV,34 obese subjects,31 and patients with hypertension33 and increasing in patients with preeclampsia,35 chronic heart failure,36 and acute kidney disease or CKD.11–13 Using sera from patients with ESRD, we showed, in cell culture assays, that circulating AZGP1 has important antifibrotic properties. Furthermore, increasing circulating AZGP1 by injection of recombinant protein during UUO rescued the profibrotic phenotype of AZGP1-deficient mice. Although the situation in various human diseases is likely to be more complex, our data suggest that patients with lower AZGP1 levels might have increased responsiveness to TGF-β and a higher susceptibility for fibrosis. This hypothesis is supported by a study by Gangadharan et al.37 showing a significant negative correlation between circulating AZGP1 levels and the degree of liver fibrosis in patients with hepatitis C. Along these lines, our data are compatible with the notion that increased levels of AZGP1 may have a protective function in patients with ESRD.15 In this patient population, volume expansion and pressure overload trigger progressive cardiomyopathy, contributing to an exceedingly high risk for cardiovascular events and heart failure.38 We found that AZGP1 may limit myocardial fibrosis in a model of experimental pressure–overload cardiomyopathy. It is, thus, conceivable that AZGP1 may partially counterbalance detrimental effects of ESRD-associated factors, such as pressure overload and circulating molecules of the uremic milieu (e.g., fibroblast growth factor 23 or parathyroid hormone), which promote abnormal myocardial morphology and cardiac fibrosis.39
Mechanistic information regarding the molecular mode of action of AZGP1 is controversial. In adipocytes, it has been suggested that AZGP1 elicits lipolytic effects through β3-AR stimulation.7,25 However, newer results failed to confirm typical β3-AR agonist characteristics.40 In our analysis, we were unable to establish a link between AZGP1 and β3-AR. Instead, we found that AZGP1 attenuated TGF-β–induced canonical Smad2 and Smad3 signaling. AZGP1 also inhibited TGF-β noncanonical ERK phosphorylation, mirroring findings by Kong et al.24 Additionally, we observed that blockade of AZGP1 internalization by genistein or caveolin-1 knockdown resulted in a significant reduction of TGF-β inhibition. Additional studies will be necessary to elucidate the underlying mechanisms that regulate this interplay.
Taken together, our data suggest that AZGP1 is a novel player in the complex regulation of TGF-β signaling, inhibiting uncontrolled epithelial dedifferentiation and attenuating fibroblast activation. Consequently, AZGP1-deficient mice are susceptible to the development of renal and cardiac fibrosis. Our study was restricted to kidney and heart fibrosis, but AZGP1 might have similar antifibrotic effects in other organs. These findings open up the possibility that recombinant AZGP1 may represent a potential novel strategy for antifibrosis therapy.
Concise Methods
UUO
AZGP1-deficient mice were generated and genotyped as previously described.5 Male wild-type, AZGP1-deficient, and heterozygote littermates (10–12 weeks old) were subjected to UUO as previously reported (n=8 for each condition and group).41 Mice were anesthetized and underwent left proximal ureteral ligation through a midline abdominal incision. Mice were euthanized 7 or 14 days after UUO, and kidneys were analyzed. Representative slices of each kidney (including cortex and medulla) were frozen in liquid nitrogen or fixed in 4% paraformaldehyde (PFA). For the rescue experiment, AZGP1-deficient mice were treated with systemic injections of AZGP1 (200 µg intravenously; approximately 8 mg/kg) on 5 consecutive days. For functional studies, blood samples were taken, and renal function was estimated by serum creatinine and urea measurements as well as measurement of triglycerides using an automated method (Beckman Analyzer; Beckman Instruments GmbH, Munich, Germany). All experimental procedures were in agreement with institutional and legislator regulations and approved by the local authorities.
AAN
For AAN, 10 mg/kg aristolochic acid (Sigma-Aldrich, St. Louis, MO) was injected intraperitoneally into male 10- to 12-week-old mice as previously described (n=8 for each condition and group).42 The control group was injected with the vehicle solution comprising of DMSO. Mice were euthanized 28 days after injection. Representative slices of each kidney (including cortex and medulla) were frozen in liquid nitrogen or fixed in 4% PFA.
Cardiac Fibrosis (TAC Model)
A cardiac fibrotic response was induced by a model of cardiac pressure overload caused by TAC as previously described (n=8 for each condition and group).43 Male AZGP1–deficient mice and heterozygote littermates (10–12 weeks old) were used for this purpose. Sham-operated animals served as controls. Hearts were explanted after 4 weeks of TAC and prepared for histologic and biochemical analysis.
Histologic and Immunohistochemical Staining
Obstructed and contralateral kidneys were dissected, fixed in 4% PFA, and embedded in paraffin. Four-micrometer sections were used for hematoxylin/eosin staining, Picrosirius Red staining, and immunohistochemistry. Immunohistochemistry was performed using the following primary antibodies: rat anti-mouse F4/80 (Serotec, Oxford, UK), mouse anti-αSMA, clone 1A4 (Sigma-Aldrich), rat anti-mouse CD45 (BD Bioscience), and anti-Collagen I (Calbiochem, Darmstadt, Germany). Biotinylated LTL was purchased from Vector Laboratories (Burlingame, CA). Deparaffinized kidney sections were boiled for 20 minutes in citrate buffer for antigen-retrieval, blocked with 5% milk, and incubated overnight with primary antibodies. Staining was visualized with Alexa 488/Alexa 547 secondary antibodies (Molecular Probes/Invitrogen, Carlsbad, CA) or the ABC Vectastain Kit (Vector Laboratories). For Picrosirius Red staining, deparaffinized kidney sections were stained in Picrosirius Red staining solution (Sigma-Aldrich) for 1 hour, washed in acidified water, and mounted. The staining was analyzed under polarized light, considering birefringence as a positive signal. Quantification of F4/80- and CD45-expressing cells was done by counting the positive cells in 10 randomly chosen, nonoverlapping fields (×200 magnification) in the outer medulla. αSMA, LTL, and Picrosirius Red staining was quantified using Photoshop-based image analysis (Adobe Photoshop software; Adobe Systems, San Jose, CA).
Western Blot and Coimmunoprecipitation
Western analysis was performed as previously described.2 In brief, a representative part of each kidney (kidney slices containing cortical and medullary tissues) was frozen immediately after harvesting in liquid nitrogen. Tissue was later homogenized, and after protein electrophoresis, proteins were transferred to polyvinylidene difluoride membranes, blocked with 5% milk in phosphate buffered saline/tween, and probed overnight at 4°C with primary antibodies: anti-αSMA (Sigma-Aldrich), rabbit anti-human KIM-1 (Novus Biologicals, Littleton, CO), goat anti-mouse Lipocalin2/NGAL (R&D Systems, Minneapolis, MN), rabbit anti-human E-Cadherin (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-human Vimentin (Santa Cruz Biotechnology), goat anti-human Fibronectin (Santa Cruz Biotechnology), rabbit anti–rat-ERK1/2 (p44/42 mitogen-activated protein kinase), rabbit anti-human phospho-ERK1/2 (phospho-p44/42 mitogen-activated protein kinase; Cell Signaling Technology, Danvers, MA), rabbit anti-Smad2 (Cell Signaling Technology), rabbit antiphospho-Smad2 (Cell Signaling Technology), rabbit anti-Smad3 (Cell Signaling Technology), rabbit antiphospho-Smad3 (Cell Signaling Technology), rabbit anti-Smad5 (Cell Signaling Technology), rabbit antiphospho-Smad1/5/9 (Cell Signaling Technology), goat anti-mouse AZGP1 (Zag [E-20]; Santa Cruz Biotechnology), and mouse anti-human AZGP1 (Zag [1D4]; Santa Cruz Biotechnology). horseradish peroxidase–conjugated secondary antibodies were purchased from Santa Cruz Biotechnology. Antibody binding was visualized by chemiluminescence (SuperSignal West Pico Chemiluminescent; Thermo Fisher Scientific, Rockford, IL). Rabbit anti-mouse glyceraldehyde-3-phosphate dehydrogenase (Sigma-Aldrich) was used as an internal loading control and normalization of protein quantification. For coimmunoprecipitation, protein lysate was incubated overnight with anti-AZGP1 (H-123 and E-20), anti–TGF-β, and anti–TGF-β receptor-II antibody (Santa Cruz Biotechnology) followed by the addition of Protein G PLUS-Agarose beads (Santa Cruz Biotechnology) for 4 hours at 4°C. Beads were washed and boiled in 4× gel-loading dye and analyzed by immunoblotting for anti-AZGP1, anti–TGF-β, and anti–TGF-β receptor-II antibody (same antibodies as for immunoprecipitation).
Cell Culture
Normal rat fibroblasts (NRK-49F; ATCC) as well as mPT epithelial cells (provided by Lloyd G. Cantley, Yale School of Medicine, New Haven, CT) were cultured in DMEM/F12 with 10% FCS. Cells were treated with human recombinant TGF-β (2 ng/ml; R&D Systems) and human recombinant AZGP1 (25 ng/ml; R&D Systems) for 6, 24, or 48 hours. Before treatment, cells were starved in DMEM/F12 without FCS for 16 hours. Fibroblasts and epithelial cells were transfected with expression or reporter plasmids using TransIT-LT1 transfection reagent (Mirus Bio LLC) according to the manufacturer’s instructions. For blockage of endocytosis, NRK-49F cells were transfected with 10 nM caveolin-1 siRNA (Qiagen, Hilden, Germany) using Lipofectamine RNAiMAX transfection reagent (Life Technologies, Darmstadt, Germany) for 48 hours before FITC–labeled recombinant AZGP1 was added (knockdown efficiency was confirmed by quantitative PCR showing a 70%–80% reduction of caveolin-1 mRNA) or preincubated with Genistein (200 µM; Sigma-Aldrich) for 30 minutes. Cells were fixed after 15 minutes or 2 hours of exposure. Uptake of labeled AZGP1 was quantified by analyzing cytoplasmic fluorescent pixels using confocal laser–scanning microphotographs and National Institutes of Health ImageJ software. For inhibition of β-adrenoreceptor, activation cells were preincubated with 10 µM propanolol (Sigma-Aldrich) for 30 minutes and harvested after 24 hours. Sera from patients with ESRD (n=11) were diluted 1:20 in culture medium.
Luciferase Assay
NRK-49F cells were transfected with Smad luciferase reporter construct SBE-Luc vector 16527 from Addgene (Cambridge, MA) and treated with TGF-β and AZGP1 as described above. After 24 hours, luciferase activity was measured using the Dual-Luciferase Reporter Assay (Promega, Madison, WI) according to the manufacturer’s instructions. Firefly luciferase activity was normalized to cotransfected Renilla luciferase activity (pRL-TK; Promega).
Production and Purification of AZGP1
HEK293-F cells were seeded in FreeStyle Medium (Gibco; Life Technologies, Carlsbad, CA) and transfected with expression vector containing mouse AZGP1. Harvesting and purification of recombinant AZGP1 was done as previously described by Russell and Tisdale9 using centrifugal concentrating filters (Amicon, Billerica, MA) with a 10-kD cutoff. Concentrated medium was mixed 1 g per 10 ml diethylaminoethyl cellulose (Sigma-Aldrich) in 10 mM Tris (pH 8.8) to allow binding of negatively charged AZGP1. Diethylaminoethyl cellulose was subsequently centrifuged, and AZGP1 was eluted by adding 10 mM Tris/0.3 M NaCl. The supernatant was concentrated using Amicon centrifugal filters with a cutoff of 30 kD. Protein concentration was measured by Bradford Assay. For in vivo injection, the protein was diluted in 0.9% NaCl. For fluorescent labeling of AZGP1, recombinant mouse AZGP1 was labeled with the Atto 488 Protein Labeling Kit (Sigma-Aldrich) according to the manufacturer’s instructions.
Quantitative Real–Time PCR
RNA was isolated from NRK-49F cells and frozen kidney tissue (kidney slices containing cortical and medullary tissue) using the RNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions. Reverse transcription was performed with M-MLV Reverse Transcription (Promega). cDNA was used as a template for quantitative PCR. The levels of mRNA expression were determined by quantitative real-time PCR using a Roche Lightcycler 480 System with SYBR green master mix and specific primers: NGAL forward: TGA AGG AAC GTT TCA CCC GCT TTG, NGAL reverse: ACA GGA AAG ATG GAG TGG CAG ACA; KIM-1 forward: AAA CCA GAG ATT CCC ACA CG, KIM-1 reverse: GTC GTG GGT CTT CCT GTA GC; αSMA forward: GTG CTA TGT CGC TCT GGA CTT TGA; αSMA reverse: ATG AAA GAT GGC TGG AAG AGG GTC; Collagen I forward: TGT CCC AAC CCC CAA AGA C, Collagen I reverse: CCC TCG ACT CCT ACA TCT GA; Fibronectin forward: CGG TAG GAC CTT CTA TTC CT, Fibronectin reverse: GAT ACA TGA CCC CTT CAT TG; E-cadherin forward: AGT CCC GGC TTC AGT TCC, E-cadherin reverse: CTG TGA TGG TGC CGT CTG T; Vimentin forward: CAC ACG CAC CTA CAG TCT G, Vimentin reverse: GTC CAC CGA GTC TTG AAG C; IL-1β forward: AGGTCCACGGGAAAGACACAGG, IL-1β reverse: GGGCTGCTTCCAAACCTTTGAC; MCP1 forward: TCATGCTTCTGGGCCTGC, MCP1 reverse: CAGCCTACTCATTGGGATCATC; and Actin forward: AGC CAT GTA CGT AGC CAT CC, Actin reverse: CTC TCA GCT GTG GTA A. Melting curves were examined to verify that a single product was amplified. For quantitative analysis, all samples were normalized to Actin gene expression using the ΔΔCT (cycle threshold) value method.
Serum Depletion
Sera from 11 patients on maintenance hemodialysis were collected after the long weekly interval as previously described.13 All patients consented in writing to donating blood for scientific evaluation. The protocol was approved by the Institutional Review Board at Hannover Medical School. AZGP1 was depleted from serum using a standard immunoprecipitation protocol. In brief, serum was incubated with 200 µg/ml AZGP1 antibody (Zag [H123]; Santa Cruz Biotechnology) at 4°C with rotation. Protein A/G Plus beads (Santa Cruz Biotechnology) were added and incubated overnight at 4°C with rotation. Beads were precipitated, and the supernatant was used for the treatment of NRK-49F cells. AZGP1 concentration was determined by ELISA (Zinc-Alpha2 Glycoprotein Human ELISA; Biovendor GmbH, Heidelberg, Germany).
Statistical Analyses
Results are expressed as means±SEMs. Statistical significance among multiple groups was determined by one-way ANOVA tests, with a post hoc Bonferroni test to determine significance between groups. To determine significance for comparisons between two groups, a t test was used. P<0.05 was considered to be statistically significant. Prism 4.0 (GraphPad Software, San Diego, CA) was used to perform statistical tests.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank H. Ricci and B. Gewecke for technical assistance.
This work was supported by Deutsche Forschungsgemeinschaft Grant SCHM 2146/3-1 and the 7th Framework Program of the European Union (FIBROTARGET).
Parts of this work have been presented at the American Society of Nephrology Renal Week, November 16–21, 2010 in Denver, CO.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014050485/-/DCSupplemental.
References
- 1.Farris AB, Colvin RB: Renal interstitial fibrosis: Mechanisms and evaluation. Curr Opin Nephrol Hypertens 21: 289–300, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmitt R, Marlier A, Cantley LG: Zag expression during aging suppresses proliferation after kidney injury. J Am Soc Nephrol 19: 2375–2383, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burgi W, Schmid K: Preparation and properties of Zn-alpha 2-glycoprotein of normal human plasma. J Biol Chem 236: 1066–1074, 1961 [PubMed] [Google Scholar]
- 4.Hassan MI, Waheed A, Yadav S, Singh TP, Ahmad F: Zinc alpha 2-glycoprotein: A multidisciplinary protein. Mol Cancer Res 6: 892–906, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Rolli V, Radosavljevic M, Astier V, Macquin C, Castan-Laurell I, Visentin V, Guigné C, Carpéné C, Valet P, Gilfillan S, Bahram S: Lipolysis is altered in MHC class I zinc-alpha(2)-glycoprotein deficient mice. FEBS Lett 581: 394–400, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Hirai K, Hussey HJ, Barber MD, Price SA, Tisdale MJ: Biological evaluation of a lipid-mobilizing factor isolated from the urine of cancer patients. Cancer Res 58: 2359–2365, 1998 [PubMed] [Google Scholar]
- 7.Russell ST, Tisdale MJ: Role of β-adrenergic receptors in the anti-obesity and anti-diabetic effects of zinc-α2-glycoprotien (ZAG). Biochim Biophys Acta 1821: 590–599, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Bing C, Bao Y, Jenkins J, Sanders P, Manieri M, Cinti S, Tisdale MJ, Trayhurn P: Zinc-alpha2-glycoprotein, a lipid mobilizing factor, is expressed in adipocytes and is up-regulated in mice with cancer cachexia. Proc Natl Acad Sci U S A 101: 2500–2505, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russell ST, Tisdale MJ: Mechanism of attenuation of skeletal muscle atrophy by zinc-alpha2-glycoprotein. Endocrinology 151: 4696–4704, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Ekman R, Johansson BG, Ravnskov U: Renal handling of Zn-alpha2-glycoprotein as compared with that of albumin and the retinol-binding protein. J Clin Invest 57: 945–954, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leal VO, Lobo JC, Stockler-Pinto MB, Farage NE, Velarde GC, Fouque D, Leite M, Jr., Mafra D: Zinc-α2-glycoprotein: Is there association between this new adipokine and body composition in hemodialysis patients? Ren Fail 34: 1062–1067, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Philipp A, Kralisch S, Bachmann A, Lossner U, Kratzsch J, Blüher M, Stumvoll M, Fasshauer M: Serum levels of the adipokine zinc-α2-glycoprotein are increased in chronic hemodialysis. Metabolism 60: 669–672, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Sörensen-Zender I, Beneke J, Schmidt BM, Menne J, Haller H, Schmitt R: Zinc-alpha2-glycoprotein in patients with acute and chronic kidney disease. BMC Nephrol 14: 145, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pelletier CC, Koppe L, Croze ML, Kalbacher E, Vella RE, Guebre-Egziabher F, Géloën A, Badet L, Fouque D, Soulage CO: White adipose tissue overproduces the lipid-mobilizing factor zinc α2-glycoprotein in chronic kidney disease. Kidney Int 83: 878–886, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leal VO, Lobo JC, Stockler-Pinto MB, Farage NE, Abdalla DS, Leite M, Jr., Mafra D: Is zinc-α2-glycoprotein a cardiovascular protective factor for patients undergoing hemodialysis? Clin Chim Acta 413: 616–619, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Zeisberg M, Kalluri R: Cellular mechanisms of tissue fibrosis. 1. Common and organ-specific mechanisms associated with tissue fibrosis. Am J Physiol Cell Physiol 304: C216–C225, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lan HY: Diverse roles of TGF-β/Smads in renal fibrosis and inflammation. Int J Biol Sci 7: 1056–1067, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koesters R, Kaissling B, Lehir M, Picard N, Theilig F, Gebhardt R, Glick AB, Hähnel B, Hosser H, Gröne HJ, Kriz W: Tubular overexpression of transforming growth factor-beta1 induces autophagy and fibrosis but not mesenchymal transition of renal epithelial cells. Am J Pathol 177: 632–643, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishibe S, Cantley LG: Epithelial-mesenchymal-epithelial cycling in kidney repair. Curr Opin Nephrol Hypertens 17: 379–385, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Lapointe J, Li C, Higgins JP, van de Rijn M, Bair E, Montgomery K, Ferrari M, Egevad L, Rayford W, Bergerheim U, Ekman P, DeMarzo AM, Tibshirani R, Botstein D, Brown PO, Brooks JD, Pollack JR: Gene expression profiling identifies clinically relevant subtypes of prostate cancer. Proc Natl Acad Sci U S A 101: 811–816, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang CY, Zhao JJ, Lv L, Chen YB, Li YF, Jiang SS, Wang W, Pan K, Zheng Y, Zhao BW, Wang DD, Chen YM, Yang L, Zhou ZW, Xia JC: Decreased expression of AZGP1 is associated with poor prognosis in primary gastric cancer. PLoS ONE 8: e69155, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parris TZ, Kovács A, Aziz L, Hajizadeh S, Nemes S, Semaan M, Forssell-Aronsson E, Karlsson P, Helou K: Additive effect of the AZGP1, PIP, S100A8 and UBE2C molecular biomarkers improves outcome prediction in breast carcinoma. Int J Cancer 134: 1617–1629, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Brysk MM, Lei G, Adler-Storthz K, Chen Z, Brysk H, Tyring SK, Arany I: Zinc-alpha2-glycoprotein expression as a marker of differentiation in human oral tumors. Cancer Lett 137: 117–120, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Kong B, Michalski CW, Hong X, Valkovskaya N, Rieder S, Abiatari I, Streit S, Erkan M, Esposito I, Friess H, Kleeff J: AZGP1 is a tumor suppressor in pancreatic cancer inducing mesenchymal-to-epithelial transdifferentiation by inhibiting TGF-β-mediated ERK signaling. Oncogene 29: 5146–5158, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Russell ST, Tisdale MJ: Role of β-adrenergic receptors in the oral activity of zinc-α2-glycoprotein (ZAG). Endocrinology 153: 4696–4704, 2012 [DOI] [PubMed] [Google Scholar]
- 26.De Wever O, Mareel M: Role of tissue stroma in cancer cell invasion. J Pathol 200: 429–447, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Bascands JL, Schanstra JP: Obstructive nephropathy: Insights from genetically engineered animals. Kidney Int 68: 925–937, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou L, Fu P, Huang XR, Liu F, Chung AC, Lai KN, Lan HY: Mechanism of chronic aristolochic acid nephropathy: Role of Smad3. Am J Physiol Renal Physiol 298: F1006–F1017, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Xia Y, Lee K, Li N, Corbett D, Mendoza L, Frangogiannis NG: Characterization of the inflammatory and fibrotic response in a mouse model of cardiac pressure overload. Histochem Cell Biol 131: 471–481, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buijs JT, Henriquez NV, van Overveld PG, van der Horst G, ten Dijke P, van der Pluijm G: TGF-beta and BMP7 interactions in tumour progression and bone metastasis. Clin Exp Metastasis 24: 609–617, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Selva DM, Lecube A, Hernández C, Baena JA, Fort JM, Simó R: Lower zinc-alpha2-glycoprotein production by adipose tissue and liver in obese patients unrelated to insulin resistance. J Clin Endocrinol Metab 94: 4499–4507, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Gong FY, Zhang SJ, Deng JY, Zhu HJ, Pan H, Li NS, Shi YF: Zinc-alpha2-glycoprotein is involved in regulation of body weight through inhibition of lipogenic enzymes in adipose tissue. Int J Obes (Lond) 33: 1023–1030, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Zhu HJ, Wang XQ, Pan H, Gong FY, Zhang DX, Li NS, Wang LJ, Yang HB: Serum levels of the adipokine zinc- α 2-glycoprotein are decreased in patients with hypertension. ISRN Endocrinol 2014: 374090, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ceperuelo-Mallafré V, Escoté X, Viladés C, Peraire J, Domingo P, Solano E, Sirvent JJ, Pastor R, Tinahones F, Leal M, Richart C, Vendrell J, Vidal F, Alba V, Aguilar A, Auguet T, Chacón MR, López-Dupla M, Megia A, Miranda M, Olona M, Saurí A, Vargas M, Velasco I, Veloso S, Fontanet A, Gutiérrez M, Mateo G, Muñoz J, Sambeat MA, HIV-1 Lipodystrophy Study Group : Zinc alpha-2 glycoprotein is implicated in dyslipidaemia in HIV-1-infected patients treated with antiretroviral drugs. HIV Med 13: 297–303, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Stepan H, Philipp A, Roth I, Kralisch S, Jank A, Schaarschmidt W, Lössner U, Kratzsch J, Blüher M, Stumvoll M, Fasshauer M: Serum levels of the adipokine zinc-α2-glycoprotein are increased in preeclampsia. J Endocrinol Invest 35: 562–565, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Tedeschi S, Pilotti E, Parenti E, Vicini V, Coghi P, Montanari A, Regolisti G, Fiaccadori E, Cabassi A: Serum adipokine zinc α2-glycoprotein and lipolysis in cachectic and noncachectic heart failure patients: Relationship with neurohormonal and inflammatory biomarkers. Metabolism 61: 37–42, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Gangadharan B, Antrobus R, Dwek RA, Zitzmann N: Novel serum biomarker candidates for liver fibrosis in hepatitis C patients. Clin Chem 53: 1792–1799, 2007 [DOI] [PubMed] [Google Scholar]
- 38.McIntyre CW, Odudu A: Hemodialysis-associated cardiomyopathy: A newly defined disease entity. Semin Dial 27: 87–97, 2014 [DOI] [PubMed] [Google Scholar]
- 39.Silver J, Rodriguez M, Slatopolsky E: FGF23 and PTH—double agents at the heart of CKD. Nephrol Dial Transplant 27: 1715–1720, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wargent ET, O’Dowd JF, Zaibi MS, Gao D, Bing C, Trayhurn P, Cawthorne MA, Arch JR, Stocker CJ: Contrasts between the effects of zinc-α2-glycoprotein, a putative β3/2-adrenoceptor agonist and the β3/2-adrenoceptor agonist BRL35135 in C57Bl/6 (ob/ob) mice. J Endocrinol 216: 157–168, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Sörensen I, Susnik N, Inhester T, Degen JL, Melk A, Haller H, Schmitt R: Fibrinogen acting as a mitogen for tubulointerstitial fibroblasts, promotes renal fibrosis. Kidney Int 80: 1035–1044, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Susnik N, Sörensen-Zender I, Rong S, von Vietinghoff S, Lu X, Rubera I, Tauc M, Falk CS, Alexander WS, Melk A, Haller H, Schmitt R: Ablation of proximal tubular suppressor of cytokine signaling 3 enhances tubular cell cycling and modifies macrophage phenotype during acute kidney injury. Kidney Int 85: 1357–1368, 2014 [DOI] [PubMed] [Google Scholar]
- 43.Rockman HA, Ross RS, Harris AN, Knowlton KU, Steinhelper ME, Field LJ, Ross J, Jr., Chien KR: Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc Natl Acad Sci U S A 88: 8277–8281, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.