Abstract
Placental growth factor (PlGF) contributes to atherogenesis through vascular inflammation and plaque destabilization. High levels of PlGF may be associated with mortality and cardiovascular disease, but the relationship between PlGF level and adverse outcomes in patients with CKD is unclear. We conducted a prospective cohort study of 1351 consecutive participants with CKD enrolled in the Novel Assessment of Risk management for Atherosclerotic diseases in CKD (NARA-CKD) study between April 1, 2004, and December 31, 2011. During a median follow-up of 3 years, 199 participants died and 383 had cardiovascular events, defined as atherosclerotic disease or heart failure requiring hospitalization. In adjusted analyses, mortality and cardiovascular risk increased in each successive quartile of serum PlGF level; hazard ratios (HRs) (95% confidence intervals [95% CIs]) for mortality and cardiovascular risk, respectively, were 1.59 (0.83 to 3.16) and 1.55 (0.92 to 2.66) for the second quartile, 2.97 (1.67 to 5.59) and 3.39 (2.20 to 5.41) for the third quartile, and 3.87 (2.24 to 7.08) and 8.42 (5.54 to 13.3) for the fourth quartile. The composite end point of mortality and cardiovascular events occurred during the study period in 76.4% of patients in both the highest PlGF quartile (≥19.6 pg/ml) and the lowest eGFR tertile (<30 ml/min per 1.73 m2). The association between PlGF and mortality or cardiovascular events was not attenuated when participants were stratified by age, sex, traditional risk factors, and eGFR. These data suggest elevated PlGF is an independent risk factor for all-cause mortality and cardiovascular events in patients with CKD.
Keywords: cardiovascular disease, VEGF, cardiovascular events
CKD is increasingly recognized as a major public health problem that is linked to poor clinical outcomes. Cardiovascular disease is the leading cause of death in patients with CKD, at a much higher rate than in the general population.1–4 Despite this growing recognition of the cardio-renal connection, conventional treatment strategies, such as modification of traditional risk factors, are unlikely to contribute substantially to cardiovascular disease risk reduction in this population.5 Little is known about nontraditional risk factors and biomarkers identifying patients with CKD at a higher risk of cardiovascular disease, but we have recently proposed that placental growth factor (PlGF) signaling is involved in the aggravation of atherosclerotic disease associated with decreased renal function.6,7
PlGF, with a similar sequence to vascular endothelial growth factor (VEGF),8,9 is a prototypical pleiotropic cytokine capable of stimulating angiogenesis and inducing atherosclerosis by binding and activating its membrane-bound receptor, fms-like tyrosine kinase-1 (Flt-1).10–13 PlGF expression in atherosclerotic lesions regulates and activates monocytes and macrophages, which subsequently produces inflammatory and angiogenic mediators, resulting in an increased risk of plaque rupture.14,15 Conversely, inhibition of PlGF through genetic approaches and use of anti-PlGF antibodies reduces the size of atherosclerotic plaques.16
In clarifying the role of PlGF signaling in humans, accumulating evidence suggests that elevated PlGF is an independent predictor of cardiovascular risk and mortality among women in the general population and in patients with diabetes and acute coronary syndrome.17–21 However, because other investigators have not found an independent association between PlGF and mortality in patients with heart failure and suspected myocardial infarction,22,23 the effect of PlGF on mortality and cardiovascular disease remains unclear. We recently found a significant inverse relationship between PlGF production and renal function7 and that the PlGF/Flt-1 signaling pathway is partly involved in the pathogenesis of CKD-associated atherosclerosis. However, the association between circulating levels of PlGF and cardiovascular events in patients with CKD is less clear.
Therefore, we conducted a longitudinal study to test the hypothesis that PlGF is a strong and useful predictor of all-cause mortality and cardiovascular events in patients with CKD who participated in the Novel Assessment of Risk management for Atherosclerotic diseases in Chronic Kidney Disease (NARA-CKD) study.
Results
Baseline Characteristics
A total of 1351 patients met the inclusion criteria (Figure 1). The median age (interquartile range) of all participants was 65 years (52–74 years), and 825 (61%) of the participants were men. The distribution of serum PlGF levels was skewed to the right, with a median level of 14.5 pg/ml (interquartile range, 10.1–19.6 pg/ml). Baseline characteristics are presented in Table 1. Factors associated with PlGF in the univariate analysis are described in Supplemental Table 1. Multivariate linear regression revealed that high levels of PlGF were independently associated with more advanced CKD stage, high levels of C-reactive protein, low levels of serum albumin and HDL, and use of antiplatelet agents (Supplemental Table 2).
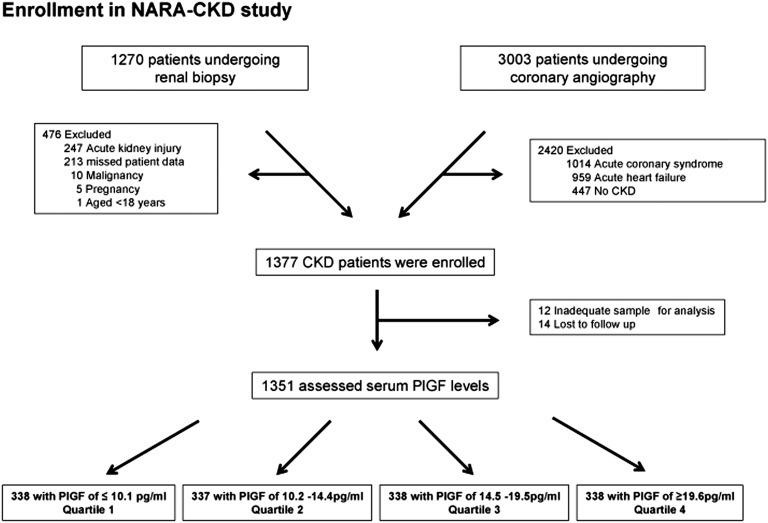
Figure 1.
We enrolled patients who underwent renal biopsy or coronary angiography in our department at Nara Medical University and four referral hospitals from April 1, 2004, to December 31, 2011. Data were ultimately analyzed for 1351 subjects and were divided into four categories according to serum PlGF levels. Flow diagram of the NARA-CKD study.
Table 1.
Baseline characteristics of study participants by serum PlGF quartile
| Characteristic | PlGF (pg/ml) | ||||
|---|---|---|---|---|---|
| Quartile 1≤10.1 (n=338) | Quartile 2 10.2–14.4 (n=337) | Quartile 3 14.5–19.5 (n=338) | Quartile 4≥19.6 (n=338) | P Value | |
| Demographics and risk factors | |||||
| Age (y) | 59 (36–69) | 64 (52–74) | 67 (56–74) | 68 (60–75) | <0.001 |
| Sex, male | 201 (59.5) | 206 (61.1) | 209 (61.8) | 209 (61.8) | 0.91 |
| Body mass index (kg/m2) | 22.8 (20.9–25.4) | 23.4 (20.7–25.4) | 23.3 (20.8–25.9) | 22.9 (20.6–25.8) | 0.53 |
| Diabetes | 79 (23.4) | 95 (28.2) | 108 (32.0) | 133 (39.3) | <0.001 |
| Hypertension | 168 (49.7) | 197 (58.5) | 227 (67.2) | 245 (72.5) | <0.001 |
| GNa | 179 (53.0) | 145 (43.0) | 131 (38.9) | 125 (37.0) | 0.001 |
| Dyslipidemia | 118 (34.9) | 157 (46.6) | 146 (43.2) | 133 (39.3) | 0.01 |
| Obesity | 92 (27.2) | 102 (30.3) | 114 (33.7) | 104 (30.8) | 0.33 |
| Smoking | 143 (42.3) | 174 (51.6) | 166 (49.1) | 187 (55.3) | 0.01 |
| Previous CAD | 44 (12.7) | 67 (19.9) | 74 (21.9) | 78 (23.1) | 0.002 |
| Previous stroke | 13 (3.8) | 17 (5.0) | 30 (8.9) | 30 (8.9) | 0.01 |
| Current medications | |||||
| RAS blocker | 135 (39.9) | 162 (48.1) | 166 (49.1) | 176 (52.1) | 0.01 |
| CCB | 84 (24.9) | 123 (36.5) | 138 (40.8) | 141 (41.7) | <0.001 |
| β-blocker | 38 (11.2) | 45 (13.4) | 55 (16.3) | 63 (18.6) | 0.03 |
| MR antagonist | 20 (5.9) | 15 (4.5) | 26 (7.7) | 25 (7.4) | 0.27 |
| Lipid-lowering agent | 65 (19.2) | 81 (24.0) | 80 (23.7) | 81 (24.0) | 0.37 |
| Diuretic | 58 (17.2) | 67 (19.9) | 90 (26.6) | 101 (29.9) | 0.001 |
| Antiplatelet agent | 71 (21.0) | 103 (30.6) | 117 (34.6) | 131 (38.8) | <0.001 |
| Laboratory results | |||||
| Serum creatinine (mg/dl) | 0.95 (0.72–1.30) | 1.05 (0.73–1.52) | 1.15 (0.90–2.04) | 1.56 (1.00–5.83) | <0.001 |
| eGFR (ml/min per 1.73 m2) | |||||
| Overall | 57.7 (41.3–86.0) | 53.5 (32.1–73.7) | 46.4 (24.4–59.3) | 32.2 (7.6–52.9) | <0.001 |
| >60 | 156 (46.2) | 127 (37.7) | 79 (23.4) | 55 (16.3) | |
| 30–60 | 127 (37.6) | 130 (38.6) | 156 (46.2) | 122 (36.1) | |
| <30 | 45 (13.3) | 63 (18.7) | 58 (17.2) | 73 (21.6) | |
| Dialysis, n (%) | 10 (3.0) | 17 (5.0) | 45 (13.3) | 88 (26.0) | <0.001 |
| BUN (mg/dl) | 18 (13–24) | 19 (15–28) | 22 (16–36) | 26 (18–44) | <0.001 |
| Proteinuriab | 234 (63.8) | 197 (54.9) | 206 (60.2) | 194 (68.1) | 0.001 |
| Uric acid (mg/dl) | 6.2 (5.0–7.7) | 6.4 (5.1–7.5) | 6.4 (5.3–7.7) | 6.5 (5.1–7.8) | 0.43 |
| Albumin (g/dl) | 4.1 (3.4–4.4) | 4.0 (3.4–4.4) | 3.9 (3.3–4.3) | 3.7 (3.0–4.2) | <0.001 |
| Calcium (mg/dl) | 9.4 (9.0–9.7) | 9.3 (9.0–9.6) | 9.3 (8.9–9.5) | 9.3 (9.0–9.7) | 0.01 |
| Phosphorus (mg/dl) | 3.6 (3.2–4.0) | 3.5 (3.1–4.0) | 3.5 (3.1–4.1) | 3.7 (3.3–4.4) | 0.001 |
| CRP (mg/dl) | 0.1 (0.1–0.3) | 0.1 (0.1–0.3) | 0.2 (0.1–0.6) | 0.2 (0.1–0.9) | <0.001 |
| Hemoglobin (g/dl) | 13.2 (11.9–14.6) | 12.9 (11.3–14.5) | 12.3 (10.6–13.8) | 11.7 (10.1–13.5) | <0.001 |
| HbA1c (%) | 5.7 (5.4–6.2) | 5.7 (5.4–6.2) | 5.8 (5.4–6.4) | 5.8 (5.5–6.4) | 0.13 |
| LDL cholesterol (mg/dl) | 116 (90–144) | 114 (87–146) | 115 (92–143) | 108 (83–146) | 0.24 |
| HDL cholesterol (mg/dl) | 56 (45–72) | 55 (44–70) | 50 (39–63) | 50 (44–64) | <0.001 |
| Triglycerides (mg/dl) | 115 (79–165) | 117 (86–168) | 116 (85–166) | 123 (89–172) | 0.43 |
| BNP (pg/ml)c | 29.4 (12.3–107.2) | 53.3 (19.4–217.3) | 69.8 (31.4–239.8) | 126 (47.4–346.7) | <0.001 |
| VEGF (pg/ml)d | 274 (144–461) | 298 (159–478) | 287 (162–535) | 310 (174–563) | 0.10 |
Data are expressed as medians (interquartile ranges) or n (%). eGFR was calculated using the MDRD equation modified for Japan. CAD, coronary artery disease; CCB, calcium channel blocker; MR, mineralocorticoid receptor; CRP, C-reactive protein; HbA1c, glycated hemoglobin.
The percentage of patients with GN was calculated from the 750 participants who underwent renal biopsy.
Data on proteinuria was available in 1209 participants.
Plasma BNP levels were available in 1175 participants.
Serum VEGF levels were available in 1327 participants.
PlGF and Risk of All-Cause Mortality
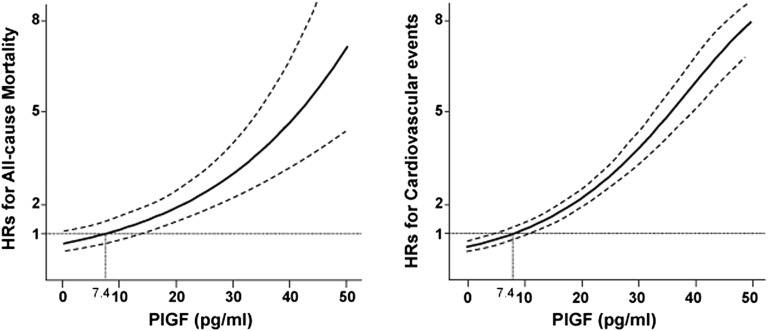
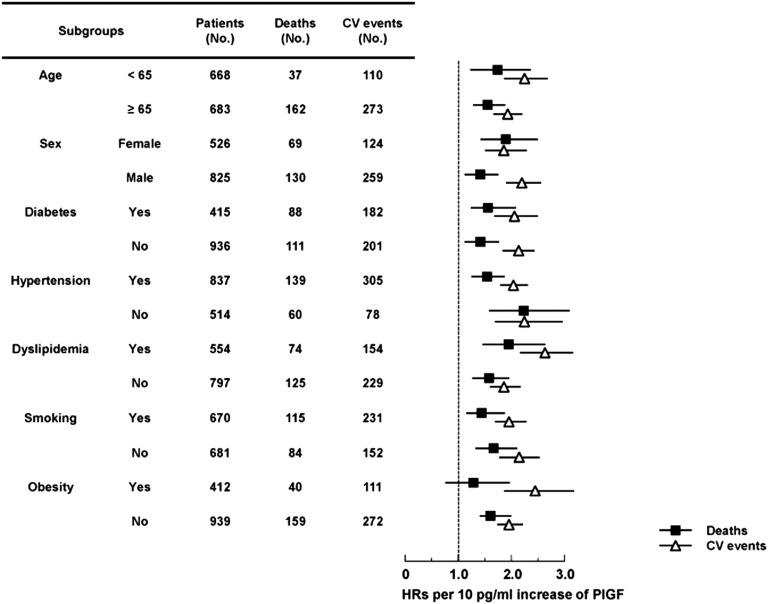
During a median follow-up of 3.3 years, 199 participants died (44.5/1000 person years), including 62 from sudden death (31%), 32 from heart failure (16%), 31 from malignancy (16%), 30 from infection (15%), 13 from stroke (6.5%), and six from acute myocardial infarction (3.0%). Median PlGF levels were significantly higher in those who died (19.1 pg/ml; interquartile range, 14.6–24.6 pg/m) than those who survived (13.8 pg/ml; interquartile range, 9.6–18.9 pg/ml) (P<0.001). Higher serum PlGF levels were associated with a greater risk of all-cause mortality in the crude Kaplan–Meier analysis (Supplemental Figure 1A) (P<0.001 by the log-rank test for trend). Adjusted hazard ratios (HRs) are presented in Table 2 for each PlGF quartile. Participants in the higher quartiles had a greater risk of all-cause mortality. Compared with patients in the lowest quartile, patients in the highest quartile had a 3.87-fold greater mortality risk in models adjusting for clinical demographics, risk factors, current medications, and laboratory results. The HR for all-cause mortality for each 10 pg/ml increase in PlGF was attenuated by the addition of more covariates; however, PlGF remained independently associated with all-cause mortality in model 4 (HR, 1.61; 95% confidence interval [95% CI], 1.37 to 1.89; P<0.001). The graph of HRs for different levels of PlGF is an upward sloping curve, with higher PlGF levels showing a consistently increased risk of mortality in the fully adjusted model (Figure 2). This relationship was similar within predefined subgroups stratified by age, sex, diabetes, hypertension, dyslipidemia and smoking, but not obesity (Figure 3).
Table 2.
Risk of various outcomes by baseline PlGF level
| Models | Unadjusted | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 |
|---|---|---|---|---|---|---|
| All-cause mortality | ||||||
| Quartile 1 | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Quartile 2 | 2.01 (1.12 to 3.77) | 1.55 (0.86 to 2.90) | 1.54 (0.84 to 2.91) | 1.52 (0.82 to 2.90) | 1.59 (0.83 to 3.16) | 1.72 (0.84 to 3.68) |
| Quartile 3 | 4.08 (2.41 to 7.32) | 2.73 (1.61 to 4.92) | 2.74 (1.59 to 4.99) | 2.85 (1.65 to 5.19) | 2.97 (1.67 to 5.59) | 2.55 (1.39 to 4.92) |
| Quartile 4 | 6.97 (4.22 to 12.3) | 4.76 (2.87 to 8.42) | 4.25 (2.53 to 7.61) | 4.47 (2.65 to 8.03) | 3.87 (2.24 to 7.08) | 3.37 (1.90 to 6.34) |
| For each 10 pg/ml increase | 1.87 (1.65 to 2.09) | 1.90 (1.65 to 2.16) | 1.76 (1.51 to 2.02) | 1.80 (1.55 to 2.09) | 1.61 (1.37 to 1.89) | 1.53 (1.30 to 1.81) |
| Cardiovascular events | ||||||
| Quartile 1 | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Quartile 2 | 1.71 (1.06 to 2.82) | 1.37 (0.85 to 2.26) | 1.31 (0.80 to 2.18) | 1.37 (0.83 to 2.29) | 1.55 (0.92 to 2.66) | 1.42 (0.83 to 2.50) |
| Quartile 3 | 4.82 (3.20 to 7.55) | 3.63 (2.40 to 5.71) | 3.48 (2.29 to 5.50) | 3.64 (2.38 to 5.77) | 3.39 (2.20 to 5.41) | 2.80 (1.80 to 4.50) |
| Quartile 4 | 11.6 (7.87 to 17.9) | 9.17 (6.19 to 14.2) | 7.41 (4.95 to 11.6) | 7.97 (5.31 to 12.5) | 8.42 (5.54 to 13.3) | 7.25 (4.72 to 11.6) |
| For each 10 pg/ml increase | 2.22 (2.03 to 2.42) | 2.24 (2.04 to 2.46) | 2.09 (1.88 to 2.31) | 2.08 (1.87 to 2.31) | 2.03 (1.82 to 2.26) | 1.97 (1.76 to 2.20) |
Values are HRs (95% CIs). Model 1 adjusted for age and sex. Model 2 adjusted for covariates in model 1 plus risk factors, including diabetes, hypertension, dyslipidemia, obesity, smoking, previous coronary artery disease, previous stroke, and CKD stage. Model 3 adjusted for covariates in model 2 plus current medications, including RAS blockers, calcium channel blockers, β-blockers, mineralocorticoid receptor antagonists, lipid-lowering agents, diuretics, and antiplatelet agents. Model 4 adjusted for covariates in Model 3 plus laboratory data, including hemoglobin, serum albumin, serum calcium, phosphorus, serum C-reactive protein, HbA1c, LDL cholesterol, HDL cholesterol, and triglycerides. Model 5 adjusted for covariates in model 4 plus BNP. Ref, reference.
Figure 2.
Multivariable-adjusted HRs for all-cause mortality and for cardiovascular events over the distribution of serum PlGF concentrations. The median of the lowest quartile of PlGF concentration served as the reference group (7.4 pg/ml). The Cox model was adjusted for age, sex, diabetes, hypertension, dyslipidemia, obesity, smoking, previous coronary artery disease, previous stroke, CKD stage, hemoglobin, serum albumin, calcium, phosphorus, C-reactive protein, HbA1c, LDL cholesterol, HDL cholesterol, triglycerides, and use of RAS blockers, calcium channel blockers, β-blockers, mineralocorticoid receptor antagonists, lipid-lowering agents, diuretics, and antiplatelet agents. The solid lines show HRs for mortality and cardiovascular events. Dashed lines indicate 95% CIs.
Figure 3.
Subgroup analysis of the association between PlGF levels with all-cause mortality and cardiovascular events. Multivariable-adjusted HRs (95% CIs) for all-cause mortality and cardiovascular events for each 10 pg/ml increase in PlGF levels within subgroups stratified by age, sex, and traditional risk factors. Black squares indicate HRs for mortality. White triangles indicate HRs for cardiovascular events. Solid lines indicate 95% CIs. CV, cardiovascular.
PlGF and Risks of Cardiovascular Events
During the median follow-up of 2.93 years, a total of 383 cardiovascular events (96.8/1000 person years) occurred, defined as atherosclerotic disease or heart failure requiring hospitalization. In detail, 255 and 176 participants developed new atherosclerotic disease and congestive heart failure during the study period, respectively, and of these, 48 patients had both new atherosclerotic disease and congestive heart failure. Atherosclerotic disease consisted of new development of ischemic heart disease and acute coronary syndrome in 115 patients (30%), cardiac sudden death in 58 patients (15%), stroke in 49 patients (13%), and peripheral artery disease in 29 patients (8%). Median PlGF levels were significantly higher in patients who experienced cardiovascular events (20.0 pg/ml; interquartile range, 15.8–24.6 pg/ml) than those who remained event-free (12.8 pg/ml; interquartile range, 9.1–16.5 pg/ml) (P<0.001). Crude Kaplan–Meier analysis revealed that higher PlGF levels were significantly associated with a greater risk of cardiovascular events, as shown in Supplemental Figure 1B (P<0.001). Participants in the two lowest PlGF quartiles had a similar risk of cardiovascular events, whereas those in the two highest quartiles had a 3.39- and 8.42-fold greater risk of cardiovascular events, respectively, compared with those in the first quartile (Table 2). When analyzing atherosclerotic disease and heart failure separately, unadjusted and adjusted multivariate analysis showed that being in the two highest PlGF quartiles was a strong risk factor of atherosclerotic disease and heart failure (Supplemental Figure 2, Supplemental Table 3). In the fully adjusted model, each 10 pg/ml increase in PlGF was independently associated with a greater risk of cardiovascular events (HR, 2.03; 95% CI, 1.82 to 2.26, P<0.001). Furthermore, the risk of cardiovascular events increased with increasing PlGF levels (Figure 2), and this association was observed in all of the subgroups analyzed (Figure 3).
Stratified by eGFR
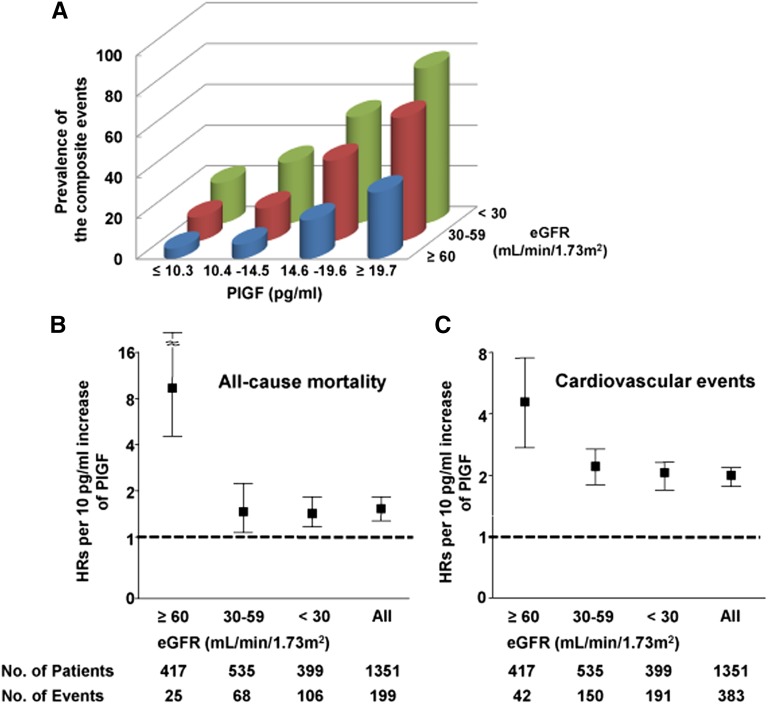
A significant inverse relationship between serum levels of PlGF and eGFR was observed (P<0.001); the median (interquartile range) PlGF concentrations were 11.9 pg/ml (8.2–15.6 pg/ml), 14.8 pg/ml (10.3–19.3 pg/ml), and 18.1 pg/ml (13.0–23.1 pg/ml) in patients with eGFR of ≥60, 30–59, and <30 ml/min per 1.73 m2, respectively. Patients in the highest PlGF quartile and the lowest eGFR tertile comprised 76.4% of the composite end points of all-cause mortality and cardiovascular events, whereas patients in the lowest quartile of PlGF and the highest tertile of eGFR accounted for 5.1%. The combination of reduced eGFR and elevated PlGF was associated with an increased risk of the composite end point in an additive manner (Figure 4A). In the multivariable-adjusted model, each 10 pg/ml increase in PlGF was independently associated with all-cause mortality and cardiovascular events when patients were stratified by eGFR (Figure 4, B and C); this relationship was observed even in patients with severe renal dysfunction.
Figure 4.
PlGF levels and the risk of mortality and cardiovascular events on the basis of renal function. (A) The prevalence of the composite end point of mortality and cardiovascular events according to categories of PlGF and eGFR. (B) and (C) Multivariable-adjusted HRs (95% CIs) for all-cause mortality and cardiovascular events for each 10 pg/ml increase in PlGF levels on the basis of renal function. Bars represent 95% CIs.
Sensitivity Analysis
Results of sensitivity analyses were similar to those of our main analysis when we substituted eGFR using the Modification of Diet in Renal Disease (MDRD) equation and CKD-EPI equation instead of CKD stage; when we excluded very elderly patients; when we excluded patients on maintenance dialysis and patients who began dialysis during the follow-up period; when we restricted the analysis to participants who were followed for at least 2 years after enrollment; when proteinuria was included in the fully adjusted model; when plasma levels of brain natriuretic peptide (BNP) were included in the fully adjusted model; and when serum levels of VEGF were included in the fully adjusted model (Table 3).
Table 3.
Sensitivity analysis
| Analysis | All-Cause Mortality | Cardiovascular Events | |||
|---|---|---|---|---|---|
| No. of Patients | No. of Events | HR (95% CI)e | No. of Events | HR (95% CI)e | |
| All participants | 1351 | 199 | 1.61 (1.29 to 1.62) | 383 | 2.04 (1.82 to 2.27) |
| eGFR using the MDRD equationa | 1191 | 156 | 1.74 (1.43 to 2.10) | 287 | 2.04 (1.80 to 2.31) |
| eGFR using the CKD-EPI equationa | 1191 | 156 | 1.74 (1.43 to 2.10) | 287 | 2.04 (1.80 to 2.31) |
| Excluding very elderly participants | 1210 | 172 | 1.71 (1.44 to 2.01) | 332 | 2.11 (1.88 to 2.37) |
| Excluding dialysis and predialysis patients | 1144 | 143 | 1.93 (1.56 to 2.36) | 258 | 2.06 (1.80 to 2.36) |
| Follow-up of at least 2 yr | 1159 | 172 | 1.65 (1.36 to 1.99) | 325 | 2.08 (1.85 to 2.34) |
| Fully adjusted model including proteinuriab | 1209 | 160 | 1.80 (1.48 to 2.17) | 296 | 2.09 (1.84 to 2.36) |
| Fully adjusted model including BNPc | 1175 | 189 | 1.53 (1.30 to 1.81) | 372 | 1.97 (1.76 to 2.20) |
| Fully adjusted model including VEGFd | 1327 | 195 | 1.63 (1.38 to 1.91) | 377 | 2.02 (1.80 to 2.25) |
eGFR was substituted to CKD stage as an adjusted covariate.
Also adjusted for proteinuria when data were available.
Also adjusted for BNP when data were available.
Also adjusted for VEGF when data were available.
Adjusted HRs for 10 pg/ml increase are shown. All categories were adjusted for age, sex, diabetes, hypertension, dyslipidemia, obesity, smoking, previous coronary artery disease, previous stroke, CKD stage, RAS blocker, calcium channel blocker, β-blocker, mineralocorticoid receptor antagonist, lipid-lowering agent, diuretic, antiplatelet agent, hemoglobin, serum albumin, serum calcium, serum phosphorus, serum C-reactive protein, HbA1c, LDL cholesterol, HDL cholesterol, and triglycerides.
VEGF and Risks of All-Cause Mortality and Cardiovascular Events
In addition to PlGF, we measured the serum level of VEGF, which is another ligand against Flt-1, in 1327 participants. The median levels of VEGF were 290 pg/ml (interquartile range, 160–508 pg/ml), and serum levels of VEGF were not significantly correlated with eGFR (r=0.01, P=0.84) unlike PlGF (r=–0.34, P<0.001). VEGF levels at baseline were significantly associated with a greater risk of mortality in the unadjusted model, but the association was attenuated in the fully adjusted model (Supplemental Figure 3, Table 4). Similar results were obtained in the relationship between VEGF and cardiovascular events.
Table 4.
Risk of various outcomes by baseline VEGF level
| Models | Unadjusted | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 |
|---|---|---|---|---|---|---|
| All-cause mortality | ||||||
| Quartile 1 (<160 pg/ml) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Quartile 2 (160–290 pg/ml) | 1.04 (0.68–1.59) | 1.06 (0.69–1.62) | 1.08 (0.70–1.68) | 0.94 (0.60–1.48) | 0.96 (0.60–1.53) | 0.92 (0.57–1.49) |
| Quartile 3 (291–507 pg/ml) | 1.11 (0.90–1.37) | 1.10 (0.90–1.36) | 1.18 (0.95–1.46) | 1.19 (0.96–1.48) | 1.13 (0.90–1.42) | 1.13 (0.90–1.42) |
| Quartile 4 (≥508pg/ml) | 1.20 (1.06–1.37) | 1.22 (1.07–1.39) | 1.25 (1.09–1.45) | 1.24 (1.04–1.43) | 1.14 (0.98–1.32) | 1.18 (1.01–1.37) |
| For each 100 pg/ml increase | 1.04 (1.02–1.06) | 1.04 (1.02–1.06) | 1.04 (1.02–1.07) | 1.04 (1.01–1.06) | 1.00 (0.97–1.03) | 1.00 (0.97–1.04) |
| Cardiovascular events | ||||||
| Quartile 1 (<160 pg/ml) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Quartile 2 (160–290 pg/ml) | 0.97 (0.72–1.31) | 0.95 (0.70–1.29) | 0.93 (0.69–1.27) | 1.01 (0.74–1.37) | 0.99 (0.72–1.36) | 1.05 (0.76–1.44) |
| Quartile 3 (291–507 pg/ml) | 1.09 (0.94–1.26) | 1.08 (0.93–1.25) | 1.12 (0.87–1.30) | 1.13 (0.98–1.31) | 1.13 (0.97–1.31) | 1.14 (0.98–1.34) |
| Quartile 4 (≥508pg/ml) | 1.11 (1.01–1.22) | 1.11 (1.01–1.22) | 1.15 (1.04–1.26) | 1.23 (1.13–1.30) | 1.12 (1.02–1.24) | 1.14 (1.03–1.27) |
| For each 100 pg/ml increase | 1.03 (1.01–1.05) | 1.02 (1.00–1.04) | 1.04 (1.02–1.06) | 1.05 (1.02–1.06) | 1.03 (1.01–1.05) | 1.03 (1.01–1.05) |
Model 1 adjusted for age and sex. Model 2 adjusted for covariates in model 1 plus risk factors, including diabetes, hypertension, dyslipidemia, obesity, smoking, previous coronary artery disease, previous stroke, and CKD stage. Model 3 adjusted for covariates in model 2 plus current medications, including RAS blockers, calcium channel blockers, β-blockers, mineralocorticoid receptor antagonists, lipid-lowering agents, diuretics, and antiplatelet agents. Model 4 adjusted for covariates in model 3 plus laboratory data, including hemoglobin, serum albumin, serum calcium, phosphorus, serum C-reactive protein, HbA1c, LDL cholesterol, HDL cholesterol, and triglycerides. Model 5 adjusted for covariates in model 4 plus BNP. Ref, reference.
Combination Use of PlGF and BNP
As shown in Table 2, although HR in all-cause mortality and cardiovascular events became lower when adjusted by other factors, including BNP, PlGF levels remained a strong predictor of both mortality and cardiovascular events. We compared predictive values for all-cause mortality and cardiovascular events among PlGF alone, BNP alone, and combination use of PlGF and BNP. For all-cause mortality, combination use of BNP and PlGF did not significantly improve the c statistics compared with BNP alone (from 0.70 to 0.69, P=0.87) but did improve integrated discrimination improvement (IDI) (0.04; 95% CI, 0.02 to 0.06; P<0.001) and category-free net reclassification improvement (NRI) (0.49; 95% CI, 0.31 to 0.67; P<0.001) (Supplemental Figure 4, Table 5). For cardiovascular events, the combination use significantly improved the c statistics (from 0.76 to 0.80, P=0.011), IDI (0.14; 95% CI, 0.12 to 0.17; P<0.001), and category-free NRI (0.84; 95% CI, 0.71 to 0.96; P<0.001) compared with BNP alone.
Table 5.
Improvement in predicting study outcomes by combination use of PlGF and BNP
| Biomarker | c Statistics | P Valuea | IDI | P Value | NRI | P Value |
|---|---|---|---|---|---|---|
| All-cause mortality | ||||||
| PlGF | 0.67 (0.62–0.72) | |||||
| BNP | 0.70 (0.65–0.74) | |||||
| PlGF+BNP | 0.69 (0.65–0.74) | |||||
| PlGF+BNP versus PlGF | 0.01 | 0.01 (–0.001 to 0.02) | 0.08 | 0.35 (0.18–0.52) | <0.001 | |
| PlGF+BNP versus BNP | 0.87 | 0.04 (0.02 to 0.06) | <0.001 | 0.49 (0.31–0.67) | <0.001 | |
| Cardiovascular events | ||||||
| PlGF | 0.77 (0.74–0.81) | |||||
| BNP | 0.76 (0.73–0.79) | |||||
| PlGF+BNP | 0.80 (0.77–0.83) | |||||
| PlGF+BNP versus PlGF | <0.001 | 0.04 (0.02 to 0.05) | <0.001 | 0.46 (0.34–0.58) | <0.001 | |
| PlGF+BNP versus BNP | 0.01 | 0.14 (0.12 to 0.17) | <0.001 | 0.84 (0.71–0.96) | <0.001 |
P value for c statistics.
Discussion
In this study we first evaluated the effect of serum PlGF concentrations on all-cause mortality and cardiovascular events in patients with CKD. We demonstrated that higher PlGF levels were associated with both all-cause mortality and cardiovascular events, independent of clinical demographic parameters and traditional risk factors. This trend remained after adjustment for current medications for preventing atherosclerosis and heart failure and laboratory data related to cardiovascular disease. Of note, this association of PlGF with adverse outcomes was stronger than traditional and CKD-specific risk factors. Subgroup and sensitivity analysis further strengthen the robustness of our findings.
Recently, a growing body of clinical evidence suggests that PlGF alone or in combination with other biomarkers is a powerful predictor of survival or cardiovascular events in patients with stable and unstable coronary artery disease17–20,24; however, some studies have not demonstrated that PlGF is independently associated with survival in patients with chronic heart failure or suspected acute myocardial infarction.22,23 More recently, a single-center study showed that elevated PlGF level is an independent predictor of increased mortality but not cardiovascular events in patients with CKD not yet on dialysis.25 Therefore, the relationship between circulating PlGF and outcomes remains unclear. However, in our setting of CKD, we found for the first time, to our knowledge, an increased risk of both mortality and cardiovascular events with increasing PlGF levels, with patients in the highest quartile having a 3.87- and 8.42-fold increased risk compared with those in the lowest quartile, respectively. On the basis of these results, we suggest that PlGF is a useful and practical tool for cardiovascular risk stratification in patients with CKD, more so than in patients with heart failure or acute coronary syndrome. We hypothesize that PlGF signaling is involved in the pathogenesis of CKD-associated atherosclerosis. An inverse relationship between PlGF and eGFR in this and other studies support this hypothesis.7,22
PlGF, originally discovered in the placenta, has also been found in the heart, lung, thyroid, and endothelial cells. As an intrinsic mediator of vascular inflammation, PlGF is responsible for promoting angiogenesis and destabilizing plaques through mediating macrophage accumulation in atherosclerotic lesions via Flt-1.26 In contrast, in one animal study, neutralization of PlGF reduced plaque size and the severity of macrophage infiltration.15 The combination of vascular inflammation and malnutrition, known as the malnutrition-inflammation-atherosclerosis syndrome, worsens outcomes in patients with CKD through aggravation of heart failure and accelerated atherosclerosis.27,28 Indeed, in our patient population, PlGF was independently correlated with CKD severity, increased levels of C-reactive protein, and decreased levels of albumin and HDL. Taken together, PlGF may play a pivotal role in the pathogenesis of malnutrition-inflammation-atherosclerosis syndrome, which helps to explain the high mortality rates among patients with CKD.
In the VEGF family system, VEGF is another powerful ligand for Flt-1. However, in this study, VEGF was not correlated with CKD severity and was less associated with all-cause mortality and cardiovascular events, suggesting that PlGF could be a more important predictor of mortality and cardiovascular events in patients with CKD than VEGF and thereby clinically relevant to the pathology of CKD-associated atherosclerosis.
The mechanisms underlying PlGF elevation in patients with CKD are largely unclear, but we have three hypotheses. First, our recent work7 demonstrated that exposure to serum from patients with advanced CKD increases PlGF production in human endothelial cells and is associated with increased endothelial dysfunction and oxidative stress. Circulating humoral factors in CKD, such as uremic toxins, may contribute to the upregulation of PlGF in CKD. Second, in addition to a circulating form in the peripheral blood, soluble Flt-1 is stored in the vascular wall and antagonizes PlGF.29,30 A marked decrease in sum of soluble Flt-1 is associated with lower eGFR, which may relatively increase circulating levels of PIGF and augment its biologic effects in CKD.6,7 Third, because the expression of PlGF in vascular cells is enhanced by angiotensin II and aldosterone, PlGF is thought to be a downstream target of the renin-angiotensin system (RAS) and mineralocorticoid receptor.31–33 In CKD, there is isolated or synergistic RAS and mineralocorticoid receptor hyperactivity, which is possibly mediated by upregulation of PlGF.34,35 Further clinical research is needed to investigate the association between PlGF levels and RAS.
Serum levels of PlGF in our patients were considerably lower than those in pregnant women, suggesting that abundant PlGF might be needed to develop and maintain the placental vascular system compared with the adult cardiovascular system. We think that the circulating level of PlGF is generally much lower than the tissue level of PlGF, but it is a surrogate marker of the tissue level of PlGF in patients with CKD, whereas PlGF itself may act as a paracrine factor and cause the development of atherosclerotic lesions.
Of note, patients with CKD with higher levels of PlGF had a greater risk of heart failure requiring hospitalization. Participants in the highest versus the lowest PlGF quartile appeared to have a higher HR for heart failure than for atherosclerotic disease. The use of PlGF alone and BNP alone had similar diagnostic accuracy for prediction of cardiovascular events, and the combination use of PlGF and BNP had higher diagnostic accuracy than either biomarker alone. A recent study has shown that PlGF is a strong predictor of an elevated left ventricular mass index in patents with CKD,36 suggesting that PlGF may be involved in the development of cardiac hypertrophy.
The present results suggest that PlGF can potentially serve as a novel therapeutic target for the treatment of CKD-associated cardiovascular disease. Indeed, we have observed that administration of soluble Flt-1 reduces atherosclerotic plaque areas in remnant kidneys in mice.6 In humans, aflibercept (VEGF Trap) and the anti-PlGF antibody TB-403, can be new antiangiogenic and antiatherogenic drugs that antagonize PlGF signaling with already well established safety and tolerability.37,38 Further interventional trials of these agents are required to determine whether reducing serum PlGF levels provides cardioprotective effects in patients with CKD.
Several limitations of this study should be noted. First, it is an observational study; therefore, the cause-effect relationship between serum PlGF levels and adverse outcomes is not evident. Second, because the study participants are residents of a single Japanese prefecture, the results may not be generalizable to other populations. Third, the population enrolled in this study consists of patients with CKD who underwent renal biopsy or coronary angiography and may not be representative of all patients with CKD. Fourth, only baseline samples were analyzed and changes of circulating levels of PlGF over time were not addressed. Fifth, we did not measure other biomarkers associated with mortality and cardiovascular events in CKD, such as troponin T and fibroblast growth factor-23.39–41 However, adjusting for levels of BNP, another biomarker of mortality and cardiovascular events, did not decrease the magnitude of the association between PlGF and mortality and cardiovascular events, respectively.
In conclusion, PlGF is strongly and independently associated with not only all-cause mortality but also cardiovascular events in patients with CKD, suggesting that PlGF is a novel prognostic biomarker in CKD. Further research is required to investigate the mechanisms underlying the effect of higher serum PlGF levels on adverse outcomes and determine whether therapeutic strategies that lower PlGF levels can improve outcomes in patients with CKD.
Concise Methods
Patients
NARA-CKD is a multicenter prospective cohort study designed to investigate the prognostic value of PlGF as a risk factor of all-cause mortality and cardiovascular disease in patients with CKD. The study population consisted of consecutive patients who underwent renal biopsy or coronary angiography in our department at Nara Medical University Hospital and four referral hospitals between April 1, 2004, and December 31, 2011. We screened 1270 patients who underwent renal biopsy and 3003 patients who underwent the first examination of coronary angiography.
The exclusion criteria consisted of (1) previous or current acute coronary syndrome and heart failure within 3 months; (2) previous or current malignancy requiring therapy within the last 5 years; (3) AKI; (4) pregnancy; (5) age <18 years; and (6) unwillingness to participate. Among the 1270 patients who underwent renal biopsy, there were 476 patients excluded, including 247 because of AKI, 213 because of missing data, 10 because of malignancy, five because of pregnancy, and one because they were aged <18 years. There were 3003 patients who underwent coronary angiography, of which 2420 were excluded. These included 1014 patients with acute coronary syndrome, 959 with acute heart failure, and 447 who did not met CKD criteria, defined as an eGFR<60 ml/min per 1.73 m2 of body surface area or continuous proteinuria over 3 months (Figure 1).
For all eligible participants, we collected data on demographic characteristics, medical and family history, and current medications. A physical examination was performed at baseline, during which blood samples were collected for evaluation of laboratory parameters, including serum levels of PlGF. In total, 1377 enrolled participants completed the baseline examination, of whom 14 were lost to follow-up and 12 did not have sufficient sample volume for measurement, leaving 1351 patients for the analysis. Participants were followed until death or withdrawal from the study.
The institutional review board or ethics committee at each site approved the protocol, and all patients provided written informed consent.
Measurement
Serum samples were immediately centrifuged and were kept frozen at −80°C until assayed. Serum levels of PlGF and VEGF were measured with a commercially available sandwich ELISA kit (R&D Systems, Minneapolis, MN), according to the manufacturer’s instructions.
Clinical Definitions
The following clinical definitions were adopted. (1) eGFR was calculated using the MDRD equation and the CKD Epidemiology Collaboration (CKD-EPI) equation modified for Japan as previously described.42,43 (2) CKD stage was categorized according to a modified National Kidney Foundation classification system as follows: stage 1 (eGFR≥90 ml/min per 1.73 m2); stage 2 (eGFR, 60–89 ml/min per 1.73 m2); stage 3a (eGFR, 45–59 ml/min per 1.73 m2); stage 3b (eGFR, 30–44 ml/min per 1.73 m2); stage 4 (eGFR, 15–29 ml/min per 1.73 m2); stage 5 (eGFR<15 ml/min per 1.73 m2); and stage 5D (on dialysis). (3) Hypertension was defined as systolic BP≥140 mmHg, diastolic BP≥90 mmHg, or use of oral antihypertensive medications. (4) Diabetes was defined as fasting glucose ≥126 mg/dl or use of oral hypoglycemic agents or insulin. (5) Dyslipidemia was defined as LDL cholesterol ≥140 mg/dl or use of lipid-lowering medications. (6) Obesity was defined as body mass index of ≥25 kg/m2. (7) Smoking was defined as former or current smoking of more than one cigarette per day. (8) Previous coronary artery disease was defined as a history of myocardial infarction, angina pectoris, or coronary artery bypass grafting. (9) Previous stroke was defined as a history of cerebral infarction or hemorrhage not caused by trauma or infection. (10) Patients were diagnosed with proteinuria if urine dipstick test results were ≥1+ for protein.
Clinical Outcomes
The primary outcomes were time to death from any cause and time to a cardiovascular event, defined as any atherosclerotic disease or heart failure requiring hospitalization. Atherosclerotic disease was a composite of cardiovascular death and nonfatal ischemic heart disease, stroke, aortic disease, and peripheral artery disease.
Cardiovascular death was defined as death because of due to myocardial infarction or stroke, or documented sudden cardiac death. Ischemic heart disease was defined as myocardial infarction or coronary artery disease with >75% luminal narrowing in one of the three major coronary arteries or major branches requiring percutaneous coronary intervention or coronary artery bypass surgery. Stroke was defined as a new fixed neurologic deficit caused by cerebral infarction or hemorrhage not known to be caused by brain trauma, tumor, infection, or other identifiable causes. Cerebrovascular disease requiring surgical or percutaneous revascularization in the cerebrovascular circulation was also included. Aortic disease included aortic dissection or rupture not secondary to trauma or collagen disease. Peripheral artery disease was defined as amputation caused by vascular disease or peripheral surgical or percutaneous revascularization. Heart failure was defined according to Framingham criteria.44
All participants were followed for at least 1 year after enrollment. All events were confirmed through medical records and self-reporting. Participants who visited the hospital every 1–3 months reported on their general health and conditions, including cardiovascular events, to attending physicians blinded to serum PlGF levels. Any event of participants without regular hospital visits was obtained by blinded clinicians through an in-person or telephone interview.
Statistical Analyses
Baseline characteristics, risk factors for cardiovascular disease, current medications, and laboratory findings were compared across quartiles of serum PlGF levels using the chi-squared test for categorical data and the Kruskal–Wallis test for skewed continuous variables. Spearman’s rank correlation coefficient was used to assess the correlation between eGFR and PlGF and VEGF. Univariate and multivariate linear regression were used to identify clinical characteristics with an independent association with PlGF.
The effect of PlGF on all-cause mortality and cardiovascular events was estimated using crude Kaplan–Meier analysis according to PlGF quartiles, and differences were assessed with the log-rank test. A Cox hazard regression model was used to assess for unadjusted and adjusted associations between serum PlGF levels and clinical outcomes. The lowest quartile of PlGF levels was defined as the reference group.
Candidate variables for adjustment were chosen because of their close association with all-cause mortality or cardiovascular events. We initially adjusted for patient demographics, including age and sex, in model 1. Model 2 consisted of model 1 plus traditional risk factors, including diabetes, hypertension, dyslipidemia, smoking, obesity, previous coronary artery disease, previous stroke, and CKD stage. Model 3 consisted of model 2 plus current medications for preventing atherosclerosis and heart failure, including RAS blockers (angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and direct renin inhibitors), calcium channel blockers, β-blockers, mineralocorticoid receptor antagonists, lipid-lowering agents, diuretics (loop and thiazide diuretics), and antiplatelet agents. Model 4 consisted of model 3 plus laboratory findings associated with cardiovascular events, including hemoglobin, serum albumin, serum C-reactive protein, serum calcium, serum phosphorus, glycated hemoglobin, LDL cholesterol, and HDL cholesterol. Model 5 consisted of model 4 plus plasma levels of BNP.
Because clinical demographics, traditional coronary risk factors, and reduced renal function are independent risk factors for death and atherosclerotic disease, we examined whether the association between PlGF levels and each outcome was similar in subgroups stratified by age, sex, diabetes, hypertension, dyslipidemia, smoking, obesity, and baseline renal function. Baseline renal function was categorized into three groups according to eGFR. Dialysis patients were included in the subgroup with eGFR<30 ml/min per 1.73 m2.
We further assessed the robustness of our results using sensitivity analysis. Multivariate-adjusted hazard analysis for all-cause mortality and cardiovascular events was repeated with the following five sensitivity analyses. The first sensitivity analysis used alternative measurements of renal function as adjusted covariates. The CKD-EPI equation more accurately categorizes mortality risk than the MDRD equation45; therefore, CKD stage was replaced by eGFR estimated using both the MDRD equation and CKD-EPI equation. The second analysis did not include very elderly participants aged ≥80, and the third analysis did not include participants who were receiving maintenance dialysis at baseline or began dialysis during the study period.46,47 The fourth analysis was restricted to participants with at least 2 years of follow-up, and the fifth analysis included proteinuria and BNP, when available, in the fully adjusted model as risk factors for death and cardiovascular events because both are established biomarkers of cardiovascular events in patients with CKD.48,49 The sixth analysis included VEGF, which is another angiogenic factor relevant with PlGF, when available, in the fully adjusted model.
To evaluate the discriminatory ability of joint effects of PlGF and BNP, we calculated the c statistic, IDI, and category-free NRI at 2-year follow-up in the subset of participants in whom plasma levels of BNP were measured. The differences between the c statistics were tested by Delong methods to examine whether combination use of PlGF and BNP improved the discrimination ability for study outcomes compared with either biomarker alone. Bootstrap CIs were reported for the area under the receiver-operating characteristics curves, IDI, and NRI. A two-sided P<0.05 was considered statistically significant. JMP10.0.2 (SAS Institute Inc., Cary, NC) and SAS version 9.4 (SAS Institute Inc.) were used to perform all statistical analyses.
Disclosures
Dr. Saito received lecture fees from Merck, Takeda Pharmaceutical Company, Novartis Pharma KK, Daiichi Sankyo Company, Mitsubishi Tanabe Pharma Corp., Pfizer Japan, Otsuka Pharmaceutical, and research funding from Japan Heart Foundation and the Naito Foundation. Dr. Saito belongs to the endowed Department (the Department of Regulatory Medicine of Blood Pressure) sponsored by Merck.
Supplementary Material
Acknowledgments
We acknowledge M. Fujii, M. Sugimoto, S. Ishihara, D. Kamon, T. Kanki, T. Nakano, F. Kengo, Y. Sugawara, K. An, S. Yoshimoto, and K. Mondori for patient data collection. We also acknowledge S. Yoshimura and A. Okuda for assay of PlGF levels and S. Somekawa for his helpful advice.
This work was supported in part by Grants-in-Aid from the Ministry of Health, Labour and Welfare of Japan, and the Takeda Science Foundation.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014080772/-/DCSupplemental.
References
- 1.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT, Chronic Kidney Disease Prognosis Consortium : Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 375: 2073–2081, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hemmelgarn BR, Manns BJ, Lloyd A, James MT, Klarenbach S, Quinn RR, Wiebe N, Tonelli M, Alberta Kidney Disease Network : Relation between kidney function, proteinuria, and adverse outcomes. JAMA 303: 423–429, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Di Angelantonio E, Chowdhury R, Sarwar N, Aspelund T, Danesh J, Gudnason V: Chronic kidney disease and risk of major cardiovascular disease and non-vascular mortality: Prospective population based cohort study. BMJ 341: c4986, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Hage FG, Venkataraman R, Zoghbi GJ, Perry GJ, DeMattos AM, Iskandrian AE: The scope of coronary heart disease in patients with chronic kidney disease. J Am Coll Cardiol 53: 2129–2140, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Onoue K, Uemura S, Takeda Y, Somekawa S, Iwama H, Imagawa K, Nishida T, Morikawa Y, Takemoto Y, Asai O, Soeda T, Okayama S, Ishigami K, Nakatani K, Kawata H, Horii M, Nakajima T, Akai Y, Iwano M, Saito Y: Reduction of circulating soluble fms-like tyrosine kinase-1 plays a significant role in renal dysfunction-associated aggravation of atherosclerosis. Circulation 120: 2470–2477, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Matsui M, Takeda Y, Uemura S, Matsumoto T, Seno A, Onoue K, Tsushima H, Morimoto K, Soeda T, Okayama S, Somekawa S, Samejima K, Kawata H, Kawakami R, Nakatani K, Iwano M, Saito Y: Suppressed soluble Fms-like tyrosine kinase-1 production aggravates atherosclerosis in chronic kidney disease. Kidney Int 85: 393–403, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Maglione D, Guerriero V, Viglietto G, Delli-Bovi P, Persico MG: Isolation of a human placenta cDNA coding for a protein related to the vascular permeability factor. Proc Natl Acad Sci U S A 88: 9267–9271, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller YA, Christinger HW, Keyt BA, de Vos AM: The crystal structure of vascular endothelial growth factor (VEGF) refined to 1.93 A resolution: Multiple copy flexibility and receptor binding. Structure 5: 1325–1338, 1997 [DOI] [PubMed] [Google Scholar]
- 10.Park JE, Chen HH, Winer J, Houck KA, Ferrara N: Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. J Biol Chem 269: 25646–25654, 1994 [PubMed] [Google Scholar]
- 11.Luttun A, Tjwa M, Moons L, Wu Y, Angelillo-Scherrer A, Liao F, Nagy JA, Hooper A, Priller J, De Klerck B, Compernolle V, Daci E, Bohlen P, Dewerchin M, Herbert JM, Fava R, Matthys P, Carmeliet G, Collen D, Dvorak HF, Hicklin DJ, Carmeliet P: Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat Med 8: 831–840, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Pilarczyk K, Sattler KJ, Galili O, Versari D, Olson ML, Meyer FB, Zhu XY, Lerman LO, Lerman A: Placenta growth factor expression in human atherosclerotic carotid plaques is related to plaque destabilization. Atherosclerosis 196: 333–340, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, Wu Y, Bono F, Devy L, Beck H, Scholz D, Acker T, DiPalma T, Dewerchin M, Noel A, Stalmans I, Barra A, Blacher S, VandenDriessche T, Ponten A, Eriksson U, Plate KH, Foidart JM, Schaper W, Charnock-Jones DS, Hicklin DJ, Herbert JM, Collen D, Persico MG: Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med 7: 575–583, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Luttun A, Tjwa M, Carmeliet P: Placental growth factor (PlGF) and its receptor Flt-1 (VEGFR-1): Novel therapeutic targets for angiogenic disorders. Ann N Y Acad Sci 979: 80–93, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Roncal C, Buysschaert I, Gerdes N, Georgiadou M, Ovchinnikova O, Fischer C, Stassen JM, Moons L, Collen D, De Bock K, Hansson GK, Carmeliet P: Short-term delivery of anti-PlGF antibody delays progression of atherosclerotic plaques to vulnerable lesions. Cardiovasc Res 86: 29–36, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Khurana R, Moons L, Shafi S, Luttun A, Collen D, Martin JF, Carmeliet P, Zachary IC: Placental growth factor promotes atherosclerotic intimal thickening and macrophage accumulation. Circulation 111: 2828–2836, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Cassidy A, Chiuve SE, Manson JE, Rexrode KM, Girman CJ, Rimm EB: Potential role for plasma placental growth factor in predicting coronary heart disease risk in women. Arterioscler Thromb Vasc Biol 29: 134–139, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lenderink T, Heeschen C, Fichtlscherer S, Dimmeler S, Hamm CW, Zeiher AM, Simoons ML, Boersma E, CAPTURE Investigators : Elevated placental growth factor levels are associated with adverse outcomes at four-year follow-up in patients with acute coronary syndromes. J Am Coll Cardiol 47: 307–311, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Glaser R, Peacock WF, Wu AH, Muller R, Möckel M, Apple FS: Placental growth factor and B-type natriuretic peptide as independent predictors of risk from a multibiomarker panel in suspected acute coronary syndrome (Acute Risk and Related Outcomes Assessed With Cardiac Biomarkers [ARROW]) study. Am J Cardiol 107: 821–826, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Oemrawsingh RM, Lenderink T, Akkerhuis KM, Heeschen C, Baldus S, Fichtlscherer S, Hamm CW, Simoons ML, Boersma E, CAPTURE investigators : Multimarker risk model containing troponin-T, interleukin 10, myeloperoxidase and placental growth factor predicts long-term cardiovascular risk after non-ST-segment elevation acute coronary syndrome. Heart 97: 1061–1066, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Theilade S, Lajer M, Jorsal A, Tarnow L, Parving HH, Rossing P: Evaluation of placental growth factor and soluble Fms-like tyrosine kinase 1 as predictors of all-cause and cardiovascular mortality in patients with type 1 diabetes with and without diabetic nephropathy. Diabet Med 29: 337–344, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Ky B, French B, Ruparel K, Sweitzer NK, Fang JC, Levy WC, Sawyer DB, Cappola TP: The vascular marker soluble fms-like tyrosine kinase 1 is associated with disease severity and adverse outcomes in chronic heart failure. J Am Coll Cardiol 58: 386–394, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hochholzer W, Reichlin T, Stelzig C, Hochholzer K, Meissner J, Breidthardt T, Reiter M, Duehsler B, Freidank H, Winkler K, Twerenbold R, Mueller C: Impact of soluble fms-like tyrosine kinase-1 and placental growth factor serum levels for risk stratification and early diagnosis in patients with suspected acute myocardial infarction. Eur Heart J 32: 326–335, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto T, Uemura S, Takeda Y, Matsui M, Okada S, Nishida T, Soeda T, Okayama S, Somekawa S, Ishigami K, Onoue K, Kawata H, Kawakami R, Horii M, Saito Y: An elevated ratio of placental growth factor to soluble fms-like tyrosine kinase-1 predicts adverse outcomes in patients with stable coronary artery disease. Intern Med 52: 1019–1027, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Rambod M, Heine GH, Seiler S, Dominic EA, Rogacev KS, Dwivedi R, Ramezani A, Wing MR, Amdur RL, Fliser D, Raj DS: Association of vascular endothelial factors with cardiovascular outcome and mortality in chronic kidney disease patients: A 4-year cohort study. Atherosclerosis 236: 360–365, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Selvaraj SK, Giri RK, Perelman N, Johnson C, Malik P, Kalra VK: Mechanism of monocyte activation and expression of proinflammatory cytochemokines by placenta growth factor. Blood 102: 1515–1524, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Coresh J, Eustace JA, Longenecker JC, Jaar B, Fink NE, Tracy RP, Powe NR, Klag MJ: Association between cholesterol level and mortality in dialysis patients: Role of inflammation and malnutrition. JAMA 291: 451–459, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Stenvinkel P, Heimbürger O, Paultre F, Diczfalusy U, Wang T, Berglund L, Jogestrand T: Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int 55: 1899–1911, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Searle J, Mockel M, Gwosc S, Datwyler SA, Qadri F, Albert GI, Holert F, Isbruch A, Klug L, Muller DN, Dechend R, Muller R, Vollert JO, Slagman A, Mueller C, Herse F: Heparin strongly induces soluble fms-like tyrosine kinase 1 release in vivo and in vitro--brief report. Arterioscler Thromb Vasc Biol 31: 2972–2974, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Sela S, Natanson-Yaron S, Zcharia E, Vlodavsky I, Yagel S, Keshet E: Local retention versus systemic release of soluble VEGF receptor-1 are mediated by heparin-binding and regulated by heparanase. Circ Res 108: 1063–1070, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Pan P, Fu H, Zhang L, Huang H, Luo F, Wu W, Guo Y, Liu X: Angiotensin II upregulates the expression of placental growth factor in human vascular endothelial cells and smooth muscle cells. BMC Cell Biol 11: 36, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jaffe IZ, Newfell BG, Aronovitz M, Mohammad NN, McGraw AP, Perreault RE, Carmeliet P, Ehsan A, Mendelsohn ME: Placental growth factor mediates aldosterone-dependent vascular injury in mice. J Clin Invest 120: 3891–3900, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGraw AP, Bagley J, Chen WS, Galayda C, Nickerson H, Armani A, Caprio M, Carmeliet P, Jaffe IZ: Aldosterone increases early atherosclerosis and promotes plaque inflammation through a placental growth factor-dependent mechanism. J Am Heart Assoc 2: e000018, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ito S: Usefulness of RAS inhibition depends on baseline albuminuria. Nat Rev Nephrol 6: 10–11, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Bertocchio JP, Warnock DG, Jaisser F: Mineralocorticoid receptor activation and blockade: An emerging paradigm in chronic kidney disease. Kidney Int 79: 1051–1060, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Peiskerová M, Kalousová M, Danzig V, Míková B, Hodková M, Němeček E, Bani-Hani A, Ambrož D, Benáková H, Linhart A, Zima T, Tesař V: Placental growth factor may predict increased left ventricular mass index in patients with mild to moderate chronic kidney disease--a prospective observational study. BMC Nephrol 14: 142, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lockhart AC, Rothenberg ML, Dupont J, Cooper W, Chevalier P, Sternas L, Buzenet G, Koehler E, Sosman JA, Schwartz LH, Gultekin DH, Koutcher JA, Donnelly EF, Andal R, Dancy I, Spriggs DR, Tew WP: Phase I study of intravenous vascular endothelial growth factor trap, aflibercept, in patients with advanced solid tumors. J Clin Oncol 28: 207–214, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lassen U, Nielsen DL, Sørensen M, Winstedt L, Niskanen T, Stenberg Y, Pakola S, Stassen JM, Glazer S: A phase I, dose-escalation study of TB-403, a monoclonal antibody directed against PlGF, in patients with advanced solid tumours. Br J Cancer 106: 678–684, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dierkes J, Domröse U, Westphal S, Ambrosch A, Bosselmann HP, Neumann KH, Luley C: Cardiac troponin T predicts mortality in patients with end-stage renal disease. Circulation 102: 1964–1969, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutiérrez OM, Steigerwalt S, He J, Schwartz S, Lo J, Ojo A, Sondheimer J, Hsu CY, Lash J, Leonard M, Kusek JW, Feldman HI, Wolf M, Chronic Renal Insufficiency Cohort (CRIC) Study Group : Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 305: 2432–2439, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dominguez JR, Shlipak MG, Whooley MA, Ix JH: Fractional excretion of phosphorus modifies the association between fibroblast growth factor-23 and outcomes. J Am Soc Nephrol 24: 647–654, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A, Collaborators developing the Japanese equation for estimated GFR : Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53: 982–992, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Horio M, Imai E, Yasuda Y, Watanabe T, Matsuo S: Modification of the CKD epidemiology collaboration (CKD-EPI) equation for Japanese: Accuracy and use for population estimates. Am J Kidney Dis 56: 32–38, 2010 [DOI] [PubMed] [Google Scholar]
- 44.Ho KK, Pinsky JL, Kannel WB, Levy D: The epidemiology of heart failure: The Framingham Study. J Am Coll Cardiol 22[Suppl A]: 6A–13A, 1993 [DOI] [PubMed] [Google Scholar]
- 45.Matsushita K, Mahmoodi BK, Woodward M, Emberson JR, Jafar TH, Jee SH, Polkinghorne KR, Shankar A, Smith DH, Tonelli M, Warnock DG, Wen CP, Coresh J, Gansevoort RT, Hemmelgarn BR, Levey AS, Chronic Kidney Disease Prognosis Consortium : Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA 307: 1941–1951, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kalogeropoulos A, Georgiopoulou V, Kritchevsky SB, Psaty BM, Smith NL, Newman AB, Rodondi N, Satterfield S, Bauer DC, Bibbins-Domingo K, Smith AL, Wilson PW, Vasan RS, Harris TB, Butler J: Epidemiology of incident heart failure in a contemporary elderly cohort: The health, aging, and body composition study. Arch Intern Med 169: 708–715, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herzog CA, Ma JZ, Collins AJ: Poor long-term survival after acute myocardial infarction among patients on long-term dialysis. N Engl J Med 339: 799–805, 1998 [DOI] [PubMed] [Google Scholar]
- 48.Sakuma M, Nakamura M, Tanaka F, Onoda T, Itai K, Tanno K, Ohsawa M, Sakata K, Yoshida Y, Kawamura K, Makita S, Okayama A: Plasma B-type natriuretic peptide level and cardiovascular events in chronic kidney disease in a community-based population. Circ J 74: 792–797, 2010 [DOI] [PubMed] [Google Scholar]
- 49.Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Hallé JP, Young J, Rashkow A, Joyce C, Nawaz S, Yusuf S, HOPE Study Investigators : Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 286: 421–426, 2001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.