Abstract
Urinary levels of C-X-C motif chemokine 9 (CXCL9) and CXCL10 can noninvasively diagnose T cell–mediated rejection (TCMR) of renal allografts. However, performance of these molecules as diagnostic/prognostic markers of antibody-mediated rejection (ABMR) is unknown. We investigated urinary CXCL9 and CXCL10 levels in a highly sensitized cohort of 244 renal allograft recipients (67 with preformed donor–specific antibodies [DSAs]) with 281 indication biopsy samples. We assessed the benefit of adding these biomarkers to conventional models for diagnosing/prognosing ABMR. Urinary CXCL9 and CXCL10 levels, normalized to urine creatinine (Cr) levels (CXCL9:Cr and CXCL10:Cr) or not, correlated with the extent of tubulointerstitial (i+t score; all P<0.001) and microvascular (g+ptc score; all P<0.001) inflammation. CXCL10:Cr diagnosed TCMR (area under the curve [AUC]=0.80; 95% confidence interval [95% CI], 0.68 to 0.92; P<0.001) and ABMR (AUC=0.76; 95% CI, 0.69 to 0.82; P<0.001) with high accuracy, even in the absence of tubulointerstitial inflammation (AUC=0.70; 95% CI, 0.61 to 0.79; P<0.001). Although mean fluorescence intensity of the immunodominant DSA diagnosed ABMR (AUC=0.75; 95% CI, 0.68 to 0.82; P<0.001), combining urinary CXCL10:Cr with immunodominant DSA levels improved the diagnosis of ABMR (AUC=0.83; 95% CI, 0.77 to 0.89; P<0.001). At the time of ABMR, urinary CXCL10:Cr ratio was independently associated with an increased risk of graft loss. In conclusion, urinary CXCL10:Cr ratio associates with tubulointerstitial and microvascular inflammation of the renal allograft. Combining the urinary CXCL10:Cr ratio with DSA monitoring significantly improves the noninvasive diagnosis of ABMR and the stratification of patients at high risk for graft loss.
Keywords: kidney transplantation, noninvasive diagnosis, biomarker, antibody-mediated rejection
The current gold standard test for the diagnosis of renal allograft dysfunction is histologic examination of the allograft biopsy. Unfortunately, this test has many limitations and pitfalls; specifically, the biopsy procedures are invasive, complications can occur, and sampling errors may bias the histologic diagnosis.1 The costs of this procedure need to be considered as well. Recent studies have focused on noninvasive tests that rely on easily accessible biologic fluids, such as urine and peripheral blood, that could ultimately be used for noninvasive serial monitoring, which is not possible with biopsies. For example, mRNA and/or protein biomarkers have been successfully developed for use in the diagnosis of T cell–mediated rejection (TCMR) of renal allografts. Suthanthiran et al.2 recently showed that the urinary cell mRNA profile can serve as a diagnostic and prognostic biomarker of TCMR. Protein markers have also been assessed for their ability to predict both clinical3–7 and subclinical TCMR.4,8–11 C-X-C motif chemokine 9 (CXCL9) and CXCL10 are IFNγ–dependent, C-X-C motif chemokine receptor 3–binding chemokines that are secreted by infiltrating inflammatory cells and renal tubular and mesangial cells. Schaub et al.10 first reported the association of CXCL9 and CXCL10, normalized to urine creatinine, with tubulitis.10 A recent multicenter observational trial in 280 patients with kidney transplants reported that the CXCL9 protein level had a strong predictive value for noninvasively diagnosing TCMR.5
Although there have been improvements in diagnosing TCMR using these new methods, it is noteworthy that, at the same time, the clinical presentation of renal allograft rejection has changed dramatically, with the incidence of TCMR progressively decreasing to 10%–15% using tacrolimus-based immunosuppression. For example, in the clinical trials in organ transplantation 4 (CTOT-4) Study, only 36 of 385 patients (9.3%) developed TCMR at 1 year post-transplant.2 In contrast, antibody-mediated rejection (ABMR) has become a major concern and is now recognized as the main cause of late allograft loss.12 Very few studies have focused on urinary biomarkers of ABMR. In the CTOT-01 Study, among 150 indication biopsies, only two ABMRs and four mixed rejections were diagnosed.5 More recently, Matignon et al.13 focused on urinary cell mRNA biomarkers of acute kidney graft dysfunction and developed a six-gene diagnostic signature to differentiate acute rejection (AR; both TCMR and ABMR) from acute tubular injury and a five-gene signature to differentiate ABMR from TCMR.
Microarray analysis of biopsy samples showing ABMR identified transcriptomic signatures of ABMR that included transcripts associated with IFNγ production and IFNγ-inducible transcripts.14 We hypothesized that this intragraft IFNγ signature could be detected noninvasively by quantification of the IFNγ–dependent chemokines CXCL9 and CXCL10 in urine samples. To test this hypothesis, we studied the accuracy of urinary CXCL9 and CXCL10 levels in the diagnosis of ABMR at the time of a clinically indicated biopsy in a cohort of 244 renal allograft recipients that included 67 patients with high immunologic risk and preformed donor–specific antibodies (DSAs) at time of transplantation.15 The urinary chemokine profile was also used combined with conventional clinical and immunologic features (i.e., presence of anti-HLA DSAs) to evaluate the added benefit of urinary chemokines with respect to the diagnosis and prognosis of ABMR.
Results
Patient and Biopsy Characteristics
From February of 2011 to January of 2013, 290 matched clinically indicated biopsies and urine samples were collected from 247 kidney transplant recipients in our center. These biopsies were indicated for acute renal dysfunction (n=240), proteinuria (n=25), identification of de novo DSAs (n=13), or positive BK virus viremia (n=12).
After the exclusion of 9 biopsies from patients with BK virus nephropathy, which is known to alter urinary inflammatory signatures2 and for which a noninvasive biomarker would not obviate a biopsy in the presence of BK viremia,13 our final study sample included 281 matched biopsy/urine samples from 244 patients (Table 1). Sixty-seven patients had known DSAs before transplantation.
Table 1.
Patient demographics
| Variables | n=244 |
|---|---|
| Recipient characteristics | |
| Men, n (%) | 150 (61.5) |
| Age (yr) at transplantation, mean±SD | 46.5±16.5 |
| Cause of ESRD, n (%) | |
| GN | 53 (21.8) |
| Diabetes | 22 (9.1) |
| Cystic/hereditary/congenital | 42 (17.3) |
| Secondary GN | 10 (4.1) |
| Hypertension | 22 (9.1) |
| Interstitial nephritis | 42 (17.3) |
| Miscellaneous conditions | 8 (3.3) |
| Neoplasm | 0 (0.0) |
| Etiology uncertain | 44 (18.1) |
| Transplant variables | |
| Donor age (yr), mean±SD | 54.8±17.3 |
| Deceased donor, n (%) | 187 (76.6) |
| Living donor, n (%) | 57 (23.4) |
| Expanded criteria donor,a n (%) | 94 (51.6) |
| Retransplantation, n (%) | 42 (17.2) |
| Cold ischemia time (h),a mean±SD | 21.8±7.7 |
| Delayed graft function,a n (%) | 62 (34.3) |
| Preformed DSAs with MFI>1000,b n (%) | 67 (33.7) |
| Immunosuppressive protocol | |
| Induction therapy, n (%) | 220 (98.7) |
| Basiliximab/thymoglobuline, n (%) | 116 (52.7)/104 (47.3) |
| Calcineurin inhibitor–based therapy, n (%) | 223 (91.4) |
| Cyclosporin/tacrolimus, n (%) | 51 (22.9)/172 (77.1) |
| Purine synthesis inhibitor, n (%) | 229 (93.9) |
| Azathioprine/mycophenolic acid, n (%) | 15 (6.6)/214 (93.4) |
| Mammalian target of rapamycin inhibitor, n (%) | 16 (6.6) |
| Steroid, n (%) | 232 (95.1) |
| Outcome | |
| Serum creatinine (µmol/L) at last follow-up, mean±SD | 206±157 |
| Patient survival at last follow-up, n (%) | 233 (95.5) |
| Graft survival at last follow-up, n (%) | 215 (88.1) |
| Mean follow-up (d), mean±SD | 1914±1762 |
In deceased donor grafts only.
Assessment of DSAs in 229 kidney transplant recipients.
At the time of the biopsy, which was performed at a median time of 10.6 months post-transplant (interquartile range=1.3–55.2), the serum creatinine level was 213±125 μmol/L (Table 2); 78 biopsies (27.8%) revealed AR (68 ABMRs [24.2%], including 37 pure ABMRs and 31 mixed rejections, and 10 TCMRs [3.6%]). The other biopsies, subsequently classified as dysfunction with no rejection (DNR), revealed acute tubular necrosis/minimal lesions (n=43 [15%]), isolated interstitial fibrosis/tubular atrophy (n=140 [50%]), borderline lesions (n=17 [6%]), or a primary diagnosis of recurrent disease (n=3 [1%]) (Table 2). Supplemental Table 1 describes the histologic parameters in the different biopsy groups.
Table 2.
Characteristics of 281 clinically indicated biopsies
| Variables | Total (n=281) | DNR Group (n=203) | TCMR (n=10) | Pure ABMRa (n=37) | Mixed Rejectionb (n=31) |
|---|---|---|---|---|---|
| Biopsy characteristics | |||||
| Time (mo) after transplantation, mean±SD | 38.0±56.9 | 30.7±52.7 | 11.8±20.4 | 70.0±54.8 | 56.2±74.7 |
| Number of glomeruli, mean±SD | 15.5±7.7 | 16.0±8.0 | 14.2±4.2 | 15.6±6.0 | 12.5±7.3 |
| Indication of biopsy, n (%) | |||||
| Acute allograft dysfunction | 236 (84.0) | 172 (84.7) | 10 (100.0) | 27 (73.0) | 27 (87.1) |
| Proteinuria | 25 (8.9) | 19 (9.4) | 0 (0) | 3 (8.1) | 3 (9.7) |
| BK virus viremia | 7 (2.5) | 7 (3.4) | 0 (0) | 0 (0) | 0 (0) |
| Anti-HLA DSAs | 13 (4.6) | 5 (2.5) | 0 (0) | 7 (18.9) | 1 (3.2) |
| Biology at the time of biopsy | |||||
| Serum creatinine (μmol/L), mean±SD | 213±125 | 208±119 | 232±100 | 205±132 | 250±157 |
| Proteinuria (g/L), mean±SD | 0.6±1.2 | 0.6±1.2 | 0.7±1.4 | 0.9±1.2 | 0.9±1.6 |
| Urine protein-to-creatinine ratio (g/mmoL), mean±SD | 0.1±0.2 | 0.1±0.2 | 0.1±0.2 | 0.2±0.2 | 0.1±0.2 |
| Pathologic primary diagnosis | |||||
| TCMR, n (%) | 20 (7.1) | 0 (0) | 10 (100) | 0 (0) | 10 (32) |
| ABMR, n (%) | 68 (24.2) | 0 (0) | 0 (0) | 37 (100) | 31 (100) |
| DNR, n (%) | 203 (72.2) | 203 (100) | 0 (0) | 0 (0) | 0 (0) |
| Acute tubular injury | 43 (15.3) | 43 (21.2) | 0 (0) | 0 (0) | 0 (0) |
| Isolated interstitial fibrosis/tubular atrophy | 140 (49.8) | 140 (69) | 0 (0) | 0 (0) | 0 (0) |
| Borderline lesions | 17 (6.0) | 17 (8.4) | 0 (0) | 0 (0) | 0 (0) |
| Recurrent disease | 3 (1.1) | 3 (1.5) | 0 (0) | 0 (0) | 0 (0) |
Pure ABMR were defined as ABMR with no tubulointerstitial inflammation (i+t score =0).
Mixed rejections include all ABMR with i+t score ≠0, including primarily ABMR with minimal tubulointerstitial inflammation (n=21) and ABMR with TCMR≥1a (n=10).
Association of Acute Banff Elementary Lesions with the Urinary Biomarkers
We assessed the level of CXCL9 and CXC10 proteins in urine samples and studied the association of CXCL9 and CXCL10 levels and values normalized to urine creatinine (CXCL9:Cr and CXCL10:Cr) with the histologic findings in 281 biopsies (Supplemental Table 2).
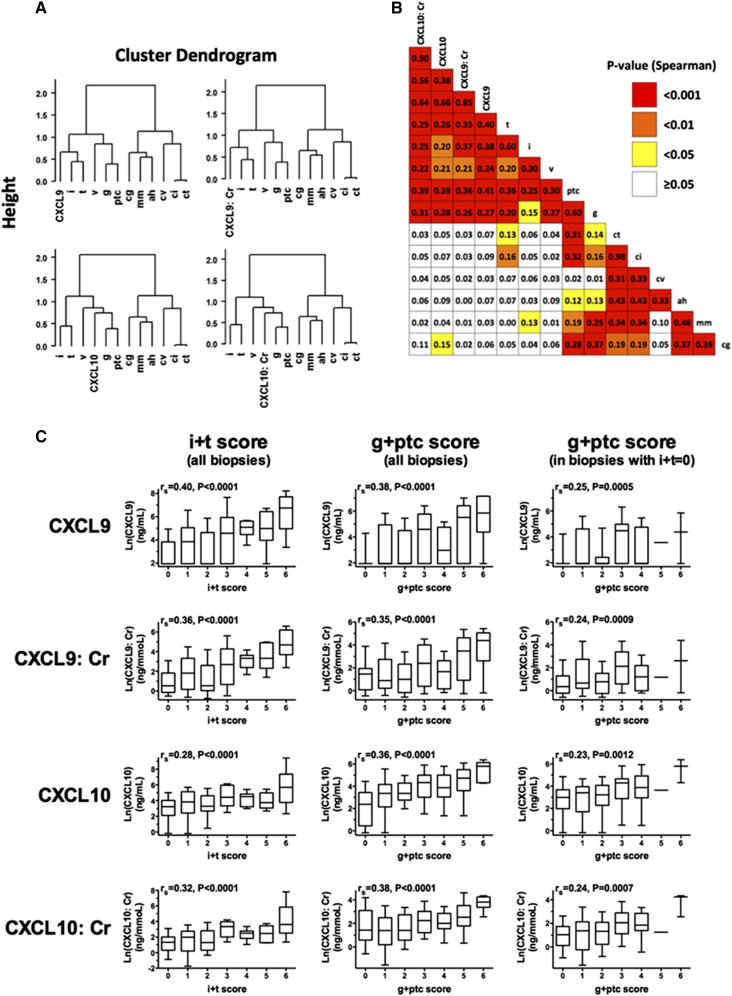
Unsupervised hierarchical clustering analysis of Banff scores and protein biomarkers (Figure 1A) showed that both CXCL9 and CXCL10 levels, normalized (Figure 1A, left panels) or not (Figure 1A, right panels) to urine creatinine, were highly associated with all acute Banff scores. CXCL10 and CXCL10:Cr clustered closer to microcirculation inflammatory scores (g and ptc), whereas CXCL9 and CXCL9:Cr strongly clustered with tubulointerstitial inflammatory scores (i and t).
Figure 1.
Urinary CXCL9 and CXCL10 levels, normalized or not by urine creatinine, correlated well with the extent of tubulointerstitial inflammation and microvascular inflammation. (A) Dendrogram representations of unsupervised hierarchical clustering analysis of acute and chronic Banff elementary scores of 281 biopsies and CXCL9 and CXCL10 levels normalized or not with urine creatinine. The vertical axis of the dendrogram represents the distance or dissimilarity between clusters. The dendrograms show a high association of CXCL9 and CXCL10, normalized or not with urine creatinine, with all acute Banff scores (i, t, g, ptc, and v). CXCL10 and CXCL10:Cr were found to be most significantly correlated with the microcirculation scores (g and ptc), whereas CXCL9 and CXCL9:Cr correlated most significantly with the cellular inflammation scores (i and t). (B) Spearman correlation matrix of Banff elementary lesions and urinary biomarkers. The color of each box indicates the P value. (C) Correlation of four urinary biomarker levels with the tubulointerstitial inflammation burden (indicated by the g+ptc score) with or without concomitant tubulointerstitial inflammation. Box-and-whisker plots show the log (natural)–transformed urinary biomarker levels. The horizontal line within each box represents the median, the bottom and top of each box represent the 25th and 75th percentile values, respectively, and the I bars represent the 10th and 90th percentile values. Spearman’s correlation coefficient (rs) and corresponding P values are shown.
Spearman’s correlation (Figure 1B) confirmed that acute Banff scores (g, ptc, i, and t) were strongly associated with urinary CXCL9, CXCL9:Cr, CXCL10, and CXCL10:Cr (all P<0.01), with Spearman’s correlation coefficients, rs, between 0.20 and 0.41. Conversely, there was no correlation between the four protein biomarkers and the chronic Banff scores. Urinary CXCL9 and CXCL10 levels, normalized or not to urine creatinine, correlated well with tubulointerstitial inflammation burden (i+t score; all P<0.001) (Figure 1C). In addition, urinary CXCL9 and CXCL10 levels, normalized or not to urine creatinine, correlated well with microvascular inflammation burden (g+ptc score; all P<0.001), even after exclusion of patients with tubulointerstitial inflammation (Figure 1C).
Because acute Banff elementary lesions were all closely correlated, multivariate linear regression was used to quantify the strength of the relationship between the urinary biomarkers and the Banff elementary lesions and identify independent Banff scores associated with the urinary biomarkers. Multivariate regression analysis revealed that both tubulointerstitial inflammation (i and/or t scores) and microvascular inflammation (ptc score) were significantly and independently associated with urinary biomarker levels (Table 3). The values of the regression coefficient-β suggested that CXCL9 and the CXCL9:Cr ratio were mainly associated with tubulointerstitial inflammation, whereas CXCL10 and the CXCL10:Cr ratio were mainly associated with peritubular capillaritis (Table 3). Glomerulitis did not seem to be an independent factor associated with the urinary biomarkers.
Table 3.
Association of Banff scores with urinary biomarker levels on the basis of multivariate linear regression analyses
| Independent Banff Variables | Coefficient-β | 95% CI | P Value |
|---|---|---|---|
| Model 1: Ln(CXCL10) | |||
| t | 0.300 | 0.11 to 0.50 | 0.002 |
| ptc | 0.632 | 0.42 to 0.85 | <0.001 |
| Model 2: Ln(CXCL10:Cr) | |||
| t | 0.313 | 0.14 to 0.49 | <0.001 |
| ptc | 0.590 | 0.40 to 0.78 | <0.001 |
| Model 3: Ln(CXCL9) | |||
| i | 0.474 | 0.13 to 0.82 | <0.01 |
| t | 0.383 | 0.16 to 0.60 | <0.001 |
| ptc | 0.529 | 0.32 to 0.74 | <0.001 |
| Model 4: Ln(CXCL9:Cr) | |||
| i | 0.620 | 0.27 to 0.98 | <0.001 |
| t | 0.343 | 0.12 to 0.57 | 0.003 |
| ptc | 0.485 | 0.28 to 0.70 | <0.001 |
Urinary Biomarkers Are Diagnostic of Both TCMR and ABMR
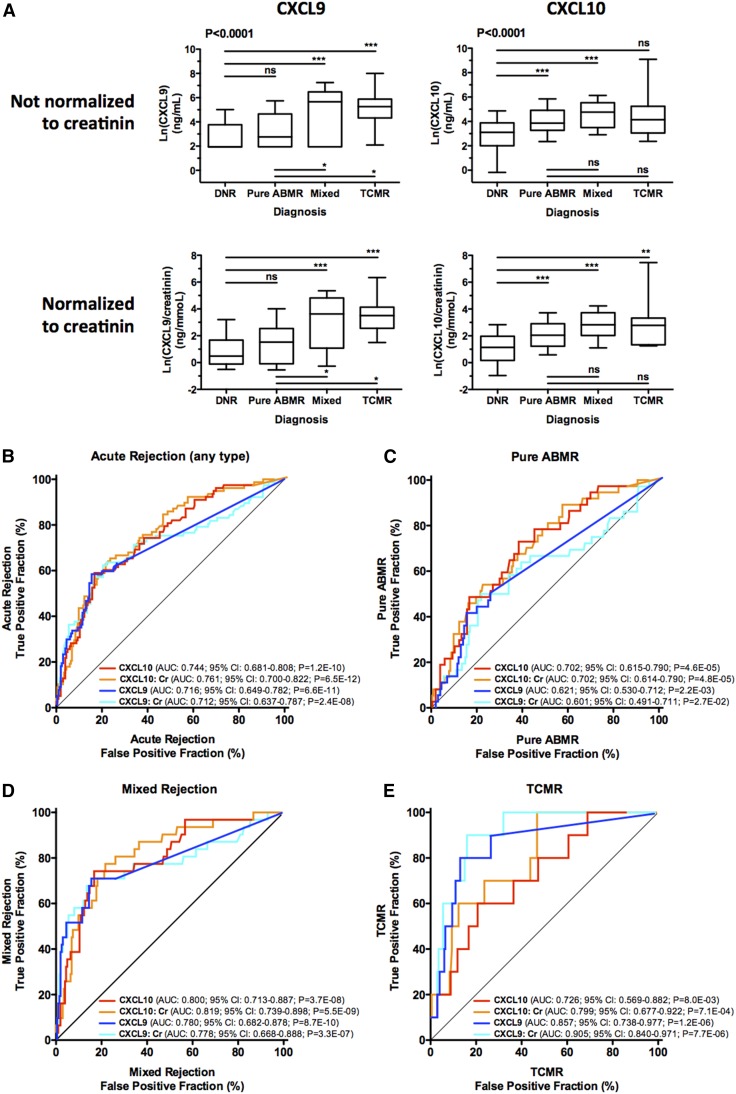
As illustrated in Figure 2A, urinary levels of four protein biomarkers were significantly different between the diagnostic groups (all P<0.001, Kruskal–Wallis test) (Supplemental Table 2). Compared with DNR, CXCL10 and the CXCL10:Cr ratio significantly increased in pure ABMRs (both P<0.001, Dunn’s post-test) and mixed rejections (both P<0.001, Dunn’s post-test). CXCL10 and the CXCL10:Cr ratio were similar in all rejection groups; however, CXCL9 and the CXCL9:Cr ratio were increased in mixed rejections (both P<0.001, Dunn’s post-test) and TCMRs (both P<0.001, Dunn’s post-test) but not in pure ABMRs (Figure 2A). We repeated the analysis while distinguishing borderline changes from other DNR diagnoses, and highly consistent results were found with similar biomarker levels in the DNR and the borderline groups (Supplemental Figure 1).
Figure 2.
Urinary biomarkers are diagnostic of both TCMR and ABMR. (A) Box-and-whisker plots show the log (natural)–transformed urinary biomarker levels in 203 matched urine/biopsy samples from patients with allograft DNR, 10 matched urine/biopsy samples from patients with TCMR, 37 matched urine/biopsy samples from patients with pure ABMR, and 31 matched urine/biopsy samples from patients with mixed rejection (mixed). P values are on the basis of the Kruskal–Wallis test. Asterisks depict pairwise group comparisons by means of Dunn’s post-test. **P<0.01; ***P<0.001. (B–E) ROC curves for the urinary chemokines. The fraction of true-positive results (sensitivity) and the fraction of false-positive results (1− specificity) for urinary CXCL9 and CXCL10 levels, normalized or not by urine creatinine, as diagnostic biomarkers of AR (both TCMR and ABMR) compared with the fractions in the group of patients with (B) DNR, (C) pure ABMR compared with DNR, (D) mixed rejection compared with DNR, and (E) TCMR compared with DNR. 95% CIs were generated by 2000 stratified bootstrap replicates.
Receiver operating characteristic (ROC) curve analysis was performed for each biomarker to evaluate its performance in the diagnosis of AR (Figure 2B), pure ABMR (Figure 2C), mixed rejection (Figure 2D), and TCMR (Figure 2E) compared with the diagnosis of DNR. The diagnostic performances of CXCL9, CXCL9:Cr, CXCL10, and CXCL10:Cr in predicting any type of AR were similar (Figure 2B, Supplemental Table 3). Figure 2E shows that CXCL9 (area under the curve [AUC]=0.86; 95% confidence interval [95% CI], 0.74 to 0.98; P<0.001) and CXCL9:Cr (AUC=0.90; 95% CI, 0.84 to 0.97; P<0.001) were strong predictors of TCMR and that CXCL9 and CXCL9:Cr were numerically better than CXCL10 and CXCL10:Cr in the noninvasive diagnosis of TCMR (Figure 2E, Supplemental Table 4). Mixed rejection was also strongly predicted by urinary chemokine levels with CXCL10 (AUC=0.80; 95% CI, 0.71 to 0.89; P<0.001) and CXCL10:Cr (AUC=0.82; 95% CI, 0.74 to 0.90; P<0.001), yielding the best AUC values (Figure 2D). Importantly, as shown in Figure 2C, urinary chemokine levels also associated with the diagnosis of pure ABMR and again, CXCL10 (AUC=0.70; 95% CI, 0.61 to 0.79; P<0.001) and CXCL10:Cr (AUC=0.70; 95% CI, 0.61 to 0.79; P<0.001) yielded the best AUC values.
C statistics and bootstrap validation showed that CXCL10 and the CXCL10:Cr ratio were strongly associated with ABMR, with modest positive predictive values and high negative predictive values (NPVs; >91% for pure ABMR and >95% for mixed rejection), suggestive of a very low false-negative rate (Table 4).
Table 4.
Diagnostic performances of the urinary biomarkers in detecting any type of AR, TCMR, all ABMR (i.e., ABMR with or without associated tubulointerstitial inflammation), mixed rejection (i.e., ABMR with i+t≠0), and pure ABMR (i.e., ABMR with i+t=0)
| Biomarker | ROC–Based Discrimination Measures | PPV | NPV | |||
|---|---|---|---|---|---|---|
| AUC (95% CI)a | AUC P Value | Sensitivity | Specificity | |||
| AR | ||||||
| CXCL10 | 74.4 (68.1 to 80.8) | <0.001 | 59.0 | 83.3 | 57.5 | 84.1 |
| CXCL10:Cr | 76.1 (70.0 to 82.2) | <0.001 | 65.4 | 76.4 | 51.5 | 85.2 |
| CXCL9 | 71.6 (64.9 to 78.2) | <0.001 | 58.4 | 84.5 | 59.2 | 84.1 |
| CXCL9:Cr | 71.2 (63.7 to 78.7) | <0.001 | 63.6 | 78.0 | 52.7 | 84.8 |
| TCMR | ||||||
| CXCL10 | 72.6 (56.9 to 88.2) | <0.01 | 60.0 | 79.3 | 12.5 | 97.6 |
| CXCL10:Cr | 79.9 (67.7 to 92.2) | <0.001 | 70.0 | 76.4 | 12.7 | 98.1 |
| CXCL9 | 85.7 (73.8 to 97.7) | <0.001 | 80.0 | 87.0 | 23.5 | 98.9 |
| CXCL9:Cr | 90.5 (84.0 to 97.1) | <0.001 | 90.0 | 84.0 | 22.0 | 99.4 |
| All ABMR | ||||||
| CXCL10 | 74.7 (68.0 to 81.4) | <0.001 | 60.3 | 83.3 | 54.7 | 86.2 |
| CXCL10:Cr | 75.5 (69.0 to 82.1) | <0.001 | 64.7 | 77.3 | 48.9 | 86.7 |
| CXCL9 | 69.4 (62.3 to 76.6) | <0.001 | 55.2 | 84.5 | 54.4 | 84.9 |
| CXCL9:Cr | 68.3 (60.0 to 76.5) | <0.001 | 59.7 | 78.0 | 47.6 | 85.2 |
| Mixed rejection | ||||||
| CXCL10 | 80.0 (71.3 to 88.7) | <0.001 | 74.2 | 83.3 | 40.4 | 95.5 |
| CXCL10:Cr | 81.9 (73.9 to 89.8) | <0.001 | 77.4 | 78.3 | 35.3 | 95.8 |
| CXCL9 | 78.0 (68.2 to 87.8) | <0.001 | 71.0 | 84.5 | 41.5 | 94.9 |
| CXCL9:Cr | 77.8 (66.8 to 88.8) | <0.001 | 71.0 | 84.5 | 41.5 | 94.9 |
| Pure ABMR | ||||||
| CXCL10 | 70.2 (61.5 to 79.0) | <0.001 | 73.0 | 61.6 | 25.7 | 92.6 |
| CXCL10:Cr | 70.2 (61.4 to 79.0) | <0.001 | 67.6 | 62.1 | 24.5 | 91.3 |
| CXCL9 | 62.1 (53.0 to 71.2) | 0.002 | 50.0 | 74.0 | 25.7 | 89.2 |
| CXCL9:Cr | 60.1 (49.1 to 71.1) | 0.03 | 50.0 | 78.0 | 29.0 | 89.7 |
The C statistics, sensitivity, specificity, positive predictive values (PPVs), and NPVs are shown.
95% CIs were generated by 2000 stratified bootstrap replicates.
In a sensitivity analysis, we assessed the robustness of our study results by investigating the diagnostic accuracy of urine biomarkers separately in the subgroup of nonsensitized patients (e.g., patients with no identified preformed DSA) and a subgroup analysis that included only the first biopsy of each patient, and highly consistent results were found (Supplemental Material, Supplemental Figure 2). In another subgroup analysis, ROC curve analysis was performed to evaluate the performance of the CXCL10:Cr ratio in the diagnosis of pure ABMR compared with the DNR diagnosis. This analysis showed that the CXCL10:Cr ratio remains diagnostic of ABMR, even in the total absence of associated tubulointerstitial inflammation (AUC=0.70; 95% CI, 0.61 to 0.79; P<0.001).
Evaluation of the chemokine levels, with and without normalization to urine creatinine (Table 4, Supplemental Material), revealed that the CXCL10:Cr ratio was the best marker of ABMR, and the results for this biomarker will be reported below. Potential confounding factors were assessed, and leukocyturia was identified as significant (Supplemental Table 4).
Urinary CXCL10:Cr Independently Improves Noninvasive Diagnosis of ABMR
The association of clinical, biologic, immunologic, and histologic factors with the risk of ABMR was evaluated by univariate and multivariate logistic regression analyses. Univariate analysis showed that recipient age, use of standard criteria donor kidneys, cold ischemia time, donor age, transplantation rank, time post-transplantation, proteinuria, Ln(CXCL9), Ln(CXCL9:Cr), Ln(CXCL10), Ln(CXCL10:Cr), DSAs at time of biopsy, and mean fluorescence intensity (MFI) of the immunodominant DSA (iDSA) were associated (P<0.10) with ABMR (Supplemental Table 5).
Multivariate logistic regression analysis showed that the MFI of the iDSA at biopsy (odds ratio [OR], 2.2 for MFI<1000; 95% CI, 0.8 to 6.0; P=0.13; OR, 3.6 for MFI=1000–3000; 95% CI, 1.1 to 10.7; P=0.02; OR, 10.4 for MFI>3000; 95% CI, 4.7 to 24.0; P<0.001) and Ln(CXCL10:Cr) (OR, 1.9; 95% CI, 1.5 to 2.5; P<0.001) were independently associated with the diagnosis of ABMR (Table 5).
Table 5.
Factors associated with the diagnosis of ABMR (multivariate logistic regression analysis)
| Variable | OR (95% CI) | P Value |
|---|---|---|
| Ln(CXCL10:Cr) | 1.90 (1.46 to 2.54) | <0.001 |
| MFI of iDSAs at biopsy | ||
| <1000 | 2.21 (0.75 to 6.02) | 0.13 |
| 1000–3000 | 3.58 (1.14 to 10.74) | 0.02 |
| >3000 | 10.44 (4.74 to 24.04) | <0.001 |
A 10-fold cross-validation strategy was used to internally validate the model used to associate the MFI of iDSA with Ln(CXCL10:Cr). The predicted probability for each patient from the cross-validation was used to construct an ROC curve. The cross-validated estimate of the AUC was 0.82 (95% CI, 0.81 to 0.83; P<0.001). This estimate is the expected value of the AUC in an independent sample.
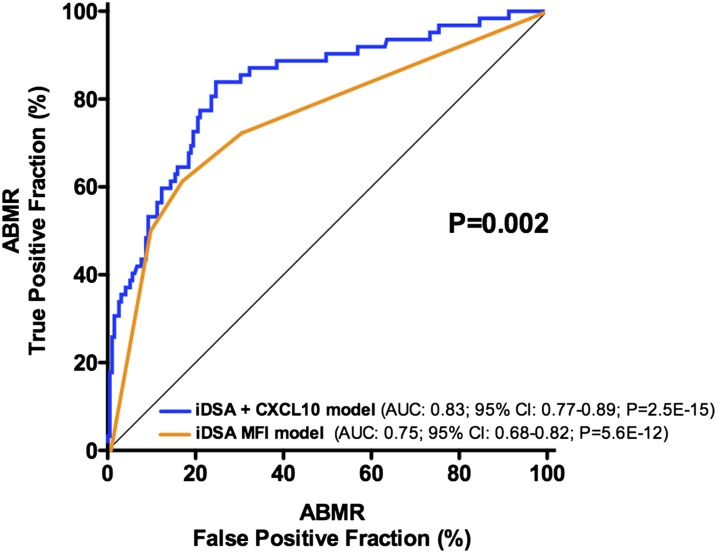
The inclusion of the CXCL10:Cr ratio in the reference model that used only the iDSA was found to significantly improve the noninvasive diagnosis of ABMR, with the C statistic increasing from 0.75 to 0.83 (P=0.002) (Figure 3) with a bootstrap mean difference of 0.075 (95% CI, 0.08 to 0.08; P<0.001). Similarly, the inclusion of the CXCL10:Cr ratio in the reference model adequately reclassified patients at lower (no event) or higher (event) risk of ABMR, which was shown by a continuous net reclassification index of 0.6718 (95% CI, 0.40 to 0.94; P<0.01). The addition of the CXCL10:Cr ratio reclassified 74 of 194 patients (38%) in the right direction in the no event group, whereas it reclassified 18 of 62 patients (29%) in the event group. The integrated discrimination improvement was 0.0854 (95% CI, 0.05 to 0.12; P<0.01).
Figure 3.
Urinary CXCL10:Cr ratio improves noninvasive diagnosis of ABMR. Shown are the ROC curves of a conventional noninvasive diagnostic model on the basis of the MFI of the iDSA and a model integrating urine CXCL10:Cr ratio into the previous model. The provided P value indicates the significant difference between the two AUCs.
CXCL10 expression is not specific for ABMR and also increases in other types of inflammation; therefore, we repeated the analysis and addressed the improvement of prediction of any type of AR by adding urinary CXCL10 expression to the DSA measurement. The MFI of the iDSA at biopsy still predicted AR (AUC=0.72; 95% CI, 0.65 to 0.79; P<0.001), and the inclusion of the CXCL10:Cr ratio in the model that used only the iDSA was found to significantly improve the noninvasive diagnosis of AR, with the C statistic increasing from 0.72 to 0.82 (P<0.001; bootstrap mean difference=0.0985; 95% CI, 0.10 to 0.10; P<0.001).
CXCL10:Cr Ratio Is Associated with Death–Censored Graft Loss after ABMR
Among 68 patients with ABMR, 14 lost their graft after a median follow-up of 6 months (interquartile range=2–19). Death–censored graft survival after the diagnosis of ABMR was 87%, 77%, and 75% at 1, 2, and 3 years, respectively. Univariate Cox analysis of the conventional features of graft loss showed that the recipient age, donor age, serum creatinine, proteinuria, and v, i, and interstitial fibrosis/tubular atrophy scores were associated (P≤0.10) with graft loss. Urinary CXCL9:Cr and CXCL10:Cr ratios were also associated with graft failure (Supplemental Table 6). Univariate analysis revealed that increased urinary CXCL10:Cr ratios, divided into quartiles at the time of the biopsy, correlated with graft survival in a dose-dependent manner (hazard ratio [HR], 1, 3.2 (95% CI, 0.3 to 30.6), 6.0 (95% CI, 0.7 to 51.0), and 10.0 (95% CI, 1.1 to 87.0) for quartile 1 [Q1], Q2, Q3, and Q4, respectively).
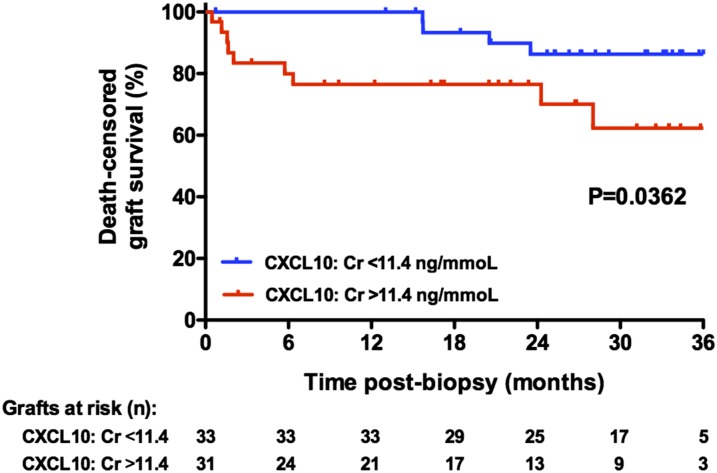
A multivariate Cox model identified two factors that were independently associated with graft loss, namely proteinuria (HR, 1.7; 95% CI, 1.3 to 2.2; P<0.001) and CXCL10:Cr (HR, 2.2; 95% CI, 1.2 to 4.0; P<0.01) (Table 6). A Kaplan–Meier analysis of post-ABMR, death–censored graft survival showed that increased urinary CXCL10:Cr ratios at the time of biopsy correlated with graft survival (Figure 4).
Table 6.
Determinants of kidney transplant graft outcome after acute ABMR (multivariate Cox proportional hazards model)
| Variable | HR (95% CI) | P Value |
|---|---|---|
| Proteinuria at biopsy, g/L | 1.72 (1.34 to 2.22) | <0.001 |
| Ln(CXCL10:Cr) | 2.21 (1.23 to 4.00) | <0.01 |
Figure 4.
CXCL10:Cr ratio is associated with death-censored graft loss after ABMR. Death–censored graft survival postbiopsy showing ABMR on the basis of the median urinary CXCL10:Cr value at the time of biopsy. Estimates were obtained using the Kaplan–Meier method and compared using the log-rank test.
Discussion
Urinary biomarkers, including mRNA and protein biomarkers, have been extensively evaluated as noninvasive biomarkers of TCMR of kidney allografts. A similar strategy, however, is missing with regard to the noninvasive diagnosis of ABMR, which is currently the main cause of late allograft loss. The main observations of this study were that urinary CXCL10 levels correlated with ongoing ABMR in a large cohort of highly sensitized and well phenotyped kidney transplant recipients, that combining the urinary CXCL10:Cr ratio with iDSA levels significantly improved the noninvasive diagnosis of ABMR in patients with kidney transplants, and that the CXCL10:Cr ratio at the time of biopsy stratified patients who were at risk of graft loss.
The chemokines CXCL9 and CXCL10 have been extensively associated with T cell–infiltrate burden, particularly tubulitis, and our demonstration that they have an increased urinary level during ABMR may be surprising. One simplistic explanation for this result could be that our ABMRs have a significant T cell–infiltrate burden, therefore contributing to an IFNγ signature. Although low–grade interstitial infiltrate is often present in biopsies showing ABMR, this does not constitute the main explanation for the above-mentioned result, as shown by restricting the analysis to patients with ABMR with complete absence of interstitial infiltrate and tubulitis (i+t=0) (Figure 2A). In addition, when studying the molecular signature of biopsy samples showing ABMR, Sellares et al.14 showed a clear IFNγ signature, with CXCL10 being one of the top ABMR classifier genes.16 In a rat model of acute antibody–mediated endothelial injury, among several chemokines and chemokine receptors, CXCL10 was, by far, the most upregulated chemokine (119-fold), a result that also supports our findings.17
Our multivariate linear regression analysis of the association of Banff scores with urinary biomarker levels revealed that, among microvascular inflammatory lesions, the peritubular capillaritis score but not the glomerulitis score seemed independently and significantly associated with the urinary chemokine level (Table 3). This result suggests the prominent role of peritubular capillaritis in triggering increased levels of urinary chemokines, which was already suggested by Panzer et al.,17 who found a huge upregulation of CXCL10 in the peritubular capillaries but not in the glomerular endothelial cells in their rat model of acute antibody–mediated endothelial injury.
The accuracy of CXCL9 and CXCL10 proteins in the diagnosis of ABMR has never been thoroughly evaluated. For example, in the extensive analysis conducted by the Winnipeg group, the urine CXCL10 level was associated with tubulitis, a feature of TCMR.4,10,11 In addition, in the recent CTOT-01 Study that validated urinary CXCL9 as a diagnostic biomarker of AR, only six biopsies showed evidence of ABMR. Urinary CXCL9 and CXCL10 levels have also been shown to be diagnostic of BK virus–associated nephropathy.8
Our analysis shows that urinary CXCL9 level is increased in TCMR and mixed rejection, confirming its close association with tubulointerstitial inflammation. If urinary CXCL10 level is increased in TCMR and mixed rejection, we also describe, for the first time, its association with microvascular inflammation, mainly peritubular capillaritis, even in the total absence of tubulointerstitial inflammation, a result that supports the view that increased CXCL10 urinary levels may relate to any type of alloimmune injury. Therefore, acute allograft dysfunction with a concomitant high level of urinary CXCL10 is not synonymous with TCMR, and a biopsy is required to assess the mechanism of injury and define adequate therapeutic interventions. This relative lack of specificity in addition to the relatively low positive predictive values and high NPVs indicate that noninvasive biomarkers may be more useful for avoiding biopsies in patients with low levels of urinary biomarkers, in whom AR is highly unlikely, than replacing biopsy in patients in whom AR would be anticipated on the basis of a positive urinary marker.
Currently, the main available biomarker of ABMR is the presence of anti-HLA DSAs in the serum, and the majority of ABMRs is associated with these circulating antibodies.18 However, DSAs are only a risk factor for ABMR, and many patients bear DSAs without evidence of antibody-mediated injury. For instance, in our cohort, 56 of 244 patients (23%) had DSAs at time of biopsy without any histologic features of ABMR. As suggested by the recent consensus guidelines on the testing and clinical management issues associated with anti-HLA antibodies in transplantation, the identification of DSAs should prompt an allograft biopsy.19 In this respect, the implementation of markers with high NPVs has the potential to avoid a large number of biopsies. Our results suggest that a single urinary chemokine, evaluated by a simple ELISA technique, conveys an NPV of >90%. Whether this strategy may avoid biopsies in DSA–positive renal transplant recipients will need to be evaluated prospectively.
Our study also highlights how innovative biomarkers may be implemented in the clinic to improve prognostic models that use more conventional markers. We used recommended methodologic approaches for the performance and reclassification analyses20 to evaluate the benefit of the urinary biomarkers when added to the conventional features, and we found that the inclusion of urinary CXCL10:Cr ratios in a conventional assessment (i.e., DSA monitoring) of patients with kidney transplants improved the noninvasive diagnosis of ABMR. Molecular markers have the potential to aid in refining the diagnosis and prognosis of renal allograft outcome. Loupy et al.16 recently showed that adding the molecular microscope strategy to a conventional analysis of biopsy samples from patients with ABMR significantly improved prognostication with regard to the risk of subsequent allograft loss. Interestingly, CXCL10 mRNA was one of the top ABMR classifier genes. Our results suggest that urine measurement of CXCL10 may constitute a noninvasive surrogate of the ABMR molecular score and that the urinary CXCL10 protein level outperforms histologic lesions with regard to the prediction of graft outcome. We believe that the strategy of adding innovative biomarkers, including urinary protein markers, to those already available clinically (i.e., clinical phenotyping, DSA monitoring, and BK virus PCR analysis) would also establish robust strategies for noninvasive diagnostics and prognostics.
One third of patients included in this study had identified pretransplant DSAs, thus explaining the high rate of ABMR, which may question the generalizability of the study results. Sensitivity and specificity are known to be fixed properties of a diagnostic test, whereas prevalence affects positive predictive values and NPVs.21 The high rate of ABMR in our cohort was, therefore, the only way to show the lack of specificity of urinary chemokines in diagnosing TCMR. In addition, disease prevalence affects predictive values in that the rarer the abnormality, the greater assurance that a negative test indicates no abnormality. This finding suggests that, when applied to a more conventional cohort of kidney transplant recipients, the NPV of urinary chemokines in predicting ABMR would probably be >90%. This point should stimulate the evaluation of these noninvasive biomarkers in a more standard population.
The CXCL10:Cr ratio is associated with an ABMR diagnosis, although the AUC of the ROC curve of 0.75 for the diagnosis of ABMR, including pure ABMR and mixed rejection, is modest, making this assay less than ideal as a diagnostic test if considered alone and at a single time point. However, combined with the DSA level, AUC significantly improved to 0.83 and adequately reclassified patients at lower (no event) or higher (event) risk of ABMR, which was shown by a significant continuous net reclassification index and integrated discrimination improvement. Additional studies with serial urine monitoring in independent cohorts of kidney transplant recipients with more conventional immunologic risk are required to assess the clinical application of this biomarker in a real-life setting. In addition, our reported NPVs of CXCL10:Cr of 91% for the diagnosis of pure ABMR and 96% for the diagnosis of mixed rejection (Table 4) are on the basis of a single measurement of the urinary biomarker. Repeated measures and changes in the biomarker profile over time may improve the prediction of allograft status, an assumption that needs to be evaluated prospectively.
Overall, in addition to confirming their potential for the noninvasive diagnosis of tubulointerstitial inflammation, our results show, for the first time, that urinary CXCL10 levels are significantly associated with ABMR. In view of its high NPV, the CXCL10:Cr ratio will need to be evaluated in independent cohorts to assess its ability to avoid biopsies in patients who are DSA positive with low levels of urinary biomarkers, in whom ABMR is highly unlikely. In addition to its diagnostic potential, the CXCL10:Cr ratio, measured at the time of a biopsy showing ABMR, also identifies patients at high risk for kidney allograft loss. Overall, our results indicate that urinary CXCL10 protein is a valuable, noninvasive diagnostic and prognostic marker in kidney transplant recipients with ABMR and provides insight beyond that provided by the classic risk stratification approach that is on the basis of DSAs and biopsy.
Concise Methods
Study Population
Beginning in February of 2011, midstream urine samples were obtained immediately before clinically indicated renal allograft biopsies. This single-center study was approved by the ethics committee of Ile-de-France XI (13016), and all participating patients provided written informed consent. All patients with indication biopsy and a corresponding urine specimen were prospectively included from February of 2011 to February of 2013. Patients with inconclusive biopsies were excluded.
Sixty-seven patients had DSAs at time of transplantation and had been included in our high–risk transplant program.15 All of these high-risk patients received an induction therapy by either rabbit ATG (Thymoglobuline, Sanofi, France; n=56) or Basiliximab (Simulect; Novartis Pharma AG, Basel, Switzerland; n=11) and four courses of intravenous immunoglobulins in addition to a conventional triple–drug immunosuppressive regimen consisting of calcineurin inhibitors, mycophenolic acid, and prednisone. From 2006 onward, patients with DSA at day 0 received additional prophylactic rituximab therapy (Mabthera; Roche Pharmaceuticals, Basel, Switzerland) together with plasmapheresis.
Most of the remaining patients received a conventional triple–drug calcineurin inhibitor–based immunosuppressive regimen. A detailed description of the immunosuppressive regimen is provided in Table 1.
Acute TCMRs were treated with high-dose steroids. Patients with acute ABMR received rituximab (325 mg/m2) and high-dose steroids and underwent plasma exchanges followed by four courses of intravenous immunoglobulins.
DSAs were present at the time of biopsy in 41% (110 of 268) of patients; 30% (33 of 110) of iDSAs were anti-class I DSAs, and 70% (77 of 110) were anti-class II DSAs. Mean (± SEM) MFI of the iDSA was 5074±543 at time of biopsy (2645±394 for anti–class I DSAs and 6172±734 for anti–class II DSAs).
Urine Sample Collection
Urine specimens were collected immediately before the clinically indicated biopsy and centrifuged at 1000×g for 10 minutes within 4 hours of collection. The supernatant was collected after centrifugation and stored with protease inhibitors at −80°C.
Urine Protein Analyses
Frozen aliquots of urine supernatants were used without any dilution and tested by ELISA for CXCL10 (IP10 Quantikine ELISA, DIP100; R&D Systems) according to the manufacturer’s instructions and CXCL9 (Human CXCL9/MIG DuoSet; R&D Systems) as recommended by the CTOT-01 Study.5 The mean minimum detectable level in the ELISA assay was 1.67 pg/ml for CXCL10, and urine samples with a chemokine concentration below this value were included in the analysis as one half the detection limit. For CXCL9, the urine samples with chemokine levels below the detection limit were included as one half the minimum value detected (7 pg/ml).
Measurement of urine creatinine was performed in the same sample using a Hitachi 917 Analyzer (Roche Diagnostics). The results were normalized or not to the urinary creatinine level, and consequently, four biomarkers were analyzed: CXCL10 (picograms per milliliter), CXCL10:Cr ratio (nanograms protein per millimole urine creatinine), CXCL9 (picograms per milliliter), and CXCL9:Cr ratio (nanograms protein per millimole).
Urinalysis was systematically performed at the time of urine collection, and leukocyturia was recorded and classified into four categories (≤104, 105, 106, and >106 leukocytes/ml).
Renal Allograft Biopsy Histology
Clinically indicated biopsy specimens were fixed in formalin, acetic acid, and alcohol and embedded in paraffin. Tissue sections were stained with hematoxylin and eosin, Masson trichrome, periodic acid–Schiff reagent, and Jones for light microscopy evaluation. C4d immunohistochemical staining was systematically performed (rabbit anti-human monoclonal anti-C4d; 1/200 dilution; Clinisciences).
Renal allograft biopsies were classified using the Banff 2007 update of the Banff 1997 classification.22 For the purpose of this study, biopsies were categorized into one of four groups according to the histologic diagnosis: pure ABMR, mixed rejection, TCMR, or DNR. Pure ABMR was defined as ABMR with no tubulointerstitial inflammation (i+t score=0). Mixed rejections include all ABMRs with i+t score≠0, including primarily ABMR with minimal tubulointerstitial inflammation (n=21) and ABMR with TCMR≥1a (n=10). In several analyses, mixed rejections were included in the ABMR category, because they required the same clinical management and therapeutic interventions as ABMR.
DSAs
The presence of circulating DSAs at the time of biopsy was analyzed using single–antigen flow bead assays (One Lambda, Canoga Park, CA) on the Luminex platform as previously described.23 Beads showing a normalized MFI>500 were considered positive. For each patient, we recorded the number, class, specificity, and MFI of all DSAs. HLA typing of donors and recipients was performed using DNA typing (Innolipa HLA Typing Kit; Innogenetics). DSA assessment was available for 229 of 244 (94%) patients, and HLA typing of donors was not available in 15 patients. Among 68 patients with ABMR, 60 were shown to have anti-HLA DSAs. The remaining eight patients did not have available results for circulating anti-HLA DSAs and should be categorized as suspicious for ABMR on the basis of Banff classification.
For the analysis, class (I or II) and MFI of the iDSAs were recorded. The MFI was divided into three categories: <1000, 1000–3000, and >3000.
Statistical Methods
The results are presented as the means±SDs for continuous variables. Frequencies of categorical variables are presented as numbers and percentages. The distribution of each protein biomarker exhibited considerable positive skewness, which was substantially reduced by use of a natural logarithm transformation.
For evaluation of the correlation of the different Banff elementary lesions and urinary biomarkers, Spearman's rank correlation coefficient (rs) was used, and unsupervised ascendant hierarchical clustering analysis was applied to the dataset to enable visualization of the overall pattern of histologic lesions and urinary biomarkers without any a priori sample classification.
We compared the levels of urinary protein biomarkers across the different diagnostic categories (i.e., ABMR, TCMR, and DNR) using the Kruskal–Wallis test followed by Dunn’s post-test.
Multivariate linear regression was used to identify the Banff scores that were independently associated with urinary biomarker levels. The variables tested were i, t, v, g, ptc, cg, mm, ci, ct, cv, and ah. In a stepwise bidirectional elimination analysis, the best model according to the Bayesian Information Criterion was retained.
ROC curves were used to illustrate the diagnostic performance of urinary biomarkers and classifier models. The discrimination ability of urinary biomarkers and the incremental value of urinary biomarkers to conventional models were evaluated by C statistics.20
The association of clinical, biologic, and immunologic variables with the diagnosis of ABMR was evaluated by univariate and multivariate logistic regression analysis. The identified factors (i.e., all covariates associated at the P<0.10 level in the univariate analysis) were included in a final multivariate model using stepwise backward elimination. The additive predictive value of CXCL10:Cr combined with the reference risk model was evaluated using C statistics.
This analysis was internally validated using a 10-fold cross–validation bootstrap method. The original sample was randomly partitioned into 10 equally sized subsamples. Of 10 subsamples, 1 subsample was retained as the validation set used to test the model, and the remaining 9 subsamples were used as the training set. The cross-validation process was then repeated 10 times, with each of 10 subsamples used exactly one time as the validation data. The 10 results obtained from each cross-validation were averaged to produce a single estimation.
The discrimination ability and incremental value of CXCL10:Cr were evaluated by C statistics. This analysis was repeated 1000 times using bootstrap samples to derive 95% CIs for the difference in the C statistic between models.
Cox proportional hazard analysis was used to associate the CXCL10:Cr ratio with death–censored graft survival. A Cox proportional hazards model was used to quantify the HRs and 95% CIs for the factors associated with postbiopsy kidney graft loss. Within each group of factors (i.e., clinical, biologic, immunologic, histologic, and protein biomarkers), we performed univariate and multivariate analyses with backward variable selection (parameters with P<0.20 entered into the multivariate analysis). The selected factors were entered into a single multivariate Cox model to identify the most predictive independent factors for kidney graft loss. The Kaplan–Meier method was used to estimate the cumulative incidence of graft loss, with a timescale of years since study entry (i.e., time since initial biopsy showing ABMR). In the survival analyses, graft survival was censored at 3 years after the index biopsy, recipient death, or last visit until April of 2014.
Analyses were performed with R software (version 3.1.0) and GraphPad Prism (version 5.00; GraphPad Software, San Diego, CA).
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Laila Aouni and Lucie Le Vaillant for their valuable technical assistance.
This research was supported, in part, by funding from Astellas France, Amgen France, Roche France, and the Fondation du Rein and grants from the Centaure Foundation. L.A. was supported by French National Institute of Health and Medical Research.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Urine CXCL10/IP-10 Fingers Ongoing Antibody-Mediated Kidney Graft Rejection,” on pages 2607–2609.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014080797/-/DCSupplemental.
References
- 1.Anglicheau D, Suthanthiran M: Noninvasive prediction of organ graft rejection and outcome using gene expression patterns. Transplantation 86: 192–199, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suthanthiran M, Schwartz JE, Ding R, Abecassis M, Dadhania D, Samstein B, Knechtle SJ, Friedewald J, Becker YT, Sharma VK, Williams NM, Chang CS, Hoang C, Muthukumar T, August P, Keslar KS, Fairchild RL, Hricik DE, Heeger PS, Han L, Liu J, Riggs M, Ikle DN, Bridges ND, Shaked A, Clinical Trials in Organ Transplantation 04 (CTOT-04) Study Investigators : Urinary-cell mRNA profile and acute cellular rejection in kidney allografts. N Engl J Med 369: 20–31, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hauser IA, Spiegler S, Kiss E, Gauer S, Sichler O, Scheuermann EH, Ackermann H, Pfeilschifter JM, Geiger H, Gröne HJ, Radeke HH: Prediction of acute renal allograft rejection by urinary monokine induced by IFN-gamma (MIG). J Am Soc Nephrol 16: 1849–1858, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Ho J, Rush DN, Karpinski M, Storsley L, Gibson IW, Bestland J, Gao A, Stefura W, HayGlass KT, Nickerson PW: Validation of urinary CXCL10 as a marker of borderline, subclinical, and clinical tubulitis. Transplantation 92: 878–882, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Hricik DE, Nickerson P, Formica RN, Poggio ED, Rush D, Newell KA, Goebel J, Gibson IW, Fairchild RL, Riggs M, Spain K, Ikle D, Bridges ND, Heeger PS, CTOT-01 consortium : Multicenter validation of urinary CXCL9 as a risk-stratifying biomarker for kidney transplant injury. Am J Transplant 13: 2634–2644, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu H, Aizenstein BD, Puchalski A, Burmania JA, Hamawy MM, Knechtle SJ: Elevation of CXCR3-binding chemokines in urine indicates acute renal-allograft dysfunction. Am J Transplant 4: 432–437, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Hu H, Kwun J, Aizenstein BD, Knechtle SJ: Noninvasive detection of acute and chronic injuries in human renal transplant by elevation of multiple cytokines/chemokines in urine. Transplantation 87: 1814–1820, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Jackson JA, Kim EJ, Begley B, Cheeseman J, Harden T, Perez SD, Thomas S, Warshaw B, Kirk AD: Urinary chemokines CXCL9 and CXCL10 are noninvasive markers of renal allograft rejection and BK viral infection. Am J Transplant 11: 2228–2234, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matz M, Beyer J, Wunsch D, Mashreghi MF, Seiler M, Pratschke J, Babel N, Volk HD, Reinke P, Kotsch K: Early post-transplant urinary IP-10 expression after kidney transplantation is predictive of short- and long-term graft function. Kidney Int 69: 1683–1690, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Schaub S, Nickerson P, Rush D, Mayr M, Hess C, Golian M, Stefura W, Hayglass K: Urinary CXCL9 and CXCL10 levels correlate with the extent of subclinical tubulitis. Am J Transplant 9: 1347–1353, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Hirt-Minkowski P, Amico P, Ho J, Gao A, Bestland J, Hopfer H, Steiger J, Dickenmann M, Burkhalter F, Rush D, Nickerson P, Schaub S: Detection of clinical and subclinical tubulo-interstitial inflammation by the urinary CXCL10 chemokine in a real-life setting. Am J Transplant 12: 1811–1823, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Sellarés J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, Hidalgo LG, Famulski K, Matas A, Halloran PF: Understanding the causes of kidney transplant failure: The dominant role of antibody-mediated rejection and nonadherence. Am J Transplant 12: 388–399, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Matignon M, Ding R, Dadhania DM, Mueller FB, Hartono C, Snopkowski C, Li C, Lee JR, Sjoberg D, Seshan SV, Sharma VK, Yang H, Nour B, Vickers AJ, Suthanthiran M, Muthukumar T: Urinary cell mRNA profiles and differential diagnosis of acute kidney graft dysfunction. J Am Soc Nephrol 25: 1586–1597, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sellares J, Reeve J, Loupy A, Mengel M, Sis B, Skene A, de Freitas DG, Kreepala C, Hidalgo LG, Famulski KS, Halloran PF: Molecular diagnosis of antibody-mediated rejection in human kidney transplants. Am J Transplant 13: 971–983, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Anglicheau D, Loupy A, Suberbielle C, Zuber J, Patey N, Noël LH, Cavalcanti R, Le Quintrec M, Audat F, Méjean A, Martinez F, Mamzer-Bruneel MF, Thervet E, Legendre C: Posttransplant prophylactic intravenous immunoglobulin in kidney transplant patients at high immunological risk: A pilot study. Am J Transplant 7: 1185–1192, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Loupy A, Lefaucheur C, Vernerey D, Chang J, Hidalgo LG, Beuscart T, Verine J, Aubert O, Dubleumortier S, Duong van Huyen JP, Jouven X, Glotz D, Legendre C, Halloran PF: Molecular microscope strategy to improve risk stratification in early antibody-mediated kidney allograft rejection. J Am Soc Nephrol 25: 2267–2277, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panzer U, Steinmetz OM, Reinking RR, Meyer TN, Fehr S, Schneider A, Zahner G, Wolf G, Helmchen U, Schaerli P, Stahl RA, Thaiss F: Compartment-specific expression and function of the chemokine IP-10/CXCL10 in a model of renal endothelial microvascular injury. J Am Soc Nephrol 17: 454–464, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Colvin RB: Antibody-mediated renal allograft rejection: Diagnosis and pathogenesis. J Am Soc Nephrol 18: 1046–1056, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Tait BD, Süsal C, Gebel HM, Nickerson PW, Zachary AA, Claas FH, Reed EF, Bray RA, Campbell P, Chapman JR, Coates PT, Colvin RB, Cozzi E, Doxiadis II, Fuggle SV, Gill J, Glotz D, Lachmann N, Mohanakumar T, Suciu-Foca N, Sumitran-Holgersson S, Tanabe K, Taylor CJ, Tyan DB, Webster A, Zeevi A, Opelz G: Consensus guidelines on the testing and clinical management issues associated with HLA and non-HLA antibodies in transplantation. Transplantation 95: 19–47, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Parikh CR, Thiessen-Philbrook H: Key concepts and limitations of statistical methods for evaluating biomarkers of kidney disease. J Am Soc Nephrol 25: 1621–1629, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altman DG, Bland JM: Diagnostic tests 2: Predictive values. BMJ 309: 102, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, Halloran PF, Baldwin W, Banfi G, Collins AB, Cosio F, David DS, Drachenberg C, Einecke G, Fogo AB, Gibson IW, Glotz D, Iskandar SS, Kraus E, Lerut E, Mannon RB, Mihatsch M, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Roberts I, Seron D, Smith RN, Valente M: Banff 07 classification of renal allograft pathology: Updates and future directions. Am J Transplant 8: 753–760, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Lefaucheur C, Loupy A, Hill GS, Andrade J, Nochy D, Antoine C, Gautreau C, Charron D, Glotz D, Suberbielle-Boissel C: Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J Am Soc Nephrol 21: 1398–1406, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.