Abstract
The myeloid differentiation protein 88 (MyD88) adapter protein is an important mediator of kidney allograft rejection, yet the precise role of MyD88 signaling in directing the host immune response toward the development of kidney allograft rejection remains unclear. Using a stringent mouse model of allogeneic kidney transplantation, we demonstrated that acute allograft rejection occurred equally in MyD88-sufficient (wild-type [WT]) and MyD88−/− recipients. However, MyD88 deficiency resulted in spontaneous diminution of graft infiltrating effector cells, including CD11b−Gr-1+ cells and activated CD8 T cells, as well as subsequent restoration of near-normal renal graft function, leading to long-term kidney allograft acceptance. Compared with T cells from WT recipients, T cells from MyD88−/− recipients failed to mount a robust recall response upon donor antigen restimulation in mixed lymphocyte cultures ex vivo. Notably, exogenous IL-6 restored the proliferation rate of T cells, particularly CD8 T cells, from MyD88−/− recipients to the proliferation rate of cells from WT recipients. Furthermore, MyD88−/− T cells exhibited diminished expression of chemokine receptors, specifically CCR4 and CXCR3, and the impaired ability to accumulate in the kidney allografts despite an otherwise MyD88-sufficient environment. These results provide a mechanism linking the lack of intrinsic MyD88 signaling in T cells to the effective control of the rejection response that results in spontaneous resolution of acute rejection and long-term graft protection.
Keywords: acute allograft rejection, kidney transplantation, lymphocytes, renal, function, transgenic mouse
Alloimmune-mediated injuries remain important predictors for late kidney allograft loss.1,2 While the host adaptive immune response is primarily responsible for allograft rejection, emerging evidence supports an important role of innate immunity in the development of allograft rejection.
Innate immune response to toll-like receptor (TLR) activation has been shown to be an important modulator of the adaptive immune response.3–5 Myeloid differentiation protein 88 (MyD88) is a cytoplasmic adaptor protein responsible for signal transduction through most TLRs, as well as the IL-1R/IL-18R family.5–7 Activation of the MyD88-dependent pathway triggers a downstream signaling cascade that leads to NF-κB activation and production of inflammatory cytokines, such as IL-6 and TNF-α, as well as chemokine ligand 2 (CCL2), which is important for leukocyte recruitment.8,9 While the significance of MyD88 has been more thoroughly examined in the innate immune system, recent studies have shown that MyD88 plays a T cell–intrinsic role in regulating the survival and accumulation of antigen-specific effector T cells during bacterial and viral infections.10
The role of MyD88 in transplant rejection was first studied in a mouse skin transplant model by MyD88 genetic deletion.11 This study demonstrated that MyD88-dependent signaling was critical for the rejection of minor antigen mismatched skin allografts by regulating dendritic cell (DC) maturation and alloreactive T cell priming. Subsequent studies suggested that MyD88 plays distinct roles depending on the specific organ or tissue transplanted. For example, MyD88 signaling was dispensable for rejection in a fully mismatched skin transplant model despite Th1 cytokines being diminished and only marginally responsible for prolonged cardiac allograft survival.12 In contrast, abrogation of MyD88 facilitated donor-specific tolerance to kidney allografts.13 Consistent with the role of MyD88 in promoting kidney allograft injury, endogenous TLR ligands were upregulated in kidney allografts.14 Clinically, MyD88 expression in peripheral blood mononuclear cells was significantly increased in patients with chronic rejection compared with those operationally tolerant.15 These findings highlight the important role of MyD88 in promoting kidney allograft inflammation and injury. However, the precise role of MyD88 signaling in the development of kidney allograft rejection remains elusive.
The present study examined whether recipient MyD88 is required for acute kidney allograft rejection and whether T cell intrinsic MyD88 signaling regulates the host immune response following kidney transplantation. We demonstrate that recipient MyD88 was not required for the initiation of an alloimmune response against kidney allografts but rather was critical in sustaining the progression of this response. Lack of intrinsic T cell MyD88 signaling contributed to compromised T cell secondary expansion, their chemokine receptor expression, and accumulation in the kidney allografts.
Results
Recipient MyD88 Deficiency Protects Full MHC-Mismatched Kidney Allografts
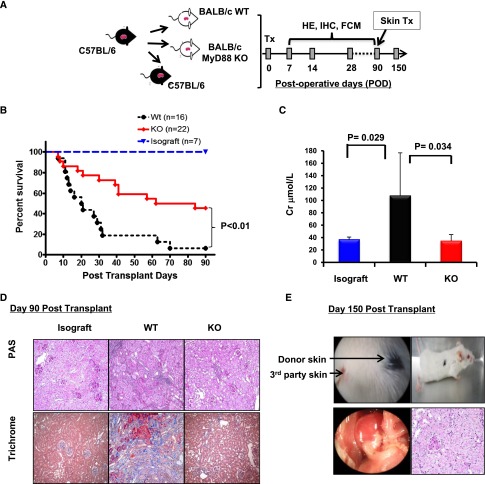
We utilized a life supporting kidney transplant model in which B6 kidneys were transplanted into full MHC-mismatched (allografts), bilaterally nephrectomized BALB/c WT, or MyD88−/− recipients or syngeneic B6 control mice (isografts) (Figure 1A) without additional immunotherapy. Consistent with previous reports,16–18 most (11/16) WT recipients died of renal allograft failure within postoperative day (POD) 10–30, with a median survival time of 20.5 days (Figure 1B). In contrast, fewer MyD88−/− recipients (5/22) experienced graft failure within POD30 (median survival time, 84 days; P≤0.001 versus WT recipients), and remarkably, approximately 45% of the MyD88−/− recipients (10 of 22) lived beyond POD90 with creatinine levels similar to the isografts (Figure 1C). Histologic examination revealed that at POD90, kidney allografts from MyD88−/− recipients had minimal cellular infiltration and preserved tubular and glomerular integrity (Figure 1D). In contrast, allografts from the remaining WT recipients showed severe ongoing cellular rejection as demonstrated by marked mononuclear cellular infiltration, tubulits, and early signs of chronic rejection, including tubular atrophy and increased collagen deposition indicating glomerular and interstitial fibrosis.
Figure 1.
MyD88 deficiency in kidney allograft recipients results in long-term graft acceptance. (A) Experimental design: WT B6 kidneys were transplanted into binephrectomized allogeneic WT BALB/c or MyD88 KO BALB/c mice (allografts), or syngeneic WT B6 mice (isografts). The recipients were analyzed for survival, renal function (RF), histology (HE), and immunohistochemistry (IHC) at the preselected day after transplant as indicated. Additional recipients surviving at POD90 received donor-matched skin grafts from B6 mice or unmatched skin grafts (third party) from C3H mice. (B) Kaplan-Meier survival curve, comparing survival time of kidney allograft recipients. Three groups of mice received kidney transplants: (1) B6 to WT: B6 donor kidney transplanted to WT BALB/c mice, (2) B6 to knockout (KO): B6 donor kidney to MyD88−/− BALB/c mice, (3) WT isograft: B6 donor kidney to B6 mice. (C) Serum creatinine levels at the endpoint of rejection (severe sickness) or at 90 days after transplantation. (D) Representative histologic sections of periodic acid-Schiff (PAS) and trichrome staining of isografts, and allografts from WT or MyD88−/− recipients. Original magnification, ×20. Histology is representative of allografts from 3 WT recipients and 9 Myd88−/− recipients. (E) Donor-matched skin allografts transplanted on the backs of MyD88−/− recipients that had accepted a kidney for 150 days. Macroscopic image and representative histologic section of periodic acid-Schiff staining of kidney allografts harvested from MyD88−/− recipients that accepted the kidneys for 150 days. Results are representative of two independent experiments.
To examine whether long-term surviving MyD88−/− recipients developed donor-specific tolerance, donor (B6) or third-party (C3H) skin allografts were transplanted onto MyD88−/− recipients (n=3) with functioning B6 kidney allografts at POD90. While all third-party skin allografts were readily rejected by POD14, MyD88−/− recipients accepted donor-matched skin allografts for >60 days after skin transplant with intact kidney allografts, reflected by normal gross appearance and histology of the grafts at POD150 (Figure 1E), indicating a state of donor-specific tolerance.
Early Inflammation and Compromised Kidney Allograft Function Are Observed in Both WT and MyD88−/− Recipients
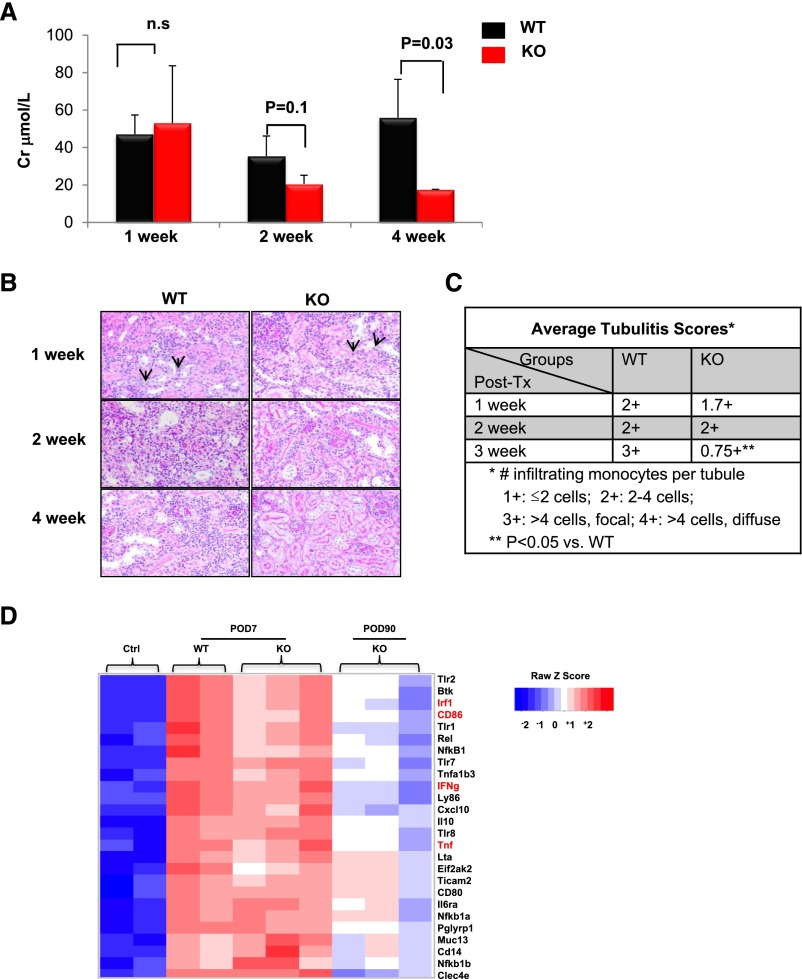
To investigate the effect of recipient MyD88 in the development of kidney allograft rejection, we conducted sequential analyses of kidney allograft function and morphology (Figure 2, A and B). Creatinine levels were equally elevated in WT and MyD88−/− recipients at 1 week after transplant. However, the MyD88−/− recipients exhibited a gradual decline of creatinine levels with significantly decreased creatinine levels by 4 weeks after transplant. Parallel to the functional change, kidney allografts in WT and MyD88−/− recipients exhibited similar patterns of acute rejection, as evidenced by diffuse mononuclear cellular infiltration and tubulitis at 1-week after transplant, and the extent of the rejection was progressively increased in WT recipients at 2 and 4 weeks. Conversely, in MyD88−/− recipients, cellular infiltration was noticeably reduced starting from 2 weeks after transplant, and acute rejection was minimized by 4 weeks after transplant, as indicated by significantly decreased tubulitis scores (Figure 2C).
Figure 2.
Early immune response is similar in both WT and MyD88−/− allografts. (A) Serum creatinine (Cr) levels in WT or MyD88−/− knockout (KO) recipient mice 1, 2, or 4 weeks after transplant. Results are expressed as the mean (±SEM) of at least 4 independent experiments. n.s., not significant. (B) Representative histologic sections (periodic acid-Schiff staining) of kidney graft infiltrating leukocytes in WT or MyD88−/− allograft mice 1, 2, or 4 weeks after transplant. Original magnification, ×20. n=≥4 mice per group. (C) Tubulitis scores as determined by the number of infiltrating cells per tubule. (D) Heat map representing color-coded expression levels of different genes within the allograft (up- or downregulated >2-fold) from four groups: naive kidneys, not transplanted (ctrl 1 and 2), allografts from WT recipients 7 days after transplantation (WT D7 1 and 2), allografts from MyD88−/− recipients 7 days after transplantation (KO D7 1, 2 and 3), and allografts from MyD88−/− recipients 90 days after transplantation (KO D90 1, 2 and 3). Red indicates overexpressed genes (expression levels over the median) and blue indicates under-expressed genes (expression levels under the median; see legend). Data are shown in a matrix format: Each row represents a single gene, and each column represents a mouse. Because few WT recipients ever survived beyond POD90, they were not included in the analysis.
Furthermore, we compared targeted gene expression patterns in kidney allografts and found that the gene expression profiles were similar in kidney allografts from WT and MyD88−/− recipients at POD7 (Figure 2C). Specifically, the intragraft expression of the inflammatory genes Irf1 (Ifn regulatory factor 1), IFN-γ (interferon γ), tnf , and CD86 were upregulated at POD7 in both MyD88−/− and WT recipients. However, in long-term protected kidney allografts of MyD88−/− recipients at POD90, the gene expression profile returns to resemble that of a control kidney in syngeneic recipients. The intragraft expression of Irf1, IFN-γ, tnf and CD86 were all significantly downregulated by POD90 in MyD88−/− recipients to levels close to those observed in the controls.
Collectively, these results suggest that recipient MyD88 deficiency does not prevent the initiation of acute kidney allograft rejection; rather, it promotes a spontaneous resolution of acute rejection.
MyD88−/− Recipients Have Significantly Reduced Numbers of Kidney Graft–Infiltrating Cells Following Initial Influx
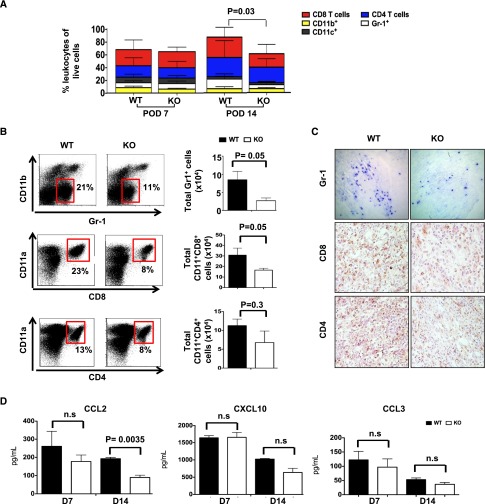
To determine the cellular basis for the clearing of infiltration in the MyD88−/− recipients, we performed phenotypic analysis on the graft-infiltrating cells. As shown in Figure 3A, both CD8 and CD4 T lymphocytes were the major graft-infiltrating populations, as expected. Other graft-infiltrating cells include CD11b+ myeloid cells, CD11b−Gr1+ granulocytes and CD11c+ DCs. At POD7, the composition and the number of total graft-infiltrating leukocytes were similar between WT and MyD88−/− recipients. However, The total number of graft-infiltrating leukocytes was significantly decreased in MyD88−/− recipients at POD14 (Figure 3A). Specifically, the numbers of granulocytes and activated CD8 T cells (CD8+CD11a+), but not CD4 T cells, were significantly decreased in the grafts from MyD88−/− recipients (Figure 3B). These findings were confirmed by immunohistochemistry (Figure 3C). No difference was observed in the spleens of WT and MyD88−/− recipients (not shown). We further investigated whether chemokines, including CCL2 (T cells and granulocytes), CXCL10 (T cells), and CCL3 (granulocytes), were implicated in the diminished allograft-infiltrating T cells and granulocytes in MyD88−/−recipients. As shown in Figure 3D, kidney allografts from MyD88−/− recipients at POD14 had a markedly diminished level of CCL2, but not CXCL10 or CCL3 compared with WT recipients.
Figure 3.
MyD88−/− allograft recipients have significantly decreased cellular infiltration 14 days after transplantation. Kidney grafts from WT or MyD88−/− recipients were harvested 7 or 14 days after transplantation as indicated. For flow cytometry, single cell suspensions were made following collagenase treatment. (A) Bar graphs showing the immune cell composition of kidney allografts from WT and MyD88−/− recipients 7 days and 14 days after transplantation. Results are expressed as the mean of at least three different experiments. (B) The percentages of CD8+CD11a+ T cells (top left panels), CD4+CD11a+ T cells (middle left panels), or CD11b-Gr-1+ cells (bottom left panels) among total lymphocytes and corresponding absolute cell number for each of the three populations per kidney in WT or MyD88−/− recipients (right panels). (C) Representative histologic sections of immunochemistry staining for CD4+, CD8+, or Gr-1+ cells in allografts from WT or MyD88−/− recipients. Original magnification, ×20. (D) Graft chemokine (CCL2, CXCL10, and CCR3) detection by Luminex bead assay at 7 or 14 days after transplantation. Data shown are representative of at least three independent experiments, with one to three mice per group. KO, knockout.
MyD88−/− Recipients Exhibit Compromised T Cell Recall Response
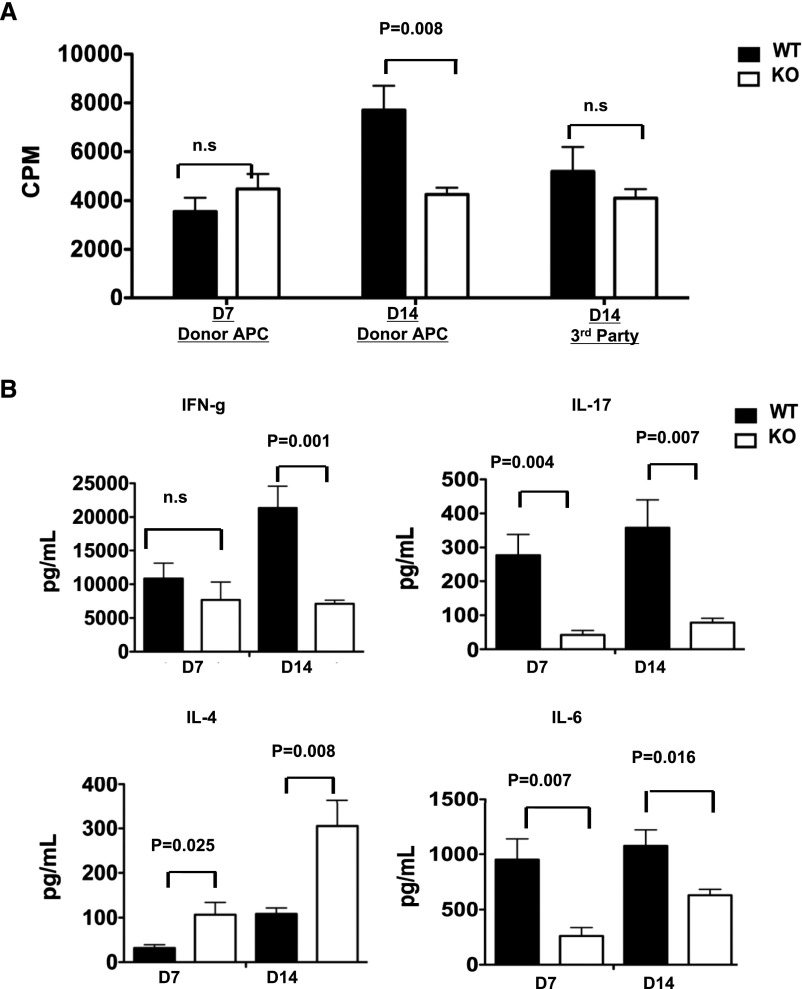
To determine whether lack of MyD88 signaling alters allogeneic responses, we performed ex vivo allogenic MLR analyses using WT donor antigen-presenting cells (APCs) as stimulators. Naive T cells from MyD88−/− or WT untransplanted mice showed no difference in their proliferation capabilities or cytokine production responding to WT stimulators (Supplemental Figure 1). We therefore hypothesized that MyD88 deficiency primarily influences secondary but not primary T cell responses to donor antigens, therefore permitting the initial antidonor priming, but subsequently compromising the recall response and secondary expansion and thus persistent intragraft accumulation of effector cells. To test this, we examined T cell recall responses from MyD88−/− and WT recipients to the WT donor APCs. As shown in Figure 4A, T cells from MyD88−/− and WT recipients at POD7 demonstrated similar proliferation capabilities to donor APCs. However, at POD14, the MyD88−/− T cells exhibited a hypo-proliferative response to donor APCs compared with the WT T cells, but not to third-party APCs (Figure 4A). Notably, supernatant from the MLRs showed that at POD7, the MyD88−/− T cells already exhibited markedly diminished IL-17 and IL-6 production but heightened IL-4 production (Figure 4B). This trend persisted at POD14, with an additional decrease in IFN-γ production by the MyD88−/− T cells. These findings indicate that intrinsic T cell MyD88 signaling is not required for the primary alloantigen-mediated T cell response, but plays a role in sustaining the secondary T cell expansion and effector function.
Figure 4.
T cells from MyD88−/− recipient mice display decreased proliferative capacity and effector function at 14 days, but not 7 days, after transplantation. (A) MLRs were set up using T cells isolated from the spleens of WT or MyD88−/− kidney allograft recipients 7 or 14 days after transplantation. Responding T cells were stimulated with donor B6 APCs or third-party SJL APCs as indicated. Proliferation was assessed by [3H] thymidine uptake during the last 18 hours of a 5-day MLR. (B) Culture supernatants from the MLR stimulated with donor APCs as in part A were collected immediately before thymidine pulse and analyzed for the presence of IFN-γ, IL-4, IL-6, and IL-17 by Luminex bead assay. Results represent two independent experiments with two to three mice per group. KO, knockout.
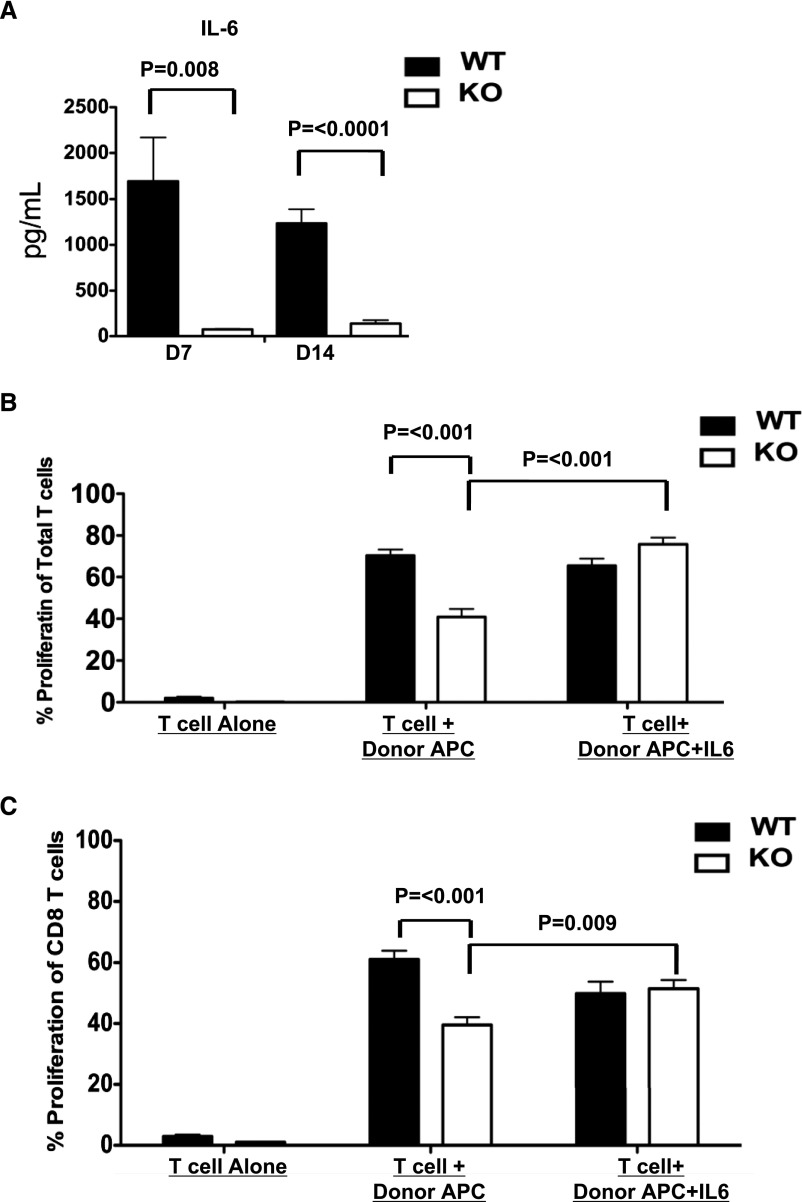
Lack of Intrinsic IL-6 Contributes to Defects in Sustained T cell Proliferation and Accumulation in the Kidney Allografts of MyD88−/− Recipients
IL-6, a cytokine produced downstream from MyD88 signaling after NF-κB activation, was shown to play a critical role in expansion and survival of activated T cells.19 Consistent with the diminished IL-6 production by T cells from MyD88−/− recipients in MLRs (Figure 4B), the intragraft IL-6 expression was also significantly decreased in the kidney allografts in MyD88−/− recipients compared with the allografts from WT at both POD7 and 14 (Figure 5A). To examine whether compromised IL-6 production underlies the hypoproliferative response seen in T cells from MyD88−/− recipients, we added IL-6 to the MLR as in Figure 4A. The exogenous IL-6 restored the proliferation of T cells (Figure 5B), specifically CD8 T cells (Figure 5C), from MyD88−/− recipients to that of T cells from WT recipients. Because the APCs in the MLRs were from MyD88 sufficient donor B6 mice, these data suggest that a T cell intrinsic defect in IL-6 production contributes to the compromised recall response of the MyD88−/− T cells.
Figure 5.
IL-6 is decreased in the grafts of MyD88−/− recipient mice and exogenous IL-6 restores the proliferative capacity of T cells from MyD88−/− recipients. (A) Kidney allografts were removed from WT or MyD88−/− recipients 7 and 14 days after transplantation. Tissue extracts were prepared from homogenized tissue for detection of IL-6 by Luminex bead assay. (B and C) Mixed lymphocyte reactions were setup using CFSE-labeled T cells (B) or MACS purified CD8 T cells (C) isolated from the spleens of WT or MyD88−/− kidney allograft recipients 14 days after transplantation. T cells cultured alone from WT or MyD88−/− recipients without any stimulation were used as negative control. The T cells were stimulated with donor B6 APCs alone or donor B6 APCs +rIL-6 (10 ng/ml). Cultures were harvested on day 6, and cell proliferation was quantified by CFSE dilution. Data shown are representative of three independent experiments. KO, knockout.
Intrinsic MyD88 Signaling in CD8 T Cells Is Required for Chemokine Receptor CCR4 and CXCR3 Expression and Effector Cell Accumulation in Kidney Allografts
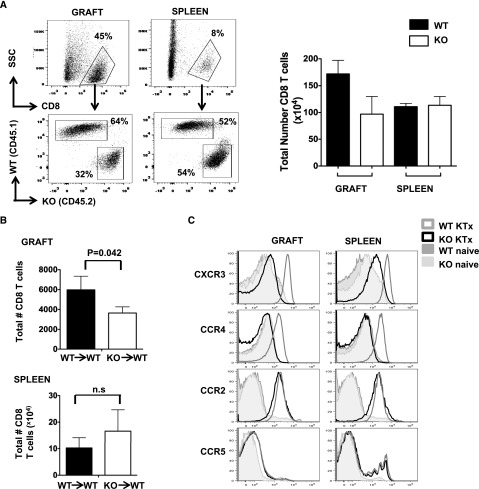
To further determine whether T cell intrinsic MyD88 signaling controls the intragraft effector cell accumulation, 5,6-carboxyfluoresceine diacetate succinimidyl ester (CFSE)–labeled naive BALB/c WT (CD45.1+) and MyD88−/− (CD45.2+) CD8 T cells were cotransferred at a 1:1 ratio into naive untransplanted BALB/c RAG1−/− mice (CD45.2+, lacking T and B cells) or BALB/c RAG1−/− recipients of B6 kidney allografts, and examined at 7 and 10 days after adoptive transfer. In the untransplanted RAG1−/− mice, both MyD88−/− and WT CD8 T cells were equally activated and proliferated in the spleens, as indicated by loss of CFSE staining (data not shown), downregulated CD62L expression, and a similar number of CD8 T cells (Supplemental Figure 2, A and B), likely due to the homeostatic expansion commonly observed in RAG−/− mice.20 Negligible numbers of CD8 T cells were recovered from the kidneys of the untransplanted mice (Supplemental Figure 2B). In contrast, allogeneic kidney transplant markedly increased the number of total CD8 T cells in spleens of the RAG−/− recipients by approximately 5-fold compared with the untransplanted mice, although no difference was observed between MyD88−/− and WT CD8 T cells at day 7 (Supplemental Figure 3) or day 10 (Figure 6A). Among the large number of CD8 T cells observed in the kidney allografts, MyD88−/− CD8 T cell accumulation was significantly diminished compared with that of WT CD8 T cells (Figure 6A) at day 10, even though similar infiltration was observed at 7 days after transplant. Similar findings were also observed in the immune-competent (as opposed to RAG−/−) BALB/c recipients (CD45.1) that were transferred with CD45.2 WT CD8 T cells or MyD88−/− CD8 T cells (Figure 6B). These data suggest that CD8 T cell intrinsic MyD88 signaling is important for their accumulation and retention in the kidney allografts.
Figure 6.
CD8 T cells from MyD88−/− mice are defective in homing to the kidney allograft. (A) RAG−/− mice on a BALB/c background (CD45.2+) received kidney allografts from B6 donor mice. The mice were allowed to recover for 14 days before CFSE-labeled naive BALB/c WT (CD45.1+) and MyD88−/− (CD45.2+) CD8 T cells were cotransferred at a 1:1 ratio (5×106 WT and 5×106 MyD88−/− cells/mouse intravenously). Ten days after adoptive transfer, the kidney grafts and spleens were harvested and the percentage (representative dot plots on left) and absolute number (bar graph on right) of congenically marked WT and MyD88−/− CD8 T cells were determined by flow cytometry. Results are representative of two to three independent experiments. (B) CFSE labeled congenic CD8 T cells derived from naive WT (CD45.1) or naive MyD88−/− (CD45.2) mice were adoptively transferred into the indicated WT or MyD88−/− immunocompetent BALB/c recipient mice (5×106/mouse intravenously). Twenty-four hours after adoptive transfer, the recipient mice received B6 kidney allografts. Ten to 12 days after transplant, the kidney grafts and spleens were harvested and the absolute number of congenically marked donor CD8 T cells were determined. Results are representative of four independent experiments. (C) Adoptively transferred CD8 T cells present in the spleens or kidney allografts from RAG−/− recipient mice as in part A were analyzed for chemokine expression. For each histogram, cells were first gated on total CD8 T cells followed by gating of the specific congenic markers CD45.1+ (WT) or CD45.2+ (MyD88−/−). Naive CD8 T cells before adoptive transfer are shown for comparison (open dark gray histogram, WT; shaded histogram, MyD88−/−). Results are representative of three independent experiments. KO, knockout; KTx, kidney transplant.
Chemokine receptors (CCR2, CCR4, CCR5, and CXCR3) are differentially expressed on T cells upon activation and regulate T cell trafficking, differentiation, and survival.21–24 To determine whether MyD88 deficiency altered chemokine receptor expression, adoptively transferred CD8 T cells from the RAG1−/− recipients were analyzed at 10 days after adoptive transfer. Consistent with previous reports,25,26 CXCR3, CCR4, CCR2, and CCR5 were not detected on naive CD8 T cells before adoptive transfer, regardless of MyD88 expression (Figure 6C). Upon allogeneic kidney transplantation, WT CD8 T cells significantly upregulated their expression of CXCR3 and CCR4 in the spleens as well as in the kidney allografts. In contrast, MyD88−/− CD8 T cells failed to induce expression of CXCR3 and CCR4 in spleens or kidney allografts. No difference was observed in CCR2 and CCR5 expression. These results indicate that intrinsic MyD88 expression in CD8 T cells is essential for CXCR3 and CCR4 expression in response to allogeneic stimulation.
Discussion
MyD88 is known to indirectly regulate T cell responses through influencing inflammatory cytokine production and alloantigen presentation by innate immune cells.11,12,27 In this study we hypothesized a direct role for MyD88, specifically in recipient CD8 T cells, in controlling rejection response to kidney allografts. We demonstrate that recipient MyD88 deficiency does not influence the initial rejection response against the kidney allografts. Abrogation of recipient MyD88, however, results in a diminished accumulation of allograft-infiltrating effector cells, leading to a spontaneous resolution of acute rejection and long-term graft acceptance. MyD88 is not required for primary T cell activation and proliferation but is critical for T cells to mount robust recall responses. The adoptively transferred MyD88−/− CD8 T cells failed to accumulate in the kidney allograft of the WT recipients, supporting that an intrinsic defect of the MyD88−/− CD8 T cells likely contributes to their spontaneous diminution. Therefore, this study highlights the important role of MyD88 in directly regulating T cell responses in kidney allograft rejection.
Essential to the development of acute rejection, effector T cells, particularly activated CD8 T cells, are rapidly generated and continuously recruited to the kidney allografts. Sustained T cell responses are necessary to perpetuate allograft injury. Our findings suggest that T cell intrinsic defects in sustained expansion and effector function may be a mechanism underlying their compromised accumulation in MyD88−/− recipients following the initial influx. We show that naive MyD88−/− T cells proliferated in the same capacity as WT T cells to alloantigen stimulation, whereas T cells from MyD88−/− kidney transplant recipients exhibited compromised proliferation and effector cytokine production upon alloantigen restimulation. These findings indicate that MyD88 deficiency influences the quality of T cell activation and their recall response rather than their primary response. Corroborating our findings, others have shown that MyD88 signaling within CD8 T cells is required for their survival and clonal expansion upon viral infection.20,27 Furthermore, we demonstrated that exogenous IL-6 rescued the proliferation of Myd88−/− T cells to restimulation, suggesting that the impaired T cell recall response may be attributed to a defect in IL-6 production.28 IL-6 is a pleiotropic cytokine that can be induced via MyD88 signaling in response to several stimuli, such as IL-1, TNF-α, and LPS.29–34 Elevated IL-6 in blood and urine was correlated with rejection in transplant recipients.35,36 IL-6 is produced by a variety of cells, such as APCs, endothelial cells (ECs), and activated T cells.31,37 Because the APCs used in our ex vivo IL-6 culture system were identical for stimulating WT or MyD88−/− T cells, we speculate that the observed defect in IL-6 production was intrinsic to the MyD88−/− T cells.
Generally, recruitment and retention of effector T cells into transplanted organs are governed in a complex manner by surface expression of chemokine receptors and specific ligands.38,39 Using the adoptive-transfer approach, we show that compromised accumulation of MyD88−/− CD8 T cells coincided with a lack of CXCR3 and CCR4 expression on these cells. CXCR3, a receptor for inflammatory chemokines CXCL10 (also known as interferon γ-induced protein 10, or IP-10) and CXCL9 (also known as monokine induced by γ interferon, or MIG),40 is preferentially expressed on activated CD8 T cells and Th1 cells, whereas CCR4 is mainly expressed on Th2 cells and may participate in skin-specific lymphocyte recruitment through its ligand thymus and activation-regulated chemokine.41 CXCR3−/− effector CD8 T cells show a significant defect in migrating to inflamed organs, including allografts.42,43 In addition, CXCR3 signaling plays a role in the development and survival of effector T cells.44,45 We postulate that the defect in the chemokine receptor expression may contribute to the diminished accumulation of allograft-infiltrating CD8 T cells in MyD88−/− recipients through influencing retention or survival, rather than recruitment of activated CD8 T cells because initial infiltration of these cells was not affected by MyD88 deficiency. Mechanisms by which MyD88 regulates the chemokine receptor expression and function warrant further investigation.
An alternative explanation for the diminished graft-infiltrating cells in MyD88−/− recipients lies in the function of the innate cells in the grafts. IL-6 activates DCs and ECs to induce their production of chemokines.46 IL-6−/− DCs induced less proliferation of T cells than WT DCs.47 Therefore, the decreased intragraft IL-6 (Figure 5A) might alter the function of DCs and ECs, which in turn fail to stimulate T cells. Consistent with this notion, CD11b−Gr-1+ granulocyte infiltration was reduced in the grafts of MyD88−/− recipients, preceding the decrease in CD8 T cells. Moreover, chemokine CCL2 level was significantly reduced in the kidney allografts. CCL2 is critical in neutrophil recruitment to sites of inflammation,48 as well as in the recruitment and differentiation of antigen-specific CD8 T cells.49 Collectively, our data point to the possibility that downregulation of intragraft CCL2 in MyD88−/− recipients impairs Gr-1+ cell recruitment followed by a decrease of graft-infiltrating CD8 T cells.
Mechanisms of action responsible for MyD88-dependent immune regulation may be influenced by genetic backgrounds of the recipients.50 Wu et al. showed that MyD88 deficiency led to an altered balance of Tregs over Th17 cells when B6 mice were used as recipients of kidney allografts.13 Data from our study using BALB/c recipients corroborate their findings but further demonstrate a relatively intact early alloimmune response followed by a spontaneous control of this response associated with an intrinsic defect in the MyD88−/− T cells.
In summary, recipient MyD88 deficiency results in spontaneous resolution of acute rejection of the kidney allografts and long-term donor-specific tolerance. Extending from previous reports, this study supports a T cell intrinsic role of MyD88 in the secondary expansion of allo-activated T cells and their accumulation in the kidney allograft. Targeting MyD88 signaling thus offers a potential tailored antirejection therapy for kidney transplant patients.
Concise Methods
Mice
Male BALB/c (H2d), B6 (H2b) donor, C3H (H2k), and SJL (H-2s) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). MyD88−/− on the BALB/c background was originally obtained from Dr. Shizuo Akira, MD, PhD (Osaka University, Osaka, Japan). Breeders of CD45.1/Ly5.1 and RAG-2−/−, both on the BALB/c background, were purchased from The Jackson Laboratory. All mice were used according to protocols approved by the Internal Animal Care and Use Committee of Northwestern University.
Kidney Transplantation
Kidneys from WT B6 donor mice were transplanted into binephrectomized WT BALB/c or MyD88−/− recipients, respectively, following the procedures previously described.51 Briefly, the donor aorta and inferior vena cava were anastomosed end to side to the recipient abdominal aorta and inferior vena cava below the level of the native renal vessels, respectively. The donor bladder patch was anastomosed dome to dome to the bladder of the recipient. The native right kidney was removed before revascularization while the recipients’ left kidney was removed immediately before the wound closure; therefore, recipients’ survival depends on the transplanted kidney. No immunosuppressive therapy was administered.
Assessment of Renal Graft Function and Rejection
Recipients were monitored daily for survival and renal function. Renal function was assessed by measuring whole blood creatinine levels by using an I-STAT handset analyzer (Abaxis, United City, CA). The endpoint of study was defined as >90 days of survival, recipient death, or when recipients developed clinical signs of renal failure or severe rejection. Tissues and blood samples were collected at the end of the study for renal function and pathohistologic examinations. Additional transplant recipients were euthanized at preselected earlier time points for sequential functional, immunologic, and pathohistologic analysis.
Skin Transplantation
Full-thickness tail skin grafts (from B6 donor or third-party SJL mice) were placed on the backs of MyD88−/− recipients that had accepted kidney allografts for ≥90 days. Grafts were covered with protective bandages for 7 days. Grafts were examined daily and considered rejected when >80% necrosis was observed.
Cell Purification and Mixed Lymphocyte Reactions
To obtain single cell-suspensions from kidney allografts, kidneys were minced up before being subject to digestion with collagenase IV (Worthington Biochemical Corp.). Cells were washed and filtered once more before being used in the assays as described. Spleen cells were also isolated using standard methods.52
Mixed lymphocyte reactions (MLRs) were set up using 1×105 splenic T cells (responders, purity >85%) from naive mice as well as MyD88−/− or WT transplant recipient mice. To purify T cells, splenocytes from these mice were negative-selected by exclusion of NK1.1+, Gr-1+, CD11b+, CD11c+, and B220+ cells. A total of 5×105 irradiated B6 (donor) or SJL (third-party) splenic APCs per well were added to responder T cells and cocultured for 5 days in complete RPMI-1640 medium with 10% FCS. Proliferation was assessed by [3H] thymidine uptake (1 mCi per well; PerkinElmer) during the last 18 hours of a 3- or 5-day MLR or by CFSE (Molecular Probes, Life Sciences) dilution. In some instances, 10 ng/ml mouse rIL-6 was added on day 0 of coculture (R&D Systems). For IFN-γ, IL-4, IL-6, and IL-17 cytokine analysis, MLR supernatant was analyzed by Luminex bead assay (EMD Millipore) as described below.
Adoptive Transfer
CD8 T cells from the spleens of unmanipulated congenically marked WT BALB/c (CD45.1 or CD45.2) or MyD88−/− (CD45.2) mice were purified through a MACS column (Miltenyi) by excluding for CD25+, NK1.1+, Gr-1+, CD11b+, CD4+, CD11c+, and B220+ cells. The purified BALB/c WT (CD45.1) or MyD88−/− (CD45.2) CD8 T cells (purity >90%) were labeled with 5 μM CFSE (Molecular Probes) and were injected into WT BALB/c recipients (CD45.2) at a dose of 5×106 cells/mouse via the tail vein at 24 hours before receiving B6 kidney transplants. Ten days after transplantation the recipient spleens and kidneys were harvested and analyzed by flow cytometry. In a separate experiment, 1:1 mixed naive MyD88−/− (CD45.2) and CD8 WT T cells (CD45.1) were injected into naive RAG-1−/− mice or RAG-1−/− recipients with kidney transplant at 14 days after transplant and analyzed at 10 days after transplant.
Real-Time PCR-based Super Array and Heat Map Generation
Kidney grafts from WT or MyD88−/− recipients were harvested at 7 or 90 days after transplant and snap-frozen in liquid nitrogen and stored at −80°C for RNA analysis. Naive nontransplanted kidneys were also included as controls. Total RNA was extracted with the RNeasy mini kit (Qiagen). cDNA was synthesized from DNase-treated RNA by reverse transcription, in accordance with the manufacture’s protocol, using first strand cDNA synthesis kits (C-02, Super Array Bioscience Corp.). The ΔCT values of the super array data were normalized to the intra-array Hprt1 control gene as this gene has the least variation among the three control genes. Differential gene expression between the treatment conditions was assessed based on a linear model in which the pooled SEM was estimated. Bonferroni-corrected P values were computed and used to identify differentially expressed genes (P<0.05). For the differentially expressed genes between the control group and the other treatment groups, their expressions were visualized with a heat map using the gene-wise mean ΔCT values of the control groups as the reference. Specifically, the differences between the Δvalues of the genes in all the samples and the gene-wise mean ΔCT values of the control groups were calculated and plotted in the heat map.
Antibodies and Flow Cytometry
At the indicated times after transplantation, lymphocytes were isolated and cell staining was performed with the specific indicated antibodies (1 μg/106 cells) at 4°C for 30 minutes. Cells were analyzed using the FACSCantoII flow cytometer (BD Bioscience). Data were analyzed using FlowJo software (TreeStar Inc.).
Biotinylated mAbs for CD25 (7D4), Ly-76 (Ter-119), CD49b/Pan-NK (DX5), B220 (RA3–682), Gr1 (RB6–8C5), and CD11b (M1/70), PercpCy5.5 conjugated CD45.1 (A20), APC conjugated CD45.2 (104), PE conjugated CD4 (GK1.5), PE-conjugated Gr-1 (RB6–8C5), PE-CY7 conjugated CD11c (N418), and PE-CY7 conjugated CD11a (2D7) were purchased from BD Biosciences. PerCPCy5.5 conjugated CD8α (53–6.7), Pacific Blue conjugated CD4 (GK1.5), APC-eflour-780 conjugated CD11b (M1/70) and PE-CY7 conjugated CD44 (IM7) were all from eBiosciences.
Luminex Assay
Culture supernatants or homogenized tissue was collected and cytokines were quantitated using LiquiChip Mouse 22-cytokine assay kit (EMD Millipore). Assays were conducted according to the manufacturer’s instructions and analyzed on a LiquiChip 200 (Qiagen). Results were normalized to total protein by Bradford analysis (Bio-Rad).
Histology and Immunohistochemistry
Kidney samples were bisected transversely and placed in phosphate-buffered 10% formalin for 10–12 hours. The tissues were embedded in melted paraffin using plastic cassettes. The sections were stained with hematoxylin and eosin or periodic acid-Schiff or trichome Masson for morphologic evaluation. The kidney sections were evaluated blindly by a pathologist for the morphologic characteristics of acute rejection (tubulitis, interstitial mononuclear cell infiltration, vasculitis, glomerular hypercellularity) and chronic rejection (interstitial fibrosis, glomerular sclerosis, and arterial intimal hyperplasia). The severity of tubulitis was scored on the periodic acid-Schiff–stained sections of kidney allografts as 0 (normal) to 4+ (severest), depending on the number of infiltrating monocytes per tubule in the kidney grafts. Tubulitis score was analyzed by Fisher exact test.
Additional graft tissue was snap frozen in optimal cutting temperature compound with liquid nitrogen. Sections (4 μm thick) were stained with anti-mouse CD4 mAb (clone H129.19, BD Biosciences), anti-mouse CD8 (clone 53–6.7BD Biosciences), or anti-mouse Ly-6G/Ly-6C (clone RB6–8C5, Biolegend). Sections were stained with biotinylated goat anti-rat immunoglobulin (1:200, goat immunoglobulin clone polyclonal; BD Biosciences). Visualization was accomplished with Vectastain ABC-AP kit and Vector Blue substrate kit (Vector Laboratories).
Statistical Analyses
All data are expressed as the mean±SD. Graft survival times were compared using Kaplan-Meier survival curves with the log-rank test. Statistical significance between two groups was determined by Wilcoxon nonparametric tests or by an unpaired t test. The data representing more than two groups was analyzed with one-way ANOVA. Tukey procedure was used for the post hoc multiple testing adjustment. All statistical analyses were performed using GraphPad Prism 5 software; the exception was the tubulitis scores, which were analyzed by Fisher exact test using the R statistical computing package. P<0.05 was considered to represent a statistically significant difference.
Disclosure
None.
Supplementary Material
Acknowledgments
The authors thank Jie Yang for technical assistant.
This work is supported by Microsurgery Core, the comprehensive transplant center and Northwestern University Mouse Histology and Phenotyping Laboratory. In addition, N.L. was supported by the National Institutes of Health (NIH) Training Grant T32-DK077662, X.L. was supported by the NIH Directors New Innovator Award DP2-DK083099, and Y.S.K. was supported by the NIH grant DK60635.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014080813/-/DCSupplemental.
REFERENCES
- 1.Sarwal M, Chua MS, Kambham N, Hsieh SC, Satterwhite T, Masek M, Salvatierra O, Jr: Molecular heterogeneity in acute renal allograft rejection identified by DNA microarray profiling. N Engl J Med 349: 125–138, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Jevnikar AM, Mannon RB: Late kidney allograft loss: What we know about it, and what we can do about it. Clin J Am Soc Nephrol 3[Suppl 2]: S56–S67, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwasaki A, Medzhitov R: Toll-like receptor control of the adaptive immune responses. Nat Immunol 5: 987–995, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Barton GM, Medzhitov R: Control of adaptive immune responses by Toll-like receptors. Curr Opin Immunol 14: 380–383, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Akira S, Takeda K: Toll-like receptor signalling. Nat Rev Immunol 4: 499–511, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Akira S: TLR signaling. Curr Top Microbiol Immunol 311: 1–16, 2006 [DOI] [PubMed] [Google Scholar]
- 7.O’Neill LA, Bowie AG: The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol 7: 353–364, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Bosschaerts T, Guilliams M, Stijlemans B, Morias Y, Engel D, Tacke F, Hérin M, De Baetselier P, Beschin A: Tip-DC development during parasitic infection is regulated by IL-10 and requires CCL2/CCR2, IFN-gamma and MyD88 signaling. PLoS Pathog 6: e1001045, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Subramanian M, Thorp E, Hansson GK, Tabas I: Treg-mediated suppression of atherosclerosis requires MYD88 signaling in DCs. J Clin Invest 123: 179–188, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rahman AH, Zhang R, Blosser CD, Hou B, Defranco AL, Maltzman JS, Wherry EJ, Turka LA: Antiviral memory CD8 T-cell differentiation, maintenance, and secondary expansion occur independently of MyD88. Blood 117: 3123–3130, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldstein DR, Tesar BM, Akira S, Lakkis FG: Critical role of the Toll-like receptor signal adaptor protein MyD88 in acute allograft rejection. J Clin Invest 111: 1571–1578, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tesar BM, Zhang J, Li Q, Goldstein DR: TH1 immune responses to fully MHC mismatched allografts are diminished in the absence of MyD88, a toll-like receptor signal adaptor protein. Am J Transplant 4: 1429–1439, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Wu H, Noordmans GA, O’Brien MR, Ma J, Zhao CY, Zhang GY, Kwan TK, Alexander SI, Chadban SJ: Absence of MyD88 signaling induces donor-specific kidney allograft tolerance. J Am Soc Nephrol 23: 1701–1716, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang S, Schmaderer C, Kiss E, Schmidt C, Bonrouhi M, Porubsky S, Gretz N, Schaefer L, Kirschning CJ, Popovic ZV, Gröne HJ: Recipient Toll-like receptors contribute to chronic graft dysfunction by both MyD88- and TRIF-dependent signaling. Dis Model Mech 3: 92–103, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Braudeau C, Ashton-Chess J, Giral M, Dugast E, Louis S, Pallier A, Braud C, Moreau A, Renaudin K, Soulillou JP, Brouard S: Contrasted blood and intragraft toll-like receptor 4 mRNA profiles in operational tolerance versus chronic rejection in kidney transplant recipients. Transplantation 86: 130–136, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Guan Q, Lan Z, Li S, Ge W, Chen H, Nguan CY, Du C: Prolonged renal allograft survival by donor interleukin-6 deficiency: Association with decreased alloantibodies and increased intragraft T regulatory cells. Am J Physiol Renal Physiol 302: F276–F283, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Zhang Z, Lazarovits A, Grant D, Garcia B, Stiller C, Zhong R: CD45RB monoclonal antibody induces tolerance in the mouse kidney graft, but fails to prevent small bowel graft rejection. Transplant Proc 28: 2514, 1996 [PubMed] [Google Scholar]
- 18.Lazarovits AI, Poppema S, Zhang Z, Khandaker M, Le Feuvre CE, Singhal SK, Garcia BM, Ogasa N, Jevnikar AM, White MH, Singh G, Stiller CR, Zhong RZ: Prevention and reversal of renal allograft rejection by antibody against CD45RB. Nature 380: 717–720, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Rochman I, Paul WE, Ben-Sasson SZ: IL-6 increases primed cell expansion and survival. J Immunol 174: 4761–4767, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Quigley M, Martinez J, Huang X, Yang Y: A critical role for direct TLR2-MyD88 signaling in CD8 T-cell clonal expansion and memory formation following vaccinia viral infection. Blood 113: 2256–2264, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murdoch C, Finn A: Chemokine receptors and their role in inflammation and infectious diseases. Blood 95: 3032–3043, 2000 [PubMed] [Google Scholar]
- 22.Wolpe SD, Sherry B, Juers D, Davatelis G, Yurt RW, Cerami A: Identification and characterization of macrophage inflammatory protein 2. Proc Natl Acad Sci U S A 86: 612–616, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deshmane SL, Kremlev S, Amini S, Sawaya BE: Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res 29: 313–326, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fiorenza S, Kenna TJ, Comerford I, McColl S, Steptoe RJ, Leggatt GR, Frazer IH: A combination of local inflammation and central memory T cells potentiates immunotherapy in the skin. J Immunol 189: 5622–5631, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakajima C, Mukai T, Yamaguchi N, Morimoto Y, Park WR, Iwasaki M, Gao P, Ono S, Fujiwara H, Hamaoka T: Induction of the chemokine receptor CXCR3 on TCR-stimulated T cells: dependence on the release from persistent TCR-triggering and requirement for IFN-gamma stimulation. Eur J Immunol 32: 1792–1801, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Morimoto Y, Bian Y, Gao P, Yashiro-Ohtani Y, Zhou XY, Ono S, Nakahara H, Kogo M, Hamaoka T, Fujiwara H: Induction of surface CCR4 and its functionality in mouse Th2 cells is regulated differently during Th2 development. J Leukoc Biol 78: 753–761, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Zhou S, Kurt-Jones EA, Cerny AM, Chan M, Bronson RT, Finberg RW: MyD88 intrinsically regulates CD4 T-cell responses. J Virol 83: 1625–1634, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Longhi MP, Wright K, Lauder SN, Nowell MA, Jones GW, Godkin AJ, Jones SA, Gallimore AM: Interleukin-6 is crucial for recall of influenza-specific memory CD4 T cells. PLoS Pathog 4: e1000006, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rose-John S, Neurath MF: IL-6 trans-signaling: The heat is on. Immunity 20: 2–4, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Hou T, Tieu BC, Ray S, Recinos A III, Cui R, Tilton RG, Brasier AR: Roles of IL-6-gp130 signaling in vascular inflammation. Curr Cardiol Rev 4: 179–192, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamimura D, Ishihara K, Hirano T: IL-6 signal transduction and its physiological roles: the signal orchestration model. Rev Physiol Biochem Pharmacol 149: 1–38, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Naugler WE, Karin M: The wolf in sheep’s clothing: The role of interleukin-6 in immunity, inflammation and cancer. Trends Mol Med 14: 109–119, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Baldwin AS, Jr: The NF-kappa B and I kappa B proteins: New discoveries and insights. Annu Rev Immunol 14: 649–683, 1996 [DOI] [PubMed] [Google Scholar]
- 34.O’Hara A, Lim FL, Mazzatti DJ, Trayhurn P: Stimulation of inflammatory gene expression in human preadipocytes by macrophage-conditioned medium: upregulation of IL-6 production by macrophage-derived IL-1β. Mol Cell Endocrinol 349: 239–247, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Perez-Villa F, Benito B, Llancaqueo M, Cuppoletti A, Roig E: Elevated levels of serum interleukin-6 are associated with low grade cellular rejection in patients with heart transplantation. Transplant Proc 38: 3012–3015, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Waiser J, Budde K, Katalinic A, Kuerzdörfer M, Riess R, Neumayer HH: Interleukin-6 expression after renal transplantation. Nephrol Dial Transplant 12: 753–759, 1997 [DOI] [PubMed] [Google Scholar]
- 37.Van Snick J: Interleukin-6: An overview. Annu Rev Immunol 8: 253–278, 1990 [DOI] [PubMed] [Google Scholar]
- 38.Bromley SK, Mempel TR, Luster AD: Orchestrating the orchestrators: Chemokines in control of T cell traffic. Nat Immunol 9: 970–980, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Kunkel EJ, Boisvert J, Murphy K, Vierra MA, Genovese MC, Wardlaw AJ, Greenberg HB, Hodge MR, Wu L, Butcher EC, Campbell JJ: Expression of the chemokine receptors CCR4, CCR5, and CXCR3 by human tissue-infiltrating lymphocytes. Am J Pathol 160: 347–355, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Groom JR, Luster AD: CXCR3 ligands: Redundant, collaborative and antagonistic functions. Immunol Cell Biol 89: 207–215, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Campbell JJ, Haraldsen G, Pan J, Rottman J, Qin S, Ponath P, Andrew DP, Warnke R, Ruffing N, Kassam N, Wu L, Butcher EC: The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature 400: 776–780, 1999 [DOI] [PubMed] [Google Scholar]
- 42.Hokeness KL, Deweerd ES, Munks MW, Lewis CA, Gladue RP, Salazar-Mather TP: CXCR3-dependent recruitment of antigen-specific T lymphocytes to the liver during murine cytomegalovirus infection. J Virol 81: 1241–1250, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hancock WW, Lu B, Gao W, Csizmadia V, Faia K, King JA, Smiley ST, Ling M, Gerard NP, Gerard C: Requirement of the chemokine receptor CXCR3 for acute allograft rejection. J Exp Med 192: 1515–1520, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whiting D, Hsieh G, Yun JJ, Banerji A, Yao W, Fishbein MC, Belperio J, Strieter RM, Bonavida B, Ardehali A: Chemokine monokine induced by IFN-gamma/CXC chemokine ligand 9 stimulates T lymphocyte proliferation and effector cytokine production. J Immunol 172: 7417–7424, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Rosenblum JM, Zhang QW, Siu G, Collins TL, Sullivan T, Dairaghi DJ, Medina JC, Fairchild RL: CXCR3 antagonism impairs the development of donor-reactive, IFN-gamma-producing effectors and prolongs allograft survival. Transplantation 87: 360–369, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohsugi Y: Recent advances in immunopathophysiology of interleukin-6: an innovative therapeutic drug, tocilizumab (recombinant humanized anti-human interleukin-6 receptor antibody), unveils the mysterious etiology of immune-mediated inflammatory diseases. Biol Pharm Bull 30: 2001–2006, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Bleier JI, Pillarisetty VG, Shah AB, DeMatteo RP: Increased and long-term generation of dendritic cells with reduced function from IL-6-deficient bone marrow. J Immunol 172: 7408–7416, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Dunay IR, Damatta RA, Fux B, Presti R, Greco S, Colonna M, Sibley LD: Gr1(+) inflammatory monocytes are required for mucosal resistance to the pathogen Toxoplasma gondii. Immunity 29: 306–317, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luther SA, Cyster JG: Chemokines as regulators of T cell differentiation. Nat Immunol 2: 102–107, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Zhang Z, Zhu L, Quan D, Garcia B, Ozcay N, Duff J, Stiller C, Lazarovits A, Grant D, Zhong R: Pattern of liver, kidney, heart, and intestine allograft rejection in different mouse strain combinations. Transplantation 62: 1267–1272, 1996 [DOI] [PubMed] [Google Scholar]
- 51.Zhang Z, Schlachta C, Duff J, Stiller C, Grant D, Zhong R: Improved techniques for kidney transplantation in mice. Microsurgery 16: 103–109, 1995 [DOI] [PubMed] [Google Scholar]
- 52.Masopust D, Vezys V, Marzo AL, Lefrançois L: Preferential localization of effector memory cells in nonlymphoid tissue. Science 291: 2413–2417, 2001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.