Abstract
Ischemia-reperfusion injury (IRI) is the leading cause of ARF. A pathophysiologic role of the coagulation system in renal IRI has been established, but the functional relevance of thrombomodulin (TM)-dependent activated protein C (aPC) generation and the intracellular targets of aPC remain undefined. Here, we investigated the role of TM-dependent aPC generation and therapeutic aPC application in a murine renal IRI model and in an in vitro hypoxia and reoxygenation (HR) model using proximal tubular cells. In renal IRI, endogenous aPC levels were reduced. Genetic or therapeutic reconstitution of aPC efficiently ameliorated renal IRI independently of its anticoagulant properties. In tubular cells, cytoprotective aPC signaling was mediated through protease activated receptor-1- and endothelial protein C receptor-dependent regulation of the cold-shock protein Y-box binding protein-1 (YB-1). The mature 50 kD form of YB-1 was required for the nephro- and cytoprotective effects of aPC in vivo and in vitro, respectively. Reduction of mature YB-1 and K48-linked ubiquitination of YB-1 was prevented by aPC after renal IRI or tubular HR injury. aPC preserved the interaction of YB-1 with the deubiquitinating enzyme otubain-1 and maintained expression of otubain-1, which was required to reduce K48-linked YB-1 ubiquitination and to stabilize the 50 kD form of YB-1 after renal IRI and tubular HR injury. These data link the cyto- and nephroprotective effects of aPC with the ubiquitin-proteasome system and identify YB-1 as a novel intracellular target of aPC. These insights may provide new impetus for translational efforts aiming to restrict renal IRI.
Keywords: cell signaling, ischemic renal failure, renal proximal tubule cell, acute renal, failure, ischemia-reperfusion, thrombosis
Ischemia-reperfusion injury (IRI) is the leading cause of ARF. The consequences of renal IRI can be dramatic, resulting in a marked decline of renal function and high mortality rates. A number of candidate mechanisms involved in renal IRI have been identified in preclinical studies, including mechanisms such as protein ubiquitination, endothelial dysfunction, or coagulation activation.1–5 Whether and how these intra- and extracellular signaling mechanisms may interact in renal IRI remains unknown.
Tissue factor-dependent coagulation and thrombin activation aggravate tubular injury through protease activated receptor-1 (PAR1)–dependent signaling.5 Unlike thrombin, the anticoagulant serine protease activated protein C (aPC) is nephroprotective, ameliorating chronic6,7 and acute8,9 renal injury. The opposing effects of thrombin and aPC are largely controlled by thrombomodulin (TM).10 TM is a type 1 transmembrane receptor which binds thrombin, directing its activity toward protein C (PC) activation, therefore providing a functional switch between the serine proteases thrombin and aPC.10 In preclinical studies, therapeutic application of recombinant soluble TM ameliorates IRI in rats.11,12 However, whether TM-dependent PC activation and signaling through aPC’s pivotal receptors PAR1 and endothelial protein C receptor (EPCR) are required for TM-dependent nephroprotection after IRI remains unknown.13,14 In addition, although aPC mediated cytoprotection after hepatic IRI has been demonstrated,15 the role of aPC in renal IRI or aPC’s intracellular targets in IRI remains unknown.
One potential intracellular target of coagulation proteases in renal IRI is the cold-shock protein Y-box binding protein-1 (YB-1) because (1) YB-1 activity is regulated by thrombin, (2) YB-1 is regulated in cardiac IRI, and (3) YB-1 has an established role in renal diseases other than IRI.16–19 YB-1 is a highly conserved protein involved in the regulation of inflammatory processes, including sterile inflammation in renal diseases.19 YB-1 conveys multiple functions through its interaction with DNA, mRNA, and proteins.20,21 The diverse functions of YB-1 are in part regulated by its subcellular localization and post-translational modifications, including ubiquitination.20,21 Subcellular localization of YB-1 is furthermore regulated by the coagulation protease thrombin, which induces a partial degradation of YB-1 and nuclear translocation of the N-terminal YB-1 fragment in endothelial cells.22,23 However, it remains unknown which receptors mediate these effects and whether other coagulation proteases regulate YB-1. Furthermore, the mechanisms through which coagulation proteases modulate partial YB-1 degradation (e.g., relevance of the proteasome or ubiquitination) remain unknown. We speculated that TM, coagulation proteases, and their receptors control renal IRI injury by modulating YB-1 function and that the partial degradation of YB-1 is controlled by the ubiquitin-proteasome system. Hence, we evaluated the effect of TM-dependent aPC generation on YB-1 in renal IRI in this study.
Results
Activated PC Maintains Renal YB-1 Levels during IRI
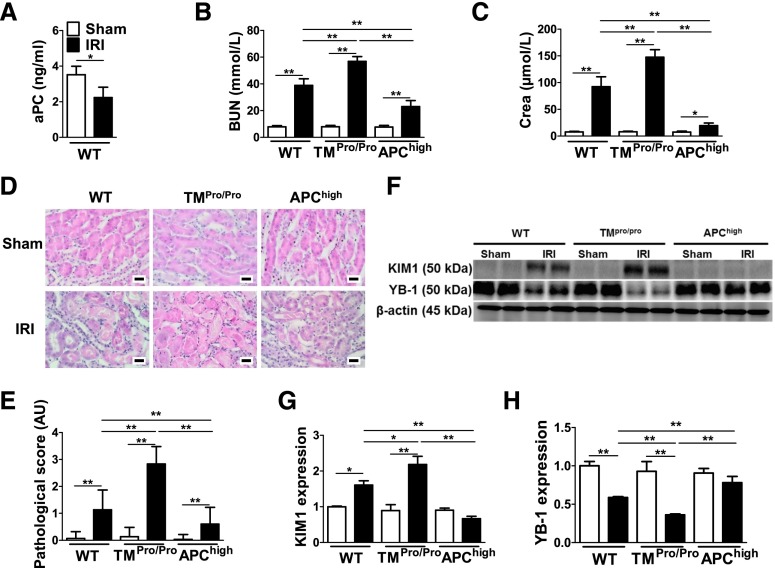
Renal IRI was induced in mice to evaluate a possible interaction between the TM-PC system and YB-1. First, we established that renal IRI impairs in vivo PC activation (Figure 1A), reflecting impairment of the endogenous TM-PC system in this model. To evaluate the mechanistic relevance of altered endogenous PC activation, we used genetically modified mice with impaired TM-dependent PC activation (TMPro/Pro mice), resulting in low blood levels of aPC, or mice expressing a hyperactivatable PC mutant, resulting in high blood levels of aPC (APChigh mice).6,24 Although no differences were observed in sham-operated mice, BUN, creatinine, tubular injury, and expression of the kidney injury marker kidney-injury-molecule 1 (KIM1) were significantly induced in wild-type (WT) mice and further increased in TMPro/Pro IRI mice (Figure 1, B–G, Supplemental Figure 1A). Of note, these markers were markedly reduced in APChigh IRI mice in comparison with WT IRI mice (Figure 1, B–G). The expression of YB-1 was likewise significantly altered in mice with IRI. YB-1 levels declined in WT IRI and to an even larger extent in TMPro/Pro IRI mice, but they were preserved in APChigh mice (Figure 1, F and H).
Figure 1.
Protection from renal IRI by TM-dependent PC activation is associated with sustained YB-1 expression. (A) Plasma levels of aPC are reduced in WT mice after renal IRI (black bars) compared with control (sham, open bars) mice. BUN (B) and creatinine (Crea) (C) in control (Sham) (open bars) and experimental (IRI) (black bars) WT, TMPro/Pro, and APChigh mice. Exemplary images of H&E-stained kidney section from WT, TMPro/Pro, and APChigh mice without (Sham) or with IRI (D) and a bar graph summarizing results of pathologic scores (E). TM-dependent PC activation modulates KIM1 and YB-1 expression during renal IRI in vivo; representative immunoblots (F) and bar graphs summarizing results [(G) and (H)]. Mean±SD values of at least six mice per group [(A)–(C), (E), (G), and (H)]; size bar: 20 µm (D); *P<0.05; **P<0.01 [(A): t test; (B), (C), (E), (G), and (H): ANOVA]. H&E, hematoxylin and eosin.
To determine the therapeutic potential of aPC and the relevance of aPC’s anticoagulant function, we next treated mice with exogenous aPC (Supplemental Figure 1B). A subgroup of mice received aPC preincubated with an antibody HAPC1573, which specifically inhibits aPC’s anticoagulant, but not its cytoprotective effects.25 Both aPC and the aPC-HAPC1573 complex were equally protective in the IRI model (Supplemental Figure 1, C–G), and both maintained YB-1 levels (Supplemental Figure 1, F–H). Taken together, impaired TM-dependent PC activation is causally linked to renal IRI, but can be compensated by restoring aPC levels. The nephroprotective effect of aPC in IRI is independent of its anticoagulant function and is associated with sustained YB-1 expression.
Activated PC Maintains YB-1 Levels in Tubular Cells after Hypoxia Reoxygenation
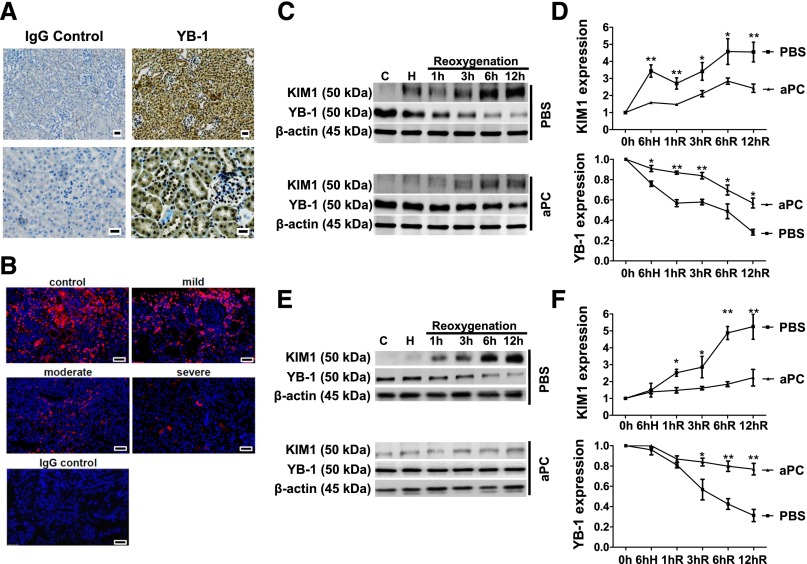
YB-1 is predominately expressed in tubular cells (Figure 2A). After IRI, tubular YB-1 expression is diminished in WT and TMPro/Pro but not in APChigh mice (Supplemental Figure 2A). Interestingly, a severe reduction of renal YB-1 expression is likewise observed in human renal biopsies after acute renal injury (Figure 2B, Supplemental Figure 2B). To evaluate whether aPC cell autonomously maintains YB-1 expression in tubular cells, we analyzed differentiated BUMPT cells (a conditionally immortalized mouse proximal tubular cell line26,27) or primary renal proximal tubular epithelial cells challenged by hypoxia and reoxygenation (HR). Pretreatment of these cells with aPC reduced KIM1 expression at all time points after reoxygenation in comparison with PBS-treated control cells (Figure 2, C–F, Supplemental Figure 2, C–F). In agreement with the opposite expression pattern of KIM1 and YB-1 in vivo, YB-1 levels declined after HR, but this effect was significantly ameliorated after pretreatment with aPC (Figure 2, C–F). Therefore, aPC preserves YB-1 protein levels during tubular hypoxia-reoxygenation injury in a cell-autonomous fashion.
Figure 2.
aPC maintains YB-1 expression in tubular cells after HR. (A) YB-1 is strongly expressed in renal tubular cells. Exemplary immunohistochemical YB-1 staining of renal paraffin-embedded tissue sections from healthy WT mice (right), IgG control (left), and YB-1 antigen detected by HRP-DAB reaction (brown) and hematoxylin counterstain (blue); overview (top) and tissue section at higher magnification (bottom); scale bar: 20 µm. (B) Expression of YB-1 (red) in human renal biopsies is reduced after acute renal injury. Exemplary immunofluorescent staining of renal paraffin-embedded tissue biopsies from patients with AKI graded as mild, moderate, or severe and control tissue sections; scale bar: 20 µm. Time-dependent expression of KIM1 and YB-1 in mouse tubular cells (BUMPT) [(C) and (D)] and in primary renal proximal tubular epithelial cells (rTEC) [(E) and (F)] in vitro at baseline, after 6 hours of hypoxia (H) (1% O2 for 6 hours), and at various time points (1–12 hours) after reoxygenation (21% O2). Pretreatment with aPC (20 nM) diminishes the increase of KIM1 and the loss of YB-1 expression compared with PBS-treated control cells; representative immunoblots of whole-cell lysates [(C) and (E)] and line graph [(D) and (F)] summarizing results. Mean±SD value of at least three independent experiments [(D) and (F)]; *P<0.05; **P<0.01 (ANOVA).
Protective Effect of aPC in Renal IRI Depends on YB-1
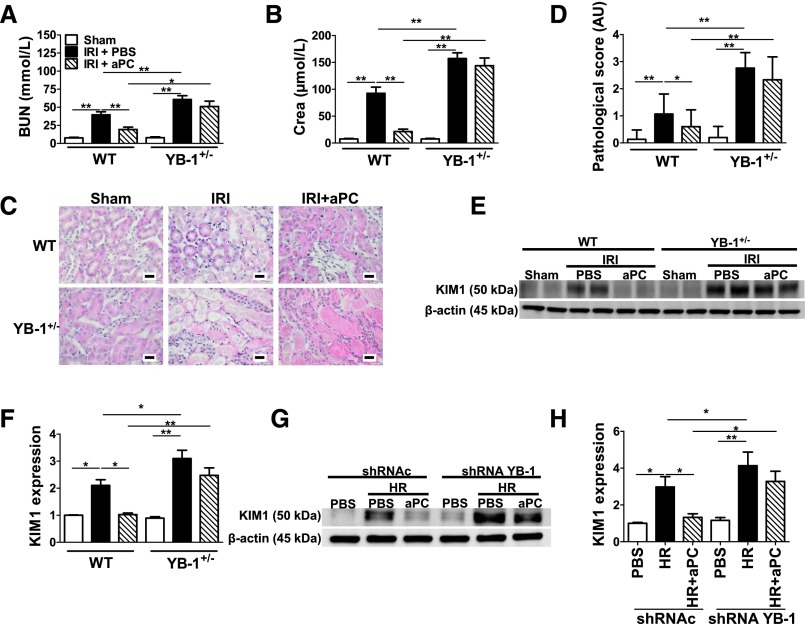
To determine the functional relevance of YB-1 for aPC mediated nephroprotection in renal IRI we used heterozygous YB-1 mice (YB-1+/−; homozygous YB-1 deficiency is embryonic-lethal), which express 50% of YB-1 compared with WT mice.28,29 BUN, creatinine, histopathologic changes, and KIM1 expression were all increased in YB-1+/− compared with WT mice undergoing IRI (Figure 3, A–F). Although treatment with aPC improved these renal injury indices in WT IRI mice, aPC failed to provide renal protection in YB-1+/− IRI mice (Figure 3, A–F). Likewise, pretreatment of tubular cells with aPC failed to reduce KIM1 expression in YB-1 knockdown cells, whereas aPC efficiently reduced KIM1 expression in control cells transfected with a nonspecific shRNA (Figure 3, G and H, Supplemental Figure 3). These data establish that aPC’s cytoprotective effect depends at least partially on YB-1 in renal IRI.
Figure 3.
Reduced YB-1 expression abolishes the protective effect of aPC in renal IRI. (A)–(F) Renal IRI was induced in WT and heterozygous YB-1 (YB-1+/−) mice. BUN (A), creatinine (Crea) (B), exemplary images of H&E-stained kidney section (C), bar graph summarizing results of pathologic scores (D), representative immunoblots of kidney lysates (E), and bar graph (F) summarizing results of KIM1 protein levels normalized to β-actin in control (Sham) (open bars) and experimental mice without (IRI) (black bars) or with aPC pretreatment (IRI+aPC) (striped bars; aPC: 0.5 mg/kg i.p.). Treatment with aPC normalizes BUN, Crea, tissue injury, and KIM1 expression in WT mice, but not in YB-1+/− mice. (G) and (H) Expression of KIM1 in BUMPT cells stably expressing control (shRNAc) or YB-1-specific (shRNA YB-1) shRNA after HR. Pretreatment of cells with aPC (aPC) (20 nM, 30 minutes before hypoxia) normalizes KIM1 expression in controls, but not in YB-1 knockdown cells exposed to 6 hours of hypoxia (1% O2) followed by 6 hours of reoxygenation (21% O2). Representative immunoblot of KIM1 and β-actin in whole-cell lysates (G) and bar graphs (H) summarizing results. Mean±SD value of at least six mice per group [(A), (B), (D), and (F)] or of at least three independent experiments (H); *P<0.05; **P<0.01 (ANOVA). H&E, hematoxylin and eosin.
Activated PC Regulates YB-1 Levels via the Ubiquitin Proteasome System
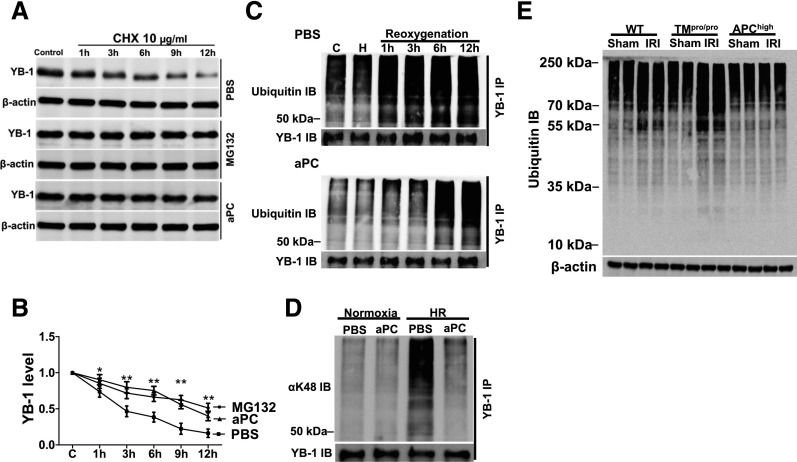
To evaluate the mechanism through which aPC maintains YB-1 protein levels we first determined YB-1 protein stability by blocking its resynthesis using cycloheximide (CHX).30 This revealed a time-dependent decay of the mature (50 kD) YB-1 protein, which was diminished by the proteasome inhibitor MG132 (Figure 4, A and B). Treatment of CHX-exposed tubular cells with aPC likewise diminished the YB-1 decay (Figure 4, A and B), raising the question as to whether aPC regulates YB-1 stability via ubiquitin-proteasome system. Because proteasomal degradation is regulated by protein ubiquitination, in particular by K48-linked ubiquitination,31 we next analyzed the extent and kinetics of YB-1 ubiquitination in HR-challenged tubular cells without and with aPC treatment. Treatment with aPC delayed and reduced YB-1 ubiquitination after tubular cell HR injury (Figure 4C). Of note, aPC markedly reduced K48-linked ubiquitination of YB-1 (Figure 4D). To ascertain the effect of the TM-PC system on ubiquitination in vivo, we analyzed renal tissue extracts from the aforementioned IRI experiments. Ubiquitination was increased in WT IRI mice and increased even further in TMPro/Pro IRI mice, whereas no increase was observed in APChigh IRI mice (Figure 4E). These data establish that aPC modulates ubiquitination and suggests that aPC regulates YB-1 protein levels via K48-dependent ubiquitination.
Figure 4.
aPC suppresses YB-1 ubiquitination. (A) and (B) aPC increases the protein stability of YB-1. After treatment of BUMPT cells with the protein synthesis inhibitor CHX (10 µg/ml) YB-1 protein levels markedly decline. Treatment of BUMPT cells with the proteasome inhibitor MG132 (10 µM) or aPC (20 nM) stabilizes YB-1 levels when compared with PBS-treated control cells; representative immunoblots (IBs) of YB-1 in whole-cell lysates (A) and line graphs (B) summarizing results. (C) YB-1 is rapidly ubiquitinated after in vitro reoxygenation (top, PBS-treated control). Pretreatment with aPC (20 nM, bottom) delays and reduces YB-1 ubiquitination. Immunoprecipitation (IP) of YB-1 from whole-cell lysates and representative images of ubiquitin IBs and YB-1 IB as input control. (D) HR (6 hours postreoxygenation) induces YB-1 K48–linked ubiquitination in BUMPT cells, which is efficiently suppressed by aPC treatment (20 nM). IP of YB-1 from whole-cell lysates and representative images of IBs using an antibody against K48-linked ubiquitin (top, K48-linked ubiquitin IB; bottom, YB-1 IB as input control). (E) After IRI, protein ubiquitination is increased in WT mice, and to a larger extent in TMPro/Pro, but not in APChigh mice. Representative IB of ubiquitinated proteins (top) in kidney lysates; β-actin as loading control (bottom). Mean±SD value of at least three independent experiments (B); *P<0.05; **P<0.01 (ANOVA).
Cytoprotective Effect of aPC Is Impaired in Heterozygous OTUB1 Mice
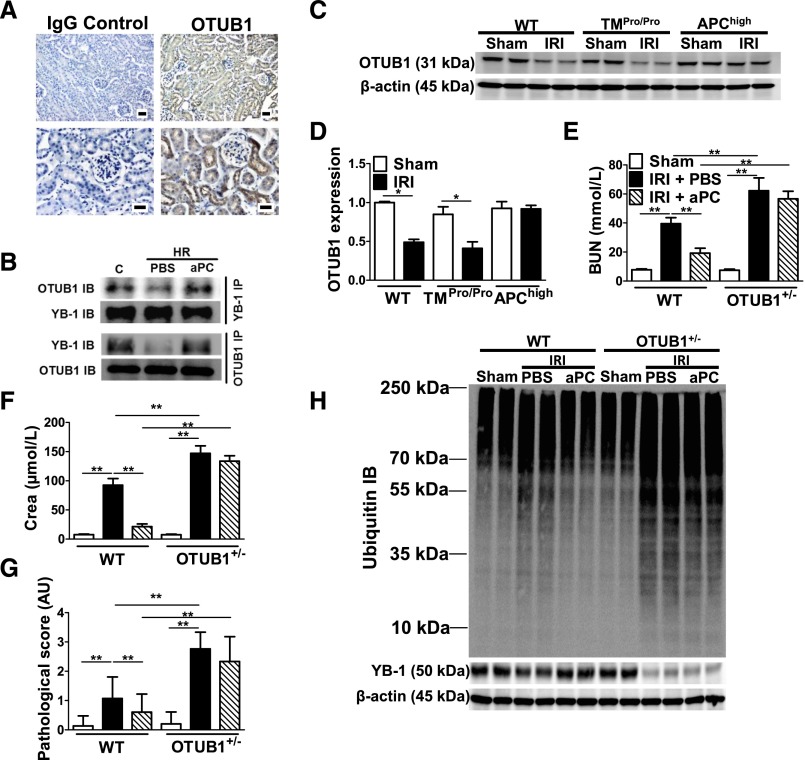
To evaluate the mechanism through which aPC reduces YB-1 ubiquitination, we immunoprecipitated YB-1 from tubular cells and identified proteins interacting with YB-1 using LC-MS/MS. Among 685 proteins immunoprecipitated, we identified 6 DUBs (otubain-1 [OTUB1], OTUD4, UBP4, UBP5, UBP7, UBP10). Because reduced expression has been previously demonstrated for OTUB1 in a model of renal tubulointerstitial injury32 and because OTUB1 is predominantly expressed in tubular cells (Figure 5A) (human protein atlas: http://www.proteinatlas.org), we focused on this DUB. The interaction of OTUB1 with YB-1 was confirmed by immunoprecipitation (Figure 5B, Supplemental Figure 4, A and B). This interaction was reduced after HR injury, but could be preserved by aPC despite HR injury of tubular cells (Figure 5B). Next, to ascertain whether the TM-PC system regulates OTUB1 in vivo, we determined OTUB1 expression. In control mice (sham group), OTUB1 expression did not differ between WT, TMPro/Pro, and APChigh mice (Figure 5 C and D). However, after renal IRI, OTUB1 expression was reduced in WT IRI mice and TMPro/Pro IRI mice, but it was maintained in APChigh IRI mice (Figure 5, C and D, Supplemental Figure 4C). To determine whether the regulation of OTUB1 by aPC has functional relevance for renal IRI, YB-1 expression, and ubiquitination, we used heterozygous OTUB1 (OTUB1+/−) (Supplemental Figure 5) mice. We used OTUB1+/− mice because homozygous OTUB1−/− mice are not viable (D. Schlüter, unpublished data). Renal expression of OTUB1 was reduced to about 50% in heterozygous OTUB1 mice (Supplemental Figure 5, D and E). Renal injury, as reflected by serum BUN and creatinine, and histopathologic injury were aggravated in OTUB1+/− IRI mice compared with WT IRI mice. Unlike in WT IRI mice, aPC failed to improve renal injury in OTUB1+/− IRI mice (Figure 5, E–G). Renal protein ubiquitination was increased in OTUB1+/− IRI mice compared with WT IRI mice (Figure 5H). Although exogenous aPC treatment normalizes ubiquitination in WT IRI mice, it failed to do so in OTUB1+/− IRI mice (Figure 5H). Concomitantly, YB-1 levels were markedly reduced in OTUB1+/− IRI mice. Unlike in WT IRI mice, this reduction persisted despite aPC treatment (Figure 5H). These data imply that YB-1, OTUB1, and aPC interact in renal IRI, which may be mechanistically relevant for the regulation of YB-1 ubiquitination by aPC.
Figure 5.
aPC-mediated renal protection and sustained YB-1 expression after IRI depends on OTUB1. (A) OTUB1 is predominately expressed in tubular cells; immunohistochemical detection of OTUB1 in paraffin-embedded tissue sections of WT mouse kidney (right) and IgG control (left); OTUB1 antigen detected by HRP-DAB reaction (brown) and hematoxylin counterstain (blue); overview (top) and tissue section at higher magnification (bottom); scale bar: 20 µm. (B) The interaction of YB-1 and OTUB1 is impaired after HR injury in tubular cells. Detection of OTUB1 by immunoblotting after immunoprecipitation of YB-1 (top; YB-1 IB: input control) or of YB-1 after immunoprecipitation of OTUB1 (bottom; OTUB1 IB: input control) from whole-cell lysates of control (C) mouse tubular cells or tubular cells after in vitro HR without (PBS) or with aPC treatment (aPC) (20 nM); representative images of immunoblots. Expression of OTUB1 is maintained in APChigh mice after IRI. OTUB1 protein levels in kidney lysates were determined by immunoblotting and normalized to β-actin; representative immunoblots (C) and bar graph summarizing results (D). BUN (E), creatinine (Crea) (F), and tissue injury (pathologic score) (G) in control (Sham, open bars) and experimental mice without (IRI) (black bars) or with aPC pretreatment (IRI+aPC) (striped bars; aPC: 0.5 mg/kg i.p.). (H) Immunoblot of ubiquitinated proteins (top) and YB-1 (middle) and β-actin as loading control (bottom) in WT and heterozygous OTUB1 (OTUB1+/−) mice. Control mice (Sham) or mice with IRI without (PBS) or with (aPC) (0.5 mg/kg i.p.) treatment. Mean±SD value of at least six mice per group [(D)–(G)]; *P<0.05; **P<0.01 (ANOVA).
Reduction of YB-1 Ubiquitination by aPC Requires OTUB1
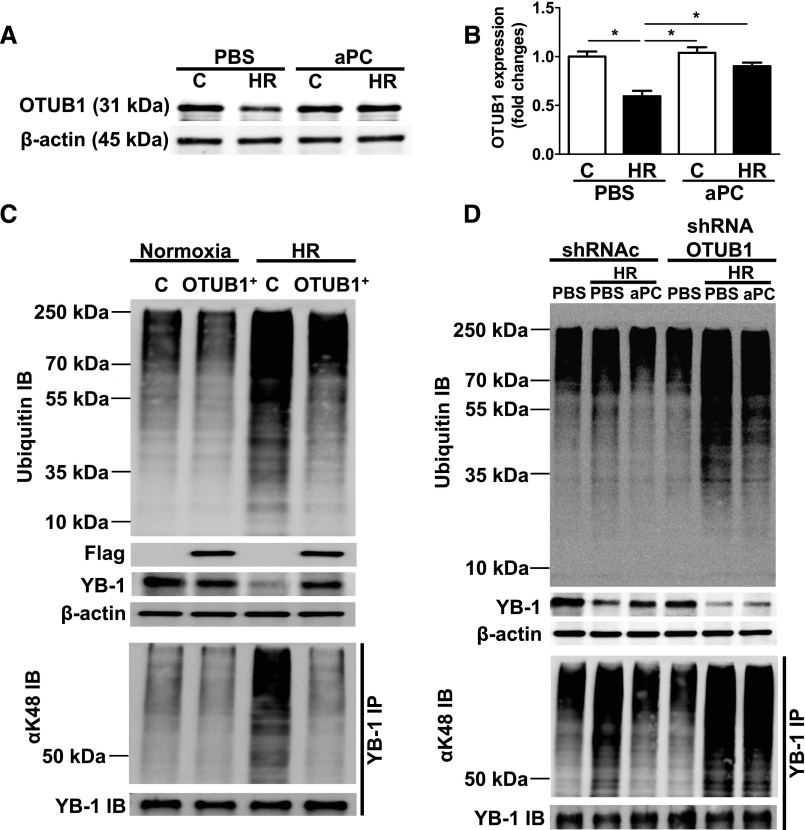
To determine the mechanistic relevance of OTUB1 for the aPC-dependent ubiquitination of YB-1, we next conducted in vitro studies. In line with the in vivo results, HR reduced OTUB1 expression in tubular cells (Figure 6, A and B). The HR-induced reduction of OTUB1 was prevented by aPC (Figure 6, A and B). To determine whether modulation of OTUB1 expression is sufficient to alter ubiquitination and expression of YB-1 after HR, we next used loss and gain of function approaches in tubular cells. Overexpression of OTUB1 was sufficient to prevent excess polyubiquitination, K48-linked YB-1 ubiquitination, and loss of YB-1 expression in HR-challenged tubular cells (Figure 6C), demonstrating that high OTUB1 expression is sufficient to reduce ubiquitination, including K48-linked YB-1 ubiquitination, and loss of YB-1 in tubular cells. Next, we analyzed tubular cells with reduced OTUB1 expression (OTUB1 knockdown [OTUB1KD]) (Supplemental Figure 6). Loss of OTUB1 did not increase polyubiquitination or K48-linked YB-1 ubiquitination and did not reduce YB-1 expression at baseline (Figure 6D). However, after HR injury, polyubiquitination and K48-linked YB-1 ubiquitination were markedly increased, whereas YB-1 expression was reduced in OTUB1KD tubular cells. Importantly, aPC failed to prevent the increased polyubiquitination, K48-linked ubiquitination, and loss of YB-1 expression in OTUB1KD tubular cells (Figure 6D), demonstrating that the effect of aPC on YB-1 stability, K48-linked YB-1 ubiquitination, and polyubiquitination depends on OTUB1. Taken together, increased expression of OTUB1 is sufficient to maintain YB-1 protein levels in tubular cells, and loss of OTUB1 abolishes the protective effect of aPC in regard to YB-1 expression.
Figure 6.
Inhibition of YB-1 ubiquitination by aPC depends on OTUB1. (A) and (B) HR (6 hours of hypoxia and 6 hours of reoxygenation; C: normoxic control) decreases OTUB1 levels in BUMPT cells in vitro. Treatment of BUMPT cells with aPC (20 nM) conserves OUTB1 expression despite HR. Representative IBs (A) and bar graph summarizing the results (B). (C) Ubiquitination in BUMPT cells stably expressing empty vector (C) or an expression construct for flag tagged OTUB1 (OTUB1+). Overexpression of OUTB1 reduces HR-induced protein ubiquitination (top; ubiquitin IB), maintains YB-1 expression (middle; β-actin as loading control), and reduces YB-1 K48–linked ubiquitination (bottom, αK48 IB: IP of YB-1 followed by K48-linked ubiquitin IB; YB-1 IB shown as input control). Flag IB reflecting efficient overexpression of flag-tagged OTUB1. (D) OTUB1 deficiency impairs aPC’s effect on ubiquitination and YB-1 expression. The aPC mediated reduction of protein ubiquitination (C, top) and YB-1 K48-linked ubiquitination (C, bottom) and the sustained YB-1 expression (C, middle, β-actin as loading control) are impaired in stable OTUB1 knockdown tubular cells (shRNA OTUB1) when compared with control transfected cells (shRNAc). YB-1 K48–linked ubiquitination is determined by IP of YB-1 followed by K48 immunoblotting (αK48 IB); YB-1 IB shown as input control. Mean±SD value of three independent experiments (B); *P<0.05, (ANOVA); representative IBs of at least three independent experiments [(A), (C), and (D)]. IB, immunoblot; IP, immunoprecipitation.
Activated PC Maintains OTUB1 and YB-1 Expression via PAR1 and EPCR in Tubular Cells
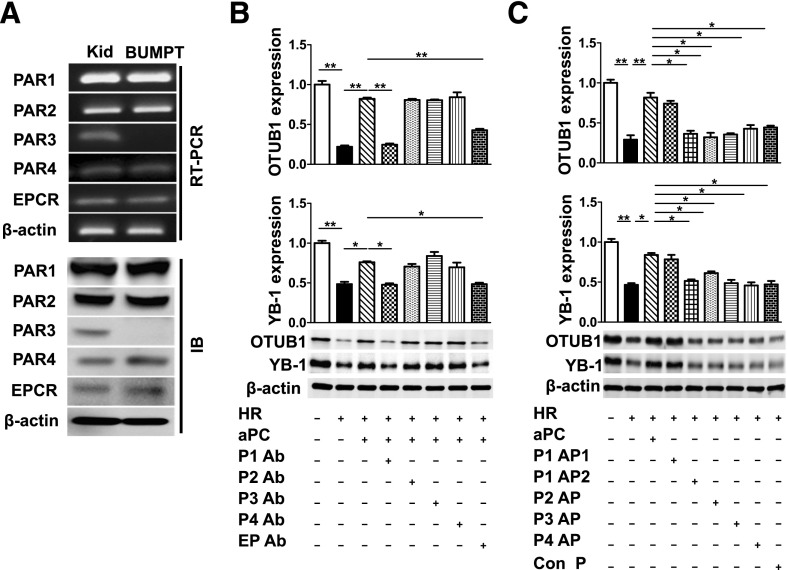
To identify the receptors through which aPC maintains YB-1 and OTUB1 expression after hypoxia, we first determined the expression of PARs and EPCR in tubular cells. PAR1, PAR2, PAR4, and EPCR, but not PAR3, were readily detectable in BUMPT cells (Figure 7A). To determine the functional relevance of receptors expressed by tubular cells, we next preincubated tubular cells with receptor blocking antibodies. Inhibition of PAR1 or EPCR efficiently abolished aPC’s effect on OTUB1 and YB-1 expression, whereas blocking PAR2 or PAR4 had no effect (Figure 7B). In agreement with these blocking studies, only the aPC-specific PAR1 agonist peptide maintained OTUB1 and YB-1 expression despite HR injury, whereas the thrombin-specific PAR1 or the other PAR agonist peptides failed to do so (Figure 7C).33 Therefore, aPC maintains OTUB1 and YB-1 expression in tubular cells exposed to HR injury via PAR1 and EPCR.
Figure 7.
aPC regulates OTUB1 and YB-1 expression in tubular cells via PAR1 and EPCR. (A) PAR1, PAR2, PAR4, and EPCR are expressed in BUMPT cells. Representative images showing expression of PARs and EPCR in BUMPT cells and mouse kidney. (Kid, positive control; semi-quantitative RT-PCR, top, and immunoblot [IB] bottom). OTUB1 and YB-1 levels in HR-stressed BUMPT cells incubated with PAR (P1-P4) or EPCR (EP) blocking antibodies (Ab) before aPC treatment (B) or treated with PAR-agonist peptides (P1-P4 AP) (C). Blocking PAR1 or EPCR, but not PAR2 or PAR4, abolishes aPC’s effect in regard to OTUB1 and YB-1 expression. Activation of PAR1 by the aPC-specific agonist peptide (P1AP1: NPNDKYEPFWEDEEKNESGL), but not by the thrombin-specific agonist peptide (P1AP2: TFLLR) prevents the HR-induced loss of OTUB1 and YB1. Representative immunoblots showing OTUB1 [(B) and (C) top] and YB-1 [(B) and (C), bottom] expression in whole-cell lysates and bar graph summarizing results. Mean±SD value of at least three independent experiments [(B) and (C)]; *P<0.05; **P<0.01 (ANOVA).
Discussion
Within this study we identify a novel mechanism through which coagulation proteases modulate renal IRI. Specifically, the coagulation protease aPC conveys nephroprotection by sustaining protein stability of YB-1. Several studies demonstrated a role of aPC in modulating mRNA expression by regulating transcription factor activity, mRNA stability, or through epigenetic mechanisms.7,34,35 Within this study we establish that aPC additionally modulates protein stability via the ubiquitin-proteasome system. We show that aPC maintains the 50 kD form of full length YB-1 protein by decreasing the K48-linked YB-1 ubiquitination through an OTUB1-dependent mechanism. These effects of aPC, the sustained expression of OTUB1, the diminished YB-1 ubiquitination, and the preservation of the 50 kD form of YB-1 are required for aPC-mediated nephroprotection after renal IRI. This newly identified nephroprotective mechanism, which depends on the functional interaction of aPC, YB-1, and OTUB1, may lay ground for new translational approaches to renal IRI.
The effect of aPC on YB-1 in renal IRI is independent of its anticoagulant function but requires TM and signaling via the PAR1-EPCR heterodimer.33 Considering the established role of TM in modulating thrombin and aPC activity and previous data, which established a detrimental role of thrombin and PAR1 in renal IRI, we conclude that TM provides a functional switch between thrombin- and aPC-dependent signaling in the context of renal IRI (and current data).5,10 Of note, soluble recombinant TM, but not the direct thrombin inhibitor argatroban, likewise protect kidneys from IRI,12 supporting the notion that a TM-mediated switch from thrombin- to aPC-dependent signaling is required, whereas inhibition of thrombin is not sufficient for nephroprotection in renal IRI. The efficacy of soluble TM in renal IRI suggests that the nephroprotective pathway identified within this study may be amendable to therapeutic interventions not only by aPC, but also by soluble TM.
Previously, the partial thrombin-induced proteolytical degradation of YB-1 was proposed to be independent of ubiquitination.36 However, studies analyzing proteasome-mediated YB-1 degradation have been conducted in a cell-free system, and we acknowledge that evaluation of their findings in cellular systems or in vivo is required.36 Using an in vitro hypoxia-reoxygenation model and the in vivo model of renal IRI, we reveal that YB-1 is ubiquitinated (including K48-linked ubiquitination) and that this is relevant for the regulation of the 50 kD form of YB-1. Polyubiquitination and proteasomal degradation of YB-1 have been previously demonstrated in HEK 293T cells.37,38 Therefore, the adaptor protein F-box protein 33 can bind to YB-1 and integrate it into an SCF E3 ligase complex composed of SKP1, CUL1, and ROC1. This E3 ligase complex induces polyubiquitination and proteasomal degradation of YB-1.37 In addition, the RING finger containing protein retinoblastoma binding protein 6 promotes polyubiquitination and proteasomal destruction of YB-1.38 However, modulation of YB-1 ubiquitination by coagulation proteases controls partial rather than complete proteasomal degradation, suggesting the involvement of a distinct mechanism. Consistently, we demonstrate that aPC modulates YB-1 ubiquitination and stability in part by regulating its interaction with the deubiquitinating enzyme OTUB1. OTUB1 can inhibit ubiquitination of target molecules by two distinct mechanisms. First, OTUB1 can directly remove ubiquitin chains from target molecules, including TRAF3, TRAF6, or c-IAP1.39,40 Second, OTUB1 can segregate E2-E3 complexes and inhibit the transfer of ubiquitin molecules from E2 conjugating enzymes to E3 ligases independent of its catalytic activity.41–43 Although our immunoprecipitation experiments demonstrate that OTUB1 and YB-1 act in close proximity to each other, we currently cannot differentiate which of these two alternative mechanisms inhibit ubiquitination of YB-1. Interestingly, OTUB1 has been proposed to preferentially inhibit K48-linked ubiquitination,40,44 which may specifically avert proteasomal degradation of target proteins, such as YB-1, while still allowing other ubiquitination-dependent processes. Future studies are required to decipher the concise molecular nature of the OTUB1 and YB-1 interaction and the consequences for YB-1 abundance, localization, and activity.
In this study we observed robust expression of YB-1 in tubular cells at baseline, which is contrary to the general perception that YB-1 is a stress-induced gene with low basal expression levels. The high expression of YB-1 within the tubular compartment is consistent with public data (http://www.proteinatlas.org/ENSG00000065978/tissue/kidney) and previous reports.45–47 Despite its high basal renal expression level heterozygosity for YB-1, resulting in a roughly 50% reduction of YB-1 expression,18,29 it did not result in an obvious renal defect. Therefore, YB-1 heterozygosity is compatible with normal renal function in the absence of additional stimuli. However, the marked phenotype in YB-1 heterozygous mice after renal IRI establishes that a partial reduction of YB-1 is sufficient to aggravate renal injury, emphasizing the potential pathophysiologic role of YB-1 in the kidney.
The mechanism through which YB-1 conveys nephroprotection after renal IRI remains to be shown, but it may be related to its function in regulating cell-cycle progression. Therefore, YB-1 induces expression of cyclin A and B1,28,29,48 and its expression is increased in regenerating liver49 or in the proliferating compartment of the colorectal mucosa.50 Renal recovery after IRI is associated with tubular cell proliferation and hence it seems possible, but remains to be shown, that YB-1 promotes renal recovery by promoting tubular proliferation.51 Alternatively, YB-1 has been proposed to block translation of oxidatively modified RNA by binding and sequestering such RNA species.52 Through the preferential binding to oxidized RNA, YB-1 may therefore prevent erroneous protein biosynthesis after renal IRI. The identification of the precise mechanism through which YB-1 conveys nephroprotection after IRI may identify new therapeutic targets for this frequent renal disease.
Taken together, we identify a new nephroprotective pathway, linking the extracellular protease aPC with cytoprotective effects of YB-1. Considering the established relevance of coagulation protease-dependent signaling in various tissues and the broad expression of YB-1, we postulate that the identified mechanism may be relevant in other tissues and disease models. Furthermore, as new and signaling-specific aPC variants (e.g., 3K3A-aPC, soluble TM, PAR agonists) are being evaluated in clinical and preclinical studies, this pathway may be therapeutically amendable in the future.53,54
Concise Methods
Animal Model for Renal IRI
APChigh, TMPro/Pro, and YB-1+/− mice have been previously described.6,18,24 To obtain OTUB1-deficient mice, we generated C57BL/6N-derived Art B6/3.6 embryonic stem cells with a conditional OTUB1 allele by flanking exons 2 and 3 with loxP sites (Supplemental Figure 5). The selection markers neomycin and puromycin were removed from correctly targeted clones by flp-mediated recombination, generating OTUB1loxP/wt mice in which exon 2 and 3 of OTUB1 were flanked by loxP-sites. OTUB1loxP/wt mice were crossed with C57BL/6 Rosa 26-Cre+/− mice to delete exons 2 and 3, resulting in out-of-frame translation and germline inactivation of OTUB1. Offsprings were crossed with WT C57BL/6 mice to generate Cre-negative OTUB1loxP/wt mice (designated as OTUB1+/− mice), which were maintained by breeding with C57BL/6 WT mice. Homozygous OTUB1−/− mice were embryonic lethal and will be published independently. Mice were backcrossed onto the C57BL/6 background for at least four generations. OTUB1+/+ littermates were used for direct comparison.
APChigh mice, TMPro/Pro mice, and YB-1+/− were backcrossed onto the C57BL/6 background for at least eight generations before renal IRI experiments. In all experiments, littermates were used and randomly assigned to experimental groups. Male mice, 8–10 weeks old, underwent bilateral renal pedicle occlusion (30 minutes) and reperfusion for 24 hours (Supplemental Figure 1B, Supplemental Material).55 aPC (0.5 mg/kg body wt, i.p.) was injected 30 minutes before IRI in a subgroup of mice. Control mice underwent sham operation omitting only pedicle clamping. All surgical instruments were obtained from F.S.T (Heidelberg, Germany). Animal experiments were conducted following principles of laboratory animal care and procedures approved by the local Animal Care and Use Committee (Regierungspräsidium Sachsen-Anhalt, Germany).
Preparation of aPC
The aPC used in this study was generated as previously described with slight modification.56,57 See the Supplemental Material for further details.
In vivo aPC Capture Assay
The in vivo aPC activation and capture assay were conducted as previously described.6 See the Supplemental Material for further details.
Determination of Serum BUN and Creatinine
Serum BUN and creatinine were analyzed using commercially available assays. See the Supplemental Material for further details.
Histology and Immunohistochemistry
Tubular injury was determined using a histologic score obtained from hematoxylin and eosin stained images. Expression of YB-1 and OTUB1 was determined by immunohistochemistry following established protocols.6,58 See the Supplemental Material for further details.
Cell Culture and in vitro Model HR
BUMPT cells were cultured according to an established protocol.26 Mouse primary renal proximal tubular epithelial cells were isolated and cultured according to established protocols.59,60 For in vitro HR injury, confluent cells were serum-deprived overnight and then maintained in HBSS (Life Technologies, Darmstadt, Germany) in a hypoxic atmosphere containing 1% O2, 94% N2, and 5% CO2 for 6 hours. For reoxygenation, cells were returned to complete medium and 21% O2. Control cells were serum-starved and maintained in HBSS for 6 hours, but they were continuously exposed to 21% O2. Efficient hypoxic stress was ascertained by determining HIF-1α expression (Supplemental Figure 2, E and F). Cells were harvested at various time points after reoxygenation for RNA and protein isolation. For further details, including pretreatment regimens, see the Supplemental Material.
Immunoblotting and Immunoprecipitation
Immunoblotting and immunoprecipitation were conducted as previously described.6,25 See the Supplemental Material for further details.
RT-PCR
RT-PCR and quantitative RT-PCR were conducted essentially as previously described.6,25,61 For further information see the Supplemental Material.
Generation of Knockdown Cell Lines
For in vitro transfection, undifferentiated BUMPT cells were seeded in six well plates and transfected with YB-1, OTUB1, or control shRNA plasmids (pLKO.1-puro based TRC clones) according to the manufacturer’s instructions (Thermo Fisher Scientific, Waltham, MA). For transfection, 4 µg of shRNA plasmid DNA and 6 μl of Turbofect were added to each well in a final volume of 1.5 ml Opti-MEM. After 6 hours, the medium was replaced by fresh culture medium. Stably transfected cell lines were selected in the presence of 1 µg/ml puromycin. After 7–10 days of selection, cells were subcloned by seeding individual clones into 96 well plates. Approximately 30 clones each were isolated and analyzed by quantitative RT-PCR. Clones with low expression of target genes were selected for further experiments.
Statistical Analyses
The data are expressed as mean±SD. Statistical analyses were performed with the unpaired t test and ANOVA, as appropriate. Posthoc comparisons of ANOVA were corrected with the method of Tukey. statistiXL (www.statistixl.com) and Prism 5 (www.graphpad.com) software were used for statistical analyses. Statistical significance was accepted at values of P<0.05.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank John H. Schwartz (Boston University School of Medicine, Boston) for providing immortalized mouse proximal tubular cell line. We thank Kathrin Deneser, Julia Judin, Juliane Friedrich, René Rudat, and Rumiya Makarova for excellent technical support.
Supported by grants from the Deutsche Forschungsgemeinschaft (IS 67/2-4 to B.I., TH 1789/1-1 to T.M., Me1365/9-1 to P.R.M., SFB 854 and TP01 to P.R.M., TP05 to D.S., TP26 to B.I.), the European Foundation for the Study of Diabetes (to B.I.), the Stiftung für Pathobiochemie und Molekulare Diagnostik (to F.B. and T.M.), and a travel grant from Boehringer Ingelheim (to F.B.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “A Friend in Need: Activated Protein C Stabilizes YB-1 during Renal Ischemia Reperfusion Injury,” on pages 2605–2607.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014080846/-/DCSupplemental.
References
- 1.Luong A, Fragiadaki M, Smith J, Boyle J, Lutz J, Dean JL, Harten S, Ashcroft M, Walmsley SR, Haskard DO, Maxwell PH, Walczak H, Pusey C, Evans PC: Cezanne regulates inflammatory responses to hypoxia in endothelial cells by targeting TRAF6 for deubiquitination. Circ Res 112: 1583–1591, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eltzschig HK, Eckle T: Ischemia and reperfusion--from mechanism to translation. Nat Med 17: 1391–1401, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu S, Soong Y, Seshan SV, Szeto HH: Novel cardiolipin therapeutic protects endothelial mitochondria during renal ischemia and mitigates microvascular rarefaction, inflammation, and fibrosis. Am J Physiol Renal Physiol 306: F970–F980, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Lu CY, Winterberg PD, Chen J, Hartono JR: Acute kidney injury: A conspiracy of toll-like receptor 4 on endothelia, leukocytes, and tubules. Pediatr Nephrol 27: 1847–1854, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sevastos J, Kennedy SE, Davis DR, Sam M, Peake PW, Charlesworth JA, Mackman N, Erlich JH: Tissue factor deficiency and PAR-1 deficiency are protective against renal ischemia reperfusion injury. Blood 109: 577–583, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Isermann B, Vinnikov IA, Madhusudhan T, Herzog S, Kashif M, Blautzik J, Corat MA, Zeier M, Blessing E, Oh J, Gerlitz B, Berg DT, Grinnell BW, Chavakis T, Esmon CT, Weiler H, Bierhaus A, Nawroth PP: Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat Med 13: 1349–1358, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Bock F, Shahzad K, Wang H, Stoyanov S, Wolter J, Dong W, Pelicci PG, Kashif M, Ranjan S, Schmidt S, Ritzel R, Schwenger V, Reymann KG, Esmon CT, Madhusudhan T, Nawroth PP, Isermann B: Activated protein C ameliorates diabetic nephropathy by epigenetically inhibiting the redox enzyme p66Shc. Proc Natl Acad Sci U S A 110: 648–653, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta A, Gerlitz B, Richardson MA, Bull C, Berg DT, Syed S, Galbreath EJ, Swanson BA, Jones BE, Grinnell BW: Distinct functions of activated protein C differentially attenuate acute kidney injury. J Am Soc Nephrol 20: 267–277, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta A, Williams MD, Macias WL, Molitoris BA, Grinnell BW: Activated protein C and acute kidney injury: Selective targeting of PAR-1. Curr Drug Targets 10: 1212–1226, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Weiler H, Isermann BH: Thrombomodulin. J Thromb Haemost 1: 1515–1524, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Sharfuddin AA, Sandoval RM, Berg DT, McDougal GE, Campos SB, Phillips CL, Jones BE, Gupta A, Grinnell BW, Molitoris BA: Soluble thrombomodulin protects ischemic kidneys. J Am Soc Nephrol 20: 524–534, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozaki T, Anas C, Maruyama S, Yamamoto T, Yasuda K, Morita Y, Ito Y, Gotoh M, Yuzawa Y, Matsuo S: Intrarenal administration of recombinant human soluble thrombomodulin ameliorates ischaemic acute renal failure. Nephrol Dial Transplant 23: 110–119, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Conway EM, Van de Wouwer M, Pollefeyt S, Jurk K, Van Aken H, De Vriese A, Weitz JI, Weiler H, Hellings PW, Schaeffer P, Herbert JM, Collen D, Theilmeier G: The lectin-like domain of thrombomodulin confers protection from neutrophil-mediated tissue damage by suppressing adhesion molecule expression via nuclear factor kappaB and mitogen-activated protein kinase pathways. J Exp Med 196: 565–577, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van de Wouwer M, Collen D, Conway EM: Thrombomodulin-protein C-EPCR system: Integrated to regulate coagulation and inflammation. Arterioscler Thromb Vasc Biol 24: 1374–1383, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Park SW, Chen SW, Kim M, D’Agati VD, Lee HT: Human activated protein C attenuates both hepatic and renal injury caused by hepatic ischemia and reperfusion injury in mice. Kidney Int 76: 739–750, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Raffetseder U, Wernert N, Ostendorf T, van Roeyen C, Rauen T, Behrens P, Floege J, Mertens PR: Mesangial cell expression of proto-oncogene Ets-1 during progression of mesangioproliferative glomerulonephritis. Kidney Int 66: 622–632, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Raffetseder U, Liehn EA, Weber C, Mertens PR: Role of cold shock Y-box protein-1 in inflammation, atherosclerosis and organ transplant rejection. Eur J Cell Biol 91: 567–575, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Raffetseder U, Rauen T, Boor P, Ostendorf T, Hanssen L, Floege J, En-Nia A, Djudjaj S, Frye BC, Mertens PR: Extracellular YB-1 blockade in experimental nephritis upregulates Notch-3 receptor expression and signaling. Nephron Exp Nephrol 118: e100–e108, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Hanssen L, Alidousty C, Djudjaj S, Frye BC, Rauen T, Boor P, Mertens PR, van Roeyen CR, Tacke F, Heymann F, Tittel AP, Koch A, Floege J, Ostendorf T, Raffetseder U: YB-1 is an early and central mediator of bacterial and sterile inflammation in vivo. J Immunol 191: 2604–2613, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Eliseeva IA, Kim ER, Guryanov SG, Ovchinnikov LP, Lyabin DN: Y-box-binding protein 1 (YB-1) and its functions. Biochemistry (Mosc) 76: 1402–1433, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Lindquist JA, Brandt S, Bernhardt A, Zhu C, Mertens PR: The role of cold shock domain proteins in inflammatory diseases. J Mol Med (Berl) 92: 207–216, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Stenina OI, Poptic EJ, DiCorleto PE: Thrombin activates a Y box-binding protein (DNA-binding protein B) in endothelial cells. J Clin Invest 106: 579–587, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stenina OI, Shaneyfelt KM, DiCorleto PE: Thrombin induces the release of the Y-box protein dbpB from mRNA: A mechanism of transcriptional activation. Proc Natl Acad Sci U S A 98: 7277–7282, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiler-Guettler H, Christie PD, Beeler DL, Healy AM, Hancock WW, Rayburn H, Edelberg JM, Rosenberg RD: A targeted point mutation in thrombomodulin generates viable mice with a prethrombotic state. J Clin Invest 101: 1983–1991, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madhusudhan T, Wang H, Straub BK, Gröne E, Zhou Q, Shahzad K, Müller-Krebs S, Schwenger V, Gerlitz B, Grinnell BW, Griffin JH, Reiser J, Gröne HJ, Esmon CT, Nawroth PP, Isermann B: Cytoprotective signaling by activated protein C requires protease-activated receptor-3 in podocytes. Blood 119: 874–883, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sinha D, Wang Z, Price VR, Schwartz JH, Lieberthal W: Chemical anoxia of tubular cells induces activation of c-Src and its translocation to the zonula adherens. Am J Physiol Renal Physiol 284: F488–F497, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Takacs-Jarrett M, Sweeney WE, Avner ED, Cotton CU: Morphological and functional characterization of a conditionally immortalized collecting tubule cell line. Am J Physiol 275: F802–F811, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Uchiumi T, Fotovati A, Sasaguri T, Shibahara K, Shimada T, Fukuda T, Nakamura T, Izumi H, Tsuzuki T, Kuwano M, Kohno K: YB-1 is important for an early stage embryonic development: Neural tube formation and cell proliferation. J Biol Chem 281: 40440–40449, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Lu ZH, Books JT, Ley TJ: YB-1 is important for late-stage embryonic development, optimal cellular stress responses, and the prevention of premature senescence. Mol Cell Biol 25: 4625–4637, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klinge S, Voigts-Hoffmann F, Leibundgut M, Arpagaus S, Ban N: Crystal structure of the eukaryotic 60S ribosomal subunit in complex with initiation factor 6. Science 334: 941–948, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Pickart CM, Fushman D: Polyubiquitin chains: Polymeric protein signals. Curr Opin Chem Biol 8: 610–616, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Petrova DT, Brehmer F, Schultze FC, Asif AR, Gross O, Oellerich M, Brandhorst G: Differential kidney proteome profiling in a murine model of renal fibrosis under treatment with mycophenolate mofetil. Pathobiology 78: 162–170, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Mosnier LO, Sinha RK, Burnier L, Bouwens EA, Griffin JH: Biased agonism of protease-activated receptor 1 by activated protein C caused by noncanonical cleavage at Arg46. Blood 120: 5237–5246, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joyce DE, Grinnell BW: Recombinant human activated protein C attenuates the inflammatory response in endothelium and monocytes by modulating nuclear factor-kappaB. Crit Care Med 30[Suppl]: S288–S293, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Brueckmann M, Marx A, Weiler HM, Liebe V, Lang S, Kaden JJ, Zieger W, Borggrefe M, Huhle G, Konstantin Haase K: Stabilization of monocyte chemoattractant protein-1-mRNA by activated protein C. Thromb Haemost 89: 149–160, 2003 [PubMed] [Google Scholar]
- 36.Sorokin AV, Selyutina AA, Skabkin MA, Guryanov SG, Nazimov IV, Richard C, Th’ng J, Yau J, Sorensen PH, Ovchinnikov LP, Evdokimova V: Proteasome-mediated cleavage of the Y-box-binding protein 1 is linked to DNA-damage stress response. EMBO J 24: 3602–3612, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lutz M, Wempe F, Bahr I, Zopf D, von Melchner H: Proteasomal degradation of the multifunctional regulator YB-1 is mediated by an F-Box protein induced during programmed cell death. FEBS Lett 580: 3921–3930, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Chibi M, Meyer M, Skepu A, G Rees DJ, Moolman-Smook JC, Pugh DJ: RBBP6 interacts with multifunctional protein YB-1 through its RING finger domain, leading to ubiquitination and proteosomal degradation of YB-1. J Mol Biol 384: 908–916, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Lawson EA, Miller KK, Bredella MA, Phan C, Misra M, Meenaghan E, Rosenblum L, Donoho D, Gupta R, Klibanski A: Hormone predictors of abnormal bone microarchitecture in women with anorexia nervosa. Bone 46: 458–463, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goncharov T, Niessen K, de Almagro MC, Izrael-Tomasevic A, Fedorova AV, Varfolomeev E, Arnott D, Deshayes K, Kirkpatrick DS, Vucic D: OTUB1 modulates c-IAP1 stability to regulate signalling pathways. EMBO J 32: 1103–1114, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Juang YC, Landry MC, Sanches M, Vittal V, Leung CC, Ceccarelli DF, Mateo AR, Pruneda JN, Mao DY, Szilard RK, Orlicky S, Munro M, Brzovic PS, Klevit RE, Sicheri F, Durocher D: OTUB1 co-opts Lys48-linked ubiquitin recognition to suppress E2 enzyme function. Mol Cell 45: 384–397, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sato Y, Yamagata A, Goto-Ito S, Kubota K, Miyamoto R, Nakada S, Fukai S: Molecular basis of Lys-63-linked polyubiquitination inhibition by the interaction between human deubiquitinating enzyme OTUB1 and ubiquitin-conjugating enzyme UBC13. J Biol Chem 287: 25860–25868, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wiener R, Zhang X, Wang T, Wolberger C: The mechanism of OTUB1-mediated inhibition of ubiquitination. Nature 483: 618–622, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edelmann MJ, Iphöfer A, Akutsu M, Altun M, di Gleria K, Kramer HB, Fiebiger E, Dhe-Paganon S, Kessler BM: Structural basis and specificity of human otubain 1-mediated deubiquitination. Biochem J 418: 379–390, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Ito K, Tsutsumi K, Kuzumaki T, Gomez PF, Otsu K, Ishikawa K: A novel growth-inducible gene that encodes a protein with a conserved cold-shock domain. Nucleic Acids Res 22: 2036–2041, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mastrangelo MA, Kleene KC: Developmental expression of Y-box protein 1 mRNA and alternatively spliced Y-box protein 3 mRNAs in spermatogenic cells in mice. Mol Hum Reprod 6: 779–788, 2000 [DOI] [PubMed] [Google Scholar]
- 47.van Roeyen CR, Eitner F, Martinkus S, Thieltges SR, Ostendorf T, Bokemeyer D, Lüscher B, Lüscher-Firzlaff JM, Floege J, Mertens PR: Y-box protein 1 mediates PDGF-B effects in mesangioproliferative glomerular disease. J Am Soc Nephrol 16: 2985–2996, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Jurchott K, Bergmann S, Stein U, Walther W, Janz M, Manni I, Piaggio G, Fietze E, Dietel M, Royer HD: YB-1 as a cell cycle-regulated transcription factor facilitating cyclin A and cyclin B1 gene expression. J Biol Chem 278: 27988–27996, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Ladomery M, Sommerville J: A role for Y-box proteins in cell proliferation. Bioessays 17: 9–11, 1995 [DOI] [PubMed] [Google Scholar]
- 50.Shibao K, Takano H, Nakayama Y, Okazaki K, Nagata N, Izumi H, Uchiumi T, Kuwano M, Kohno K, Itoh H: Enhanced coexpression of YB-1 and DNA topoisomerase II alpha genes in human colorectal carcinomas. Int J Cancer 83: 732–737, 1999 [DOI] [PubMed] [Google Scholar]
- 51.Canaud G, Bonventre JV: Cell cycle arrest and the evolution of chronic kidney disease from acute kidney injury [published online ahead of print July 12, 2014]. Nephrol Dial Transplant [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hayakawa H, Uchiumi T, Fukuda T, Ashizuka M, Kohno K, Kuwano M, Sekiguchi M: Binding capacity of human YB-1 protein for RNA containing 8-oxoguanine. Biochemistry 41: 12739–12744, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Lyden P, Levy H, Weymer S, Pryor K, Kramer W, Griffin JH, Davis TP, Zlokovic B: Phase 1 safety, tolerability and pharmacokinetics of 3K3A-APC in healthy adult volunteers. Curr Pharm Des 19: 7479–7485, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang L, Jiang R, Sun XL: Recombinant thrombomodulin of different domains for pharmaceutical, biomedical, and cell transplantation applications. Med Res Rev 34: 479–502, 2014 [DOI] [PubMed] [Google Scholar]
- 55.Wei Q, Dong Z: Mouse model of ischemic acute kidney injury: Technical notes and tricks. Am J Physiol Renal Physiol 303: F1487–F1494, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Esmon CT, Esmon NL, Le Bonniec BF, Johnson AE: Protein C activation. Methods Enzymol 222: 359–385, 1993 [DOI] [PubMed] [Google Scholar]
- 57.Taylor FB, Jr, Chang A, Esmon CT, D’Angelo A, Vigano-D’Angelo S, Blick KE: Protein C prevents the coagulopathic and lethal effects of Escherichia coli infusion in the baboon. J Clin Invest 79: 918–925, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang H, Vinnikov I, Shahzad K, Bock F, Ranjan S, Wolter J, Kashif M, Oh J, Bierhaus A, Nawroth P, Kirschfink M, Conway EM, Madhusudhan T, Isermann B: The lectin-like domain of thrombomodulin ameliorates diabetic glomerulopathy via complement inhibition. Thromb Haemost 108: 1141–1153, 2012 [DOI] [PubMed] [Google Scholar]
- 59.Sheridan AM, Schwartz JH, Kroshian VM, Tercyak AM, Laraia J, Masino S, Lieberthal W: Renal mouse proximal tubular cells are more susceptible than MDCK cells to chemical anoxia. Am J Physiol 265: F342–F350, 1993 [DOI] [PubMed] [Google Scholar]
- 60.Breggia AC, Himmelfarb J: Primary mouse renal tubular epithelial cells have variable injury tolerance to ischemic and chemical mediators of oxidative stress. Oxid Med Cell Longev 1: 33–38, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kashif M, Hellwig A, Hashemolhosseini S, Kumar V, Bock F, Wang H, Shahzad K, Ranjan S, Wolter J, Madhusudhan T, Bierhaus A, Nawroth P, Isermann B: Nuclear factor erythroid-derived 2 (Nfe2) regulates JunD DNA-binding activity via acetylation: A novel mechanism regulating trophoblast differentiation. J Biol Chem 287: 5400–5411, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.