Abstract
Podocyte injury and loss mark an early step in the pathogenesis of various glomerular diseases, making these cells excellent targets for therapeutics. However, cell–based high–throughput screening assays for the rational development of podocyte-directed therapeutics are currently lacking. Here, we describe a novel high–content screening–based phenotypic assay that analyzes thousands of podocytes per assay condition in 96-well plates to quantitatively measure dose-dependent changes in multiple cellular features. Our assay consistently produced a Z′ value >0.44, making it suitable for compound screening. On screening with >2100 pharmacologically active agents, we identified 24 small molecules that protected podocytes against injury in vitro (1% hit rate). Among the identified hits, we confirmed an β1-integrin agonist, pyrintegrin, as a podocyte-protective agent. Treatment with pyrintegrin prevented damage-induced decreases in F-actin stress fibers, focal adhesions, and active β1-integrin levels in cultured cells. In vivo, administration of pyrintegrin protected mice from LPS–induced podocyte foot process effacement and proteinuria. Analysis of the murine glomeruli showed that LPS administration reduced the levels of active β1 integrin in the podocytes, which was prevented by cotreatment with pyrintegrin. In rats, pyrintegrin reduced peak proteinuria caused by puromycin aminonucleoside–induced nephropathy. Our findings identify pyrintegrin as a potential therapeutic candidate and show the use of podocyte–based screening assays for identifying novel therapeutics for proteinuric kidney diseases.
Keywords: glomerular epithelial cells, glomerular disease, adhesion molecule
Podocytes are terminally differentiated visceral epithelial cells that wrap the glomerular capillaries with foot processes (FPs) constituting essential components for kidney structure and function.1–3 Intracellular F-actin–based cytoskeleton stabilizes FPs and is further linked through focal contacts to the highly surface–expressed adhesion receptors, such as integrins-α3β1 and -αvβ3, which allows podocytes to form strong adhesive bonds with the glomerular basement membrane (GBM).4 FP effacement is an early finding in many glomerular diseases and accompanies loss of podocyte slit diaphragm and occurrence of proteinuria. Progressive FP effacement leads to podocyte loss, ultimately leading to glomerulosclerosis.5 Thus, agents that directly target podocytes and protect them from FP effacement can be developed into targeted therapeutics against glomerular diseases. Indeed, many currently used renoprotective drugs, such as glucocorticoids and antirejection agents, have been shown to impart their in vivo efficacy, in part, by directly targeting signaling pathways in podocytes.6 However, a key hurdle in the identification and rational development of specific podocyte–directed therapeutics is a lack of automated cell–based screening assays.7
Studies with cultured podocytes in vitro have shown that the injury–induced cytoskeletal changes, such as the loss of F-actin stress fibers and the changes in lamellipodia, mimic the podocyte changes in vivo.2,4,7,8 Furthermore, the rescue of these cellular phenotypes in vitro by genetic or chemical means correlates with protection from FP effacement and proteinuria in vivo. This suggests that assays quantifying such phenotypic changes in podocytes could be useful for the discovery of novel podocyte protective therapeutics.7 Here, we describe a novel podocyte cell–based phenotypic assay for use in an high throughput screening (HTS) environment and apply it to identify novel podocyte–protective small molecules. The assay uses an automated microscopy–based high–content screening (HCS) platform to quantify changes in a variety of cellular features, such as cell size and morphology, F-actin fibers, fiber intensity, and focal adhesions, with direct relevance for the treatment of human glomerular diseases.
Results
Design of a Podocyte HCS Assay
We designed a new cell imaging–based phenotypic assay with cultured podocytes (schematically described in Figure 1A) to quantify cellular changes and determined whether such an assay would provide unbiased, reproducible, and quantitative measurements of phenotypic changes in cells for use in an HTS environment. We chose the immortalized murine podocytes9 as a robust cell line for the initial assay development rather than the immortalized human podocytes10 or primary cells, because not all primary or human cells in the population elaborate F-actin structure uniformly as well as the murine cells. We also slightly modified the existing cell culture protocol to obtain similar numbers of differentiated podocytes in each well of a 96-well plate and reduce well-to-well variability. We found that culturing podocytes at 37°C for the first 7 days of differentiation in large tissue culture flasks and subsequently reseeding and further culturing them at 37°C in the 96-well assay plates for the next 4–6 days provided us with low well-to-well variability in cell number in the assay plates and healthy podocytes expressing specific and well characterized podocyte markers synaptopodin and podocin (Supplemental Figure 1).
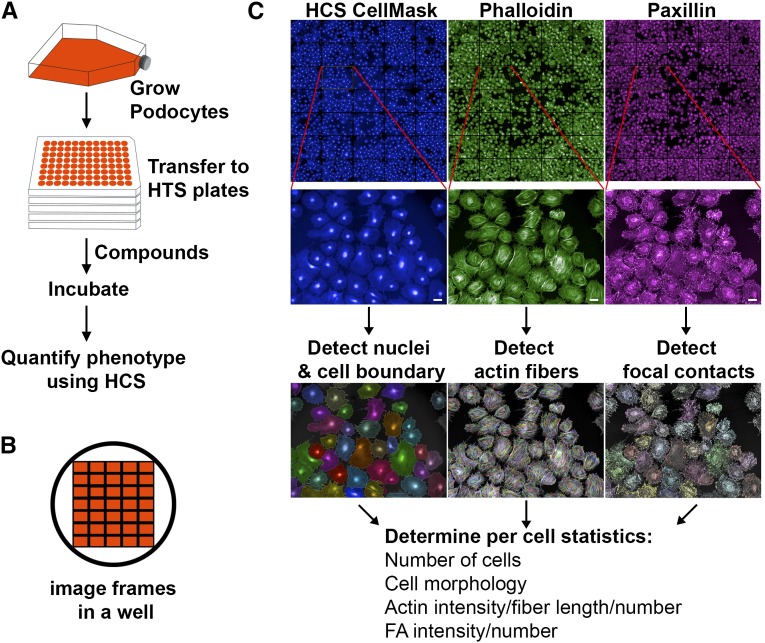
Figure 1.
High content imaging and automated analysis can be used to design a podocyte phenotypic assay. (A) Schematic of the assay design. Immortalized podocytes were proliferated under permissive conditions (33°C) in large tissue culture flasks and thermoshifted to 37°C to induce differentiation according to published protocols.9,10 After 7 days in large flasks, cells were trypsinized, counted, and transferred to multiwell plates, and they were further cultured at 37°C for another 4 days and subsequently used in an HCS assay. (B) Schematic of a well of a 96-well optical plate and the layout of various imaging frames that were typically captured using an HCS imaging system. (C) HCS–based image analysis of a podocyte phenotypic assay. Podocytes in multiwell optical plates were washed, fixed with paraformaldehyde, fluorescently stained with various markers, and imaged using a PerkinElmer Opera HCS microscope. Images show a representative composite montage of multiple frames (three different channels) from a well of an optical multiwell plate after staining podocytes with HCS CellMask Blue, phallodin, and paxillin, which are labeled with different fluorophores. Zoomed-in images from single frames are shown below. Automated detection of nuclei and assignment of cellular regions to nuclei were performed (using Columbus Analysis System) from the segmented CellMask Blue images. Morphology properties were then calculated to determine cell area and roundness. Images from phalloidin-stained cells were used to identify actin fibers, and from cells stained with antipaxillin (or antivinculin), antibodies were used to find focal adhesions (FA). Subsequently, the detected nuclei and cell regions were merged with the detected actin fibers and focal adhesions to quantify the various phenotypic parameters on a per-cell basis. Scale bar, 50 μm.
Next, we tested the applicability of image–based HCS assays for quantifying podocyte phenotypes using a series of steps as described in Figure 1. Podocytes were cultured in 96-well optical plates, fixed using paraformaldehyde, and stained with a variety of markers. Then, multichannel fluorescent images of multiple fields in each well were obtained using the automated confocal microscopy–based Opera HCS System. Images acquired on staining with HCS CellMask Blue were used for automated detection of cellular nuclei, intercell boundaries, definition of cellular regions, and the level of background using the Find Nuclei and Find Cytoplasm modules (Columbus Image Analysis Suite). Cellular properties, such as cell number, size, and roundness, were obtained using the Calculate Morphology Properties tool. Next, the Find Spots analysis module was used with Alexa568–labeled phalloidin–stained cells to detect and quantify F-actin fibers. Similarly, cells immunostained with anti-paxillin (or anti-vinculin) antibodies were used to detect focal adhesions. After the detection, the number of F-actin fibers and the number of focal adhesions were calculated on a per-cell basis using Columbus Image Analysis Suite. This provided us with a method for robust cell segmentation and the quantification of the cell shape and morphology, the number and intensity of actin cytoskeletal fibers, and the number and intensity of focal adhesions (and other markers) with high accuracy and low variability for every cell in a frame and from multiple frames per well (Figure 1C).
Development of a Podocyte–Based HCS Assay
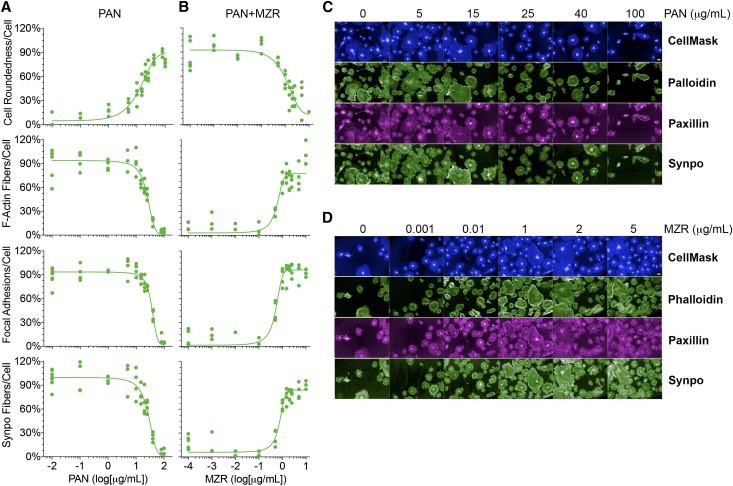
To quantitatively determine changes in podocyte phenotype with injury, we used puromycin aminonucleoside (PAN) as the damaging agent. PAN-induced nephropathy in rats causes podocyte damage, resulting in podocyte FP effacement and high-grade proteinuria, and is a widely used experimental model for human minimal change disease and/or FSGS.1,11–14 In vitro, PAN causes podocyte blebbing and rounding, loss of actin stress fibers, reduced focal contacts and adhesion, reactive oxygen species–mediated damage, and eventually, cell death.15,16 We treated podocytes with various doses of PAN and quantified changes in cellular features using our HCS assay system (Figure 2). This comprehensive cellular analysis used images of hundreds of cells per well to quantify various cellular features in an automated and nonbiased fashion. PAN also resulted in increased cell roundness. It shows that a PAN concentration of 16 μg/ml caused half-maximal damage (half–maximal inhibitory concentration), and that 30 μg/ml PAN was sufficient to cause significant podocyte injury. Mizoribine (MZR) is an imidazole nucleoside immunosuppressive agent that has been used in renal transplantation and treatment of steroid–resistant nephrotic syndrome.17 MZR has also been shown to protect podocytes from PAN injury.18 Treatment of podocytes with MZR showed a dose-dependent protection from PAN-induced injury (30 μg/ml), which was determined from an increase in F-actin fiber count, increased focal adhesions, increased synaptopodin levels, and decreased cell roundness. An MZR concentration of 1.4 μg/ml was sufficient to reduce PAN damage by one half (half–maximal effective concentration) and afforded complete protection of podocytes from PAN injury at 5 μg/ml, which is in good agreement with the reported values in the literature.18 Furthermore, to study if other known podocyte-protective agents would provide similar readouts in our novel assay, we investigated the in vitro effects of glucocorticoid dexamethasone (DEX) on podocytes. Glucocorticoids are widely used to treat a variety of glomerular diseases, and studies have shown that DEX also directly targets and protects podocytes from injury.19–22 Analysis of PAN-injured podocytes treated with DEX showed that DEX provides dose-dependent protection of podocytes from PAN-induced injury (30 μg/ml), which was determined from an increase in F-actin fiber count (Supplemental Figure 2), and that DEX as low as 0.1 μM was able to protect cells from PAN damage, which is in excellent agreement with the published literature.21 This suggests that this phenotypic HCS assay is quite reliable and not limited to a single agent to quantitatively differentiate damaged podocytes from healthy cells.
Figure 2.
The novel assay quantitatively measures phenotypic changes in podocytes. (A) PAN induces dose–dependent podocyte damage. Podocytes in 96-well optical plates were treated with an increasing dose of PAN at 37°C for 48 hours, and the cellular damage was assessed on staining podocytes with CellMask Blue (to measure cell morphology), phalloidin (to quantify F-actin fibers), anti-paxillin antibody (to quantify focal adhesions), and anti-synaptopodin (anti-synpo) antibody (to quantify synaptopodin levels) and analyzing them using the newly developed HCS assay. Dose-response curves showing the effects of increasing concentrations of PAN on four different cellular parameters (cell morphology [as defined by cell roundness], the number of actin fibers per cell, the number of focal adhesions per cell, and the number of synpo fibers per cell) as a way to measure PAN–induced podocyte injury. The x axis represents PAN concentration, and the y axis shows quantification of each of four parameters at a defined dose of PAN. Data shown are means±SEMs per cell from a single-assay well (n=500–1000 cells) performed in three replicate wells. (B) MZR dose dependently protects podocyte from PAN injury. Podocytes in 96-well optical plates were cotreated with PAN (30 μg/ml) and an increasing concentration of MZR at 37°C for 48 hours, and the cellular damage was assessed using an HCS system. Dose-response curves showing the protective effects of increasing concentration of MZR on various cellular parameters (as with PAN treatment) are presented. Data shown are means±SEMs per cell from a single-assay well (n=500–1000 cells) performed in three replicate wells. (C) Representative fluorescence images of cells treated with increasing doses of PAN (as shown), stained with CellMask Blue, phalloidin, antipaxillin antibody, or antisynpo antibody, and quantified as shown in A. Scale bar, 50 μm. (D) Representative fluorescence images of cells cotreated with PAN (30 μg/ml) and an increasing dose of MZR (as shown), stained with CellMask Blue, phalloidin, anti-paxillin antibody, or anti-synpo antibody, and quantified as shown in B. Scale bar, 50 μm.
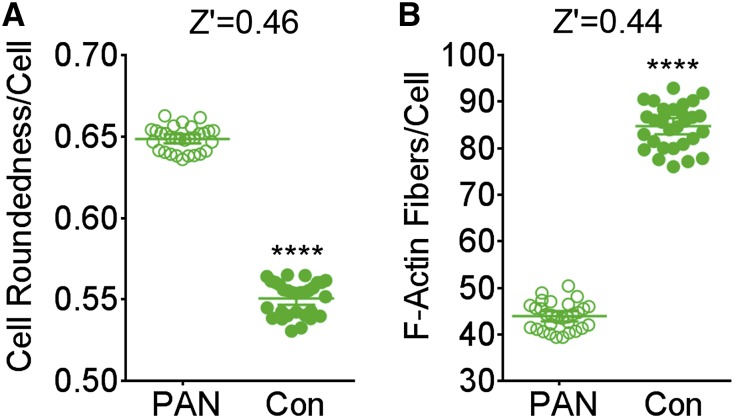
To determine the robustness of our optimized assay for use in a screening environment, we evaluated the reproducibility and variability in the assay and analyses by determining the Z′ values across multiple replicates on a plate.23 Podocytes in multiple wells were either cultured in the cell culture media or treated with PAN (16 μg/ml) for 48 hours, and the phenotypic parameters for cell roundness and F-actin fibers from each of the wells were quantified. Results show a statistically significant difference (P<0.001) between the PAN and the healthy control cells (Figure 3). Additionally, the 95% confidence intervals show nice separation for both of the phenotypes. It also shows that the Z′ value for quantified change in a simple cell morphology phenotype (cell roundness) was ≥0.46, suggesting that this simple parameter presented low variability and high compatibility with the HCS environment and high enough robustness for the screening assay. Quantification of F-actin fibers per cell per well provided us with a similar Z′ value (0.44). We also performed a multiparametric analysis using a machine learning algorithm (Linear Classifier in Columbus Analysis Software) to define and phenotypically segment damaged cells from healthy cells within a population (Supplemental Figure 3). It also provided highly robust dose-response curves for PAN injury versus healthy controls (Supplemental Figure 3B) and an assay Z′ value of 0.69 (Supplemental Figure 3C). All of these analyses suggest that a podocyte–based phenotypic assay is amenable to the HCS methodology. On the basis of the data presented above, we chose to use cell roundness as the phenotype for our primary HCS assay with a chemical library to minimize the imaging and image analysis time. Additionally, the podocytes were treated with PAN alone (15–30 μg/ml; negative control) or PAN with 5 μg/ml MZR (positive control) for 48 hours as the optimum conditions for detailed high–content analysis to ensure the capture of a broad range of phenotypic responses.
Figure 3.
Podocyte phenotypic assay has low variability. Graphs showing analysis of assay variability of the newly developed podocyte cell–based assay using automated microscope–based quantification of (A) cell roundness (morphology) or (B) F-actin fibers. Each graph shows quantified per-cell parameters from individual wells per condition (n=500–1000 cells per well) and the calculated means±95% confidence intervals across 30 wells and is representative of at least two independent assays. The calculated Z' value between cells treated with PAN alone (damaged) or media alone (Con) is also shown. In both types of analyses, Z' value is >0.44. ****P<0.001.
HCS Assay–Based Identification of Novel Agents That Protect Podocytes from PAN Injury
To identify small molecules that protect podocytes from PAN injury, we screened a chemical library of 2121 pharmacologically active compounds (Supplemental Table 1). Podocytes were treated with compounds from the chemical library in the presence of PAN (16 μg/ml) at 37°C for 48 hours. The assay plates also contained eight positive (PAN and MZR treatment) and eight negative (PAN treatment) control wells. Wells were imaged and analyzed, and the mean cell roundness per cell was calculated for each well. Mean cell roundness was normalized using plate–based positive and negative controls, and a Z score for the change in cell morphology on treatment with each compound (SD away from the plate mean) was calculated. The results from the screen are presented as a graph in Figure 4A. Wells with nuclei counts >200 were used in the assay (1985 compounds; average of 576 cells per well) (Figure 4B), and the rest (136 compounds) were flagged and removed from analyses as potentially highly toxic compounds (frequency plot presented in Supplemental Figure 4A shows a bell-shaped curve bottoming out at nuclei count <200). The distribution of the Z-score values was bell shaped (frequency plot is shown in Supplemental Figure 4B). Primary hits (compounds that protected podocytes from PAN damage) were identified from the assay using a Z-score cutoff of ≤−1.28 corresponding to the 10% population on one side of the bell-shaped curve. As a reference, MZR showed a Z score of −2.31±0.2 in the assay. Our analysis resulted in an initial identification of 34 compounds as primary hits (Table 1). Given that our assay is image based, the images from all initial hits were visually inspected manually, which eliminated 10 compounds as nonhits. This resulted in a curated primary hit list of 24 compounds (approximately 1% hit rate). Representative images are shown in Supplemental Figure 5. The identified list of primary hit compounds included three Rho kinase inhibitors (Y27632, GSK 429286, and Thiazovivin) and a p38 kinase inhibitor (SB 203580). Inhibition of both Rho kinase and the p38 mitogen-activated protein kinase (MAPK) has previously been shown to protect podocytes from injury in vitro and ameliorate proteinuria in vivo,24–26 which provides us with excellent internal positive controls and assay validation.
Figure 4.
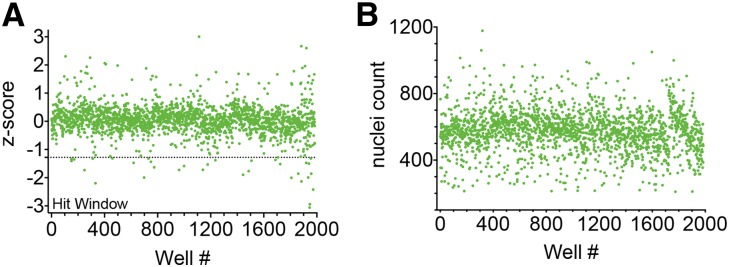
HCS assay using a library of bioactive compounds identifies novel podocyte-protective agents. (A) A graph showing results from the screening of a chemical library using the podocyte HCS assay. Podocytes in 96-well optical plates were treated with PAN (16 μg/ml) at 37°C for 48 hours, and 2121 pharmacologically active compounds were screened against it in the phenotypic assay. Each data point represents measured activity of each compound on cell roundness phenotype and its calculated Z score (Z score=0 means inactive compound, a positive value represents compounds that produce worse phenotypes, and a negative Z score represents active compounds). A dotted line marks the Z-score threshold of ≤−1.28 that was applied to obtain 34 primary hits in the hit window. Visual confirmation led to a final list of 24 active compounds. (B) A graph showing the nuclei count in each of the assay wells from the primary screen presented in A. Compounds resulting in a nuclei count of <200 were removed from the analyses as potentially cytotoxic agents.
Table 1.
List of primary hits from phenotypic screen with podocytes
| Compound Name | Molecular Weight | Known Bioactivity | Z Score | Confirmation on Visual Check? |
|---|---|---|---|---|
| Agelasine | 458.1 | Cytotoxic, antineoplastic | −1.40 | Yes |
| Altrenogest | 310.4 | Progestin, antineoplastic | −1.44 | Yes |
| Antimycin A | 534.6 | Antifungal, depletes ATP, interferes with cytochrome oxidation | −1.77 | Yes |
| Apigenin | 270.2 | CK-II inhibitor, induces autophagy | −1.50 | Yes |
| Berberine chloride | 371.8 | Antiarrhythmic, α2-agonist, cholinesterase | −1.89 | Yes |
| Bupropion | 276.2 | Antidepressant | −1.63 | Yes |
| Chloroguanide.HCl | 290.2 | Antimalarial | −1.99 | Yes |
| Derrusnin | 356.3 | Mechanism of action unknown | −1.52 | Yes |
| Diosmetin | 300.3 | CYP1A1 inhibitor, antioxidant | −1.72 | Yes |
| Doxazosin mesylate | 547.6 | Antihypertensive | −1.53 | Yes |
| GSK 429286 | 432.4 | ROCK inhibitor | −2.94 | Yes |
| GSK3b inhibitor | 327.2 | GSK-3b inhibitor | −3.06 | Yes |
| Mundoserone | 342.4 | Natural product, rotenone analog, mechanism of action unknown | −1.72 | Yes |
| Mundulone acetate | 476.5 | Mechanism of action unknown | −1.28 | Yes |
| Norgestimate | 369.5 | Progestin | −1.32 | Yes |
| Papaverine hydrochloride | 375.9 | Muscle relaxant (smooth), cerebral vasodilator | −1.41 | Yes |
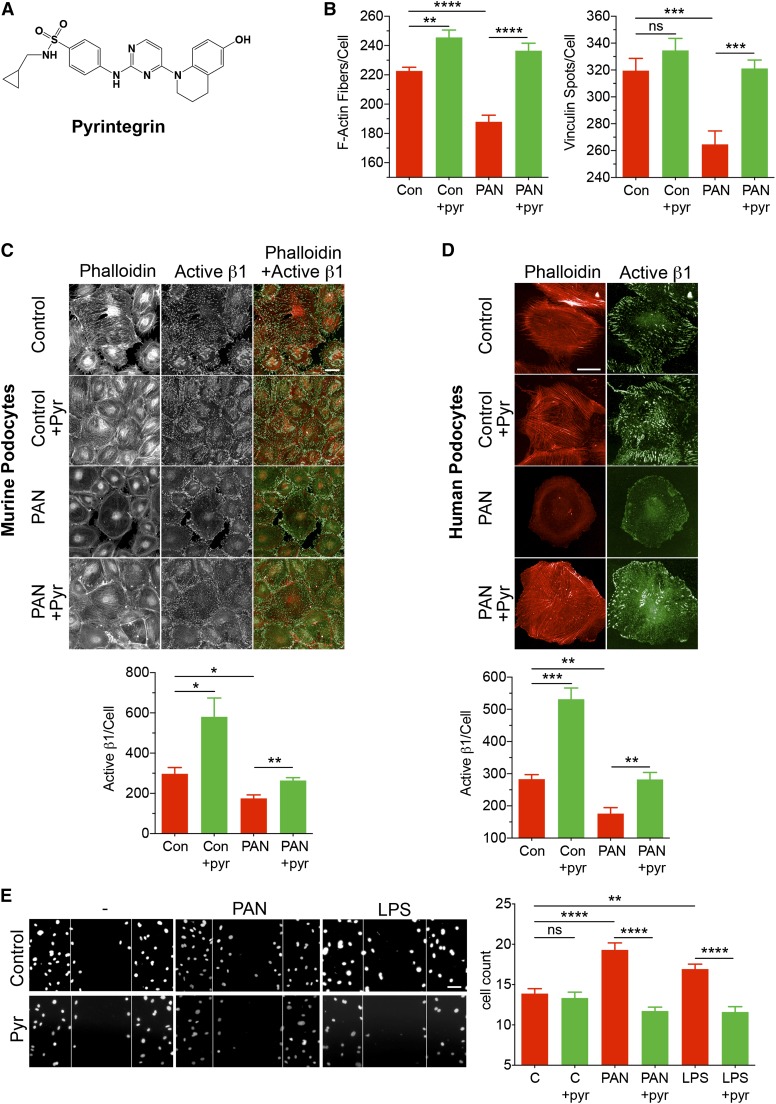
| Pyrintegrin | 451.5 | β1-Integrin agonist | −1.86 | Yes |
| Quinamide isopropylidene | 231.3 | Mechanism of action unknown | −1.59 | Yes |
| Rosolic acid | 290.3 | Diagnostic aid | −1.47 | Yes |
| Salicyl alcohol | 124.1 | Anesthetic (local), anti-inflammatory | −1.32 | Yes |
| SB 203580 | 377.4 | p38 MAPK inhibitor | −2.13 | Yes |
| Thiazovivin | 311.4 | ROCK inhibitor | −2.42 | Yes |
| Thioguanine | 167.2 | Antineoplastic, purine antimetabolite | −2.20 | Yes |
| Y27632 | 320.3 | ROCK inhibitor | −1.99 | Yes |
| Alsterpaullone | 293.3 | CDK inhibitor | −1.32 | No |
| Aurothioglucose | 392.2 | Antirheumatic | −1.73 | No |
| Cloxyquin | 179.6 | Antibacterial, antifungal | −1.35 | No |
| Cryptotanshinone | 296.4 | Angiogenesis inhibitor | −1.37 | No |
| Hinokitiol | 164.2 | Antifungal, mettaloprotease inhibitor, DNA synthesis inhibitor | −2.00 | No |
| Osajin | 404.5 | Antioxidant | −1.38 | No |
| Oxyquinoline hemisulfate | 243.2 | Anti-infectant | −1.34 | No |
| Quercetin tetramethylether | 358.4 | AD-R ligand, antibacterial | −1.86 | No |
| SB 202190 | 331.3 | p38 MAPK inhibitor | −1.47 | No |
| Trimethoprim | 290.3 | Antibacterial | −1.32 | No |
HCS assay–based identification of compounds that protect podocytes from injury. List of compounds identified as hits in the primary screen. The table shows compound name, its molecular weight, known biologic activity, and the Z score in the HCS assay. The last column shows whether visual inspection of the images of wells verified the compound as a hit in the primary screen. CK-II, casein kinase II; CYP1A1, cytochrome p450, family 1, subfamily A, polypeptide 1; ROCK, Rho-associated protein kinase; CDK, cyclin-dependent kinase; AD-R, adenosine receptor.
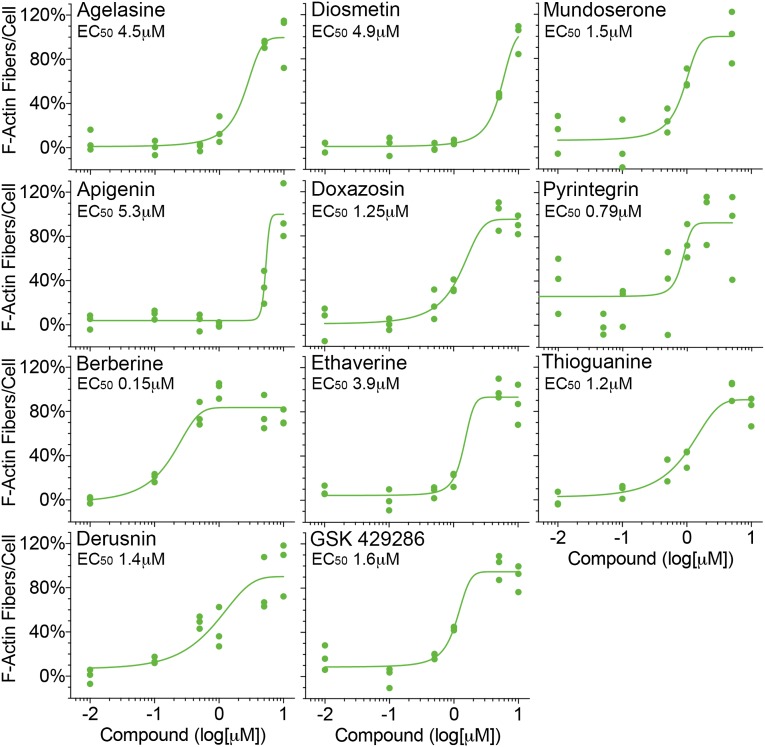
Next, to validate the hit compounds identified from our screen, selected primary hits were reanalyzed using independently obtained powder forms of the compounds. All of the chosen hits provided dose-dependent protection of podocytes from PAN injury (Figure 5), showing the reliability and robustness of the assay.
Figure 5.
Independent assays with select primary hits show dose-dependent protection of podocytes, confirming their validity as a hit. Graphs showing dose-dependent protection of podocytes from PAN injury by compounds identified in the primary screen. The name of each of the selected active compounds is shown on each graph. Podocytes in 96-well optical plates were incubated with PAN (16 μg/ml) at 37°C for 48 hours in the presence of increasing doses of each of the selected compounds, and the cellular damage was quantified by measuring change in F-actin fibers per cell. F-actin fiber counts from podocytes treated with PAN alone (16 μg/ml; negative control) or PAN (16 μg/ml) and MZR (5 μg/ml; positive control) were set at 0% and 100%, respectively, to normalize the data. Each data point on the graphs represents normalized per-cell F-actin fiber count from individual wells per condition (n=500–1000 cells per well). Curve fitting was used to calculate apparent half–maximal effective concentration (EC50) values (green line) and shows a dose-dependent protection of podocytes from PAN damage by each compound.
A Small Molecule Integrin-β1 Agonist Protects Podocytes from Injury
Integrin-α3β1 is highly expressed in podocytes and primarily binds to the GBM–expressed ligand laminin, thereby mediating stable adhesion of podocytes to the GBM.27 Functional α3β1 is essential for podocyte health and function and maintaining the integrity of podocytes and the glomerular filtration barrier.28–33 PAN treatment also reduces expression of α3β1 in podocytes.32 Damaging agents, protein mutations, or deletions that destabilize active β1 by reducing either its activation or its expression also result in podocyte damage and cause proteinuria in animals and patients.30–33 Therefore, we chose to validate a top primary hit in our podocyte–based HCS screen (i.e., the small molecule β1-agonist pyrintegrin).34
To test, we investigated the efficacy of pyrintegrin (chemical structure shown in Figure 6A) in vitro. Pyrintegrin dose dependently protected podocytes from PAN-induced damage, with an apparent half–maximal effective concentration of 0.8 μM (Figure 5), and showed protection of a variety of cellular markers (Supplemental Figure 6). Pyrintegrin also protected podocytes from the loss of actin stress fibers and focal adhesion complexes on PAN-induced injury (Figure 6B). Interestingly, treatment of healthy cells with pyrintegrin also showed a small but significant increase in actin stress fibers. Given that pyrintegrin affects integrin-β1,34 we quantified the level of β1 in treated cells. We found that PAN reduced β1 levels in podocytes, which were preserved on cotreatment with pyrintegrin (Supplemental Figure 6). Furthermore, we also studied the level of active β1 using antibody 9EG7, which specifically recognizes an activation epitope in β1 integrin.35 Results showed that PAN caused a significant loss of active β1 levels in podocytes but not on treatment with pyrintegrin (Figure 6C). Treatment of healthy cells with pyrintegrin also showed a significant increase in active β1 levels. Pyrintegrin also showed a similar protection of podocytes from LPS-mediated damage (Supplemental Figure 7).36 Finally, to determine whether hits identified from our HCS assay, which is on the basis of murine podocytes, would be applicable to the human system, we tested the efficacy of pyrintegrin in protecting human podocytes from damage. As shown in Figure 6D, treatment of human podocytes with PAN also produced a significant reduction in the level of active β1 integrin (as measured by staining with antibody against activation-dependent epitope in human β1 integrin 12G1037) that was rescued on treatment with pyrintegrin, suggesting that pyrintegrin is equally effective on human podocytes.
Figure 6.
An β1-integrin agonist pyrintegrin (pyr) protects podocytes from damage. (A) The published chemical structure of pyr.34 (B–D) Pyr protects podocytes from PAN-induced loss of F-actin fibers and focal adhesions and enhances active β1-integrin levels. Podocytes in 96-well optical plates were cultured at 37°C for 48 hours in the absence (control [Con]) or presence of PAN (30 μg/ml) and cotreated with vehicle (DMSO; 1%) or pyr (1 μM). The cellular damage was assessed after staining the cells with phalloidin, anti-vinculin, and anti-active β1-integrin antibodies and quantified using the HCS system. (B) Graphs showing the effect of pyr on the number of F-actin fibers per cell and the number of vinculin spots per cell under each treatment condition. Data shown are means±SEMs per cell from three replicate wells (n=500–1000 cells per well). **P<0.01; ***P<0.001; ****P<0.001. (C) Representative fluorescence images of murine podocytes after various treatments and after staining with phalloidin and antiactive β1 antibody. Two–color costained images show cells stained with phalloidin (red) and anti-active β1 antibody 9EG7 (green). Images were acquired using the Opera HCS System. The graph (lower panel) shows quantification of the active β1-integrin spot intensity per cell under each treatment condition. Data shown are means±SEMs per cell from four to five replicate wells (n=500–1000 cells per well). Scale bar, 50 μm. *P<0.01; **P<0.001. (D) Representative fluorescence images of human podocytes after various treatments as shown. Images show cells stained with phalloidin (red) and anti-active β1-integrin antibody 12G10 (green). Images were acquired using the Opera HCS System. The graph (lower panel) shows quantification of the active β1-integrin spots per cell under each treatment condition. Data shown are medians±SEMs per cell from four to five replicate wells (n=500–1000 cells per well). Scale bar, 50 μm. **P<0.01; ***P<0.001. (E) Pyr reduces injury-mediated increase in podocyte migration in a scratch wound–healing assay. Representative images (left panel) and a bar graph (right panel) showing confocal microscopy–based quantitation of the number of migrating podocytes in a scratch wound–healing assay. Wounds were created in podocyte monolayers using sterile pipette tips, and the cells were incubated in the absence (−) or presence of PAN or LPS at 37°C for 48 hours. One set of wounds was cotreated with pyr. Subsequently, cells were fixed and stained with 4′,6-diamidino-2-phenylindole (DAPI), and the cells migrating inside the edge of the wounds were quantified. Lines represent the wound margins at the beginning of the experiment (0 hours). The data presented in the graph are plotted as means±SEMs (n≥15). C, control. Scale bar, 50 μm. **P<0.01; ****P<0.001.
Pyrintegrin Blocks PAN– and LPS–Induced Podocyte Migration
Next, we assessed the efficacy of this small molecule in a functional assay. In vitro, PAN- or LPS-mediated injury promotes directional migration and wound closure by podocytes in a scratch wound–healing assay.38 Whereas both PAN and LPS treatments significantly increased migration of treated podocytes after 48 hours (Figure 6E), pyrintegrin cotreatment significantly reduced podocyte motility and wound closure under both conditions. This suggests that pyrintegrin treatment protects podocytes from injury through integrin-β1.
Pyrintegrin Ameliorates Albuminuria In Vivo
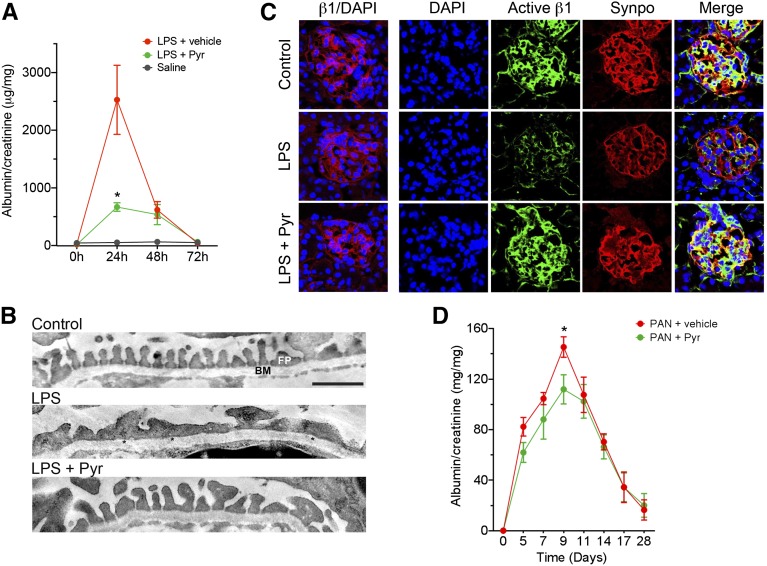
Low-dose LPS decreases β1 integrin activation in podocytes and causes podocyte damage and proteinuria in mice.30 Here, we used this model to test whether pyrintegrin, by preserving active β1 integrin in podocytes, is able to suppress podocyte FP effacement and the development of proteinuria in live animals. LPS administration into C57BL/6 mice resulted in a strong induction of proteinuria after 24 hours (2.5±0.6 mg albumin per 1 mg creatinine) compared with saline-injected animals (0.052±0.022) (Figure 7A). Ultrastructural analysis showed that it also produced podocyte FP effacement (Figure 7B). However, coadministration of the β1 agonist pyrintegrin (10 mg/kg) provided a significant protection for these animals from LPS-induced proteinuria (0.668±0.074) (Figure 7A) and FP effacement (Figure 7B). Analysis of glomeruli from control animals using confocal microscopy and the β1 activation–specific antibody 9EG7 (and costaining with the podocyte–specific marker synaptopodin39) showed high expression and localization in the podocytes (Figure 7C). LPS treatment resulted in decreased active β1 integrin and synaptopodin staining in the glomeruli, with very little change in the level of total β1 integrin. In contrast, animals treated with pyrintegrin showed a significant protection of the active β1 levels, similar to its levels in saline–treated control mice. This suggests that the newly identified podocyte–protective compound pyrintegrin is active in vivo and protects animals from proteinuric kidney injury by activation of podocyte β1 integrin.
Figure 7.
β1-Integrin agonist protects animals against proteinuria. (A–C) Pyrintegrin (Pyr) protects mice from LPS-induced proteinuria. (A) Graph showing the ratio of albumin to creatinine in the urine of mice at the indicated time points after LPS administration and treatment with either Pyr (LPS+Pyr) or vehicle alone (LPS+vehicle). Control animals were administered saline alone. Data shown are means±SEMs (n=6–13 per group). *P<0.05. (B) Pyr protects against LPS–induced podocyte FP effacement. Representative electron microscopy image of mouse glomeruli treated with saline alone (control), LPS and vehicle (LPS), or LPS and Pyr (LPS+Pyr). *Effaced FPs in LPS-treated sections. BM, basement membrane. Scale bar, 1 μm. (C) Pyr preserves the level of active β1-integrin expression in the glomeruli. Representative confocal microscopy images of immunofluorescently labeled glomeruli from animals 24 hours after LPS administration. Frozen kidney sections from animals treated with saline alone (control), LPS and vehicle (LPS) or LPS and Pyr (LPS+Pyr) were imaged after staining with 4′,6-diamidino-2-phenylindole (DAPI) and antibodies against total β1 integrin (β1/DAPI), active β1 integrin (active β1), or synaptopodin (synpo). Merged active β1 and synpo channels are also shown (merge column). Scale bar, 25 μm. (D) Pyr protects rats from PAN-induced nephropathy. Graph showing the ratio of albumin to creatinine in the urine of rats at the indicated time points after PAN administration and treatment with either Pyr (PAN+Pyr) or vehicle alone (PAN+vehicle). Data shown are means±SEMs (n=4–6 per group). *P<0.05.
Given that pyrintegrin protected podocytes from PAN-induced damage in vitro, we also tested the efficacy of pyrintegrin in a PAN injury model. Pyrintegrin (10 mg/kg) significantly reduced albuminuria caused by PAN-induced nephropathy in rats (Figure 7D), further validating the in vitro results, although the level of protection from albuminuria was not as high as that observed in the LPS model system.
Discussion
Podocyte drug discovery is hampered, because the techniques for rapid identification of podocyte-targeting agents have been lacking. Here, we describe the first podocyte cell–based phenotypic assay for high–throughput HCS of agents for the identification of podocyte-protective therapeutics. This assay uses a confocal microscopy–based HCS system to image and measure phenotypic changes in cultured podocytes, such as cell morphology, F-actin cytoskeleton, and focal adhesions, that have been used in the past to define healthy podocytes to provide a robust, unbiased, and quantitative readout that has established relevance to podocyte function in vivo.4 A few attempts in the past at designing HTS assays relevant to podocytes used stably transfected cells with a nephrin promoter–based reporter for the identification of a few small molecules that modulate the nephrin expression.21 However, cell–based phenotypic assays have significant advantages over more target–based approaches and have been more successful in generating novel Food and Drug Administration–approved therapeutics for various indications.40,41
Using PAN-induced injury as a model system, we show that the assay provides quantitative assessment of podocyte damage and has low variability and high robustness for use in an HTS environment. Furthermore, on screening with a >2100 compound chemical library, we identified 24 small molecules that protected podocytes from PAN injury. Inhibition of signaling proteins Rho-associated protein kinase (ROCK) and p38 MAPK using small molecule antagonists has previously been shown to protect against podocyte injury in vitro and proteinuria in vivo.24,25,42–46 Among the primary hits, we found compounds that inhibit ROCK (Y27632, GSK 429286, and Thiazovivin) or p38 MAPK (SB 203580), which provided us with excellent validation for the screening assay. Furthermore, the assays showed Z′ values of 0.44 and 0.46, respectively, in two independent primary assay readouts and Z′>0.65 for a multiparametric classifier assay readout. Although Z′ values >0.5 are generally required for use of a biochemical assay in an HTS campaign, HCS assays are considered ready for HTS with lower Z′ values because of the variability and complexities of the multiple image–based readouts.47,48 Additionally, although the primary screen presented in this report used cells of the murine origin, which show a more homogenous phenotype for better quantification in a phenotypic assay, the assay methodology described here is applicable to human podocytes as well (Figure 6D).
The β1 integrin α3β1 is highly relevant for podocytes and the integrity of the filtration barrier in vivo.27–29 Global or podocyte-specific deletion of either subunit leads to proteinuria and early death in animals.28,29,49 Additionally, we and others have recently shown that podocyte injury leads to reduced levels of active β1 in mice and patients,30,33 which suggests that preventing loss of active β1-integrin levels in podocytes might be protective. Using novel small molecule agonists of β2 integrin CD11b/CD18, we have previously shown that such pharmacologic activation of integrins in vivo is a new, therapeutically relevant mechanism for targeting this family of adhesion receptors.50 Identified by our screen, pyrintegrin provided dose-dependent protection to cells from PAN injury in vivo and in vitro. Most importantly, it protected mice from LPS-induced proteinuria and podocyte FP effacement. Pyrintegrin administration for 14 days also significantly reduced peak proteinuria in PAN-induced nephropathy, albeit with smaller effects than observed in the LPS model. A reason for this effect could be the low solubility of pyrintegrin in the aqueous media, suggesting that future optimization of pyrintegrin might be warranted for chronic delivery. Furthermore, additional work is also needed to confirm whether β1 integrin activation alone is sufficient as a therapeutic strategy against glomerular diseases. It will also be important to study progressive models of glomerular disease and evaluate effects of permanent versus pulsatile activation of β1 integrin activation.
In addition to pyrintegrin, we identified inhibitor totaling 24 primary hits, including apigenin (a flavone that may induce autophagy), agelasine (inhibitor of Na/K-ATPase), antimycin A (antifungal; depletes ATP and has been shown to induce F-actin polymerization51), berberine (antifungal isoquinoline alkaloid with wide activities), doxazocin (a quinazoline antihypertensive α1–selective α–blocker), and thioguanine (antiproliferative and cytotoxic). We also identified two synthetic progestins altrenogest and norgestimate. Podocytes express hormone receptors, including for progesterone and estrogen, and 17β-estradiol has been shown to stabilize actin cytoskeleton and protect podocytes against oxidant-induced injury.52–54 The diversity of the identified hits signifies the possibility of identifying a number of relevant signaling pathways through the presented phenotypic assay that may be targeted for protecting podocytes from injury.
Concise Methods
Podocyte HCS Assay
Podocytes were cultured in 96-well optical plates (PerkinElmer, Waltham, MA). Cells were treated with PAN (Sigma-Aldrich, St. Louis, MO), MZR (Sigma-Aldrich), or LPS (Sigma-Aldrich) in cell culture media for 48 hours at 37°C and analyzed using the Opera HCS System (PerkinElmer). Subsequently, cells were rinsed with PBS and fixed using a PBS solution containing 4% paraformaldehyde and 2% sucrose. The cells were permeabilized and stained with Alexa568-labeled phalloidin (Life Technologies, Carlsbad, CA), HCS CellMask Blue (Life Technologies), and antibodies against paxillin (EMD Millipore, Billerica, MA), vinculin (Sigma-Aldrich), podocin (Santa Cruz Biotechnology, Dallas, TX), synaptopodin (Santa Cruz Biotechnology), and β1 integrin (Biolegend, San Diego, CA and BD Biosciences, San Jose, CA). Appropriate secondary antibodies (fluorescently labeled; Life Technologies) were used for fluorescent visualizations. High–throughput confocal microscopy was performed using the Opera LX (PerkinElmer) with filters, and exposure times used were according to the manufacturer’s instructions. Images were quantified for various cellular parameters using Columbus Analysis System (PerkinElmer).
Disclosures
S.S. and J.R. are cofounders and advisors of TRISAQ, a biotechnology company designed to develop novel therapeutics for kidney disease. S.S., J.R., and V.G. have the potential for financial benefit from product commercialization. J.R. and V.G. are inventors on pending patent applications related to this study.
Supplementary Material
Acknowledgments
We thank Dony Maiguel, Tristan Hays, Alex Braley, Salman Jaffer, and Isabel Fernandez for technical help with the podocyte cell–based assays and helpful discussions. We also thank Daniel Chow for help with the animal experiments.
This work was supported, in part, by National Institutes of Health Grants DK073495 (to J.R.), DK089394 (to J.R.), DK101350 (to J.R.), DK084195 (to V.G.), and HL109582 (to V.G.); the Nephcure Foundation; and resources from the Rush University Medical Center.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014090859/-/DCSupplemental.
References
- 1.Pavenstädt H, Kriz W, Kretzler M: Cell biology of the glomerular podocyte. Physiol Rev 83: 253–307, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Faul C, Asanuma K, Yanagida-Asanuma E, Kim K, Mundel P: Actin up: Regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol 17: 428–437, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Huber TB, Benzing T: The slit diaphragm: A signaling platform to regulate podocyte function. Curr Opin Nephrol Hypertens 14: 211–216, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Reiser J, Sever S: Podocyte biology and pathogenesis of kidney disease. Annu Rev Med 64: 357–366, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiggins RC: The spectrum of podocytopathies: A unifying view of glomerular diseases. Kidney Int 71: 1205–1214, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Mathieson PW: The podocyte as a target for therapies—new and old. Nat Rev Nephrol 8: 52–56, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Reiser J, Gupta V, Kistler AD: Toward the development of podocyte-specific drugs. Kidney Int 77: 662–668, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faul C, Donnelly M, Merscher-Gomez S, Chang YH, Franz S, Delfgaauw J, Chang JM, Choi HY, Campbell KN, Kim K, Reiser J, Mundel P: The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med 14: 931–938, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mundel P, Reiser J, Zúñiga Mejía Borja A, Pavenstädt H, Davidson GR, Kriz W, Zeller R: Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res 236: 248–258, 1997 [DOI] [PubMed] [Google Scholar]
- 10.Saleem MA, O’Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, Xing CY, Ni L, Mathieson PW, Mundel P: A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 13: 630–638, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Frenk S, Antonowicz I, Craig JM, Metcoff J: Experimental nephrotic syndrome induced in rats by aminonucleoside; renal lesions and body electrolyte composition. Proc Soc Exp Biol Med 89: 424–427, 1955 [DOI] [PubMed] [Google Scholar]
- 12.Caulfield JP, Reid JJ, Farquhar MG: Alterations of the glomerular epithelium in acute aminonucleoside nephrosis. Evidence for formation of occluding junctions and epithelial cell detachment. Lab Invest 34: 43–59, 1976 [PubMed] [Google Scholar]
- 13.Whiteside CI, Cameron R, Munk S, Levy J: Podocytic cytoskeletal disaggregation and basement-membrane detachment in puromycin aminonucleoside nephrosis. Am J Pathol 142: 1641–1653, 1993 [PMC free article] [PubMed] [Google Scholar]
- 14.Pippin JW, Brinkkoetter PT, Cormack-Aboud FC, Durvasula RV, Hauser PV, Kowalewska J, Krofft RD, Logar CM, Marshall CB, Ohse T, Shankland SJ: Inducible rodent models of acquired podocyte diseases. Am J Physiol Renal Physiol 296: F213–F229, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Fishman JA, Karnovsky MJ: Effects of the aminonucleoside of puromycin on glomerular epithelial cells in vitro. Am J Pathol 118: 398–407, 1985 [PMC free article] [PubMed] [Google Scholar]
- 16.Marshall CB, Pippin JW, Krofft RD, Shankland SJ: Puromycin aminonucleoside induces oxidant-dependent DNA damage in podocytes in vitro and in vivo. Kidney Int 70: 1962–1973, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Kawasaki Y: Mizoribine: A new approach in the treatment of renal disease. Clin Dev Immunol 2009: 681482, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takeuchi S, Hiromura K, Tomioka M, Takahashi S, Sakairi T, Maeshima A, Kaneko Y, Kuroiwa T, Nojima Y: The immunosuppressive drug mizoribine directly prevents podocyte injury in puromycin aminonucleoside nephrosis. Nephron, Exp Nephrol 116: e3–e10, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Wada T, Pippin JW, Marshall CB, Griffin SV, Shankland SJ: Dexamethasone prevents podocyte apoptosis induced by puromycin aminonucleoside: Role of p53 and Bcl-2-related family proteins. J Am Soc Nephrol 16: 2615–2625, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Ransom RF, Lam NG, Hallett MA, Atkinson SJ, Smoyer WE: Glucocorticoids protect and enhance recovery of cultured murine podocytes via actin filament stabilization. Kidney Int 68: 2473–2483, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Yamauchi K, Takano Y, Kasai A, Hayakawa K, Hiramatsu N, Enomoto N, Yao J, Kitamura M: Screening and identification of substances that regulate nephrin gene expression using engineered reporter podocytes. Kidney Int 70: 892–900, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Xing CY, Saleem MA, Coward RJ, Ni L, Witherden IR, Mathieson PW: Direct effects of dexamethasone on human podocytes. Kidney Int 70: 1038–1045, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Zhang JH, Chung TD, Oldenburg KR: A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen 4: 67–73, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Wang L, Ellis MJ, Fields TA, Howell DN, Spurney RF: Beneficial effects of the Rho kinase inhibitor Y27632 in murine puromycin aminonucleoside nephrosis. Kidney Blood Press Res 31: 111–121, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koshikawa M, Mukoyama M, Mori K, Suganami T, Sawai K, Yoshioka T, Nagae T, Yokoi H, Kawachi H, Shimizu F, Sugawara A, Nakao K: Role of p38 mitogen-activated protein kinase activation in podocyte injury and proteinuria in experimental nephrotic syndrome. J Am Soc Nephrol 16: 2690–2701, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Pengal R, Guess AJ, Agrawal S, Manley J, Ransom RF, Mourey RJ, Benndorf R, Smoyer WE: Inhibition of the protein kinase MK-2 protects podocytes from nephrotic syndrome-related injury. Am J Physiol Renal Physiol 301: F509–F519, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pozzi A, Zent R: Integrins in kidney disease. J Am Soc Nephrol 24: 1034–1039, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pozzi A, Jarad G, Moeckel GW, Coffa S, Zhang X, Gewin L, Eremina V, Hudson BG, Borza DB, Harris RC, Holzman LB, Phillips CL, Fassler R, Quaggin SE, Miner JH, Zent R: Beta1 integrin expression by podocytes is required to maintain glomerular structural integrity. Dev Biol 316: 288–301, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanasaki K, Kanda Y, Palmsten K, Tanjore H, Lee SB, Lebleu VS, Gattone VH, Jr., Kalluri R: Integrin beta1-mediated matrix assembly and signaling are critical for the normal development and function of the kidney glomerulus. Dev Biol 313: 584–593, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu CC, Fornoni A, Weins A, Hakroush S, Maiguel D, Sageshima J, Chen L, Ciancio G, Faridi MH, Behr D, Campbell KN, Chang JM, Chen HC, Oh J, Faul C, Arnaout MA, Fiorina P, Gupta V, Greka A, Burke GW, 3rd, Mundel P: Abatacept in B7-1-positive proteinuric kidney disease. N Engl J Med 369: 2416–2423, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tian X, Kim JJ, Monkley SM, Gotoh N, Nandez R, Soda K, Inoue K, Balkin DM, Hassan H, Son SH, Lee Y, Moeckel G, Calderwood DA, Holzman LB, Critchley DR, Zent R, Reiser J, Ishibe S: Podocyte-associated talin1 is critical for glomerular filtration barrier maintenance. J Clin Invest 124: 1098–1113, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krishnamurti U, Zhou B, Fan WW, Tsilibary E, Wayner E, Kim Y, Kashtan CE, Michael A: Puromycin aminonucleoside suppresses integrin expression in cultured glomerular epithelial cells. J Am Soc Nephrol 12: 758–766, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Potla U, Ni J, Vadaparampil J, Yang G, Leventhal JS, Campbell KN, Chuang PY, Morozov A, He JC, D’Agati VD, Klotman PE, Kaufman L: Podocyte-specific RAP1GAP expression contributes to focal segmental glomerulosclerosis-associated glomerular injury. J Clin Invest 124: 1757–1769, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu Y, Zhu X, Hahm HS, Wei W, Hao E, Hayek A, Ding S: Revealing a core signaling regulatory mechanism for pluripotent stem cell survival and self-renewal by small molecules. Proc Natl Acad Sci U S A 107: 8129–8134, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lenter M, Uhlig H, Hamann A, Jenö P, Imhof B, Vestweber D: A monoclonal antibody against an activation epitope on mouse integrin chain beta 1 blocks adhesion of lymphocytes to the endothelial integrin alpha 6 beta 1. Proc Natl Acad Sci U S A 90: 9051–9055, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reiser J, von Gersdorff G, Loos M, Oh J, Asanuma K, Giardino L, Rastaldi MP, Calvaresi N, Watanabe H, Schwarz K, Faul C, Kretzler M, Davidson A, Sugimoto H, Kalluri R, Sharpe AH, Kreidberg JA, Mundel P: Induction of B7-1 in podocytes is associated with nephrotic syndrome. J Clin Invest 113: 1390–1397, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alachkar N, Carter-Monroe N, Reiser J: Abatacept in B7-1-positive proteinuric kidney disease. N Engl J Med 370: 1263–1264, 2014 [DOI] [PubMed] [Google Scholar]
- 38.Wei C, Möller CC, Altintas MM, Li J, Schwarz K, Zacchigna S, Xie L, Henger A, Schmid H, Rastaldi MP, Cowan P, Kretzler M, Parrilla R, Bendayan M, Gupta V, Nikolic B, Kalluri R, Carmeliet P, Mundel P, Reiser J: Modification of kidney barrier function by the urokinase receptor. Nat Med 14: 55–63, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Mundel P, Heid HW, Mundel TM, Krüger M, Reiser J, Kriz W: Synaptopodin: An actin-associated protein in telencephalic dendrites and renal podocytes. J Cell Biol 139: 193–204, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swinney DC, Anthony J: How were new medicines discovered? Nat Rev Drug Discov 10: 507–519, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Lee JA, Berg EL: Neoclassic drug discovery: The case for lead generation using phenotypic and functional approaches. J Biomol Screen 18: 1143–1155, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Hidaka T, Suzuki Y, Yamashita M, Shibata T, Tanaka Y, Horikoshi S, Tomino Y: Amelioration of crescentic glomerulonephritis by RhoA kinase inhibitor, Fasudil, through podocyte protection and prevention of leukocyte migration. Am J Pathol 172: 603–614, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kanda T, Wakino S, Hayashi K, Homma K, Ozawa Y, Saruta T: Effect of fasudil on Rho-kinase and nephropathy in subtotally nephrectomized spontaneously hypertensive rats. Kidney Int 64: 2009–2019, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Nishikimi T, Matsuoka H: Molecular mechanisms and therapeutic strategies of chronic renal injury: Renoprotective effect of rho-kinase inhibitor in hypertensive glomerulosclerosis. J Pharmacol Sci 100: 22–28, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Sakurai N, Kuroiwa T, Ikeuchi H, Hiramatsu N, Takeuchi S, Tomioka M, Shigehara T, Maeshima A, Kaneko Y, Hiromura K, Kopp JB, Nojima Y: Fluvastatin prevents podocyte injury in a murine model of HIV-associated nephropathy. Nephrol Dial Transplant 24: 2378–2383, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Shibata S, Nagase M, Fujita T: Fluvastatin ameliorates podocyte injury in proteinuric rats via modulation of excessive Rho signaling. J Am Soc Nephrol 17: 754–764, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Quintavalle M, Elia L, Price JH, Heynen-Genel S, Courtneidge SA: A cell-based high-content screening assay reveals activators and inhibitors of cancer cell invasion. Sci Signal 4: ra49, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh S, Carpenter AE, Genovesio A: Increasing the content of high-content screening: An overview. J Biomol Screen 19: 640–650, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sachs N, Claessen N, Aten J, Kreft M, Teske GJ, Koeman A, Zuurbier CJ, Janssen H, Sonnenberg A: Blood pressure influences end-stage renal disease of Cd151 knockout mice. J Clin Invest 122: 348–358, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maiguel D, Faridi MH, Wei C, Kuwano Y, Balla KM, Hernandez D, Barth CJ, Lugo G, Donnelly M, Nayer A, Moita LF, Schürer S, Traver D, Ruiz P, Vazquez-Padron RI, Ley K, Reiser J, Gupta V: Small molecule-mediated activation of the integrin CD11b/CD18 reduces inflammatory disease. Sci Signal 4: ra57, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Atkinson SJ, Hosford MA, Molitoris BA: Mechanism of actin polymerization in cellular ATP depletion. J Biol Chem 279: 5194–5199, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Catanuto P, Doublier S, Lupia E, Fornoni A, Berho M, Karl M, Striker GE, Xia X, Elliot S: 17 beta-estradiol and tamoxifen upregulate estrogen receptor beta expression and control podocyte signaling pathways in a model of type 2 diabetes. Kidney Int 75: 1194–1201, 2009 [DOI] [PubMed] [Google Scholar]
- 53.Doublier S, Lupia E, Catanuto P, Periera-Simon S, Xia X, Korach K, Berho M, Elliot SJ, Karl M: Testosterone and 17β-estradiol have opposite effects on podocyte apoptosis that precedes glomerulosclerosis in female estrogen receptor knockout mice. Kidney Int 79: 404–413, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Catanuto P, Fornoni A, Pereira-Simon S, Wu F, Burnstein KL, Xia X, Conti F, Lenzi A, Elliot S: In vivo 17β-estradiol treatment contributes to podocyte actin stabilization in female db/db mice. Endocrinology 153: 5888–5895, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.