Abstract
Patients on dialysis are 20 times more likely to have a cardiac arrest compared with the general population. We considered whether inherited factors associate with cardiac arrest among patients on dialysis. From a sample of 647,457 patients on chronic dialysis, we identified 5117 pairs of patients who came from the same family. These patients were each matched to a control subject from the same population. McNemar’s tests were used to compare the risk of cardiac arrest between the familial related and unrelated pairs. Genetically related family members who did not cohabitate had an odds ratio of 1.88 (95% confidence interval [95% CI], 1.25 to 2.84) for cardiac arrest compared with their phenotypically matched unrelated controls. Genetically related family members who lived together in the same environment had an odds ratio of 1.66 (95% CI, 1.20 to 2.28). Spouses, who are genetically unrelated but live together in the same environment, had an odds ratio of 0.95 (95% CI, 0.60 to 1.59) for cardiac arrest. The risk of cardiac arrest in patients on dialysis may be attributable to inherited factors. Additional studies are needed to identify such candidate genes that modify cardiovascular risk in ESRD.
Keywords: cardiovascular, dialysis, epidemiology and outcomes
Patients on dialysis silently live with the age-adjusted risk of cardiac death that is 3.5 times higher than that in the general population.1 Although cardiac arrest, commonly caused by ventricular arrhythmias, can be mostly attributed to coronary artery disease in the general population, ESRD–related cardiac arrest may be mediated by a different mechanism.2 Retrospective adjudication of 476 dialysis deaths found no significant difference in the prevalence of coronary artery disease, decreased left ventricular ejection fraction, valvular heart disease, or left ventricular hypertrophy between patients who died of cardiac death versus those who died of another cause.3 As such, we do not fully understand the pathophysiology of cardiac arrest in ESRD, and there are no accurate instruments for risk stratification or recommendations for the primary prevention of cardiac arrest in the dialysis population.4
The discovery of predictive markers for cardiac arrest in ESRD could alter the practice of nephrology, because the risk of cardiac arrest is 5% per year in the dialysis population.2,5 In the general population, genome–wide association studies have identified genetic polymorphisms that significantly increase the risk of cardiac arrest6–8; however, their clinical application has been limited, because the rate of cardiac arrest is only 0.24% per year.9 As such, a doubling of risk in the general population would still be too low to warrant empirical primary prevention therapy. In the patient on dialysis, a genetic marker that doubles the risk of cardiac arrest would increase the absolute risk enough to possibly justify primary prophylactic therapies. However, the inherited risk for cardiac arrest among patients on dialysis has not been well established, and we have not discovered specific genetic polymorphisms that independently associate with an increased risk for cardiac arrest in ESRD.
We postulated that inherited factors could substantially modify the risk of cardiac arrest in ESRD and tested this hypothesis in a large cohort of patients on maintenance dialysis.
Results
Description of the Study Cohort: Family Members on Dialysis
Among a population of 647,457 patients with ESRD drawn from chronic dialysis facilities, we identified 5117 pairs of patients who came from the same family; 4053 of these pairs were genetically related and further classified into pairs that cohabitated (n=2449) and pairs that lived in separate houses (n=1604), and 1064 of these pairs were nongenetically related spouses.
Compared with the full dialysis population, families on dialysis were more likely to be African American (58.4% versus 30.4%, respectively), younger (58.4 versus 61.8 years old, respectively), and diabetic (30.3% versus 27.8%, respectively).
Heritability of Cardiac Arrest
Individuals in the 5117 family pairs were data matched on 26 characteristics to control subjects. After matching, the patient characteristics between the family pairs and their control pairs seemed to be well balanced (Table 1).
Table 1.
Patient characteristics of family members on dialysis (A+B) and their matched unrelated controls
| Patient Characteristic | Unrelated Match to A (n=5117) | Related Pair | Unrelated Match to B (n=5117) | |
|---|---|---|---|---|
| Family Member A (n=5117) | Family Member B (n=5117) | |||
| Age (yr) | 60.1 | 59.0 | 56.4 | 58.1 |
| Sex (% men) | 48.7 | 48.7 | 46.7 | 46.7 |
| Time on dialysis (yr) | 3.8 | 3.9 | 6.2 | 5.7 |
| Follow-up time (yr) | 3.4 | 3.5 | 5.4 | 5.0 |
| Time period | August of 2008 | August of 2008 | November of 2005 | November of 2005 |
| Race (%) | ||||
| Caucasian | 35.1 | 35.1 | 35.3 | 35.3 |
| African American | 57.1 | 57.1 | 57.5 | 57.5 |
| Hispanic Latino | 0.7 | 0.7 | 1.1 | 1.1 |
| Cause of ESRD (%) | ||||
| Diabetes | 35.5 | 35.5 | 25.0 | 25.0 |
| HTN | 25.3 | 25.3 | 21.4 | 21.4 |
| Access (%) | ||||
| Fistula | 38.1 | 38.1 | 29.9 | 29.9 |
| Graft | 24.9 | 24.9 | 32.4 | 32.4 |
| Catheter | 31.9 | 31.9 | 33.0 | 33.0 |
| PD | 5.1 | 5.1 | 4.6 | 4.6 |
| BMI (kg/m2) | 28.8 | 28.8 | 29.3 | 29.5 |
| BP (mmHg) | 139/73 | 139/73 | 139/74 | 139/74 |
| Albumin (g/dl) | 3.6 | 3.6 | 3.6 | 3.6 |
| Hemoglobin (g/dl) | 11.0 | 11.0 | 11.0 | 11.0 |
| EPO (units per tx) | 6770 | 8083 | 7528 | 6407 |
| Potassium (mEq/L) | 4.6 | 4.7 | 4.7 | 4.7 |
| Bicarbonate (mEq/L) | 22.2 | 22.1 | 20.3 | 20.4 |
| Calcium (mg/dl) | 9.0 | 9.0 | 9.0 | 9.1 |
| Magnesium (mg/dl) | 1.9 | 1.9 | 1.9 | 1.9 |
| Dialysate K (mEq/L) | 2.2 | 2.1 | 2.1 | 2.1 |
| Dialysate Ca (mEq/L) | 2.5 | 2.5 | 2.5 | 2.5 |
| CAD (%) | 31.1 | 31.1 | 31.1 | 31.1 |
| CHF (%) | 36.0 | 36.0 | 38.4 | 38.4 |
| CVA (%) | 9.2 | 10.9 | 10.0 | 9.7 |
| ASA (%) | 51.6 | 51.6 | 49.8 | 49.8 |
| ACEI-ARB (%) | 62.9 | 62.9 | 60.7 | 60.7 |
| β-Blocker (%) | 47.4 | 47.4 | 45.0 | 45.0 |
| Longitude (°) | −86.9 | −86.2 | −85.9 | −86.6 |
| Latitude (°) | 35.5 | 35.1 | 34.9 | 35.2 |
HTN, hypertension; PD, peritoneal dialysis; BMI, body mass index; EPO erythropoietin; tx, tx treatment; CAD, coronary artery disease; CHF, congestive heart failure; CVA, stroke; ASA, aspirin; ACEI-ARB, angiotensin–converting enzyme inhibitor–angiotensin receptor blocker.
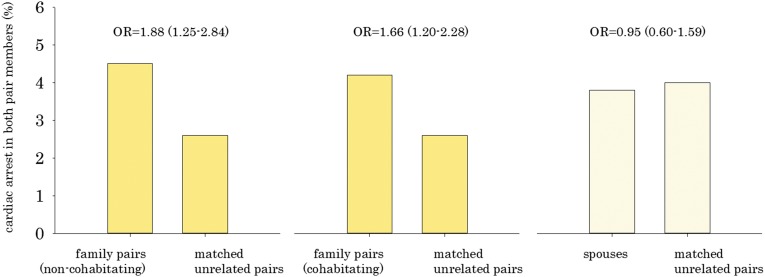
We compared the intrapair prevalence of cardiac arrest between nonspouse family pairs and control pairs (Figure 1). In 4.3% of family pairs, both members died of a cardiac arrest compared with 2.6% in the control pairs (odds ratio [OR], 1.74; 95% confidence interval [95% CI], 1.35 to 2.23). For family pairs who did not cohabitate, the OR for dual cardiac arrest was 1.88 (95% CI, 1.25 to 2.84), whereas the OR for family pairs who lived together was 1.66 (95% CI, 1.20 to 2.28) compared with their control pairs. OR estimates for all-cause and noncardiovascular mortality were also calculated for family pairs referent to matched unrelated pairs (Table 2).
Figure 1.
Higher prevalence of intrafamily cardiac arrest (patients) compared with matched unrelated pairs (controls). Family pairs: parent/child (n=1695), siblings (n=1602), cousin/uncle (n=756), or spouses (n=1064).
Table 2.
Proportion of family and unrelated pairs where both subjects died by cause of death
| Outcome | Dual Event (%) | OR (95% CI) | |
|---|---|---|---|
| Family Pairs | Unrelated Pairs | ||
| Fatal cardiac arrest | |||
| Family pairs (noncohabitating) | 4.5 | 2.6 | 1.88 (1.25 to 2.84) |
| Family pairs (cohabitating) | 4.2 | 2.6 | 1.66 (1.20 to 2.28) |
| Spouses | 3.8 | 4.0 | 0.95 (0.60 to 1.59) |
| All-cause mortality | |||
| Family pairs (noncohabitating) | 14.3 | 13.6 | 1.07 (0.86 to 1.32) |
| Family pairs (cohabitating) | 16.7 | 13.6 | 1.32 (1.12 to 1.57) |
| Spouses | 21.7 | 14.9 | 1.68 (1.32 to 2.13) |
| Noncardiovascular mortality | |||
| Family pairs (noncohabitating) | 4.0 | 3.9 | 1.02 (0.71 to 1.46) |
| Family pairs (cohabitating) | 4.9 | 4.2 | 1.17 (0.90 to 1.53) |
| Spouses | 7.9 | 4.8 | 1.75 (1.21 to 2.53) |
Spouses, who cohabitate but do not inherit genes from a common ancestor, both died of cardiac arrest in 3.8% of the patients relative to 4.0% of the patients in their control pairs. This relationship was not statistically significant (OR, 0.95; 95% CI, 0.60 to 1.59).
Effect Size of Heritable and Acquired Factors Associated with Sudden Cardiac Arrest
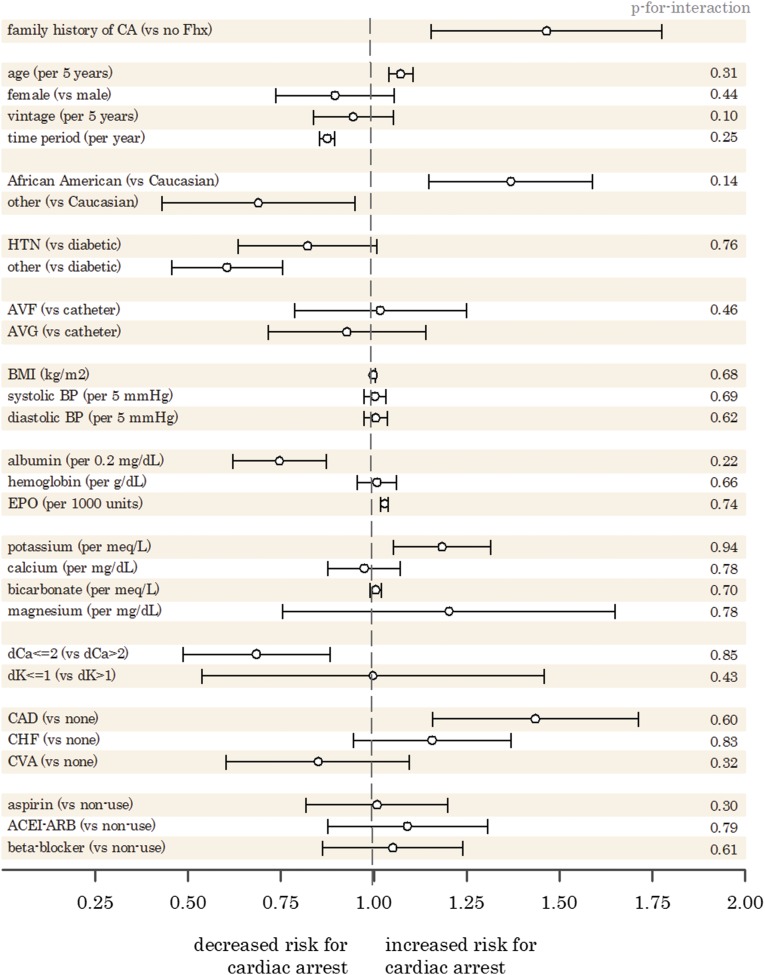
For the secondary analysis, we isolated index family patients (family member A column in Table 1) into those who had a cardiac arrest in their family and those who did not per family member B in Table 1. Multivariable logistic modeling was used to determine the effect size of inherited and acquired factors associated with cardiac arrest. The results are displayed in Forest plot format (Figure 2).
Figure 2.
Forest plot of adjusted OR for fatal cardiac arrest by risk factor. ACEI-ARB, angiotensin–converting enzyme inhibitor–angiotensin receptor blocker; AVF, arteriovenous fistula; AVG, arteriovenous graft; BMI, body mass index; CA, cardiac arrest; CAD, coronary artery disease; CHF, congestive heart failure; CVA, stroke; dCa, dialysate calcium; dK, dialysate potassium; EPO erythropoietin; Fhx, family history; HTN, hypertension.
Patients with a nonspouse relative who previously died from a cardiac arrest were at increased odds (OR, 1.45; 95% CI, 1.19 to 1.75) of dying from a cardiac arrest relative to patients without a family history. Other significant factors associated with an increased risk of cardiac arrest included age (OR, 1.07 per 5 years; 95% CI, 1.04 to 1.11), African-American race (OR, 1.37; 95% CI, 1.15 to 1.63 referent to Caucasian), serum potassium level (OR, 1.19 per mEq/L; 95% CI, 1.07 to 1.32), erythropoietin dose (1.03 per 1000 units; 95% CI, 1.02 to 1.04), and documented coronary artery disease (OR, 1.44; 95% CI, 1.20 to 1.71). Protective factors associated with cardiac arrest included time period (OR, 0.88 per year; 95% CI, 0.86 to 0.90), higher albumin levels (OR, 0.75 per 0.2 mg/dl; 95% CI, 0.64 to 0.87), and a lower (dialysate calcium ≤2) calcium dialysate bath (OR, 0.69; 95% CI, 0.53 to 0.88) referent to dialysate calcium >2. There were no statistically significant effect modifications between inherited and acquired factors (Supplemental Table 1).
Discussion
In this study of family members on dialysis, we uncovered findings that suggest that heritable factors contribute to the risk of cardiac arrest in patients receiving dialysis. Compared with phenotypically matched unrelated controls, genetically related family members had a 1.7 odds increase of cardiac arrest, whereas there was no risk increase among spouses who were family members without genetic overlap.
The annual mortality rate for United States patients on dialysis has been reported to be 18 per 100 patient-years according to the 2013 US Renal Data Systems.5 Among these deaths, cardiac arrest was reported to be the largest cause at five events per 100 patient-years. The pathophysiology of ESRD–related cardiac arrest remains unclear but is likely an interaction between multiple factors, such as inflammatory, cardiac, and electrolyte abnormalities, combined with a host of biologic mechanisms that remain undiscovered.2 In our study, we identified significant associations that suggest that the risk of cardiac arrest in ESRD maybe inheritable.
Taken altogether, our study suggests that family history significantly increases the risk of fatal cardiac arrest by approximately 70% among patients on dialysis. This magnitude of risk increase is comparable with the results reported in inheritance studies of cardiac arrest in the general population.10,11 We saw no increase in risk for cardiac arrest among spouses with no genetic overlap, and we did not see a large change in risk among family members who cohabited or lived apart.
To date, no genome–wide association studies have been done in the dialysis population to identify specific single–nucleotide polymorphisms (SNPs) that may identify particular patients with ESRD with the highest risk of cardiac arrest. In the general population, multiple SNPs have been identified that associate with an increased risk of cardiac arrest.6–8 As such, it is possible that these same SNPs occur with increased frequency or have a magnified effect size in patients on dialysis as an explanation for the increased rate of cardiac arrest reported in the dialysis population. Alternatively, novel SNPs specific to patients with ESRD may explain the excess cardiovascular mortality.
Clinically, patients on dialysis have a similar risk for cardiac arrest as patients who fulfill the criteria for prophylactic implantation of a cardioverter defibrillator (ICD)12; however, multiple retrospective analyses have reported that ICDs would likely provide reduced survival benefits to patients on dialysis compared with the general population.13–15 This is likely, because patients with ESRD have many comorbidities that compete for patient mortality. Taken all together, ICD implantation for the primary prevention of cardiac arrest in patients on dialysis may be effective but is currently unfeasible because of the high costs, limited reduction in absolute risk, and unproven efficacy. Genetic markers that identify patients on dialysis at the highest cardiac risk could help distinguish which patients may sufficiently benefit from prophylactic ICD placement.
We acknowledge several limitations of our study. Although validation studies have shown that ESRD 2746 forms provide reasonable accuracy for the identification of fatal cardiac arrests, there still remains variation in the exact definition of cardiac arrest.16,17 This heterogeneity may have biased our effect estimates and decreased our power to detect more granular outcomes. Although our matching included criteria to choose subjects in close geographic proximity to each other, we were not able to restrict case-control matching within the clinic, because some facilities had too few control subjects. We also acknowledge the potential for misclassification in the process to identify familial relationships and cohabitation status. For example, we could not know if siblings were adopted, in whom there would be minimal genetic overlap, and the use of phone numbers may not precisely identify patients and cohabitation status, especially in those who interchange mobile and landlines as their home telephone number. Finally, as in all observational studies, there is potential for confounding from unmeasured factors.
In conclusion, heritable factors may contribute to the risk of cardiac arrest in hemodialysis. Additional genome–wide association studies are needed to identify novel candidate genes that identify patients with ESRD at the highest risk for cardiac arrest who may warrant therapeutic intervention.
Concise Methods
Description of the Data Sources
We drew on a large cohort of patients with ESRD to identify family members on dialysis with matched pairs of unrelated control individuals. We compared the intrafamily and intracontrol prevalence of cardiac arrest to determine the presence and estimate the magnitude of possible genetic factors associated with cardiac arrest in ESRD. The study protocol was approved and granted a waiver of informed consent by the New England Institutional Review Board.
Data for the study were abstracted from the Fresenius Medical Care North America (FMCNA) ESRD Database, which prospectively captures clinically relevant and standardized data elements for the purpose of conducting outcomes studies in the dialysis population. Over 2000 clinics in 48 states, the District of Columbia, and the Territory of Puerto Rico are represented in the database, which corresponds to approximately 40% of the United States chronic dialysis population.
All subjects registered in the database are followed longitudinally; data are actively collected and entered at the point of care for each patient at every visit (typically three times per week). Demographic, comorbid, medication, vascular access, and dialysis treatment data are charted on standardized electronic flow sheets provided on touchscreen monitors located at each treatment station. The data collection tool includes predefined logic features and user alerts to check data as they are entered. Required fields are structured so that valid data must be entered before the system can authorize the hemodialysis treatment to begin. At the end of each dialysis shift, automatic edit checks are executed to identify inconsistent or out-of-range data that require reconciliation by the facility charge nurse before the shift can be formally closed and submitted for billing. Laboratory testing was performed by a single accredited provider (Spectra Laboratories, Rockleigh, NJ), and the results were directly downloaded into the database.
Definition of the Study Cohort
From an ESRD population of >600,000 subjects from 1994 to 2014, we identified probable family members on dialysis through the linkage of emergency contact phone numbers provided by each patient on dialysis. As an example, if patient X on dialysis listed person A as the person (mother) to contact for an emergency and patient Y on dialysis also listed person A as the person (mother) to contact for an emergency, then we deduced that patients X and Y were related as siblings. Similarly, if patient X listed person A as mother and patient Y also listed person A as wife, then we deduced that patients X and Y were related as child and father. Moreover, if patient X listed patient Y as spouse, then patients X and Y were married. We validated this linkage algorithm by verifying that family member pairs were, indeed, related through the review of published obituaries. Among a sample of 75 family member pairs, the familial accuracy was found to be 97%. Family members were identified as cohabiting if they both provided the same home landline telephone number.
Using the above approach, we identified all potential pairs of family members who received chronic RRT within the FMCNA network of dialysis clinics. Each potential family pair with their corresponding emergency contact names was reviewed and validated for accuracy by K.E.C. before inclusion in the analysis. Family pairs could be genetically related, such as in siblings and parent/child, or nongenetically related, such as in spouses. In families with more than two genetically related members, the most contemporary family member was declared as A, whereas the remaining family members formed pairwise groups as B. Family pairs were also classified by cohabitation status depending on whether they lived in the same household.
A case-control study cohort was then generated by matching each individual family (n=10,234) member to another patient on dialysis (n=10,234) who was similar in demographics, vascular access, laboratory, comorbidity, follow-up time, geography, dialysate prescription, and medication characteristics at the time that the patient was discharged from the Fresenius Dialysis Network (Table 1 has a full list of matching parameters). Matching was executed through a greedy algorithm supplied by Kosanke and Bergstralh.18 After matching, family members were then paired together so that they could be compared with their paired unrelated controls. Thus, our final study cohort was comprised of 5117 pairs of family members and for comparison, 5117 pairs of phenotypically similar but unrelated patients with ESRD; all patients were drawn from the full FMCNA cohort.
Study Outcomes
The primary study outcome was fatal cardiac arrest as determined by the cardiac arrhythmia or cardiac arrest, cause unknown categories from Form 2746-ESRD death notification. This form is mandated by The Centers for Medicare and Medicaid Services for all dialysis deaths. Validation studies of 2746 cardiac arrest outcomes against clinically adjudicated outcomes reported accuracy rates of 71%–84%.16,17 The secondary outcomes of the study included all-cause mortality and noncardiovascular death.
Patient Characteristics
Baseline patient characteristics of family members and their matched unrelated controls were tabulated for comparison. Characteristics were ascertained as the most recent values before the patient died or was discharged from the Fresenius Dialysis Network. Time on dialysis or vintage was calculated as the difference between the date that the patient was discharged from the Fresenius Dialysis Network and the date that the patient was declared to have ESRD.
Statistical Analyses
Each pair of family members and unrelated control subjects was considered an independent observation for the purpose of the primary statistical analysis. Using a case-control design, we used McNemar’s test to compare the proportion of pairs where both subjects died of a cardiac arrest in the family group versus the control group. A higher prevalence of dual cardiac arrests within family pairs (versus control pairs) would be consistent with the heritability of cardiac arrest. McNemar’s testing was further repeated in subpopulations of family pairs by cohabitation status and among spouse pairs.
For the secondary analysis, we isolated 5117 family subjects (only subjects in the family member A column in Table 1) and classified them according to whether their former relative died of a cardiac arrest or not (per family member B in Table 1). In other words, member A was current, and their inherited risk of cardiac arrest was on the basis of the outcome of their past relative B. A multivariable logistic model was used to determine the association between cardiac arrest with 26 measured factors. All continuous variables in the logistic model were tested for linearity using the Box–Tidwell test.19 Continuous variables that did not pass the test of linearity were quartiled for transformation into a categorical variable. Models were also stratified by cohabitation status. Effect modification between the inherited risk of cardiac arrest and each acquired factor was tested through inclusion of interaction terms in the logistic model. All statistical analyses were executed using SAS, version 9.3 (Cary, NC).
Disclosures
K.E.C. and F.W.M. are Medical Officers of Fresenius Medical Care North America.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014090881/-/DCSupplemental.
References
- 1.Consensus Development Conference Panel : Morbidity and mortality of renal dialysis: An NIH consensus conference statement. Ann Intern Med 121: 62–70, 1994 [DOI] [PubMed] [Google Scholar]
- 2.Herzog CA, Mangrum JM, Passman R: Sudden cardiac death and dialysis patients. Semin Dial 21: 300–307, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Genovesi S, Valsecchi MG, Rossi E, Pogliani D, Acquistapace I, De Cristofaro V, Stella A, Vincenti A: Sudden death and associated factors in a historical cohort of chronic haemodialysis patients. Nephrol Dial Transplant 24: 2529–2536, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Green D, Roberts PR, New DI, Kalra PA: Sudden cardiac death in hemodialysis patients: An in-depth review. Am J Kidney Dis 57: 921–929, 2011 [DOI] [PubMed] [Google Scholar]
- 5.US Renal Data Systems : USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institute of Diabetes and Digestive and Kidney Disease, 2013 [Google Scholar]
- 6.Arking DE, Junttila MJ, Goyette P, Huertas-Vazquez A, Eijgelsheim M, Blom MT, Newton-Cheh C, Reinier K, Teodorescu C, Uy-Evanado A, Carter-Monroe N, Kaikkonen KS, Kortelainen M-L, Boucher G, Lagacé C, Moes A, Zhao X, Kolodgie F, Rivadeneira F, Hofman A, Witteman JCM, Uitterlinden AG, Marsman RF, Pazoki R, Bardai A, Koster RW, Dehghan A, Hwang S-J, Bhatnagar P, Post W, Hilton G, Prineas RJ, Li M, Köttgen A, Ehret G, Boerwinkle E, Coresh J, Kao WHL, Psaty BM, Tomaselli GF, Sotoodehnia N, Siscovick DS, Burke GL, Marbán E, Spooner PM, Cupples LA, Jui J, Gunson K, Kesäniemi YA, Wilde AA, Tardif JC, O’Donnell CJ, Bezzina CR, Virmani R, Stricker BH, Tan HL, Albert CM, Chakravarti A, Rioux JD, Huikuri HV, Chugh SS: Identification of a sudden cardiac death susceptibility locus at 2q24.2 through genome-wide association in European ancestry individuals. PLoS Genet 7: e1002158, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bezzina CR, Pazoki R, Bardai A, Marsman RF, de Jong JSSG, Blom MT, Scicluna BP, Jukema JW, Bindraban NR, Lichtner P, Pfeufer A, Bishopric NH, Roden DM, Meitinger T, Chugh SS, Myerburg RJ, Jouven X, Kääb S, Dekker LRC, Tan HL, Tanck MWT, Wilde AA: Genome-wide association study identifies a susceptibility locus at 21q21 for ventricular fibrillation in acute myocardial infarction. Nat Genet 42: 688–691, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kao WHL, Arking DE, Post W, Rea TD, Sotoodehnia N, Prineas RJ, Bishe B, Doan BQ, Boerwinkle E, Psaty BM, Tomaselli GF, Coresh J, Siscovick DS, Marbán E, Spooner PM, Burke GL, Chakravarti A: Genetic variations in nitric oxide synthase 1 adaptor protein are associated with sudden cardiac death in US white community-based populations. Circulation 119: 940–951, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertoia ML, Allison MA, Manson JE, Freiberg MS, Kuller LH, Solomon AJ, Limacher MC, Johnson KC, Curb JD, Wassertheil-Smoller S, Eaton CB: Risk factors for sudden cardiac death in post-menopausal women. J Am Coll Cardiol 60: 2674–2682, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Friedlander Y, Siscovick DS, Weinmann S, Austin MA, Psaty BM, Lemaitre RN, Arbogast P, Raghunathan TE, Cobb LA: Family history as a risk factor for primary cardiac arrest. Circulation 97: 155–160, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Jouven X, Desnos M, Guerot C, Ducimetière P: Predicting sudden death in the population: The Paris Prospective Study I. Circulation 99: 1978–1983, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML, Multicenter Automatic Defibrillator Implantation Trial II Investigators : Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 346: 877–883, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Robin J, Weinberg K, Tiongson J, Carnethon M, Reddy M, Ciaccio C, Quadrini M, Hsu J, Fan J, Choi P, Kadish A, Goldberger J, Passman R: Renal dialysis as a risk factor for appropriate therapies and mortality in implantable cardioverter-defibrillator recipients. Heart Rhythm 3: 1196–1201, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Wase A, Basit A, Nazir R, Jamal A, Shah S, Khan T, Mohiuddin I, White C, Saklayen M, McCullough PA: Impact of chronic kidney disease upon survival among implantable cardioverter-defibrillator recipients. J Interv Card Electrophysiol 11: 199–204, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Eckart RE, Gula LJ, Reynolds MR, Shry EA, Maisel WH: Mortality following defibrillator implantation in patients with renal insufficiency. J Cardiovasc Electrophysiol 17: 940–943, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rocco MV, Yan G, Gassman J, Lewis JB, Ornt D, Weiss B, Levey AS, Hemodialysis Study Group. The National Institutes of Health-funded Hemodialysis. Health Care Financing Administration : Comparison of causes of death using HEMO Study and HCFA end-stage renal disease death notification classification systems. Am J Kidney Dis 39: 146–153, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Pun PH, Herzog CA, Middleton JP: Improving ascertainment of sudden cardiac death in patients with end stage renal disease. Clin J Am Soc Nephrol 7: 116–122, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kosanke J, Bergstralh E: Gmatch: SAS Macro, 2008. Available at: http://mayoresearch.mayo.edu/mayo/research/biostat/upload/gmatch.sas. Accessed November X, 2013
- 19.Osborne JW: Best Practices in Logistic Regression, Louisville, KY, Sage, 2014 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.