Abstract
Rapidly rising global rates of chronic diseases portend a consequent rise in ESRD. Despite this, kidney disease is not included in the list of noncommunicable diseases (NCDs) targeted by the United Nations for 25% reduction by year 2025. In an effort to accurately report the trajectory and pattern of global growth of maintenance dialysis, we present the change in prevalence and incidence from 1990 to 2010. Data were extracted from the Global Burden of Disease 2010 epidemiologic database. The results are on the basis of an analysis of data from worldwide national and regional renal disease registries and detailed systematic literature review for years 1980–2010. Incidence and prevalence estimates of provision of maintenance dialysis from this database were updated using a negative binomial Bayesian meta-regression tool for 187 countries. Results indicate substantial growth in utilization of maintenance dialysis in almost all world regions. Changes in population structure, changes in aging, and the worldwide increase in diabetes mellitus and hypertension explain a significant portion, but not all, of the increase because increased dialysis provision also accounts for a portion of the rise. These findings argue for the importance of inclusion of kidney disease among NCD targets for reducing premature death throughout the world.
Keywords: chronic dialysis, diabetes mellitus, ESRD
There is growing emphasis throughout the world on understanding the effects of chronic diseases on population health. Recent advances in treating and preventing communicable diseases coupled with the dramatic rise in the prevalence of diabetes and hypertension have initiated a shift in focus to the relationship between NCDs and morbidity, most recently in developing regions of the world.1–4 This is exemplified by the United Nations 2012 Summit 2025 Initiative, which focuses on decreasing the burden of premature mortality to NCDs by 25% by year 2025.
The recent publication of the 2010 Global Burden of Disease Study (GBD) offers a systematic analysis of the contribution of disease and injury to morbidity and early mortality throughout the world.1 The 2010 GBD is the first edition to include CKD among the chronic diseases assessed and ranks it as the 18th most common cause of death, a substantial increase from its 27th ranking two decades before.1 These rankings illustrate the significant and increasing effect of CKD on global health.
The 2010 GBD makes it now possible for the first time to calculate the change in prevalence and incidence of provision of maintenance dialysis for 187 countries from 1990 to 2010. These estimates provide timely information and are highly relevant for countries striving to develop programs and strategies for addressing the needs of a quickly growing population of individuals that require the costly medical intervention of RRT.5,6
Global Burden of Maintenance Dialysis
In 2010, throughout the world, we have estimated 284 individuals per million population (pmp) to be undergoing maintenance dialysis (concise methods are included in Supplemental Appendix 1). In 2010, >60 countries provided universal access to maintenance dialysis (Supplemental Table 1). These countries accounted for 70% of prevalent maintenance dialysis and 60% of incident dialysis population worldwide (Figures 1 and 2, Tables 1 and 2).
Figure 1.
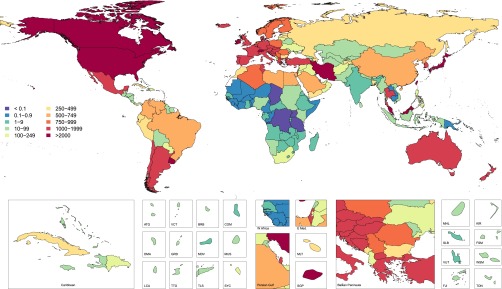
Age-standardized prevalence per million population of maintenance dialysis in year 2010 for 187 countries. ATG, Antigua and Barbuda; BRB, Barbados; COM, Comoros; DMA, Dominica; E. Med, Eastern Mediterranean; FJI , Fiji; FSM, Federated States of Micronesia; GRD, Grenada and Trinidad; KIR, Kiribati; LCA, Saint Lucia; MDV, Maldives; MHL, Marshall Islands; MUS, Mauritius; SGP, Singapore; SLB, Solomon Islands; SYC, Seychelles; TLS, Timor-Leste; TTO, Trinidad and Tobago; TUN, Tunisia; VCT, Saint Vincent and Grenadines; VUT, Vanuata; WSM, Samoa.
Figure 2.
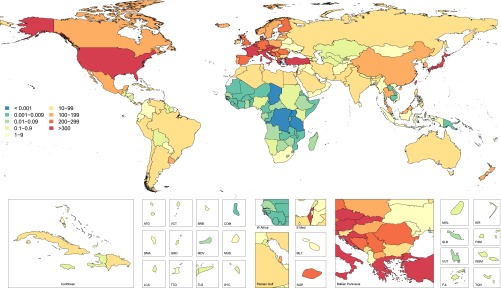
Age-standardized incidence rate per million population of maintenance dialysis in year 2010 for 187 countries. ATG, Antigua and Barbuda; BRB, Barbados; COM, Comoros; DMA, Dominica; E. Med, Eastern Mediterranean; FJI , Fiji; FSM, Federated States of Micronesia; GRD, Grenada and Trinidad; KIR, Kiribati; LCA, Saint Lucia; MDV, Maldives; MHL, Marshall Islands; MUS, Mauritius; SGP, Singapore; SLB, Solomon Islands; SYC, Seychelles; TLS, Timor-Leste; TTO, Trinidad and Tobago; TUN, Tunisia; VCT, Saint Vincent and Grenadines; VUT, Vanuata; WSM, Samoa.
Table 1.
Decomposition analysis of the change in global dialysis prevalence rates and counts from 1990 to 2010 stratified by sex and provision
| Stratification According to Dialysis Provision | Men | Women | Total |
|---|---|---|---|
| Global 1990 prevalence rate (pmp) | 180 (169, 191) | 151 (143, 160) | 165 (158, 172) |
| Global 2010 prevalence rate (pmp) | 301 (293, 310) | 269 (262, 276) | 284 (279, 289) |
| Countries with universal maintenance dialysis access | |||
| Year 1990 prevalent patients | 254,593 | 236,213 | 490,806 |
| No. of patients in 2010 expected secondary to population growth | 295,018 | 271,522 | 566,577 |
| No. of patients in 2010 expected secondary to population aging | 367,291 | 327,809 | 692,991 |
| Year 2010 prevalent patients | 656,850 | 667,030 | 1,323,880 |
| Percentage change between 1990 and 2010 because of | |||
| Growth | 16 | 15 | 15 |
| Aging | 28 | 24 | 26 |
| Rate | 114 | 144 | 129 |
| Percentage total change, 1990–2010 | 158 | 182 | 170 |
| Countries with partial maintenance dialysis access | |||
| Year 1990 prevalent patients | 112,569 | 100,050 | 212,619 |
| No. of patients in 2010 expected secondary to population growth | 135,138 | 118,675 | 253,761 |
| No. of patients in 2010 expected secondary to population aging | 175,418 | 155,014 | 330,396 |
| Year 2010 prevalent patients | 281,899 | 258,863 | 540,761 |
| Percentage change between 1990 and 2010 because of | |||
| Growth | 20 | 19 | 19 |
| Aging | 36 | 36 | 36 |
| Rate | 95 | 104 | 99 |
| Percentage total change, 1990–2010 | 150 | 159 | 154 |
All number sets in parentheses in indicate 95% uncertainty intervals.
Table 2.
Decomposition analysis of the change in global dialysis incidence rates and counts from 1990 to 2010 stratified by sex and provision
| Stratification According to Dialysis Provision | Men | Women | Total |
|---|---|---|---|
| Global 1990 incidence rate (pmp) | 54 (49, 59) | 36 (33, 39) | 44 (41, 47) |
| Global 2010 incidence rate (pmp) | 112 (107, 116) | 76 (73, 79) | 93 (90, 95) |
| Countries with universal maintenance dialysis access | |||
| Year 1990 incident patients | 56,020 | 46,563 | 102,583 |
| No. of patients in 2010 expected secondary to population growth | 65,417 | 53,908 | 119,358 |
| No. of patients in 2010 expected secondary to population aging | 80,753 | 63,868 | 143,916 |
| Year 2010 incident patients | 197,478 | 168,821 | 366,300 |
| Percentage change between 1990 and 2010 because of | |||
| Growth | 17 | 16 | 16 |
| Aging | 27 | 21 | 24 |
| Rate | 208 | 225 | 217 |
| Percentage total change, 1990–2010 | 253 | 263 | 257 |
| Countries with partial maintenance dialysis access | |||
| Year 1990 incident patients | 52,657 | 33,217 | 85,874 |
| No. of patients in 2010 expected secondary to population growth | 63,063 | 39,308 | 102,281 |
| No. of patients in 2010 expected secondary to population aging | 84,315 | 52,574 | 136,738 |
| Year 2010 incident patients | 146,738 | 95,585 | 242,323 |
| Percentage change between 1990 and 2010 because of | |||
| Growth | 20 | 18 | 19 |
| Aging | 40 | 40 | 40 |
| Rate | 119 | 130 | 123 |
| Percentage total change, 1990–2010 | 179 | 188 | 182 |
All number sets in parentheses in indicate 95% uncertainty intervals.
Global Prevalence and Incidence
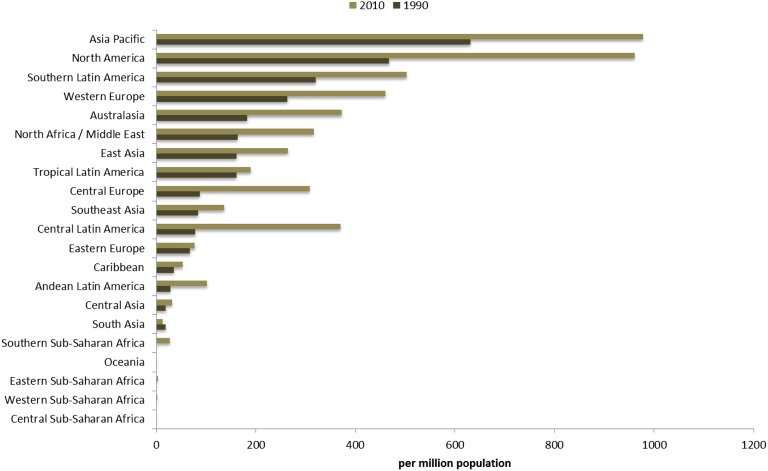
The global prevalence of maintenance dialysis has increased 1.7 times from 165 pmp patients in 1990 to 284 pmp in 2010. There was a 170% increase in prevalence of patients treated with maintenance dialysis in countries that provided universal access and a 154% increase in the last two decades for countries still working toward universal access (Table 1).
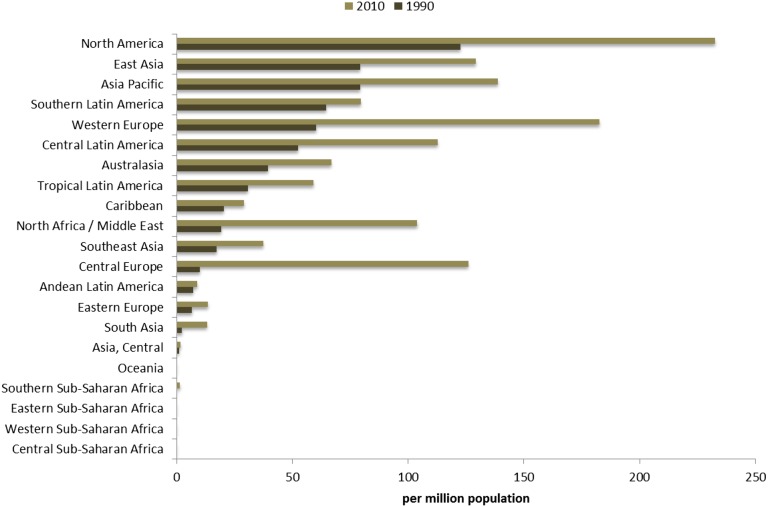
The rise in incidence was even more notable. The global incidence more than doubled from 44 pmp incidents in 1990 to 93 pmp in 2010 (Table 2). When stratifying the world by universal and partial dialysis provision, the percent change in incidence for both men and women was approximately 250% among countries that provided universal access and approximately 180% among countries with partial access (Table 2).
The strongest contributor to a larger increase in incidence rate than prevalence is the continued expansion of programs that have recently granted universal or partial access to maintenance dialysis in low- and middle-income countries. In contrast, recent data for developed nations, such as the United States and Western Europe, actually demonstrate a stabilizing trajectory of dialysis initiation in recent years.7,8 Furthermore, in some countries, many patients are offered maintenance dialysis only as a bridge to kidney transplantation; this, in turn, could explain the higher increase in incidence rates when compared with the change in prevalence. Although mortality rates for patients undergoing maintenance dialysis have decreased, mortality is still significantly higher among dialysis patients when compared with the general population, which would also affect the prevalence over the incidence.7,9,10
Patterns of Change: Geography and Population Structure
Assessing patterns of change at a more granular geographic level allows for identification of regions where dramatic change has occurred and other parts of the world where provision of maintenance dialysis has remained stable during the last two decades. North America and Pacific Asian regions had the highest prevalence for maintenance dialysis in both 1990 and 2010, followed by world regions of Europe, Australasia, and portions of Latin America (Figure 1). The incidence of provision of maintenance dialysis has followed a similar geographic pattern over time (Figure 2). World regions with the consistently lowest estimates include Sub-Saharan Africa and South and Central Asia.
Access to Maintenance Dialysis in Developing Nations
The incidence and prevalence of maintenance dialysis are not equivalent to the burden of ESRD. In many countries in Africa and South Asia, chronic comorbidities contribute to the societal burden of ESRD as also infections such as malaria, schistosomiasis, HIV, and chronic hepatitis.11–14 Hence, there is likely a large, untreated burden of ESRD within these regions.15 These countries face the difficult task of allocating adequate resources for the care of this condition from the large economic strain likely to be imposed by universal provision of maintenance dialysis.14,16 The resultant rationing of maintenance dialysis often selects against the impoverished and socially marginalized groups, such as the elderly and chronically infirm, sections of society known to generally have higher rates of CKD.16–19 A study of >2000 patients with ESRD in South Africa between 1988 and 2003 revealed that more than half of these patients were not offered dialysis secondary to rationing of RRT. Factors that weighed into the decision to provide dialysis centered on patient access to transportation, degree of comorbidity, and social stability.20 Therefore, this continued depressed activity within these countries indicates continued limited regional ability to provide RRT rather than a lack of disease burden.
Population Structure
To assess the effect of changes in population structure over time on the burden of treated ESRD, we modeled projected estimates of the prevalence expected if all contributing factors aside from population growth and aging remained constant (Table 1). Within countries with universal dialysis access in 2010, population growth and aging should have contributed to a 41% increase in prevalent dialysis and a 55% increase among nations with limited dialysis access. Countries with universal access sustained a growth rate of 114% over and above the projected 41% increase, whereas countries with limited access sustained a growth rate of 99% over and above growth anticipated secondary to changes in population structure. These data indicate that although changes in population structure over time play an important role in explaining the dramatic increase in maintenance dialysis throughout the world, this is only a partial explanation.
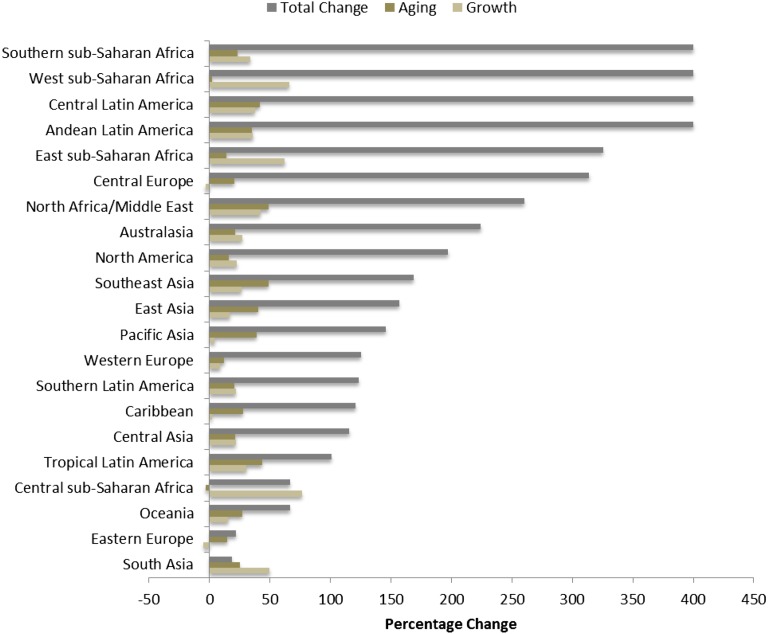
Within low-income countries, general improved health conditions, such as prevalence of childhood malnutrition, water purity, sanitation, and improved treatment of infectious diseases (e.g., HIV),21,22 have contributed to more stable population growth and aging, as indicated by population-based ESRD estimates for these regions (Figure 3).21–23 The seemingly enormous growth rate in provision of maintenance dialysis above population estimates for these regions should be interpreted with caution (Figure 3). Regions in Sub-Saharan Africa likely experienced such a growth rate because maintenance dialysis in 1990 was largely nonexistent. In comparison, there has been measurable growth in Central Latin America and Eastern Europe over the last two decades by making significant progress toward increasing dialysis access to substantial portions of the population (Figure 4).
Figure 3.
Contributions of changes in population aging and growth between years 1990 and 2010 to dialysis prevalence per million population for 21 world regions.
Figure 4.
Age-standardized maintenance dialysis incidence rate per million population for 21 world regions in years 1990 and 2010.
Factors for Change: Population Burden of Diabetes Mellitus and Hypertension
There is a robust literature evidencing the increase in diabetes mellitus and hypertension throughout the world, thought secondary to increasing life span, westernization of diet, and the rising tide of obesity and consequent metabolic syndrome.1,3,24–31 Because diabetes and hypertension are leading causes of ESRD, we determined the contribution of the global rise in these diseases to the rise in prevalence and incidence of maintenance dialysis (Figures 4 and 5). Results among countries providing partial versus universal dialysis were remarkably similar. We estimated an anticipated growth of approximately 50% in maintenance dialysis patients secondary to diabetes within the general population. Within countries providing universal dialysis access, there was a total increase of 184% and a 188% increase among countries providing partial dialysis access (Tables 3 and 4). A similar pattern emerged when assessing the increasing burden of hypertension among the general population relative to the growth in maintenance dialysis (Table 4). These percentages illustrate that even though the growth of diabetes mellitus and hypertension within the global population plays a large role in the increasing burden of maintenance dialysis, these drivers also do not explain the total rise in rates. The greatest contributor to markedly increased rates of dialysis provision remains the expansion of or governmental support for dialysis programs.
Figure 5.
Age-standardized maintenance dialysis prevalence per million population for 21 world regions in years 1990 and 2010.
Table 3.
Decomposition analysis of the change in global dialysis prevalence rates and counts for patients receiving dialysis secondary to diabetes mellitus from 1990 to 2010 stratified by sex
| Stratification Based on Dialysis Provision | Men | Women | Total |
|---|---|---|---|
| Countries with universal maintenance dialysis access | |||
| Year 1990 maintenance dialysis patients secondary to DM | 80,453 | 86,392 | 166,846 |
| Patients expected in 2010 secondary to increase of DM in general population | 125,457 | 119,261 | 244,718 |
| Year 2010 maintenance dialysis patients secondary to DM | 222,782 | 250,886 | 473,668 |
| Percentage of dialysis prevalence increase because of increase of DM prevalence in the population | 56 | 38 | 47 |
| Percentage total change | 177 | 190 | 184 |
| Countries with partial maintenance dialysis access | |||
| Year 1990 maintenance dialysis patients secondary to DM | 18,878 | 19,018 | 37,896 |
| Patients expected in 2010 secondary to increase of DM in general population | 30,712 | 29,212 | 59,924 |
| Year 2010 maintenance dialysis patients secondary to DM | 53,138 | 56,022 | 109,160 |
| Percentage of dialysis prevalence increase because of increase of DM prevalence in the population | 63 | 54 | 58 |
| Percentage total change | 181 | 195 | 188 |
DM, diabetes mellitus.
Table 4.
Decomposition analysis of the change in global dialysis prevalence and prevalent count for patients receiving dialysis secondary to hypertension from 1990 to 2010 stratified by sex
| Stratification Based on Dialysis Provision | Men | Women | Total |
|---|---|---|---|
| Countries with universal maintenance dialysis access | |||
| Year 1990 maintenance dialysis patients secondary to hypertension | 55,868 | 46,809 | 102,677 |
| Patients expected in 2010 secondary to increase of hypertension in general population | 74,930 | 59,072 | 134,002 |
| Year 2010 maintenance dialysis patients secondary to hypertension | 149,499 | 138,746 | 288,244 |
| Percentage of dialysis prevalence increase because of increase of hypertension prevalence in the population | 34 | 26 | 31 |
| Percentage total change | 168 | 196 | 181 |
| Countries with partial maintenance dialysis access | |||
| Year 1990 maintenance dialysis patients secondary to hypertension | 23,362 | 19,063 | 42,425 |
| Patients expected in 2010 secondary to increase of hypertension in general population | 36,835 | 28,909 | 65,744 |
| Year 2010 maintenance dialysis patients secondary to hypertension | 60,817 | 51,159 | 111,976 |
| Percentage of dialysis prevalence increase because of increase of hypertension prevalence in the population | 58 | 52 | 55 |
| Percentage total change | 160 | 168 | 164 |
Patterns of Growth: Sex Differences
Sex imbalances in kidney transplantation are well described within the literature, where women are more often known to serve as living donors, whereas men are more likely to be in receipt of kidney transplants.32–35 Sex imbalances in the provision of maintenance dialysis are less well described. In limited-resource countries, the sex differences in provision of maintenance dialysis are similar to that for kidney transplantation, with a higher incidence in men than in women. Other factors that may play a role in sex imbalances within resource-limited settings may involve prioritization of men in patriarchal societies and family prioritization of men on the basis of earning potential.
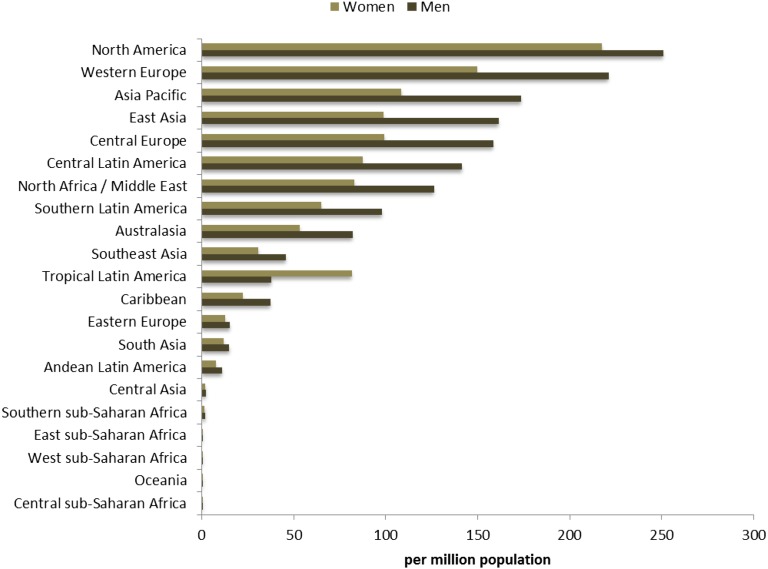
Our analysis indicates that at the global level, in both years 1990 and 2010, men were in greater receipt of maintenance dialysis than women, but dialysis rates for women increased more than men (Table 1). At the regional level, in 2010, men were in receipt of dialysis to a greater degree than women in all world regions except tropical Latin America, with most notable differences in regions of Australasia, South Asia, and Oceania (Figure 6). These results indicate that although social structures and limited resources likely play a role, there may also be a biologic explanation for sex disparities because there is a higher incidence of maintenance dialysis in men in nearly all societies.
Figure 6.
Sex-stratified age-standardized maintenance dialysis incidence rate per million population for 21 world regions in year 2010.
Drivers of ESRD Burden: CKD Detection, Progression, and Death
Within the last decade, advances in detection of CKD through widespread implementation of creatinine and GFR measurements have enhanced our ability to detect kidney disease.36 Specifically, the conceptual model of CKD developed in 2002 by the National Kidney Foundation Kidney Disease Outcomes Quality Initiative has aided our ability to quantify the burden of various stages of CKD and evaluate risk factors and outcomes for the CKD population. Such advances may have had a complex effect on ESRD burden. First, detecting earlier stage CKD allows for time to determine possible etiologies and factors contributing to CKD progression that has the potential to decrease the societal burden of ESRD. Alternatively, detecting later-stage CKD allows for time to prepare for initiation of maintenance dialysis before death. This increased ability to detect CKD at earlier stages and monitor disease progression (subsequently discussed) has possibly contributed to the growth in the ESRD population between 1990 and 2010.
CKD Progression
Understanding risk factors for CKD progression is pivotal to understanding drivers for perspective ESRD increase and for targeting ways in which risk factors for incidence and disease progression can be altered.37 Although certain determinants of CKD progression are unalterable, such as age, sex, race, and in some circumstances, CKD etiology, some interventions have the potential to affect the rate of progression.37 As previously stated, diabetes mellitus and hypertension have become the leading drivers for the growing ESRD population within high-, middle-, and low-income countries. Numerous studies indicate the benefit of glucose control and BP control on delaying progression of CKD.38–47 These facts highlight the importance of early CKD detection to allow for time to implement such disease-altering strategies.
Other contributors to CKD progression include episodes of AKI, which is hypothesized to initiate an inflammatory response that continues after the AKI episode has resolved.48–50 Causes of AKI vary geographically. The causes for AKI in the developed world regions include imagining contrast, surgery, toxicity from medications, critical illness, and complications to chronic diseases, such as cardiac and liver disease.51–53 Within developing world regions, causes can include toxicity from herbal treatments and complications during perinatal events.54–56 Again, the relationship between AKI episodes and CKD progression highlights the importance of early CKD detection to increase the likelihood of protecting remaining kidney function from such events.
Within the last decade, evidence for the relationship between CKD and cardiovascular (CV) events highlights the importance of treatment of modifiable CV risk factors within this population, such as lipid management and smoking cessation.7,57,58 Of concern are studies indicating that among patients with CKD, such targets are often not met.59,60 Foster et al. highlight suboptimal lipid control among patients with CKD in a cross-sectional National Health and Nutrition Examination Survey study of United States adults. The study illustrated the increased CV risk burden among patients with CKD.59
Pre-ESRD CKD Mortality
Another contributor to rising ESRD rates is improved survival of patients with CKD. Individuals with pre-ESRD CKD are known to experience a significantly higher likelihood of all-cause mortality than the general population.61–63 The United States Renal Data System indicates that between 1995 and 2012, mortality for individuals >65 with and without CKD has decreased, but by 42% for those with CKD compared with 16% for those without. This decline in risk of death within the CKD population persists even when adjusting for demographic factors such as age, race, and sex.61 This success in mitigating premature death within the pre-ESRD CKD population within the last two decades may also have contributed to growth of the ESRD population because patients with CKD live long enough to progress to end stage.
Growing ESRD Population: Future Projections
Studies have projected growth of CKD and ESRD populations beyond year 2010. Within the United States, it is projected that by 2020 there will be 150,000 incident ESRD patients and 785,000 prevalent patients.7 Total Medicare spending for this 2020 ESRD population is estimated to approach $53.6 billion in comparison with expenditure in 2012 of $28.6 billion, which was 5.6% of total Medicare costs.7 Other countries have performed similar estimations of projected ESRD growth.64,65 By 2020, it is estimated that Greece’s ESRD prevalent population will grow to 15,147, with an annual incident increase of 2%.65 The projected growth of ESRD has also been modeled for Australia, which anticipates a 29% growth in the ESRD population by 2020. This growth is projected to require an average annual RRT per capita expenditure increase of 16%.64
Economic Effect of Provision of Maintenance Dialysis
Considering the economic implications of growth in the maintenance dialysis population throughout the world, we have modeled the percentage of total health care expenditure that would need to be allocated to the provision of maintenance dialysis for a portion of the national prevalent CKD stage 5 population under two different scenarios. The first considers 2010 costs for maintenance dialysis for the prevalent population using Thailand’s 2010 spending adjusted for national gross domestic product (Figure 7). The second considers the estimated annual per capita spending in the United States for hemodialysis patients in 2010 (Figure 8). When using the Thailand’s reimbursement rate for peritoneal dialysis, our results illustrate that of the 14 countries with >1% of the total health care expenditure allocated to maintenance dialysis, ten of these countries are located in Asia and North Africa/Middle East regions (Figures 7 and 8). Applying Thailand’s peritoneal dialysis reimbursement rate, all countries ranged between 0.1% and 3.4% of total health care expenditure attributed to maintenance dialysis, except for Suriname with 47% (not shown in Figures 7 and 8). Using the per capita reimbursement for hemodialysis patients, world countries ranged from 0.2% to 4% of total health care expenditure in 2010 (Figure 8). The results of this analysis should be interpreted with caution because it assumes care delivery comparable with the referent country (i.e., United States, Thailand). What this analysis illustrates is that there are regional patterns, and regions with high prevalent ESRD burden and high rates of health care spending are most likely to be heavily affected financially by further increasing rates of treated ESRD.
Figure 7.
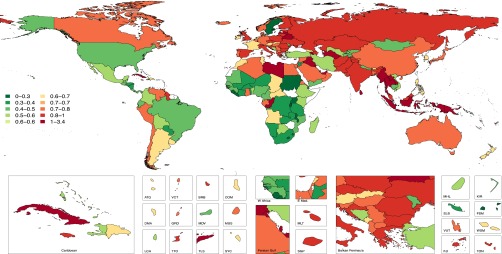
Estimated percentage of country-specific total health expenditure allocated to prevalent maintenance dialysis using the Thailand dialysis reimbursement paradigm. ATG, Antigua and Barbuda; BRB, Barbados; COM, Comoros; DMA, Dominica; E. Med, Eastern Mediterranean; FJI , Fiji; FSM, Federated States of Micronesia; GRD, Grenada and Trinidad; KIR, Kiribati; LCA, Saint Lucia; MDV, Maldives; MHL, Marshall Islands; MUS, Mauritius; SGP, Singapore; SLB, Solomon Islands; SYC, Seychelles; TLS, Timor-Leste; TTO, Trinidad and Tobago; TUN, Tunisia; VCT, Saint Vincent and Grenadines; VUT, Vanuata; WSM, Samoa.
Figure 8.
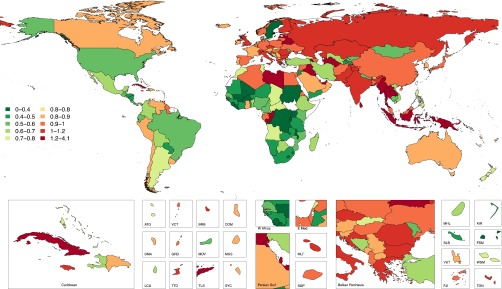
Estimated percentage of country-specific total health expenditure allocated to prevalent maintenance dialysis using the United States dialysis reimbursement paradigm. ATG, Antigua and Barbuda; BRB, Barbados; COM, Comoros; DMA, Dominica; E. Med, Eastern Mediterranean; FJI , Fiji; FSM, Federated States of Micronesia; GRD, Grenada and Trinidad; KIR, Kiribati; LCA, Saint Lucia; MDV, Maldives; MHL, Marshall Islands; MUS, Mauritius; SGP, Singapore; SLB, Solomon Islands; SYC, Seychelles; TLS, Timor-Leste; TTO, Trinidad and Tobago; TUN, Tunisia; VCT, Saint Vincent and Grenadines; VUT, Vanuata; WSM, Samoa.
Addressing the Burden: Dialysis Modalities, Transplant, and CKD Screening
Dialysis Modalities
Alleviating the expected increase in the global economic burden from the growth in the prevalent ESRD population will necessitate capitalizing on all forms of treatment beyond in-center hemodialysis. Within high- and middle-income countries, home-based RRTs, such as peritoneal dialysis and home hemodialysis, are feasible and defray the substantial costs of infrastructure maintenance and staffing and offer patient autonomy.66–69 Within the United States, there has been a 35% increase in home-based RRT between 2002 and 2012, most of which were peritoneal dialysis.7 Thailand has a universal health care structure meant to provide health coverage to individuals not covered by existing systems, such as social security. Maintenance dialysis was incorporated into universal coverage in 2007.70 This program prioritizes initiation with peritoneal dialysis and transition to hemodialysis for those deemed unsuitable for peritoneal dialysis.70 Within developing countries, the financial gains of home-based therapies are less clear.71 Costs and supplies vary on the basis of regional production of solutions for peritoneal dialysis versus importation; therefore, home-based treatments, such as peritoneal dialysis, may not always be more cost-effective.71 Within such regions, country-level analyses are needed to determine unique barriers for broad-based provision of RRT for the largest segment of the population at the lowest cost.
Renal Transplantation
Renal transplantation offers clear benefits in terms of quality of life and survival and societal economic benefit. Within the last two decades, advances in immunosuppression have led to improved allograft and patient survival. Within high-income countries, advances in ability to transplant across blood groups, paired kidney donation programs, and preemptive renal transplants have collectively increased rates of renal transplantations.72 However, resource scarcity limits this treatment modality in both high- and low-income regions. Low-income countries experience added limitations of infrastructure and shortages of surgical expertise. Resultant transplant tourism and organ trafficking that occur in such settings to address deceased donor organ shortages often target the most impoverished members of such populations.72 Further, financial constraints secondary to lack of governmental financial support for transplant programs lead to early discontinuation of immunosuppressive therapy, leading to premature graft loss.73
Population-level CKD Screening
The most effective prevention against further ESRD growth is CKD prevention.74 Systematic population-based CKD screening should be considered by societies around the world to determine the portion of the population at risk versus those affected by CKD.75–77 Currently, few societies implement systematic screening for adults unless they have risk factors, such as diabetes mellitus or advanced age.78 Considering that early- to moderate stage CKD is relatively asymptomatic, screening has multiple advantages. First, screening identifies individuals with CKD that might not be diagnosed until late stage when symptoms manifest, but when options for retarding progression are few. Second, screening for CKD may facilitate detection of undiagnosed diabetes, hypertension, and CV disease.79–82 Third, as CV risk factors are suboptimally controlled within the CKD population, screening and detection of CKD allow for better treatment of CV risk factors. Fourth, identification of the portion of the population with kidney disease further delineates the remaining population that could serve as potential living kidney donors. When assessing the wide variation in the treatment of ESRD in Asia, such as differences between India, Pakistan, and China compared with Taiwan, one can deduce the magnitude of burden of untreated ESRD within densely populated regions of the world (Figures 1 and 2). It will be challenging to plan to address the anticipated increase in the burden of ESRD solely with renal transplantation and maintenance dialysis, for both high- and low-income world regions. Systematic early CKD detection combined with detection and prevention of drivers, such as diabetes, hypertension, and CV disease, are critical.
Long-reaching Effect: ESRD and the Global Burden of NCDs
The recent 2012 United Nations Summit held to address the global burden of NCDs concluded with the formation of the 2025 Initiative.83 This initiative resolves to decrease the global burden of NCDs by 25% by 2025. Although an important initiative that focuses global attention on the leading causes of premature mortality, the initiative focuses specifically on diabetes, cancer, CV disease, respiratory diseases, and neurologic disease. In light of the rising burden of both pre-ESRD CKD and ESRD, omission of CKD as an independent cause of premature life loss will inevitably detract from the overall success of the initiative. Furthermore, with success in decreasing the burden of these specifically targeted NCDs, the consequent increased survival may potentially cause a further increase in the prevalence of CKD globally. As noted, our estimates illustrate such a continued rise in both CKD and ESRD, as survival within the CKD population also improves, and countries expand their ability to provide RRT to the ESRD population.
Study Strengths and Limitations
The considerable strengths of the study involve its scope both in terms of geography and time course for comparison, methods applied to estimate provision of maintenance dialysis for countries for which no previous estimates exist, and ability to model how growth, aging, and prevalence of diabetes and hypertension account for the growth in the dialysis population over time. The limitations include an under-representation of data from countries of the world with limited registry information. When projecting estimates for country-years with limited information, the meta-regression tool borrows strength from data for other countries in the same region, or other regions in the same super-region, which relies on the assumption that similar countries have similar dialysis rates. The possible inaccuracy of this assumption is a limitation of this study and may also account for some of the differences from estimates in the literature. However, this report includes the results of an extensive literature review to detect all possible nationally representative data for countries for which no registry data exist to address such deficits. The results of our analysis also may not reflect the precise results of national registries for two reasons. First, in specific instances a national registry may have chosen a denominator population for a region that differed from the national denominator used in this analysis. Second, our regression analysis estimates epidemiologic parameters for 1990 and 2010 on the basis of data gathered from available time points closest to those years.
Summary
To our knowledge, this study is the first analysis of sufficient scope to describe the global prevalence and incidence of maintenance dialysis treatment for ESRD. Comparing 1990 and 2010, there has been a general trend of substantially increased provision of maintenance dialysis in both men and women above what was anticipated secondary to population growth, aging, and increase in prevalence of diabetes and hypertension, with most notable increases occurring in parts of Australasia, Asia, North America, and Western Europe. Oceania and portions of Sub-Saharan Africa have maintained a low prevalence of maintenance dialysis when compared with most of the world; however, growth in these regions has occurred. With the exception of tropical Latin America, men were more likely to receive dialysis than women; however, the global change in prevalence and incidence for treated women in the last two decades has surpassed that of men.
Maintenance dialysis is a costly treatment for ESRD with economic implications for both high- and low-income world regions. Within high-income regions of the world, efforts are needed to address drivers of increase in ESRD, such as obesity, diabetes mellitus, and hypertension. Research is needed to further evaluate reasons for sex differences within the ESRD population. Within regions of the world with emerging economies, expansion of dialysis to larger portions of the population is urgently needed, especially within Sub-Saharan Africa and Oceania, to save lives and deter the difficult task of patient selection when resources are limited.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Hideki Higashi and Joseph Dieleman from the Institute for Health Metrics and Evaluation, University of Washington, for their assistance with the economic analysis.
We acknowledge funding from the Bill and Melinda Gates Foundation for this research study.
The results of this study do not reflect the views of the foundation.
We would like to thank the International Society of Nephrology for coordinating the members of the 2010 Global Burden of Disease Genitourinary Expert Group, namely, Boris Bikbov, Claudia Cella, Monica Cortinovis, Karen Courville de Vaccaro, William G. Couser, Patricia Espindola, Flavio Gaspari, Valeria Miglioli, Bishnu Pahari, Andrea Panozo, Norberto Perico, Estaban Porrini, Giuseppe Remuzzi, Felipe Rodriguez De Leon, Bernadette Thomas, Marcello Tonelli, and Natasha Wiebe.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Meeting the World’s Need for Maintenance Dialysis,” on pages 2601–2603.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014101017/-/DCSupplemental.
References
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O’Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA, 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA: Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2095–2128, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogedegbe G, Gyamfi J, Plange-Rhule J, Surkis A, Rosenthal DM, Airhihenbuwa C, Iwelunmor J, Cooper R: Task shifting interventions for cardiovascular risk reduction in low-income and middle-income countries: A systematic review of randomised controlled trials. BMJ Open 4: e005983, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas B, van Pelt M, Mehrotra R, Robinson-Cohen C, LoGerfo J: An estimation of the prevalence and progression of chronic kidney disease in a rural diabetic cambodian population. PLoS One 9: e86123, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abboud H, Labreuche J, Arauz A, Bryer A, Lavados PG, Massaro A, Munoz Collazos M, Steg PG, Yamout BI, Vicaut E, Amarenco P, OPTIC Registry Investigators : Demographics, socio-economic characteristics, and risk factor prevalence in patients with non-cardioembolic ischaemic stroke in low- and middle-income countries: The OPTIC registry. Int J Stroke 8[Suppl A100]: 4–13, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Lysaght MJ: Maintenance dialysis population dynamics: Current trends and long-term implications. J Am Soc Nephrol 13[Suppl 1]: S37–S40, 2002 [PubMed] [Google Scholar]
- 6.Grassmann A, Gioberge S, Moeller S, Brown G: ESRD patients in 2004: Global overview of patient numbers, treatment modalities and associated trends. Nephrol Dial Transplant 20: 2587–2593, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Johansen K, Kasiske B, Kutner N, Liu J, St Peter W, Guo H, Gustafson S, Heubner B, Lamb K, Li S, Li S, Peng Y, Qiu Y, Roberts T, Skeans M, Snyder J, Solid C, Thompson B, Wang C, Weinhandl E, Zaun D, Arko C, Chen SC, Daniels F, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Constantini E, Everson S, Eggers P, Agodoa L: United States Renal Data System 2011 Annual Data Report: Atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis 59[1 Suppl 1]: A7, e1–e420 [DOI] [PubMed] [Google Scholar]
- 8.Gansevoort RT, van der Heij B, Stegeman CA, de Charro FT, Nieuwenhuizen MG, de Zeeuw D, de Jong PE: Trends in the incidence of treated end-stage renal failure in The Netherlands: Hope for the future? Kidney Int Suppl (92): S7–S10, 2004. [DOI] [PubMed] [Google Scholar]
- 9.van Walraven C, Manuel DG, Knoll G: Survival trends in ESRD patients compared with the general population in the United States. Am J Kidney Dis 63: 491–499, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Steenkamp R, Shaw C, Feest T: UK Renal Registry 15th annual report: Chapter 5 survival and causes of death of UK adult patients on renal replacement therapy in 2011: national and centre-specific analyses. Nephron Clin Pract 123[Suppl 1]: 93–123, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Fogazzi GB, Castelnovo C: Maintenance dialysis in patients from developing countries: the experience of an Italian center. J Nephrol 17: 552–558, 2004 [PubMed] [Google Scholar]
- 12.Naicker S: End-stage renal disease in sub-Saharan and South Africa. Kidney Int Suppl (83): S119–S122, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Katz I: Kidney and kidney related chronic diseases in South Africa and chronic disease intervention program experiences. Adv Chronic Kidney Dis 12: 14–21, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Naicker S: Burden of end-stage renal disease in sub-Saharan Africa. Clin Nephrol 74[Suppl 1]: S13–S16, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Liyanage T, Ninomiya T, Jha V, Neal B, Patrice HM, Okpechi I, Zhao MH, Lv J, Garg AX, Knight J, Rodgers A, Gallagher M, Kotwal S, Cass A, Perkovic V: Worldwide access to treatment for end-stage kidney disease: A systematic review [published online ahead of print March 13, 2015]. Lancet 10.1016/S0140-6736(14)61601-9 [DOI] [PubMed]
- 16.Jha V, Chugh KS: The practice of dialysis in the developing countries. Hemodial Int 7: 239–249, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Dirks JH, Levin NW: Dialysis rationing in South Africa: A global message. Kidney Int 70: 982–984, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Lugon JR, Strogoff de Matos JP: Disparities in end-stage renal disease care in South America. Clin Nephrol 74[Suppl 1]: S66–S71, 2010 [PubMed] [Google Scholar]
- 19.Sakhuja V, Kohli HS: End-stage renal disease in India and Pakistan: Incidence, causes, and management. Ethn Dis 16[2 Suppl 2]: S2–S20–S3, 2006 [PubMed] [Google Scholar]
- 20.Moosa MR, Kidd M: The dangers of rationing dialysis treatment: The dilemma facing a developing country. Kidney Int 70: 1107–1114, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Hankins C: Overview of the current state of the epidemic. Curr HIV/AIDS Rep 10: 113–123, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdool Karim Q: The global HIV epidemic: Current status and challenges. Curr HIV/AIDS Rep 10: 111–112, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD, 3rd, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd Hanafiah K, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA, 3rd, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stöckl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M, AlMazroa MA, Memish ZA: A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2224–2260, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wild S, Roglic G, Green A, Sicree R, King H: Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 27: 1047–1053, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Zabetian A, Sanchez IM, Narayan KM, Hwang CK, Ali MK: Global rural diabetes prevalence: A systematic review and meta-analysis covering 1990-2012. Diabetes Res Clin Pract 104: 206–213, 2014 [DOI] [PubMed] [Google Scholar]
- 26.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J: Global burden of hypertension: Analysis of worldwide data. Lancet 365: 217–223, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Corriere M, Rooparinesingh N, Kalyani RR: Epidemiology of diabetes and diabetes complications in the elderly: An emerging public health burden. Curr Diab Rep 13: 805–813, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meigs JB: Epidemiology of the metabolic syndrome, 2002. Am J Manag Care 8[11 Suppl]: S283–S292, quiz S293–S296, 2002 [PubMed] [Google Scholar]
- 29.Danaei G, Singh GM, Paciorek CJ, Lin JK, Cowan MJ, Finucane MM, Farzadfar F, Stevens GA, Riley LM, Lu Y, Rao M, Ezzati M, Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group : The global cardiovascular risk transition: Associations of four metabolic risk factors with national income, urbanization, and Western diet in 1980 and 2008. Circulation 127: 1493–1502, 1502e1–1502e8, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Basu S, Millett C: Social epidemiology of hypertension in middle-income countries: Determinants of prevalence, diagnosis, treatment, and control in the WHO SAGE study. Hypertension 62: 18–26, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Bromfield S, Muntner P: High blood pressure: The leading global burden of disease risk factor and the need for worldwide prevention programs. Curr Hypertens Rep 15: 134–136, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zimmerman D, Donnelly S, Miller J, Stewart D, Albert SE: Gender disparity in living renal transplant donation. Am J Kidney Dis 36: 534–540, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Bloembergen WE, Port FK, Mauger EA, Briggs JP, Leichtman AB: Gender discrepancies in living related renal transplant donors and recipients. J Am Soc Nephrol 7: 1139–1144, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Segev DL, Kucirka LM, Oberai PC, Parekh RS, Boulware LE, Powe NR, Montgomery RA: Age and comorbidities are effect modifiers of gender disparities in renal transplantation. J Am Soc Nephrol 20: 621–628, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Couchoud C, Bayat S, Villar E, Jacquelinet C, Ecochard R, REIN registry : A new approach for measuring gender disparity in access to renal transplantation waiting lists. Transplantation 94: 513–519, 2012 [DOI] [PubMed] [Google Scholar]
- 36.National Kidney Foundation : K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39[2 Suppl 1]: S1–S266, 2002 [PubMed] [Google Scholar]
- 37.Rosansky SJ: Renal function trajectory is more important than chronic kidney disease stage for managing patients with chronic kidney disease. Am J Nephrol 36: 1–10, 2012 [DOI] [PubMed] [Google Scholar]
- 38.The Diabetes Control and Complications (DCCT) Research Group : Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. Kidney Int 47: 1703–1720, 1995 [DOI] [PubMed] [Google Scholar]
- 39.Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group : Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: The Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA 290: 2159–2167, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Breyer JA, Bain RP, Evans JK, Nahman NS, Jr, Lewis EJ, Cooper M, McGill J, Berl T, The Collaborative Study Group : Predictors of the progression of renal insufficiency in patients with insulin-dependent diabetes and overt diabetic nephropathy. Kidney Int 50: 1651–1658, 1996 [DOI] [PubMed] [Google Scholar]
- 41.de Boer IH, Sun W, Cleary PA, Lachin JM, Molitch ME, Steffes MW, Zinman B, DCCT/EDIC Research Group : Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med 365: 2366–2376, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hovind P, Rossing P, Tarnow L, Smidt UM, Parving HH: Remission and regression in the nephropathy of type 1 diabetes when blood pressure is controlled aggressively. Kidney Int 60: 277–283, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Hovind P, Rossing P, Tarnow L, Smidt UM, Parving HH: Progression of diabetic nephropathy. Kidney Int 59: 702–709, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Lewis JB, Berl T, Bain RP, Rohde RD, Lewis EJ, Collaborative Study Group : Effect of intensive blood pressure control on the course of type 1 diabetic nephropathy. Am J Kidney Dis 34: 809–817, 1999 [DOI] [PubMed] [Google Scholar]
- 45.Mogensen CE: Progression of nephropathy in long-term diabetics with proteinuria and effect of initial anti-hypertensive treatment. Scand J Clin Lab Invest 36: 383–388, 1976 [DOI] [PubMed] [Google Scholar]
- 46.Mulec H, Blohmé G, Grände B, Björck S: The effect of metabolic control on rate of decline in renal function in insulin-dependent diabetes mellitus with overt diabetic nephropathy. Nephrol Dial Transplant 13: 651–655, 1998 [DOI] [PubMed] [Google Scholar]
- 47.Parving HH, Andersen AR, Smidt UM, Svendsen PA: Early aggressive antihypertensive treatment reduces rate of decline in kidney function in diabetic nephropathy. Lancet 1: 1175–1179, 1983 [DOI] [PubMed] [Google Scholar]
- 48.D'Hoore E, Neirynck N, Schepers E, Vanholder R, Verbeke F, Van Thielen M, Van Biesen W: Chronic kidney disease progression is mainly associated with non-recovery of acute kidney injury [published online ahead of print February 21, 2015]. J Nephrol [DOI] [PubMed]
- 49.Heung M, Chawla LS: Predicting progression to chronic kidney disease after recovery from acute kidney injury. Curr Opin Nephrol Hypertens 21: 628–634, 2012 [DOI] [PubMed] [Google Scholar]
- 50.Jones J, Holmen J, De Graauw J, Jovanovich A, Thornton S, Chonchol M: Association of complete recovery from acute kidney injury with incident CKD stage 3 and all-cause mortality. Am J Kidney Dis 60: 402–408, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsai TT, Patel UD, Chang TI, Kennedy KF, Masoudi FA, Matheny ME, Kosiborod M, Amin AP, Messenger JC, Rumsfeld JS, Spertus JA: Contemporary incidence, predictors, and outcomes of acute kidney injury in patients undergoing percutaneous coronary interventions: Insights from the NCDR Cath-PCI registry. JACC Cardiovasc Interv 7: 1–9, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cerdá J, Bagga A, Kher V, Chakravarthi RM: The contrasting characteristics of acute kidney injury in developed and developing countries. Nat Clin Pract Nephrol 4: 138–153, 2008 [DOI] [PubMed] [Google Scholar]
- 53.Kume K, Yasuoka Y, Adachi H, Noda Y, Hattori S, Araki R, Kohama Y, Imanaka T, Matsutera R, Kosugi M, Sasaki T: Impact of contrast-induced acute kidney injury on outcomes in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Cardiovasc Revasc Med 14: 253–257, 2013 [DOI] [PubMed] [Google Scholar]
- 54.Godara SM, Kute VB, Trivedi HL, Vanikar AV, Shah PR, Gumber MR, Patel HV, Gumber VM: Clinical profile and outcome of acute kidney injury related to pregnancy in developing countries: A single-center study from India. Saudi J Kidney Dis Transpl 25: 906–911, 2014 [DOI] [PubMed] [Google Scholar]
- 55.Bentata Y, Housni B, Mimouni A, Azzouzi A, Abouqal R: Acute kidney injury related to pregnancy in developing countries: Etiology and risk factors in an intensive care unit. J Nephrol 25: 764–775, 2012 [DOI] [PubMed] [Google Scholar]
- 56.Jha V, Parameswaran S: Community-acquired acute kidney injury in tropical countries. Nat Rev Nephrol 9: 278–290, 2013 [DOI] [PubMed] [Google Scholar]
- 57.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 58.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American Heart Association Statistics Committee and Stroke Statistics Subcommittee : Heart disease and stroke statistics--2013 update: A report from the American Heart Association. Circulation 127: e6–e245, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Foster MC, Rawlings AM, Marrett E, Neff D, Willis K, Inker LA, Coresh J, Selvin E: Cardiovascular risk factor burden, treatment, and control among adults with chronic kidney disease in the United States. Am Heart J 166: 150–156, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Plantinga LC, Miller ER, 3rd, Stevens LA, Saran R, Messer K, Flowers N, Geiss L, Powe NR, Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team : Blood pressure control among persons without and with chronic kidney disease: US trends and risk factors 1999-2006. Hypertension 54: 47–56, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Ishani A, Johansen K, Kasiske BL, Kutner N, Liu J, St Peter W, Guo H, Hu Y, Kats A, Li S, Li S, Maloney J, Roberts T, Skeans M, Snyder J, Solid C, Thompson B, Weinhandl E, Xiong H, Yusuf A, Zaun D, Arko C, Chen SC, Daniels F, Ebben J, Frazier E, Johnson R, Sheets D, Wang X, Forrest B, Berrini D, Constantini E, Everson S, Eggers P, Agodoa L: US Renal Data System 2013 Annual Data Report. Am J Kidney Dis 63[1 Suppl]: A7, 2014 [DOI] [PubMed] [Google Scholar]
- 62.Lin CM, Yang MC, Hwang SJ, Sung JM: Progression of stages 3b-5 chronic kidney disease--preliminary results of Taiwan national pre-ESRD disease management program in Southern Taiwan. J Formos Med Assoc 112: 773–782, 2013 [DOI] [PubMed] [Google Scholar]
- 63.Wen CP, Cheng TY, Tsai MK, Chang YC, Chan HT, Tsai SP, Chiang PH, Hsu CC, Sung PK, Hsu YH, Wen SF: All-cause mortality attributable to chronic kidney disease: A prospective cohort study based on 462 293 adults in Taiwan. Lancet 371: 2173–2182, 2008 [DOI] [PubMed] [Google Scholar]
- 64.Tucker PS, Kingsley MI, Morton RH, Scanlan AT, Dalbo VJ: The increasing financial impact of chronic kidney disease in australia. Int J Nephrol 2014: 120537, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rodina-Theocharaki A, Bliznakova K, Pallikarakis N: Markov Chain Monte Carlo simulation for projection of end stage renal disease patients in Greece. Comput Methods Programs Biomed 107: 90–96, 2012 [DOI] [PubMed] [Google Scholar]
- 66.Agar JW, Knight RJ, Simmonds RE, Boddington JM, Waldron CM, Somerville CA: Nocturnal haemodialysis: An Australian cost comparison with conventional satellite haemodialysis. Nephrology (Carlton) 10: 557–570, 2005 [DOI] [PubMed] [Google Scholar]
- 67.Liu FX, Treharne C, Culleton B, Crowe L, Arici M: The financial impact of increasing home-based high dose haemodialysis and peritoneal dialysis. BMC Nephrol 15: 161, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Treharne C, Liu FX, Arici M, Crowe L, Farooqui U: Peritoneal dialysis and in-centre haemodialysis: A cost-utility analysis from a UK payer perspective. Appl Health Econ Health Policy 12: 409–420, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Palmer SC, Palmer AR, Craig JC, Johnson DW, Stroumza P, Frantzen L, Leal M, Hoischen S, Hegbrant J, Strippoli GF: Home versus in-centre haemodialysis for end-stage kidney disease. Cochrane Database Syst Rev 11: CD009535, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tantivess S, Werayingyong P, Chuengsaman P, Teerawattananon Y: Universal coverage of renal dialysis in Thailand: Promise, progress, and prospects. BMJ 346: f462, 2013 [DOI] [PubMed] [Google Scholar]
- 71.Just PM, de Charro FT, Tschosik EA, Noe LL, Bhattacharyya SK, Riella MC: Reimbursement and economic factors influencing dialysis modality choice around the world. Nephrol Dial Transplant 23: 2365–2373, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jafar TH: Organ trafficking: Global solutions for a global problem. Am J Kidney Dis 54: 1145–1157, 2009 [DOI] [PubMed] [Google Scholar]
- 73.Jha V: End-stage renal care in developing countries: The India experience. Ren Fail 26: 201–208, 2004 [DOI] [PubMed] [Google Scholar]
- 74.Molitoris BA: Screening: Screening for kidney disease--a lost opportunity. Nat Rev Nephrol 10: 6–8, 2014 [DOI] [PubMed] [Google Scholar]
- 75.Komenda P, Ferguson TW, Macdonald K, Rigatto C, Koolage C, Sood MM, Tangri N: Cost-effectiveness of primary screening for CKD: A systematic review. Am J Kidney Dis 63: 789–797, 2014 [DOI] [PubMed] [Google Scholar]
- 76.Kondo M, Yamagata K, Hoshi SL, Saito C, Asahi K, Moriyama T, Tsuruya K, Yoshida H, Iseki K, Watanabe T: Cost-effectiveness of chronic kidney disease mass screening test in Japan. Clin Exp Nephrol 16: 279–291, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.de Jong PE, van der Velde M, Gansevoort RT, Zoccali C: Screening for chronic kidney disease: Where does Europe go? Clin J Am Soc Nephrol 3: 616–623, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Berns JS: Routine screening for CKD should be done in asymptomatic adults... selectively. Clin J Am Soc Nephrol 9: 1988–1992, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Atthobari J, Asselbergs FW, Boersma C, de Vries R, Hillege HL, van Gilst WH, Gansevoort RT, de Jong PE, de Jong-van den Berg LT, Postma MJ, PREVEND IT Study Group : Cost-effectiveness of screening for albuminuria with subsequent fosinopril treatment to prevent cardiovascular events: A pharmacoeconomic analysis linked to the prevention of renal and vascular endstage disease (PREVEND) study and the prevention of renal and vascular endstage disease intervention trial (PREVEND IT). Clin Ther 28: 432–444, 2006 [DOI] [PubMed] [Google Scholar]
- 80.Özyilmaz A, de Jong PE, Gansevoort RT: Screening for chronic kidney disease can be of help to prevent atherosclerotic end-organ damage. Nephrol Dial Transplant 27: 4046–4052, 2012 [DOI] [PubMed] [Google Scholar]
- 81.Cravedi P, Sharma SK, Bravo RF, Islam N, Tchokhonelidze I, Ghimire M, Pahari B, Thapa ?, Basnet A, Tataradze A, Tinatin D, Beglarishvili L, Fwu CW, Kopp JB, Eggers P, Ene-Iordache B, Carminati S, Perna A, Chianca A, Couser WG, Remuzzi G, Perico N: Preventing renal and cardiovascular risk by renal function assessment: insights from a cross-sectional study in low-income countries and the USA. BMJ Open 2(5); pii, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sharma SK, Ghimire A, Carminati S, Remuzzi G, Perico N: Management of chronic kidney disease and its risk factors in eastern Nepal. Lancet Glob Health 2(9): e506–507, 2014 [DOI] [PubMed] [Google Scholar]
- 83.Beaglehole R, Bonita R, Ezzati M, Alleyne G, Dain K, Kishore SP, Horton R: NCD Countdown 2025: Accountability for the 25 × 25 NCD mortality reduction target. Lancet 384: 105–107, 2014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.