Abstract
Information about environmental exposure to melamine and renal injury in adults is lacking. We investigated this relationship in 44 workers at two melamine tableware manufacturing factories in Taiwan (16 manufacturers, eight grinders, ten packers, and ten administrators) and 105 nonexposed workers (controls) at one shipbuilding company who were enrolled in August–December of 2012. For melamine workers, personal and area air samples were obtained at the worksite over 1 workweek (Monday–Friday). In the same week, pre- and post-shift one-spot urine samples were collected each workday and one first-spot urine sample was collected on each weekend morning and the following Monday morning. For each control, a one-spot urine sample was collected on Friday morning. A blood sample was also obtained from each participant at this time. Melamine levels were measured in air, urine, and serum, and early renal injury biomarkers were measured in urine. Urinary melamine concentrations in manufacturers increased sharply between pre- and post-shift measurements on Monday, remained significantly elevated throughout the workweek, and decreased over the weekend; changes in urinary melamine concentrations were substantially lower for other melamine workers. Manufacturers were exposed to the highest concentrations of ambient melamine and had significantly higher urinary and serum melamine concentrations than did the controls (P<0.001). Urinary melamine levels were positively associated with urinary N-acetyl β-d-glucosaminidase (NAG) levels but not microalbumin levels, and the detectable β2-microglobulin rate increased in the manufacturers group. In conclusion, ambient melamine exposure may increase the levels of urinary biomarkers of renal tubular injury in this occupational setting.
Keywords: chronic kidney disease, chronic renal failure, clinical epidemiology, nephrotoxicity, biomarkers of renal tubular injury
Kidney disease diagnosed with objective measures of kidney injury and dysfunction has been recognized as a major public health burden in the past few decades.1 The population prevalence of chronic kidney disease exceeds 10% and is more than 50% in high-risk subpopulations worldwide. Accumulative evidence suggests that kidney injury causes numerous systematic complications and has even increased all-cause and cardiovascular mortality.2 Before the development of renal function impairment or renal failure, several biomarkers such as microalbumin, N-acetyl β-d-glucosaminidase (NAG), and β2-microglobulin (β2-MG) in urine were used as early biologic indicators to monitor or predict its worsening3,4 Besides the well known independent risks for kidney disease such as age, sex, ethnic group, comorbidity (e.g., hypertension and diabetes), genetic components, reduced glomerular filtration rate, and increased urinary albumin excretion, newly emerging environmental chemicals such as melamine are also considered as additional crucial hazards.1,5
The melamine chemical is still ubiquitously present in our environment, even after the 2008 melamine incident in China that caused kidney-related diseases and kidney failure in children.6 In 2012, Panuwet et al. reported that 76% of 492 urine samples from the general United States population were detectable for melamine (>0.66 ng/ml of method of detection limit [MDL]).7 Our recent data of 87 urine samples from 22 study children and 70 urine samples from their parents in one Taiwanese community revealed that in only two urine samples could melamine not be detected at a MDL of 0.8 ng/mL.8 The aforementioned results indicated that both of these different ethnic populations are continuously exposed to melamine from the general environment.
One of the probable exposure sources of melamine chemical is from the wide and daily use of melamine-made tableware, which can migrate substantial amounts of melamine, especially at high temperatures or high acidity.9,10 These wares are made of a mixture of melamine and formaldehyde that forms a polymer resin. Because of the approval of the US Food and Drug Administration to allow the use of melamine-formaldehyde in the manufacture of food packaging, melamine tableware manufacturing has become a fast-growing business.11
Some previous occupational epidemiologic studies have found that melamine-formaldehyde resin-related workers suffered from respiratory and dermal adverse health effects, probably due to ambient formaldehyde exposure.12–14 In contrast, no one has examined the effect of melamine exposure on early renal injury in the same workplace. Thus we designed time-series and cross-sectional studies in melamine tableware manufacturing factories to investigate whether melamine exposure causes early renal injury by measuring several candidate markers for glomerular and renal tubular functions. In order to increase the incentive of workers to participate in this study, we also provided free health check-ups for this occupational population.
Results
Study Subjects
All 44 melamine tableware manufacturing workers (melamine workers), including 16 from manufacturing areas, eight from grinding areas, ten from packing areas, and ten from administrative areas, who worked for ≥1 year in Factories A and B were recruited (Table 1). Another 105 office staff from Factory C served as the comparison group.
Table 1.
Demographic characteristics in melamine tableware manufacturing workers by worksites and their comparison group
| Variables | Exposed Workers (n=44) | Nonexposed workers Controls (N=105) | P valuea (Overall) | P valueb(Manufacturers versus Controls) | |||
|---|---|---|---|---|---|---|---|
| Manufacturers (N=16) | Grinders (N=8) | Packers (N=10) | Administrators (N=10) | ||||
| N (%) | |||||||
| Factory | |||||||
| A | 7 | 4 | 4 | 8 | — | ||
| B | 9 | 4 | 6 | 2 | |||
| Gender | |||||||
| Male | 12 (75.0) | 3 (37.0) | 2 (20.0) | 4 (40.0) | 97 (92.0) | <0.001 | 0.053 |
| Female | 4 (25.0) | 5 (62.0) | 8 (80.0) | 6 (60.0) | 8 (8.0) | ||
| Education (year) | |||||||
| ≤9 | 12 (75.0) | 5 (63.0) | 6 (60.0) | 2 (20.0) | 2 (2.0) | <0.001 | <0.001 |
| >9 | 4 (25.0) | 3 (38.0) | 4 (40.0) | 8 (80.0) | 103 (98.0) | ||
| Personal habits | |||||||
| Current smokers | 4 (25.0) | 2 (25.0) | 1 (10.0) | 2 (20.0) | 13 (12.4) | 0.580 | 0.164 |
| Current betel chewers | 2 (12.5) | 0 | 0 | 0 | 6 (5.7) | 0.523 | 0.285 |
| Current drinkers | 4 (25.0) | 1 (12.5) | 1 (10.0) | 3 (30.0) | 50 (47.6) | 0.029 | 0.075 |
| Mean±SD (median, IQR) | |||||||
| Age (years) | 47.3±6.2 | 43.9±8.43 | 38.4±9.9 | 36.5±8.5 | 48.8±9.0 | <0.001 | 0.119 |
| (49.0, 40.8–52.5) | (48.0, 34.0–50.8) | (38.5, 29.8–48.5) | (34.0, 30.3–42.0) | (51.0, 48.0–55.0) | |||
| Year of employment (years) | 18.0±11.7 | 6.9±9.0 | 7.1±6.1 | 8.9±5.3 | — | 0.014 | — |
| (18.0, 7.0–26.0) | (2.5, 1.0–10.0) | (5.5, 1.8–14.3) | (10.0, 3.5–12.3) | — | |||
| BMI | 23.1±4.5 | 22.6±3.4 | 22.5±2.5 | 25.4±7.1 | 23.7±2.7 | 0.482 | 0.603 |
| (23.7, 20.0–25.4) | (21.7, 19.7–4.9) | (22.3, 20.8–23.9) | (22.6, 20.8–29.4) | (23.2, 22.0–25.5) | |||
| WHR | 0.9±0.1 | 0.8±0.1 | 0.8±0.0 | 0.9±0.1 | 1.6±7.2 | 0.058 | 1.000 |
| (0.9, 0.8–0.9) | (0.8, 0.8–0.9) | (0.8, 0.8–0.9) | (0.8, 0.8–0.9) | (0.9, 0.8–0.9) | |||
IQR, interquartile range; BMI, body mass index; WHR, waist-to-hip ratio.
Kruskal–Wallis test or chi-squared test.
Wilcoxon rank-sum test or Fisher’s exact test.
Time-Series Study
Among the 44 melamine workers, 14 were measured for ambient personal melamine and formaldehyde for 5 consecutive days during the working week (from Monday to Friday) and 35 had one-spot urine samples collected consecutively pre- and post-shift from Monday to the end-of-shift on Friday. In addition, three first-spot urine samples on the mornings of Saturday and Sunday and the following Monday were collected (Figure 1).
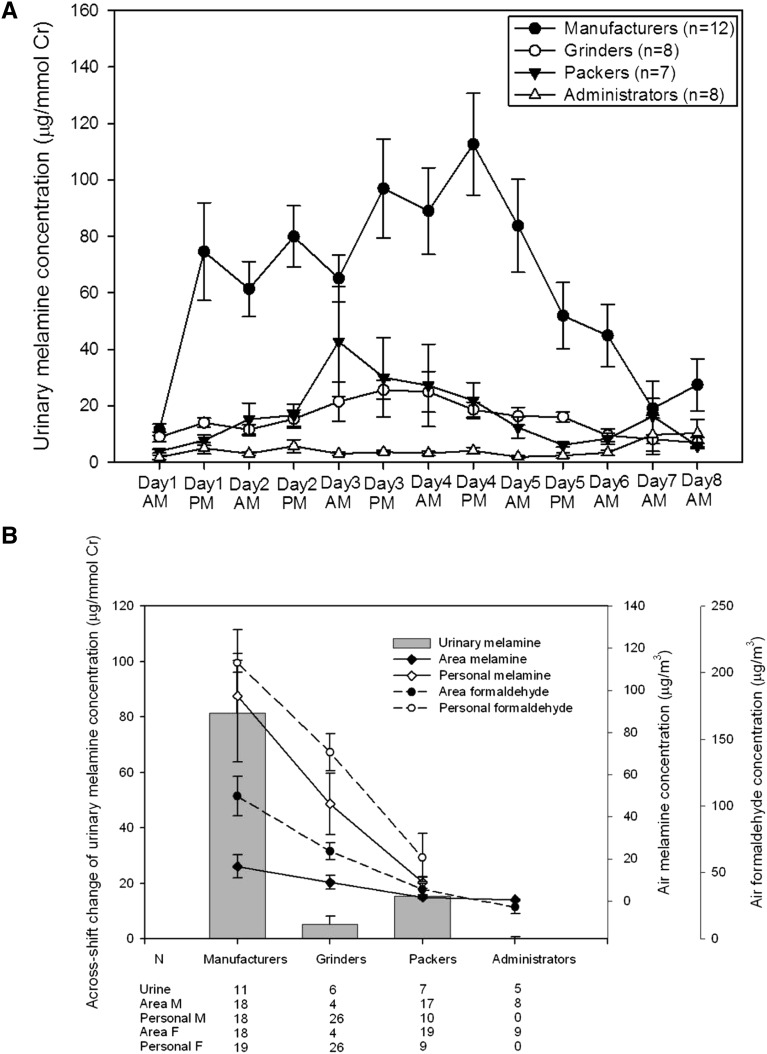
Figure 1.
Melamine and formaldehyde concentration in different specimens by worksites. (A) Temporal changes of urinary melamine concentrations by worksites. (B) Mean (±SEM) across-changes of urinary melamine concentration, air area, and personal melamine and formaldehyde concentrations by worksites. Cr, creatinine.
Urinary melamine concentrations in manufacturers increased sharply during Monday post-shift and the highest levels were recorded on Thursday post-shift. The levels then decreased during the weekend, returning to similar concentrations by Monday pre-shift (Figure 1A, Supplemental Table 3). Mean ambient melamine concentrations and across-shift change of urinary melamine concentrations were highest in manufacturers, followed by grinders or packers, and the lowest levels were in office staff (Figure 1A, Supplemental Table 4). A similar pattern was present for ambient formaldehyde (Supplemental Table 4). The real-time ambient particle concentrations were also higher in the manufacturing area than in the administrative area (Supplemental Figure 4, Supplemental Table 5).
Generalized linear mixed models using the generalized estimating equation found that the daily difference of urinary melamine concentrations was significantly higher in manufacturers than in nonmanufacturers (Supplemental Figure 5A, Supplemental Table 6). In addition, urinary melamine concentrations increased in both manufacturers and nonmanufacturers during the work and returned to baseline on the weekend. Ambient melamine but not ambient formaldehyde concentration was significantly correlated with the daily difference in the pre- and post-shift urinary melamine concentrations (P=0.034) (Supplemental Table 6).
Cross-Sectional Study
Melamine exposure markers and renal injury indicators categorized by worksites are shown in Table 2. Because of menstruation, two, one, one, and one female melamine workers in manufacturing, grinding, packing, and administrative areas, respectively, did not provide urine specimens for urinary melamine and renal injury indicators. In the nonexposed workers, we randomly selected one-spot urine samples from 42 for urinary melamine measurement.
Table 2.
Melamine concentrations and renal function indicators in different specimens in melamine tableware manufacturing workers by worksites and their comparison group
| Variables | Exposed Workers (n=44) | Nonexposed Workers Controls (N=105) | P valuea (Overall) | P valueb (Manufacturers versus Controls) | |||
|---|---|---|---|---|---|---|---|
| Manufacturers (N=16) | Grinders (N=8) | Packers (N=10) | Administrators (N=10) | ||||
| Mean±SD (median, IQR) | |||||||
| Exposure markers | |||||||
| Urine | |||||||
| Melamine (ng/ml) | 917.6±516.4 | 188.0±107.4 | 292.6±363.6 | 109.8±214.7 | 8.1±9.1 | <0.001 | <0.001 |
| (943.0, 438.0–1251.8) | (206.3, 64.7–291.6) | (252.6, 42.4–390.3) | (18.2, 12.6–101.3) | (4.3, 1.6–11.2)c | |||
| Melamine (μg/mmol Cr) | 84.4±47.4 | 18.4±9.7 | 24.2±23.0 | 4.6±6.8 | 0.7±0.9 | <0.001 | <0.001 |
| (80.5, 51.3–112.9) | (16.2, 12.3–29.5) | (15.9, 3.9–46.7) | (1.9, 1.0–5.2) | (0.3, 0.1–1.0)c | |||
| Serum | |||||||
| Melamine (ng/ml), N (%) | |||||||
| Non-detectabled | 0 | 5 (62.5) | 7 (70.0) | 7 (70.0) | — | <0.001 | |
| Detectable | 16 (100.0) | 3 (37.5) | 3 (30.0) | 3 (30.0) | — | ||
| 7.2±4.6 | 2.4±0.3 | 3.6±2.2 | 1.7±0.4 | — | |||
| (6.7, 4.0–9.6) | (2.4, 2.0–) | (2.4, 2.2–) | (1.6, 1.5–) | — | |||
| Nephrolithiasis markers | |||||||
| Urine | |||||||
| Microalbumin (mg/mmol Cr) | 14.8±49.2 | 1.0±0.6 | 0.8±0.5 | 1.5±2.7 | 1.9±6.8 | 0.486 | 0.248 |
| (0.8, 0.4–2.5) | (0.5, 0.5–1.7) | (0.7, 0.4–1.5) | (0.4, 0.3–1.5) | (0.6, 0.4–0.9) | |||
| NAG (IU/mmol Cr) | 1.8±3.5 | 0.9±0.8 | 0.6±0.4 | 0.6±0.3 | 0.4±0.2 | 0.002 | 0.002 |
| (0.9, 0.4–1.4) | (0.8, 0.3–0.9) | (0.5, 0.3–0.9) | (0.6, 0.3–0.9) | (0.4, 0.3–0.5) | |||
| β2-microglobulin, N (%) | |||||||
| Nondetectablee | 10 (62.5) | 6 (75.0) | 9 (90.0) | 9 (90.0) | 101 (96.2) | 0.006 | 0.007 |
| Detectable | 4 (25.0) | 1 (12.5) | 0 | 0 | 4 (3.8) | ||
| Missing | 2 (12.0) | 1 (12.5) | 1 (10.0) | 1 (10.0) | 0 | ||
| Laboratory data | |||||||
| eGFR | 83.4±14.0 | 81.0±12.1 | 82.7±8.2 | 80.0±10.1 | 77.8±9.7 | 0.383 | 0.246 |
| (79.6, 72.7–91.5) | (82.0, 70.9–88.1) | (86.0, 75.1–88.6) | (79.9, 73.3–88.5) | (77.4, 71.4–84.7) | |||
| eCCR | 80.8±12.0 | 78.9±14.0 | 82.0±15.4 | 98.1±35.4 | 78.4±13.2 | 0.518 | 0.375 |
| (79.7, 75.5–90.6) | (79.7, 68.0–86.7) | (79.3, 69.6–96.8) | (84.0, 73.6–117.9) | (77.6, 68.6–88.0) | |||
| eGFR<60 mL/min/1.73 m2, N (%) | 0 | 0 | 0 | 0 | 4 (3.8) | — | — |
| Urine, mg/dl | |||||||
| Creatinine | 142.9±64.1 | 123.1±60.2 | 140.7±52.0 | 185.2±92.2 | 143.7±78.3 | 0.706 | 0.843 |
| (139.4, 81.0–202.7) | (112.9, 95.7–145.3) | (139.5, 87.2–181.7) | (150.9, 116.1–276.8) | (124.9, 83.0–202.9) | |||
| Serum, mg/dl | |||||||
| BUN | 15.1±4.6 | 14.0±3.1 | 13.2±3.2 | 14.1±3.6 | 13.1±2.7f | 0.541 | 0.141 |
| (15.4, 11.5–18.1) | (14.0, 11.7–16.9) | (12.6, 10.9–15.6) | (13.6, 11.8–17.8) | (13.0, 10.7–14.8) | |||
| Creatinine | 1.0±0.2 | 0.9±0.2 | 0.9±0.1 | 1.0±0.1 | 1.1±0.1f | <0.001 | 0.116 |
| (1.0, 0.8–1.1) | (0.9, 0.8–1.0) | (0.9, 0.8–1.0) | (1.0, 0.9–1.0) | (1.1, 1.0–1.1) | |||
| Uric acid | 5.7±1.6 | 5.7±2.6 | 5.1±1.4 | 6.8±1.2 | 6.0±1.3f | 0.061 | 0.162 |
| (5.6, 4.4–6.5) | (5.4, 3.7–7.6) | (5.1, 4.0–6.1) | (6.6, 5.6–8.0) | (5.9, 5.1–7.0) | |||
IQR, interquartile range; Cr, creatinine; eGFR, estimated glomerular filtration rate calculated by the Modification of Diet in Renal Disease calculator-extended version (adjusted by age, gender, serum creatinine, and race), unit as ml/min/1.73 m2; eCCr, estimated creatinine clearance rate calculated by the Modification of the Cockcroft–Gault Calculator (adjusted by age, gender, serum creatinine, and weight), unit as ml/min.
Kruskal–Wallis test or chi-squared test.
Wilcoxon rank-sum test or Fisher’s exact test.
n=42.
MDL of melamine in serum: 1.33 ng/ml.
MDL for β2-microglobulin: 0.206 mg/l.
Missing data, n=2.
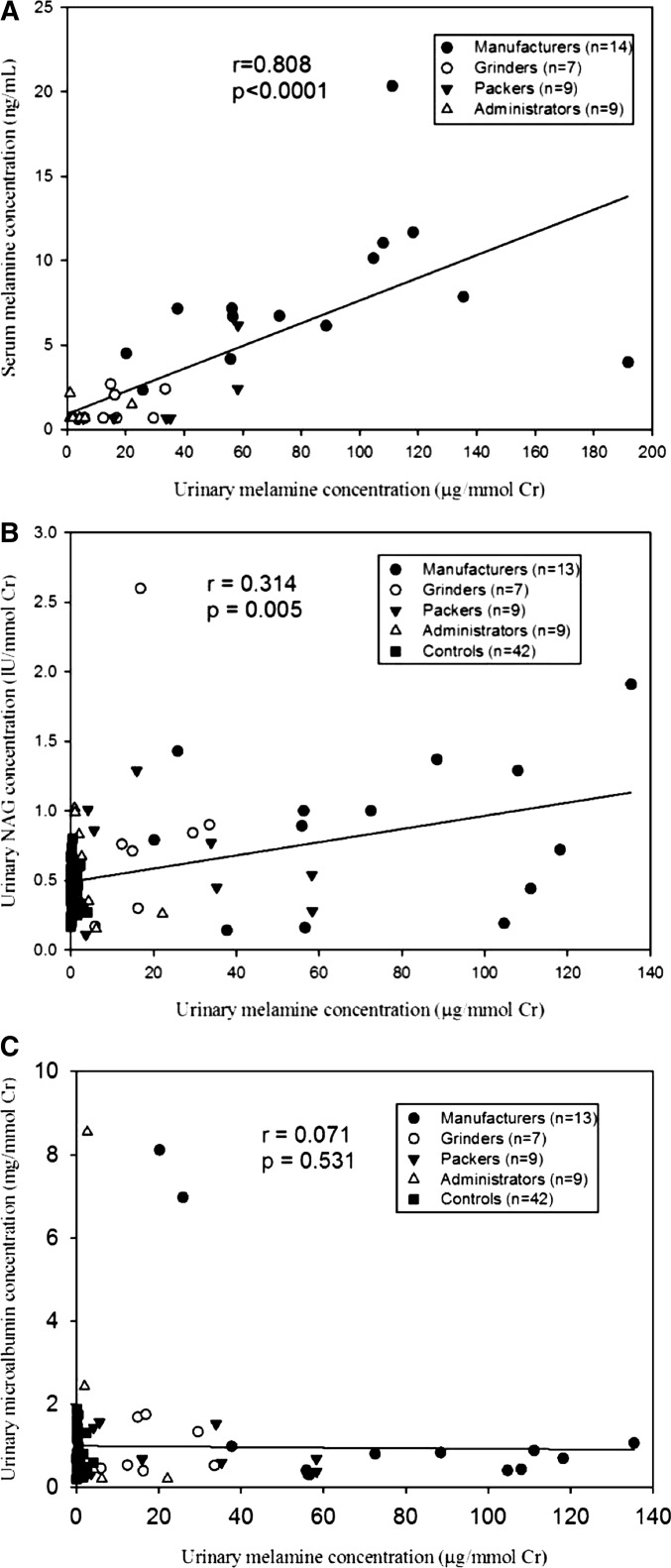
We found that manufacturers had the highest urinary melamine concentrations with or without creatinine correction, followed by grinders and packers (Table 2). The lowest concentration was in the controls. For serum melamine, all were detectable in manufacturers. A high correlation was found between urinary and serum melamine in 39 melamine workers (Spearman correlation coefficients r=0.808, P<0.001) (Figure 2A).
Figure 2.
Spearman correlation between urinary and serum melamine concentrations as well as urinary melamine concentrations and early renal tubular injury markers in urine by worksites. (A) Urinary and serum melamine concentrations (n=39). (B) Urinary melamine concentrations and NAG levels (n=80). (C) Urinary melamine concentrations and microalbumin levels (n=80). Cr, creatinine.
For urinary renal injury indicators, we found that manufacturers had the highest NAG levels and the highest detectable β2-MG rate and the nonexposed workers had the lowest NAG levels (Table 2, Supplemental Figure 6A). However, this significant pattern was not found for urinary microalbumin (Supplemental Figure 6A). The results remained similar even after excluding one outlier (Figure 2, A and C). Other serum renal function markers and laboratory data were not significantly different between manufacturers and nonexposed workers (Table 2, Supplemental Table 7).
After controlling for other covariates, we found that urinary melamine levels were significantly and positively associated with NAG levels and the detectable rate of β2-MG (Table 3). The results remained significant even after further adjusting for hypertension (Supplemental Table 8). Categorized by worksites, both significant levels were also present in manufacturers when compared with nonexposed workers (Table 3). In contrast, a significant level was not found in urinary microalbumin (Supplemental Table 8).
Table 3.
Relationship of urinary renal injury indicators with urinary melamine levels or worksites in multivariate regression models
| N | Mean±SD | Median, IQR | Crude | Adjustedb | Adjustedc | ||||
|---|---|---|---|---|---|---|---|---|---|
| β (±SEM) | P value | β (±SEM) | P value | β (±SEM) | P value | ||||
| Log10 NAGa | |||||||||
| Model 1 | |||||||||
| Urinary melamine (μg/mmol Cr) | 81 | 0.7±1.5 | 0.5, 0.3–0.8 | 0.004 (0.001) | <0.001 | 0.004 (0.001) | 0.001 | 0.004 (0.001) | 0.001 |
| Model 2a | |||||||||
| Nonexposed workers | 105 | 0.4±0.2 | 0.4, 0.3–0.5 | 1 | — | 1 | — | 1 | — |
| Administrators | 9 | 0.6±0.3 | 0.6, 0.4–0.8 | 0.140 (0.094) | 0.138 | 0.097 (0.106) | 0.365 | 0.148 (0.106) | 0.165 |
| Grinders and packers | 16 | 0.7±0.6 | 0.7, 0.3–0.9 | 0.181 (0.072) | 0.014 | 0.065 (0.097) | 0.507 | 0.090 (0.096) | 0.350 |
| Manufacturers | 14 | 1.8±3.5 | 0.9, 0.4–1.4 | 0.344 (0.077) | <0.001b | 0.217 (0.107) | 0.044 | 0.213 (0.105) | 0.044c |
| N | Normal N (%) | Abnormal N (%) | Crude OR (95% CI) | Adjusted OR (95% CI)d | Adjusted OR (95% CI)e | ||||
| β2-microglobulinf | |||||||||
| Model 1 | |||||||||
| Urinary melamine (μg/mmol Cr) | 81 | 75 (92.6) | 6 (7.4) | 1.03 (1.01–1.04) | 1.03 (1.01–1.06) | 1.03 (1.01–1.06) | |||
| Model 2a | |||||||||
| Nonexposed workers | 105 | 101 (96.2) | 4 (3.8) | 1 | 1 | 1 | |||
| Administrators | 9 | 9 (100.0) | 0 | — | — | — | |||
| Grinders and packers | 16 | 15 (93.8) | 1 (6.2) | 1.68 (0.18–16.01) | 0.76 (0.04–13.67) | 0.76 (0.04–15.16) | |||
| Manufacturers | 14 | 10 (71.4) | 4 (28.6) | 10.10 (2.19–46.67) | 30.18 (1.59–573.90)g | 27.94 (1.28–611.62)h | |||
OR, odds ratio; 95% CI, 95% confidence interval.
Missing data, n=1 for office staff, n=2 for grinders and packers, and n=2 for manufacturers.
P value for trend: P<0.001.
P value for trend: P=0.047.
Adjusting for age, sex, BMI, educational level, and cigarette smoking.
Adjusting for age, sex, BMI, educational level, cigarette smoking, and serum uric acid.
Multiple linear regression or logistic regression.
P value for trend: P=0.021.
P=0.047.
Discussion
This study found the temporal changes of urinary melamine concentrations in melamine workers. In addition, air, serum, and urine melamine levels were highly correlated. Melamine workers in manufacturing areas were exposed to the highest melamine levels. The higher the urinary melamine levels, the significantly higher the urinary NAG levels and the greater the detectable rate of urinary β2-MG noted.
Melamine tableware consists of 70%–75% melamine-formaldehyde resin and 25%–30% refined wood pulp, and the rest is tiny amounts of aluminum stearate and magnesium stearate. Thus, melamine-formaldehyde resin, a dry white condensate of melamine and formaldehyde, is the main raw material to produce melamine tableware. During the manufacturing of melamine tableware, the workplace is full of coarse and fine particles containing melamine. Previous studies have only focused on the effect of air formaldehyde exposure on respiratory and dermal health due to the unknown health impact of melamine (Supplemental Table 9).12–17
Ours is the first study to comprehensively examine the exposure assessment of melamine and its health effect in the occupational setting. Two prior studies only measured ambient melamine exposure either from one laboratory chamber or from the field with different exposure sources.18,19 Pukkila et al. first measured ambient melamine concentration in the process of epoxy powder coating curing in the laboratory and the highest melamine concentration was found in 400 μg/m3.18 Recently, Yassine et al. measured ambient melamine emission from a diesel engine employing selective catalytic reduction technology and found the exposure level to be nondetectable to 11.0 μg/L.19
A wide range of urinary melamine concentrations from nondetection up to approximately 2000 μg/mmol was found in infants or children who were exposed to melamine-tainted milk in China, Taiwan, and Hong Kong (Supplemental Table 10).20–23 Our previous study has suggested that the half-life of melamine excretion in human urine is relatively short, approximately 6 h.10 This study further demonstrated that the exposure pattern in melamine workers, particularly manufacturers, showed an accelerating increase in the afternoon and then slightly declined the next morning, and finally sharply decreased during the weekend. Thus, the low-melamine concentrations in urine of affected children in previous studies were probably due to delayed urine collection. In addition, the study found that urinary melamine concentrations in melamine workers can reach approximately 100 μg/mmol. For the nonexposed workers, their urinary melamine concentrations, mostly <2 μg/mmol, were similar to the general Caucasian and Asian populations.7,8 However, urinary melamine concentrations in melamine workers in administrative areas were slightly higher than those in the general population, probably due to the adjacency of the workplace of the manufacturing process and consequent melamine particular “drift.”
Markers representing early renal injury such as microalbumin, NAG, and β2-MG have been well recognized in many review articles.24,25 Microalbuminuria is a well known early marker of renal glomerular damage in the general population, whereas NAG and β2-MG have been recognized to be useful clinical markers for early renal tubular damage.8 This study found that melamine exposure was associated with NAG and β2-MG but not microalbumin, suggesting that the initial damage by melamine is mainly in renal tubular cells and is consistent with our previous in vitro study that showed that low and chronic melamine insult in human renal proximal tubular cells can increase cellular reactive oxygen species, decrease antiapoptotic/proapoptotic protein ratio, and eventually result in cell apoptosis prior to renal morphologic change.26 Thus, the proposed mechanism in this study is quite different from that in the 2008 toxic milk scandal from China in which affected children suffered from acute renal injury directly caused by the formation of renal stones from the cocrystallization of melamine and other body ions, such as phosphate or uric acid, during urine filtration due to the large consumption of melamine-tainted baby milk formula.
Melamine chemical is present in our environment, even after the wind-down of the melamine incident in China.7,8 One of the main sources is from the universal use of melamine tableware and kitchenware,9,10 which are produced by melamine workers. Although the melamine workers in this study did not have abnormal clinical data of renal function, including serum BUN, creatinine, uric acid, eGFR and estimated creatinine clearance rate, and prominent abnormal renal morphology or increased renal stones by renal echo, preclinical renal injury markers have changed, as has been demonstrated in this study. Two possible explanations are that (1) early renal injury caused by melamine in the study may precede the impairment of clinical renal data and (2) the health workers’ effect whereby workers exposed to high doses of melamine who developed clinical symptoms and signs might have left the workplace and not be recruited in this cross-sectional study; this might cause an underestimation. In contrast, this study also found that the control group had a 10% incidence of kidney stones, possibly due to different etiologies in different occupational settings. Although the general population is probably not exposed to melamine levels as high as those in melamine workers, the health impact for low-dose long-term melamine exposure in vulnerable groups, such as children and pregnant women, still needs attention.17
The design is mainly cross-sectional and thus a reverse causality of melamine exposure and early renal injury is likely. However, if this is true, we probably also observed increased excretion of microalbumin accompanied by high urinary melamine excretion.27 However, this study only showed that melamine exposure is significantly and positively associated with NAG and β2-MG, but not microalbumin in urine, suggesting that the probability of reverse causality is minimal. Because ambient formaldehyde concentrations and ambient melamine concentrations were highly correlated by worksites (Figure 1), we cannot adjust for the impact of formaldehyde on biomarkers of renal tubular injury to avoid collinearity, which has the potential to introduce confounding bias. Another limitation is that measures of other environmental and occupational nephrotoxins, such as lead and cadmium etc., were not available in this study. In addition, we did not measure cyanuric acid, because these two factories did not use any cyanuric acid or urea to substitute melamine based on workplace inspection. However, one previous study has shown that gut microbiota in rats has the ability to transform melamine into cyanuric acid.28 Thus, the effect of cyanuric acid on renal function in this population cannot be ignored. Again, no information about microscopic hematuria was available in this population; thus, we cannot explore the interactive effect of hematuria and melamine on biomarkers of early renal tubular injury. Finally, the sample size was relatively small and the generalizability of this occupational study to the public is necessarily cautionary.
We conclude that exposure to air melamine may increase the levels of urinary biomarkers of early renal tubular injury in this occupational setting. However, the significance of these biomarkers with regard to the progressive loss of kidney function or long-term renal damage remains unclear. A long-term follow-up study in this population can clarify the relationship between continuous melamine exposure and chronic kidney diseases.
Concise Methods
Study Population and Study Design
This study followed the guidelines of STROBE (Supplemental Table 1).29 The cross-sectional study was carried out at two local melamine tableware manufacturing factories (Factories A and B) in southwestern Taiwan (Supplemental Figure 1). The study population consisted of melamine workers who had a current occupational exposure to different concentrations of melamine from raw materials of melamine-formaldehyde powder in the air and who had a work history of ≥1 years (exposed group). Factories A and B were estimated to use approximately 1000 kg and approximately 500 kg of raw materials daily, respectively. According to the workplace inspection, melamine workers mainly work in four different sites: the manufacturing and molding area (manufacturing area), the grinding and polishing area (grinding area), the packing area, and the administrative area. All exposure monitoring, personal information, and health check-ups, performed by the Department of Occupational Medicine, Kaohsiung Municipal Hsiao-Kang Hospital (KMHKH), were completed between August and December of 2012.
In order to compare this exposed group, we recruited eligible workers who worked as office staff from another large company, had no current or past exposure to melamine or other chemicals known to cause renal injury, came to the KMHKH for health check-ups in the same month as the exposed workers, and agreed to participate in this study. The final comparison group (nonexposed workers) was from one shipbuilding company (Factory C). All study workers in these three factories worked weekdays from Monday to Friday, 8:00 am to 5:00 pm, and were off on the weekend (Saturday and Sunday). Because the business was temporarily down in Factory B, that company took one more day off (Friday) to reduce the making of products during the collection of their biospecimens. This study was approved by the Institutional Review Board of Kaohsiung Medical University Hospital and all study subjects gave written informed consent.
Collection of Air, Urine, and Blood Samples
Continuous Air Samplings for 5 Days
Among the melamine workers, personal breathing-zone and area air samples were used to measure particular-phase melamine and gaseous-phase formaldehyde by using AirLite SKC air sampling pumps (SKC Ltd., Dorset, UK) with glass fiber filters (0.7 μm pore size) for melamine and 2,4-DNPH cartridges (Supelco, PA) for formaldehyde at a flow rate of 2.0 l/min for approximately 8 h per day during working hours. The collection points of both personal and area samples (2–3 samples) was randomly selected in four main worksites of Factories A and B and both measurements were from Monday to Friday in 1 week.
In order to measure the ambient distribution of dust particle sizes (PM10, PM2.5, and PM1) in a real-time status (one measurement every minute) for 8 h, we simultaneously placed one portable laser aerosol spectrometer and one dust monitor (Model 1.108/1.109; GRIMM Aerosol Technik GmbH & Co. KG, Ainring, Germany) in the manufacturing area and administrative area in Factory A from Monday to Friday during the study period. This dust monitor is a compact portable device, which is built for continuous measurement of airborne particles as well as for measuring the particle count distribution.
A Series Collection of Urine Samples
We first collected consecutively one-spot urine samples from the melamine workers both pre- and post-shift starting from the first working day after 2 days off (Monday) to the end-of-shift on the fifth working day (Friday), and additional three first-spot urine samples on the mornings of the weekend (Saturday and Sunday) and the following Monday, of which the total samples were 13 (Supplemental Figure 1). All urine samples were stored at −20°C until analysis.
One-Time Blood and Urine Samples
One-time blood and urine samples were collected on the morning of the fifth working day after physical examination by occupational physicians. Each blood sample was divided into three separate samples: one for blood routine, one for serum biochemistry such as liver function, cardiometabolic function, and renal function (BUN, creatinine, and uric acid), and the other for measuring serum melamine. The one-spot urine sample was taken for routine urinary analysis and measured for early biomarkers of renal injury, including microalbumin, NAG, and β2-MG in both exposed and nonexposed workers. Serum was stored at −80°C until analysis.
Analytical Methods of Exposure and Outcome Indices
Melamine in air, serum, and urine was measured by a liquid chromatography-electrospray ionization–tandem mass spectrometry assay method, whereas formaldehyde in air was measured by the method of high-performance liquid chromatography with ultraviolet detection (see Supplemental Materials). All markers of early renal injury and other biochemistry were analyzed at the KMHKH.
Statistical Analysis
In the time-series study, we first plotted the temporal change of ambient melamine and formaldehyde and the daily pre- and post-shift urinary melamine concentrations from the four worksites. Then, Spearman rank correlation coefficients were used to study the correlation of the across-shift change of urinary melamine concentrations defined as either post-shift melamine on the fifth day (Factory A) or fourth day (Factory B) minus pre-shift melamine on the first day with air average melamine (5-day and 4-day average for Factories A and B, respectively).
The generalized estimating equation was used to investigate the relationship of the daily difference of urinary melamine concentration (post-shift minus pre-shift in each day from the first to the fifth day) to the daily ambient melamine concentrations (first to fifth day) or by worksites (manufacturers versus nonmanufacturers), after adjusting for appropriate covariates. The confounding effect of each working day and weekend was also considered. Because melamine and formaldehyde in air were highly correlated, we ran the two parameters separately.
In the cross-sectional study, we tabulated and compared demographic and clinical characteristics across four worksites and nonexposed workers, as well as between manufacturers and nonexposed workers, by using parametric and nonparametric statistics whenever appropriate. The Spearman correlation was used to examine the relationship of urinary melamine with serum melamine and urinary microalbumin and NAG. If necessary, urinary markers were corrected by urinary creatinine before statistical analysis.
Multiple linear regression or logistic regression was used to examine the relationship of each renal injury indicator with urinary melamine levels as continuous variable or categorical variable after adjusting for other covariates. If any continuous variable of renal injury marker was not normally distributed, log10-transformation was performed. For covariates in the model, we first included the variable of serum uric acid, because it was the most important risk factor of urolithiasis in previous studies, including ours.30,31 Then, other covariates were added to models in a forward stepwise selection and were finally included if they altered the association between melamine exposure and risk of renal injury indicators by greater than 10%. The additional covariates included age, sex, education, body mass index, smoking status, and serum uric acid. The data were analyzed using the Statistical Analysis System statistical package. All P values were two-sided and statistical significance was defined as P<0.05.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Mr. Steve Tredrea for assisting in the editing of the manuscript.
This work was supported by Kaohsiung Medical University (grant numbers KMU-DT103004 and KMU-TP103A23), the Taiwan Ministry of Science and Technology (grant numbers NSC 101-2314-B-037-037-MY3), and Taiwan’s National Health Research Institutes (grant number NHRI-EX104-10209PI), none of which had any role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014121233/-/DCSupplemental.
References
- 1.Eckardt KU, Coresh J, Devuyst O, Johnson RJ, Köttgen A, Levey AS, Levin A: Evolving importance of kidney disease: From subspecialty to global health burden. Lancet 382: 158–169, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, Saran R, Wang AY, Yang CW: Chronic kidney disease: Global dimension and perspectives. Lancet 382: 260–272, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Price RG: The role of NAG (N-acetyl-beta-D-glucosaminidase) in the diagnosis of kidney disease including the monitoring of nephrotoxicity. Clin Nephrol 38[Suppl 1]: S14–S19, 1992 [PubMed] [Google Scholar]
- 4.Dieterle F, Perentes E, Cordier A, Roth DR, Verdes P, Grenet O, Pantano S, Moulin P, Wahl D, Mahl A, End P, Staedtler F, Legay F, Carl K, Laurie D, Chibout SD, Vonderscher J, Maurer G: Urinary clusterin, cystatin C, beta2-microglobulin and total protein as markers to detect drug-induced kidney injury. Nat Biotechnol 28: 463–469, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Wu Y, Zhang Y: Analytical chemistry, toxicology, epidemiology and health impact assessment of melamine in infant formula: recent progress and developments. Food Chem Toxicol 56: 325–335, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Bhalla V, Grimm PC, Chertow GM, Pao AC: Melamine nephrotoxicity: An emerging epidemic in an era of globalization. Kidney Int 75: 774–779, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Panuwet P, Nguyen JV, Wade EL, D’Souza PE, Ryan PB, Barr DB: Quantification of melamine in human urine using cation-exchange based high performance liquid chromatography tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 887-888: 48–54, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Lin YT, Tsai MT, Chen YL, Cheng CM, Hung CC, Wu CF, Liu CC, Hsieh TJ, Shiea J, Chen BH, Wu MT: Can melamine levels in 1-spot overnight urine specimens predict the total previous 24-hour melamine excretion level in school children? Clin Chim Acta 420: 128–133, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Chien CY, Wu CF, Liu CC, Chen BH, Huang SP, Chou YH, Chang AW, Lee HH, Pan CH, Wu WJ, Shen JT, Chang MY, Huang CH, Shiea J, Hsieh TJ, Wu MT: High melamine migration in daily-use melamine-made tableware. J Hazard Mater 188: 350–356, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Wu CF, Hsieh TJ, Chen BH, Liu CC, Wu MT: A crossover study of noodle soup consumption in melamine bowls and total melamine excretion in urine. JAMA Intern Med 173: 317–319, 2013 [DOI] [PubMed] [Google Scholar]
- 11.U.S. Food and Drug Administration: (FDA). Available at: http://www.fda.gov/Food/ResourcesForYou/Consumers/ucm199525.htm [Accessed June 20, 2014]
- 12.Srivastava AK, Gupta BN, Gaur JS, Bihari V: Clinical evaluation of workers handling melamine formaldehyde resin. J Toxicol Clin Toxicol 30: 677–681, 1992 [DOI] [PubMed] [Google Scholar]
- 13.Isaksson M, Zimerson E, Bruze M: Occupational dermatoses in composite production. J Occup Environ Med 41: 261–266, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Neghab M, Soltanzadeh A, Choobineh A: Respiratory morbidity induced by occupational inhalation exposure to formaldehyde. Ind Health 49: 89–94, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Marsh GM, Stone RA, Henderson VL: Lung cancer mortality among industrial workers exposed to formaldehyde: A Poisson regression analysis of the National Cancer Institute Study. Am Ind Hyg Assoc J 53: 681–691, 1992 [DOI] [PubMed] [Google Scholar]
- 16.Aalto-Korte K, Jolanki R, Estlander T: Formaldehyde-negative allergic contact dermatitis from melamine-formaldehyde resin. Contact Dermat 49: 194–196, 2003 [DOI] [PubMed] [Google Scholar]
- 17.García Gavin J, Loureiro Martinez M, Fernandez-Redondo V, Seoane MJ, Toribio J: Contact allergic dermatitis from melamine formaldehyde resins in a patient with a negative patch-test reaction to formaldehyde. Dermatitis 19: E5–E6, 2008 [PubMed] [Google Scholar]
- 18.Pukkila J, Peltonen K, Savolainen T: Determination of melamine in air by high-performance liquid chromatography with ultraviolet detection. J Chromatogr A 411: 409–414, 1987 [DOI] [PubMed] [Google Scholar]
- 19.Yassine MM, Dabek-Zlotorzynska E, Celo V: Development of a hydrophilic interaction liquid chromatography-mass spectrometry method for detection and quantification of urea thermal decomposition by-products in emission from diesel engine employing selective catalytic reduction technology. J Chromatogr A 1229: 208–215, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Lam CW, Lan L, Che X, Tam S, Wong SS, Chen Y, Jin J, Tao SH, Tang XM, Yuen KY, Tam PK: Diagnosis and spectrum of melamine-related renal disease: plausible mechanism of stone formation in humans. Clin Chim Acta 402: 150–155, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Cheng WC, Chen SK, Lin TJ, Wang IJ, Kao YM, Shih DY: Determination of urine melamine by validated isotopic ultra-performance liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 23: 1776–1782, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Zhang M, Li S, Yu C, Liu G, Jia J, Lu C, He J, Ma Y, Zhu J, Yu C: Determination of melamine and cyanuric acid in human urine by a liquid chromatography tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 878: 758–762, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Kong AP, Choi KC, Ho CS, Chan MH, Wong CK, Liu EK, Chu WC, Chow VC, Lau JT, Chan JC: Hong Kong Chinese school children with elevated urine melamine levels: a prospective follow up study. BMC Public Health 11: 354, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fassett RG, Venuthurupalli SK, Gobe GC, Coombes JS, Cooper MA, Hoy WE: Biomarkers in chronic kidney disease: A review. Kidney Int 80: 806–821, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Siew ED, Ware LB, Ikizler TA: Biological markers of acute kidney injury. J Am Soc Nephrol 22: 810–820, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Hsieh TJ, Hsieh PC, Tsai YH, Wu CF, Liu CC, Lin MY, Wu MT: Melamine induces human renal proximal tubular cell injury via transforming growth factor-β and oxidative stress. Toxicol Sci 130: 17–32, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Trasande L, Sathyanarayana S, Trachtman H: Dietary phthalates and low-grade albuminuria in US children and adolescents. Clin J Am Soc Nephrol 9: 100–109, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng X, Zhao A, Xie G, Chi Y, Zhao L, Li H, Wang C, Bao Y, Jia W, Luther M, Su M, Nicholson JK, Jia W: Melamine-induced renal toxicity is mediated by the gut microbiota. Sci Transl Med 5: 172ra22, 2013 [DOI] [PubMed] [Google Scholar]
- 29.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative : The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 370: 1453–1457, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Taylor EN, Stampfer MJ, Curhan GC: Obesity, weight gain, and the risk of kidney stones. JAMA 293: 455–462, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Liu CC, Wu CF, Chen BH, Huang SP, Goggins W, Lee HH, Chou YH, Wu WJ, Huang CH, Shiea J, Lee CH, Wu KY, Wu MT: Low exposure to melamine increases the risk of urolithiasis in adults. Kidney Int 80: 746–752, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.