Abstract
The practice of intravenous iron supplementation has grown as nephrologists have gradually moved away from the liberal use of erythropoiesis-stimulating agents as the main treatment for the anemia of CKD. This approach, together with the introduction of large-dose iron preparations, raises the future specter of inadvertent iatrogenic iron toxicity. Concerns have been raised in original studies and reviews about cardiac complications and severe infections that result from long-term intravenous iron supplementation. Regarding the iron preparations specifically, even though all the currently available preparations appear to be relatively safe in the short term, little is known regarding their long-term safety. In this review we summarize current knowledge of iron metabolism with an emphasis on the sources and potentially harmful effects of labile iron, highlight the approaches to identifying labile iron in pharmaceutical preparations and body fluids and its potential toxic role as a pathogenic factor in the complications of CKD, and propose methods for its early detection in at-risk patients.
Keywords: chronic renal disease, anemia, mortality risk, labile iron, inflammation
The anemia of CKD is routinely treated with erythropoiesis-stimulating agents (ESAs) and iron supplements, mostly via the intravenous route. In the last decade, several randomized controlled trials consistently indicated adverse cardiovascular outcomes with hemoglobin levels targeted above 13 g/dl.1–4 Therefore, current KDIGO guidelines for CKD recommend hemoglobin levels of 10 (or possibly less) to 11.5 g/dl.5 As a result of the renewed doubt regarding the long-term safety of ESAs, as well as the recently instituted ‘bundling’ of dialysis services to include both ESAs and intravenous iron (IVI), nephrologists have gradually moved away from the liberal use of ESA in CKD anemia toward more IVI supplementation. This policy, together with the introduction of large-dose iron preparations, has raised the future specter of potential inadvertent iatrogenic iron toxicity. Although in the short term, all currently available iron preparations appear to be safe,6 little is known regarding the long-term safety of repeated IVI.7 The purpose of this review is to summarize current knowledge of iron biology, with an emphasis on the metal’s potentially harmful effects, highlight the role of iron toxicity as a pathogenic factor in the complications of CKD and propose methods for its early detection in at-risk patients. The role of iron in kidney injury will not be addressed and the reader is referred to an excellent comprehensive review of this subject.8
Iron Biology
The classic perception of iron in the biomedical field has been of a Janus-faced or double-edged sword element essential for life but life-threatening if not properly controlled.9 Thus, inherent to cell10,11 and system12 is the maintenance of a pool of iron available for biosynthetic purposes. In body fluids such a pool is associated with plasma transferrin (Tf) molecules that safely carry the metal and supply it to cells ‘on demand’, i.e., as per the level of expression of cell Tf receptors. In cells, most of the iron is stably associated with proteins or cofactors, but their manufacture or degradation also involve labile forms of the metal that are both redox-active and ligand-exchangeable. This poses a constant demand on cells to prevent labile Fe2+/Fe3+ from promiscuous generation of toxically reactive O species (ROS) from reactive O intermediates (ROI, such as superoxide and hydrogen peroxide), that normally represent up to 1% of O2 consumed by the respiratory chain (Figure 1).13,14 To cope with such natural imperfections, mammalian cells rely on two complementary defense strategies: (1) control of labile cell iron (LCI) levels by coordinately balancing iron uptake versus utilization and/or storage, and (2) control of ROS formation by eliminating ROIs with superoxide dismutases and various peroxidases, as well as by antioxidant molecules. However, whenever those protective measures fail or become insufficient, as in siderosis or inflammatory crisis, cell oxidative damage ensues, often leading to necrotic cell death or to more complex death paths such as ferroptosis or oxytosis, if rescuable by iron chelators or antioxidants, respectively.14,15 Pathologic scenarios develop in the two main types of siderosis: systemic siderosis (inherited or iatrogenic), whereby cells are overwhelmed by excessive ingress of non-physiologic forms of plasma iron and in regional siderosis (inherited or acquired) where damage results from maldistribution of the metal within cell compartments.16,17
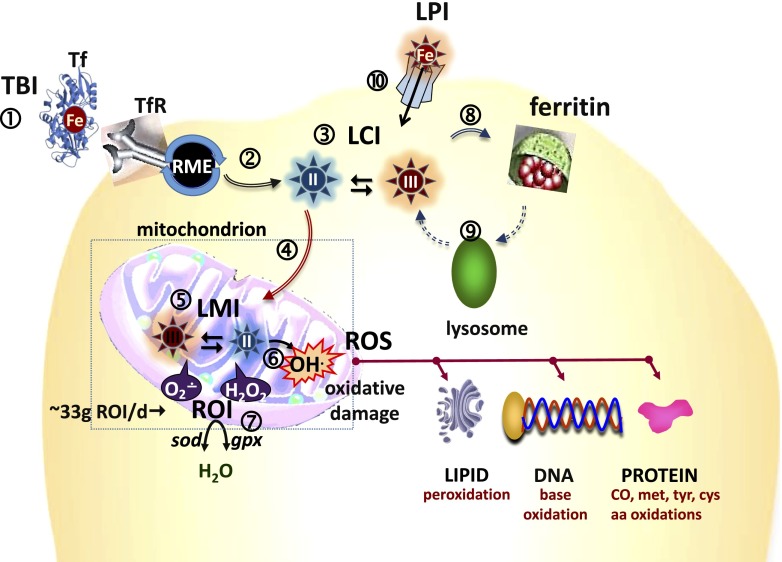
Figure 1.
Where and how does labile iron cause cell damage? Schematic representation of iron distribution among cell compartments (see number 1 in circle). Transferrin (Tf) bound iron (TBI) is the physiologic source of circulating Fe that is captured by cells via RME (see number 2 in circle), whereby the TBI is delivered into cells and the metal is released into the cytosol as LCI (see number 3 in circle) that is comprised of Fe (II and III). LCI can permeate into mitochondria (see number 4 in circle) where it serves as a metal source for heme and iron-sulfur cluster proteins. Labile iron in mitochondria (LMI) (see number 5 in circle) or in cytosol (not shown) can catalyze formation of ROS (e.g., OH•) from ROI (see number 6 in circle) (e.g., O2•− and H2O2, approximately 33 g/day from respiration) that are normally eliminated by cell enzymes such as superoxide dismutase (sod) and glutathione peroxidases (gpx) or catalase (see number 7 in circle). ROS can chemically damage membrane components such as lipids by peroxidation, proteins by oxidation of amino acids (AA) such as tyrosine (tyr), methionine (met), lysine (lys) and cysteine (cys), or via CO formation and nucleic acids by base modification and DNA breaks. Excess LCI is generally absorbed into ferritin iron shells (see number 8 in circle) that then can be dissolved by proteolytic cleavage of the protein in lysosomes (see number 9 in circle) whereby iron can be recycled into the LCI pool. In systemic iron overload, LPI forms (which are non-TBI, NTBI) (see number 10 in circle) can infiltrate cells, raise LCI and LMI and cause cell iron overload.
Tissue Iron Accumulation and Siderotic Damage
The identification of tissue iron overload (siderosis) in experimental and clinical settings has largely relied on the detection of iron agglomerates by histochemical stains18 or magnetic resonance imaging (MRI).19 Although often perceived as deleterious, the mere presence of iron agglomerates in cells is not to be tacitly taken as indicating siderotic damage, insofar as the metal is chemically shielded within ferritin shells.11 The etiopathology of siderotic damage is most likely associated with ROS formation catalyzed by LCI.14 This relies largely on the fact that permeant iron chelators can confer direct protection from ROS-mediated oxidations by demonstrably complexing LCI and thereby rendering it non-labile.2 LCI can also be reduced by overexpression of ferritin molecules.20 These two features, redox activity and susceptibility to specific iron chelators, define LCI19 (also called labile iron pool, LIP) which is a physiologic component that is maintained homeostatically by most cell types.21–23 The emerging issue is in which conditions does LCI attain levels that implicate it in siderotic damage or, conversely, in iron deprivation, which can also be deleterious.21 A related issue is how do different cell types cope when exposed to non-physiologic iron forms that appear in plasma of patients with systemic iron overload or with iatrogenic iron forms that are delivered parenterally as iron supplements?
Labile Cell Iron
LCI was hypothesized as a transitory pool of labile iron that is at the crossroads of cell metabolism.21 Experimentally, it was demonstrated in living cells with the aid of fluorescent probes that either sense labile iron per se or monitor its propensity to generate ROS when prompted by pro-oxidants but curbed by membrane permeant chelators. LCI is maintained in metabolically active cells at submicromolar to low micromolar level, representing 1%–2% of the total cell iron content.21 Those homeostatic levels reflect a balance between metal uptake and utilization versus the iron-absorptive capacity of cell ferritin, but also the cell metal ligand composition and the redox activity of the cell compartment in question. As mentioned above, the link between labile iron and biologic damage has largely leaned on the demonstrable ability of permeant iron chelators to protect or ameliorate cell functions affected by siderosis and, thereby, rescue cells from entrance into a death path.24–26 That has provided a rationale for treating siderotic disorders of systemic or regional character with chelators, while taking into consideration that the benefits expected from regional iron detoxification by chelation need to be balanced against the possible systemic depletion of an essential metal.
Labile Plasma Iron
Unlike LCI, plasma normally has spare Tf iron binding capacity for effectively binding incoming labile iron (from absorption by the gut or recycling by the reticuloendothelial system (RES)) and thereby rendering it non-labile, i.e., non-redox-active and non-transferable to potential iron acceptors (e.g., citrate, nucleotides) and even to some chelators in pharmacologic use. Physiologically, after being taken up by cells via Tf receptor-mediated endocytosis, Tf will release the protein-occluded metal once exposed to the acidic milieu of the cell endosome. Under normal conditions, plasma Tf saturation (TSAT) is maintained in the 20%–40% range, rarely exceeding 60%, except in systemic iron overload,18 which results from hyperabsorption of iron (as in hereditary hemochromatosis), increased erythrophagocytosis (as in transfusional siderosis), or hypotransferrinemia. In those conditions, an outpouring of iron that is not matched by sufficient local plasma unsaturated iron binding capacity, will lead to the formation of non-transferrin bound iron (NTBI), a group of iron forms initially found in plasma (serum) of patients with TSAT near or above 100% (i.e., approximately 0 unsaturated iron binding capacity).27–29 NTBI detection by colorimetric assays or by HPLC was made possible by extraction of sera with chelating/mobilizing agents followed by protein exclusion-filtration.30 The fact that NTBI was detected in high TSAT sera gave the parameter credibility as a genuine clinical indicator of systemic iron overload, although some overestimations caused by mobilization of iron from Tf-Fe by harsh extraction conditions have been noted.31 Conceptually, the term NTBI, which ‘defines something just by what it is not’ (known classically as an apophasis) is rather misleading, particularly when used to define potentially toxic iron species in plasma and/or designate it as the source of tissue iron overload. Firstly, not all iron species in plasma that qualify stricto senso as NTBI need to be toxic or even reflective of iron overload (e.g., plasma ferritin in inflammation, iron chelates in chelated individuals or polymeric iron particles (PIPs) in intravenous supplemented individuals). Secondly, studies attempting to demonstrate tissue iron overload associated with NTBI uptake have rarely been performed in pathophysiologically-relevant media or with genuine NTBI (from iron-overloaded patients). Thirdly, as plasma NTBI composition varies with the degree and/or the source of iron overload, its potential toxicity cannot be reliably estimated just from concentration. For example, in chronic diabetes, persistent plasma NTBI is detectable (even at TSAT as low as approximately 50%), but in the absence of overt tissue iron overload.32 In chelated patients, some NTBI assays do not necessarily distinguish between genuine NTBI and iron chelates, leading to erroneous assessment of iron overload and of chelation efficacy. Last, but not least, the association of NTBI forms with plasma albumin might not only blunt the detection of NTBI but also affect it as a source of tissue siderosis.33 A typical misusage of the term NTBI is found with PIPs that generate in plasma mM concentrations of NTBI. However, unlike the NTBI detected in systemic siderosis, iatrogenic NTBI derived from most PIP formulations is ‘mostly’ non-toxic, ‘safely’ acquired and processed by the RES macrophages.34,35
Thus, while the introduction of the term NTBI was a landmark in understanding the pathophysiology of systemic iron overload,27–29 its indiscriminate use as an indicator of toxic forms of iron in plasma turned out to be a source of confusion. A more reliable indicator of pathologic forms of iron in biologic fluids is the catalytically-active component of NTBI, which can be revealed by physiologic reductants such as ascorbic acid. In early studies, bleomycin was used in conjunction with high concentrations of ascorbate to trigger the oxidation of sugars (free or as part of polymers such as DNA) and, thereby, rendering them detectable with thiobarbituric acid.36 However, the detection of seemingly catalytic metal in iron chelates and in plasma of patients with TSAT <50%–60% apparently indicates a tendency for the bleomycin assay to overestimate levels of genuine redox-active iron in biologic samples. A parameter that defines redox-active components specifically associated with iron but also chelatable is labile plasma iron (LPI).21,28 This parameter is assayable in biologic fluids and provides a clinically-relevant indicator of iron chelation efficacy or adequacy.21,37,38 This is of particular importance for evaluating treatments that can confer round-the-clock prevention of LPI appearance as, by daily containment of LPI, it is feasible to reduce and possibly eliminate impending tissue iron overload. Moreover, LPI can also be used as a diagnostic indicator for initiating chelation therapy in naïve polytransfused thalassemia children.38
Iron in the Pathogenesis of CKD Complications
On a backdrop of CKD, a condition of underlying oxidative stress, there is clear scope for excess iron-associated adverse outcomes via ROS-induced damage which could contribute to endothelial dysfunction, inflammation, and immune dysfunction.18,39 Also, iron is required for bacterial growth and part of the human antibacterial armamentarium relies on iron mobilization from plasma into sanctuaries associated with the RES. However, iron-laden RES cells become vulnerable to intracellular pathogens.40 Antibacterial host defenses also depend on iron-catalyzed formation of ROS, which is critical for normal phagocytic function. Conversely, excess iron has been linked to impaired neutrophil and T-cell function, thereby promoting bacterial growth both in vivo and in vitro, at least in short term (2–3 day) studies.41 In addition there is evidence from the pre-ESA period for impaired neutrophil function in dialysis patients with iron overload and, subsequently, for abnormal T-cell function in mice iron-overloaded by intraperitoneal injection of iron dextran. In the latter experiments, the mice failed to mount a Th1-mediated protective response to C. albicans infection, but were rescued with the iron chelator deferioxamine.42
Against this theoretical background, observational studies linking amounts of administered iron to adverse outcomes in CKD have steadily increased in recent years, but with inconsistent results. Because the link between administered iron and outcomes has been comprehensively reviewed in a recent JASN article,43 we briefly summarize earlier data, address in more detail studies that have appeared since that publication and offer our own views on the subject.
As reported, observational studies have shown a weak association between amounts of iron administered and increased mortality or cardiovascular events,44–52 incidence of bacterial infection53,54 and a somewhat stronger association between ferritin levels and carotid-intimal thickness.55
Recently two Japanese studies and an international one have provided support for an association between iron dosage and all-cause mortality in ESRD.47,48 In the Japanese study a highly significant association was observed between rising serum ferritin (>100 versus <100 ng/ml) and mortality.48 These intriguing results are difficult to interpret in light of ferritin levels that would be considered frank iron deficiency in the Western world. Nevertheless, mortality in Japanese ESRD and other patient groups is persistently lower than in Western countries. Although tempting to invoke a contribution of minimalist IVI protocols to this phenomenon, Japanese and Western populations differ in many other respects, besides IVI protocols, including prolonged genetic isolation, different dietary intake, and body habitus. Therefore, we reserve judgement on this issue.
In the recently published Dialysis Outcomes and Practice Patterns Study, involving 32,435 patients from 32 countries, IVI doses >300 mg monthly, as compared with lower doses, were associated with higher all-cause, cardiovascular, and infectious mortality, as well as hospitalization. Moreover, this association was seen not only when hemoglobin was >12 g/dl, as observed previously,1,3,4 but even within the guideline-recommended range of 10–12 g/dl, although not with values <10 g/dl.49 These studies were performed on prevalent dialysis populations and, despite multiple adjustments for confounding, using sophisticated contemporary statistical techniques, could still suffer from undetected residual confounding and be subject to survivor bias. The only study to date on incident hemodialysis patients, also just published, showed that administration of ≤1050 mg IVI in 3 months or 2100 mg in 6 months was not associated with all-cause, cardiovascular, or infection-related mortality. However, non-statistically significant findings suggested the possibility of infection-related mortality with receipt of >1050 mg in 3 months or >2100 mg in 6 months.50
Only two randomized controlled trials (RCTs) have addressed the association between iron dosage and infection and then only as a secondary end point. In the Dialysis Patients’ Response to IVI with Elevated Ferritin (DRIVE) study,56 patients who were randomized to iron gluconate had a similar incidence of bacterial infections as the placebo group, both at the end of the 6-week study period and after a further 6-week observation period (DRIVE II).57 However, this trial was too short and the sample size insufficient for assessing the likelihood of infrequent events or medium-term safety. Similar results were seen in the second RCT, which used iron dextran.58
Iron dosing method and polymer composition may also contribute to infection risk. Bolus dosing has been associated with a higher infection risk than maintenance dosing53 and iron sucrose with a greater risk than dextran or gluconate.54 In this regard, newer preparations, such as ferric carboxymaltose and the recently FDA-approved ferric citrate (oral preparation),59 have yet to be assessed. Oral ferric citrate is of particular interest because it is marketed as a phosphate binder, but some iron is also absorbed. As shown in a recently published study, patients receiving ferric citrate not only had good phosphate control, but also their requirements for IVI were reduced compared with placebo controls. In addition, the favorable safety data obtained from the 56-week follow-up period are reassuring.60 The potential benefit of orally delivered iron is that absorption may be regulated more physiologically, if hepcidin-induced resistance to iron absorption is sufficiently countered. Conversely, we cannot exclude unregulated absorption and longer-term safety issues, including generation of ROS and its adverse consequences.
The discrepant results elucidated above may be explained by different follow-up times, variable underlying comorbidities, inflammatory status (as reflected, at least in part, by varying serum ferritin levels) and oxidative stress. Other biomarkers, such as hepcidin, also suffer from this limitation of reflecting inflammation as well as iron overload.61 Taken together, the epidemiologic evidence for iron-associated adverse effects in CKD is, at best, unconvincing, most probably due to the many confounding factors involved. Nevertheless, some experts believe that indiscriminate use of IVI to achieve guideline-recommended hemoglobin targets in ESA hyporesponsive patients is likely to be detrimental.62,63
LPI, Tissue Iron Deposition, Oxidative Stress, and Damage in CKD
The generation of LPI in ESRD patients supplemented with parenteral PIPs has been proposed in various studies that dealt with the appearance of oxidized plasma components55,64–74 and demonstrated by direct measurements of LPI.35,75
Both ours35 and other groups75,76 have reported LPI detection in approximately 10% of hemodialysis patients receiving IVI, but, by 48 h after administration, LPI was apparently no longer detectable. Although several abnormalities detected during or shortly after iron administration have been reported, they are apparently also of transient nature. However, the critical and still outstanding question is whether relatively short but repetitive exposures to LPI will over time pose the risk of oxidative tissue damage caused either by cumulative LPI infiltration into cells or via plasma oxidation products.68,77,78
Several studies have reported high tissue iron levels in CKD detected either histologically or by MRI.79,80 In the pre-ESA era, ferritin levels of >4000 ng/ml were reported to be associated with end-organ damage.81 In the ESA era, a dramatic example was demonstrated by T2* MRI of iron deposits in the livers of hemodialysis patients with ferritin levels >2000 ng/ml.82 Although this observation directly reflects iron deposition as hemosiderin, it gives no information regarding iron-mediated tissue damage. IVI has also been implicated in the propagation of renal tissue damage in animal models of CKD.83 Moreover, in a very recent JASN paper, the first evidence for direct IVI involvement in atherogenesis in CKD was reported. Using an in vitro model of leukocyte–endothelium interactions and a mouse remnant kidney model, iron sucrose was shown to accelerate early atherogenesis by upregulating the expression of intracellular cell adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) through enhanced NADPH-oxidase and NFκB signaling. Furthermore, this iron preparation exacerbated atherosclerosis in an apolipoprotein-E deficient mouse. In patients with CKD, IVI sucrose increased circulating mononuclear superoxide production, expression of soluble ICAM-1, VCAM-1 and mononuclear-endothelial adhesion compared with healthy subjects or untreated CKD patients.64 These data complement earlier reports of an association between ferritin levels and carotid-intimal thickness,55 a surrogate marker of atherosclerosis, and iron dextran-induced oxidative stress in rat aorta and heart.84
Taken together, data are converging on an important role for IVI in the two major CKD-related complications, cardiovascular disease and infection. Clearly, as succinctly stated in a recent editorial, the ground is set for multi-center RCTs on both CKD dialysis and non-dialysis patients, comparing different iron-dosing regimens, with adequate long-term follow-up. The study should be sufficiently powered to avoid imbalances in the type of anemia, degree of inflammation, dialysis modality where appropriate, and available iron preparations.63 We would also emphasize the need for hard end points in these trials and look to the ongoing UK Multicentre Open-label Randomized Controlled Trial Of IV Iron Therapy In Incident Haemodialysis Patients (Proactive IV irOn therapy for HaemodiALysis patients trial) to provide important answers, at least with regard to ferric carboxymaltose versus small doses of iron sucrose (EudraCT Number: 2013-002267-25).
Potential Contamination of Intravenous Iron Formulations with Labile Iron
Most IVI formulations in clinical use are nanoparticles comprised of a polyiron oxide/hydroxide core coated with carbohydrate (Table 1).85 It is generally accepted that these formulations, prepared as PIPs, supply metabolic iron to circulating Tf by a mechanism that recapitulates physiologic iron recycling via erythrophagocytosis (Figure 2A). With few exceptions,86 PIPs are regarded as ‘relatively’ free of labile iron87 and stable in circulation until endocytosed and processed by macrophages by mechanisms that are only partly understood (Figure 2B). However, an impending issue is the possible contamination of the newer IVI formulations designed for intensive iron supplementation, ferric carboxymaltose, ferumoxytol and iron isomaltoside, with labile iron88 and the subsequent generation of LPI following massive intravenous administration of these polymers. This is of particular importance for quality control of the formulations themselves, as well as for their fate in plasma of patients with CKD and other chronic inflammatory diseases.89 With the wider and more intense application of chemically diverse IVI supplements, it would seem prudent to obtain more information about the safety of the various products in terms of: (1) propensity to generate labile iron under different storage conditions and (2) possible adverse reactions related to transient rises in LPI as a function of delivery rates and dosage.90 The latter is especially relevant for patients with high plasma levels of (1) pro-oxidants, e.g., chronic patients with diabetes91; (2) hepcidin due to an inflammatory state or mutations affecting its expression; or (3) low ferroportin activity, induced either directly by elevated hepcidin levels or, independently of hepcidin, via toll-like receptors 2 and 6 signaling.92,93 These phenomena, in turn, could cause excessive splenic or hepatic retention of PIPs as such or as elevated ferritin but also perhaps as potentially toxic levels of LCI. The recent introduction of methods to measure the LPI potentially contained in IVI preparations will allow the systematic testing of our hypothesis that LPI could be formed in highly oxidized plasma or plasma with little antioxidant capacity and/or high redox-active groups (e.g., advanced-glycation end products) as found in patients with CKD.
Table 1.
Selected properties of polymeric iron preparations
| Iron Preparation | LMW Iron Dextran (INFeD® USA; Cosmofer® Europe) | HMW Iron Dextran (Dexferrum®) | Iron Sucrose (Venofer®) | Sodium Ferric Gluconate (Ferrlecit®) | Ferumoxytol (Feraheme®) | Ferric Carboxymaltose (Ferinject®) | Iron Isomaltoside 1000 (Monofer®)a |
|---|---|---|---|---|---|---|---|
| Potential for LI release | ++ | ++ | +++ | +++ | ± | ± | ± |
| Maximum approved dose | 100 mg IV push | 100 mg IV push | 100–200 mg IV over 2–5 min | 125 mg IV over 2–5 min | 510 mg IV over 1 min | 750 mg IV push/infusion over 15 min | 100–200 mg IV bolus or 1000 mg by infusion over 1 h |
LI, labile iron; LMW, low molecular weight; HMW, high molecular weight.
Not available in the United States.
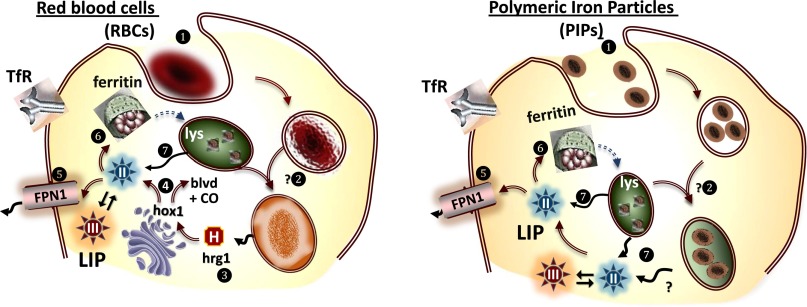
Figure 2.
Macrophage processing of red blood cells (RBCs) (left) and of PIPs (right). Left. Macrophages phagocytose aged RBCs (see number 1 enclosed in a circle). into a phagosome that after acquisition of hydrolytic enzymes from fusion processes (see number 2 enclosed in a circle) disrupt the cell and proteolyse Hb leading to release of the heme moiety (H) and its transfer across the cytosol via hrg1 (see number 3 enclosed in a circle) to the endoplasmic reticulum, where heme oxygenase 1 (hox1) (see number 4 enclosed in a circle) cleaves heme and releases biliverdin (BLVD), Fe(II) and CO. The Fe(II) can be exported from the cells into the plasma via ferroportin1 (FPN1) (see number 5 enclosed in a circle) where it can be incorporated into transferrin or stored as ferritin (see number 1 enclosed in a circle) that eventually can enter the lysosomal pathway of degradation (see number 7 enclosed in a circle) and release Fe(II) into the LCI pool. Right. Same as for RBCs, except that the hydrolytically processed PIP Fe(II) is released into the cytosol. Hb, hemoglobin.
Conclusions and Future Directions
Iron-induced cell damage or death, resulting from the intrinsic inflammatory nature of CKD and, possibly, exacerbated by repeated infusions of IVI in our patients remains a relatively unexplored field. The recent upsurge in the use of IVI following reports incriminating excessive ESA dosing as being associated with increased mortality, behooves nephrologists to take heed of the potential for replacing ESA toxicity with iron toxicity. This potential is highlighted by the intermittent detection of labile iron in both CKD patients’ plasma and currently available IVI preparations. In addition, the recent promotion of preparations containing up to 500 mg of iron per dose, although by the nature of their structure thought to be safe, further enhances the risk of iatrogenic iron toxicity. Given this combination of factors, we propose the following two-pronged strategy: (1) multi-center, international RCTs comparing different iron-dosing regimens in both CKD and ESRD patients, with adequate long-term follow-up; (2) the systematic monitoring of LPI during and post administration, which could serve as a promising biomarker of impending iron toxicity.
Disclosures
Z.I.C. received consulting/lecture fees from Aferrix, Ltd., Tel Aviv, Israel, Hinoman, Ltd., Or-Yehudah, Israel, and Apopharma, Toronto, Canada.
Acknowledgments
Unrestricted grant support was received by Z.I.C. from the Adelina and Massimo Della Pergola Chair in Life Sciences, Hebrew University of Jerusalem and I.S. from Medison Pharmaceuticals, Israel.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, Schwab SJ, Goodkin DA: The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 339: 584–590, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Drüeke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, Burger HU, Scherhag A, CREATE Investigators : Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 355: 2071–2084, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D, CHOIR Investigators : Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 355: 2085–2098, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill JB, McMurray JJ, Parfrey P, Parving HH, Remuzzi G, Singh AK, Solomon SD, Toto R, TREAT Investigators : A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 361: 2019–2032, 2009 [DOI] [PubMed] [Google Scholar]
- 5.KDIGO Anemia Work Group : KDIGO Clinical Practice Guidelines for Anemia in Chronic Kidney Disease. Kidney Int Suppl 2: 1–335, 2012 [Google Scholar]
- 6.Auerbach M, Macdougall IC: Safety of intravenous iron formulations: facts and folklore. Blood Transfus 12: 296–300, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fishbane S, Mathew AT, Wanchoo R: Intravenous iron exposure and outcomes in patients on hemodialysis. Clin J Am Soc Nephrol 9: 1837–1839, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martines AM, Masereeuw R, Tjalsma H, Hoenderop JG, Wetzels JF, Swinkels DW: Iron metabolism in the pathogenesis of iron-induced kidney injury. Nat Rev Nephrol 9: 385–398, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Crichton R: Inorganic Biochemistry of Iron Metabolism: From Molecular Mechanisms to Clinical Consequences, 2nd Ed., Chichester, Wiley, 2014 [Google Scholar]
- 10.Wang J, Pantopoulos K: Regulation of cellular iron metabolism. Biochem J 434: 365–381, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rouault TA: The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nat Chem Biol 2: 406–414, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Ganz T: Systemic iron homeostasis. Physiol Rev 93: 1721–1741, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Gammella E, Buratti P, Cairo G, Recalcati S: Macrophages: central regulators of iron balance. Metallomics 6: 1336–1345, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Halliwell B, Gutteridge JM: Free Radicals in Biology and Medicine, 4th Ed., London, Oxford University Press, 2007 [Google Scholar]
- 15.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B, 3rd, Stockwell BR: Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149: 1060–1072, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cabantchik ZI, Sohn YS, Breuer W, Esposito B: The molecular and cellular basis of iron toxicity in iron overload disorders. Diagnostic and therapeutic approaches. Thalassemia Rep 3: e3, 2013 [Google Scholar]
- 17.Camaschella C: Hereditary sideroblastic anemias: pathophysiology, diagnosis, and treatment. Semin Hematol 46: 371–377, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Fleming RE, Ponka P: Iron overload in human disease. N Engl J Med 366: 348–359, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Wood JC: Magnetic resonance imaging measurement of iron overload. Curr Opin Hematol 14: 183–190, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Picard V, Epsztejn S, Santambrogio P, Cabantchik ZI, Beaumont C: Role of ferritin in the control of the labile iron pool in murine erythroleukemia cells. J Biol Chem 273: 15382–15386, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Cabantchik ZI: Labile iron in cells and body fluids: physiology, pathology, and pharmacology. Front Pharmacol 5: 45, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobs A: Low molecular weight intracellular iron transport compounds. Blood 50: 433–439, 1977 [PubMed] [Google Scholar]
- 23.Kruszewski M: Labile iron pool: the main determinant of cellular response to oxidative stress. Mutat Res 531: 81–92, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Glickstein H, El RB, Shvartsman M, Cabantchik ZI: Intracellular labile iron pools as direct targets of iron chelators: a fluorescence study of chelator action in living cells. Blood 106: 3242–3250, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Kakhlon O, Manning H, Breuer W, Melamed-Book N, Lu C, Cortopassi G, Munnich A, Cabantchik ZI: Cell functions impaired by frataxin deficiency are restored by drug-mediated iron relocation. Blood 112: 5219–5227, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Sohn YS, Breuer W, Munnich A, Cabantchik ZI: Redistribution of accumulated cell iron: a modality of chelation with therapeutic implications. Blood 111: 1690–1699, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Hershko C, Graham G, Bates GW, Rachmilewitz EA: Non-specific serum iron in thalassaemia: an abnormal serum iron fraction of potential toxicity. Br J Haematol 40: 255–263, 1978 [DOI] [PubMed] [Google Scholar]
- 28.Breuer W, Hershko C, Cabantchik ZI: The importance of non-transferrin bound iron in disorders of iron metabolism. Transfus Sci 23: 185–192, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Brissot P, Ropert M, Le Lan C, Loréal O: Non-transferrin bound iron: a key role in iron overload and iron toxicity. Biochim Biophys Acta 1820: 403–410, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Singh S, Hider RC, Porter JB: A direct method for quantification of non-transferrin-bound iron. Anal Biochem 186: 320–323, 1990 [DOI] [PubMed] [Google Scholar]
- 31.Kolb AM, Smit NP, Lentz-Ljuboje R, Osanto S, van Pelt J: Non-transferrin bound iron measurement is influenced by chelator concentration. Anal Biochem 385: 13–19, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Lee DH, Liu DY, Jacobs DR, Jr, Shin HR, Song K, Lee IK, Kim B, Hider RC: Common presence of non-transferrin-bound iron among patients with type 2 diabetes. Diabetes Care 29: 1090–1095, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Silva AM, Hider RC: Influence of non-enzymatic post-translation modifications on the ability of human serum albumin to bind iron. Implications for non-transferrin-bound iron speciation. Biochim Biophys Acta 1794: 1449–1458, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Kooistra MP, Kersting S, Gosriwatana I, Lu S, Nijhoff-Schutte J, Hider RC, Marx J-JM: Nontransferrin-bound iron in the plasma of haemodialysis patients after intravenous iron saccharate infusion. Eur J Clin Invest 32[Suppl 1]: 36–41, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Espósito BP, Breuer W, Slotki I, Cabantchik ZI: Labile iron in parenteral iron formulations and its potential for generating plasma nontransferrin-bound iron in dialysis patients. Eur J Clin Invest 32[Suppl 1]: 42–49, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Halliwell B, Aruoma OI, Mufti G, Bomford A: Bleomycin-detectable iron in serum from leukaemic patients before and after chemotherapy. Therapeutic implications for treatment with oxidant-generating drugs. FEBS Lett 241: 202–204, 1988 [DOI] [PubMed] [Google Scholar]
- 37.Zanninelli G, Breuer W, Cabantchik ZI: Daily labile plasma iron as an indicator of chelator activity in Thalassaemia major patients. Br J Haematol 147: 744–751, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Danjou F, Cabantchik ZI, Origa R, Moi P, Marcias M, Barella S, Defraia E, Dessì C, Foschini ML, Giagu N, Leoni GB, Morittu M, Galanello R: A decisional algorithm to start iron chelation in patients with beta thalassemia. Haematologica 99: e38–e40, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Buren P, Velez RL, Vaziri ND, Zhou XJ: Iron overdose: a contributor to adverse outcomes in randomized trials of anemia correction in CKD. Int Urol Nephrol 44: 499–507, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gordon S, Taylor PR: Monocyte and macrophage heterogeneity. Nat Rev Immunol 5: 953–964, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Ishida JH, Johansen KL: Iron and infection in hemodialysis patients. Semin Dial 27: 26–36, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mencacci A, Cenci E, Boelaert JR, Bucci P, Mosci P, Fè d’Ostiani C, Bistoni F, Romani L: Iron overload alters innate and T helper cell responses to Candida albicans in mice. J Infect Dis 175: 1467–1476, 1997 [DOI] [PubMed] [Google Scholar]
- 43.Charytan DM, Pai AB, Chan CT, Coyne DW, Hung AM, Kovesdy CP, Fishbane S, on behalf of the Dialysis Advisory Group of the American Society of Nephrology : Considerations and challenges in defining optimal iron utilization in hemodialysis [published online ahead of print xxxx xx, 2014]. J Am Soc Nephrol pii: ASN.2014090922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feldman HI, Santanna J, Guo W, Furst H, Franklin E, Joffe M, Marcus S, Faich G: Iron administration and clinical outcomes in hemodialysis patients. J Am Soc Nephrol 13: 734–744, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Feldman HI, Joffe M, Robinson B, Knauss J, Cizman B, Guo W, Franklin-Becker E, Faich G: Administration of parenteral iron and mortality among hemodialysis patients. J Am Soc Nephrol 15: 1623–1632, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Kalantar-Zadeh K, Regidor DL, McAllister CJ, Michael B, Warnock DG: Time-dependent associations between iron and mortality in hemodialysis patients. J Am Soc Nephrol 16: 3070–3080, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Kuo KL, Hung SC, Lin YP, Tang CF, Lee TS, Lin CP, Tarng DC: Intravenous ferric chloride hexahydrate supplementation induced endothelial dysfunction and increased cardiovascular risk among hemodialysis patients. PLoS ONE 7: e50295, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuragano T, Matsumura O, Matsuda A, Hara T, Kiyomoto H, Murata T, Kitamura K, Fujimoto S, Hase H, Joki N, Fukatsu A, Inoue T, Itakura I, Nakanishi T: Association between hemoglobin variability, serum ferritin levels, and adverse events/mortality in maintenance hemodialysis patients. Kidney Int 86: 845–854, 2014 [DOI] [PubMed] [Google Scholar]
- 49.Bailie GR, Larkina M, Goodkin DA, Li Y, Pisoni RL, Bieber B, Mason N, Tong L, Locatelli F, Marshall MR, Inaba M, Robinson BM: Data from the Dialysis Outcomes and Practice Patterns Study validate an association between high intravenous iron doses and mortality. Kidney Int 87: 162–168, 2015 [DOI] [PubMed] [Google Scholar]
- 50.Miskulin DC, Tangri N, Bandeen-Roche K, Zhou J, McDermott A, Meyer KB, Ephraim PL, Michels WM, Jaar BG, Crews DC, Scialla JJ, Sozio SM, Shafi T, Wu AW, Cook C, Boulware LE, Developing Evidence to Inform Decisions about Effectiveness (DEcIDE) Network Patient Outcomes in End Stage Renal Disease Study Investigators : Intravenous iron exposure and mortality in patients on hemodialysis. Clin J Am Soc Nephrol 9: 1930–1939, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kshirsagar AV, Freburger JK, Ellis AR, Wang L, Winkelmayer WC, Brookhart MA: Intravenous iron supplementation practices and short-term risk of cardiovascular events in hemodialysis patients. PLoS ONE 8: e78930, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goodkin DA, Bragg-Gresham JL, Koenig KG, Wolfe RA, Akiba T, Andreucci VE, Saito A, Rayner HC, Kurokawa K, Port FK, Held PJ, Young EW: Association of comorbid conditions and mortality in hemodialysis patients in Europe, Japan, and the United States: the Dialysis Outcomes and Practice Patterns Study (DOPPS). J Am Soc Nephrol 14: 3270–3277, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Brookhart MA, Freburger JK, Ellis AR, Wang L, Winkelmayer WC, Kshirsagar AV: Infection risk with bolus versus maintenance iron supplementation in hemodialysis patients. J Am Soc Nephrol 24: 1151–1158, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sirken G, Raja R, Rizkala AR: Association of different intravenous iron preparations with risk of bacteremia in maintenance hemodialysis patients. Clin Nephrol 66: 348–356, 2006 [DOI] [PubMed] [Google Scholar]
- 55.Drüeke T, Witko-Sarsat V, Massy Z, Descamps-Latscha B, Guerin AP, Marchais SJ, Gausson V, London GM: Iron therapy, advanced oxidation protein products, and carotid artery intima-media thickness in end-stage renal disease. Circulation 106: 2212–2217, 2002 [DOI] [PubMed] [Google Scholar]
- 56.Coyne DW, Kapoian T, Suki W, Singh AK, Moran JE, Dahl NV, Rizkala AR, DRIVE Study Group : Ferric gluconate is highly efficacious in anemic hemodialysis patients with high serum ferritin and low transferrin saturation: results of the Dialysis Patients’ Response to IV Iron with Elevated Ferritin (DRIVE) Study. J Am Soc Nephrol 18: 975–984, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Kapoian T, O’Mara NB, Singh AK, Moran J, Rizkala AR, Geronemus R, Kopelman RC, Dahl NV, Coyne DW: Ferric gluconate reduces epoetin requirements in hemodialysis patients with elevated ferritin. J Am Soc Nephrol 19: 372–379, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Besarab A, Amin N, Ahsan M, Vogel SE, Zazuwa G, Frinak S, Zazra JJ, Anandan JV, Gupta A: Optimization of epoetin therapy with intravenous iron therapy in hemodialysis patients. J Am Soc Nephrol 11: 530–538, 2000 [DOI] [PubMed] [Google Scholar]
- 59.Auryxia (ferric citrate) tablets. 2015. 11-1-2015
- 60.Lewis JB, Sika M, Koury MJ, Chuang P, Schulman G, Smith MT, Whittier FC, Linfert DR, Galphin CM, Athreya BP, Nossuli AK, Chang IJ, Blumenthal SS, Manley J, Zeig S, Kant KS, Olivero JJ, Greene T, Dwyer JP, Collaborative Study Group : Ferric citrate controls phosphorus and delivers iron in patients on dialysis. J Am Soc Nephrol 26: 493–503, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mercadel L, Metzger M, Haymann JP, Thervet E, Boffa JJ, Flamant M, Vrtovsnik F, Houillier P, Froissart M, Stengel B, NephroTest Study Group : The relation of hepcidin to iron disorders, inflammation and hemoglobin in chronic kidney disease. PLoS ONE 9: e99781, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vaziri ND: Understanding iron: promoting its safe use in patients with chronic kidney failure treated by hemodialysis. Am J Kidney Dis 61: 992–1000, 2013 [DOI] [PubMed] [Google Scholar]
- 63.Weiss G, Kronenberg F: Intravenous iron administration: new observations and time for the next steps. Kidney Int 87: 10–12, 2015 [DOI] [PubMed] [Google Scholar]
- 64.Kuo KL, Hung SC, Lee TS, Tarng DC: Iron sucrose accelerates early atherogenesis by increasing superoxide production and upregulating adhesion molecules in CKD. J Am Soc Nephrol 25: 2596–2606, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lim PS, Wei YH, Yu YL, Kho B: Enhanced oxidative stress in haemodialysis patients receiving intravenous iron therapy. Nephrol Dial Transplant 14: 2680–2687, 1999 [DOI] [PubMed] [Google Scholar]
- 66.Descamps-Latscha B, Witko-Sarsat V: Importance of oxidatively modified proteins in chronic renal failure. Kidney Int Suppl 78: S108–S113, 2001 [DOI] [PubMed] [Google Scholar]
- 67.Anraku M, Kitamura K, Shinohara A, Adachi M, Suenga A, Maruyama T, Miyanaka K, Miyoshi T, Shiraishi N, Nonoguchi H, Otagiri M, Tomita K: Intravenous iron administration induces oxidation of serum albumin in hemodialysis patients. Kidney Int 66: 841–848, 2004 [DOI] [PubMed] [Google Scholar]
- 68.Michelis R, Gery R, Sela S, Shurtz-Swirski R, Grinberg N, Snitkovski T, Shasha SM, Kristal B: Carbonyl stress induced by intravenous iron during haemodialysis. Nephrol Dial Transplant 18: 924–930, 2003 [DOI] [PubMed] [Google Scholar]
- 69.Himmelfarb J, McMonagle E, McMenamin E: Plasma protein thiol oxidation and carbonyl formation in chronic renal failure. Kidney Int 58: 2571–2578, 2000 [DOI] [PubMed] [Google Scholar]
- 70.Sezer MT, Akin H, Demir M, Erturk J, Aydin ZD, Savik E, Tunc N: The effect of serum albumin level on iron-induced oxidative stress in chronic renal failure patients. J Nephrol 20: 196–203, 2007 [PubMed] [Google Scholar]
- 71.Pai AB, Boyd AV, McQuade CR, Harford A, Norenberg JP, Zager PG: Comparison of oxidative stress markers after intravenous administration of iron dextran, sodium ferric gluconate, and iron sucrose in patients undergoing hemodialysis. Pharmacotherapy 27: 343–350, 2007 [DOI] [PubMed] [Google Scholar]
- 72.Pai AB, Conner T, McQuade CR, Olp J, Hicks P: Non-transferrin bound iron, cytokine activation and intracellular reactive oxygen species generation in hemodialysis patients receiving intravenous iron dextran or iron sucrose. Biometals 24: 603–613, 2011 [DOI] [PubMed] [Google Scholar]
- 73.Parkkinen J, von Bonsdorff L, Peltonen S, Grönhagen-Riska C, Rosenlöf K: Catalytically active iron and bacterial growth in serum of haemodialysis patients after i.v. iron-saccharate administration. Nephrol Dial Transplant 15: 1827–1834, 2000 [DOI] [PubMed] [Google Scholar]
- 74.Barton Pai A, Pai MP, Depczynski J, McQuade CR, Mercier RC: Non-transferrin-bound iron is associated with enhanced Staphylococcus aureus growth in hemodialysis patients receiving intravenous iron sucrose. Am J Nephrol 26: 304–309, 2006 [DOI] [PubMed] [Google Scholar]
- 75.Rangel EB, Espósito BP, Carneiro FD, Mallet AC, Matos AC, Andreoli MC, Guimarães-Souza NK, Santos BF: Labile plasma iron generation after intravenous iron is time-dependent and transitory in patients undergoing chronic hemodialysis. Ther Apher Dial 14: 186–192, 2010 [DOI] [PubMed] [Google Scholar]
- 76.Van Wyck D, Anderson J, Johnson K: Labile iron in parenteral iron formulations: a quantitative and comparative study. Nephrol Dial Transplant 19: 561–565, 2004 [DOI] [PubMed] [Google Scholar]
- 77.Rooyakkers TM, Stroes ES, Kooistra MP, van Faassen EE, Hider RC, Rabelink TJ, Marx JJ: Ferric saccharate induces oxygen radical stress and endothelial dysfunction in vivo. Eur J Clin Invest 32[Suppl 1]: 9–16, 2002 [DOI] [PubMed] [Google Scholar]
- 78.Tovbin D, Mazor D, Vorobiov M, Chaimovitz C, Meyerstein N: Induction of protein oxidation by intravenous iron in hemodialysis patients: role of inflammation. Am J Kidney Dis 40: 1005–1012, 2002 [DOI] [PubMed] [Google Scholar]
- 79.Canavese C, Bergamo D, Ciccone G, Longo F, Fop F, Thea A, Martina G, Piga A: Validation of serum ferritin values by magnetic susceptometry in predicting iron overload in dialysis patients. Kidney Int 65: 1091–1098, 2004 [DOI] [PubMed] [Google Scholar]
- 80.Rostoker G, Griuncelli M, Loridon C, Couprie R, Benmaadi A, Bounhiol C, Roy M, Machado G, Janklewicz P, Drahi G, Dahan H, Cohen Y: Hemodialysis-associated hemosiderosis in the era of erythropoiesis-stimulating agents: a MRI study. Am J Med 125: 991–999. e1, 2012 [DOI] [PubMed] [Google Scholar]
- 81.McLaren GD, Muir WA, Kellermeyer RW: Iron overload disorders: natural history, pathogenesis, diagnosis, and therapy. Crit Rev Clin Lab Sci 19: 205–266, 1983 [DOI] [PubMed] [Google Scholar]
- 82.Ghoti H, Rachmilewitz EA, Simon-Lopez R, Gaber R, Katzir Z, Konen E, Kushnir T, Girelli D, Campostrini N, Fibach E, Goitein O: Evidence for tissue iron overload in long-term hemodialysis patients and the impact of withdrawing parenteral iron. Eur J Haematol 89: 87–93, 2012 [DOI] [PubMed] [Google Scholar]
- 83.Johnson AC, Becker K, Zager RA: Parenteral iron formulations differentially affect MCP-1, HO-1, and NGAL gene expression and renal responses to injury. Am J Physiol Renal Physiol 299: F426–F435, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lim CS, Vaziri ND: The effects of iron dextran on the oxidative stress in cardiovascular tissues of rats with chronic renal failure. Kidney Int 65: 1802–1809, 2004 [DOI] [PubMed] [Google Scholar]
- 85.Fütterer S, Andrusenko I, Kolb U, Hofmeister W, Langguth P: Structural characterization of iron oxide/hydroxide nanoparticles in nine different parenteral drugs for the treatment of iron deficiency anaemia by electron diffraction (ED) and X-ray powder diffraction (XRPD). J Pharm Biomed Anal 86: 151–160, 2013 [DOI] [PubMed] [Google Scholar]
- 86.Jahn MR, Andreasen HB, Fütterer S, Nawroth T, Schünemann V, Kolb U, Hofmeister W, Muñoz M, Bock K, Meldal M, Langguth P: A comparative study of the physicochemical properties of iron isomaltoside 1000 (Monofer), a new intravenous iron preparation and its clinical implications. Eur J Pharm Biopharm 78: 480–491, 2011 [DOI] [PubMed] [Google Scholar]
- 87.Scheiber-Mojdehkar B, Lutzky B, Schaufler R, Sturm B, Goldenberg H: Non-transferrin-bound iron in the serum of hemodialysis patients who receive ferric saccharate: no correlation to peroxide generation. J Am Soc Nephrol 15: 1648–1655, 2004 [DOI] [PubMed] [Google Scholar]
- 88.Macdougall IC: Evolution of iv iron compounds over the last century. J Ren Care 35[Suppl 2]: 8–13, 2009 [DOI] [PubMed] [Google Scholar]
- 89.Bishu K, Agarwal R: Acute injury with intravenous iron and concerns regarding long-term safety. Clin J Am Soc Nephrol 1[Suppl 1]: S19–S23, 2006 [DOI] [PubMed] [Google Scholar]
- 90.Malindretos P, Sarafidis PA, Rudenco I, Raptis V, Makedou K, Makedou A, Grekas DM: Slow intravenous iron administration does not aggravate oxidative stress and inflammatory biomarkers during hemodialysis: a comparative study between iron sucrose and iron dextran. Am J Nephrol 27: 572–579, 2007 [DOI] [PubMed] [Google Scholar]
- 91.Styskal J, Van Remmen H, Richardson A, Salmon AB: Oxidative stress and diabetes: what can we learn about insulin resistance from antioxidant mutant mouse models? Free Radic Biol Med 52: 46–58, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guida C, Altamura S, Klein FA, Galy B, Boutros M, Ulmer AJ, Hentze MW, Muckenthaler MU: A novel inflammatory pathway mediating rapid hepcidin-independent hypoferremia. Blood 125: 2265–2275, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Finberg KE: Ironing out the role of Toll-like receptors. Blood 125: 2183–2184, 2015 [DOI] [PubMed] [Google Scholar]