Abstract
Background/Aims
We experimented with different ablation methods and two types of microwave antennas to determine whether microwave ablation (MWA) increases intrahepatic pressure and to identify an MWA protocol that avoids increasing intrahepatic pressure.
Methods
MWA was performed using either a single-step standard ablation or a stepwise increment ablation paired with either a 16-gauge (G) 2-cm antenna or a 14G 4-cm antenna. We compared the maximum pressures and total ablation volumes.
Results
The mean maximum intrahepatic pressures and ablation volumes were as follows: 16G single-step: 37±33.4 mm Hg and 4.63 cm3; 16G multistep: 31±18.7 mm Hg and 3.75 cm3; 14G single-step: 114±45.4 mm Hg and 15.33 cm3; and 14G multistep: 106±43.8 mm Hg and 10.98 cm3. The intrahepatic pressure rose during MWA, but there were no statistically significant differences between the single and multistep methods when the same gauge antennae were used. The total ablation volume was different only in the 14G groups (p<0.05).
Conclusions
We demonstrated an increase in intrahepatic pressure during MWA. The multistep method may be used to prevent increased intrahepatic pressure after applying the proper power.
Keywords: Microwave ablation, Intrahepatic pressure, Ex vivo, Liver
INTRODUCTION
Radiofrequency ablation (RFA) has been accepted clinically as a useful local treatment for hepatocellular carcinoma (HCC).1,2 But intrahepatic dissemination,3,4 extrahepatic seeding,5 and rapidly growing recurrences6,7 have recently been reported.
Several reports suggested that these recurrences might be attributed to increased intratumoral pressure. Portolani et al.8 documented four cases of aggressive recurrence after RFA of liver tumors. Seki et al.9 have also reported a case with rapid progression of numerous tumors around the ablation zone after RFA. They suggested that an increase in local pressure induced by RFA caused the unwanted tumor dissemination. They hypothesize that RFA during energy application may increase intratu-moral pressure, aggravating intravascular spread of the tumor to the surrounding tissues. Kotoh et al.10,11 subsequently reported that liver parenchymal pressure increased during RFA, and they developed a multistep incremental expansion method for RFA in vitro and in vivo that prevented increases in pressure. Kawamoto et al.12 reported that hepatic parenchymal pressure increased during RFA, and suggested that rapid tumor heating may lead to an unpredicted increase in intratumoral pressure. However, increased intrahepatic pressure can be controlled using multistep methods. In particular, LeVeen needle multistep methods should be used to prevent increased intrahepatic pressure during RFA.
Microwave ablation (MWA) is the recent development in the field of tumor ablation7,13–15 and is expected to be as effective as RFA for local treatment. Microwave energy is found along the spectrum of electromagnetic radiation, heating the target tissue. Electromagnetic microwaves heat matter by agitating water molecules in the adjacent tissue, producing friction and heat and therefore inducing cellular death via coagulation necrosis.16 Microwave energy has several benefits over radiofrequency energy for tumor ablation, and it has been accepted as a useful local treatment for thermal coagulation.16–18 However, intratu-moral pressure is expected to rise during MWA because microwave energy uses heating to achieve its aims, alike RFA.3
No studies exist on MWA-induced increased intrahepatic pressure and the potential for an associated increase in intratumoral pressure during MWA. Variety of conditions cause pressure to rise and its relation to ablation volume such as type and length of antenna, power, or duration of ablation time. We hypothesize that during MWA, intrahepatic pressure arises, but can be controlled using multistep methods.11 Significant research is required to determine if MWA, like RFA, increases intratumoral pressure. Therefore, we used ex vivo experiments to determine whether intrahepatic pressure rises during MWA, which conditions affect the pressure rise, and how such conditions influence the ablation volume.
MATERIALS AND METHODS
1. Study design
Ex-vivo bovine liver model was used as a model of a HCC tumor. To mimic the temperature of the human body, we soaked a block of cow liver in a water tank and heated it wrapped in a plastic case in 40°C water until just before the start of the experiment about 30 minutes. There was about 7 to 8 hours time interval between organ harvest and experiment.
After placing a heated cow liver on a flat plate, we inserted a microwave antenna (MedWaves, San Diego, CA, USA) (Fig. 1) and pressure sensing needle (STARmed, Seoul, Korea) (Fig. 2) perpendicularly into the cow liver. A 16-gauge (G) antenna with a 2-cm active tip and a 14G antenna with a 4-cm tip were used. The tips of the antennae and pressure sensing needle were fixed at the proximal portion (Fig. 3). The width between the two tips was maintained within 1 to 2 mm. The average depth of insertion was 6 cm for the 14G antenna and 4 cm for the 16G antenna.
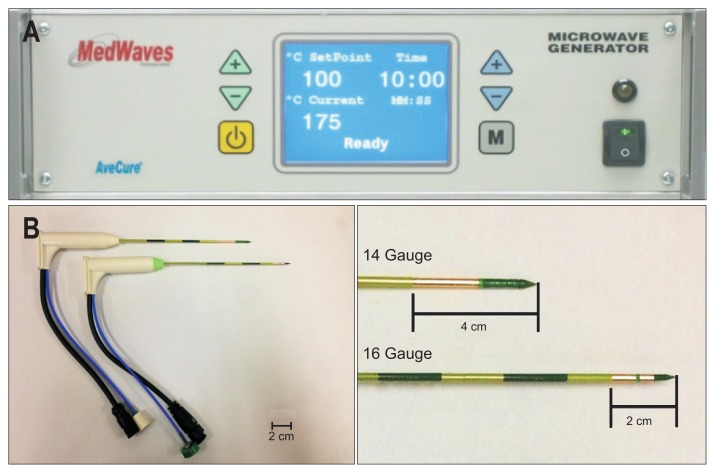
Fig. 1.
Microwave generator and needle antenna (AveCure Microwave Generator; MedWaves). (A) We used a microwave generator to create a dynamic frequency range (902 to 908 MHz) capable of adapting to tissue permittivity changes during ablation. It monitors power levels automatically and adjusts the frequency to maximize power delivery to the targeted tissue. (B) A 2-cm active tip with a 16-gauge (G) (lower figure) antenna and a 4-cm tip with a 14G antenna (upper figure) were used.
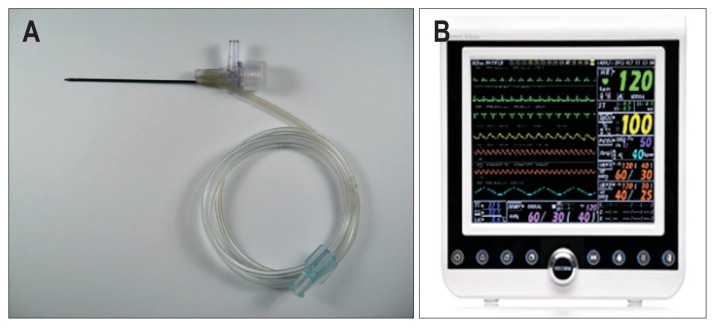
Fig. 2.
Pressure sensing needle (STARmed) and pressure monitoring device (VP-1000; VOTEM Co., Ltd.). (A) A percutaneous pressure-sensing needle was inserted into the cow liver. The pressure rise in the cow liver can be determined by measuring the pressure of saline in the transparent line, which is connected to the pressure monitoring device. (B) Pressure monitoring device (VP-1000).
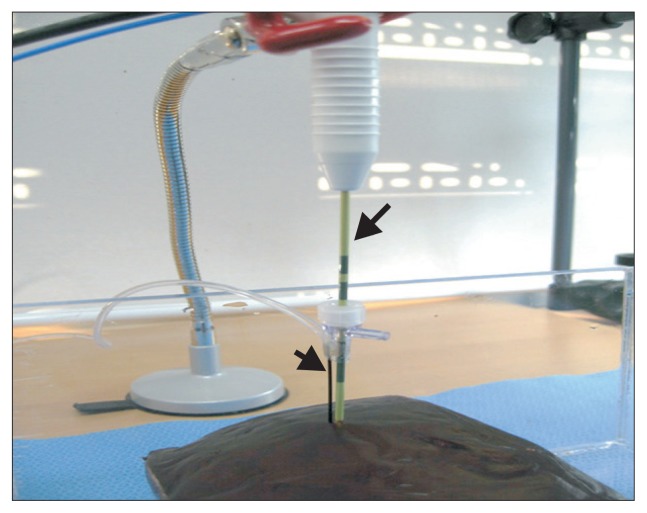
Fig. 3.
Setting of microwave antenna and pressure-sensing needle. The microwave antenna (long arrow) and the pressure-sensing needle (short arrow) were perpendicularly inserted into the liver, parallel with one another. These two tips were fixed and immobilized around the hub. The width between the two tips was maintained between 1 and 2 mm. The antenna tip was located 6 cm from the liver surface for the 14-gauge (G) antenna and 4 cm for the 16G antenna.
The microwave antenna was connected to a microwave generator (AveCure Microwave Generator; MedWaves) (Fig. 1). This microwave generator uses a dynamic frequency range (902 to 908 MHz) capable of adapting to tissue permittivity changes during ablation. It can monitor power levels automatically and adjusts frequency to maximize power delivery to the targeted tissue.
We monitored intrahepatic pressure using a 19G pressure sensing needle (1.06 mm) connected to a pressure measuring machine (VP-1000; VOTEM Co., Ltd., Seoul, Korea) (Fig. 2).
2. MWA protocol
MWA was performed using standard single-step ablation with a16G antenna, stepwise multistep ablation with a 16G antenna, standard single-step ablation with a 14G antenna, and stepwise multistep ablation with a 14G antenna. Ten pieces of cow liver were used in each group and 40 pieces were used in total. The maximum intrahepatic pressure at each pulsing were measured during the ablation process and were recorded.
In the group with standard single-step ablation with a 16G antenna (n=10), the power was applied at 24 W. In the group that received stepwise multistep ablation with a 16G antenna (n=10), the power was gradually increased from14 W to 16 W, 18 W, 20 W, and finally 24 W at 1 minute intervals (Table 1).
Table 1.
Four Protocols for Microwave Ablation
| Electrode | Step | Start watt, W | Condition |
|---|---|---|---|
| 16G antenna | Single | 24 | Maintain 24 W |
| Multi | 14 | 5 Steps with14, 16, 18, 20, 24 W | |
| 14G antenna | Single | 28 | Maintain 28 W |
| Multi | 16 | 5 Steps with 16, 18, 20, 24, 28 W |
G, gauge; W, watt.
In the group that received standard single-step ablation with a 14G antenna (n=10), the power was applied at 28 W and maintained. In the stepwise multistep ablation with a 14G antenna (n=10), the power was gradually increased from 16 W to 18 W, 20 W, 24 W, and finally 28 W at 1 minute intervals (Table 1). MWA protocol was different between 16G antenna and 14G antenna. Start watt should be same but we followed optimized condition in each antenna which was proposed by manufacturer.
3. Measurement of volume of ablation zone
To reduce bias, we used a method that measured the distance through the photo taken of the specimens. We measured the length and depth of a longitudinal section and the length and width of a cross section. We then transformed the real numeric value using a survey program (Image J program). Ablation volume was calculated by following equation:
4. Statistical analysis
Maximum intrahepatic pressures obtained under each MWA protocol and ablation volume were compared using the Kruskal-Wallis test and Tukey test using ranks. A p-value lower than 0.05 was considered statistically significant.
RESULTS
The maximal intrahepatic pressure and mean ablation volume in each group are summarized in Table 2. We also presented peak pressure measurement of all 40 data in Table 3 and Fig. 4. Average temperature of cow liver tissue was 18°C which had discrepancy of water temperature.
Table 2.
Maximum Pressure and Mean Ablation Volume of the Four Groups
| Electrode | Step | Case | Temperature of the liver, °C | Maximal pressure, mm Hg | Longitudinal section, cm | Cross section, cm | Mean ablation volume, cm3 | Ablation time, sec | ||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Width | Depth | Short axis | Long axis | |||||||
| 16G | Single | 10 | 19.0 | 37±33.4 | 1.74 | 2.81 | 1.72 | 1.84 | 4.63±0.5 | 675 |
| Multi | 10 | 18.2 | 31±18.7 | 1.69 | 2.70 | 1.56 | 1.69 | 3.75±0.8 | 401 | |
| 14G | Single | 10 | 19.7 | 114±45.4 | 2.23 | 4.99 | 2.21 | 2.38 | 15.33±3.4 | 339 |
| Multi | 10 | 19.0 | 106±43.8 | 1.96 | 4.34 | 1.92 | 2.14 | 10.98±2.5 | 306 | |
G, gauge.
Table 3.
Peak Pressure Measurement of All 40 Data Measures per Group
| 16G | 14G | |||
|---|---|---|---|---|
|
|
|
|||
| Single | Multi | Single | Multi | |
| Maximal pressure per case, mm Hg | 13 | 21 | 140 | 137 |
| 33 | 32 | 129 | 126 | |
| 104 | 56 | 139 | 100 | |
| 89 | 63 | 84 | 75 | |
| 13 | 17 | 192 | 122 | |
| 20 | 38 | 61 | 100 | |
| 23 | 23 | 55 | 107 | |
| 47 | 43 | 96 | 141 | |
| 14 | 13 | 79 | 77 | |
| 13 | 6 | 161 | 164 | |
| Average±SD | 37±33.4 | 31±18.7 | 114±45.4 | 106±43.8 |
G, gauge; SD, standard deviation.
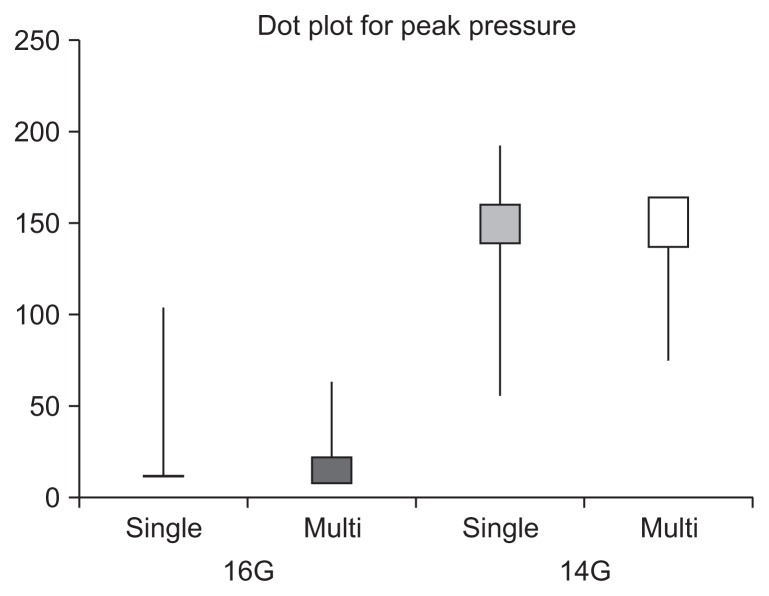
Fig. 4.
A dot plot for visual comparison. G, gauge.
1. Intrahepatic pressure
The average maximum intrahepatic pressure was as follows: standard single-step ablation with 16G antenna, 37±33.4 mm Hg; stepwise multistep ablation with 16G antenna, 31±18.7 mm Hg; standard single-step ablation with 14G antenna, 114±45.4 mm Hg; stepwise multistep ablation with 14G antenna, 106±43.8 mm Hg. Standard single-step ablation with a 14G antenna resulted in the highest peak pressure among the groups.
We measured the rise of intrahepatic pressure clearly during MWA, although explosive sounds was not heard during MWA procedures. We found that hepatic parenchymal pressure increased more in groups treated with a 14G antenna than those treated with a 16G antenna in both single and stepwise treatments. There were no statistically significant differences in the rise of pressure between single-step ablation and stepwise ablation if the antenna gauge remained constant (Kruskal Wallis test and Tukey test using ranks, p>0.05) (Table 4).
Table 4.
Comparison of Intrahepatic Pressure for Four Groups (Tukey Test Using Ranks)
| 14G single | 16G single | 14G multi | 16G multi | |
|---|---|---|---|---|
| 14G single | - | - | - | - |
| 16G single | <0.0005* | - | - | - |
| 14G multi | 0.9647† | 0.0018 | - | - |
| 16G multi | 0.0003 | 0.9967† | 0.0010‡ | - |
Maximum pressures of single 14-gauge (G) group and single 16G group were significantly different;
There were no significant differences in the rise of pressure between single-step ablation and stepwise ablation within the same gauge groups;
Maximum pressures of stepwise 14G group and stepwise 16G group were significantly different.
2. Ablation volume
The average value of ablation volume was as follows: standard single-step ablation with 16G antenna, 4.63±0.5 cm3; stepwise multistep ablation with 16G antenna, 3.75±0.8 cm3; standard single-step ablation with 14G antenna, 15.33±3.4 cm3; stepwise multistep ablation with 14G antenna, 10.98±2.5 cm3. The ablation areas in each groups appeared oval or spherical in shape (Fig. 5). The ablation volume of groups treated with a 14G antenna was larger than that of groups treated with a 16G antenna, and the ablation volume of single-step groups was larger than that of stepwise groups in each gauge. There was statistical difference in ablation volume between single-step ablation and stepwise multistep ablation in the 14G groups. There was also a statistical difference in ablation volume between 14G and 16G antennae in single and stepwise groups. However, there was no statistical difference in ablation volume between single-step ablation and stepwise ablation in the 16G group (Kruskal Wallis test and Tukey test using ranks, p<0.05) (Table 5).
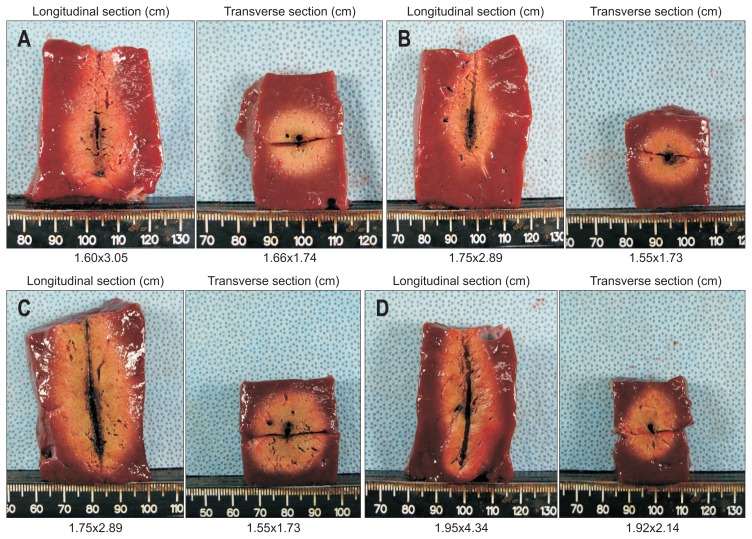
Fig. 5.
Cross-sectional image. The depth of a longitudinal section and the longest and shortest axes (means) of the ablation area were measured. The ablation areas appeared uniformly oval in four groups: (A) Cross-section of ablation zone by single-step ablation with a 16-gauge (G) antenna. (B) Cross-section of ablation zone by multistep ablation with a 16G antenna. (C) Cross-section of ablation zone by single-step ablation with a 14G antenna. (D) Cross-section of ablation zone by multistep ablation with a 14G antenna.
Table 5.
Comparison of Ablation Volume for Four Groups (Tukey Test Using Ranks)
| 14G single | 16G single | 14G multi | 16G multi | |
|---|---|---|---|---|
| 14G single | - | - | - | - |
| 16G single | <0.0001* | - | - | - |
| 14G multi | 0.0243† | <0.0001 | - | - |
| 16G multi | <0.0001 | 0.0682‡ | <0.0001§ | - |
Ablation volumes of single 14-gauge (G) group and single 16G group were significantly different;
Ablation volumes of single 14G group and stepwise 14G group were significantly different;
Ablation volumes of single 16G group and stepwise 16G group were not significantly different;
Ablation volumes of stepwise 14G group and stepwise 16G group were significantly different.
3. Ablation time
The ablation time was as follows: standard single-step ablation with 16G antenna, 675 seconds; stepwise multistep ablation with 16G antenna, 401 seconds; standard single-step ablation with 14G antenna, 339 seconds; stepwise multistep ablation with 14G antenna, 306 seconds. The reason why there was a difference in running time was that microwave generator was stopped for a while and was given repeatedly when microwave rises more than target watt, so MWA was applied intermittently.
DISCUSSION
RFA is becoming an accepted treatment modality for many tumors of the liver and is being explored for tumors in the lung, kidney, and bone.1 While RF energy is the most familiar heat source for tissue ablation, it has several limitations.2 Kotoh et al.3 and Seki et al.9 reported rapid and scattered recurrences and suggested that increased intratumoral pressure might be the cause.19 Explosive sounds like a “pop” are often heard during RFA procedures, and microbubbles are frequently observed by ultrasonography.20 During exposure to a high-frequency current, the tumor is heated and generates air bubbles throughout the treatment period.21 These bubbles enter the blood vessels, and rapid tumor heating may lead to unpredicted increases in intrahepatic pressure.22
Microwave energy may be a better source for tissue ablation.13 A MWA is essentially induced dielectric heating using oscillation of water molecules.23 When an oscillating electric charge from radiation interacts with a water molecule, it causes the molecule to flip.
The technique may be useful for flexible approaches to treatment, including percutaneous, laparoscopic, and open surgical approximation. With imaging guidance, the tumor is localized and a thin microwave antenna is placed directly into the target lesion.13,15–18,23–27 A microwave generator emits an electromagnetic wave through the exposed, noninsulated portion of the antenna. Electromagnetic microwaves induce water molecules in the surrounding tissue, producing friction and heat and causing cellular death via coagulation necrosis.26 MWA has promising potential in the treatment of primary and secondary liver disease, primary and secondary lung malignancies, renal and adrenal tumors, and bone metastases.15
The major difference between MWA and RFA is that MWA occurs in a volume around the applicator antenna, while RFA is restricted to areas of high current density. RFA requires an electrically conductive path, while microwaves do not; thus, microwaves are capable of propagating through materials with low or zero conductivity. This means that low conductivity tissues inhibit RF current flow, but allow better microwave propagation. One important factor when comparing RFA and MWA is the tumor’s host organ and location within the organ, since the tissue properties of normal liver, lung, kidney, and bone are quite different.13,26
Intratumoral pressure is expected to rise during ablation, due to the heating of the tissue. No previous studies have investigated intratumoral pressure during MWA. Kotoh et al.10 recently reported an evaluation of liver parenchymal pressure during RFA in an in vivo porcine model.
Our results showed that increased intrahepatic pressure is evident during MWA, which was observed when both 16G and 14G antenna were used; however, the increase in pressure was much more prominent when the 14G antenna was used, regardless of the single-step versus multistep protocol. Multistep protocols produced a slightly smaller intrahepatic pressure rise than single-step protocols, but this was not statistically significant. We hypothesize that MWA, especially when used in a multistep protocol, produced less pressures increases. This might be due to differences in the lower ablation time and even distribution of the MWA (Table 2).
The present experiment found that the size and the volume of ablation zone tended to be small when compared with the general RFA experiment. Sixteen-gauge antennas created smaller zones of ablation than 14G antennas, and multistep protocols created smaller ablation zones than single-step protocols (Table 2). The reason why smaller ablation volume when using 16G antennas or multistep protocols compared with 14G antennas or single-step protocols was water vaporization process due to the high heat generation from MWA due to more increased intra-hepatic pressure at latter conditions. These results may originate from the low power protocol (902 to 928 MHz) generator used in our experiment.28 However, there was no statistical difference in ablation volume between the multistep groups and single-step groups using a 14G antenna.
And actual temperature of cow liver tissue was inappropriate due to indirect method for warming. Even ex vivo study at room temperature produce ablation zone larger compared to in vivo. Thus, preheating the tissue is not warranted as it will further amplify the ablation zone compared to in vivo.
In this study, we found that varying settings of the MWA device could influence the pressure during ablation. The multistep method required a slightly lower pressure during ablation than the single-step method. That is, the single-step procedure requires continuous ablation until the entire targeted region becomes necrotic. In contrast, in the multistep procedure, the region of ablation is confined in each step.
There were several limitations to this study that have to be acknowledged. The first limitation concerns the bovine liver model. These livers were pure parenchyma and contained no tumor, and it is likely that there are differences between normal liver and tumor. As we used ex vivo bovine liver, we found it difficult to maintain body temperature because vapor produced during MWA leaked through vessels or fissure, exposing the cut surface of the liver. Therefore, a further in vivo study will be necessary. Second, we were also limited by the small sample size of each group, in which only 10 pieces of cow liver were used. If larger samples were used, more results may have been statistically significant. Third, we only used peak pressure measurement. Tracking the changes in pressure over time might reveal more about how the gas bubbles form. Third, the pressure was measured while the bovine liver was in open space, whereas previous ex vivo study used a sealed box.11 Finally, this study was limited by the use of technology still in development. RFA devices are evolving rapidly, and the MWA device used in this study are undergoing continued development. In particular, the MWA generator used in this experiment does not have a large ablation zone due to a low power protocol (902 to 928 MHz).28 Therefore, more research is needed to assess its mechanism of action in a more powerful MWA device.
In this study, we showed that the standard single-step method might entail a risk of an extreme increase in intratumoral pressure under some conditions, which could result in intrahepatic metastasis or rapid progression after the procedure. However, intratumoral pressure can be reduced by adopting an incremental, multistep method during MWA. We believe that this multistep method might be useful as a standard clinical procedure for MWA, after optimization of the MWA power.
In conclusion, MWA resulted in increases in intrahepatic pressure. Single-step methods resulted in larger pressure increases than multistep methods. Unfortunately, increased intrahepatic pressure could not be controlled using multistep methods. Further study is needed to prove that multistep methods may be able to be used to prevent increased intrahepatic pressure during MWA after applying the proper power.
ACKNOWLEDGEMENTS
This study was supported by Samsung Medical Center grant (#GFO1130071) and Samsung Biomedical Research Institute grant (#CB11261).
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Rossi S, Garbagnati F, Rosa L, Azzaretti A, Belloni G, Quaretti P. Radiofrequency thermal ablation for treatment of hepatocellular carcinoma. Int J Clin Oncol. 2002;7:225–235. doi: 10.1007/s101470200034. [DOI] [PubMed] [Google Scholar]
- 2.Tateishi R, Shiina S, Teratani T, et al. Percutaneous radiofrequency ablation for hepatocellular carcinoma: an analysis of 1000 cases. Cancer. 2005;103:1201–1209. doi: 10.1002/cncr.20892. [DOI] [PubMed] [Google Scholar]
- 3.Kotoh K, Enjoji M, Arimura E, et al. Scattered and rapid intrahepatic recurrences after radio frequency ablation for hepatocellular carcinoma. World J Gastroenterol. 2005;11:6828–6832. doi: 10.3748/wjg.v11.i43.6828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicoli N, Casaril A, Abu Hilal M, et al. A case of rapid intrahepatic dissemination of hepatocellular carcinoma after radiofrequency thermal ablation. Am J Surg. 2004;188:165–167. doi: 10.1016/j.amjsurg.2003.12.061. [DOI] [PubMed] [Google Scholar]
- 5.Shirato K, Morimoto M, Tomita N, et al. Hepatocellular carcinoma: a case of extrahepatic seeding after percutaneous radiofrequency ablation using an expandable needle electrode. Hepatogastroenterology. 2002;49:897–899. [PubMed] [Google Scholar]
- 6.Takada Y, Kurata M, Ohkohchi N. Rapid and aggressive recurrence accompanied by portal tumor thrombus after radiofrequency ablation for hepatocellular carcinoma. Int J Clin Oncol. 2003;8:332–335. doi: 10.1007/s10147-003-0328-6. [DOI] [PubMed] [Google Scholar]
- 7.Vergara Gómez M, Miquel Planas M, Gil Prades M, et al. Rapid progression of hepatocellular carcinoma after surgery: apropos of a case and review of the literature. Gastroenterol Hepatol. 2010;33:569–573. doi: 10.1016/j.gastrohep.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Portolani N, Tiberio GA, Ronconi M, et al. Aggressive recurrence after radiofrequency ablation of liver neoplasms. Hepatogastroenterology. 2003;50:2179–2184. [PubMed] [Google Scholar]
- 9.Seki T, Tamai T, Ikeda K, et al. Rapid progression of hepatocellular carcinoma after transcatheter arterial chemoembolization and percutaneous radiofrequency ablation in the primary tumour region. Eur J Gastroenterol Hepatol. 2001;13:291–294. doi: 10.1097/00042737-200103000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Kotoh K, Morizono S, Kohjima M, Enjoji M, Sakai H, Nakamuta M. Evaluation of liver parenchymal pressure and portal endothelium damage during radio frequency ablation in an in vivo porcine model. Liver Int. 2005;25:1217–1223. doi: 10.1111/j.1478-3231.2005.01167.x. [DOI] [PubMed] [Google Scholar]
- 11.Kotoh K, Nakamuta M, Morizono S, et al. A multi-step, incremental expansion method for radio frequency ablation: optimization of the procedure to prevent increases in intra-tumor pressure and to reduce the ablation time. Liver Int. 2005;25:542–547. doi: 10.1111/j.1478-3231.2005.01051.x. [DOI] [PubMed] [Google Scholar]
- 12.Kawamoto C, Yamauchi A, Baba Y, Kaneko K, Yakabi K. Measurement of intrahepatic pressure during radiofrequency ablation in porcine liver. J Gastroenterol. 2010;45:435–442. doi: 10.1007/s00535-009-0156-1. [DOI] [PubMed] [Google Scholar]
- 13.Brace CL. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: what are the differences? Curr Probl Diagn Radiol. 2009;38:135–143. doi: 10.1067/j.cpradiol.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shiozawa K, Watanabe M, Takahashi M, Wakui N, Iida K, Sumino Y. Analysis of patients with rapid aggressive tumor progression of hepatocellular carcinoma after percutaneous radiofrequency ablation. Hepatogastroenterology. 2009;56:1689–1695. [PubMed] [Google Scholar]
- 15.Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: principles and applications. Radiographics. 2005;25( Suppl 1):S69–S83. doi: 10.1148/rg.25si055501. [DOI] [PubMed] [Google Scholar]
- 16.Laeseke PF, Lee FT, Jr, Sampson LA, van der Weide DW, Brace CL. Microwave ablation versus radiofrequency ablation in the kidney: high-power triaxial antennas create larger ablation zones than similarly sized internally cooled electrodes. J Vasc Interv Radiol. 2009;20:1224–1229. doi: 10.1016/j.jvir.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knavel EM, Hinshaw JL, Lubner MG, et al. High-powered gas-cooled microwave ablation: shaft cooling creates an effective stick function without altering the ablation zone. AJR Am J Roentgenol. 2012;198:W260–W265. doi: 10.2214/AJR.11.6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lubner MG, Brace CL, Hinshaw JL, Lee FT., Jr Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol. 2010;21:S192–S203. doi: 10.1016/j.jvir.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eick OJ, Gerritse B, Schumacher B. Popping phenomena in temperature-controlled radiofrequency ablation: when and why do they occur? Pacing Clin Electrophysiol. 2000;23:253–258. doi: 10.1111/j.1540-8159.2000.tb00807.x. [DOI] [PubMed] [Google Scholar]
- 20.Livraghi T, Goldberg SN, Lazzaroni S, et al. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology. 2000;214:761–768. doi: 10.1148/radiology.214.3.r00mr02761. [DOI] [PubMed] [Google Scholar]
- 21.Iida H, Aihara T, Ikuta S, Yamanaka N. Effectiveness of impedance monitoring during radiofrequency ablation for predicting popping. World J Gastroenterol. 2012;18:5870–5878. doi: 10.3748/wjg.v18.i41.5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernandes ML, Lin CC, Lin CJ, Chen WT, Lin SM. Prospective study of a ‘popping’ sound during percutaneous radiofrequency ablation for hepatocellular carcinoma. J Vasc Interv Radiol. 2010;21:237–244. doi: 10.1016/j.jvir.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 23.Brace CL. Microwave ablation technology: what every user should know. Curr Probl Diagn Radiol. 2009;38:61–67. doi: 10.1067/j.cpradiol.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bui JT, Gaba RC, Knuttinen MG, et al. Microwave lung ablation complicated by bronchocutaneous fistula: case report and literature review. Semin Intervent Radiol. 2011;28:152–155. doi: 10.1055/s-0031-1280654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Baere T. New techniques of tumor ablation (microwaves, electroporation) J Radiol. 2011;92:789–795. doi: 10.1016/j.jradio.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 26.Dupuy DE. Microwave ablation compared with radiofrequency ablation in lung tissue-is microwave not just for popcorn anymore? Radiology. 2009;251:617–618. doi: 10.1148/radiol.2513090129. [DOI] [PubMed] [Google Scholar]
- 27.Jiao DC, Zhou Q, Han XW, et al. Microwave ablation treatment of liver cancer with a 2,450-MHz cooled-shaft antenna: pilot study on safety and efficacy. Asian Pac J Cancer Prev. 2012;13:737–742. doi: 10.7314/APJCP.2012.13.2.737. [DOI] [PubMed] [Google Scholar]
- 28.Hoffmann R, Rempp H, Erhard L, et al. Comparison of four microwave ablation devices: an experimental study in ex vivo bovine liver. Radiology. 2013;268:89–97. doi: 10.1148/radiol.13121127. [DOI] [PubMed] [Google Scholar]