Abstract
Solvents play an important role in protein folding, protein-protein associations, stability, and specificity of recognition as in the case of antibody-antigen interactions through hydrogen bonds. One of the underappreciated features of protein-associated waters is that it weakens inter- and intra-molecular interactions by modulating electrostatic interactions and influencing conformational changes. Such observations demonstrate the direct relationship between macroscopic solvent effects on protein-protein interactions and atom-scale solvent-protein interactions. Although crystallographic solvents do explain some aspects of solvent-mediated interactions, molecular simulation allows the study of the dynamic role of solvents. Thus, analysis of conformations from molecular simulations are employed to understand the role of solvent on the inherent polyspecificity of a Lewis Y reactive germline gene relative to its expanded hybridomas and a humanized anti-Lewis Y antibody. Our analysis reveals that solvent mediates critical contacts through charged residues to facilitate cross-reactivity to carbohydrate antigens, but also increases the flexibility of some anti-Lewis Y antibodies concomitant with mutations (amino acid substitutions) to the germline antibody. Such flexibility might better allow for recognition and binding of internal structures of extended carbohydrate structures on tumor cells.
Introduction
The first order function of immune molecules in the immune system is to distinguish self from non-self. Among regulatory antigens, glycans/carbohydrates emerge as post-translational modifications recognized by B cells(1) and sometimes T cells.(1,2) Carbohydrate antigens are complex and heterogeneous structural species. While various processes of antigen processing are associated with adaptive immunity, the first contact of glycans is with antibodies of germline lineage as part of the innate surveillance system.(3) Germline genes that define carbohydrate-reactive antibodies are known to sculpt antibody-combining sites containing key innate side-chain contacts that define the antigen recognition step.(4,5) Much like lectin-glycan interactions, antibody-glycan binding is typically stabilized in two ways: by hydrogen bonding between amino acids in the carbohydrate recognition domain and the glycan hydroxyl groups, and by Van der Waals packing of the hydrophobic glycan face against aromatic amino acid side chains. At the same time, the typical germline antibody paratope has evolved to accommodate diverse epitopes.(6,7) Flexibility in antigen recognition is a hallmark of the germline antibodies manifesting as polyspecificity. Among the various components of molecular interactions that define specific binding of antigens by antibodies, electrostatics is of special importance because of their long-range nature and their influence on polar or charged molecules.(8) Solvent plays a role in molecular recognition, modulating a direct relationship between macroscopic solvent effects on protein-protein interactions and atom-scale solvent-protein interactions. Water, the predominant solvent, mediated interactions are as important as direct hydrogen bonds in the stability and specificity of recognition in antibody-antigen interactions. Solvent also weakens interactions by modulating electrostatic interactions that can influence the flexibility of molecules. The inclusion of a solvent in the calculations reaffirms that low levels of electrostatic interactions are associated with conformational flexibility.(9)
Here, we further characterize how antibodies that are close in sequence to germline genes can distinguish glycans considering the effect of solvent on the recognition process. We are interested in directly testing the hypothesis of whether the processes of affinity maturation involve tailoring antibody flexibility and conformational heterogeneity in the context of solvation. We principally focus on the monoclonal antibody BR55-2 reactive with the neolactoseries antigen Lewis Y (LeY). The LeY antigen is a carbohydrate structure found on type 2 blood group chains of glycoproteins and glycolipids and long recognized as a potential target for immunotherapy because it is expressed in 70–90% of tumors of epithelial origin.(10,11) Originally detected and characterized as an oligosaccharide expressed on mucins of an ovarian cyst adenoma,(12) the LeY determinant is considered an oncofetal carbohydrate antigen related to ontogenesis,(13) cellular apoptosis,(14,15) and human cancer.(16) The LeY core is highly restricted in its conformational properties associated with the H-type 2 determinant, L-Fuc–α1, 2Gal-β1,4GlcNAc.(17) LeY shares structural similarities with the murine autoantigen antigen SSEA-1, defined as a Lewis X carbohydrate structure.(18) The carrier carbohydrate chain bearing these epitopes may vary in length. This difference in the length of the oligosaccharide side chain has important implications because certain cancer cells have the rather unique ability to synthesize extended type 2 chain antigens.(19,20)
Although solvents from crystallographic studies highlight the enthalpic component of the interactions, it does not provide a complete picture of solvents' dynamic nature; alternatively nuclear magnetic resonance (NMR) can provide insight. In the absence of any insight from NMR, we decided to test the hypothesis using molecular simulation. Therefore, we utilized molecular simulations to investigate the effect of an explicit solvent model, on the flexibility and modulation of the electrostatic interaction of BR55-2 in comparison to other anti-LeY antibodies to determine if solvent stabilizes the complex of antibodies upon LeY binding. BR55-2 is suggested to emerge from the VH7183.a13.20 germline for its heavy chain and Vκ cr1 for its light chain, displaying the least number of mutations of the several anti-LeY antibodies described in the literature.(21) BR55-2 was generated from mice immunized with MCF-7 human breast carcinoma cells and was identified to react with LeY.(22) In this context, it is of interest to determine what regulates BR55-2 specificity for the LeY/LeX nominal antigen. Not surprisingly, our studies suggest that solvent primarily modulates the electrostatic interactions between antibody and antigen, and in some cases solvent enhances the flexibility of a complex over its non-solvated model. We find that solvent increases the flexibility of BR55-2 relative to its germline, which might expand its antigen reactivity patterns and participates in further stabilizing the LeY core recognition through the addition of several hydrogen bonds.
Methods
Energy minimization and molecular dynamics (MD)
The starting structures for the solvated MD simulation were crystal structures and, in some cases, structures modeled in the lab as described previously.(21) Briefly, crystallographic water molecules, ions, and other heteroatoms except for the bound Lewis Y were removed. Hydrogen atom positions were assigned using the biopolymer module of Discovery Studio. Solvated MD was performed under periodic boundary conditions as an nPT ensemble where partial charges on atoms were eliminated. Each antibody-Lewis Y complex was placed in a box of TIP3P water molecules with a minimum distance between the solute surface and the box face of 8 Å. For each system, initial solvated conformations were subjected to 20,000 cycles of energy minimization consisting of 15,000 cycles of steepest descent (SD) and 5,000 cycles of conjugate gradient (CG), restraining the solute coordinates.
Simulated annealing was performed on the solvent box by heating the system to 300 K from 5 K in 25 ps steps, maintaining the system at 300 K for 25 ps and then annealed (i.e., cooling back the system) to 5 K over 25 ps steps. This was then followed by minimization (15,000 SD; 5,000 CG) of the entire system. Next, the entire system was re-simulated (reheated) from 5 to 300 K over 25 ps and maintained there for an equilibration (using the CPT algorithm – constant pressure of 1 atmosphere, Langevin piston mass of 1000 amu, constant temperature of 300 K) period of 250 ps. The standard 1–4 electrostatic and Van der Waals interactions were used with a weak harmonic restraint (restraint weight = 22) applied to all cα positions during MD simulations.(16) A 2 fs time step was used and long-range electrostatic interactions were computed using the particle mesh Ewald option, with a 10 Å cut-off for non-bonded interactions.(23)
The resulting ensemble of structures was evaluated for total energy and proximity to the initial starting structure based on an all-atom RMSD calculation. The lowest energy structure with low RMSD was selected for further analyses. The Van der Waals contacts for the final structures obtained from molecular dynamics were determined using a cut-off of 4 Å. The potential intermolecular hydrogen bonds were listed using a cut-off of 3.6 Å with the intermolecular hydrogen bond monitor in Discovery Studio and also visually confirmed.
The RMS fluctuation with respect to the average structure was also calculated for each antibody-LeY complex(21) and was compared against the germline behavior. Fluctuations of individual residues that bind to the cognate antigen was also calculated for each antibody-LeY complex and compared against each other.
Results
Solvation effects on the recognition for Lewis Y at the binding site
In vacuo analysis of binding profiles of lactoseries isomeric structures by BR55-2 suggest that the binding epitope includes the OH-4 and OH-3 groups of the β-D-galactose unit, the 6-CH3 groups of the two fucose units and the N-acetyl group of the subterminal β-D-N-acetylglucosamine (β-DglcNAc).(17) Specificity for LeY over the Lewis B (LeB) antigen stems from the relative spatial orientation of the β–DglcNAc moiety.(10) Based upon LeY-crystal structures and sequence homologies, a model for BR55-2 was constructed and the molecular complex of LeY and BR55-2 was further optimized.(21) In the absence of solvent, the BR55-2 makes polar contacts involving hydrogen-bonding residues that include the side-chain interactions of L-His 27D (Kabat numbering for CDR2) and H-Tyr 35 contacting Gal; side-chain interactions of L-Asn 28 contacting Fuc1; the side chain of H-Tyr33 and its backbone atoms contacting Nag along with H-Asp 97; and the backbone interactions of H-Tyr 96, H-Gly 99, and H-Ala 100 contacting Fuc2 (Table 1). In this context, a central feature of the BR55-2 paratope seems to be a pocket for the Gal moiety to be built as a main prerequisite for bridging the germ-line heavy and light chains. Built around this pocket, the side-chain hydrogen bonds involving L-Asn28 and H-Tyr33 define the recognition of the trisaccharide core Fuc(1,2)-Galβ1-2GlcNAc, whereas the Fuc2 structure is stabilized by a series of backbone interactions that are predicated on a non-canonical conformation of CDR3-H.
Table 1.
Intermolecular Hydrogen-bonding Scheme
| Residue contacts involving solvent | Residue contacts without solvent | Recognition unit of LeY tetrasaccharide core |
|---|---|---|
| L-Ser27E | Fuc1 | |
| L-Asn28 | L-Asn28 | Fuc1 |
| L-His27D | Fuc1 | |
| L-His27D | L His 27D | Gal |
| H-Tyr35 | H-Tyr35 | Gal |
| H-Tyr33 | H-Tyr33 | Nag |
| H-Asp97 | H-Asp97 | Nag |
| H-Tyr96 | H-Tyr96 | Fuc2 |
| H-Gly99 | H-Gly99 | Fuc2 |
| H-Ala100 | H-Ala100 | Fuc2 |
| H-Trp100A | Fuc2 |
BR55-2-LeY complex at binding site for MD simulations carried out with/without solvent environment; donor and acceptor atoms. Fuc1 denotes Fuc residue linked to Gal via the (α1->2) linkage; Fuc 2 denotes Fuc residue linked to GlcNAc via the (α1->3) linkage. Standard three-letter amino acid nomenclature and carbohydrate numbering scheme in use. Fuc(α1->2)Gal(β1->4)[Fuc(α1->3)]GlcNAc represents the Lewis tetrasaccharide determinant. Additional interactions with LeY in the solvated environment are shown in bold.
Solvation effects on interaction energy
The LeY recognition from in vacuo dynamics are largely preserved in the solvated complex, except for a few additional interactions involving L-Ser27E and L-His 27D interacting with Fuc1 and H-Trp 100a interacting with Fuc 2 (Table 1). Solvation dynamics results show that a large number of water molecules (TIP3P) interact with each unit of the LeY tetrasaccharide core. To further appreciate the general influence of solvent on anti-LeY antibodies, we performed dynamics calculations with the TIP3P model on other anti-LeY antibodies described in previous studies; the murine VL and the VH domains of the four antibodies considered here—BR55-2 (IgG3), B3 (IgG3), BR96 (IgG3), and mu3S193 (IgG3)—are highly homologous with the VH7183.a13.20/VH50.1 and Vκ cr1 germ-line gene families, and the humanized from of mu3S193, hu3S193, was also included. Simulating these antibodies with explicit water diminishes the electrostatic contribution to the overall total interaction energy, as expected (Table 2). The solvated models of huS193 and BR55-2 and the germ-line antibodies had no effect on the van der Waals contribution to the total energy. In contrast, the solvated models of BR96 and B3 showed increased van der Waals contribution, and muS193 showed reduced van der Waals contribution to the total energy.
Table 2.
Difference in Antigen-LeY Total Interaction Energy
| Antigen-antibody complex | Total interaction energy (in Kcal/mol) | Total Van der Waal's energy (in Kcal/mol) | Total electrostatic energy (in Kcal/mol) | KD (in M) |
|---|---|---|---|---|
| mu3S193 (solvent) | −159.00 | −11.49 | −147.53 | 5.3 × 10−9(38,39) |
| mu3S193 (non-solvent) | −226.87 | −15.18 | −211.69 | |
| hu3S193 (solvent) | −146.95 | −14.65 | −132.28 | 2.4 × 10−7(38,39) |
| hu3S193 (non-solvent) | −220.15 | −15.61 | −204.54 | |
| Germline (solvent) | −141.76 | −15.38 | −126.37 | N/A |
| Germline (non-solvent) | −212.84 | −15.25 | −197.59 | |
| B3 (solvent) | −139.02 | −20.21 | −118.81 | N/A |
| B3 (non-solvent) | −211.64 | −13.37 | −198.27 | |
| BR55-2 (solvent) | −117.53 | −11.41 | −106.12 | 8 × 10−6(40) |
| BR55-2 (non-solvent) | −188.16 | −10.54 | −177.62 | |
| BR96 (solvent) | −90.36 | −16.21 | −74.15 | 9.9 × 10−6(41) |
| BR96 (non-solvent) | −213.13 | −11.56 | −201.56 |
Calculations were conducted under solvated and unsolvated environments in MD simulations.(21) Total interaction energy is the sum of total Van der Waals energy and total electrostatic energy. Note that total Van der Waals energy component of total interaction energy remains optimized under both situations whereas total electrostatic interactions plays a major role in validating stability of the antibody-carbohydrate complex. Experimentally determined dissociation constant or KD values are listed to show robustness of in silico molecular calculations.
Further analysis of the energetic components of these anti-LeY antibodies with LeY in the presence of solvent also indicates that solvent diminishes the electrostatic contribution for each of the antibodies. In contrast, the van der Waals energy deviates from 1–7 Kcal/mol among the solvent/in vacuo complexes. From the interaction energy calculations, it is observed that the van der Waals interactions are still relatively optimized when compared to in vacuo calculations. This suggests that maturation process is not necessarily consistent in how it optimizes the electrostatic components mediated by solvent.
To validate our in silico observations, we have compared the interaction energy with experimentally determined dissociation constants (KD) (Table 2). It is important to review KD as it correlates with the functionality of the antibody. Our studies show that the total interaction energy of the unsolvated complex follows the trend of experimental KD ranking of antibodies (i.e., muS193>huS193>BR96>BR55-2). Unexpectedly, with inclusion of the solvents, the KD trend for BR55-2 and BR96 are reversed. The effect of the solvents was reflected in the reduction of total energy of interaction ranging from 67–73 Kcal/mol for mu3S193, hu3S193, germline, B3, and BR55-2; the decrease was much larger for BR96 (123 Kcal/mol) than for BR55-2 (-70.63 Kcal/mol). The large energy difference in BR96 is due to unfavorable steric interactions at the binding site, which arise from interacting residues belonging to BR96 under solvated conditions and reorganization of packing interactions since solvent seems to have increased the Van der Waals interaction in the LeY interaction by almost 6 Kcal/mol (Table 2).
Solvation effects on antibody complex flexibility
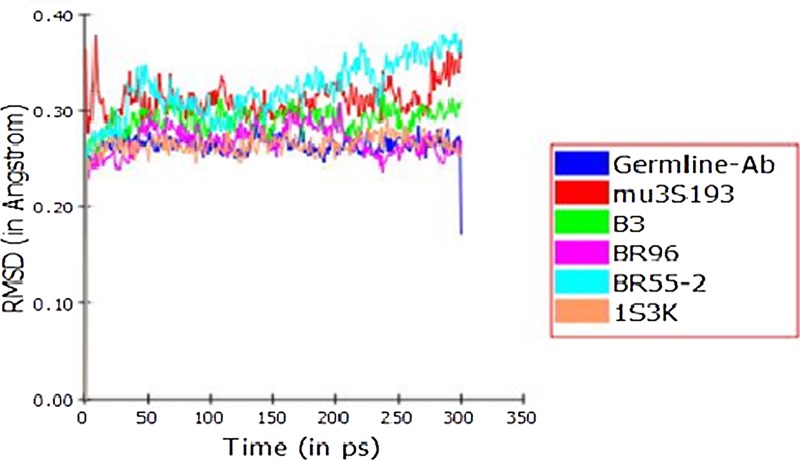
Following the discordant energetics between calculated and experimental studies, to determine if the explicit solvation model lends to increased entropy, we compared the degree of conformational diversity in the intermediate states of the antibodies generated during the MD simulation. Analyzing the backbone RMSD from the average structure over the course of the trajectory illustrates an antibody's stability and plasticity. The RMS fluctuations for the mature antibody-LeY complexes were compared individually with respect to the RMS fluctuation curve of the germ-line antibody-LeY complex over time. As shown in Figure 1, the germ-line complex displayed relatively smaller atomic fluctuations (RMS 0.25Å–0.28 Å) than those in the mature LeY complex (0.23–0.39 Å). The humanized 3s193 antibody and the germ-line antibody overlap and B3 and BR96 display smaller atomic flexibility.
FIG. 1.
RMS fluctuations of germ-line and mature antibody-LeY complexes in solvated environment. RMS fluctuations are calculated after superimposing the backbone atoms of each conformer (as generated from the MD production run) against the average structure for each antibody-LeY complex.
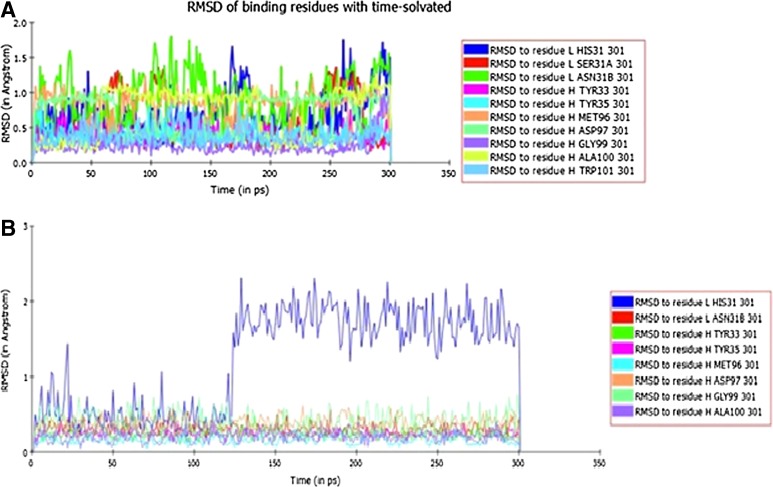
The RMS fluctuations of individual binding residues were also calculated for BR55-2 complexed with LeY in its physiological (solvated) and in vacuo environment (Fig. 2). The purpose of this analysis was to investigate the contribution of individual residues in binding LeY under different simulation conditions. While there are additional binding interactions at the molecular level, the epitope largely remains conserved for both the conditions. Under solvated conditions, overall fluctuations show a stable behavior. Light chain binding residues (L:His 27D, Ser 27E, and Asn 28) exhibited wider fluctuations compared to their heavy chain counterparts, suggesting that light chain CDR residues at the binding site are more susceptible to domain adjustment when binding to the cognate antigen under solvation. Light chain residue His 27D is distinct from the rest of the binding site residues in its wider distribution of RMS fluctuations (0.3–2.1 Å) under in vacuo simulation conditions; perhaps by having histidine and depending on its protonation state in CDR2L in Lewis Y, it may also recognize Fuc moiety (Table 1). Also, the contribution of other binding residues of BR55-2 in stabilizing interaction with LeY is unequivocally established from this analysis.
FIG. 2.
Residue RMS deviations. RMS fluctuations of individual residues of BR55-2 with respect to their average structure that participates in contacting Lewis Y. (A) Under solvated conditions (B) under in vacuo conditions (L:His 31 is L:His 27D, L:SER 31A is L:SER 27E, L:ASN 31B is L:ASN 28 in Kabath nomenclature).
Specificity of BR55-2 for glycan array
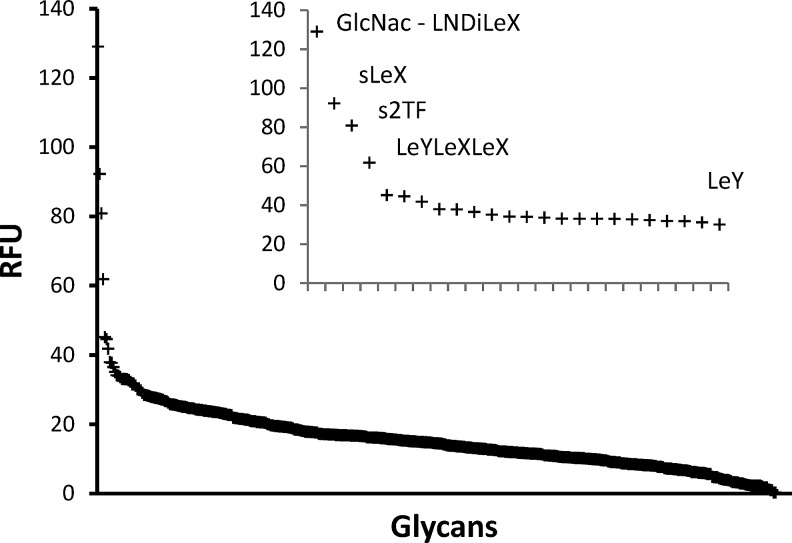
Glycans often achieve biologically significant binding via multivalent avidity, with their interaction involving more than one pair of partners in close physical proximity. The increased flexibility of BR55-2 might translate to interacting with elongated forms of Galβ1- >4GlcNAc structures on the tumor surface. To test this hypothesis, a glycan array screening experiment was performed with BR55-2 at The Consortium for Functional Glycomics. The possible limitations of glycan arrays might be a restricted flexibility in terms of assay. In keeping with the suggested flexibility of BR55-2 in solvent, BR55-2 appears to recognize a host of glycans with the Galβ1- >4GlcNAc structure, however BR55-2 appears to bind to s2FT that has a Galβ1-3(Neu5Acα2-6)GalNAc structure. The glycan array data (Table 3, Fig. 3) shows that it has high binding specificity measured in terms of relative fluorescence unit (RFU) to GlcNac—LNDiLeX, s2TF (disialylated Thomsen-Friedenreich antigen), LeYLeXLeX, and GM1-like antigens, types I, II, and IIH precursors. It has been observed that the molecular representation of these glycans bearing the Galβ1- >4GlcNAc and Galβ1- >3GalNAc signature are very similar as is observed in the super positioning Lewis b and Lewis Y isomers.(17) Therefore, a change in the linkage from 1- >3 to 1- >4 does not alter the shared molecular topology, and BR55-2 easily accommodates both linkages.(17) Most notably, BR55-2 recognizes these glycans as extended structures, either as larger moieties than the core tetrasaccharide or with extended linker on the glycan array, which affects conformational presentation of the glycan.(24)
Table 3.
BR55-2 Binding to Top Glycans in Glycan Array Screening
| Glycan no. | Name of glycan | Glycan | RFU | % CV |
|---|---|---|---|---|
| 1 | GlcNac-LNDiLeX | GlcNAcα1-4Galβ1-4GlcNAcb1-3Galβ1-4(Fucα1-3)GlcNAcb1-3Galβ1-4(Fucα1-3)GlcNAcb-Sp0 | 129 | 43 |
| 2 | sLeX | Neu5Acα2-3Galβ1-4(Fucα1-3)GlcNAcβ1-3GalNAcα-Sp14 | 92 | 38.14 |
| 3 | s2TF | Neu5Acα2-3Galβ1-3(Neu5Acα2-6)GalNAcα-Sp14 | 81 | 46.97 |
| 4 | LeYLeXLeX | Fucα1-2Galβ1-4(Fucα1-3)GlcNAcβ1-3Galβ1-4(Fucα1-3)GlcNAcβ1-3Galβ1-4(Fucα1-3)GlcNAcβ-Sp0 | 62 | 49.86 |
| 11 | LeY | Fucα1-2Galβ1-4(Fucα1-3)GlcNAcβ-Sp0 | 24 | 4.15197 |
LeY has been included for comparison purpose. Glycans are ranked in order of highest binding specificity to BR55-2.
FIG. 3.
Polyspecificity of BR55-2. Distribution of intensity of BR55-2 binding to glycans in glycan array screening experiment. Inset shows data for the top binding glycans. Apparently, presentation greatly affects the binding since LeY alone was bound with much lower affinity than the longer chain of the complex glycan LeYLeXLeX.
Discussion
Molecular recognition processes depend on the ability of the molecules to adapt structural complementarity to increase the binding affinity to their interacting partner. Conformational flexibility is an inherent feature of proteins and is essential to structurally reorganize and achieve maximal complementarity. While shape complementarity is essential, a biophysical characteristic that distinguishes polyspecificity from specificity is flexibility.(25) Decreased flexibility is therefore, in principle, associated with increased specificity and affinity for an antigen. Thus, affinity maturation of antibodies involves an increase in antibody specificity and affinity for a given antigen as antibodies expand from a germ-line origin.(26,27) Affinity maturation involves the evolution of an antibody paratope to reduce its inherent flexibility and toward epitope complementarity, resulting in a well-defined binding site for the immunogen in mature antibodies.(28–30)
Despite several studies on antibody-antigen complexes, the functional role and the structural basis of conformational change in antibody binding, in particular anti-carbohydrate antibodies, remains unpredictable, and the structural and thermodynamic determinants of antibody specificity and affinity are still not completely understood. The BR55-2 monoclonal antibody is very close in sequence to its germ-line gene, yet solvent affects its flexibility more so than the germline. BR55-2 specifically detected glycolipids with the Y determinant Fuc-α 1- >2Gal-βl- >4GlcNAc (3- >1 alpha Fuc)-β1- >3Galβ1- >4Glcβ1- >1 Cer and the Y-related B-active difucosylated determinant Gal-α1- >3Gal(2- >1α-Fuc) β1- >4GlcNAc(3- >1-α-Fuc) β-1- >3Gal β-1- >4Glc β-1- >1 Cer, but was suggested not to be reactive with related monofucosylated glycolipids of type 2 chain (X-antigen, blood group H), type 1 chain (LeA antigen, blood group H and B), or with difucosylated type 2 and type 1 chain structures (A blood group antigen or blood group B and LeB, respectively).
The availability of crystal structures (found in the public repository of crystal structures, Protein Data Bank) of different antibody complexes against the same antigen, but preserving the germ-line signature, has enabled the mapping of antigenic determinants and the fine specificity for LeY epitopes. While the crystal structures provide a detailed understanding of the complex, it provides limited information on the dynamic nature of the complex. Molecular simulations provide the complementing view for a molecular level interpretation of the changes induced by affinity maturation of an antibody. Molecular dynamics simulations of Fab fragments of antibodies reflect different levels in the maturation process and reveal a network of residue interactions that mediate the flexibility changes accompanying maturation.(25,31) Besides structural flexibility, water molecules play an important role in the complex formation by acting as an intermediate of hydrogen bonds between proteins.
It is well known that structures in the crystal and in solution differ in several important respects, such as radius of gyration, solvent accessible surface, intramolecular hydrogen bonds, and orientation of surface side chains. Since single water molecules may act as intermediates of hydrogen bonds to alter the entropic contribution of the complex formation, it is imperative to include solvent in the simulation to understand flexibility of an antibody. In fact, flexible protein simulated in vacuo tends to squeeze and maximize the intramolecular contacts. Solvation plays an important role in stabilizing carbohydrate-protein interactions at the binding site, as has also been observed in the LeY reactive antibody repertoire. It is well known that polar interactions including salt bridges that are electrostatically driven limit flexibilities through geometric constraints. As the free energy of binding is enthalpy driven, there is always an enthalpic gain and less entropic penalty for solvation at the binding cavity.(23,32)
Molecular mechanisms underlying conformational shifts are largely controlled by alterations within the H-bond network, the link between structure, and network rigidity, and dynamics remain largely nebulous, presumably due to the non-additive and long-range nature of network rigidity.(33) Therefore, while solvent often participates in defining the recognition elements for antigen binding of antibodies, it might play a role to restrict the flexibility of antibodies or it might increase flexibility due to mediating particular energetic considerations (e.g., dielectric effect). Molecular models generated from solvated simulation dynamics give an estimation of the antigenic interaction at the binding pocket. Positional fluctuations in the RMS curve for individual binding residues help us form an idea about their temporal behavior under solvation conditions and provide important evidence to altered electrostatics and lower binding enthalpy. Even though the epitope is largely preserved, there are additional interactions and minor variations at the atomic level. This can be attributed to the electrostatic component of the time-dependent interaction energy, altered by pocket water molecules.
A comparison of in vacuo and solvation dynamics calculations strongly suggest that polar contacts including hydrogen bonds and salt bridges are important features required for specificity of LeY. BR55-2 and mu3s193 are more flexible compared to their antibody counterparts. For mu3s193, this result is rather surprising since this complex is observed to be the most stable of all the complexes (Table 2). In the absence of solvation, BR55-2 and mu3S193 displayed restricted fluctuations(21) whereas the trend is reversed in the presence of solvent. For mu3S193, the reduced van der Waals interaction (5 Kcal/mol; see Table 2) under solvated conditions might lend to it being more flexible because hydrophobic interactions in the core of a protein plays a key role in enhancing protein stability. In the case of BR55-2, it is unclear what the source of flexibility might be. It is possible that increased flexibility results in part from reduced interaction of charged and/or polar residues with BR55-2 because of the dielectric screening by solvent. The dependence of flexibility on solvation of charged residues suggest a possible mechanism for increased polyspecificity of some antibodies after treatment with low pH.(34) It also draws attention to the fact that the changes in paratope flexibility depend on the context (solvation, electrostatics, etc.) so the results of the affinity maturation are dependent on this context. Thus, a mature antibody put in an environment of different context may prove with a (partially) reverted flexibility despite the results of the microevolution.
Our study suggests that the LeY reactive antibody repertoire preserves the epitope imprint in their germ-line state, which is passed on to the mature antibody forms with improved affinity to the cognate antigen following the accumulation of amino acid residue mutations; this accumulation of mutations impacts the electrostatic interaction contribution to specificity while keeping the non-polar contribution within constant levels (∼20 Kcal). The ambiguous nature of the fate of these clones, leading to the accumulation of some somatic mutations as well as their TI-2 (thymus independent type 2) character, precludes general conclusions about the process of affinity maturation. No clear trend is observed as to the flexibility of the antibody paratope and the number of mutations and BR55-2 are still relatively polyspecific. At the same time, the few somatic mutations do optimize for the interaction with the LeY antigen, suggesting that either these B cells have gone through only a transient follicular cycle, or a low intensity mutation and selection process outside germinal centers through many cycles of restimulation yielded the desired specificity. It is interesting to what extent such a hypothetical process contributes to self/non-self discrimination of carbohydrate epitopes by modulating structural flexibility and electrostatic interactions with the LeY antigen.
“Weak” interactions are commonplace and water molecules readily adapt to variations in protein-ligand interactions. Detailed structural interpretation of protein-ligand interactions requires consideration of a complex set of many subtle interactions, especially where modifications of interacting groups are to be analyzed. Solvent interactions can reduce Van der Waals interactions, which might result in increased movement of internal groups and therefore increase the flexibility of a complex. In this context, we suggest that solvent might act both as a “plasticizer” to increase flexibility and as a solvent to affect dielectrically screen electrostatic interactions between charged and/or polar residues within an antibody.
It is generally believed that high expression of aberrant glycosylation can protect cells from external assault, such as cell-mediated cytotoxicity from immune cells.(35) Depending on the distribution of a glycan, glycolipid, or glycoprotein, antibodies display different functional properties, which reflect how far away from the membrane surface the glycan is presented.(36) Mammalian glycan chain elongation is mostly based on extending the type 2 chain, Galβ1-4GlcNAc, whereas the corresponding type 1 chain, Galβ1-3GlcNAc, is not normally extended.(19,20) Different monoclonal antibodies to LeY display differing LeY recognition patterns, with those that recognize extended structures being more discriminate against tumor tissue than normal tissue.(37) Consequently, BR55-2, being more flexible, might in fact be more discriminatory toward tumor cells because of LeY presentation.
In summary, these results suggest that even though the molecular footprint for antigen recognition does not change among the murine and human antibodies, there are readjustment processes at the molecular level within the three dimensional structure of the antibodies under solvated environment. Flexibility at the antigen-binding site, mediated by water structures, is an essential element during the evolution of antigen recognition, in particular, involving antigens such as carbohydrates. Polyspecificity during this process is poorly understood. Our analysis suggests that water may play a dynamic and intermediate role in this process, highlighting the importance of the consideration of solvent to improve the success of the structure-based design of antigens by detailed structural characterization of the subtle interactions characteristic of antibodies, including any complicating features such as flexibility.
Acknowledgment
This work was supported by a Clinical Translational Award from the Department of Defense Breast Cancer Program (W81XWH-06-1-0542). We wish to acknowledge and thank the Consortium for Functional Glycomics for screening BR55-2 against the glycan array developed under NIH support (grant nos. GM62116 and GM098791).
Author Disclosure Statement
The authors have no financial interests to disclose.
References
- 1.Purcell AW, van Driel IR, and Gleeson PA: Impact of glycans on T-cell tolerance to glycosylated self-antigens. Immunol Cell Biol 2008;86:574–579. doi: 510.1038/icb.2008.1048. Epub 2008 Jul 1015. [DOI] [PubMed] [Google Scholar]
- 2.Schneider C, Smith DF, Cummings RD, Boligan KF, Hamilton RG, Bochner BS, Miescher S, Simon HU, Pashov A, Vassilev T, and von Gunten S: The human IgG anti-carbohydrate repertoire exhibits a universal architecture and contains specificity for microbial attachment sites. Sci Transl Med 2015;7:269ra261. doi: 210.1126/scitranslmed.3010524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartz-Albiez R: Naturally occurring antibodies directed against carbohydrate tumor antigens. Adv Exp Med Biol 2012;750:27–43. 10.1007/1978-1001-4614-3461-1000_1003. [DOI] [PubMed] [Google Scholar]
- 4.Evans DW, Muller-Loennies S, Brooks CL, Brade L, Kosma P, Brade H, and Evans SV: Structural insights into parallel strategies for germline antibody recognition of lipopolysaccharide from Chlamydia. Glycobiology 2011;21:1049–1059. Epub 2011 May 1044. [DOI] [PubMed] [Google Scholar]
- 5.Gerstenbruch S, Brooks CL, Kosma P, Brade L, Mackenzie CR, Evans SV, Brade H, and Muller-Loennies S: Analysis of cross-reactive and specific anti-carbohydrate antibodies against lipopolysaccharide from Chlamydophila psittaci. Glycobiology 2010;20:461–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cameron DJ, and Erlanger BF: Evidence for multispecificity of antibody molecules. Nature 1977;268:763–765 [DOI] [PubMed] [Google Scholar]
- 7.Zhou ZH, Zhang Y, Hu YF, Wahl LM, Cisar JO, and Notkins AL: The broad antibacterial activity of the natural antibody repertoire is due to polyreactive antibodies. Cell Host Microbe 2007;1:51–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ren P, Chun J, Thomas DG, Schnieders MJ, Marucho M, Zhang J, and Baker NA: Biomolecular electrostatics and solvation: a computational perspective. Q Rev Biophys 2012;45:427–491. doi: 410.1017/S003358351200011X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinha N, Mohan S, Lipschultz CA, and Smith-Gill SJ: Differences in electrostatic properties at antibody-antigen binding sites: implications for specificity and cross-reactivity. Biophys J 2002;83:2946–2968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shetye J, Christensson B, Rubio C, Rodensjo M, Biberfeld P, and Mellstedt H: The tumor-associated antigens BR55-2, GA73-3 and GICA 19-9 in normal and corresponding neoplastic human tissues, especially gastrointestinal tissues. Anticancer Res 1989;9:395–404 [PubMed] [Google Scholar]
- 11.Steplewski Z, Lubeck MD, Scholz D, Loibner H, McDonald Smith J, and Koprowski H: Tumor cell lysis and tumor growth inhibition by the isotype variants of MAb BR55-2 directed against Y oligosaccharide. In Vivo 1991;5:79–83 [PubMed] [Google Scholar]
- 12.Lloyd KO, Kabat EA, Layug EJ, and Gruezo F: Immunochemical studies on blood groups. XXXIV. Structures of some oligosaccharides produced by alkaline degradation of blood group A, B, and H substances. Biochemistry 1966;5:1489–1501 [DOI] [PubMed] [Google Scholar]
- 13.Miyake M, Zenita K, Tanaka O, Okada Y, and Kannagi R: Stage-specific expression of SSEA-1-related antigens in the developing lung of human embryos and its relation to the distribution of these antigens in lung cancers. Cancer Res 1988;48:7150–7158 [PubMed] [Google Scholar]
- 14.Hiraishi K, Suzuki K, Hakomori S, Adachi M: Le(y) antigen expression is correlated with apoptosis (programmed cell death). Glycobiology 1993;3:381–390 [DOI] [PubMed] [Google Scholar]
- 15.Yamada T, Ohwada S, Saitoh F, Adachi M, Morishita Y, and Hozumi M: Induction of Ley antigen by 5-aza-2'-deoxycytidine in association with differentiation and apoptosis in human pancreatic cancer cells. Anticancer Res 1996;16:735–740 [PubMed] [Google Scholar]
- 16.Yin BW, Finstad CL, Kitamura K, Federici MG, Welshinger M, Kudryashov V, Hoskins WJ, Welt S, and Lloyd KO: Serological and immunochemical analysis of Lewis y (Ley) blood group antigen expression in epithelial ovarian cancer. Int J Cancer 1996;65:406–412 [DOI] [PubMed] [Google Scholar]
- 17.Blaszczyk-Thurin M, Murali R, Westerink MA, Steplewski Z, Co MS, and Kieber-Emmons T: Molecular recognition of the Lewis Y antigen by monoclonal antibodies. Protein Eng 1996;9:447–459 [DOI] [PubMed] [Google Scholar]
- 18.Hirashima K, Zenita K, Takada A, Kitahara A, Ishihara G, Harada R, Ohmori K, Hirohashi S, Kyoizumi S, Akiyama M, et al. : High idiotypic connectivity of the VH7183-encoded antibodies directed to a murine embryonic carbohydrate antigen, Lewis Y, as ascertained by syngenic anti-idiotype monoclonal antibodies. J Immunol 1990;145:224–232 [PubMed] [Google Scholar]
- 19.Fan YY, Yu SY, Ito H, Kameyama A, Sato T, Lin CH, Yu LC, Narimatsu H, and Khoo KH: Identification of further elongation and branching of dimeric type 1 chain on lactosylceramides from colonic adenocarcinoma by tandem mass spectrometry sequencing analyses. J Biol Chem 2008;283:16455–16468. doi: 16410.11074/jbc.M707274200. Epub 707272008 Apr 707274215. [DOI] [PubMed] [Google Scholar]
- 20.Kannagi R, Levery SB, and Hakomori S: Lea-active heptaglycosylceramide, a hybrid of type 1 and type 2 chain, and the pattern of glycolipids with Lea, Leb, X (Lex), and Y (Ley) determinants in human blood cell membranes (ghosts). Evidence that type 2 chain can elongate repetitively but type 1 chain cannot. J Biol Chem 1985;260:6410–6415 [PubMed] [Google Scholar]
- 21.Saha S, Pashov A, Siegel ER, Murali R, and Kieber-Emmons T: Defining the recognition elements of Lewis Y-reactive antibodies. PLoS One 2014;9:e104208. doi: 104210.101371/journal.pone.0104208. eCollection 0102014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blaszczyk-Thurin M, Thurin J, Hindsgaul O, Karlsson KA, Steplewski Z, and Koprowski H: Y and blood group B type 2 glycolipid antigens accumulate in a human gastric carcinoma cell line as detected by monoclonal antibody. Isolation and characterization by mass spectrometry and NMR spectroscopy. J Biol Chem 1987;262:372–379 [PubMed] [Google Scholar]
- 23.Kadirvelraj R, Foley BL, Dyekjaer JD, and Woods RJ: Involvement of water in carbohydrate-protein binding: concanavalin A revisited. J Am Chem Soc 2008;130:16933–16942. doi: 16910.11021/ja8039663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grant OC, Smith HM, Firsova D, Fadda E, and Woods RJ: Presentation, presentation, presentation! Molecular-level insight into linker effects on glycan array screening data. Glycobiology 2014;24:17–25. doi: 10.1093/glycob/cwt1083. Epub 2013 Sep 1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zimmermann J, Romesberg FE, Brooks CL, 3rd, and Thorpe IF: Molecular description of flexibility in an antibody combining site. J Phys Chem B 2010;114:7359–7370. doi: 7310.1021/jp906421v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sagawa T, Oda M, Ishimura M, Furukawa K, and Azuma T: Thermodynamic and kinetic aspects of antibody evolution during the immune response to hapten. Mol Immunol 2003;39:801–808 [DOI] [PubMed] [Google Scholar]
- 27.Kang J, and Warren AS: Thermodynamic analysis of additivity between the heavy and light chains in affinity maturation of an antibody. Mol Immunol 2008;45:304–305 [DOI] [PubMed] [Google Scholar]
- 28.Wedemayer GJ, Patten PA, Wang LH, Schultz PG, and Stevens RC: Structural insights into the evolution of an antibody combining site. Science 1997;276:1665–1669 [DOI] [PubMed] [Google Scholar]
- 29.Manivel V, Sahoo NC, Salunke DM, and Rao KV: Maturation of an antibody response is governed by modulations in flexibility of the antigen-combining site. Immunity 2000;13:611–620 [DOI] [PubMed] [Google Scholar]
- 30.Acierno JP, Braden BC, Klinke S, Goldbaum FA, and Cauerhff A: Affinity maturation increases the stability and plasticity of the Fv domain of anti-protein antibodies. J Mol Biol 2007;374:130–146. Epub 2007 Sep 2011. [DOI] [PubMed] [Google Scholar]
- 31.Thorpe IF, and Brooks CL, III: Molecular evolution of affinity and flexibility in the immune system. Proc Natl Acad Sci USA 2007;104:8821–8826. Epub 2007 May 8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tempel W, Tschampel S, and Woods RJ: The xenograft antigen bound to Griffonia simplicifolia lectin 1-B(4). X-ray crystal structure of the complex and molecular dynamics characterization of the binding site. J Biol Chem 2002;277:6615–6621. Epub 2001 Nov 6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li T, Tracka MB, Uddin S, Casas-Finet J, Jacobs DJ, and Livesay DR: Redistribution of flexibility in stabilizing antibody fragment mutants follows Le Chatelier's principle. PLoS One 2014;9:e92870. doi: 92810.91371/journal.pone.0092870. eCollection 0092014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Djoumerska-Alexieva IK, Dimitrov JD, Voynova EN, Lacroix-Desmazes S, Kaveri SV, and Vassilev TL: Exposure of IgG to an acidic environment results in molecular modifications and in enhanced protective activity in sepsis. FEBS J 2010;277:3039–3050. doi: 3010.1111/j.1742-4658.2010.07714.x. Epub 02010 Jun 07718. [DOI] [PubMed] [Google Scholar]
- 35.Madsen CB, Lavrsen K, Steentoft C, Vester-Christensen MB, Clausen H, Wandall HH, and Pedersen AE: Glycan elongation beyond the mucin associated Tn antigen protects tumor cells from immune-mediated killing. PLoS One 2013;8:e72413. doi: 72410.71371/journal.pone.0072413. eCollection 0072013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ragupathi G, Liu NX, Musselli C, Powell S, Lloyd K, and Livingston PO: Antibodies against tumor cell glycolipids and proteins, but not mucins, mediate complement-dependent cytotoxicity. J Immunol 2005;174:5706–5712 [DOI] [PubMed] [Google Scholar]
- 37.Kim YS, Yuan M, Itzkowitz SH, Sun QB, Kaizu T, Palekar A, Trump BF, and Hakomori S: Expression of LeY and extended LeY blood group-related antigens in human malignant, premalignant, and nonmalignant colonic tissues. Cancer Res 1986;46:5985–5992 [PubMed] [Google Scholar]