Summary
Until a few years ago, the possibility that glucose-lowering drugs affect glucose metabolism and fracture risk was not even considered. The increased incidence of fractures with thiazolidinediones in women was a causal finding. This phenomenon, which has been demonstrated by large-scale clinical trials, is associated with a reduction in bone density. Thiazolidinediones stimulate adipocyte differentiation, and inhibit osteoblast differentiation, from bone marrow stromal cells; other mechanisms could also be involved in the thiazolidinedione-induced reduction of bone density. Insulin has an anabolic effect on the bone, but it is nonetheless associated with an increased incidence of fractures in observational studies. Although this finding could be partly due to unaccounted confounders, it is likely that insulin-induced hypoglycemia, and consequent falls, produce a higher risk for fractures, at least in the elderly. Among older drugs, metformin and sulfonylureas do not appear to produce any beneficial or detrimental effects on the bone. Of newer agents, DPP4 inhibitors have been associated with a possible protective effect in earlier trials, but this result has not been confirmed in larger scale studies on patients with a higher level of comorbidities. Considering that the increase in active incretin levels determined by DPP4 inhibitors could theoretically improve bone density, further clinical studies are needed to assess more clearly the effect of this class of drugs. GLP-1 receptor agonists also increase bone density in experimental models, but human data are still insufficient to draw any conclusion.
Keywords: diabetes, bone fractures, osteoporosis
Although drugs for type 2 diabetes are prescribed for their effects on blood glucose, it has been known for a long time that they may have some relevant (either beneficial or detrimental) effect on extra-glycemic parameters – such as body weight, blood pressure, or lipids. However, until a decade ago the possibility that treatments for diabetes interfere with bone metabolism had not even been considered. The unexpected finding that thiazolidinediones reduce bone density and increase the risk of fractures opened a new scenario, prompting novel experimental and clinical research. This issue is clinically relevant, as the prevalence of type 2 diabetes increases with age, with the consequence that many patients receiving hypoglycemic treatments are the age range at greatest risk for bone fractures.
The bad guys: thiazolidinediones
The increased risk of bone fractures with thiazolidinediones was first reported in 2006, as an unexpected finding of ADOPT, a large-scale trial comparing rosiglitazone with metformin and glyburide monotherapy in patients with recently diagnosed type 2 diabetes (1). The principal endpoint of the study was long-term (5-year) glycemic control, and bone fractures were reported as adverse events. Women randomized to rosiglitazone showed a two-fold increase in the incidence of bone fractures in comparison with other treatment groups, whereas no difference was found in men. The increase in risk was statistically significant only in post-menopausal women, but the number of events in pre-menopausal subjects was very low (1). Post-hoc analyses showed that the increase in fracture risk became evident after the second year from randomization, with treatment groups progressively diverging afterwards (2).
When this phenomenon was reported, rosiglitazone had already been on the market for almost a decade, and in many countries it was one of the most widely used drugs for type 2 diabetes. No signal of risk had emerged during registration studies, post-marketing surveillance or subsequent trials. This provides a clear example of how a clinically relevant side effect with a long latency, if unexpected, can remain unnoticed for quite a long time, until a large-scale trial with sufficient duration is performed.
Curiously, the adverse effect can still remain undetected even after such a trial is available. A cardiovascular outcome study of adequate size and duration had been performed with pioglitazone, another drug of the same class, in 2005, but no data on bone fractures had been reported (3). A post-hoc analysis after the publication of ADOPT revealed that pioglitazone had the same effect on fractures of rosiglitazone: a doubling of risk in women, with no difference in men (4).
Those findings from clinical trials designed for other purposes prompted specifically focused experimental and clinical research. Observational studies showed that, in women, treatment with thiazolidinediones is associated with a reduced bone density (5). Several small clinical trials, performed either with pioglitazone or rosiglitazone, confirmed this phenomenon (6). Interestingly, this effect was observed also in pre-menopausal women, suggesting that the detrimental action of thiazolidinediones on bone density is independent of estrogen status (5).
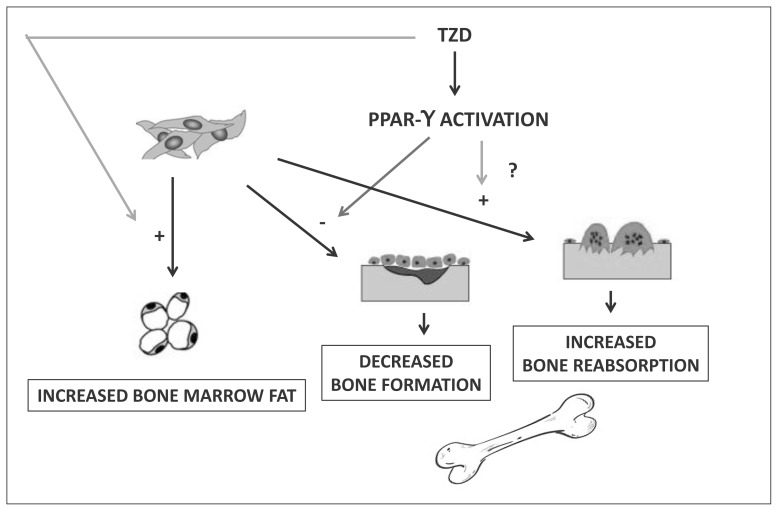
Several studies have been performed to identify the mechanisms responsible for thiazolidinedione-induced reduction of bone density (Figure 1). Thiazolidinediones are agonists of the nuclear receptor PPAR-γ, which regulates the expression of many different genes, including several loci involved in the regulation of glucose metabolism; this results in an increase of insulin sensitivity, which is believed to be the main mechanism of the glucose-lowering action of these drugs (7). Another effect of PPAR-γ activation is the stimulation of adipocyte differentiation (8). This latter action has been demonstrated in bone marrow stromal cells, which are induced by thiazolidinediones toward adipocyte, rather than osteoblastic, differentiation (9, 10). Interestingly, this action is inhibited by testosterone, accounting for the apparent lack of effect of thiazolidinediones on bone density and fracture risk in men (9). The disruption of the normal pattern of differentiation of bone marrow stromal cells determined by pharmacologic activation of PPAR-γ could lead to reduced osteoblastogenesis, accounting for decreased bone density, associated to augmented formation of bone marrow fat. In a placebo-controlled randomized trial, pioglitazone increased bone marrow fat measured with a magnetic resonance technique (11).
Figure 1.
Mechanisms responsible for thiazolidinedione-induced reduction of bone density.
Other mechanisms, beside the alteration of adipocyte and osteoblast differentiation, could be involved in the detrimental action of thiazolidinediones on bone metabolism. An increase of markers of bone turnover has been reported with both pioglitazone and rosiglitazone, compared with either metformin or placebo in randomized trials (12, 13), although some studies disagree (14). It is possible that the reduction in bone density is partly due to increased bone reabsorption, as suggested by the higher number of circulating osteoclast precursors in women treated with thiazolidinediones (15). In addition, one study also reported an increase in calcium excretion rate and an increase in parathormone levels in association with pioglitazone treatment (14), but this finding has not been replicated so far.
Although the mechanism remains controversial, the increase in fracture risk determined by long-treatment with thiazolidinediones is clinically relevant. This phenomenon suggests caution in the prescription of pioglitazone (the only drug of the class presently available in most countries) in women with known osteoporosis, although this condition does not represent an absolute contraindication. In post-menopausal women without known osteoporosis, the expected reduction in bone density should be considered in the overall assessment of the risk/benefit ratio in individual cases.
Insulin: friend or foe?
Insulin has anabolic effects on the bone, stimulating osteoblast differentiation and synthesis of bone matrix. Those actions are partly due to a direct interaction of insulin with its receptors on osteoblasts and their precursors, and partly mediated through increased mechanical load determined by greater muscle strength (16).
Despite these favorable effects, several observational studies have shown that insulin treatment is associated with an increased risk of bone fractures, not only in women, but also in men (17, 18). In fact, in cohorts of patients with type 2 diabetes, after matching for age and gender, those treated with insulin show a higher risk of fractures than those on thiazolidinediones (17). Observational data on the effects of treatment should always be considered with caution, because of prescription bias: the higher risk in insulin-treated patients could be the consequence of associated conditions prompting the prescription of insulin (e.g., renal failure, unwanted weight loss, other concomitant diseases, greater severity of diabetes, etc.), rather than the effect of insulin per se. However, the association of insulin with a higher incidence of bone fractures is maintained even after adjusting for a wide panel of confounders, in carefully matched case-control studies (18).
Interestingly, the duration of insulin treatment is not relevant with respect to the risk of fractures (18). This suggests that the possible detrimental effect of insulin is not due to adverse effects on bone metabolism. In fact, the simplest explanation is represented by hypoglycemia, which is more frequent with insulin than with any other treatment for type 2 diabetes, which is a well-known determinant of falls, particularly in the elderly, who are at higher risk for fractures (19, 20). In fact, in the ORIGIN study, the largest available randomized trial with insulin, in which the incidence of hypoglycemia was remarkably lower than in most other studies, there was no increase in the risk of bone fractures.
From a clinical standpoint, the possibility of bone fractures determined by hypoglycemia-induced falls is one of the reasons for which physicians should refrain from pursuing strict glycemic targets in insulin-treated elderly patients.
Is metformin the good guy?
Among the other older drugs used for type 2 diabetes, sulfonylureas, glinides, and α-glucosidase inhibitors have not been reported to affect, neither favorably nor unfavorably, bone metabolism.
Some experimental data suggest that metformin, via the activation of the orphan nuclear receptor SHP, stimulates osteoblast differentiation (21). However, no studies have been performed to verify the effect of metformin treatment on bone density. In addition, in observational studies metformin is not associated with a reduction in the incidence of bone fractures (18). In the largest available randomized trial with metformin reporting data on fractures, the ADOPT study, the risk is lower than that of rosiglitazone, but not different from sulfonylureas.
Taken together, those data show that, although some minor effect of metformin on bone metabolism cannot be excluded, the actions of this drug on the bone are not sufficient to modify the incidence of fractures. For clinical practice, metformin can be considered substantially neutral with respect to bone health.
New drugs, new hopes, new concerns
In recent years, three classes of drugs have been introduced for the treatment of type 2 diabetes: Dipeptidyl Peptidase-4 (DPP4) inhibitors, Glucagon-Like Peptide-1 (GLP-1) receptor agonists, and Sodium-Glucose Transporter-2 (SGLT-2) inhibitors.
DPP4 inhibitors reduce the inactivation, and therefore increase the circulating concentrations, of GLP-1 and Gastric Inhibitory Polypeptide (GIP), two intestinal hormones mainly produced in the post-prandial phase, which stimulate insulin secretion in a glucose-dependent manner. GIP is known to stimulate osteoblast differentiation and type I collagen production (22). Some experimental data show that GLP-1 is also involved in the regulation of bone metabolism: GLP-1 receptor knock-out mice show extensive bone reabsorption, with increased number of osteoclasts (23), whereas treatment with GLP-1 receptor agonists increases bone density in different models of osteopenia in rodents (24, 25). In addition, studies in osteoblastic cell lines suggested that GLP-1 could concur to the regulation of osteogenesis (26, 27), although possibly via a receptor different from the “classic” cAMP-associated GLP-1 receptor (27).
The exciting hypothesis of a protective action of DPP4 inhibitors on the bone, based on those experimental data, was initially confirmed by a meta-analysis of short-term randomized trials, showing a reduction in the incidence of fractures in patients treated with those drugs (28). The interpretation of those promising results should be cautious, because the fractures considered were only those reported as serious adverse events; in addition, the limited number of events and duration of follow-up does not exclude the possibility of a causal observation. In the following years, two large-scale cardiovascular outcome trials with DPP4 inhibitors failed to detect any reduction in the risk of fractures; however, those studies enrolled patients with relevant comorbidities, such as renal impairment, which may have masked a beneficial effect of the experimental drugs (29, 30).
If the role of GLP-1 in the regulation of bone metabolism outlined by experimental studies (23–27) was relevant in humans, treatment with its receptor agonists could theoretically produce some benefit on the bone. A recent meta-analysis of short-term trials did not find any overall protective effects of GLP-1 receptor agonists with respect to bone fractures, suggesting possible differences across different molecules of the class, but the number of observed events was too small to draw any reliable conclusion (31). Unfortunately, no large-scale, long-term trials are yet available for this class, although several cardiovascular outcome trials are currently ongoing. Little evidence has been produced so far on the effects of GLP-1 receptor agonists on bone density in humans. These drugs, besides reducing blood glucose in diabetic patients, are also capable of inducing a relevant weight loss (32); this could even lead to a reduction of bone density. In a small randomized, placebo-controlled trial a GLP-1 receptor agonist did not affect bone density in patients with type 2 diabetes, but the relevant weight loss in the active treatment group makes the interpretation of results very problematic (33).
Recently, a further class of drugs, the Sodium-Glucose Transporter-2 (SGLT-2) inhibitors, has been introduced in the treatment of type 2 diabetes. These agents reduce glycemia by inhibiting tubular reabsorption of glucose in the kidney, thus inducing glycosuria. Available data seem to exclude any relevant effect of SGLT-1 inhibitors on calciuria or calcemia. In pre-registrations trials with one of the drugs of this class, dapagliflozin, a trend toward a higher incidence of bone fractures has been reported in patients with renal failure (34) – which, for other reasons, represents a contraindication to the use of SGLT-2 inhibitors. However, this phenomenon, observed in a very small number of cases, could be the effect of chance. At present, no large-scale trials are available to verify the longer-term effects of treatment with SGLT-2 inhibitors on bone metabolism (Table 1).
Table 1.
Classes of antidiabetic drugs and their effects on bone.
| Agent | Effects in vitro | Effects in vivo: animals bone density | Effects in vivo: humans bone density | Effects in vivo: humans fractures |
|---|---|---|---|---|
| Thiazolidinediones | adipogenesis | reduced | decreased | increased |
| Insulin | Increased bone matrix production | increased | increased | |
| Metformin | osteoblastogenesis | ? | ? | unchanged |
| GLP-1 receptor agonists | osteoblastogenesis, decreased osteoclast formation | increased | unchanged | ? |
| DPP-4 inhibitors | osteoblastogenesis, decreased osteoclast formation | increased | ? | decreased/unchanged |
| Sulfanylureas | ? | ? | ? | unchanged |
| SGLT-2 inhibitors | ? | ? | ? | increased (?) |
Conclusion
A growing body of evidence shows that drugs for type 2 diabetes can affect bone metabolism, modifying bone density. In addition, hypoglycemia, which is one of the most frequent adverse effects of treatment, is a risk factor for falls, which may cause bone fractures. At present, there is a clear demonstration of a detrimental action on bone metabolism of thiazolidinediones, at least in women, whereas positive expectations for some of the newer drugs still need to be confirmed.
The usual parameters considered for the choice of a glucose-lowering drug are efficacy on HbA1c, risk of hypoglycemia, effect on body weight, and action on cardiovascular risk. The effect on bone metabolism and on the risk for fractures, which have a relevant impact on overall health, deserve to be included into the evaluation of the risk-benefit ratio of drugs for diabetes.
References
- 1.Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 2.Kahn SE, Zinman B, Lachin JM, et al. Rosiglitazone-associated fractures in type 2 diabetes: an Analysis from A Diabetes Outcome Progression Trial (ADOPT) Diabetes Care. 2008;31:845–851. doi: 10.2337/dc07-2270. [DOI] [PubMed] [Google Scholar]
- 3.Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 4.Dormandy J, Bhattacharya M, van Troostenburg de Bruyn AR. Safety and tolerability of pioglitazone in high-risk patients with type 2 diabetes: an overview of data from PROactive. Drug Saf. 2009;32:187–202. doi: 10.2165/00002018-200932030-00002. [DOI] [PubMed] [Google Scholar]
- 5.Glintborg D, Andersen M, Hagen C, et al. Association of pioglitazone treatment with decreased bone mineral density in obese premenopausal patients with polycystic ovary syndrome: a randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2008;93:1696–1701. doi: 10.1210/jc.2007-2249. [DOI] [PubMed] [Google Scholar]
- 6.Zhu ZN, Jiang YF, Ding T. Risk of fracture with thiazolidinediones: an up-dated meta-analysis of randomized clinical trials. Bone. 2014;68:115–123. doi: 10.1016/j.bone.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Hauner H. The mode of action of thiazolidinediones. Diabetes Metab Res Rev. 2002;18(Suppl 2):S10–S15. doi: 10.1002/dmrr.249. [DOI] [PubMed] [Google Scholar]
- 8.Fürnsinn C, Waldhäusl W. Thiazolidinediones: metabolic actions in vitro. Diabetologia. 2002;45:1211–1223. doi: 10.1007/s00125-002-0899-1. [DOI] [PubMed] [Google Scholar]
- 9.Benvenuti S, Cellai I, Luciani P, et al. Rosiglitazone stimulates adipogenesis and decreases osteoblastogenesis in human mesenchymal stem cells. J Endocrinol Invest. 2007;30:RC26–RC30. doi: 10.1007/BF03350807. [DOI] [PubMed] [Google Scholar]
- 10.Beck GR, Jr, Khazai NB, Bouloux GF, et al. The effects of thiazolidinediones on human bone marrow stromal cell differentiation in vitro and in thiazolidinedione-treated patients with type 2 diabetes. Transl Res. 2013;161:145–155. doi: 10.1016/j.trsl.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grey A, Beckley V, Doyle A, et al. Pioglitazone increases bone marrow fat in type 2 diabetes: results from a randomized controlled trial. Eur J Endocrinol. 2012;166:1087–1091. doi: 10.1530/EJE-11-1075. [DOI] [PubMed] [Google Scholar]
- 12.Bilezikian JP, Josse RG, Eastell R, et al. Rosiglitazone decreases bone mineral density and increases bone turnover in postmenopausal women with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2013;98:1519–1528. doi: 10.1210/jc.2012-4018. [DOI] [PubMed] [Google Scholar]
- 13.van Lierop AH, Hamdy NA, van der Meer RW, et al. Distinct effects of pioglitazone and metformin on circulating sclerostin and biochemical markers of bone turnover in men with type 2 diabetes mellitus. Eur J Endocrinol. 2012;166:711–716. doi: 10.1530/EJE-11-1061. [DOI] [PubMed] [Google Scholar]
- 14.Zanchi A, Pechère-Bertschi A, Burnier M, Bonny O. Effects of pioglitazone on renal calcium excretion. J Clin Endocrinol Metab. 2011;96:E1482–E1485. doi: 10.1210/jc.2011-0373. [DOI] [PubMed] [Google Scholar]
- 15.Rubin MR, Manavalan JS, Agarwal S, et al. Effects of rosiglitazone vs metformin on circulating osteoclast and osteogenic precursor cells in postmenopausal women with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2014;99:E1933–E1942. doi: 10.1210/jc.2013-3666. [DOI] [PubMed] [Google Scholar]
- 16.Klein GL. Insulin and bone: Recent developments. World J Diabetes. 2014;5:14–16. doi: 10.4239/wjd.v5.i1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vallarino C, Perez A, Fusco G, et al. Comparing pioglitazone to insulin with respect to cancer, cardiovascular and bone fracture endpoints, using propensity score weights. Clin Drug Investig. 2013;33:621–631. doi: 10.1007/s40261-013-0106-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monami M, Cresci B, Colombini A, et al. Bone fractures and hypoglycemic treatment in type 2 diabetic patients: a case-control study. Diabetes Care. 2008;31:199–203. doi: 10.2337/dc07-1736. [DOI] [PubMed] [Google Scholar]
- 19.Signorovitch JE, Macaulay D, Diener M, et al. Hypoglycaemia and accident risk in people with type 2 diabetes mellitus treated with non-insulin antidiabetes drugs. Diabetes Obes Metab. 2013;15:3353–41. doi: 10.1111/dom.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ORIGIN Trial Investigators. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367:319–328. doi: 10.1056/NEJMoa1203858. [DOI] [PubMed] [Google Scholar]
- 21.Jang WG, Kim EJ, Bae IH, et al. Metformin induces osteoblast differentiation via orphan nuclear receptor SHP-mediated transactivation of Runx2. Bone. 2011;48:885–893. doi: 10.1016/j.bone.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Bollag RJ, Zhong Q, Phillips P, et al. Osteoblast-derived cells express functional glucose-dependent insulinotropic peptide receptors. Endocrinology. 2000;141:1228–1235. doi: 10.1210/endo.141.3.7366. [DOI] [PubMed] [Google Scholar]
- 23.Yamada C, Yamada Y, Tsukiyama K, et al. The murine glucagon-like peptide-1 receptor is essential for control of bone resorption. Endocrinology. 2008;149:574–579. doi: 10.1210/en.2007-1292. [DOI] [PubMed] [Google Scholar]
- 24.Nuche-Berenguer B, Moreno P, Portal-Nuñez S, et al. Exendin-4 exerts osteogenic actions in insulin-resistant and type 2 diabetic states. Regul Pept. 2010;159:61–66. doi: 10.1016/j.regpep.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Nuche-Berenguer B, Lozano D, Gutiérrez-Rojas I, et al. GLP-1 and ex-endin-4 can reverse hyperlipidic-related osteopenia. J Endocrinol. 2011;209:203–210. doi: 10.1530/JOE-11-0015. [DOI] [PubMed] [Google Scholar]
- 26.Pacheco-Pantoja EL, Ranganath LR, Gallagher JA, et al. Receptors and effects of gut hormones in three osteoblastic cell lines. BMC Physiol. 2011;11:12. doi: 10.1186/1472-6793-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nuche-Berenguer B, Portal-Núñez S, Moreno P, et al. Presence of a functional receptor for GLP-1 in osteoblastic cells, independent of the cAMP-linked GLP-1 receptor. J Cell Physiol. 2010;225:585–592. doi: 10.1002/jcp.22243. [DOI] [PubMed] [Google Scholar]
- 28.Monami M, Dicembrini I, Antenore A, Mannucci E. Dipeptidyl peptidase-4 inhibitors and bone fractures: a meta-analysis of randomized clinical trials. Diabetes Care. 2011;34:2474–2476. doi: 10.2337/dc11-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–1335. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 30.Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 31.Su B, Sheng H, Zhang M, et al. Risk of bone fractures associated with glucagon-like peptide-1 receptor agonists’ treatment: a meta-analysis of randomized controlled trials. Endocrine. 2014 Jul 30; doi: 10.1007/s12020-014-0361-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32.Monami M, Marchionni N, Mannucci E. Glucagon-like peptide-1 receptor agonists in type 2 diabetes: a meta-analysis of randomized clinical trials. Eur J Endocrinol. 2009;160:909–917. doi: 10.1530/EJE-09-0101. [DOI] [PubMed] [Google Scholar]
- 33.Bunck MC, Eliasson B, Cornér A, et al. Exenatide treatment did not affect bone mineral density despite body weight reduction in patients with type 2 diabetes. Diabetes Obes Metab. 2011;13:374–377. doi: 10.1111/j.1463-1326.2010.01355.x. [DOI] [PubMed] [Google Scholar]
- 34.European Medicines Agency (EMA) Committee for Medicinal Products for Human Use (CHMP) Assessment report of Forxiga (dapagliflozin), Procedure EMEA/H/C/002322. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002322/WC500136024.pdf.