Summary
Currently, the accepted “gold standard” method for bone mineral density (BMD) measurement and osteoporosis diagnosis is dual-energy X-ray absorptiometry (DXA). However, actual DXA effectiveness is limited by several factors, including intrinsic accuracy uncertainties and possible errors in patient positioning and/or post-acquisition data analysis. DXA employment is also restricted by the typical issues related to ionizing radiation employment (high costs, need of dedicated structures and certified operators, unsuitability for population screenings). The only commercially-available alternative to DXA is represented by “quantitative ultrasound” (QUS) approaches, which are radiation-free, cheaper and portable, but they cannot be applied on the reference anatomical sites (lumbar spine and proximal femur). Therefore, their documented clinical usefulness is restricted to calcaneal applications on elderly patients (aged over 65 y), in combination with clinical risk factors and only for the identification of healthy subjects at low fracture risk. Literature-reported studies performed some QUS measurements on proximal femur, but their clinical translation is mostly hindered by intrinsic factors (e.g., device bulkiness). An innovative ultrasound methodology has been recently introduced, which performs a combined analysis of B-mode images and corresponding “raw” radiofrequency signals acquired during an echographic scan of the target reference anatomical site, providing two novel parameters: Osteoporosis Score and Fragility Score, indicative of BMD level and bone strength, respectively. This article will provide a brief review of the available systems for osteoporosis diagnosis in clinical routine contexts, followed by a synthesis of the most promising research results on the latest ultrasound developments for early osteoporosis diagnosis and fracture prevention.
Keywords: osteoporosis diagnosis, ultrasound, DXA, bone mineral density, bone quality
Introduction
Osteoporosis is a highly prevalent bone disease characterized by a decrease in bone mass accompanied by microarchitectural alterations, resulting in bone fragility and increased fracture risk (1).
Almost 3 million osteoporotic fractures occur each year in Europe, causing a direct cost of about €40 billion for national healthcare systems, and these figures are expected to double by the year 2050 because of population ageing (2–4).
Vertebral and hip fractures are the most frequent, expensive and disabling osteoporotic fractures, typically leading to significant reductions in quality of life for the patients and being also responsible for high rates of morbidity and mortality (5–7). For instance, in 2010 the number of deaths causally related to osteoporotic fractures in Europe was 43,000 and almost 80% of these were due to hip or vertebral fractures (4).
It has been recently estimated that 200 millions of individuals are affected by osteoporosis worldwide (8) [4.5 million only in Italy (9)]. Unfortunately, 75% of these people represent undiagnosed cases because of the lack of reliable diagnostic tools (8). Therefore, the introduction of innovative methods aimed at improving the effectiveness of osteoporosis diagnosis and subsequent patient management is of paramount importance from clinical, social and economic points of view.
According to the operational definition provided by the World Health Organization (WHO), osteoporosis is diagnosed when bone mineral density (BMD) measured at lumbar spine or proximal femur is at least 2.5 standard deviations lower than the young adult mean (10, 11). Currently, dual-energy X-ray absorptiometry (DXA) is the most widely adopted method for osteoporosis diagnosis, since it is considered the “gold standard” reference for BMD measurements (12–14).
However, the steady increment of osteoporosis prevalence due to the demographic transition occurring worldwide is progressively emphasizing the substantial ineffectiveness of current approaches to diagnosis and clinical management of this disease. In fact, on one hand, DXA has important intrinsic limitations that prevent its use for population mass screenings (i.e., ionizing radiation employment, high costs, need of dedicated structures with certified operators) (8), and, on the other hand, BMD measurements showed a suboptimal sensitivity in the identification of patients that will suffer from an osteoporotic fracture (15, 16). Actually, fragility fractures occur in subjects with a reduced bone strength, which is determined not only by BMD (representing bone quantity), but also by a multiplicity of bone quality factors (e.g., elastic properties, microstructural parameters, etc.) that are mostly not assessed by available DXA scanners (17).
For these reasons, in the last years, increasing research efforts have been put in the development of the so-called “quantitative ultrasound” (QUS) approaches to bone health assessment and osteoporosis diagnosis (14, 18–22). In fact, ultrasound (US) waves are in principle inherently suited to probe mechanical properties of investigated bones, and the typical advantages of corresponding devices (absence of ionizing radiation, low costs, portability, wide availability) can overcome most of the DXA limitations (17).
Commercially-available QUS devices are currently employable only on peripheral bone sites (e.g., calcaneus) and several investigations of their diagnostic effectiveness with respect to DXA measurements on the central reference sites (lumbar spine and proximal femur) obtained contradictory results (14, 18, 22–29).
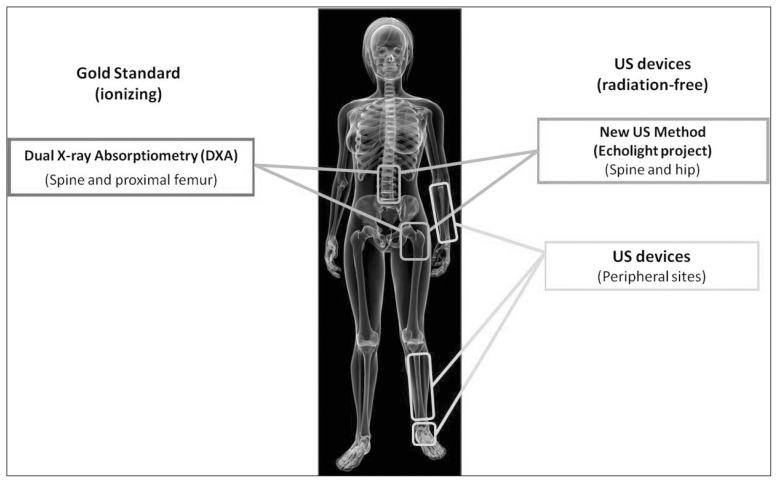
Consequently, available QUS approaches are typically used only as a pre-screening method, requiring a further DXA verification before taking therapeutic decisions (25, 27). In this context, diagnosis and management of osteoporosis are routinely based on DXA outcomes and evaluation of clinical risk factors (e.g., presence of a previous fragility fracture), resulting in the reported evidence of underdiagnosis and under-treatment (30, 31). In order to improve this situation, researchers in this field have recently turned their attention to the investigation of US approaches for osteoporosis diagnosis directly applicable on proximal femur and/or lumbar spine (32–34). A schematic summary of the existing methods for osteoporosis diagnosis is shown in Figure 1.
Figure 1.
Synthetic overview of the available systems for osteoporosis diagnosis.
This article will provide an overview of the currently available systems for osteoporosis diagnosis in clinical routine contexts (DXA and peripheral QUS), followed by a synthesis of the most significant literature-reported results involving the measurements of QUS parameters on proximal femur. The first clinical validations of a novel US approach to obtain both quantity- and quality-related bone parameters on central reference sites will be then illustrated. Finally, some concluding remarks will summarize the most promising paths towards the effective achievement of early and accurate osteoporosis diagnosis and fragility fracture prevention.
Dual-energy X-ray absorptiometry
DXA is presently recognized as the reference method to measure BMD with acceptable accuracy and reasonable reproducibility (35). This technology has been introduced in 1987 as a successor of Dual-Photon Absorptiometry.
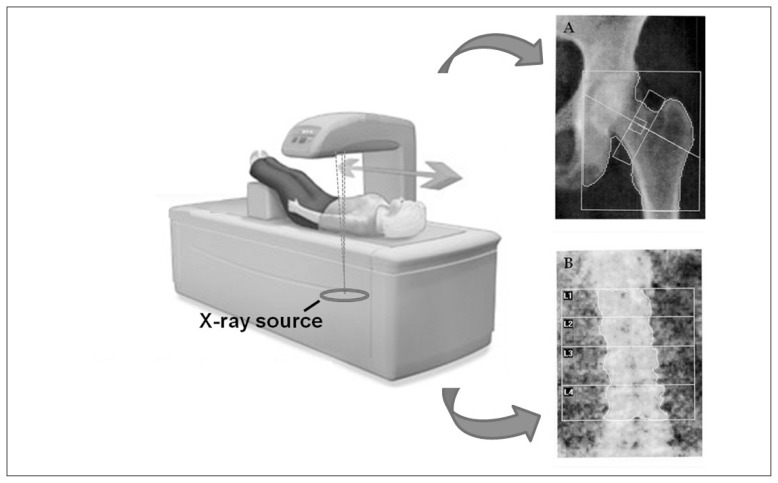
DXA scanners (Figure 2) use an X-ray beam composed of two different photon energies, in order to compensate for the different attenuation coefficients of mineralized bone and soft tissues encountered along the target path within the human body (36): the intensities of high-energy and low-energy photons that passed through the body are analyzed separately by a dedicated algorithm, which subtracts soft tissue attenuation and provides only bone attenuation values. These values are then compared with reference measurements in phantoms of known composition to obtain bone mineral content (BMC, in grams), which is finally divided by the projected area of the considered bone (in cm2) to obtain the BMD value (in g/cm2) (37). These principles are used to obtain BMD measurements on multiple skeletal sites, including hip and lumbar spine.
Figure 2.
Schematic illustration of a DXA scanner, including typical diagnostic images acquired on proximal femur (A) and lumbar spine (B).
However, as with every other diagnostic technique, the actual effectiveness of DXA systems should be critically assessed taking into account the factors that can restrict its employment and/or affect its accuracy and precision levels. These factors have been scientifically investigated and literature results are briefly reviewed and summarized in the following paragraphs.
First of all, because DXA scanners use two X-ray energies in the presence of three types of tissue (mineralized bone, lean tissue and adipose tissue), measurement errors due to nonuniform distribution of adipose tissues have been reported (38–43). The typical uncertainty level associated to both hip and spine BMD measurements is around 0.060 g/cm2 (38), which roughly corresponds to a relative error in the range 5–10% and this should be considered in evaluating the accuracy of DXA scanning.
Secondly, DXA outcome is strongly influenced by patient positioning, which should be carefully assessed by the technologist and double-checked by the clinician that interprets the test (35, 44). For instance, for correct hip positioning, the patient should keep the femur straight with the shaft parallel to the image edge and an internal rotation of 25°, obtained by the use of apposite positioning devices (45). A very recent paper (46) retrospectively reviewed 793 DXA reports, including both spine and femur investigations, and documented the presence of patient positioning errors in about 9% of femoral acquisitions and in about 8% of spinal ones.
A further source of inaccuracy in DXA scans is represented by possible post-acquisition analysis errors. Actually, DXA software typically provides an automatic identification of the regions of interest (ROIs) within the target bone district, but the technologist should make manual adjustments in order to obtain a reliable outcome (35). In the case of spine, the ROI consists of the vertebrae L1–L4 and the correct placement of “spine box” and “intervertebral lines” is critical to avoid errors in BMD measurement, especially in patients with scoliosis (35). Analogous manual adjustments are routinely required for femoral investigations. The above referenced paper (46) reported a very high rate of data analysis errors affecting the final BMD value: 64% for lumbar examinations and 48% for femoral ones.
Therefore, proper DXA employment requires well-trained personnel, since incorrect patient positioning, data analysis errors and interpretation mistakes can easily affect diagnosis and subsequent therapeutic decisions (44).
Recent literature has also questioned the intrinsic DXA suitability for osteoporotic fracture risk assessment (17, 47, 48), since, although BMD is one of the major determinant of bone strength (49), considerable overlaps in BMD values have been reported between individuals that develop fractures and those that do not (50). In order to try to overcome this important issue the trabecular bone score (TBS) based on DXA images has been recently introduced (47, 48, 51). It consists of a novel parameter based on a gray-scale textural analysis of spine DXA images, which uses variograms of 2D projection images to provide a quantitative estimate of trabecular microarchitecture status (51). TBS resulted to be independent of bone size [which, on the other hand, is known to affect DXA-measured BMD values (52)] and correlated with bone quality indicators, such as trabecular number and connectivity density, as measured by microcomputed tomography (51). In particular, TBS showed the potential to predict osteoporotic fracture risk independently of BMD and to provide lumbar DXA scans with a specific added value (48, 53). However, TBS scores are provided through an additional software module installation in the DXA systems with the consequent cost increasing of the final examinations.
Furthermore, it has to be underlined that DXA exploits ionizing radiation properties and its employment is consequently subject to the typical limitations associated to this kind of devices: high costs, possible long-term risks for patient health, limited exam repetition frequency, need for dedicated structures with certified operators (8). Because of these reasons, coupled with the mentioned sources of inaccuracy in BMD measurement, international guidelines typically recommend DXA scans for osteoporosis diagnosis only in people aged 65 and older if no other specific risk factors are present (54).
QUS approaches for peripheral sites
QUS technologies represent a group of US-based methods for bone health assessment that has gained much popularity since their introduction into clinical practice in the 1990s (55). Compared to DXA, QUS approaches offer a wider accessibility to the public, because they are portable, easier to handle, cheaper and do not use ionizing radiation (8).
From a physical point of view, QUS techniques typically involve the generation of US pulses in the frequency range between 200 kHz and 1.5 MHz, which are transmitted through the bone under investigation (56). Some devices transmit US waves parallel to the axis of the target bone (axial transmission): the same US probe contains both the pulse emitter and the pulse receiver, and this approach is adopted to investigate forearm, tibia and radius (57, 58). Nevertheless, the most common clinically-available QUS devices send US pulses perpendicularly with respect to target bone axis (transversal transmission): there are two separate probes for sending and receiving US pulses, with the investigated bone (usually the calcaneus) placed between them (56, 58).
Most of literature-available papers focused on the assessment of QUS diagnostic effectiveness involved calcaneal applications (14, 24, 26–28, 59–63). In fact, calcaneus is composed almost entirely of trabecular bone, is a weight-bearing bone and has the advantage of having two flat, parallel lateral surfaces that are very suitable to achieve a satisfactory transmission of US pulses through the bone (56). As a result, calcaneus is the only validated skeletal site for the clinical use of QUS in osteoporosis management (64).
Nevertheless, despite the huge amount of published data, the International Society for Clinical Densitometry (ISCD) restricted the actual clinical diagnostic usefulness of validated calcaneal QUS devices to patients aged 65 and older, and only in combination with clinical risk factors, in order to identify patients with very low fracture risk, requiring no further investigations (64). This is probably due to the fact that the still limited understanding of the actual interaction mechanisms between an US pulse and the complex trabecular structure does not allow a clear interpretation of the measured QUS variables, nor the identification of clear relationships between such variables and bone strength (17). Consequently, it is quite difficult the proper interpretation of specific literature-reported studies that did not find a clear diagnostic value for calcaneal QUS devices (24, 27). However, further work in this field is ongoing and interesting experimental studies aimed at elucidating the mentioned interaction mechanisms are being continuously published (65–70).
Although the most common QUS devices employ the described through transmission approach to provide parameters such as broadband US attenuation (BUA), speed of sound (SOS) and stiffness index (STI), some recent papers reported the potential of US backscatter as a new approach to osteoporosis diagnosis (34). The considered US backscatter parameters include: backscatter coefficient (71), apparent integrated backscatter (AIB) (33, 72), frequency slope of apparent backscatter (FSAB) and time slope of apparent backscatter (TSAB) (73), spectral centroid shift (72), broadband ultrasound backscatter (BUB) (74), integrated reflection coefficient (IRC) (33), mean of backscatter difference spectrum (MBDS) and slope of backscatter difference spectrum (SBDS) (75). Reported articles documented visible correlations between considered US backscatter parameters and BMD, often supporting the idea that this kind of measurements might also provide an important added value represented by the evaluation of bone microstructural properties. Encouraging clinical results were obtained by a pilot study involving multi-site measurements of AIB and IRC (33) and by a more extended clinical validation focused on calcaneal measurements of AIB and spectral centroid shift (72). Nevertheless, most of the referred papers performed only in vitro measurements on excised bone samples and, the mentioned QUS backscatter approaches are still at an experimental research level and generally suffer from the lack of appropriate clinical validation.
QUS research approaches for proximal femur
Established QUS methods are applicable only on peripheral bone sites and their limited diagnostic power derives from the absence of clear relationships with the health status of hip and/or spine, which actually represents the fracture sites carrying the largest costs and the most severe reductions in patient quality of life.
Considering that site-matched correlations between BMD and QUS parameters are typically better than the corresponding ones obtained from different sites, proximal femur has become the target of several experimental studies aimed at translating the measurement of peripheral QUS parameters to the femoral site (32, 33, 76–82). Among these, the most clinically significant results were those obtained by Barkmann et al. (2010) (32) with a “through transmission” approach for measuring SOS and BUA, and those reported by Karjalainen et al. (2012) (33) exploiting a backscatter technique to measure AIB and IRC.
A strong correlation (r=0.95) between proximal femur BMD and a linear combination of SOS and BUA was first documented in an in vitro study by Haiat et al. (2005) (81). A similar approach was then applied in vivo in 62 women (32), obtaining a significant correlation (r=0.85) between DXA-measured total hip BMD and a QUS-based estimate obtained from a linear combination of SOS values measured through different tissues (i.e., cortical bone, trabecular region, and soft tissue). In the same study, selected QUS parameters showed a performance similar to BMD, or even slightly better, in the discrimination between patients with recent hip fractures and controls. However, the adopted prototypal device was quite bulky and expensive, therefore reducing the advantages of QUS over DXA. Moreover, its translation to clinical routine was further hindered by the relatively long scan time, which was on the order of 5 to 10 minutes.
An alternative approach was employed by Karjalainen et al. (2012) (33), who, on one hand, replaced the described “through transmission” method with a more straightforward pulse-echo technique for QUS measurements at the hip, and, on the other hand, combined their QUS measurements with patient-specific information, such as weight and age, in order to obtain a more effective fracture prediction. They used single-element US transducers with a fixed focus depth, whose effective employment required a preliminary localization of the ROIs through a clinical US imaging system. Reported findings emphasized that AIB measured on femoral neck, combined with patient age and weight, provided a good performance in discriminating patients with a previous hip fracture from control subjects. In addition, AIB alone showed a statistically significant correlation with BMD measured in different femoral regions (r in the range 0.49–0.64). However, although clinical implementation of pulse-echo QUS measurements at hip should be in principle more feasible than the corresponding “through transmission” techniques, the approach adopted in the referred paper (33) suffered from the difficult applicability on obese patients. In fact, 4 out of the 30 enrolled patients (~13%) could not undergo femoral QUS measurements because of their obesity, thus reducing actual sample size to only 26 subjects. Furthermore, the described approach did not show a clear advantage over DXA and its reliability was specifically limited by a relatively low short-term reproducibility of reported results: RMS-CV for AIB measured on femoral neck was 4.6%, while the corresponding DXA values for femoral neck investigations are typically in the range 0.7–0.9% (83). As proposed by the Authors themselves (33), the mentioned issues could be partially overcome by the implementation of the adopted method on a device equipped with a phased-array US imaging transducer capable of optimizing the focal depth to bone surface, although the actual clinical usefulness of the proposed femoral measurements needs anyway to be verified on more extended study populations.
Novel echosound approaches for hip and spine
A different US methodology for osteoporosis diagnosis and fragility fracture prevention has been recently introduced by our research group (34).
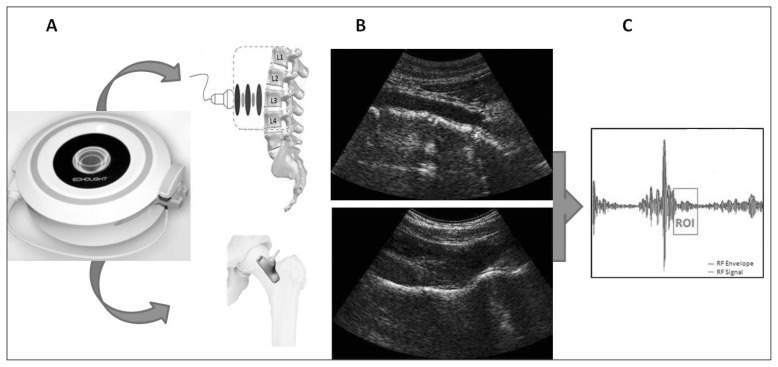
The basic idea underlying this approach is that “raw” unfiltered radiofrequency (RF) signals, acquired during an in vivo echographic scan of a target bone district, can be used to determine the health status of the considered bone through advanced comparisons with previously derived reference spectral models of the possible pathological or normal conditions. This method is natively integrated with US imaging, since B-mode echographic images are needed for two reasons: 1) the ROI for diagnostic calculations within the investigated bone is frame-by-frame identified by a fully automatic segmentation algorithm; 2) the simultaneous acquisition of several RF signals, corresponding to the echographic scan lines of the considered frame, is necessary to provide a solid and reliable statistical basis for subsequent spectral analyses. In principle, the outlined approach can be applied to any bone district to determine in what measure the spectral characteristics of corresponding RF signals are more similar to those of a pathologic bone with respect to those of a normal bone. Spinal and femoral applications of the proposed methodology are illustrated in Figure 3.
Figure 3.
Application of the novel echographic approach to lumbar spine and proximal femur: A) data acquisition; B) typical obtained B-mode images of spine (top) and femur (bottom); C) illustration of a sample automatically selected ROI for RF signal analysis.
The most important bone pathologic conditions for osteoporosis diagnosis and fracture prevention can be summarized in “low BMD” and “susceptibility to fracture”. Accordingly, two novel diagnostic parameters were introduced: the Osteoporosis Score (OS), which measures the degree of similarity to spectral models derived from subjects with a low BMD (T-score ≥ −2.5) with respect to those derived from normal subjects (T-score ≥ −1.0), and the Fragility Score (FS), which quantifies an analogous spectral similarity to subjects that reported a recent fragility fracture with respect to control subjects without fracture history. Literature-available results on the first clinical validations of OS and FS are illustrated in the next two sub-paragraphs.
Osteoporosis Score
A complete and detailed description of OS employment for spinal investigations, including construction of reference database, spectral model derivation, reproducibility assessment and diagnostic accuracy comparison with DXA, has been reported in a very recent paper involving 342 patients (34). Fundamentals of the adopted methodology and main results are briefly summarized herein.
The study included all the consecutive female patients that fulfilled the following enrollment criteria: Caucasian ethnicity, aged 51–60 y, BMI (body mass index) < 25 kg/m2, absence of previous vertebral fractures, medical prescription for a spinal DXA. All the enrolled patients (n = 342) underwent a conventional spinal DXA and an abdominal echographic scan of lumbar spine with RF signal acquisition. Patients were subdivided in two groups based on their age (51–55 y and 56–60 y) and, for each group, the first 100 patients were included in the reference database for spectral model derivation and the remaining ones represented the study population for reproducibility assessments and accuracy measurements. For both the considered age intervals, US datasets from patients included in the reference database were used to calculate the corresponding model spectra for “osteoporosis” and “healthiness” through the procedure detailed in (34). Once the reference models had been calculated, patient datasets belonging to study population were processed by the implemented algorithm in a fully automatic manner that included the following two main steps: 1) highly selective identification of vertebrae and ROIs within them; 2) statistical shape comparisons between selected RF spectra extracted from identified ROIs and the calculated reference models, providing as a final output the OS value for the considered patient. Additionally, the adopted algorithm provided the automatic identification of the “noisy” acquisitions, in which quality of RF backscatter signals was not sufficient to obtain a reliable parameter calculation and the acquisition had to be repeated [in the referred paper (34) the “noisy” acquisition rate was 3.7%].
Statistical analysis of obtained results showed that, in both the considered age ranges, OS values of patients classified as “osteoporotic” by DXA were significantly higher than the corresponding values of either “osteopenic” or “healthy” patients, with OS values of the latters being also significantly lower than those of the formers (34). Diagnostic comparison with DXA produced the following results: classification of patients as “osteoporotic”, “osteopenic” or “healthy” on the basis of OS thresholds was the same of DXA in 91.1% of considered cases (k = 0.859, p<0.0001), and OS-based BMD estimates showed a significant correlation with DXA-measured values (r = 0.84, p<0.001) (34).
The same approach was also preliminarily employed on proximal femur in a different study population (112 Caucasian women, 61–75 y, BMI<40) (84). For 81.3% of the patients US-based diagnostic classification (osteoporotic, osteopenic, healthy) coincided with the corresponding DXA one, and this accuracy level was not appreciably influenced by patient age nor by BMI in the considered ranges. Statistically significant correlations were also found between US-based BMD estimates and DXA values (r in the range 0.65–0.80, p<0.01). Therefore, the adopted approach, whose first clinical target has been osteoporosis diagnosis on spine, confirmed its feasibility on proximal femur as well. Notably, the required US scans were effectively carried out on all the enrolled patients, including the obese ones. Furthermore, some algorithm refinements specific for femoral application are currently under development, involving in particular an optimized automatic identification of bone profile and related ROIs for subsequent spectral analyses: encouraging initial results support the actual possibility of reaching a diagnostic performance at least as good as with the spinal application.
Because of its diagnostic agreement with DXA in patient classification and its proven ease of use combined with the complete absence of ionizing radiation (34), OS has an important potential to reduce the burden of osteoporosis through early diagnoses obtained from mass screenings on young populations at the primary healthcare level. In fact, OS could become particularly useful in the prompt identification of osteopenic subjects, which represents a high-priority public health issue (1) since osteopenia often evolves to osteoporosis, but, in presence of an early detection of this condition, the process can be significantly slowed or even stopped through simple modifications of lifestyle and daily habits.
Fragility Score
A different application of the theoretical concepts that guided the development of OS has led to the implementation of a further US parameter for the assessment of bone health status, FS, which quantifies the fragility of the target bone, independently on BMD level (85, 86).
Actually, the global approach to osteoporosis diagnosis and bone health assessment started to change 10–15 years ago, when the awareness that a very high percentage of fragility fractures occur in subjects without osteoporosis (16) has gradually moved the focus from the identification of patients with osteoporosis (based on BMD thresholds) to the detection of patients at high risk of fracture (87). In other words, it was progressively recognized the importance of bone quality parameters in determining actual bone strength, independently of BMD value.
This gave a further impulse to the development of QUS systems for bone status evaluation and, in particular, to focus the assessment of their diagnostic performance on the ability in discriminating between fractured and non-fractured patients (i.e., between “frail” and “non-frail” subjects) or in the prediction of actual fracture occurrence, rather than on the correlation with BMD values (24, 26, 27, 32, 33, 88). Even the described implementation of TBS calculation from DXA images was aimed at the same goal: the achievement of an osteoporotic fracture predictor that is independent of BMD (47, 48, 51).
An alternative way to obtain a tool for accurate fracture risk prediction was proposed by the WHO Collaborating Centre for Metabolic Bone Diseases at Sheffield (UK) with the implementation of a proprietary fracture risk assessment tool (FRAX®) (89). FRAX® is a software algorithm that calculates the 10-year probability of a major osteoporotic fracture (occurring at hip, clinical spine, humerus or wrist) and the 10-year probability of hip fracture. Fracture risk is calculated from age, BMI and dichotomized risk factors, including a prior fragility fracture, parental history of hip fracture, tobacco smoking, use of long-term oral glucocorticoids, rheumatoid arthritis, other causes of secondary osteoporosis and alcohol consumption; femoral neck BMD can be optionally added to improve fracture risk prediction (2). The introduction of FRAX® is based on the reported evidence that the use of clinical risk factors in conjunction with BMD and age improves BMD sensitivity in fracture prediction without adverse effects on specificity (90).
Unfortunately, the reported socio-economic data on fracture occurrence, associated costs and related projections for the next decades demonstrate that none of the above mentioned approaches to fracture risk prediction has satisfactorily reached its goal. In fact, regarding QUS approaches, their current clinical usefulness is limited to the restricted application field identified by the reported official position of ISCD (64), while a consensus still has not been reached on a wider or different application range. For what concerns TBS, although it has proven to represent an added value with respect to BMD alone (48), its employment is necessarily related to DXA use and, therefore, to the discussed limitations related to ionizing radiation, including in particular high costs, unsuitability for population screening purposes and unavailability in primary healthcare settings.
FRAX® limitations have been discussed in detail in dedicated papers (91,92). The most important weaknesses are related to the following main points: 1) the employment of dichotomized risk factors does not take into account the dose-response effects, which have been demonstrated to have appreciable influences on fracture risk (91, 93–95); 2) even taking into account the previous point, there are limited chances of improvement, since a more accurate assessment of risk factors would cause an increased difficulty in properly answering the corresponding questionnaire, thus making the method more time-consuming without beneficial effects on prediction accuracy; 3) the 10-year fracture probability calculated without femoral neck BMD has a limited reliability and it is almost useless for the clinician, while, on the other hand, the need of the DXA-measured BMD value is again associated to the typical DXA limitations.
In this context, a very recent attempt to introduce a radiation-free and easy-to-use method for bone strength estimation and fracture risk prediction was represented by the mentioned development of FS (85, 86). In its first implementation, FS was thought to be measured on lumbar spine and it was aimed at providing a quantification of the general skeletal fragility. Therefore, the corresponding analysis of RF signals acquired on a patient was tailored to quantify the spectral similarities with reference models derived from “frail” subjects (i.e., those that reported a recent non-vertebral osteoporotic fracture), compared with models of “non-frail” subjects (i.e., without fracture history). Once the target reference populations had been established, the procedure for spectral model derivation and calculation of FS values was the same that has been described for OS (see previous paragraph).
A first preliminary clinical validation of FS ability in the identification of “frail” subjects was conducted on a population of 84 postmenopausal Caucasian women (40 with a recent non-vertebral fragility fracture and 44 controls without fracture history) (85). Obtained results showed that F.S. was significantly higher in the fracture group than in the control group, and vice versa for DXA-measured BMD. ROC curve analysis documented the same discrimination power for both the techniques (AUC=0.77 for both).
In a different study, conducted on a population of 64 post-menopausal Caucasian women, we aimed at assessing the correlation between FS values and the 10-year probabilities of hip and major fragility fractures provided by FRAX® (86). FRAX® fracture probabilities, calculated including also the outcome of DXA measurements on femoral neck, resulted significantly correlated with FS measured on spine (r in the range 0.69–0.75, p<0.001).
As a general result, FS preliminarily showed the following interesting properties: 1) the same discrimination power of spinal DXA in the identification of patients that reported a recent non-vertebral osteoporotic fracture; 2) significant linear correlations with fracture risk probabilities provided by FRAX® integrated with DXA measurements at the femoral neck. Obviously, these findings will need to be confirmed on more extended study populations. However, taking into account the complete absence of ionizing radiation employment with respect to DXA, and the simplicity with respect to the combination of FRAX® and femoral DXA, the introduction of FS in clinical routine and primary healthcare settings can be envisioned with the specific aim of identifying subjects at increased fracture risk, independently of their BMD.
Future studies of this method will include the implementation of FS measurement on different anatomical sites starting from proximal femur, whose application is already under development.
Conclusions and future perspectives
In order to significantly reduce the socio-economic burden currently associated with osteoporosis and fragility fractures, the introduction of novel diagnostic approaches, specifically aimed at early disease detection through accurate population screenings, is needed. Therefore, osteoporosis assessments and evaluations should be routinely carried out in the primary healthcare settings, which require radiation-free, portable and easy-to-use systems.
Furthermore, in order to determine the actual bone strength and to obtain reliable fracture risk prediction, it is important to integrate bone quantity parameters (e.g., BMD) with bone quality indicators (e.g., microstructural properties).
The most promising approach seems to be the optimized clinical implementation of backscatter US measurements on the most disabling fracture sites (i.e., proximal femur and lumbar spine).
In this context, two novel parameters have been very recently introduced: OS, which showed significant correlations with BMD, and FS, which demonstrated a clear potential for the identification of “frail” bone structures. Their combined employment can enhance the outcome of osteoporosis diagnosis through the very early identification of both subjects with a reduced BMD level (who are prone to develop osteoporosis in the future, but can avoid this perspective through lifestyle corrections) and subjects at increased fracture risk because of a compromised bone strength (who should be considered for possible specific drug therapies).
Acknowledgements
This work was partially funded by PO FESR, Apulia Region 2007–2013, Action 1.2.4 (Grant N. 3Q5AX31: ECHOLIGHT Project).
References
- 1.Bernabei R, Martone AM, Ortolani E, et al. Screening, diagnosis and treatment of osteoporosis: a brief review. Clin Cases Miner Bone Metab. 2014;11:201–207. [PMC free article] [PubMed] [Google Scholar]
- 2.Kanis JA, McCloskey EV, Johansson H, et al. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2013;24:23–57. doi: 10.1007/s00198-012-2074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanis JA, Borgstrom F, De Laet C, et al. Assessment of fracture risk. Osteoporos Int. 2005;16:581–589. doi: 10.1007/s00198-004-1780-5. [DOI] [PubMed] [Google Scholar]
- 4.Kanis JA, Compston J, Cooper C, et al. The burden of fractures in the European Union in 2010. Osteoporos Int. 2012;23:S57. [Google Scholar]
- 5.Albanese CV, De Terlizzi F, Passariello R. Quantitative ultrasound of the phalanges and DXA of the lumbar spine and proximal femur in evaluating the risk of osteoporotic vertebral fracture in postmenopausal women. Radiol Med. 2011;116:92–101. doi: 10.1007/s11547-010-0577-1. [DOI] [PubMed] [Google Scholar]
- 6.Ensrud KE, Thompson DE, Cauley JA, et al. Prevalent vertebral deformities predict mortality and hospitalization in older women with low bone mass. Fracture Intervention Trial Research Group. J Am Geriatr Soc. 2000;48:241–249. doi: 10.1111/j.1532-5415.2000.tb02641.x. [DOI] [PubMed] [Google Scholar]
- 7.Cooper C. The crippling consequences of fractures and their impact on quality of life. Am J Med. 1997;103:12S–17S. doi: 10.1016/s0002-9343(97)90022-x. [DOI] [PubMed] [Google Scholar]
- 8.Pisani P, Renna MD, Conversano F, et al. Screening and early diagnosis of osteoporosis through X-ray and ultrasound based techniques. World J Radiol. 2013;5:398–410. doi: 10.4329/wjr.v5.i11.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Società Italiana dell’Osteoporosi, del Metabolismo Minerale e delle Malattie dello Scheletro (SIOMMMS) Linee guida per la diagnosi, prevenzione e terapia dell’osteoporosi. 2012. [Accessed January 22, 2015]. Available at: http://www.siommms.it/linee-guida-per-la-diagnosi-prevenzione-e-terapia-dellosteoporosi/ [PubMed]
- 10.Kanis JA WHO Study Group. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: Synopsis of a WHO report. Osteoporos Int. 1994;4:368–381. doi: 10.1007/BF01622200. [DOI] [PubMed] [Google Scholar]
- 11.Genant HK, Cooper C, Poor G, et al. Interim report and recommendations of the World Health Organization task force for osteoporosis. Osteoporos Int. 1999;10:259–264. doi: 10.1007/s001980050224. [DOI] [PubMed] [Google Scholar]
- 12.Baim S, Leslie WD. Assessment of fracture risk. Curr Osteoporos Rep. 2012;10:28–41. doi: 10.1007/s11914-011-0093-9. [DOI] [PubMed] [Google Scholar]
- 13.Link TM. Osteoporosis imaging: State of the art and advanced imaging. Radiology. 2012;263:3–17. doi: 10.1148/radiol.12110462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schnitzer TJ, Wysocki N, Barkema D, et al. Calcaneal quantitative ultrasound compared with hip and femoral neck dual-energy X-ray absorptiometry in people with a spinal cord injury. PM R. 2012;4:748–755. doi: 10.1016/j.pmrj.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1–129. [PubMed] [Google Scholar]
- 16.Kanis JA, Johnell O, Oden A, et al. Ten year probabilities of osteoporotic fractures according to BMD and diagnostic thresholds. Osteoporos Int. 2001;12:989–995. doi: 10.1007/s001980170006. [DOI] [PubMed] [Google Scholar]
- 17.Raum K, Grimal Q, Varga P, et al. Ultrasound to assess bone quality. Curr Osteoporos Rep. 2014;12:154–162. doi: 10.1007/s11914-014-0205-4. [DOI] [PubMed] [Google Scholar]
- 18.Breban S, Padilla F, Fujisawa Y, et al. Trabecular and cortical bone separately assessed at radius with a new ultrasound device in a young adult population with various physical activities. Bone. 2010;46:1620–1625. doi: 10.1016/j.bone.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Nayak S, Olkin I, Liu H, et al. Meta-analysis: Accuracy of quantitative ultrasound for identifying patients with osteoporosis. Ann Intern Med. 2006;144:832–841. doi: 10.7326/0003-4819-144-11-200606060-00009. [DOI] [PubMed] [Google Scholar]
- 20.Paggiosi MA, Barkmann R, Gluer CC, et al. A European multicenter comparison of quantitative ultrasound measurements variables: The OPUS study. Osteoporos Int. 2012;23:2815–2828. doi: 10.1007/s00198-012-1912-2. [DOI] [PubMed] [Google Scholar]
- 21.Pais R, Campean R, Simon SP, et al. Accuracy of quantitative ultrasound parameters in the diagnosis of osteoporosis. Centr Eur J Med. 2010;5:478–485. [Google Scholar]
- 22.Trimpou P, Bosaeus I, Bengtsson BA, et al. High correlation between quantitative ultrasound and DXA during 7 y of follow-up. Eur J Radiol. 2010;73:360–364. doi: 10.1016/j.ejrad.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 23.Dane C, Dane B, Cetin A, et al. The role of quantitative ultrasound in predicting osteoporosis defined by dual-energy X-ray absorptiometry in pre-and postmenopausal women. Climateric. 2008;11:296–303. doi: 10.1080/13697130802178471. [DOI] [PubMed] [Google Scholar]
- 24.El Maghraoui A, Morjane F, Mounach A, et al. Performance of calcaneus quantitative ultrasound and dual-energy X-ray absorptiometry in the discrimination of prevalent asymptomatic osteoporotic fractures in post-menopausal women. Rheumatol Int. 2009;29:551–556. doi: 10.1007/s00296-008-0751-0. [DOI] [PubMed] [Google Scholar]
- 25.Iida T, Chikamura C, Aoi S, et al. A study on the validity of quantitative ultrasonic measurement used the bone mineral density values on dual-energy X-ray absorptiometry in young and in middle-aged or older women. Radiol Phys Technol. 2010;3:113–119. doi: 10.1007/s12194-010-0086-x. [DOI] [PubMed] [Google Scholar]
- 26.Kwok T, Khoo CC, Leung J, et al. Predictive values of calcaneal quantitative ultrasound and dual energy X-ray absorptiometry for non-vertebral fracture in older men: results from the MrOS study (Hong Kong) Osteoporos Int. 2012;23:1001–1006. doi: 10.1007/s00198-011-1634-x. [DOI] [PubMed] [Google Scholar]
- 27.Liu JM, Ma LY, Bi YF, et al. A population-based study examining calcaneus quantitative ultrasound and its optimal cut-points to discriminate osteoporotic fractures among 9352 Chinese women and men. J Clin Endorinol Metab. 2012;97:800–809. doi: 10.1210/jc.2011-1654. [DOI] [PubMed] [Google Scholar]
- 28.Moayyeri A, Adams JE, Adler RA, et al. Quantitative ultrasound of the heel and fracture risk assessment: an updated meta-analysis. Osteoporos Int. 2012;23:143–153. doi: 10.1007/s00198-011-1817-5. [DOI] [PubMed] [Google Scholar]
- 29.Stewart A, Felsenberg D, Eastell R, et al. Relationship between risk factors and QUS in a European population: the OPUS study. Bone. 2006;39:609–615. doi: 10.1016/j.bone.2006.02.072. [DOI] [PubMed] [Google Scholar]
- 30.Curtis JR, Safford MM. Management of osteoporosis among the elderly with other chronic medical conditions. Drugs Aging. 2012;29:549–564. doi: 10.2165/11599620-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van den Bergh JP, van Geel TA, Geusens PP. Osteoporosis, frailty and fracture: implications for case finding and therapy. Nat Rev Rheumatol. 2012;8:163–172. doi: 10.1038/nrrheum.2011.217. [DOI] [PubMed] [Google Scholar]
- 32.Barkmann R, Dencks S, Laugier P, et al. Femur ultrasound (FemUS)-first clinical results on hip fracture discrimination and estimation of femoral BMD. Osteoporos Int. 2010;21:969–976. doi: 10.1007/s00198-009-1037-4. [DOI] [PubMed] [Google Scholar]
- 33.Karjalainen JP, Riekkinen O, Toyras J, et al. Multi-site bone ultrasound measurements in elderly women with and without previous hip fractures. Osteoporos Int. 2012;23:1287–1295. doi: 10.1007/s00198-011-1682-2. [DOI] [PubMed] [Google Scholar]
- 34.Conversano F, Franchini R, Greco A, et al. A novel ultrasound methodology for estimating spine mineral density. Ultrasound Med Biol. 2015;41:281–300. doi: 10.1016/j.ultrasmedbio.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 35.El Maghraoui A, Roux C. DXA scanning in clinical practice. Q J Med. 2008;101:605–617. doi: 10.1093/qjmed/hcn022. [DOI] [PubMed] [Google Scholar]
- 36.Williams JE, Wells JC, Wilson CM, et al. Evaluation of Lunar Prodigy dual-energy X-ray absorptiometry for assessing body composition in healthy persons and patients by comparison with the criterion 4-component model. Am J Clin Nutr. 2006;83:1047–1054. doi: 10.1093/ajcn/83.5.1047. [DOI] [PubMed] [Google Scholar]
- 37.Schousboe JT, Debold CR. Reliability and accuracy of vertebral fracture assessment with densitometry compared to radiography in clinical practice. Osteoporos Int. 2006;17:281–289. doi: 10.1007/s00198-005-2010-5. [DOI] [PubMed] [Google Scholar]
- 38.Tothill P, Weir N, Loveland J. Errors in dual-energy X-ray scanning of the hip because of non-uniform fat distribution. J Clin Densitom. 2014;17:91–96. doi: 10.1016/j.jocd.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 39.Tothill P, Avenell A. Errors in dual-energy X-ray absorptiometry of the lumbar spine owing to fat distribution and soft tissue thickness during weight change. Br J Radiol. 1994;67:71–75. doi: 10.1259/0007-1285-67-793-71. [DOI] [PubMed] [Google Scholar]
- 40.Svendsen OL, Hassager C, Skodt V, et al. Impact of soft tissue on in vivo accuracy of bone mineral measurements in the spine, hip, and forearm: a human cadaver study. J Bone Miner Res. 1995;10:868–873. doi: 10.1002/jbmr.5650100607. [DOI] [PubMed] [Google Scholar]
- 41.Lee DC, Wren TAL, Gilsanz V. Correcting DXA pediatric bone mineral density measurements to account for fat inhomogeneity. [Abstract] AS-BMR. 2007:W514. [Google Scholar]
- 42.Kuiper JW, van Kuijk C, Grashuis JL, et al. Accuracy and the influence of marrow fat on quantitative CT and dual-energy X-ray absorptiometry measurements of the femoral neck in vitro. Osteoporos Int. 1996;6:25–30. doi: 10.1007/BF01626534. [DOI] [PubMed] [Google Scholar]
- 43.Griffith JF, Yeung DK, Antonio GE, et al. Vertebral marrow fat content and diffusion and perfusion indexes in women with varying bone density: MR evaluation. Radiology. 2006;241:831–838. doi: 10.1148/radiol.2413051858. [DOI] [PubMed] [Google Scholar]
- 44.Watts NB. Fundamentals and pitfalls of bone densitometry using dual-energy X-ray absorptiometry (DXA) Osteoporos Int. 2004;15:847–854. doi: 10.1007/s00198-004-1681-7. [DOI] [PubMed] [Google Scholar]
- 45.Hologic. QDR series user’s guide. Hologic; Bedford: 2000. [Google Scholar]
- 46.Messina C, Bandirali M, Sconfienza LM, et al. Prevalence and type of errors in dual-energy X-ray absorptiometry. Eur Radiol. 2015 doi: 10.1007/s00330-014-3509-y. in press. [DOI] [PubMed] [Google Scholar]
- 47.Silva BC, Walker MD, Abraham A, et al. Trabecular bone score is associated with volumetric bone density and microarchitecture as assessed by central QCT and HRpQCT in Chinese-American and white women. J Clin Densitom. 2013;16:554–561. doi: 10.1016/j.jocd.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hans D, Goertzen AL, Krieg M-A, et al. Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: the Manitoba study. J Bone Miner Res. 2011;26:2762–2769. doi: 10.1002/jbmr.499. [DOI] [PubMed] [Google Scholar]
- 49.Johnell O, Kanis JA, Oden E, et al. Predictive value of BMD for hip and other fractures. J Bone Miner Res. 2005;20:1185–1194. doi: 10.1359/JBMR.050304. [DOI] [PubMed] [Google Scholar]
- 50.Hordon LD, Raisi M, Paxton S, et al. Trabecular architecture in women and men of similar bone mass with and without vertebral fracture: Part I. 2-D histology. Bone. 2000;27:271–276. doi: 10.1016/s8756-3282(00)00329-x. [DOI] [PubMed] [Google Scholar]
- 51.Hans D, Barthe N, Boutroy S, et al. Correlations between trabecular bone score, measured using anteroposterior dual-energy X-ray absorptiometry acquisition, and 3-dimensional parameters of bone microarchitecture: an experimental study on human cadaver vertebrae. J Clin Densitom. 2011;14:302–312. doi: 10.1016/j.jocd.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 52.Carter DR, Bouxsein ML, Marcus R. New approaches for interpreting projected bone densitometry data. J Bone Miner Res. 1992;7:137–145. doi: 10.1002/jbmr.5650070204. [DOI] [PubMed] [Google Scholar]
- 53.Boutroy S, Hans D, Sornay-Rendu E, et al. Trabecular bone score improves fracture risk prediction in non-osteoporotic women: the OFELY study. Osteoporos Int. 2013;24:77–85. doi: 10.1007/s00198-012-2188-2. [DOI] [PubMed] [Google Scholar]
- 54.Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359–2381. doi: 10.1007/s00198-014-2794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gluer CC. Quantitative ultrasound techniques for the assessment of osteoporosis: expert agreement on current status. The International Quantitative Ultrasound Consensus Group. J Bone Miner Res. 1997;12:1280–1288. doi: 10.1359/jbmr.1997.12.8.1280. [DOI] [PubMed] [Google Scholar]
- 56.Guglielmi G, de Terlizzi F. Quantitative ultrasound in the assessment of osteoporosis. Eur J Radiol. 2009;71:425–431. doi: 10.1016/j.ejrad.2008.04.060. [DOI] [PubMed] [Google Scholar]
- 57.Scherrer MJ, Rochat MK, Inci D, et al. Reference equations for ultrasound bone densitometry of the radius in Central European children and adolescents. Osteoporos Int. 2014;25:2617–2623. doi: 10.1007/s00198-014-2807-1. [DOI] [PubMed] [Google Scholar]
- 58.Chin K-Y, Ima-Nirwana S. Calcaneal quantitative ultrasound as a determinant of bone health status: what properties of bone does it reflect? Int J Med Sci. 2013;10:1778–1783. doi: 10.7150/ijms.6765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hans D, Dargent-Molina P, Schott AM, et al. Ultrasonographic heel measurements to predict hip fracture in elderly women: the EPIDOS prospective study. Lancet. 1996;348:511–514. doi: 10.1016/s0140-6736(95)11456-4. [DOI] [PubMed] [Google Scholar]
- 60.Bauer DC, Gluer CC, Cauley JA, et al. Broadband ultrasound attenuation predict fractures strongly and independently of densitometry in older women. A prospective study. Arch Intern Med. 1997;157:629–634. [PubMed] [Google Scholar]
- 61.Khaw KT, Reeve J, Luben R, et al. Prediction of total and hip fracture risk in men and women by quantitative ultrasound of the calcaneus: EPIC-Norfolk prospective population study. Lancet. 2004;363:197–202. doi: 10.1016/S0140-6736(03)15325-1. [DOI] [PubMed] [Google Scholar]
- 62.Marin F, Gonzalez-Macias J, Diez-Perez A, et al. Relationship between bone quantitative ultrasound and fractures: a meta-analysis. J Bone Miner Res. 2006;21:1126–1135. doi: 10.1359/jbmr.060417. [DOI] [PubMed] [Google Scholar]
- 63.Xu Y, Guo B, Gong J, et al. The correlation between calcaneus stiffness index calculated by QUS and total body BMD assessed by DXA in Chinese children and adolescents. J Bone Miner Metab. 2014;32:159–166. doi: 10.1007/s00774-013-0474-5. [DOI] [PubMed] [Google Scholar]
- 64.Official Positions of the ISCD (International Society for Clinical Densitometry) as updated in 2013. [Accessed January 22, 2015]. Available at: http://www.iscd.org/official-positions/2013-iscd-official-positions-adult/
- 65.Meziere F, Muller M, Dobigny B, et al. Simulations of ultrasound propagation in random arrangements of elliptic scatterers: occurrence of two longitudinal waves. J Acoust Soc Am. 2013;133:643–652. doi: 10.1121/1.4774276. [DOI] [PubMed] [Google Scholar]
- 66.Meziere F, Muller M, Bossy E, et al. Measurements of ultrasound velocity and attenuation in numerical anisotropic porous media compared with Biot’s and multiple scattering models. Ultrasonics. 2014;54:1146–1154. doi: 10.1016/j.ultras.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 67.Pakula M, Padilla F, Laugier P. Influence of the filling fluid on frequency-dependent velocity and attenuation in cancellous bones between 0.35 and 2.5 MHz. J Acoust Soc Am. 2009;126:3301–3310. doi: 10.1121/1.3257233. [DOI] [PubMed] [Google Scholar]
- 68.Hoffman JJ, Nelson AM, Holland MR, et al. Cancellous bone fast and slow waves obtained with Bayesian probability theory correlate with porosity from computed tomography. J Acoust Soc Am. 2012;132:1830–1837. doi: 10.1121/1.4739455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Anderson CC, Bauer AQ, Holland MR, et al. Inverse problems in cancellous bone: estimation of the ultrasonic properties of fast and slow waves using Bayesian probability theory. J Acoust Soc Am. 2010;128:2940–2948. doi: 10.1121/1.3493441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mizuno K, Somiya H, Kubo T, et al. Influence of cancellous bone microstructure on two ultrasonic wave propagations in bovine femur: an in vitro study. J Acoust Soc Am. 2010;128:3181–3189. doi: 10.1121/1.3493444. [DOI] [PubMed] [Google Scholar]
- 71.Wear KA, Nagaraja S, Dreher M, et al. Relationships of quantitative ultrasound parameters with cancellous bone microstructure in human calcaneus in vitro. J Acoust Soc Am. 2012;131:1605–1612. doi: 10.1121/1.3672701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiang Y-Q, Liu C-C, Li R-Y, et al. Analysis of apparent integrated backscatter coefficient and backscatter spectral centroid shift in calcaneus in vivo for the ultrasonic evaluation of osteoporosis. Ultrasound Med Biol. 2014;40:1307–1317. doi: 10.1016/j.ultrasmedbio.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 73.Hoffmeister BK, Johnson DP, Janeski JA, et al. Ultrasonic characterization of human cancellous bone in vitro using three different apparent backscatter parameters in the frequency range 0.6–15 MHz. IEEE Trans Ultrason Ferroelectr Freq Control. 2008;55:1442–1452. doi: 10.1109/TUFFC.2008.819. [DOI] [PubMed] [Google Scholar]
- 74.Karjalainen JP, Toyras J, Riekkinen O, et al. Ultrasound backscatter imaging provides frequency-dependent information on structure, composition and mechanical properties of human trabecular bone. Ultrasound Med Biol. 2009;35:1376–1384. doi: 10.1016/j.ultrasmedbio.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 75.Hoffmeister BK, Wilson AR, Gilbert MJ, et al. A backscatter difference technique for ultrasonic bone assessment. J Acoust Soc Am. 2012;132:4069–4076. doi: 10.1121/1.4763992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barkmann R, Laugier P, Moser U, et al. A method for the estimation of femoral bone mineral density from variables of ultrasound transmission through the human femur. Bone. 2007;40:37–44. doi: 10.1016/j.bone.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 77.Barkmann R, Laugier P, Moser U, et al. A device for in vivo measurements of quantitative ultrasound variables at the human proximal femur. IEEE Trans Ultrason Ferroelectr Freq Control. 2008;55:1197–1204. doi: 10.1109/TUFFC.2008.783. [DOI] [PubMed] [Google Scholar]
- 78.Barkmann R, Laugier P, Moser U, et al. In vivo measurements of ultrasound transmission through the human proximal femur. Ultrasound Med Biol. 2008;34:1186–1190. doi: 10.1016/j.ultrasmedbio.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 79.Dencks S, Barkmann R, Padilla F, et al. Model-based estimation of quantitative ultrasound variables at the proximal femur. IEEE Trans Ultrason Ferroelectr Freq Control. 2008;55:1304–1315. doi: 10.1109/TUFFC.2008.793. [DOI] [PubMed] [Google Scholar]
- 80.Grimal Q, Grondin J, Guerard S, et al. Quantitative ultrasound of cortical bone in the femoral neck predicts femur strength: results of a pilot study. J Bone Miner Res. 2013;28:302–312. doi: 10.1002/jbmr.1742. [DOI] [PubMed] [Google Scholar]
- 81.Haiat G, Padilla F, Barkmann R, et al. Optimal prediction of bone mineral density with ultrasonic measurements in excised human femur. Calcif Tissue Int. 2005;77:186–192. doi: 10.1007/s00223-005-0057-0. [DOI] [PubMed] [Google Scholar]
- 82.Padilla F, Jenson F, Bousson V, et al. Relationships of trabecular bone structure with quantitative ultrasound parameters: in vitro study on human proximal femur using transmission and backscatter measurements. Bone. 2008;42:1193–1202. doi: 10.1016/j.bone.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 83.Kiebzak GM, Morgan SL. Long-term versus short-term precision of dual-energy X-ray absorptiometry scans and the impact on interpreting change in bone mineral density at follow-up. J Clin Densitom. 2011;14:108–115. doi: 10.1016/j.jocd.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 84.Chiriacò F, Conversano F, Quarta E, et al. Preliminary clinical validation of a new ultrasound-based methodology for femoral neck densitometry. Proc 3rd Imeko TC13 Symp Meas Biol Med. 2014:58–61. [Google Scholar]
- 85.Pisani P, Greco A, Renna MD, et al. An innovative ultrasound-based method for the identification of patients at high fracture risk. Proc 3rd Imeko TC13 Symp Meas Biol Med. 2014:50–53. [Google Scholar]
- 86.Greco A, Pisani P, Soloperto G, et al. Comparison between ultrasound Fragility Score and FRAX® for the assessment of osteoporotic fracture risk. Proc 3rd Imeko TC13 Symp Meas Biol Med. 2014:54–57. [Google Scholar]
- 87.Kanis JA, Black D, Cooper C, et al. A new approach to the development of assessment guidelines for osteoporosis. Osteoporos Int. 2002;13:527–536. doi: 10.1007/s001980200069. [DOI] [PubMed] [Google Scholar]
- 88.Hartl F, Tyndall A, Kraenzlin M, et al. Discriminatory ability of quantitative ultrasound parameters and bone mineral density in a population-based sample of postmenopausal women with vertebral fractures: results of the Basel Osteoporosis Study. J Bone Miner Res. 2002;17:321–330. doi: 10.1359/jbmr.2002.17.2.321. [DOI] [PubMed] [Google Scholar]
- 89.Kanis JA, Johnell O, Oden A, et al. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19:385–397. doi: 10.1007/s00198-007-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kanis JA, Oden A, Johnell O, et al. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int. 2007;18:1033–1046. doi: 10.1007/s00198-007-0343-y. [DOI] [PubMed] [Google Scholar]
- 91.Kanis JA, Hans D, Cooper C, et al. Interpretation and use of FRAX in clinical practice. Osteoporos Int. 2011;22:2395–2411. doi: 10.1007/s00198-011-1713-z. [DOI] [PubMed] [Google Scholar]
- 92.Hans DB, Kanis JA, Baim S, et al. Joint official positions of the International Society for Clinical Densitometry and International Osteoporosis Foundation on FRAX®. Executive summary of the 2010 Position Development Conference on Interpretation and use of FRAX® in clinical practice. J Clin Densitom. 2011;14:171–180. doi: 10.1016/j.jocd.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 93.Kanis JA, Johansson H, Johnell O, et al. Alcohol intake as a risk factor for fracture. Osteoporos Int. 2005;16:737–742. doi: 10.1007/s00198-004-1734-y. [DOI] [PubMed] [Google Scholar]
- 94.Van Staa TP, Leufkens HG, Abenhaim L, et al. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2000;15:993–1000. doi: 10.1359/jbmr.2000.15.6.993. [DOI] [PubMed] [Google Scholar]
- 95.Kanis JA, Johnell O, Oden A, et al. Smoking and fracture risk: a meta-analysis. Osteoporos Int. 2005;16:155–162. doi: 10.1007/s00198-004-1640-3. [DOI] [PubMed] [Google Scholar]