Summary
According to European legislation, a disease can be considered rare or “orphan” when it affects less than 1 subject of 2000 (1). Often these diseases affecting the pediatric age, are complex diseases and chronically debilitating and for this motive need the intervention of multidisciplinary skills specific. Among the rare disease as affecting the skeleton more than 400 are characterized by dysplastic changes of the skeleton (2). Alongside the disorders affecting the skeleton primitively, many systemic diseases can have a bone involvement. Among these, the Gaucher disease (GD), an heterogeneous lysosomal storage determined by hereditary enzyme deficiency of β-glucosidase. Patients with this disease have skeletal disorders of varying severity (Erlenmeyer flask deformity, lytic lesions and osteonecrosis, pathological fractures) that affects both the bone marrow, both mineralized bone with progressive damage of the tissue. The bone disease is the most debilitating of GD and can have a significant impact on the quality of life of patients. Thorough evaluations by monitoring biochemical markers of bone turnover and instrumental, with a quantitative and qualitative evaluation of the bone, are of fundamental importance to intervene early so they can prevent complications irreversible.
Keywords: Gaucher disease, osteoporosis, bone crisis, osteomyelitis, avascular bone necrosis, Bone Mineral Density (BMD), multidisciplinary
Introduction
Gaucher disease (GD; OMIM#230800) is an autosomal recessively inherited lysosomal storage disorder. GD results from a deficiency of the lysosomal enzyme glucocerebrosidase (or acid β-glucosidase, EC 3.2.1.45). The enzyme is encoded on chromosome 1 (1q21) and as a consequence of the deficiency storage of its substrate, glucocerebroside, occurs in macrophages (3). Patients with non-neuronopathic (type 1) GD, the most common form, appear with hepatomegaly, splenomegaly, anemia, bleeding tendencies, thrombocytopenia, skeletal pathologies, growth retardation, and, in severe cases, pulmonary disease. The bone manifestations include bone infarcts, avascular bone necrosis, lytic lesions, osteosclerosis, fractures due to osteopenia or osteoporosis, and rarely acute osteomyelitis. Bone pain of varying intensity, fractures, and progressive joint collapses may cause impaired mobility and performances status, and increased morbidity (4). The bone involvement in GD is known to be frequent: according to literature it occurs in approximately 75% of GD type 1 (GD1) patients (4, 5). Data from the Gaucher Registry indicated that the frequency of any bone involvement in GD1 is present in more than 90% of patients (6). Bone is a highly specialized supporting framework of the body, characterized by its rigidity, hardness, and power of regeneration and repair. It protects the vital organs, provides an environment for marrow (both blood forming and fat storage), acts as a mineral reservoir for calcium homeostasis and a reservoir of growth factors and cytokines, and also takes part in acid-base balance (7). Bone constantly undergoes modeling (reshaping) during life to help it adapt to changing biomechanical forces, as well as remodeling to remove old, microdamaged bone and replace it with new, mechanically stronger bone to help preserve bone strength. The bones have two components – the cortical bone that is dense, solid, and surrounds the marrow space and the trabecular bone which is composed of a honeycomb-like network of trabecular plates and rods interspersed in the bone marrow compartment (8). Bone is composed of support cells, namely, osteoblasts and osteocytes; remodeling cells, namely, osteoclasts; and non-mineral matrix of collagen and non-collagenous proteins called osteoid, with inorganic mineral salts deposited in the matrix (Figure 1). There is a growing knowledge the achievement of optimal peak bone mass (PBM) is important for the prevention of bone loss in later life (9). Up to 25% of PBM is acquired during the 2 years of peak height velocity. By age 18, at least 90% of PBM has been acquired, while the remaining 10% will be added later in the skeletal consolidation phase. Bone mineral mass gain during childhood and adolescence is influenced by many factors. Genetic factors are estimated to determine 60–80% of peak adult bone mass, leaving environmental and lifestyle factors as important contributors of 20–40% of bone mass (like nutrition, lifestyle behaviours, exercise habits, exposition to sunlight and eventual drug administration) (Figure 2) (10, 11). In the course of the following decades (beginning from 40 years) this quantity of bone tissue tends to decrease in a progressive trend. Since the risk of fractures is in inverse relation to bone mass, the achievement of a high peak bone mass at the end of adolescence represents the best modality to prevent the onset of fractures in adulthood (12).
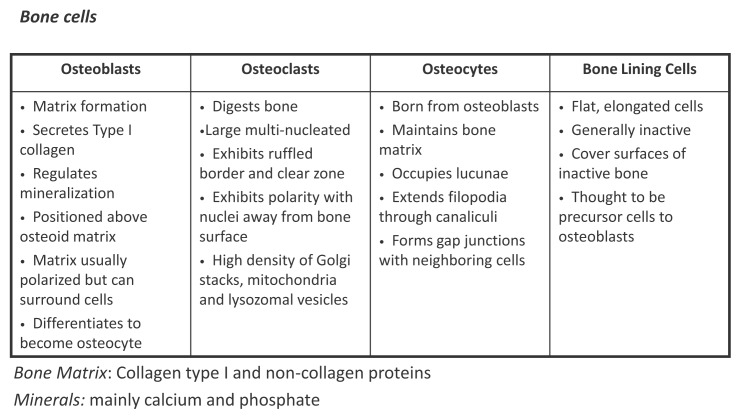
Figure 1.
Bone elements: bone is composed by cells (osteoblasts osteocytes osteoclasts, lining cells) immersed in a matrix formed by collagen type I and non-collagen proteins. The matrix is mineralized for the deposition of inorganic salts (mainly calcium and phosphate).
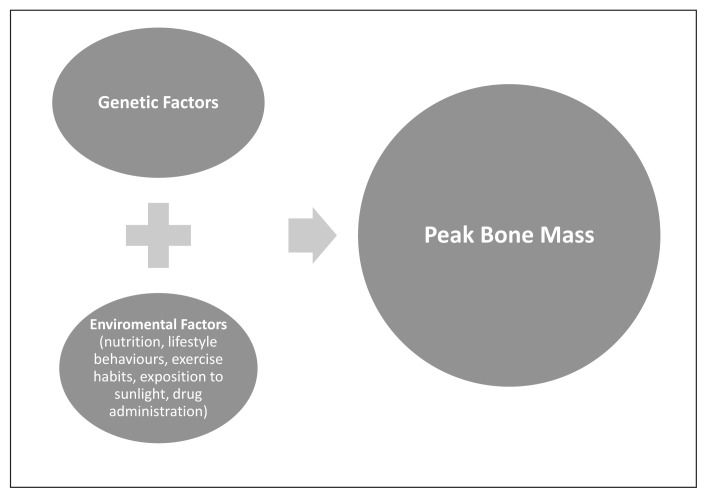
Figure 2.
Interaction of genetic and non-genetic factors on bone mass.
The risk of osteoporosis depends on the achievement of peak bone mass, which is mostly determined by complex and selective genetic, hormonal, nutritional and other environmental factors, which tightly interact.
Pathophysiology of bone diseases in GD
The skeletal manifestations of GD include a variety of bone pathologies due to several factors. Bone manifestations in GD include bone infarcts, avascular bone necrosis, cortical thinning, lytic bone lesions, osteosclerosis, fractures due to osteopenia or osteoporosis and rarely acute osteomyelitis. Bone pathologies may be divided into primary, secondary, and tertiary changes. Primary changes are likely to be due to altered cytokine expression or increased local pressure (4). Changes of cytokines including inflammatory mediators, such as interleukin (IL)-1, IL-6, and tumor necrosis factor-alpha (TNF-α), consequently influence osteoclast and osteoblast activity (4). In particular, the changes of some cytokines which increase bone resorption by osteoclasts and reduced bone formation by osteoblasts seem to be of relevance to the development of osteoporosis in GD (4, 13). Indeed, several bone resorbing cytokines have been found to be elevated in GD-patients (14, 15). Secondary changes, such as bone infarcts, may evolve out of complex pathological mechanisms including changes of cytokine release, alteration of vascularity, and increased local pressure due to extensive glucocerebroside accumulation (16). Clinically, these pathologies are acute events often accompanied by severe bone pain. Tertiary changes summarize those rather chronic pathologies seen as further deterioration evolving out of secondary, firstly acute, changes. In addition, an alteration of the cross-talk of immune cell-osteoclast/osteoblast interactions is present in GD (4). Change in total T-lymphocyte numbers with alteration CD4+/CD8+ T-cells ratios with lower CD8+T has been described in GD with bone disease (17). Cathepsin K has been identified as the principal expressed protein of the osteoclast and its pattern of expression is restricted to osteoclasts, the ovary, and colonic tissue (18). Cathepsin K is highly active in the cleavage of the bone matrix proteins collagen type 1 and osteonectin and its role in bone resorption, modeling, and turnover is clearly demonstrated by the occurrence of an osteopetrotic syndrome in mice homozygous for a disrupted allele of cathepsin K (19). An enhanced cathepsin K expression has been observed in patients with GD (20).
Bone manifestations of GD
Growth retardation
Growth retardation during childhood and an improves of bone mineral density (BMD) and growth rates in pediatric patients with GD has been observed after the enzyme replacement therapy (ERT) (21).
Erlenmeyer flask deformity
The Erlenmeyer flask deformity (EFD) describes a distinct abnormality of the distal femora or other tubular bones and in particular the proximal tibia. It occurs before puberty, develop progressively and it is present in 80% of adult patients. Radiologically is characterized by a constriction of the diaphysis, flaring of the metaphysis and progressive enlargement of the metaphyseal area (4).
Focal osteolytic lesions
They are frequently seen in GD, which may be combined with other localized pathologies such as cortical thinning. The bone has a “worm-eaten” aspect with radiological rarefied cortex and dentate endosteum (4).
Osteonecrosis
It is the most relevant and invalidating skeletal manifestation secondary to bone infarction. The most affected areas are the femoral head, proximal humerus and vertebral bodies. It is a non reversible manifestation and results in articular collapse and pathological fractures. In addition, in GD patients bone infracts may also present with sudden onset of localized pain, tenderness, erythema, and swelling. Such acute episodes of severe bone pain are frequently accompanied by fever, elevated leukocytes, and an accelerated erythrocyte sedimentation rate. This acute focal bone involvement of GD, also called “bone crisis,” can result in aseptic osteomyelitis. “True osteomyelitis” is rare in GD, although clinical differentiation between aseptic and pyogenic osteomyelitis is difficult or even impossible at the time of onset. Negative blood cultures and aspirates can exclude pyogenic osteomyelitis (4). Finally, associated with bone infarction, can be present an osteosclerosis often accompanied by severe pain (irreversible). It can result from: 1) necrotic bone marrow dystrophic calcification and 2) increased activity of periosteum above necrotic area in cases of extensive infarction (22, 23).
Osteoporosis
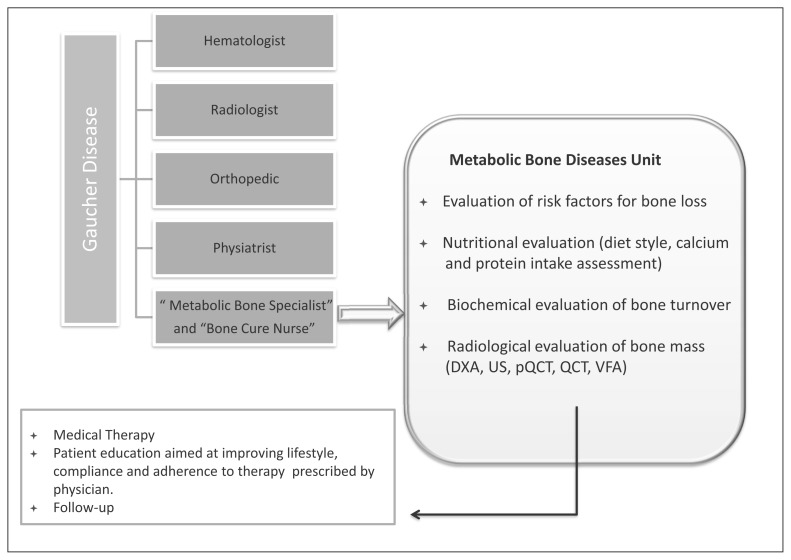
The World Health Organization (WHO) has defined osteoporosis as a systemic skeletal disease characterized by low bone mass and micro-architectural deterioration of bone tissue, with a consequent increase in bone fragility and susceptibility to fracture (24). Approximately 6% of men and 21% of women aged 50–84 years are classified as having osteoporosis. The prevalence of osteoporosis in women over the age of 50 years is 3–4 times greater than in men – comparable to the difference in lifetime risk of an osteoporotic fracture in men and women (25). Osteoporosis is manifested by fractures but the definition of an osteoporotic fracture is not straightforward. Common sites for osteoporotic fracture are the spine, hip, distal forearm and proximal humerus; however, one approach is to consider all fractures from low energy trauma as being osteoporotic. “Low energy” may variously be defined as a fall from a standing height or less, or trauma that in a healthy individual would not give rise to fracture (26). This characterization of low trauma indicates that the vast majority of hip and forearm fractures are low energy injuries or fragility fractures (27). Osteopenia and osteoporosis, is frequent in GD patients, occurring in all ages and in both genders, and progresses with age (16, 28). Low bone mass may be diffuse or localized and can affect both cortical and trabecular bone, and is suggested to occur close to the sites of Gaucher cell infiltration (16). Reduced BMD can be detected as early as 5 years of age in GD1 patients, although it is most pronounced during adolescence (29). Failure to achieve optimal bone mass is likely to affect peak bone mass and may contribute to osteoporosis and increase the risk of pathological fractures and joint collapse during adolescence (30, 31). Further studies (32–35) have confirmed these findings in GD-patients showing decreased BMD at different sites (lumbar spine, neck, trochanter and distal radius). Patients with lower BMD had 5-fold increase in risk to experience a fracture (36). In addition, Khan et al. also indicated that, in the management of patients with GD1, a spinal DXA Z-score <-1 should be a significant trigger for therapeutic intervention directed at maintaining bone mineral density above this value (36). From the metabolic point of view, Giuffrida et al. (37) in a meta-analysis observed that studies involving bone biomarkers in GD patients show variable results which do not currently support their routine use for the clinical assessment of bone status, as an indication for therapy initiation, or monitoring the response to therapy suggesting that a greater understanding of bone markers and the relation to the bone manifestations of GD is required (37). Some studies have shown a positive effect of ERT and Substrate Reduction Therapy (SRT) on the frequency of bone crisis, bone pain and on BMD (38–40). Finally, in GD patients with osteopenia/osteoporosis, few studies on the effects of bisphosphonates (BPs) have been reported in the literature (41, 42) but with etherogenicity of the results and design of study. In addition, no data on the fracture reduction are so far available and the pathophysiology of GD bone complications is not well understood (42). Biochemical markers, bone mineral quantity and quality parameters are needed in long-term studies for diagnosis, staging and monitoring of the skeletal lesions (42) in GD. In addition, an “intelligent” diagnostic approach will consider the use of anti-fracture drugs and/or GD-specific drugs for reduction of fracture risk. Recently Giuffrida et al. (43) have been reported that despite numerous papers on bone complications in patients with GD, there are no specific indications on how to assess properly bone involvement in such condition. A multidisciplinary approach to the GD patient that also includes the bone specialist is predictable. The management of the bone loss of patients with GD should be whenever possible within or in close connection with a center specialized in the diagnosis, management and therapy of metabolic bone diseases. A multidisciplinary approach could be important to better understand the complexity and the pathogenesis of bone involvement in GD. The Figure 3 indicates a possible approach to evaluate the bone metabolism change in patient with GD. By this way will be possible to refine and standardize the diagnostic and therapeutic approaches concerning the bone disease in GD.
Figure 3.
Bone metabolism evaluation.
DXA: Dual-energy X-ray absorptiometry: measurement of bone mineral density (BMD): lumbar spine, hip, wrist, total body;
QUS: Quantitative Ultrasound: heel, finger;
pQCT: Peripheral Quantitative Computed Tomography: leg, wrist;
QCT: Quantitative Computed Tomography: spine;
VFA: DXA Vertebral Fracture Assessment (VFA).
References
- 1.European Union Committee of Expert on Rare Disease. 2013 Report on the state of the art of rare disease activities in Europe. Overview of rare disease activities in Europe. Part I. pp. 1–78. http://www.eucerd.eu/upload/file/Reports/2013ReportStateofArtRDActivities.pdf.
- 2.Mäkitie O. Molecular Defects Causing Skeletal Dysplasias. In: Camacho-Hübner C, Nilsson O, SŠvendahl L, editors. Cartilage and Bone Development and Its Disorders Endocr Dev. Ch 21. Basel: Karger; 2011. pp. 78–84. [DOI] [PubMed] [Google Scholar]
- 3.van Dussen L, Biegstraaten M, Dijkgraaf MGW, et al. Modeling Gaucher disease progression: long-term enzyme replacement therapy reduces the incidence of splenectomy and bone complications. Orphanet Journal of Rare Diseases. 2014;9:112. doi: 10.1186/s13023-014-0112-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mikosch P, Hughes D. An overview on bone manifestations in Gaucher disease. Wien Med Wochenschr. 2010;160(23–24):609–624. doi: 10.1007/s10354-010-0841-y. [DOI] [PubMed] [Google Scholar]
- 5.Germain DP. Gaucher’s disease: a paradigm for interventional genetics. Clin Genet. 2004;65:77–86. doi: 10.1111/j.0009-9163.2004.00217.x. [DOI] [PubMed] [Google Scholar]
- 6.Walton-Bowen K, Mantick N. Gaucher Registry Annual Aggregate Data Report. 2000 [Google Scholar]
- 7.Taichman RS. Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem cell niche. Blood. 2005;105:2631–2639. doi: 10.1182/blood-2004-06-2480. [DOI] [PubMed] [Google Scholar]
- 8.Usha Kini, Nandeesh BN. Physiology of Bone Formation, Remodeling, and Metabolism. In: Fogelman I, et al., editors. Radionuclide and Hybrid Bone Imaging. Ch 2. Springer-Verlag; Berlin Heidelberg: 2012. pp. 29–55. [Google Scholar]
- 9.Rizzolì R, Bianchi ML, Garabédian M, et al. Maximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and the elderly. Bone. 2010;46:294–305. doi: 10.1016/j.bone.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Bachrach LK. Acquisition of optimal bone mass in childhood and adolescence. TRENDS in Endocrinol Metab. 2001;12:22–28. doi: 10.1016/s1043-2760(00)00336-2. [DOI] [PubMed] [Google Scholar]
- 11.Caradonna P, Drigante D. Bone health as a primary target in the pediatric age. European Review for Medical and Pharmacological Sciences. 2009;13:117–128. [PubMed] [Google Scholar]
- 12.Heany RP, Abrams S, Dawson-Hughes B, et al. Peak bone mass. Osteoporos Int. 2000;11:985–1009. doi: 10.1007/s001980070020. [DOI] [PubMed] [Google Scholar]
- 13.Allen MJ, Meyer BJ, Khokher AM, et al. Pro-inflammatory cytokines and the pathogenesis of Gaucher’s disease: increased release of interleukin-6 and interleukin-10. QJM. 1997;90:19–25. doi: 10.1093/qjmed/90.1.19. [DOI] [PubMed] [Google Scholar]
- 14.Barak V, Acker M, Nisman B, et al. Cytokines in Gaucher’s disease. Eur Cytokine Netw. 1999;10:205–210. [PubMed] [Google Scholar]
- 15.Breemen MJ, de Fost M, Voerman JS, et al. Increased plasma macrophage inflammatory protein (MIP)-1alpha and MIP-1beta levels in type 1 Gaucher disease. Biochim Biophys Acta. 2007;1772:788–796. doi: 10.1016/j.bbadis.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Wenstrup RJ, Roca-Espiau M, Weinreb NJ, et al. Skeletal aspects of Gaucher disease: a review. Br J Radiol. 2002;75:A2–A12. doi: 10.1259/bjr.75.suppl_1.750002. [DOI] [PubMed] [Google Scholar]
- 17.Lacerda L, Arosa FA, Lacerda R, et al. T Cell Numbers Relate to Bone Involvement in Gaucher Disease. Blood Cells Mol Dis. 1999;25:130–138. doi: 10.1006/bcmd.1999.0237. [DOI] [PubMed] [Google Scholar]
- 18.Bromme D, Okamoto K. Human cathepsin O2, a novel cysteine protease highly expressed in osteoclastomas and ovary molecular cloning, sequencing and tissue distribution. Biol Chem Hoppe Seyler. 1995;376:379–384. doi: 10.1515/bchm3.1995.376.6.379. [DOI] [PubMed] [Google Scholar]
- 19.Saftig P, Hunziker E, Wehmeyer O, et al. Impaired osteoclastic bone resorption leads to osteopetrosis in cathepsin-K-deficient mice. Proc Natl Acad Sci U S A. 1998;95:13453–13458. doi: 10.1073/pnas.95.23.13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moran MT, Schofield JP, Hayman AR, et al. Pathologic gene expression in Gaucher disease: up-regulation of cysteine proteinases including osteoclastic cathepsin K. Blood. 2000;96:1969–1978. [PubMed] [Google Scholar]
- 21.Bembi B, Ciana G, Mengel E, et al. Bone complications in children with Gaucher disease. Br J Radiol. 2002;75:A37–44. doi: 10.1259/bjr.75.suppl_1.750037. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe M, Yanagisawa M, Sonobe S, et al. An adult form of Gaucher’s disease with a huge tumour formation of the right tibia. Int Orthop. 1984;8:195–202. doi: 10.1007/BF00269916. [DOI] [PubMed] [Google Scholar]
- 23.Poll LW, Koch JA, vom Dahl S, et al. Extraosseous manifestation of Gaucher’s disease type I: MR and histological appearance. Eur Radiol. 2000;10:1660–1663. doi: 10.1007/s003300000446. [DOI] [PubMed] [Google Scholar]
- 24.Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1993;94:646–650. doi: 10.1016/0002-9343(93)90218-e. [DOI] [PubMed] [Google Scholar]
- 25.Hernlund E, Svedbom A, Ivergård M, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. Arch Osteoporos. 2013;8:136. doi: 10.1007/s11657-013-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melton LJ, III, Thamer M, Ray NF, et al. Fractures attributable to osteoporosis: report from the National Osteoporosis Foundation. J Bone Miner Res. 1997;12:16–23. doi: 10.1359/jbmr.1997.12.1.16. [DOI] [PubMed] [Google Scholar]
- 27.Mackey DC, Lui LY, Cawthon PM, et al. High-trauma fractures and low bone mineral density in older women and men. JAMA. 2007;298:2381–2388. doi: 10.1001/jama.298.20.2381. [DOI] [PubMed] [Google Scholar]
- 28.Pastores GM, Wallenstein S, Desnick RJ, et al. Bone density in Type 1 Gaucher disease. J Bone Miner Res. 1996;11:1801–1807. doi: 10.1002/jbmr.5650111125. [DOI] [PubMed] [Google Scholar]
- 29.Mistry PK, Weinreb NJ, Kaplan P, et al. Osteopenia in Gaucher disease develops early in life: response to imiglucerase enzyme therapy in children, adolescents and adults. Blood Cells Mol Dis. 2011;46:66–723. doi: 10.1016/j.bcmd.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mistry PK, Deegan P, Vellodi A, et al. Timing of initiation of enzyme replacement therapy after diagnosis of type 1 Gaucher disease: effect on incidence of avascular necrosis. Br J Haematol. 2009;147:561–570. doi: 10.1111/j.1365-2141.2009.07872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elstein D, Itzchaki M, Mankin HJ. Skeletal involvement in Gaucher’s disease. Bailliere’s Clin Haematol. 1997;10:793–816. doi: 10.1016/s0950-3536(97)80041-8. [DOI] [PubMed] [Google Scholar]
- 32.Ciana G, Addobbati R, Tamaro G, et al. Gaucher disease and bone: laboratory and skeletal mineral density variations during a long period of enzyme replacement therapy. J Inherit Metab Dis. 2005;28:723–32. doi: 10.1007/s10545-005-0032-y. [DOI] [PubMed] [Google Scholar]
- 33.Drugan C, Jebeleanu G, Grigorescu–Sido P, et al. Biochemical markers of bone turnover as tools in the evaluation of skeletal involvement in patients with type 1 Gaucher disease. Blood Cells Mol Dis. 2002;28:13–22. doi: 10.1006/bcmd.2001.0479. [DOI] [PubMed] [Google Scholar]
- 34.Schiffmann R, Mankin H, Dambrosia JM, et al. Decreased bone density in splenectomized Gaucher patients receiving enzyme replacement therapy. Blood Cells Mol Dis. 2002;28:288–96. doi: 10.1006/bcmd.2002.0517. [DOI] [PubMed] [Google Scholar]
- 35.Fiore CE, Barone R, Pennisi P, et al. Bone ultrasonometry, bone density, and bone turnover markers in type 1 Gaucher disease. J Bone Miner Metab. 2002;20:24–38. doi: 10.1007/s774-002-8444-1. [DOI] [PubMed] [Google Scholar]
- 36.Khan A, Hangartner T, Weinreb NJ, Taylor JS, Mistry PK. Risk factors for fractures and avascular osteonecrosis in type 1 Haucher Disease: a study from international Collaborative Gaucher Group (ICGG) Gaucher Registry. J Bone Min Res. 2012;27:1839–1848. doi: 10.1002/jbmr.1680. [DOI] [PubMed] [Google Scholar]
- 37.Giuffrida G, Cingari MR, Parrinello N, et al. Bone turnover markers in patients with type 1 Gaucher disease. Hematology Reports. 2012;4:e21. doi: 10.4081/hr.2012.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elstein D, Foldes AJ, Zahrieh D, et al. Significant and continuous improvement in bone mineral density among type 1 Gaucher disease patients treated with velaglucerase alfa: 69-month experience, including dose reduction. Blood Cells Mol Dis. 2011;47:56–61. doi: 10.1016/j.bcmd.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 39.Sims KB, Pastores GM, Weinreb NJ, et al. Improvement of bone disease by imiglucerase (Cerezyme) therapy in patients with skeletal manifestations of type 1 Gaucher disease: results of a 48-month longitudinal cohort study. Clin Genet. 2008;73:430–440. doi: 10.1111/j.1399-0004.2008.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deegan PB, MD, Pavlova E, et al. Osseous Manifestations of Adult Gaucher Disease in the Era of Enzyme Replacement Therapy. Medicine. 2011;90(1):52–60. doi: 10.1097/MD.0b013e3182057be4. [DOI] [PubMed] [Google Scholar]
- 41.Rossi L, Zulian F, Stirnemann J, et al. Bone involvement as presenting sign of pediatric-onset Gaucher disease. Joint Bone Spine. 2011;78:70. doi: 10.1016/j.jbspin.2010.02.045. [DOI] [PubMed] [Google Scholar]
- 42.Mikosch P. Miscellaneous non-inflammatory musculoskeletal conditions. Gaucher disease and bone. Best Pract Res Clin Rheumatol. 2011;25:665–681. doi: 10.1016/j.berh.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 43.Giuffrida G, Cappellini MD, Carubbi F, et al. Management of bone disease in Gaucher disease type 1: clinical practice. Adv Ther. 2014;31:1197–1212. doi: 10.1007/s12325-014-0174-0. [DOI] [PubMed] [Google Scholar]