Summary
Autologous bone, for its osteoconductive, osteoinductive and osteogenetic properties, has been considered to be the gold standard for maxillary sinus augmentation procedures. Autograft procedures bring also some disadvantages: sometimes the limited amount of available intraoral bone makes necessary to obtain bone from an extraoral site, and this carries an associated morbidity. To overcome this problem we started using homologous freeze-dried bone in maxillary sinus augmentation procedures. This bone is industrially processed with γ-irradiation to eliminate its disease transmission potential and it’s considered safe, but this treatment also eliminates the osteoinductive and osteogenetic properties, making it just an inert scaffold for regeneration. Mesenchymal stem cells are successfully used in and orthopedic surgery for their amplification potential of healing mechanisms. We assumed that mesenchymal stem cells can restore the osteogenetic and osteoinductive properties in homologous bone grafts. The aim of this study was an histological evaluation of bone regeneration in maxillary sinus elevation using: 1) mesenchymal stem cells engineered freeze-dried bone allografts; 2) freeze-dried bone allografts.
Twenty patients (10M, 10F) with a mean age of 55.2 years affected by severe maxillary atrophy were treated with bilateral maxillary sinus floor elevation. For each patient were randomly assigned a “test” side and a “control” side, different from each other exclusively in the composition of the graft material. The “control” sides were composed by corticocancellous freeze-dried bone chips and the “test” sides were composed by corticocancellous freeze-dried bone chips engineered in a bone marrow mesenchymal stem cells concentrate. After three months bone biopsies were performed on the grafts and histological specimens were made in order to evaluate the healed bone from an histological point of view.
Histologically all the specimens showed active remodelling signs and all the tissues were free of inflammatory cells. “Control” side specimens showed a substantial persistence of the grafted bone and, with the interposition of connective tissue, a considerable amount of newly formed bone. “Test” side specimens showed a much more represented cellular component compared to the “control” sides. The grafted bone trabeculae, when detectable, were completely imprisoned inside new formed bone, in direct contact with it and without interposition of connective tissue.
Freeze-dried bone can be used successfully as graft material in the treatment of maxillary atrophy. The same bone engineered with stem cells showed a greater histological integration potential comparable with autografts histological morphology. Further studies are needed to confirm these hypotheses.
Keywords: Mesenchimal Stem Cells, bone graft, maxillary atrophy, sinus lift
Introduction
Autologous bone grafts are currently considered the gold standard for regeneration of the hard tissue in oral surgery because of their osteoconductive, osteoinductive and osteogenic properties. However, they are limited by major disadvantages, such as lack of availability of intraoral bone, double-site surgery and postoperative morbidity, that have stimulated the research for alternative graft materials (1, 2). Various osteoconductive materials have been used for grafting but these, due to the preparation technique, are devoid of cells and growth factors (3–5) and require long times for integration.
In recent years, bone grafting techniques have taken great advantage of the establishment of Tissue Banks, which have allowed an increasing diffusion of freeze-dried bone allografts (FDBA). Freeze-drying is suitable for reducing heterologous bone tissue antigenicity without inducing biomechanical changes in the material. This procedure consists in sublimation of water from frozen bone tissue specimens, which are then vacuum-packed and made suitable for long-term storage at room temperature. On the other hand, freeze-drying destroys bone cells and soft organic matrix, thus resulting in a substantial limitation of the osteogenic and osteoinductive potential of FDBA, which can be regarded as a mere osteoconductive scaffold.
In the last years, a tissue engineering procedure has been set up in which autologous mesenchymal stem cells (MSCs) are used in combination with an osteoconductive scaffold as a graft material. MSCs are a population of bone marrow-derived, non-hematopoietic multipotent cells which can be expanded and differentiated in vitro into cells with osteogenic phenotype (6, 7). Recent studies have highlighted that MSCs are capable of regenerating large bone defects when used in combination with bone substitutes (8, 9) and increasing allograft osteointegration (10).
Another method that has been used to improve the outcome of bone grafting is based on autologous platelets. Indeed, they are a readily accessible, safe and cheap source of growth factors, such as platelet-derived growth factor (PDGF), transforming growth factor beta (TGF-b), epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), and insulin-like growth factor I (IGF-I), which play a key role in tissue homeostasis (11–13). Platelets are usually concentrated in a small volume of plasma called platelet-rich plasma (PRP), which can be an excellent addition to MSCs and freeze-dried bone grafts. In fact, MSCs express receptors for the growth factors contained in the PRP, and in vitro studies have shown that the addition of PRP promotes MSC proliferation.
Based on the above grounds, we performed the present study to test the hypothesis that grafting of FDBA in combination with autologous MSCs and PRP may lead to increased bone regeneration and reduced healing time, thus reproducing the performance of autologous bone grafting.
Materials and methods
Subjects
Twenty patients (10 males and 10 females, mean age 55.2) with severe maxillary atrophy, attributable to V class, according to Cawood and Howell (14) and requiring bilateral maxillary sinus augmentation, were enrolled in the study between September 2010 and February 2012. The clinical protocol complied with guidelines of the Declaration of Helsinki, as amended in Edinburgh, 2008, and was approved by the Ethical Committee of the Faculty of Medicine, University of Florence, Italy. The patients were thoroughly informed about the possible risks and complications related to the use of homologous grafting materials, including the rare possibility of transmission of human immunodeficiency (HIV), hepatitis C (HCV) and hepatitis B (HBV) viruses. All the enrolled patients signed a written informed consent to the treatment.
Inclusion criteria were: i) edentulous premolar and molar region and ii) presence of less than 5 mm of residual crestal bone between the sinus floor and alveolar ridge. General exclusion criteria were: acute myocardial infarction in the six months preceding the intervention, coagulation disorders, metabolic diseases (diabetes mellitus, bone diseases), psychiatric disorders, heavy smoking habits (> 10 cigarettes per day), head/neck radiotherapy in the previous 24 months, treatment with intravenous bisphosphonates. Local exclusion criteria were: disorders of the maxillary sinus, oral infections and periodontal diseases.
All patients underwent anamnesis, physical examination and panoramic radiographs. Subsequently CT investigations were performed to evaluate the best sites for implant placement, to assess the morphology of the maxillar/mandibular bone and to identify possible disorders of the maxillary sinus. According to the split-mouth design, each patient underwent two parallel treatments, as detailed below: the test quadrant was grafted with FDBA in the form of corticocancellous particulate, rehydrated in sterile saline and then absorbed with MSCs and PRP; the control quadrant was grafted with FDBA alone. Allocation concealment of the patients’ quadrants to either treatments was performed by sequentially numbered opaque sealed envelopes (SNOSE).
MSC preparation and PRP preparation
We have used a RegenKit Extracell Glue procedure (RegenLab SA Losanne, Swiss) to concentration of autologous Stem Cells
Preparation of autologous bone marrow from withdrawal of the iliac crest:
4 sampling points moving with the trocar;
filling volume in Regen THT tubes, 5 ml for tube, final volume before centrifugation approximately 20 ml;
only centrifugation 3400 rpm x 8 minutes (greater recovery of stem cells);
Centrifuge Regen Centrigel H-19 F.
Gently resuspend the cells by separating gel, concentrated and put them in a arcella for engineering, based on the final volume of autologous stem cells concentrate (about 14/15 ml) will added 5ml of gluconate calcium if the cells will need to be gelled.
Protocol of use PRP
Blood withdrawal directly with the closed tube - system, concentration of platelets with dedicated centrifuge Regen Centrigel H19 F.
Only centrifugation 3200 rpm x 12 minutes, collecting the PRP above cells selector gel. Production of Autologous Thrombin Serum with dedicated ATS tube.
Complete solution
Mix of BMC and PRP with calcium and autologous bone or substitute for osseous reconstruction.
Effects and advantages
Autologous and natural product, no risk of intolerance or allergies. Stimulate healing process and the growth of injured tissues, improves angiogenesis and rivascularisation of tissues, stimulate mesenchymal cells proliferation, stimulate both fibroblasts growth and collagen production, accelerate the development of bone tissues by osteo-inductive capacity and stimulation of osteoblast growth.
Mixing of the components
In the sterile surgical field, a container was filled with lyophilized bone tissue. The bone was then rehydrated by soaking in freshly prepared PRP and then absorbed with concentrated MSCs. Finally, autologous thrombin serum was added to induce platelet activation.
Surgical Procedure
The patients were subjected to general anesthesia in nasotracheal intubation. Intraoral and extraoral tissues were disinfected with 10% Iodopovidone (Betadine, Purdue Pharma LP, Stamford, CT, USA). The maxillary surgical sites were infiltrated with local anesthetic with vasoconstrictor (40 mg/ml D-Ultracain plus 5 mg/ml Suprarenin; Hoechst, Frankfurt am Main, Germany). The anterolateral wall of the maxillary sinus was exposed by continue crestal incision and a vertical incision in the vestibular fornix. After elevation of a mucoperiosteal flap, an oval-shaped osteotomy was made with a round bur and the Schneiderian membrane was lifted by an appropriate periosteal elevator. Then the control or test graft material was put in place, according to the randomized split-mouth protocol. Finally, the flap was sutured without tension to prevent dehiscence using multiple points in horizontal mattress sutures with 3-0 absorbable thread (Vicryl Rapid, Ethicon, Johnson & Johnson Rome, Italy).
Biopsy procedure
Three months after maxillary sinus surgery, the patients were recalled for implant placement. During this intervention, bone biopsies perpendicular to the alveolar bone were performed in the grafted region using a trephine drill (16 mm length, 5 mm outer diameter, 3.5 mm inner diameter) under constant cooling using sterile saline flow. After collection, the specimens were marked at the level of external cortical bone with India ink and immediately fixed by immersion in buffered 4% formaldehyde (pH 7.3) overnight. Then, bone samples were thoroughly washed and stored in 70% ethanol at 4° C until inclusion. The samples were included in an embedding medium composed of 80% methyl methacrylate and 20% Plastoid®. Five μm-thick sections were obtained with a microtome (Reichert-Jung, Germany), de-waxed and stained with hematoxylin and eosin.
Results
Clinical observations
No complications occurred during the surgical procedures for maxillary sinus elevation, the post-operative course and the bone biopsies performed 3 months after surgery. None of the enrolled patients complained for pain or other dysfunctional symptoms which could impair normal feeding habits. In all the patients, at the time of biopsy, the objective conditions of the control and test quadrants were deemed adequate for insertion of implants, allowing prosthetic reconstruction and rehabilitation of the dental apparatus.
Histological analysis
By light microscopy, all the samples examined showed signs of bone remodelling and no substantial infiltration by inflammatory cells. However, major histologic differences were observed between the control and test areas.
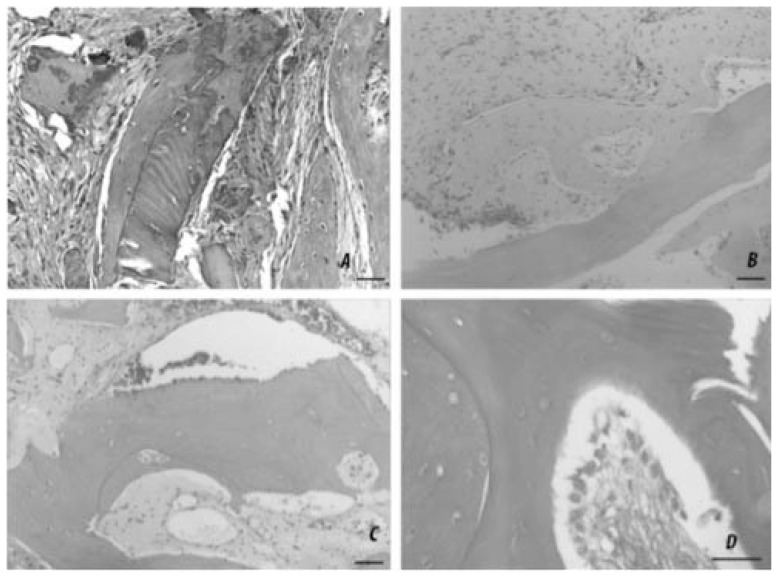
In particular, the biopsies taken from the control quadrants (Figure 1, A–D) showed numerous elongated particles of the grafted FDBA, recognizable by the presence of acellular bony lacunae and pseudo-basophilia of the extracellular matrix, intermingled with trabeculae of newly formed bone, recognizable as being populated by viable osteocytes. However, the grafted FDBA was usually separated from the trabecular bone by large amounts of interposed fibrous connective tissue. It is conceivable that this type of graft material, used alone, carries an action exclusively osteoconductive and that therefore will encounter a substitution mechanism similar to that of fresh frozen bone (FFB), described by other Authors as “creeping substitution”.
Figure 1.
A, B, C, D. Representative micrographs of decalcified samples of grafted bone in different experimental conditions at 3-month-follow-up. A) Autologous bone shows an optimal integration potential, as judged by extended, tight contact between the grafted and newly formed bone, with scarce fibrotic reaction. B) Freeze-dried bone allograft (control side) shows a moderate degree of integration, with the grafted trabeculae only partially apposed to the newly formed bone and a consistent grade of fibrosis. C) Bio-engineered bone allograft (test side) shows very good osteointegration, with the grafted trabeculae completely encircled by newly formed bone and negligible fibrosis. D) High-magnification detail of an osteointegrating area in which a continuous row of osteoblasts can be seen juxtaposed to a grafted trabecula. H&E; bars = 50 μm.
On the other hand, the biopsies taken from the test quadrants showed that the grafted FDBA was usually adjacent to or embedded within the newly formed bone, without interposition of fibrous connective tissue. This histological pattern is suggestive for successful osseointegration of the grafted FD-BA conditioned with MSCs and PRP and closely resembles that of autologous bone grafts.
For proper comparison, an archive biopsy taken from a patient who underwent grafting of autologous bone from the iliac crest was also examined. In this biopsy, an optimal integration of the grafted and endogenous bone was observed, as judged by the extended, tight contact between the two bone tissues and the absence of any fibrotic reaction. This type of healing resembles that observed in the grafts of autologous bone.
Discussion and conclusion
The increasing demand for implant-based prosthetic rehabilitation in patients with maxillary atrophy has prompted operators to search for a valuable alternative to autologous bone for grafting purposes. In this context, the use of lyophilized human bone in combination with bone-stimulating cells has been investigated as a suitable method to induce endogenous bone regeneration, in a way that can fairly reproduce the osseoinductive effects of autologous bone.
The present split-mouth study offers circumstantial evidence that a bio-engineered grafting material, composed of homologous freeze-dried bone (FDBA), absorbed with autologous MSCs and activated PRP, can induce de-novo formation of alveolar bone, thus substantially accelerating elevation of the floor of the maxillary sinus for implant placement purposes. In fact, the osseoinductive performance of the bio-engineered FDBA was significantly better than that of FDBA alone placed in the contralateral, control sites of the same patients. Clinically, 3 months after grafting, the newly-formed bone is sufficiently stable to allow safe placement of endosseous implants. On the other hand, it is conceivable that FDBA alone can have a merely osteoconductive effect, activating a slow substitution mechanism similar to that observed with fresh frozen bone (FFB) and previously termed “creeping substitution” (15). The histological analysis of bone biopsies taken 3 months after grafting confirmed the clinical data. Indeed, the degree of osteogenesis was greater in the test than in the control quadrants and clear-cut images of newly-formed bone interlocked with the grafted bone were frequently observed in the former sites. Basically, these histological pictures were nearly similar to those observed upon grafting of autologous bone taken from the ileal crest.
The present findings fit well with those of the recent literature, indicating that transplantation of MSCs and PRP can induce increased bone formation when using osteoconductive scaffold for maxillary sinus floor raising (Ohya et al., 2005, Ueda et al., 2005).
In conclusion, our study provides further support to the notion that FDBA bio-engineered with MSCs and PRP can significantly improve bone formation as compared with FDBA alone and can represent the treatment of choice for sinus floor elevation. In turn, rapid and adequate reconstitution of maxillary bone thickness can significantly improve the biomechanical performance and hence clinical outcome of dental implants. Further studies are needed to determine the long-term behaviour of this grafting material, especially concerning the possibility of delayed resorption.
References
- 1.van den Bergh JP, ten Bruggenkate CM, Groeneveld HH, Burger EH, Tuinzing DB. Recombinant human bone morphogenetic protein-7 in maxillary sinus floor elevation surgery in 3 patients compared to autogenous bone grafts. A clinical pilot study. Journal of clinical periodontology. 2000;27:627–36. doi: 10.1034/j.1600-051x.2000.027009627.x. [DOI] [PubMed] [Google Scholar]
- 2.Nkenke E, Weisbach V, Winckler E, Kessler P, Schultze-Mosgau S, Wilt-fang J, Neukam FW. Morbidity of harvesting of bone grafts from the iliac crest for preprosthetic augmentation procedures: a prospective study. International journal of oral and maxillofacial surgery. 2004;33:157–63. doi: 10.1054/ijom.2003.0465. [DOI] [PubMed] [Google Scholar]
- 3.Nasr HF, Aichelmann-Reidy ME, Yukna RA. Bone and bone substitutes. Periodontology 2000. 1999;19:74–86. doi: 10.1111/j.1600-0757.1999.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 4.Degidi M, Artese L, Rubini C, Perrotti V, Iezzi G, Piattelli A. Microvessel density in sinus augmentation procedures using anorganic bovine bone and autologous bone: 3 months results. Implant dentistry. 2007;16:317–25. doi: 10.1097/ID.0b013e3180de4c5f. [DOI] [PubMed] [Google Scholar]
- 5.Gronthos S, Akintoye SO, Wang CY, Shi S. Bone marrow stromal stem cells for tissue engineering. Periodontology 2000. 2006;41:188–95. doi: 10.1111/j.1600-0757.2006.00154.x. [DOI] [PubMed] [Google Scholar]
- 6.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell and tissue kinetics. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 7.Bruder SP, Kraus KH, Goldberg VM, Kadiyala S. The effect of implants loaded with autologous mesenchymal stem cells on the healing of canine segmental bone defects. The Journal of bone and joint surgery. American volume. 1998;80:985–96. doi: 10.2106/00004623-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 8.De Kok IJ, Drapeau SJ, Young R, Cooper LF. Evaluation of mesenchymal stem cells following implantation in alveolar sockets: a canine safety study. The International journal of oral & maxillofacial implants. 2005;20:511–8. [PubMed] [Google Scholar]
- 9.Dallari D, Fini M, Stagni C, Torricelli P, Nicoli Aldini N, Giavaresi G, Cenni E, Baldini N, Cenacchi A, Bassi A, Giardino R, Fornasari PM, Giunti A. In vivo study on the healing of bone defects treated with bone marrow stromal cells, platelet-rich plasma, and freeze-dried bone allografts, alone and in combination. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2006;24:877–88. doi: 10.1002/jor.20112. [DOI] [PubMed] [Google Scholar]
- 10.Lucarelli E, Beccheroni A, Donati D, Sangiorgi L, Cenacchi A, Del Vento AM, Meotti C, Bertoja AZ, Giardino R, Fornasari PM, Mercuri M, Picci P. Platelet-derived growth factors enhance proliferation of human stromal stem cells. Biomaterials. 2003;24:3095–100. doi: 10.1016/s0142-9612(03)00114-5. [DOI] [PubMed] [Google Scholar]
- 11.Pryor ME, Polimeni G, Koo KT, Hartman MJ, Gross H, April M, Safadi FF, Wikesjo UM. Analysis of rat calvaria defects implanted with a platelet-rich plasma preparation: histologic and histometric observations. Journal of clinical periodontology. 2005;32:966–72. doi: 10.1111/j.1600-051X.2005.00772.x. [DOI] [PubMed] [Google Scholar]
- 12.Christgau M, Moder D, Hiller KA, Dada A, Schmitz G, Schmalz G. Growth factors and cytokines in autologous platelet concentrate and their correlation to periodontal regeneration outcomes. Journal of clinical periodontology. 2006;33:837–45. doi: 10.1111/j.1600-051X.2006.00991.x. [DOI] [PubMed] [Google Scholar]
- 13.Kassolis JD, Rosen PS, Reynolds MA. Alveolar ridge and sinus augmentation utilizing platelet-rich plasma in combination with freeze-dried bone allograft: case series. Journal of periodontology. 2000;71:1654–61. doi: 10.1902/jop.2000.71.10.1654. [DOI] [PubMed] [Google Scholar]
- 14.Cawood JI, Howell RA. A classification of the edentulous jaws. Int J Oral Maxillofac Surg. 1988 Aug;17(4):232–6. doi: 10.1016/s0901-5027(88)80047-x. [DOI] [PubMed] [Google Scholar]
- 15.Acocella A, Bertolai R, Nissan J, Ellis E, 3rd, Sacco R. Maxillary sinus lift using fresh frozen bone chips in presence of sinus cyst: clinical and histological report. Cell Tissue Bank. 2012 Jun;13(2):327–32. doi: 10.1007/s10561-011-9259-z. Epub 2011 May 26. [DOI] [PubMed] [Google Scholar]