Abstract
Background
There is mixed support for the efficacy of the opioid antagonist naltrexone in the treatment of nicotine dependence. One potential unexplored mechanism underlying naltrexone’s effects in smoking cessation may be in its ability to reduce alcohol consumption.
Methods
Alcohol consumption and liver enzyme levels (aspartate aminotransferase and alanine transaminase) were examined in a sample of 78 nonalcoholic social drinking smokers (34 naltrexone, 44 placebo) enrolled in a double-blind randomized clinical trial of naltrexone in smoking cessation. Naltrexone or placebo began 3 days prior to the quit date (25 mg daily) and continued for 8 weeks (50 mg daily). All participants received nicotine patches and behavioral counseling up through 4 weeks after the quit date.
Results
Naltrexone significantly reduced weekly heavy drinking rates. This effect was associated with greater nausea and pill taking adherence within the naltrexone group. Within heavy drinkers, naltrexone also directionally improved smoking quit rates compared with placebo. Liver enzyme levels did not differ during treatment with naltrexone compared with placebo.
Conclusions
Naltrexone may reduce the frequency of heavy drinking in nonalcoholics attempting to quit smoking. Further, naltrexone may preferentially improve smoking quit rates within heavy drinkers who smoke, and further investigation in larger sample sizes is warranted.
Keywords: Naltrexone, Smoking Cessation, Alcohol, Binge Drinking, Heavy Drinking, Liver
There is a preponderance of evidence that the opioid antagonist naltrexone, compared with placebo, reduces relapse rates), in patients enrolled in alcohol dependence treatment (for review, see Pettinati et al., 2006). These data lead to FDA approval in 1994 for 50-mg oral naltrexone in the treatment of alcohol dependence. Various mechanisms have been proposed to explain naltrexone’s effect on decreasing relapse rates, including decreasing alcohol craving (O’Malley et al., 1996), attenuating the positive or euphoric effects of alcohol (Davidson et al., 1999; King et al., 1997; Swift et al., 1994; Volpicelli et al., 1995), and increasing alcohol’s sedative or aversive effects (Davidson et al., 1996, 1999; King et al., 1997; Swift et al., 1994).
While naltrexone has been established for treatment of alcohol dependence, it is unclear whether naltrexone may be efficacious for the treatment of nicotine dependence. The most recent 2006 Cochrane Report concluded that more data is needed in larger sample sizes before conclusions can be drawn about naltrexone for smoking cessation (David et al., 2006). Preclinical and clinical studies have yielded mixed results on the effects of naltrexone to alter human smoking behaviors (for review, see King et al., 2009). However, recent research suggests that naltrexone may be particularly efficacious in specific smoker subgroups who have shown greater difficulty in quitting smoking; such as women (Covey et al., 1999; King et al., 2006), those with past or current depressive symptoms (Covey et al., 1999; Walsh et al., 2008), and smokers concerned about cessation-related weight gain (King et al., 2006; O’Malley et al., 2006). Another potential subgroup of interest may be smokers who are also regular alcohol drinkers, or those with concurrent heavy drinking patterns, i.e., a historically treatment resistant group in smoking cessation (Shiffman et al., 1994). Since alcohol drinking may increase urges to smoke (Epstein et al., 2007; King and Epstein, 2005) and precipitate smoking lapses (Borland, 1990; Shiffman et al., 1996), it would follow that naltrexone might be beneficial for drinker-smokers. While smoking outcomes with naltrexone are as yet unclear, there is initial support for naltrexone to decrease smokers’ hazardous drinking rates during the first 6 weeks of smoking cessation (O’Malley et al., 2008).
One potential issue with naltrexone is its safety profile in terms of hepatic function. Past studies in obese men administered high-dose naltrexone (300 mg daily) indicated clinically significant elevations in the liver enzymes aspartate aminotransferase (AST) and alanine transaminase (ALT) (Mitchell et al., 1987; Pfohl et al., 1986). Such findings resulted in a black box warning of liver toxicity in the naltrexone package insert. Clinicians therefore may hesitate to use naltrexone due to concerns over potential liver toxicity. However, there is little data to support liver inflammation or damage in otherwise healthy alcoholics receiving the FDA-approved dose of 50 mg daily (Carmen et al., 2004; Croop et al., 1997), in fact liver enzyme levels are often lower in patients treated with naltrexone because of naltrexone-related decreases in harmful drinking (Berg et al., 1996). Cigarette smoking has been shown to induce the activity of cytochrome P450 (cyp)1A2 which can increase the clearance of certain medications (Tantcheva-Poor et al., 1999). Thus, smoking cessation in patients who are receiving medications metabolized by this system can result in elevated levels of the medication and potential toxicities. The cytochrome P450 system is not involved in naltrexone metabolism, therefore smokers involved in a smoking cessation program should not be at increased risk for naltrexone-induced liver inflammation, but data on the safety of naltrexone on hepatic function in nicotine-dependent persons are lacking. In addition to assessing drug safety for medications metabolized by the liver, liver enzymes have been used as an objective index of alcohol’s effects on liver functioning; however, there are limitations in terms of sensitivity and specificity, particularly in non-dependent samples (Allen et al., 2001; Lee et al., 2001).
In the present study, we examined the effects of naltrexone (50 mg oral daily) on alcohol drinking, including total drinks per week and heavy drinking rates in nonalcoholic social drinkers enrolled in a smoking cessation clinical trial. Smoking outcomes from the larger trial (King et al., 2006) showed that naltrexone modestly increased end-of-treatment quit rates (48%) compared with placebo (41%) but this was not statistically significant. Other outcome indicators, besides smoking quit rates, may help elucidate mechanisms underlying opioid antagonism effects to smoking and related behaviors, especially in terms of co-morbidity between alcohol and tobacco. Therefore, this exploratory study focused on naltrexone’s effects on alcohol drinking outcomes in the subset of currently drinking nonalcohol-dependent participants enrolled in the larger King and colleagues (2006) study. We also examined subjects’ liver enzyme levels at baseline and during treatment to verify the safety of 50 mg naltrexone daily in nicotine-dependent subjects.
MATERIALS AND METHODS
Participants
Participants were n = 78 nonalcoholic, current social drinking smokers desiring to quit smoking. They were taken from the larger sample of 110 participants enrolled in a double-blind clinical trial investigating the efficacy of naltrexone in smoking cessation (King et al., 2006). To meet criteria for inclusion as a current drinker in the present study, participants must have reported consuming at least one alcoholic drink during the 2-week baseline period prior to study enrollment, and must not have met criteria for current or past alcohol dependence. The justification for this criterion was to include subjects who were “current drinkers” and were recently consuming alcohol in order to capture naltrexone’s effects on current drinking behaviors. The current drinker designation from the baseline period had high concurrence (i.e. 97%) with a retrospective quantity–frequency interview of past 6 months drinking. The remaining 32 subjects from the original study were not appropriate for inclusion in this exploratory study because they were teetotalers, infrequent drinkers, or recovered alcoholics, as determined by Master’s level diagnostic interview of quantity–frequency measures and the Structured Clinical Interview for DSM-IV (First et al., 1995). Of the 78 subjects included in the current study, n = 34 were randomly assigned to naltrexone and n = 44 were randomly assigned to placebo by use of a computer number generator.
Procedure
After meeting medical, psychiatric, and smoking eligibility criteria (for details see King et al., 2006), eligible participants were enrolled in the study. There were 8 study visits over a 10-week period. All participants received the platform treatment of 6 individual weekly 45-minute behavioral counseling sessions using a standard cognitive-behavioral smoking cessation treatment manual (© King and Riley, 2001) delivered by a Master’s level clinician, and 1 month of nicotine patch (Nicoderm® CQ® patches; GlaxoSmithKline Consumer Healthcare, Moon Township, PA). The materials were focused on skills for tobacco cessation and alcohol-related counseling was not included. In fact, alcohol drinking was only mentioned briefly as part of the larger context of potential triggers for smoking relapse. Consistent with the clinical practice guidelines (Fiore et al., 2008) and with data showing potential negative or withdrawal-like effects with naltrexone only for smokers (Covey et al., 1999; Krishnan-Sarin et al., 1999), starting on the quit date, subjects received 2 weeks of nicotine patch at 21 mg daily, followed by 1 week of 14 mg daily, and then 1 week of7 mg daily.
Upon enrollment, participants attended the first 2 weekly study visits prior to drug randomization. These visits consisted of a research assessment followed by an individual behavioral counseling session to prepare the participant for the quit date. Weekly study visits continued in a similar format (assessments and counseling) on the third week, the quit date, and for 3 more weekly visits over the ensuing month. The last 2 visits were conducted every other week and included research assessments only. The study drug began 3 days prior to the quit date with an initial dose of 25 mg oral naltrexone daily that increased to 50 mg daily on the fourth day (i.e., the smoking quit date) and for the ensuing 8 weeks. Participants in the placebo group received identical placebo tablets on the same schedule. Reimbursements were provided for travel expenses and for time for completing study measures (a $35 gift card with a drawing for an additional gift card valued at either $50 or $100).
Dependent Variables
Alcohol Drinking and Smoking Measures
To determine baseline level of tobacco dependence, subjects completed the 6-item Fagerström Test for Nicotine Dependence (FTND; Heatherton et al., 1991). At baseline screening and during each study visit, the Time Line Follow Back interview (TLFB; Sobell and Sobell, 1995; Sobell et al., 1979) was used to derive daily estimates of cigarette and alcohol drinking, starting with the interval 2 weeks prior to the first study visit and continuing for the next 6 visits. This interview was conducted by a trained research assistant and subjects were asked to report daily alcohol drinking and cigarette smoking behaviors for each day since their last visit. One drink was defined as 1.5 oz of liquor, 5 oz. of wine, or 12 oz. of beer, and 1 cigarette was defined as 1 puff or more of a cigarette. Dependent variables were weekly total number of alcoholic drinks consumed and weekly heavy drinking rates (dichotomized as reporting 1 or more heavy drinking episodes). A heavy drinking episode (SAMHSA, 2005) was defined as consuming on at least 1 occasion in that week of 5 or more drinks for men, and 4 or more drinks for women. The TLFB was also used to determine the subset of heavier social drinkers (n = 36) for secondary analyses (see Data Analysis). Heavy drinkers were conservatively classified as those who reported at least 1 heavy drinking episode during the 2-week pre-enrollment baseline period; this determination was supported by a retrospective 6-month quantity–frequency interview showing that the majority of persons in this subset engaged in heavy drinking bouts at least monthly.
Smoking quit status was determined at the end of the first month of treatment and defined as not smoking even a puff in any day in each of 2 consecutive weeks or not smoking even a puff on 7 days in a row (Hughes et al., 2003), and confirmed by a carbon monoxide breath test reading of ≤10 ppm. Finally, pill and patch taking adherence was conducted by self-report and pill and patch counts at each visit. For each subject, the percentage of pills and patches reported taken weekly was computed.
Hepatic Function
During baseline screening and again approximately 1 month later, a hepatic function panel test, including analyses of AST and ALT levels were conducted. Values were considered normal for AST if between 8 and 37 U/l of serum, and normal for ALT if between 8 and 35 U/l of serum. At baseline, 4 subjects (3 randomized to naltrexone, 1 randomized to placebo) had ALT levels out of normal range, but all values were <54 IU. Three subjects (2 randomized to naltrexone, 1 randomized to placebo) had baseline AST levels out of normal range but all values were <42 IU.
Side-effects were assessed weekly by an 8-item scale (Volpicelli et al., 1992; King et al., 1997) of naltrexone-related side-effects, including nausea, vomiting, headache, light-headed, flushed/warm, vague symptoms of agitation/anxiety, increased sexual desire, and increased erections.
Statistical Analyses
Chi-square and t-tests were used, where appropriate, to compare the naltrexone and placebo groups on demographic and baseline data. A generalized equations estimation (GEE) model (Liang and Zeger, 1986) was used to compare the groups on weekly alcoholic drinks consumed and on medication compliance, and a GEE model with a logit link function was used to compare groups on weekly heavy drinking rates. Similar analyses were conducted in the subset of heavier social drinkers. Chi-square tests were used to compare the groups on 1-month smoking quit rates. A mediation analysis (Baron & Kenny, 1986) was used to examine the effect of heavy drinking days on the relationship of medication and smoking quit rates. A GEE model was used to compare groups on nausea and side-effects and also to examine mediation of these variables on the relationship between treatment (naltrexone) to drinking outcomes. Paired t-tests were used to compare groups on AST and ALT levels and chi-squared tests were used to examine percentage of subjects outside the normal range.
RESULTS
Demographic characteristics and the baseline drinking and smoking levels for naltrexone and placebo groups are shown in Table 1. The groups were similar on most background variables including age, years of education, sex, ethnic/racial composition, marital status, body mass index, and baseline weekly drinking. Cigarettes smoked per day and FTND scores were significantly higher in the naltrexone compared with placebo group (see Table 1). This difference in baseline smoking did not relate to post-treatment alcohol or smoking outcomes (ps ≥ 0.15 for cigarettes per day; ps ≥ 0.51 for FTND scores).
Table 1.
Demographic and Baseline Smoking and Drinking Characteristics
| Demographic variables | Placebo group (n = 44) |
Naltrexone group (n = 34) |
|---|---|---|
| Age (years) | 42.5 ± 1.8 | 42.2 ± 2.0 |
| Education (years) | 14.8 ± 0.3 | 14.3 ± 0.4 |
| Sex (male) | (55%) | 19 (56%) |
| Race (Caucasian) | 31 (70%) | 26 (76%) |
| Married or living with partner | 19 (43%) | 15 (44%) |
| Body mass index (kg/m2) | 25.6 ± 0.7 | 26.0 ± 0.8 |
| Baseline smoking | ||
| Average cigarettes smoked/day | 19.90 ± 1.0 | 23.4 ± 1.4* |
| Fagerström score (FTND) | 5.1 ± 0.3 | 6.5 ± 0.4** |
| Smoking duration (years) | 23.2 ± 2.0 | 24.0 ± 1.9 |
| Previous quit attempts (n) | 2.7 ± 0.2 | 4.9 ± 1.5 |
| Baseline drinking | ||
| Number of alcoholic drinks/week | 7.6 ± 1.1 | 7.5 ± 1.4 |
| Drinks per drinking day | 1.4 ± 0.1 | 1.5 ± 0.2 |
| Drinks per heavy drinking day | 5.8 ± 0.4 | 6.0 ± 0.6 |
| Heavy drinkers at baseline | 21 (48%) | 15 (44%) |
Data are mean ± SEM or count (percentage). Comparisons by t-test or chi-square, where appropriate,
p < 0.05;
p < 0.01.
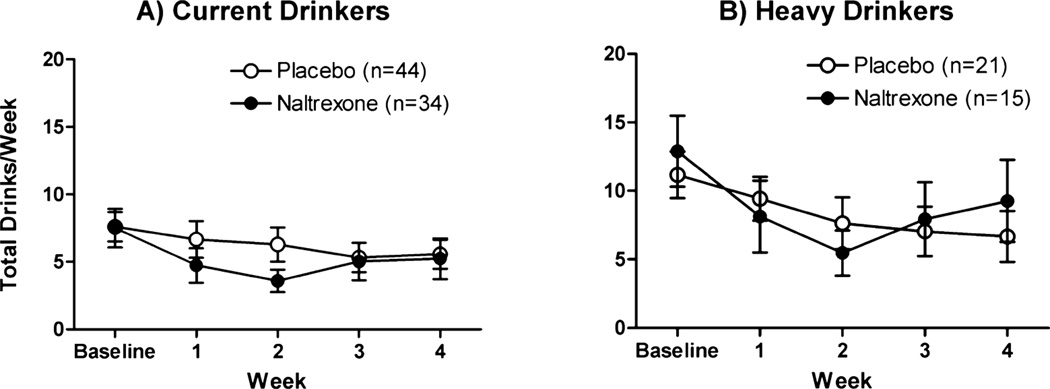
Naltrexone directionally decreased the weekly number of alcoholic drinks consumed, but this was not statistically significant (group: p = 0.10; group × time: p = 0.10; Fig. 1A). As can be seen in Fig. 1A, the largest difference in drinking between the groups occurred during the second week after the quit date, with an average of 3.6 ± 0.8 drinks consumed in the naltrexone group compared with 6.3 ± 1.3 drinks consumed in the placebo group; by the third and fourth weeks, average weekly drinking levels were similar between groups. Analysis within the subgroup of heavy drinkers only for weekly number of drinks consumed revealed a significant week by treatment group interaction [beta (SE) = 1.47 (0.72), p < 0.05; Fig. 1B], with naltrexone-treated subjects initially reporting lower weekly number of drinks in the first few weeks, but greater number of drinks during the last few weeks compared with placebo subjects. Comparison of another variable, drinks per drinking day, yielded similar results, with the naltrexone effect only evident in the subgroup of heavy drinkers [naltrexone: 4.5 ± 0.4 drinks at baseline, 3.2 ± 0.5 drinks average over treatment, t(14) = 2.78, p = 0.01; placebo: 4.0 ± 0.2 drinks at baseline, 3.6 ± 0.5 drinks average over treatment, t(20) = 1.03, p = 0.32].
Fig. 1.
Average number of drinks per week (±SEM). Baseline = mean of the 2 weeks prior to the quit date; weeks 1–4 = each week after the quit date.
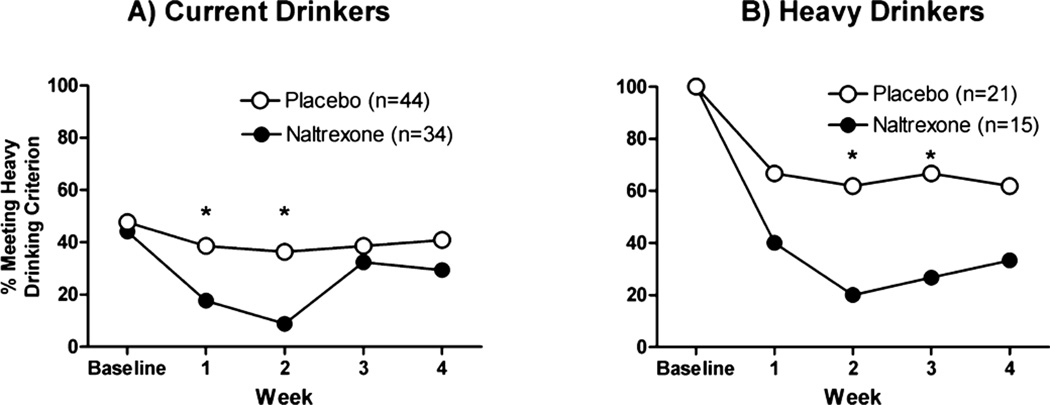
Naltrexone significantly decreased weekly heavy drinking rates [beta (SE) = −1.63 (0.66), p < 0.05; OR = 0.45 (95% CI = 0.27–0.74); Fig. 2A], and this was most evident over the first 2 weeks of treatment, i.e., at week 2, heavy drinking occurred in 10% of those on naltrexone compared with 40% on placebo. In the heavier drinkers only, naltrexone significantly attenuated weekly heavy drinking rates throughout the 4-week interval compared with placebo [group: beta (SE) = −1.44 (0.526), p = 0.01; OR = 0.24 (95%CI: 0.12–0.48); Fig. 2B]. As can be gleaned from Fig. 2, both groups showed reduction in heavy drinking frequency, but the reduction was greater in the naltrexone group over the first month (average heavy drinking rate 30% for naltrexone vs. 64% for placebo).
Fig. 2.
Heavy drinking rates per week. Baseline = 2 weeks prior to the quit date; weeks 1–4 = each week after the quit date.
Smoking quit rates were not significantly different between the naltrexone and placebo groups [quit rate: 71% vs. 66%, χ2 = 0.19, p = 0.66; OR = 1.24 (95% CI: 0.43–3.69)]. However, among heavy drinkers only, naltrexone tended to yield higher smoking quit rates compared with placebo [80% vs. 52%, χ2 = 2.89, p = 0.09; OR = 3.64 (95% CI: 0.66–25.1)]. At the end of the first month, the smoking quit rate was negatively associated with heavy drinking, such that greater success in quitting smoking related to reduced rates of heavy drinking, both for naltrexone-treated [17% heavy drinking rate in those who quit smoking vs. 60% in those who did not quit; χ2 = 6.38, p = 0.01;OR=0.13 (95%CI: 0.02–0.91)] and placebo-treated participants [24% heavy drinking rate in those who quit smoking vs. 73% in those who did not quit; χ2 = 9.89, p = 0.002; OR = 0.12 (95% CI: 0.02–0.58)]. Mediation analysis within the heavy drinkers showed that heavy drinking days mediated the relationship between medication (naltrexone) and smoking quit rates: the relationship between medication and heavy drinking days was significant (p < 0.05), and medication was no longer significant when heavy drinking days was included in the model (p = 0.38).
Adherence to pill taking was similar between the groups [week × group, beta (SE) = −0.05(1.85), p = 0.98; group: beta (SE) = 0.02 (6.59), p = 0.99], with relatively high rates in the first week, i.e., 92% of pills reported taken for both placebo and naltrexone groups, declining to 76% and 74% of pills reported taken, respectively, during the fourth week of treatment [week: beta (SE) = −4.86 (1.22), p < 0.001]. While there was no association between pill taking adherence and drinking outcomes in the placebo group, for those in the naltrexone group, pill taking adherence over time was negatively associated with heavy drinking, i.e., the better the adherence to taking naltrexone pills, the lower the rates of heavy drinking by the end of the first month [adherence × week: beta (SE) = −0.02 (0.01), p < 0.001; adherence: beta (SE) = 0.05 (0.02), p = 0.006].
The naltrexone-treated participants reported greater side-effects than the placebo group in current drinkers (p < 0.001) but not in the heavy drinkers (p = 0.13); however, the single item for nausea was greater for naltrexone-treated subjects in both current and heavy drinkers (ps < 0.05). Among the heavy drinkers, nausea mediated the effect of medication (naltrexone) on heavy drinking rates [i.e., the medication effect was no longer significant (p = 0.38) when heavy drinking days was included in the model].
Liver Enzyme Levels
Levels for AST and ALT did not differ between the naltrexone and placebo groups at baseline (naltrexone vs. placebo: AST: 20.3 ± 1.0 vs. 21.5 ± 0.9; p = 0.37; ALT: 20.6 ± 1.9 vs. 21.0 ± 1.3; p = 0.86) and again approximately 1 month later (naltrexone vs. placebo: AST: 21.3 ± 1.2 vs. 23.7 ± 1.1; p = 0.15; ALT: 23.8 ± 2.3 vs. 24.2 ± 1.8; p = 0.89). Moreover, the percentage of subjects having levels out of the normal range for AST and ALT did not differ at baseline or at repeat testing between the groups (see Table 2). Elevations, if they did occur, were mild in both groups: there were 2 subjects (1 in the naltrexone group, and 1 in the placebo group) with elevations in AST, with levels ≤40 IU, and there were 10 subjects (4 in the naltrexone group, and 6 in the placebo group) with elevations in ALT, with levels ≤58 IU.
Table 2.
Percentage of Subjects With Liver Enzyme Levels Outside Normal Range
| Placebo | Naltrexone | |||
|---|---|---|---|---|
| Liver enzyme | Baseline | Week 2 | Baseline | Week 2 |
| AST (%) | 2.7 | 2.7 | 3.6 | 3.6 |
| ALT (%) | 5.4 | 18.9 | 7.1 | 10.7 |
Change from baseline to repeat testing in placebo versus naltrexone group: AST: p = 0.99; ALT: p = 0.26. Liver enzyme levels were available on n = 65 subjects (28 in the naltrexone group and 37 in the placebo group).
DISCUSSION
In the present study, naltrexone reduced alcohol drinking levels, particularly heavy drinking rates, in nicotine-dependent persons attempting to quit smoking. Although naltrexone-related decreases in alcohol drinking behaviors have been documented previously (for meta-analysis, see Srisurapanont and Jarusuraisin, 2005), a unique aspect of this study was that participants were not alcohol dependent, and they were neither seeking nor receiving treatment for alcohol-related issues. Thus far, there has not been support for the converse, i.e., 1 study failed to show significant effects of naltrexone on cigarette smoking in alcoholic patients neither interested in nor receiving smoking counseling (Rohsenow et al., 2003). While the psychosocial intervention employed in this trial was somewhat intensive (i.e., six 45-minute individual sessions with a Master’s level counselor), the treatment was specific to smoking cessation with the topic of alcohol drinking included only within the broader topic of possible smoking-related triggers. The results support a recent preclinical study demonstrating main effects of naltrexone in decreasing alcohol drinking days in a nontreatment seeking sample of alcoholics and heavy social drinkers who did not receive alcohol counseling (Tidey et al., 2008).
While the overall effects of naltrexone may be modest for smoking quit rates among general smokers (King et al., 2006; O’Malley et al., 2006), further research with smoker subgroups has been suggested (for review, see King et al., 2009). It is possible that heavy drinking smokers may preferentially respond to more comprehensive treatment, i.e., naltrexone combined with nicotine replacement and counseling. Mediation analyses revealed that naltrexone-induced decreases in heavy drinking, particularly in persons who tend to drink heavily, could lead to improved success in quitting smoking. This is consistent with preclinical studies that indicate that the greater the alcohol consumption, the greater the urge to smoke (Epstein et al., 2007; King and Epstein, 2005) and that naltrexone may reduce smoking urges induced by alcohol at breath alcohol levels consistent with heavy but not light drinking (Ray et al., 2007). It is also possible that naltrexone-related increases in nausea may be involved in the reduction in heavy drinking; however, the current design did not allow for distinguishing nonspecific nausea versus nausea experienced when drinking alcohol. Future research is warranted to examine these potential mechanisms in more detail.
The naltrexone-induced decreases in alcohol drinking, particularly heavy drinking, observed in this study replicate and extend findings from another recent trial with naltrexone in smoking cessation (O’Malley et al., 2008). In the study by O’Malley and colleagues (2008), participants received less intensive and briefer smoking cessation counseling (15 min weekly with a bachelor’s level research assistant) and results showed that either 25 or 50 mg oral daily naltrexone, but not 100 mg, increased time to remission to daily hazardous drinking relative to placebo. In the current study, alcohol drinking outcomes were assessed with complimentary measures of weekly total number of drinks and weekly heavy drinking rates and showed similar findings, with 50 mg naltrexone attenuating the potentially more clinically relevant outcome, i.e., that of meeting criteria for heavy drinking at least once in a week, consistent with findings in alcoholics (for review, see Pettinati et al., 2006). Another point of significance in the current study is that naltrexone-related decreases in heavy drinking were noted throughout the interval when the dose of nicotine replacement was steadily declining. Although nicotinic agonists (Acheson et al., 2006; McKee et al., 2008b) and partial agonists (McKee et al., 2008a; Steensland et al., 2007), may decrease alcohol drinking, it is unlikely that the acetylcholine receptor system played a large role in the current study findings, as both groups received identical nicotine patch dosing, and naltrexone’s effects on reducing heavy drinking continued even as nicotine patch dose was declining. Finally, more adherence to pill-taking in the naltrexone group related to lower heavy drinking by the end of the first month, similar to findings on the role of compliance to the efficacy of naltrexone in alcohol dependent patients (Volpicelli et al., 1997).
Naltrexone treatment in nonalcoholic nicotine dependent participants did not produce increases in either AST or ALT levels compared with the placebo group. Though this has been reported previously in the literature for alcoholic patients treated with the 50 mg dose, our study focused on relatively healthy, nonalcoholic nicotine-dependent patients, who represent a different sample than most previous naltrexone treatment studies. These findings support the hepatic safety of naltrexone in people with nicotine dependence, and may be reassuring to treatment providers concerned about potential liver toxicity with naltrexone. Naltrexone may have further benefit in smokers, given the observations of reduced heavy drinking, which likely produces greater impairment of liver function than naltrexone at the FDA-approved dose (Berg et al., 1996; Pettinati et al., 2006). The measurement of ALT and AST are widely available and are used in clinical practice as a measure of alcohol-induced liver injury. While use of these liver enzymes may be somewhat limited as objective biomarkers of alcohol-induced liver injury particularly in nonclinical samples, future studies combining AST and ALT with other more sensitive measures, such as gamma-glutamyl transpeptidase and carbohydrate-deficient transferrin may help confirm self-report of reductions in recent heavy drinking in smoker-drinkers treated with naltrexone versus placebo.
There are several limitations in the present study worth noting. First, this study was exploratory in nature and examined a secondary outcome from a larger parent trial. Second, the sample size was relatively small, and therefore some analyses may not have been sufficiently powered. To address both these limitations, replication in larger sample sizes and examination of mediators of naltrexone response on drinking and smoking behaviors would help elucidate the role of opioid antagonism for co-morbid behaviors. Third, the study examined only 1 dose of naltrexone, and some effects of naltrexone may be dose-dependent as larger doses may increase smoking quit rates, while doses at or below that used in this study may improve other proximal outcomes such as weight gain during cessation (O’Malley et al., 2006). Fourth, the use of AST and ALT as a measure of alcohol consumption has some limitations in terms of specificity, especially given that subjects were nonalcoholics. Finally, naltrexone was examined in combination with a platform treatment of nicotine replacement and intensive individual counseling, and so dismantling the effects of each component was not possible.
In sum, naltrexone (50 mg oral) reduced heavy drinking rates in smokers enrolled in a smoking cessation trial, and the effects of naltrexone on alcohol and smoking outcomes appeared to be most evident in persons who engage in heavier social drinking. These results are significant in that heavy drinking rates were reduced in a nontreatment seeking group of heavy social drinkers attempting to quit smoking. Naltrexone did not increase liver enzyme levels (i.e., a proximal objective biomarker for self-reported drinking behavior as well as a hepatic safety indicator) in otherwise healthy nicotine-dependent subjects. In conclusion, a historically treatment resistant group, i.e., social heavy/binge drinker-smokers, may selectively respond to naltrexone to improve both smoking cessation and alcohol drinking outcomes, but further research is warranted.
ACKNOWLEDGMENTS
This research was supported by NIH #R01-DA016834 and #K08-AA00276; the University of Chicago Cancer Research Center, P30-CA14599; and the University of Chicago General Clinical Research Center, #M01-RR00055. The experiments in this study comply with the current US laws and were in compliance with the Declaration of Helsinki for human subjects.
Footnotes
Presented in part at the 2008 Research Society on Alcoholism meeting, Washington, DC.
REFERENCES
- Acheson A, Mahler SV, Chi H, de Wit H. Differential effects of nicotine on alcohol consumption in men and women. Psychopharmacology. 2006;186:54–63. doi: 10.1007/s00213-006-0338-y. [DOI] [PubMed] [Google Scholar]
- Allen JP, Litten RZ, Strid N, Sillanaukee P. The role of biomarkers in alcoholism medication trials. Alcohol Clin Exp Res. 2001;25:1119–1125. [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Berg BJ, Pettinati HM, Volpicelli JR. A risk-benefit assessment of naltrexone in the treatment of alcohol dependence. Drug Saf. 1996;15:274–282. doi: 10.2165/00002018-199615040-00005. [DOI] [PubMed] [Google Scholar]
- Borland R. Slip-ups and relapse in attempts to quit smoking. Addict Behav. 1990;15:235–245. doi: 10.1016/0306-4603(90)90066-7. [DOI] [PubMed] [Google Scholar]
- Carmen B, Angeles M, Ana M, Maria AJ. Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: a systematic review. Addiction. 2004;99:811–828. doi: 10.1111/j.1360-0443.2004.00763.x. [DOI] [PubMed] [Google Scholar]
- Covey LS, Glassman AH, Stetner F. Naltrexone effects on short-term and long-term smoking cessation. J Addict Dis. 1999;18:31–40. doi: 10.1300/J069v18n01_04. [DOI] [PubMed] [Google Scholar]
- Croop RS, Faulkner EB, Labriola DF. The safety profile of naltrexone in the treatment of alcoholism. Arch Gen Psychiatry. 1997;54:1130–1135. doi: 10.1001/archpsyc.1997.01830240090013. [DOI] [PubMed] [Google Scholar]
- David S, Lancaster T, Stead LF, Evins AE. Opioid antagonist for smoking cessation. Cochrane Database Syst Rev. 2006;4:CD0003086. doi: 10.1002/14651858.CD003086.pub2. [DOI] [PubMed] [Google Scholar]
- Davidson D, Palfai T, Bird C, Swift R. Effects of naltrexone on alcohol self-administration in heavy drinkers. Alcohol Clin Exp Res. 1999;23:195–203. [PubMed] [Google Scholar]
- Davidson D, Swift R, Fitz E. Naltrexone increases the latency to drink alcohol in social drinkers. Alcohol Clin Exp Res. 1996;20:732–739. doi: 10.1111/j.1530-0277.1996.tb01679.x. [DOI] [PubMed] [Google Scholar]
- Epstein AM, Sher TG, Young MA, King AC. Tobacco chippers show robust increases in smoking urge after alcohol consumption. Psychopharmacology. 2007;190:321–329. doi: 10.1007/s00213-006-0438-8. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, Dorfman SF, Froelicher ES, Goldstein MG, Healton CG, Henderson PN, Heyman RB, Koh HK, Kottke TE, Lando HA, Mecklenburg RE, Mermelstein RJ, Mullen PD, Orleans CT, Robinson L, Stitzer ML, Tommasello AC, Villejo L, Wewers ME. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2008. Treating Tobacco Use and Dependence: 2008 Update. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders–Patient Edition (SCID-I/P, Version 2.0) New York: Biometrics Research Department; 1995. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstromm K. The Fagerström test for nicotine dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Kelly JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendation. Nicotine Tob Res. 2003;5:13–25. [PubMed] [Google Scholar]
- King AC, de Wit H, Riley RC, Cao D, Niaura R, Hatsukami D. Efficacy of naltrexone in smoking cessation: a preliminary study and an examination of sex differences. Nicotine Tob Res. 2006;5:1–12. doi: 10.1080/14622200600789767. [DOI] [PubMed] [Google Scholar]
- King AC, Epstein AM. Alcohol dose-dependent increases in smoking urge in light smokers. Alcohol Clin Exp Res. 2005;29:547–552. doi: 10.1097/01.alc.0000158839.65251.fe. [DOI] [PubMed] [Google Scholar]
- King AC, Riley R. Stop Smoking Manual. Chicago, IL: The University of Chicago Studies on Smoking Cessation. University of Chicago; 2001. [Google Scholar]
- King AC, Torello R, Krishnan-Sarin S, O’Malley SS. Naltrexone in smoking cessation: a review of the literature and future directions, in. In: Dean R, Bilsky E, Negus S, editors. Opioid Receptors and Antagonists: From Bench to Clinic. Totowa, NJ: Humana Press; 2009. pp. 307–324. [Google Scholar]
- King AC, Volpicelli JR, Gunduz M, O’Brien CP, Kreek MJ. Naltrexone biotransformation and incidence of subjective side effects: a preliminary study. Alcohol Clin Exp Res. 1997;21:906–909. [PubMed] [Google Scholar]
- Krishnan-Sarin S, Rosen MI, O’Malley SS. Naloxone challenge in smokers: preliminary evidence of an opioid component in nicotine dependence. Arch Gen Psychiatry. 1999;56:663–668. doi: 10.1001/archpsyc.56.7.663. [DOI] [PubMed] [Google Scholar]
- Lee DH, Ha MH, Christiani DC. Body weight, alcohol consumption and liver enzyme activity—a 4-year follow-up study. Int J Epidemiol. 2001;30:766–770. doi: 10.1093/ije/30.4.766. [DOI] [PubMed] [Google Scholar]
- Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- McKee SA, Krishnan-Sarin K, O’Malley S, Harrison E, Shi J, Tetrault J, Estevez N. Effect of varenicline, a partial nicotinic agonist, on alcohol self-administration in heavy drinking smokers. Alcohol Clin Exp Res. 2008a;32:302A. [Google Scholar]
- McKee SA, O’Malley SS, Shi J, Mase T, Krishnan-Sarin S. Transdermal nicotine replacement attenuates alcohol reactivity and reduces alcohol self-administration. Psychopharmacology. 2008b;196:189–200. doi: 10.1007/s00213-007-0952-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JE, Morley JE, Levine AS, Hatsukami D, Gannon M, Pfohl D. High-dose naltrexone therapy and dietary counseling for obesity. Biol Psychiatry. 1987;22:35–42. doi: 10.1016/0006-3223(87)90127-2. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Cooney JL, Krishnan-Sarin S, Dubin JA, McKee SA, Cooney NL, Blakeslee A, Meandzija B, Romano-Dahlgard D, Wu R, Makuch R, Jatlow P. A controlled trial of naltrexone augmentation of nicotine replacement for smoking cessation. Arch Intern Med. 2006;166:667–674. doi: 10.1001/archinte.166.6.667. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Jaffe AJ, Rode S, Rounsaville BJ. Experience of a “slip” among alcoholics treated with naltrexone or placebo. Am J Psych. 1996;153:281–283. doi: 10.1176/ajp.153.2.281. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, McKee SA, Leeman RF, Cooney NL, Meandzija B, Wu R, Makuch RW. Dose-dependent reduction of hazardous alcohol use in a placebo-controlled trial of naltrexone for smoking cessation. Int J Neuropsychopharmacol. 2008;17:1–9. doi: 10.1017/S146114570800936X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettinati HM, O’Brien CP, Rabinowitz AR, Wortman SM, Oslin DW, Kampman KM, Dackis CA. The status of naltrexone in the treatment of alcohol dependence. J Clin Psychopharmacol. 2006;26:610–625. doi: 10.1097/01.jcp.0000245566.52401.20. [DOI] [PubMed] [Google Scholar]
- Pfohl DN, Allen JI, Atkinson RL, Knopman DS, Malcolm RJ, Mitchell JE, Morley JE. Naltrexone hydrochloride (Traxan): a review of serum transaminase elevations at high dosage. NIDA Res Monogr. 1986;67:66–72. [PubMed] [Google Scholar]
- Ray LA, Miranda R, Kahler CW, Leventhal AM, Monti PM, Swift R, Hutchison KE. Pharmacological effects of naltrexone and intravenous alcohol on craving for cigarettes among light smokers: a pilot study. Psychopharm. 2007;193:449–456. doi: 10.1007/s00213-007-0794-z. [DOI] [PubMed] [Google Scholar]
- Rohsenow D, Monti P, Colby S, Gulliver S, Swift R, Abrams D. Naltrexone treatment for alcoholics: effect on cigarette smoking rates. Nicotine Tob Res. 2003;5:231–236. doi: 10.1080/1462220031000073298. [DOI] [PubMed] [Google Scholar]
- SAMHSA. National Survey on Drug Use and Health. Bethesda, MD: Office of Applied Studies; 2005. [Google Scholar]
- Shiffman SM, Fischer LA, Paty J, Gnys M, Hickcox M, Kassel JD. Drinking and smoking: a field study of their association. Ann Behav Med. 1994;16:203–209. [Google Scholar]
- Shiffman S, Paty JA, Kassel JA, Hickcox M. First lapses to smoking: within-subjects analysis of real-time reports. J Consult Clin Psychol. 1996;64:366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Maisto SA, Sobell MB, Cooper AM. Reliability of alcohol abusers’ self-reports of drinking behavior. Behav Res Ther. 1979;17:157–160. doi: 10.1016/0005-7967(79)90025-1. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Alcohol Timeline Follow-Back Users’ Manual. Toronto, ON: Addiction Research Foundation; 1995. [Google Scholar]
- Srisurapanont M, Jarusuraisin N. Opioid antagonists for alcohol dependence. Cochrane Database Syst Rev. 2005;1:CD001867. doi: 10.1002/14651858.CD001867.pub2. [DOI] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. PNAS. 2007;104:12518–12523. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift RM, Whelihan W, Kuznetsov O, Buongiorno G, Hsuing H. Naltrexone-induced alterations in human ethanol intoxication. Am J Psychiatry. 1994;151:1463–1467. doi: 10.1176/ajp.151.10.1463. [DOI] [PubMed] [Google Scholar]
- Tantcheva-Poor I, Zaigler M, Rietbrocks S, Fuhr U. Estimation of cytochrome P450 CYP1A2 activity in 863 health Caucasians using a saliva-based caffeine test. Pharmacogenetics. 1999;9:131–144. [PubMed] [Google Scholar]
- Tidey JW, Monti PM, Rohsenow DJ, Gwaltney CJ, Miranda R, McGeary JE, MacKillop J, Swift RM, Abrams DB, Shiffman S, Paty JA. Moderators of naltrexone’s effects on drinking, urge, and alcohol effects in non-treatment-seeking heavy drinkers in the natural environment. Alcohol Clin Exp Res. 2008;32:58–66. doi: 10.1111/j.1530-0277.2007.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli JR, Alterman AI, Hayashida M, O’Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876–880. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Rhines KC, Rhines JS, Volpicelli LA, Alterman AI, O’Brien CP. Naltrexone and alcohol dependence: role of subject compliance. Arch Gen Psychiatry. 1997;54:737–742. doi: 10.1001/archpsyc.1997.01830200071010. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Watson NT, King AC, Sherman CE, O’Brian CP. Effect of naltrexone on alcohol “High” in alcoholics. Am J Psychiatry. 1995;152:613–615. doi: 10.1176/ajp.152.4.613. [DOI] [PubMed] [Google Scholar]
- Walsh Z, Epstein A, Munisamy G, King A. The impact of depressive symptoms on the efficacy of naltrexone in smoking cessation. J Addict Dis. 2008;27:65–72. doi: 10.1300/J069v27n01_07. [DOI] [PubMed] [Google Scholar]