Abstract
Although attention deficit/hyperactivity disorder (ADHD) is associated with impairment in working memory and short-term memory, up to half of individual children with ADHD perform within a normative range. Heterogeneity in other ADHD-related mechanisms, which may compensate for or combine with cognitive weaknesses, is a likely explanation. One candidate is the robustness of parasympathetic regulation (as indexed by respiratory sinus arrhythmia; RSA). Theory and data suggest that a common neural network is likely tied to both heart-rate regulation and certain cognitive functions (including aspects of working and short-term memory). Cardiac-derived indices of parasympathetic reactivity were collected during short-term memory (STM) storage and rehearsal tasks from 243 children (116 ADHD, 127 controls). ADHD was associated with lower STM performance, replicating previous work. In addition, RSA reactivity moderated the association between STM and ADHD – both as a category and a dimension – independent of comorbidity. Specifically, conditional effects revealed that high levels of withdrawal interacted with weakened STM but high levels of augmentation moderated a positive association predicting ADHD. Thus, variations in parasympathetic reactivity may help explain neuropsychological heterogeneity in ADHD.
Keywords: Attention-deficit/hyperactivity disorder, Psychophysiology, RSA, Working memory, Executive function
Attention-deficit/hyperactivity disorder (ADHD) is defined by symptoms of inattention, hyperactivity, and/or impulsivity to the extent that functional impairment occurs across multiple domains (American Psychiatric Association 2013). However, ADHD is a heterogeneous disorder in terms of clinical course, neuropsychological profile, and co-occurring psychopathology (Loeber et al. 2000; Nigg et al. 2005; Willcutt et al. 2012). In particular, although executive functioning (EF) is a central correlate of ADHD, heterogeneity in EF among children with ADHD is not well explained (Nigg et al. 2005; Lambek et al. 2011). That is, many children with ADHD do not have problems with effortful cognition or executive function. It may be that compensatory mechanisms closely related to effortful cognition, such as regulatory systems, are also weakened in some children, which can combine with existing cognitive risk factors to confer greater risk for ADHD.
Several theories about cognitive mechanisms underlying ADHD exist, including theories focused on behavioral inhibition, self-regulation of affect/arousal, and working memory (Barkley 1997; Nigg and Casey 2005; Rapport et al. 2001). Of these domains, working memory, and spatially-based short-term memory (STM), in particular, is among the most heavily studied cognitive features of ADHD (Lambek et al. 2011; Martinussen et al. 2005).
Complimentary theories posit different roles for working memory processes in ADHD, with some hypothesizing a relatively central role of working memory (Raiker et al. 2012) and others viewing working memory as a product of primary inhibition (Barkley 1997). According to Baddeley’s (2007) multi-component model, working memory is the limited-capacity ability to store, rehearse and manipulate multiple pieces of transitory information. In this model, spatial STM storage and rehearsal are sub-components of working memory, and are distinct factors from executive processes which inhibit extraneous demands and maintain attentional focus during complex tasks with competing stimuli1 (Engle et al. 1999).
Spatial STM storage (STM-S) refers to the ability to visually keep multiple pieces of spatial location information available. Rehearsal (STM-R) includes abilities related to maintenance and recitation of the information, and is presumed to require cognitive resources. These abilities are supported by activation of prefrontal neural networks which are innervated by dopaminergic neurons (Bunge and Wright 2007). For example, these same regions exhibit activation during memory tasks and are impacted by effective pharmacotherapies for ADHD (Arnsten and Li 2005). Reduced STM-S and STM-R performance is also associated with academic impairments in ADHD youth (Biederman et al. 2004; Gropper and Tannock 2009).
One potential reason for heterogeneity observed in the association between STM and ADHD is that other processes may compensate (or fail to compensate) for weaknesses in STM among children with ADHD. Thus, one biological approach that has been proposed to explain this heterogeneity involves disruptions to biological regulatory processes via the parasympathetic (PNS) and sympathetic nervous system (SNS). Porges (2001) Polyvagal Theory proposes that PNS output via the vagus nerve is associated with the regulation of behavior, cognition, emotion, and complex social behavior (Porges 2001, 2007). Specifically, Polyvagal theory predicts successful broad-based regulation to be associated with optimal PNS withdrawal during challenging states. This is presumed to allow for increases in metabolic resources to cope with challenge (i.e., increased heart rate, respiration, etc.; Porges 2001), such as cognitive demands. Thus, effective regulation in these domains is tied to an ideal range of PNS influence, but dysregulation of this system is predicted to compromise control over those domains.
Relevance of this theory to psychopathology is relatively well established. There is evidence that blunted PNS withdrawal (from baseline) during a challenging task predicts sub-optimal performance and/or dysregulation across a range of behaviors, cognitive functions, and emotions (Beauchaine 2001). Additionally, excessive PNS withdrawal may result in a lower threshold for fight/flight responding and therefore be related to aggressive or panicked response styles (Beauchaine 2001). With respect to ADHD, these systems are of particular interest given the increased recognition that ADHD is characterized by dysregulation of behavior, cognition and emotion coinciding with alterations in ANS functioning (for a review see Rash and Aguirre-Camacho 2012).
A primary cardiac-derived index of PNS functioning widely utilized in the literature is respiratory sinus arrhythmia (RSA). Both baseline RSA (indexing resting PNS or vagal tone) and changes in RSA during tasks (i.e. PNS reactivity) have been differentially associated with clinical, cognitive, and temperamental profiles (Beauchaine 2001; Suess et al. 1994). In particular, lower baseline RSA (implying reduced regulatory capacity) has been associated with greater risk for psychopathology, broadly. Furthermore, abnormally reduced or blunted RSA withdrawal (i.e., low PNS reactivity), which would imply failure of a regulatory response to challenge, has been associated with greater inattention and disinhibition in children (Conradt et al. 2014; Suess et al. 1994). More recently, a meta-analysis by Graziano and Derefinko (2013) revealed that children from both clinical and at-risk samples exhibited lower baseline RSA, while blunted RSA-withdrawal (i.e., PNS-based dysregulation) during challenging states was associated specifically with externalizing symptoms.
However, ADHD has been associated with both low resting RSA, as well as blunted RSA withdrawal during cognitively challenging tasks (Beauchaine et al. 2001), suggesting PNS dysregulation and difficulty recruiting SNS activation systems in these children. A recent review cites evidence for low baseline or resting RSA among children with ADHD (Rash and Aguirre-Camacho 2012). Additionally, children with ADHD exhibit blunted RSA reactivity to both cognitive (Negrao et al. 2011) and emotionally challenging tasks (Musser et al. 2011, 2014). However, differences in RSA reactivity appear to vary according to type of task utilized, with some studies showing PNS hypo-reactivity and others showing PNS hyper-reactivity in ADHD (Rash and Aguirre-Camacho 2012). Thus, PNS regulatory function – as indexed by resting RSA and RSA response to challenge – appears to be associated with a range of abilities including emotion regulation, attention, and inhibitory processes, as well as ADHD. This supports the idea that PNS regulation may be a system that can either compensate for, or disrupt, working memory in children with ADHD.
There is growing evidence that the brain regions that support PNS reactivity co-act with regions of the pre-frontal cortex that support STM and other EF (e.g., Thayer et al. 2009). For example, a study of normative adults engaged in a STM task showed concurrent reduction in heart rate variability (HRV; an index of RSA and PNS activity) and decreased activation of the anterior cingulate cortex and medial prefrontal cortex, which are also associated with STM and working memory (Gianaros et al. 2004; Wager and Smith 2003). Thus STM and PNS regulation appear to be biologically related.
Thus, it is possible to hypothesize that sub-optimal parasympathetic regulation may interact with STM deficits to result in risk for the development of ADHD. Atypical RSA profiles (i.e. abnormal PNS reactivity) may compensate for disruptions in the cognitive processes needed to support STM abilities, or in contrast, atypical RSA profiles may serve as a second and distinct risk factor, such that deficits in both PNS regulation and STM confers greatest risk. However, to our knowledge there have been no examinations of the associations between effortful cognitive processes and PNS regulation in children with ADHD. The present study aims to close that gap so as to clarify mechanisms involved in ADHD and better explain cognitive heterogeneity, focusing on aspects of working memory.
In this study, children with ADHD and typically developing children completed spatial short-term memory tasks while indices of RSA were obtained. Associations among PNS regulation and STM in predicting likelihood of ADHD group membership and parent-reported severity were examined. It was hypothesized that: (1) ADHD will be associated with STM difficulties; (2) ADHD will be associated with lower RSA at baseline; (3) ADHD will be associated with blunted RSA withdrawal during the STM task, and (4) the association between ADHD and STM performance will be moderated by RSA reactivity (i.e., PNS reactivity), such that those children with STM weakness and atypical RSA withdrawal (i.e., PNS dysregulation) will be the most likely to have ADHD. ADHD was considered both as a category and a dimension; secondary analyses examine the specificity of these findings by repeating the model with oppositional defiant disorder as the outcome.
Methods
Participants
Participants were 243 children aged 7 to 12 years; 116 met DSM-5 criteria for ADHD (86 combined presentation, 30 inattentive presentation), and 127 were non-ADHD comparison youth (American Psychiatric Association 2013). The middle childhood age range was selected as it coincides with peak identification of ADHD, as well as the period when new demands for regulation are put upon children with ADHD in school. Ethnic minority children made up a relatively small proportion of the sample (13.8 % in controls, 21.7 % in ADHD) but the sample overall is representative of region where the research was conducted. Between 9.5 % (ADHD) and 6.4 % (Controls) of families qualified as below the poverty line. Families were recruited from the community through advertisements and mass mailings in order to obtain a sample that would not be biased by clinic identification, and then ADHD caseness was established as described below. The local Institutional Review Board approved the study; all procedures conformed to the Ethical Principles of Psychologists and Code of Conduct (American Psychological Association 2002). Parents provided written informed consent and children provided written informed assent.
Recruitment and Identification
Families volunteering for the study underwent a multi-gate screening process to establish eligibility and diagnostic group assignment. The screening process for this study is identical to that described elsewhere (see Musser et al. 2014). After completing the Schedule for Affective Disorders and Schizophrenia for School-Age Children—Epidemiologic Version (KSAD-S-E), parent and teacher standardized ratings, and an IQ screen (WISC-IV; Wechsler 2003), a clinical diagnostic team comprising a board-certified psychiatrist and licensed clinical psychologist independently reviewed all case information to arrive at diagnoses using DSM-IV criteria. For both groups, diagnostic team agreement ratings were acceptable for ADHD diagnosis (k>0.74). Control cases underwent the same procedure and were required to have fewer than three ADHD symptoms endorsed. Twenty children in the ADHD group received a diagnosis of comorbid oppositional defiant disorder. Note that while DSM-IV criteria for ADHD diagnosis were utilized for assessment and grouping procedures at the outset of this study, diagnosed youth in the current sample also meet criteria according to DSM-5.
Parent ratings of child ADHD symptoms were obtained using the ADHD Rating Scale (DuPaul et al. 1998), a well-validated tool for assessing ADHD symptoms. T-scores of symptom severity were used as continuous dependent variables in follow-up analyses examining ADHD total symptoms, inattentive symptoms, and hyperactive symptoms. Cronbach’s alpha for both symptoms domains and the overall scale was excellent (all a>0.95).
Exclusion Criteria
All children (i.e., for either diagnostic group) were excluded if there was a history of neurological impairments, seizures, traumatic brain injury, or other major medical impairments by parent report. Exclusion criteria for both groups also included a full-scale IQ below 75, long-acting psychoactive medication (not including stimulants), or diagnostic team findings of current mood disorder, conduct disorder, life-time psychosis, or pervasive developmental disorder.
Medication Washout
All children were required to be medication free at the time of testing. Children taking stimulant medication underwent a 24–48 h washout (equivalent to at least seven half-lives), dependent on the type of stimulant preparation they were prescribed. However, not all children in the ADHD group were taking medication. Forty ADHD children (35 %) were prescribed stimulants, consistent with rates of treatment in the community (Froehlich et al. 2007). Therefore, prescription status (whether or not child was currently taking medication) was treated as a categorical covariate.
Procedure
Physiological Recording
Disposable silver/silver-chloride electrodes were placed in an electrocardiogram (ECG) and impedance cardiography (ICG) configuration. The ECG electrodes were placed at the right collar bone and the tenth-left rib with a ground electrode placed at the tenth-right rib. To estimate respiration rates, ICG was used with two voltage electrodes placed below the suprasternal notch and xiphoid process and two current electrodes placed along the spine 1.5 in. above and below the voltage electrodes. ECG and ICG were recorded continuously across task epochs. The R-R series was sampled at 1000 Hz. Interbeat-interval (IBI) and respiration rate data were derived using the ECG and ICG data, respectively.
Respiratory Sinus Arrhythmia (RSA)
RSA was indexed by extracting the high frequency component (>0.15 Hz) of the R-R peak time series. R-R waves were examined for artifacts and outliers using MindWare® Heart Rate Variability software V.2.6 (MindWare 2008). Artifacts were removed using the software and visual inspection completed by two raters for validity (all k>0.90). There were no between-group differences in the rate of artifacts, all p>0.50. Physiological reactivity to task demands were calculated by subtracting average RSA during the first 30 s epoch of the neutral baseline condition from average RSA during the first 30 s epoch of the STM task (described below).
RSA[Spatial Span]−RSA [Neutral baseline] = ΔRSA [reactivity]
Spatial Span
The Spatial Span task (similar to the CANTAB Spatial Span; Fray et al. 1996) forward and backward conditions were used to assess visual-spatial STM-S and STM-R respectively. The task was displayed using E-prime software. The task stimuli are presented as a series of white boxes arranged on-screen in fixed locations. The boxes light-up one at a time, followed by a tone, after which children click on the boxes in the order in which they appeared (i.e., forward/storage condition; STM-S) or in the reverse order (i.e., backward/rehearsal condition; STM-R). Before beginning the automated task, children demonstrate comprehension by correctly completing a single practice trial (span of three); no children in the current sample failed to advance to testing. During the actual task each block consists of two trials, and at least one trial within a block must be completed correctly in order to advance to the next trial, up to a maximum span of nine boxes in a given trial (i.e., the task is ended upon two incorrect trials within a span set).
Analyses utilize the number of trials completed successfully, which maintains information regarding unsuccessful trials. Although alternative scoring methods such as the partial-credit approach have demonstrated higher internal consistency, the computerized task did not provide the necessary output for calculating scores in this way. Furthermore, the approach utilized here is highly correlated with partial credit scoring (0.87 to 0.93; Conway et al. 2005). Forward and backward conditions were counter-balanced, such that approximately half the sample completed the forward condition first.
Baseline Physiological Recording
Prior to physiological recording, children’s resting RSA was assessed during a single 2 min period while in a still, seated position. Additionally, children completed a neutral baseline task before and after each condition of the Spatial Span task. This neutral baseline period accounted for the physiological response associated with orienting and attending and facilitated the return of RSA to baseline levels prior to beginning the next task. RSA during this neutral baseline task was used to calculate physiology change scores. The neutral baseline task consisted of 10 pictures from the neutral set of the International Affective Picture System (IAPS). Children were asked to complete Self-Assessment Manikin (SAM) ratings of emotional valence and arousal in response to these pictures each time and groups did not differ in their ratings of the neutral pictures (p 0.10).
Statistical Analysis
ANOVA was used to examine group differences in STM, baseline RSA, and RSA reactivity during the Spatial Span task. The PROCESS macro (Hayes 2013) was used to conduct logistic and linear regression-based moderation analyses in SPSS and examine the interaction of STM performance and RSA reactivity in predicting ADHD as a category and dimensionally. Predictor variables were mean-centered prior to analysis. The PROCESS macro can perform a number of moderation and mediation analyses, and provides conditional effects (www.processmacro.org). Johnson-Neyman conditional effects are presented for moderation results and indicate the significance of the conditional effect across a range of values of the moderator (Bauer and Curran 2005). Conditional effects at one standard deviation above and below the mean are presented in Tables 3 for ease of illustration and consistency with figures. Finally, to examine specificity of effects in relation to the most common comorbid disorder, all analyses were repeated using ODD diagnosis as the dependent variable.
Table 3.
Conditional effects of STM-R on ADHD outcome by value of the moderator
| Outcome | ΔRSA | b | SE | 95 % CI | Exp(b) |
|---|---|---|---|---|---|
| ADHD diagnosis | −1 SD | −0.413** | 0.127 | (−0.662, −0.163) | 0.661 |
| Mean | −0.188* | 0.088 | (−0.361, −0.015) | 0.829 | |
| +1 SD | 0.037 | 0.127 | (−0.212, 0.285) | 1.038 | |
| ΔR2 due to interaction: 0.020 | |||||
| Overall t-score (total) | −1 SD | −1.719** | 0.589 | (−2.879, −0.559) | |
| Mean | −0.544 | 0.413 | (−1.359, 0.271) | ||
| +1 SD | 0.631 | 0.578 | (−0.508, 1.769) | ||
| ΔR2 due to interaction: 0.019 | |||||
| Inattentive t-score | −1 SD | −1.869* | 0.636 | (−3.121, −0.617) | |
| Mean | −0.540 | 0.446 | (−1.420, 0.340) | ||
| +1 SD | 0.789 | 0.624 | (−0.441, 2.018) | ||
| ΔR2 due to interaction: 0.024 | |||||
| Hyperactive t-score | −1 SD | −1.259* | 0.545 | (−2.332, −0.186) | |
| Mean | −0.454 | 0.382 | (−1.207, 0.300) | ||
| +1 SD | 0.351 | 0.534 | (−0.702, 1.404) | ||
| ΔR2 due to interaction: 0.010 | |||||
p<0.05.
p<0.01
Results
Sample Characteristics
Table 1 provides demographic and clinical description of the sample. Reflecting ADHD’s presentation in the community, children with ADHD had lower IQ (p<0.01), more oppositional defiant disorder (ODD) symptoms (p<0.01), and were more often male (p<0.01). Such differences are representative of children with ADHD in the general population; thus, all main analyses covaried age, gender, ODD symptoms, and stimulant medication status as appropriate. There were no statistically significant between group differences in terms of age, ethnicity, or household income (all p’s>0.10). For the entire sample (both children with and without ADHD), most t-scores from the ADHD Rating Scale demonstrated skewness and kurtosis (statistic>1.96) with the exception of the Overall t-score which was skewed but not significantly kurtotic. This is not surprising given the inclusion of a control group which displayed few symptoms of ADHD by design. Therefore, primary analyses utilized either a least-squares or logistic regression approach, both of which are relatively robust against violations of normality (Cohen et al. 2003).
Table 1.
Basic demographic and clinical characteristics of sample
| Group | ||||
|---|---|---|---|---|
|
| ||||
| ADHD | Control | F/χ2 | p | |
| Demographics | ||||
| N | 116 | 127 | ||
| Age in months | 104.02 (11.68) | 103.9 (12.83) | 0.004 | 0.952 |
| Est. IQa | 111.05 (13.51) | 115.77 (12.45) | 8.104 | 0.005 |
| % Male | 69.8 % | 45.0 % | 15.386 | <0.001 |
| % White | 86.2 % | 78.3 % | 2.595 | 0.107 |
| % Low Incomeb | 9.5 % | 6.4 % | 0.787 | 0.375 |
| % Rx Stimulant | 34.5 % | 0 % | 53.162 | <0.001 |
| Inatt. t-scorec | 72.36 (11.50) | 45.48 (8.36) | 434.57 | <0.001 |
| Hyp. t-scorec | 67.54 (13.48) | 45.22 (7.38) | 280.87 | <0.001 |
| Total t-scorec | 71.49 (11.60) | 44.96 (8.01) | 432.13 | <0.001 |
| # Inatt. Symptomsd | 6.53 (2.19) | 0.39 (0.99) | 823.72 | <0.001 |
| # Hyp. Symptomsd | 5.11 (2.44) | 0.46 (1.16) | 372.89 | <0.001 |
| # ODD Symptomsd | 1.41 (1.79) | 0.29 (1.04) | 35.84 | <0.001 |
N=243
Abbreviations: ODD oppositional defiant disorder; Inatt. DSM-IV Inattentive domain; Hyp. DSM-IV Hyperactive/Impulsive domain
Estimated IQ from WISC-IV
Reported family income below $25 k/year
From ADHD Rating Scale
From KSADS
Primary Analyses Based on ADHD Diagnosis
Analyses initially focused on the ADHD syndrome as defined by DSM-5 criteria (American Psychiatric Association 2013).
Short-Term Memory According to ADHD Diagnosis
STM performance was indexed by number of trials completed successfully for the forward/storage (STM-S) and backward/rehearsal (STM-R) conditions respectively. Results supported the hypothesis that ADHD is associated with poorer STM performance. The ADHD group had fewer correct trials than the control group in the STM-S, F(1, 236)=15.68, p<0.001, and STM-R condition, F(1, 235)=6.08, p<0.05. Between-group differences in STM task performance are shown in Table 2.
Table 2.
Dependent variables
| Group | ||||
|---|---|---|---|---|
|
| ||||
| ADHD | Control | F | η 2 p | |
| Short-term memory | ||||
| # Correct Trials FWD | 4.34 (1.74) | 5.55 (1.91) | 14.687** | 0.062 |
| # Correct Trials BK | 3.66 (1.95) | 4.65 (2.09) | 8.170** | 0.025 |
| Psychophysiology data | ||||
| Resting RSA | 7.52 (1.11) | 7.21 (1.17) | 4.072 | 0.015 |
| Neutral baseline RSA | 7.08 (1.28) | 6.70 (1.32) | 6.787* | 0.028 |
| SSp Fwd RSA 30seca | 7.28 (1.18) | 6.85 (1.22) | 6.454* | 0.027 |
| SSp Bkw RSA 30seca | 7.20 (1.17) | 6.84 (1.20) | 5.999* | 0.025 |
| ΔRSA SSp FWDb | 0.20 (0.97) | 0.15 (0.83) | 0.122 | 0.001 |
| ΔRSA SSp BKb | 0.12 (0.93) | 0.15 (0.80) | 0.289 | 0.001 |
All analyses covaried for gender, age, ODD symptoms, and stimulant use status.
p<0.05.
p<0.01. η2 p, Partial Eta-squared
Mean RSA during the first 30-s epoch
Change from neutral baseline condition
RSA Baseline Effects According to ADHD Diagnosis
Mean scores of RSA were derived in 30-s epochs for the resting and neutral pictures baselines. Children with ADHD exhibited marginally higher RSA at resting baseline compared to controls (F(1, 236)=3.70, p>0.05) and significantly higher RSA during the between-task neutral condition, F(1, 236)=6.79, p<0.05. Between-group RSA effects at baseline are shown in Table 2.
RSA Reactivity Effects According to ADHD Diagnosis
In contrast to our hypothesis, one way ANOVA revealed no significant between-groups differences in RSA reactivity for either the storage or rehearsal conditions of the STM task (all p>0.70).
Test of Moderation Effects
Analyses were conducted both with categorical group variable (ADHD/non-ADHD) and with dimensional symptom scores. To test the hypothesis that the association between ADHD diagnosis and STM performance would be moderated by RSA reactivity, moderation analyses were conducted using a logistic regression-based approach (the PROCESS macro; Hayes 2013). Tables provided by Hsieh (1989) indicated sufficient power. For STM-S, the interaction was not statistically significant (p = 0.28). However, in line with our primary hypothesis, for STM-R the interaction was significant (z=2.46, b=0.26, p<0.05; see Fig. 1). McFadden’s R2 of 0.35 indicates a moderate effect, and the interaction resulted in an increased McFadden’s R2 of 0.02 compared to the model without the interaction. Specifically, conditional effects indicated that the association of STM-R with ADHD diagnosis was significant when RSA was withdrawn and also at more pronounced levels of withdrawal (see Table 3)2.
Fig. 1.
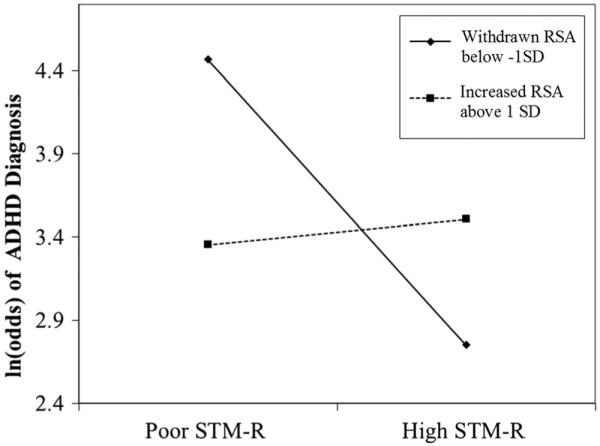
Graph of interaction of RSA reactivity and STM-R in predicting likelihood of ADHD diagnosis. Note: z=2.460, b=0.261, p<0.05
Follow-Up Analyses
Symptom domain t-scores from the ADHD-RS were highly correlated with one another (r=0.81) as well as with the Total t-score (both r>0.95). Post-hoc power analysis indicated sufficient power to detect an R2 of 0.07 given the sample size (Soper 2015).
Continuous Measures of ADHD Total T-Score
Moderation analyses were repeated using total t-scores. The interaction between STM-S performance and RSA reactivity was not significant (p > 0.99). However, the interaction between STM-R and RSA reactivity was associated with Overall t-score (t(228)=2.86, b=1.36, p<0.05), such that children with low STM-R and withdrawn RSA had significantly higher ADHD-RS total t-scores (see Fig. 2). Specifically, the conditional effect of the interaction was significant, and increasing, when RSA withdrew below Z=−0.26 as well as when RSA was extremely increased during the task (Z>1.90), but not at values in between. However, at extremely increased RSA, the relation between STM-R and ADHD t-score was positive such that better performance still predicted greater ADHD severity in the context of abnormal RSA reactivity. Notably, RSA change during the STM-R task was a significant predictor in the full model (t(229)=−3.28, b=−7.24, p<0.05) with STM-R treated as a covariate. The full model accounted for a significant portion of the variance in ADHD scores (R2 =0.46) and the interaction increased the model R2 by 0.02.
Fig. 2.
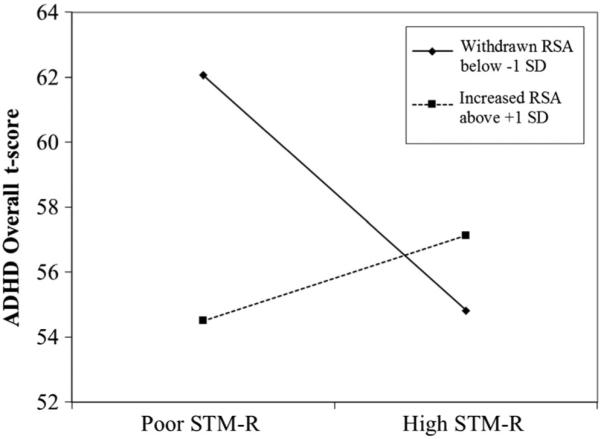
Graph of interaction of RSA reactivity and STM-R in predicting Overall ADHD t-score. Note: t(238)=2.856, b=1.358, p<0.01. The visualized moderation effect was similar for both individual symptom domains as well
Continuous Measures of Inattentive and Hyperactive/Impulsive T-Scores
Results for the interaction of STM-S and RSA withdrawal were non-significant in predicting inattentive t-scores (p=0.68) and hyperactive/impulsive t-scores as the outcome (p=0.58). However, the interaction between STM-R and RSA reactivity was significant in predicting Inattentive t-scores (t(229)=2.99, b=1.54, p<0.01), as well as hyperactive/impulsive t-scores, t (232) = 2.12, b =0.93, p<0.05. Johnson-Neyman conditional effects indicate that the association between STM-R and Inattentive t-score was similar to that for Overall t-score: significant when the RSA was withdrawn below average (Z<−0.30) and at extreme increase (Z> 1.53), but not at RSA between those values. Additionally, during extremely elevated RSA the conditional effect was positive, indicating that greater Inattentive t-score severity was positively predicted by higher STM-R. Furthermore, RSA change was a significant predictor of Inattentive ratings in the full model, t(229)=−2.04, b=−2.06, p<0.05. With hyperactive/impulsive t-score outcome, conditional effects were only significant at pronounced levels of RSA withdrawal (Z<−0.50).
Both full models, with inattentive or hyperactive t-scores as outcomes, accounted for a significant portion of the variance in domain severity (R2 =0.38 and 0.47, respectively). Specifically, the interaction terms uniquely accounted for an increase in R2 of 0.02 (inattentive t-score) and 0.01 (hyperactive t-score).
For the dimensional outcome approaches, RSA withdrawal during the STM-R task strengthened the negative association between task performance and symptom domain t-scores (see Table 3 for conditional effects). Put another way, dimensional ADHD indices were predicted by the combination of poor regulatory function and poor STM-R. Additionally, for the Overall and Inattentive indices, STM-R was a positive predictor during extreme increase in RSA, suggesting—along with multiple regression results—that abnormal RSA reactivity can be independently associated with ADHD. Thus, these results are consistent with a multiple pathway model of ADHD.
Specificity of Effects: ODD Diagnosis and Symptoms as Outcomes
In addition to including ODD symptoms as a covariate in the primary analyses, moderation analyses were conducted with the ADHD subsample utilizing both ODD diagnosis and number of ODD symptoms as outcomes in the model. The interaction of STM ability (either condition) and RSA withdrawal was not a significant predictor of ODD diagnosis or symptoms (all p’s>0.10) supporting at least some degree of specificity in the moderation effect for ADHD specifically rather than for disruptive behavior more generally. Results were similar when examining the entire sample.
Discussion
In recent decades it has become increasingly clear that ADHD is not caused by a single cognitive deficit or common developmental pathway. Research evaluating impaired executive function has revealed substantial neuropsychological heterogeneity in well-characterized samples of children with ADHD, implicating multiple developmental pathways to the disorder (Nigg et al. 2005; Sonuga-Barke et al. 2003; Willcutt et al. 2005) and indicating a need for examination of ADHD-related impairments at multiple levels of analysis (Nigg 2012). An alternative model is that ADHD is associated with the joint involvement of regulatory systems (i.e., cognitive and physiological), such that a weakness in only one area can be compensated by better functioning in another area. So called double-hit models suggest that ADHD would emerge when a single compensatory system is also weak in some but not all children.
A well-developed theory suggests that effortful cognition is supported by parasympathetic regulation (Thayer et al. 2009), so a logical proposal is that such regulation can compensate for weak STM and that, for some children, ADHD may involve their joint weakness. To our knowledge the present study is the first to formally test this hypothesis in the current form. We first consider the preliminary main effects and then the principal interaction tests.
Consistent with previous work and our first hypothesis, weakened STM performance was observed in ADHD youth when compared to typically developing children. Meta-analyses published in the last decade have consistently concluded that visual-spatial storage (indexed by both conditions) and rehearsal abilities (backward condition) are weaker in ADHD children compared to controls (although see work by Rapport et al. 2008, for important potential caveats). The association between reduced STM and ADHD remained even when statistically controlling for age, gender, ODD symptoms, and stimulant use. However, these impairments are not expected to occur in all children diagnosed with ADHD (Nigg et al. 2005) and, not surprisingly, the score distributions of the two groups in this sample did clearly overlap.
To examine variation in STM performance in children with ADHD, we turned to an examination of PNS-based regulation, as prior, mostly separate, literature suggest that 1) children with ADHD display disruptions in this domain and 2) PNS-based regulation is associated with STM abilities (Gianaros et al. 2004; Musser et al. 2011; Rash and Aguirre-Camacho 2012). The main effects of PNS regulation in the current sample were not as predicted. Compared to typically developing children, children with ADHD exhibited (non-significantly) higher levels of PNS influence at rest, and significantly higher PNS during a neutral condition, whereas we had posited they would have lower resting PNS. However, previous examinations of the associations between resting PNS and ADHD have often included samples with cooccurring conduct disorder (Crowell et al. 2006; Graziano and Derefinko 2013). Ours did not; in fact, parent-reported ODD symptoms were treated as a covariate removing any effects associated with disruptive behavior. Therefore, it may be that lower PNS influence at rest is more characteristic of behavioral dimensions linked with aggression and irritability and not ADHD.
Research in this area, particularly among children with ADHD, is just beginning to emerge, therefore precluding firm conclusions. However, it may be that children with ADHD require a higher level of PNS regulation to maintain homeostasis (i.e., while at rest) and during basic attending and responding, as well as executive tasks (i.e., during the neutral and the spatial span tasks). Conversely, children with oppositional and conduct problems appear to experience reduced PNS-regulation while at rest (Beauchaine et al. 2007), perhaps due to a reduced SNS influence broadly (Beauchaine et al. 2007; Musser et al. 2014).
When examining PNS-based reactivity to the STM tasks (i.e., RSA change from neutral baseline), children with ADHD did not differ from typically developing youth, again failing to support our initial hypothesis. However, consistent with differences at neutral baseline, the mean level of PNS activity during the STM task remained higher in ADHD children than in controls (see Table 2). Thus, between-group effects in mean PNS levels, and STM performance, were independently associated with ADHD in this sample (and ODD symptoms did not account for this effect).
Our principal focus however, was on the joint (interactive) effect of STM and RSA. Thus, the main and most novel finding here, as predicted based on our reading of Thayer et al. (2009), was that the interaction between PNS-based regulation (i.e., RSA change from neutral baseline) and STM-R performance significantly predicted ADHD diagnosis as well as ADHD symptoms measured dimensionally. ADHD as a diagnosis was only likely when both systems – STM and PNS regulation – were weak. This supports a compensatory or cascading risk model of ADHD. Specifically, when PNS control of the heart was withdrawn, worse STM-R performance predicted ADHD group membership; however, when PNS-based control was unmoved or increased, STM-R performance was not predictive of ADHD group membership.
Similar findings emerged when overall ADHD severity or either symptom domain were considered independently. Notably, however, in models with overall ADHD severity or Inattentive severity as the outcome, conditional effects were also significant for extreme RSA increase during the task, but in the positive direction. This finding indicates that inattentive symptoms and overall severity were positively predicted even when STM-R performance was not compromised, further suggesting that an abnormal PNS profile (i.e., extreme RSA augmentation) may act to hinder behavioral and/or attention regulation independently of STM.
Overall, the moderation effect held across all symptom domains, such that children were likely to have more ADHD symptoms (of either kind) when they exhibited STM weakness and PNS-based dysregulation. Practically speaking, it is possible to hypothesize that this leads to cognitive weaknesses for the ADHD child in two ways. One possibility is that exaggerated PNS responding (marked by above-average RSA withdrawal) may be a conditioned attempt to supplement deficient regulatory abilities necessary for the child to maintain STM performance. Alternatively, excessive PNS increase during a task may be disruptive to the optimal deployment of neurocognitive function via insufficient SNS activation; indeed, the relationship between excessive PNS elevation and weakened cognitive processing has been reported in young children previously (Marcovitch et al. 2010). That is, these patterns of PNS-based regulation may combine with neurocognitive factors to hinder performance or PNS-based regulation may compensate for STM difficulties or vice versa, such that the largest effects are viewed when both systems under perform.
These results indicate a potential mechanism underlying the neuropsychological heterogeneity observed in children with ADHD (Nigg et al. 2005). Specifically, these findings suggest a developmental pathway by which atypical regulation converges with other factors in neurocognitive development to exacerbate or protect WM ability among children with ADHD. Additionally, an unexpected novel finding here was that abnormally increased RSA during a challenging task may be independently associated with ADHD. Previous findings similarly showed that abnormally elevated RSA was tied to emotion regulation in ADHD children but not controls (Musser et al. 2011).
These results add to our understanding of the heterogeneity in ADHD and may help elucidate a multi-system profile predictive of greater symptom severity, consistent with a multiple pathway model of ADHD etiology (Sonuga-Barke 2005). Of course, in cross sectional data, we cannot determine the direction of effects and do not know whether other factors that cause ADHD also cause problems in STM and/or RSA.
Other limitations should be noted. First we did not examine variation by age. The age of the sample in this study represents a dynamic developmental range (7 to 11 years-old), and while age did not account for the observed effects, it will be valuable to know whether effects are similar at earlier or later developmental periods. Second, our index of short-term memory processes, particularly as they relate to working memory more broadly, should be considered in light of some limitations. The scoring procedure used (number of successful trials) is considered an “all or nothing” approach and, while it conveys more information than a simple “highest span” method, may be less sensitive than a “partial-credit” approach (see Conway et al. 2005). However, these approaches are highly correlated (Conway et al. 2005).
Finally, we should note again here that the moderation effect was only found for the rehearsal condition—important because, although the two conditions of the task load onto a single factor (Cantor et al. 1991; Engle et al. 1999), it is also clear that the conditions differ in terms of difficulty and cognitive load (Jensen and Figueroa 1975). Past research has suggested that forward span tasks are best operationalized as measures of the visual-spatial sketch pad (i.e. short-term storage). Similarly, the backward span tasks are posited to engage storage components, but also impose limited executive demands given that information must be held, protected, and then strategically re-ordered (e.g. Jensen and Figueroa 1975). Importantly, recent literature suggests that differences in central executive control of working memory may be a primary mechanism of the differences seen in ADHD groups (Rapport et al. 2008; Raiker et al. 2012). Therefore, replication of the current study with more executively demanding paradigms may inform which components of the working memory system are most associated with (or susceptible to) dysregulation at the autonomic level.
Future work should also examine how other cognitive domains may be related to, or interact with, autonomic reactivity in predicting risk, onset, and trajectory of the disorder. For example, there are still questions in this area of research concerning the role of inhibitory processes in the disorder and more specifically, the relation of those processes to working memory in ADHD (e.g., Raiker et al. 2012). In addition, examination of sympathetic nervous system (SNS) indices as potential moderators of impairment should be undertaken given the current findings and recent SNS-related findings in specific temperament-based profiles of ADHD (e.g. Musser et al. 2014; Karalunas et al. 2014). Although emerging work indicates that blunted RSA reactivity is associated with risk for externalizing behavior problems (Calkins et al. 2007; Graziano et al. 2013), additional longitudinal work in this area could elucidate which physiological and cognitive factors converge in early development to set the stage for ADHD symptoms.
In conclusion, these results help us understand the association of ADHD with cognitive deficits and can help inform models of ADHD. They suggest that ADHD is most likely in the presence of a cognitive weakness when compensating regulatory functions are also weak. This double hit conceptualization may open new avenues for conceptualizing intervention trials that strengthen a compensatory skill.
Acknowledgments
This research was supported by National Institute of Mental Health Grant R01-MH59105 awarded to Joel T. Nigg, Ph. D.
Footnotes
Extant work has suggested that many commonly used simple span tasks such as those used here consistently load on a factor typically referred to as short-term memory (i.e., storage or rehearsal abilities) while more complex tasks better index working memory (see Engle 2002). However, as evidenced in meta-analytic literature (Martinussen et al. 2005; Willcutt et al. 2005), these simple span tasks are often employed as measures of working memory. Thus, to increase the generalizability of findings to previous studies, we utilize simple span tasks but have chosen to use the terms short-term memory storage and rehearsal to reflect this advancement.
Moderation analyses were also conducted using ADHD Inattentive and Combined presentations as categorical outcomes. Findings largely mirrored those presented here and below. Specific findings are available upon request.
Conflict of Interest The authors declare that they have no conflict of interest.
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5th American Psychiatric Publishing; Arlington: 2013. [Google Scholar]
- American Psychological Association Ethical principles of psychologists and code of conduct. American Psychologist. 2002;57:1060–1073. [PubMed] [Google Scholar]
- Arnsten AF, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biological Psychiatry. 2005;57:1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. Working memory, thought and action. Oxford Univ. Press; Oxford: 2007. [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. doi:10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Bauer DJ, Curran PJ. Probing interactions in fixed and multilevel regression: inferential and graphical techniques. Multivariate Behavioral Research. 2005;40:373–400. doi: 10.1207/s15327906mbr4003_5. doi:10.1207/s15327906mbr4003_5. [DOI] [PubMed] [Google Scholar]
- Beauchaine T. Vagal tone, development, and gray’s motivational theory: toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology. 2001;13:183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Katkin ES, Strassberg Z, Snarr J. Disinhibitory psychopathology in male adolescents: discriminating conduct disorder from attention-deficit/hyperactivity disorder through concurrent assessment of multiple autonomic states. Journal of Abnormal Psychology. 2001;110:610–624. doi: 10.1037//0021-843x.110.4.610. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Gatzke-Kopp L, Mead HK. Polyvagal theory and developmental psychopathology: emotion dysregulation and conduct problems from preschool to adolescence. Biological Psychology. 2007;74:174–184. doi: 10.1016/j.biopsycho.2005.08.008. doi:10.1016/j.biopsycho.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Monuteaux MC, Doyle AE, Seidman LJ, Wilens TE, Ferrero F, Faraone SV. Impact of executive function deficits and attention-deficit/hyperactivity disorder (ADHD) on academic outcomes in children. Journal of Consulting and Clinical Psychology. 2004;72:757. doi: 10.1037/0022-006X.72.5.757. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Wright SB. Neurodevelopmental changes in working memory and cognitive control. Current Opinion in Neurobiology. 2007;17:243–250. doi: 10.1016/j.conb.2007.02.005. doi:10.1016/j.conb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Graziano PA, Keane SP. Cardiac vagal regulation differentiates among children at risk for behavior problems. Biological Psychology. 2007;74:144–153. doi: 10.1016/j.biopsycho.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor J, Engle RW, Hamilton G. Short-term memory, working memory, and verbal abilities: how do they relate? Intelligence. 1991;15:229–246. [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. Lawrence Erlbaum Associates; Mahwah: 2003. [Google Scholar]
- Conradt E, Degarmo D, Fisher P, Abar B, Lester BM, Lagasse LL. Hammond, J. The contributions of early adverse experiences and trajectories of respiratory sinus arrhythmia on the development of neurobehavioral disinhibition among children with prenatal substance exposure. A. Development and psychopathology. 2014;26:901–916. doi: 10.1017/S095457941400056X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway AR, Kane MJ, Bunting MF, Hambrick DZ, Wilhelm O, Engle RW. Working memory span tasks: a methodological review and user’s guide. Psychonomic Bulletin & Review. 2005;12:769–786. doi: 10.3758/bf03196772. [DOI] [PubMed] [Google Scholar]
- Crowell SE, Beauchaine TP, Gatzke-Kopp L, Sylvers P, Mead H, Chipman-Chacon J. Autonomic correlates of attention-deficit/hyperactivity disorder and oppositional defiant disorder in preschool children. Journal of Abnormal Psychology. 2006;115:174. doi: 10.1037/0021-843X.115.1.174. [DOI] [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD rating scale—IV: Checklists, norms, and clinical interpretation. Guilford Press; New York: 1998. [Google Scholar]
- Engle RW. Working memory capacity as executive attention. Current Directions in Psychological Science. 2002;11:19–23. [Google Scholar]
- Engle RW, Tuholski SW, Laughlin JE, Conway AR. Working memory, short-term memory, and general fluid intelligence: a latent-variable approach. Journal of Experimental Psychology: General. 1999;128:309. doi: 10.1037//0096-3445.128.3.309. [DOI] [PubMed] [Google Scholar]
- Fray PJ, Robbins TW, Sahakian BJ. Neuropsychiatric applications of CANTAB. International Journal of Geriatric Psychiatry. 1996;11:329–336. [Google Scholar]
- Froehlich TE, Lanphear BP, Epstein JN, Barbaresi WJ, Katusic SK, Kahn RS. Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of U.S. children. Archives of Pediatrics & Adolescent Medicine. 2007;161:857–864. doi: 10.1001/archpedi.161.9.857. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Van der Veen FM, Jennings JR. Regional cerebral blood flow correlates with heart period and high-frequency heart period variability during working-memory tasks: implications for the cortical and subcortical regulation of cardiac autonomic activity. Psychophysiology. 2004;41:521–530. doi: 10.1111/1469-8986.2004.00179.x. doi:10.1111/1469-8986. 2004.00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano P, Derefinko K. Cardiac vagal control and children’s adaptive functioning: a meta-analysis. Biological Psychology. 2013;94:22–37. doi: 10.1016/j.biopsycho.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gropper RJ, Tannock R. A pilot study of working memory and academic achievement in college students with ADHD. Journal of Attention Disorders. 2009;12:574–581. doi: 10.1177/1087054708320390. [DOI] [PubMed] [Google Scholar]
- Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. Guilford Press; New York: 2013. [Google Scholar]
- Hsieh FY. Sample size tables for logistic regression. Statistics in Medicine. 1989;8:795–802. doi: 10.1002/sim.4780080704. [DOI] [PubMed] [Google Scholar]
- Jensen AR, Figueroa RA. Forward and backward digit span interaction with race and IQ: predictions from Jensen’s theory. Journal of Educational Psychology. 1975;67:882. [PubMed] [Google Scholar]
- Karalunas SL, Fair D, Musser ED, Aykes K, Iyer SP, Nigg JT. Subtyping attention-deficit/hyperactivity disorder using temperament dimensions: toward biologically based nosologic criteria. JAMA Psychiatry. 2014;71:1015–1024. doi: 10.1001/jamapsychiatry.2014.763. doi:10.1001/jamapsychiatry.2014.763. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lambek R, Tannock R, Dalsgaard S, Trillingsgaard A, Damm D, Thomsen PH. Executive dysfunction in school-age children with ADHD. Journal of Attention Disorders. 2011;15:646–655. doi: 10.1177/1087054710370935. doi: 10.1177/1087054710370935. [DOI] [PubMed] [Google Scholar]
- Loeber R, Green S, Lahey B, Frick P, McBurnett K. Findings on disruptive behavior disorders from the first decade of the developmental trends study. Clinical Child and Family Psychology Review. 2000;3:37–60. doi: 10.1023/a:1009567419190. doi:10.1023/A:1009567419190. [DOI] [PubMed] [Google Scholar]
- Marcovitch S, Leigh J, Calkins SD, Leerks EM, O’Brien M, Blankson AN. Moderate vagal withdrawal in 3.5-year-old children is associated with optimal performance on executive function tasks. Developmental Psychobiology. 2010;52:603–608. doi: 10.1002/dev.20462. doi:10. 1002/dev.20462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinussen R, Hayden J, Hogg-Johnson S, Tannock R. A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:377. doi: 10.1097/01.chi.0000153228.72591.73. [DOI] [PubMed] [Google Scholar]
- MindWare . MindWare heart rate variability V.2.6 system. MindWare Technologies; Gahanna: 2008. [Google Scholar]
- Musser ED, Backs RW, Schmitt CF, Ablow JC, Measelle JR, Nigg JT. Emotion regulation via the autonomic nervous system in children with attention-deficit/hyperactivity disorder (ADHD) Journal of Abnormal Child Psychology. 2011;39:841–852. doi: 10.1007/s10802-011-9499-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser ED, Hawkey E, Kachan-Liu SS, Lees P, Roullet JB, Goddard K, Nigg JT. Shared familial transmission of autism spectrum and attention-deficit/hyperactivity disorders. Journal of Child Psychology and Psychiatry. 2014;55:819–827. doi: 10.1111/jcpp.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrao BL, Bipath P, van der Westhuizen D, Viljoen M. Autonomic correlates at rest and during evoked attention in children with attention-deficit/hyperactivity disorder and effects of methylphenidate. Neuropsychobiology. 2011;63(2):82–91. doi: 10.1159/000317548. doi:10.1159/ 00031754. [DOI] [PubMed] [Google Scholar]
- Nigg JT. Future directions in ADHD etiology research. Journal of Clinical Child & Adolescent Psychology. 2012;41:524–533. doi: 10.1080/15374416.2012.686870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, Casey BJ. An integrative theory of attention-deficit/hyperactivity disorder based on the cognitive and affective neurosciences. Development and Psychopathology. 2005;17:785–806. doi: 10.1017/S0954579405050376. doi:10.1017/S0954579405050376. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Willcutt EG, Doyle AE, Sonuga-Barke EJS. Causal heterogeneity in attention-deficit/ hyperactivity disorder: do we need neuropsychologically impaired subtypes? Biological Psychiatry. 2005;57:1224–1230. doi: 10.1016/j.biopsych.2004.08.025. doi:10.1016/j.biopsych.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Porges SW. The Polyvagal Theory: phylogenetic substrates of a social nervous system. International Journal of Psychophysiology. 2001;42:123–146. doi: 10.1016/s0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiker JS, Rapport MD, Kofler MJ, Sarver DE. Objectively-measured impulsivity and attention-deficit/hyperactivity disorder (ADHD): testing competing predictions from the working memory and behavioral inhibition models of ADHD. Journal of Abnormal Child Psychology. 2012;40:699–713. doi: 10.1007/s10802-011-9607-2. [DOI] [PubMed] [Google Scholar]
- Rapport MD, Chung KM, Shore G, Isaacs P. A conceptual model of child psychopathology: implications for understanding attention deficit hyperactivity disorder and treatment efficacy. Journal of Clinical Child Psychology. 2001;30:48–58. doi: 10.1207/S15374424JCCP3001_6. [DOI] [PubMed] [Google Scholar]
- Rapport MD, Alderson RM, Kofler MJ, Sarver DE, Bolden J, Sims V. Working memory deficits in boys with attention-deficit/hyperactivity disorder (ADHD): the contribution of central executive and subsystem processes. Journal of Abnormal Child Psychology. 2008;36:825–837. doi: 10.1007/s10802-008-9215-y. [DOI] [PubMed] [Google Scholar]
- Rash JA, Aguirre-Camacho A. Attention-deficit hyperactivity disorder and cardiac vagal control: a systematic review. ADHD Attention Deficit and Hyperactivity Disorders. 2012;4:167–177. doi: 10.1007/s12402-012-0087-1. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. Causal models of attention-deficit/hyperactivity disorder: from common simple deficits to multiple developmental pathways. Biological Psychiatry. 2005;57:1231–1238. doi: 10.1016/j.biopsych.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Dalen L, Remington B. Do executive deficits and delay aversion make independent contributions to pre-school attention-Deficit/Hyperactivity disorder symptoms? Journal of the American Academy of Child & Adolescent Psychiatry. 2003;42:1335. doi: 10.1097/01.chi.0000087564.34977.21. [DOI] [PubMed] [Google Scholar]
- Soper DS. Post-hoc statistical power calculator for multiple regression [Software] 2015 Available from http://www.danielsoper.com/ statcalc.
- Suess PE, Porges SW, Plude DJ. Cardiac vagal tone and sustained attention in school-age children. Psychophysiology. 1994;31:17–22. doi: 10.1111/j.1469-8986.1994.tb01020.x. doi:10.1111/j.1469-8986.1994.tb01020.x. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Hansen AL, Saus-Rose E, Johnsen BH. Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Annals of Behavioral Medicine. 2009;37:141–153. doi: 10.1007/s12160-009-9101-z. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory. Cognitive, Affective, & Behavioral Neuroscience. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler intelligence scale for children–Fourth Edition (WISC-IV) The Psychological Corporation; San Antonio: 2003. [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biological Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. doi:10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Nigg JT, Pennington BF, Solanto MV, Rohde LA, Tannock R, Lahey BB. Validity of DSM-IV attention deficit/hyperactivity disorder symptom dimensions and subtypes. Journal of Abnormal Psychology. 2012;121:991. doi: 10.1037/a0027347. [DOI] [PMC free article] [PubMed] [Google Scholar]


