Abstract
Chronic lymphocytic leukemia remains incurable despite availability of potent chemoimmunotherapy regimens. Allogeneic hematopoietic cell transplantation (HCT) is the only modality that offers the possibility of cure. To identify predictors of progression-free and overall survival, we evaluated outcomes of 43 consecutive patients who received an allograft for advanced CLL. The majority received a reduced intensity conditioning regimen (n = 37). Donors were HLA matched-related (n = 18), matched-unrelated (n = 15), mismatched-unrelated (n = 7), or umbilical cord blood (n = 3). The median progression-free (PFS) and overall survival (OS) were 31.4 months and 46.4 months respectively. Twenty (46.5%) patients were alive and in complete remission at a median follow-up of 31.4 months. NRM was higher than previously published series for CLL, likely due to a high burden of comorbidity (22 patients with HCT-CI ≥ 2) and a high proportion receiving HLA mismatched-unrelated donor or umbilical cord blood cells. Presence of del (11q), del(17p), or progressive disease at HCT are independent predictors of worse PFS and OS. New strategies are needed to improve survival outcomes in CLL associated with poor risk cytogenetics.
Keywords: CLL, Allogeneic hematopoietic cell transplant, Outcomes, Genomic aberrations, 17p deletion, 11q deletion
1. Introduction
Chronic lymphocytic leukemia (CLL) is the most common leukemia in Western countries. It is characterized by a variable course, ranging from an indolent to rapidly progressive [1]. Chemoimmunotherapy regimens which combine fludarabine, cyclophosphamide and rituximab induce objective responses in over 90% of patients treated in the front-line setting [2]. For patients with relapsed or refractory disease, novel therapies such as the Bruton’s tyrosine kinase inhibitor, ibrutinib, may offer higher response rates than traditional chemoimmunotherapy, even in the setting of poor risk cytogenetics [3]. Long-term follow up data is not available and it is uncertain whether these or other novel therapies can cure patients with high risk CLL [4–7].
Allogeneic hematopoietic cell transplantation (HCT) is the only known treatment modality which offers the potential for long term disease-free survival for relapsed/refractory and high risk CLL. Allogeneic HCT for patients with relapsed/refractory CLL may improve life expectancy compared to conventional chemotherapy or chemoimmunotherapy [8]. Moreover, a beneficial graft vs. leukemia (GVL) effect has been described: relapse rates are lower in patients with graft-vs.-host disease (GVHD) and donor lymphocyte infusion can induce remissions [9–14]. High non-relapse mortality (NRM), up to 40% even in fit younger patients, was observed following myeloablative conditioning (MAC) regimens [11,12]. More recently, less toxic reduced-intensity conditioning (RIC) regimens have expanded applicability of allogeneic HCT [15–19].
Various prognostic factors enable identification of CLL patients with worse anticipated outcomes such as CD38 expression, ZAP-70 expression, IgVH mutational status (unmutated), and certain genomic aberrations, such as del(11q) and del(17p) [20,21]. These factors have been integrated into decision models aimed at identifying subjects likely to benefit from hematopoietic cell allografting [22,23]. Here, we present an analysis of risk factors associated with outcome of 43 CLL patients who received HCT.
2. Materials and methods
2.1. Study design
Following approval by the Institutional Review Board of the University of South Florida, we performed a retrospective review of patients with CLL who received an allogeneic HCT at the H. Lee Moffitt Cancer Center between January 2003 and January 2011. Patients with any concurrent lymphoid malignancy at the time of transplantation were excluded except for those with previously treated Richter’s transformation in remission. All patients signed informed consent for inclusion in our long term follow-up database.
2.2. Donor selection
Adult donors were selected following high-resolution/molecular typing for HLA-A, HLA-B, HLA-C, and HLA-DRB1. Criteria for cord blood matching followed standard criteria: low-resolution at HLA-A, and HLA-B and high-resolution at HLA-DRB1. Matched related and unrelated donors were defined as those with 8/8 matches at HLA-A, HLA-B, HLA-C, and HLA-DRB1.
2.3. Conditioning regimen, stem cell collection, immunosuppression, and supportive care
Transplant regimens and GVHD prophylaxis were at the discretion of the treating physician in concordance with institutional standards [24,25]. All but three patients received G-CSF mobilized peripheral blood stem cells. All patients received GVHD prophylaxis, as outlined below. Treatment of acute GVHD was provided at the discretion of the treating physician and generally consisted of 1–2 mg/kg/day of prednisone or equivalent with taper as tolerated.
Post-transplantation G-CSF was administered only for persistent neutropenia beyond day +28. All patients received infectious prophylaxis with trimethoprim/sulfamethoxazole (or pentamidine in allergic patients) and acyclovir. Cytomegalovirus (CMV) reactivation was monitored weekly through day +90 by polymerase chain reaction (PCR) and treated accordingly with gancyclovir or fos-carnet. Blood products were administered as per institutional policies and current protocols.
2.4. Cytogenetic abnormalities
Conventional chromosome banding was performed on peripheral blood and/or bone marrow aspirates, along with fluorescence in situ hybridization (FISH) with probes for the most common cytogenetic abnormalities in CLL as per Dohner’s hierarchical group [26]. If FISH and/or band cytogenetics were not performed or not available, we considered those patients as not evaluable for cytogenetic abnormalities. The CLL FISH panel used in our institution included 11q22/ATM, 13q14/D13S319-D13S25, 17p13/TP53 DNA specific probes, chromosome 12 centromere probe, and a 14q32/IGH dual color apart probe. A test was considered adequate when it included the evaluation of at least 200 nucleated cells per probe.
2.5. Endpoint definition
The Hematopoietic Cell Transplant-Co-morbidity Index (HCT-CI) was calculated prior to transplant [27]. Acute (aGVHD) and chronic (cGVHD) GVHD were diagnosed and graded prospectively as per the Glucksberg grade (aGVHD) and the NIH consensus criteria (cGVHD), respectively [28,29]. For pre-HCT disease status, the response to last therapy was assessed according to the 2008 International Workshop on Chronic Lymphocytic Leukemia (IWCLL 2008) [21]. For post-transplant evaluation the pre-HCT staging data was considered as baseline, regardless of response to last therapy before HCT. Disease status assessments consisted of a bone marrow biopsy and aspiration as well as CT scans at 3 months, 6 months and 12 months after HCT. Patients with stable disease (SD) after transplant were not considered as progression. OS, PFS, and NRM were calculated from the day of stem cell infusion.
For univariate and multivariate analysis the predetermined covariates were: age (≥ 60 vs. <60 years old); donor type (MMUD vs. MRD/MUD); status of disease at HCT defined as response to pre-HCT therapy by IWCLL 2008 criteria (PD vs. CR/PR/SD) [21]; conditioning regimen (pentostatin + busulfan vs. other) [30]; Karnofsky performance status (KPS) (≤80 vs. >80); acute GVHD (grade II–IV vs. 0–I); 17p deletion by FISH (present vs. absent); 11q deletion by FISH (present vs. absent) [26]; number of distinct chemotherapy or immunotherapy regimens before HCT (≥3 vs. <3 regimens); number of lymph nodes (LN) groups enlarged at HCT (≥3 vs. <3 groups); bulky disease at HCT defined as lymph node/conglomerate nodes >5 cm in greatest dimension (present vs. absent); percentage of bone marrow involvement by CLL (≥30% vs.<30%) at HCT; and transplant comorbidity index (HCT-CI) (≥1 vs. 0).
2.6. Statistical analysis
Baseline patient characteristics at transplantation were summarized using descriptive statistics including mean, median, standard deviation and range for continuous measures and proportions and frequencies for categorical measures. OS and PFS were computed using the Kaplan–Meier method [31]. The cumulative incidence of relapse and NRM were computed using the Gray method [32]. The effect of covariates on OS and PFS was examined using the Cox proportional hazards model and the proportional sub-distribution hazards regression model by Fine and Gray [33]. The multivariable model was considered for each outcome, and the backward elimination method was used to select the final model. A p-value of <0.05 was considered significant.
3. Results
3.1. Patient and transplant characteristics
Patient and transplant characteristics are summarized in Table 1. Forty-three patients received an allogeneic HCT between January 2003 and January 2011 for CLL. Median interval from diagnosis to allogeneic HCT was 42.4 (8.9–154.8) months and median time from the date of first treatment to allogeneic HCT was 26.9 (2.7–100.9) months. Average hemoglobin levels and absolute lymphocyte counts prior to transplant were 12.5 g/dl (7.7–15.2) and 3 × 103 μL−1 (0.19–28.3), respectively. Two patients (4.7%) had Richter’s transformation before HCT but were in complete remission at time of HCT.
Table 1.
Patient characteristics.
| Number of patients n (%) | 43 (100) |
| Follow-up in months for survivors, median (range) | |
| From diagnosis | 73 (25–178) |
| From HCT | 31.6 (8–64) |
| Age, median (range) | |
| At diagnosis | 49 (26–65) |
| At HCT | 51.5 (35–66) |
| Gender, male (%) | 27 (62.8) |
| B symptoms, n (%) | 8 (18.6) |
| Karnofsky performance status (KPS), n (%) | |
| 80% or more | 27 (62.8) |
| Less than 80% | 16 (37.2) |
| HCT–CI score, n (%) | |
| 0 | 19 (44.2) |
| 1 | 2 (4.7) |
| 2 or more | 22 (55.1) |
| FISH CLL-Dohner hierarchy present/# tested (%) | |
| 13q deletion | 22/39 (56.4) |
| 17p deletion | 15/39 (38.5) |
| 11q deletion | 15/39 (38.5) |
| Trisomy 12 | 2/39 (5.1) |
| 2 or more genetic abnormalities | 20/39 (51.3) |
| Pre-HCT treatments, n (%) | |
| Number of regimens, median (range) | 3 (1–6) |
| Fludarabine or pentostatin based therapy | 38 (88.4) |
| Alemtuzumab | 9 (20.9) |
| Lenalidomide | 3 (7) |
| Disease status at HCT, n (%) | |
| CR | 10 (23.3) |
| PR | 14 (32.6) |
| SD | 9 (20.9) |
| PD/refractory | 10 (23.3) |
| Disease burden at HCT, n (%) | |
| Bulky disease at HCT (nodes > 5 cm) | 8 (18.6) |
| BM involvement (30% or more) | 15 (34.8) |
| LN areas enlarged at HCT (3 or more) | 16 (37.2) |
| Donor type, n (%) | |
| MRD | 18 (41.9) |
| MUD | 15 (34.9) |
| MMUD (includes 3 cord blood) | 10 (23.2) |
| Conditioning regimens, n (%) | |
| Pentostatin-Busulfan | 16 (37.2) |
| Fludarabine-Busulfan | 14 (32.6) |
| Fludarabine/TBI | 7 (16.3) |
| Other | 6 (14.1) |
HCT, allogeneic transplant; HCT-CI, hematopoietic cell transplant comorbidity index; CR, complete remission; PR, partial remission; SD, stable disease; PD, progressive disease; BM, bone marrow; MRD, matched related donor; MUD, matched unrelated donor; MMUD, mismatched unrelated donor; TBI, total body irradiation.
Nine patients (21%) received alemtuzumab as CLL therapy prior to transplant, with a median time from last alemtuzumab therapy to allografting of 180 days. Eight of the nine patients that received alemtuzumab had either 17p deletion, 11q deletion, or both. Three patients (7%) received lenalidomide prior to transplant: two as part of a trial evaluating the combination lenalidomide and rituximab. The third patient received lenalidomide as single agent.
The conditioning regimen for the majority of patients consisted of busulfan and a nucleoside analog. Fourteen patients received targeted IV busulfan (daily AUC 3500–6000 μmol min/L × 4 days), fludarabine 40 mg/m2 × 4 days and rituximab 375 mg/m2 on days +1 and +8 [34]. Sixteen received targeted busulfan on days −4 and −2 (cumulative AUC 16000 micromol min/L), pentostatin at 4 mg/m2 IV on days −28, −21, −14,−4 and −3 and rituximab 375 mg/m2 on days −21, −14, +1 and +8 [30]. Seven patients received fludarabine 25 mg/m2 × 3 days, and total body irradiation (TBI) of 1320 Gy. One patient received BCNU 300 mg/m2 on day −6, etoposide 800 mg/m2 on days −5 to −2 and cyclophosphamide 4.8 g/m2 on days −5 to −2 (CBV). Three cord blood recipients received fludarabine 25 mg/m2 × 3 days, cyclophosphamide 60 mg/kg × 2 days, and total body irradiation (TBI) of 1320 Gy, as did one patient who had an adult donor. One patient was conditioned with cyclophosphamide, carmustine and etoposide (CBV) and another with Busulfan and cyclophosphamide (BUCy) [35]. Rituximab was administered on day +1 and +8 as part of the conditioning regimen for patients that received fludarabine + busulfan. In the case of pentostatin-based RIC regimen rituximab was administered as previously described [36]. For patients who received a fludarabine plus intravenous busulfan conditioning regimen, rituximab 375 mg/m2 was administered on days +1 and +8 post-allogeneic transplantation.
Graft source consisted of G-CSF mobilized peripheral blood stem cells (PBSC) in 40 and UCB in 3 patients. The median CD34 dose was 7.67 × 106 (3–14 × 106) cells/kg for adult donor recipients. GVHD prophylaxis consisted of tacrolimus and methotrexate (n = 24), tacrolimus and mycophenolate (n = 11), tacrolimus and sirolimus (n = 2), cyclosporine and methotrexate (n = 1), cyclosporine and mycophenolate (n = 5).
At the time of transplantation, 17 patients had multiple CLL FISH abnormalities as follows: 17p, 11q and 13q deletions (n = 5); 11q and 17p deletions (n = 1); 17p and 13q deletions (n = 6); 11q and 13q deletions (n = 4); 17p and 13q deletions and trisomy 12 (n = 1). Of the fourteen patients with single FISH abnormality, one had 17p deletion and four had 11q deletion. Eight patients had a normal CLL FISH panel.
3.2. Engraftment and chimerism
For recipients of PBSC grafts, the median time to neutrophil engraftment, the first of three consecutive days of an absolute neutrophil count above 500 μL−1, was 17 (8–36) days. Median time to platelet engraftment, the first of three consecutive days of a platelet count ≥20,000 μL−1, with PBSC grafts was 18 (7–56) days. For cord blood transplant recipients, 2 of 3 died early, before neutrophil and platelet engraftment, and the third achieved neutrophil and platelet engraftment at 36 and 56 days, respectively.
Chimerism was determined by polymerase chain reaction of 16 short tandem repeat markers in the peripheral blood and whole bone marrow [37]. Median levels of peripheral-blood donor CD3, CD33, and whole marrow chimerism at day 30 were 93%, 100% and 93% and at day 90 were 96%, 100% and 95% respectively.
3.3. Disease response
At 100 days post HCT, 19/43 (44%) and 14/43 (33%) patients were in CR and PR, respectively. At 6 months the CR and PR rates were 58% and 14%, respectively. Thirty-three patients had less than CR before HCT, of these 21 (64%) achieved CR, 11 patients by day 90 and 10 more by day 180. At last follow up, 20 of the 43 transplanted patients (46.5%) remained in CR. The relapse risk as calculated by competing risk analysis was 9% at 1 year, 16% at 3 years, and 22% at 5 years.
3.4. Survival
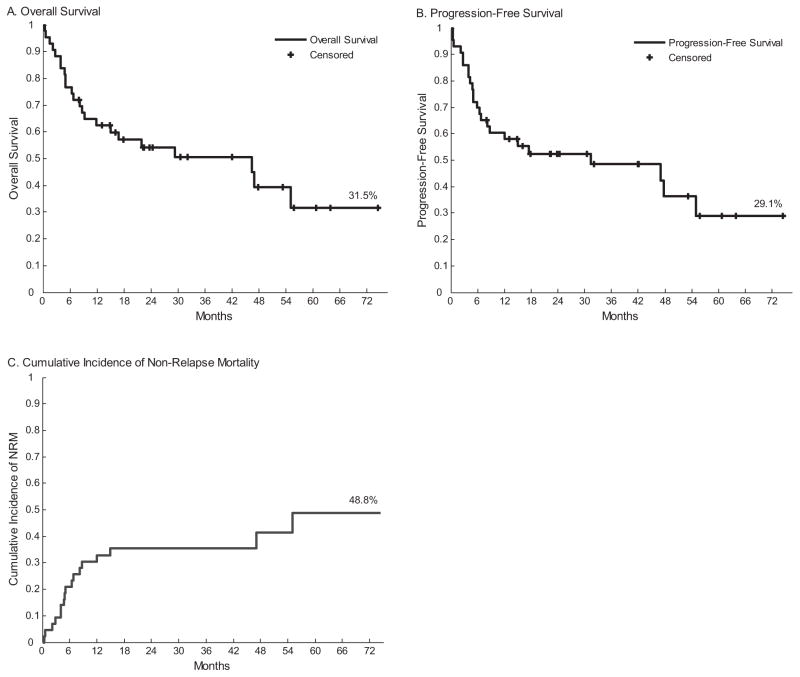
At the time of analysis the median post allogeneic HCT follow up was 2.6 years (0.7–6.2) for survivors, with 47% alive. Median overall survival from the time of diagnosis was 8.5 years. From the time of transplantation, the PFS and OS by Kaplan–Meier estimate were 31.3 months and 46.4 months, respectively (Fig. 1). The estimated 3-year PFS and OS were 48% and 51% respectively for the entire cohort. Nine patients died of CLL progression. Thirteen patients died from treatment related causes, without evidence of progressive disease. Five patients died before post-transplant day +100.
Fig. 1.
Overall survival (OS), progression free survival (PFS) and cumulative non-relapse mortality (NRM) for the whole cohort.
The median OS of the 10 patients who had PD going into HCT was 4.8 months (0–12) and none were alive at time of last follow up. Six of these patients achieved CR/PR following HCT, two of which eventually died of PD and the other four of NRM causes. The other four patients with PD at HCT did not achieve PR/CR after HCT and all died with progressive disease at or before day +90.
For each 5 years of age, the HR (95% CI) for OS and PFS were 1.59 (1.22–2.27) and 1.58 (1.13–2.22) respectively. Both of the two patients with prior, but treated, Richter’s transformation died, one of PD at day +880 and the other in CR at day +204 of infectious complications. The OS for patients treated with alemtuzumab (n = 9) vs. no alemtuzumab (n = 34) was 6.8 vs. 55 months (p = 0.005) and PFS was 6.4 vs. 55 months (p = 0.009). The OS and PFS were not affected by the HCT-CI when evaluated in different subgroups: HCT-CI score 0–1 vs. 2 or more, OS not reached vs. 16.8 months (p = 0.131). A trend toward worse OS was observed with HCT-CI score of 3 or more (55 vs. 15 months, p = 0.061). The median HCT-CI scores for patients carrying neither, either, or both Del 11q or Del 17p were 0, 1, and 3 respectively. OS and PFS survival results for patients with or without Del 17p and Del 11p are discussed below.
3.5. GVHD and NRM
Grade II–IV acute GVHD was observed in 24 (56%) patients. Of patients surviving beyond day 100, incidence of chronic GVHD (any grade) occurred in 55% (moderate/severe = 24%). Cumulative incidence of NRM at 6 months, 1-year and 5-years was 21%, 33%, and 49%, respectively (Fig. 1). NRM results for patients with or without Del 17p and Del 11p are discussed below.
Overall, thirteen patients had NRM related deaths. Nine died of infectious causes: severe fungal infections (6), bacterial sepsis (2), or bacterial pneumonia (1). Seven of these nine patients had grade II-IV GVHD at some time before or at the time of death. An additional 2 patients died of uncontrolled GVHD. One patient died of an intracranial hemorrhage and another patient died as a result of a second malignancy (lung cancer).
3.6. Preplanned univariate and multivariate analysis
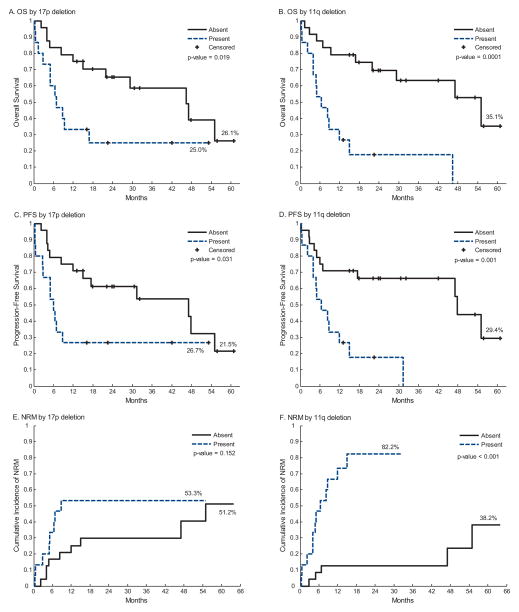
Univariate analysis results of multiple risk factors are presented in Table 2. The following factors were predictive of worse OS: age ≥60 years, progressive disease at time of transplantation, use of a MMUD donor, presence of del(17p), and presence of del(11q). These same 5 factors were also predictive of worse PFS. By Kaplan Meier estimation the median OS was worse for those with del(17p) (6.8 vs. 46.4 months, p = 0.019) and del(11q) (7 vs. 55 months, p = 0.0001).
Table 2.
Univariate analysis: the effect of transplant factors on OS and PFS.
| Variable | HR | 95% CI | p-Value | |
|---|---|---|---|---|
| Age at HCT (≥60 vs. <60 years old) | OS | 2.90 | 1.14–7.35 | 0.025a |
| PFS | 2.48 | 0.99–6.21 | 0.052 | |
| Donor status (MMUD vs. MRD/MUD) | OS | 3.25 | 1.3–8.10 | 0.012a |
| PFS | 3.17 | 1.29–7.78 | 0.012a | |
| Status of disease at HCT (PD vs. CR/PR/SD) | OS | 5.34 | 2.20–12.96 | < 0.001a |
| PFS | 7.04 | 2.85–17.38 | < 0.001a | |
| Conditioning regimen (Pento/BU vs. others) | OS | 0.45 | 0.17–1.24 | 0.124 |
| PFS | 0.5 | 0.20–1.32 | 0.168 | |
| KPS (≤80 vs. >80) | OS | 1.93 | 0.84–4.45 | 0.122 |
| PFS | 2.06 | 0.91–4.65 | 0.081 | |
| Acute GVHD (II–IV vs. 0-I) | OS | 0.86 | 0.37–1.98 | 0.718 |
| PFS | 0.83 | 0.37–1.87 | 0.703 | |
| 17p deletion (present vs. absent) | OS | 2.66 | 1.14–6.24 | 0.024a |
| PFS | 2.43 | 1.06–5.58 | 0.036a | |
| 11q deletion (present vs. absent) | OS | 5.20 | 2.08–12.99 | < 0.001a |
| PFS | 4.10 | 1.66–10.13 | 0.002a | |
| # treatments before HCT (≥3 vs. <3 regimens) | OS | 2.23 | 0.88–5.66 | 0.092 |
| PFS | 2.50 | 0.99–6.32 | 0.053 | |
| # LN areas enlarged at HCT (≥3 vs. <3 groups) | OS | 2.25 | 0.81–6.22 | 0.120 |
| PFS | 2.51 | 0.91–6.90 | 0.075 | |
| Bulky disease at HCT (present vs. absent) | OS | 1.13 | 0.42–3.05 | 0.810 |
| PFS | 1.35 | 0.51–3.63 | 0.548 | |
| % BM involvement at HCT (≥30% vs.<30%) | OS | 2.04 | 0.86–4.85 | 0.105 |
| PFS | 2.12 | 0.91–4.96 | 0.083 | |
| HCT-CI score (≥1 vs. 0) | OS | 1.80 | 0.76–4.27 | 0.186 |
| PFS | 1.46 | 0.64–3.35 | 0.374 |
MRD, matched related donor; MUD, matched unrelated donor; MMUD, mismatched unrelated donor; Pento, pentostatin; BU, busulfan; KPS, Karnofsky performance status; GVHD, graft vs. host disease; LN, lymph node; BM, bone marrow; HCT-CI, hematopoietic cell transplant comorbidity index.
Variables with significant statistical difference.
The multivariable models (Table 3) were built using the backward elimination method. Variables with a p-value <0.1 in the univariate analysis entered the initial multivariable model and remained if p < 0.05. In our model, the following 3 variables were significantly associated with worse OS: progressive disease at transplant (p = 0.003), del(17p) (p = 0.040) and del(11q) (p = 0.001). These variables along with a KPS ≤ 80 were also correlated with inferior PFS: PD (p = 0.001), del(17p) (p = 0.05), del(11q) (p = 0.003) and KPS ≤ 80 (p = 0.011).
Table 3.
Multivariate analysis: the effect of transplant factors on OS and PFS.
| HR | 95% CI | p-Value | |
|---|---|---|---|
| Overall survival (OS) | |||
| Status of disease at HCT (PD vs. CR/PR/SD) | 3.98 | 1.60–9.89 | 0.003a |
| 17p deletion (present vs. absent) | 2.48 | 1.04–5.87 | 0.040a |
| 11q deletion (present vs. absent) | 4.70 | 1.83–12.07 | 0.001a |
| Progression free survival (PFS) | |||
| Status of disease at HCT (PD vs. CR/PR/SD) | 4.89 | 1.94–12.31 | 0.001a |
| KPS (80 or less vs. 90–100) | 3.47 | 1.34–8.99 | 0.011a |
| 17p deletion (present vs. absent) | 3.35 | 1.00–5.53 | 0.050a |
| 11q deletion (present vs. absent) | 4.90 | 1.71–14.06 | 0.003a |
CR, complete remission; PR, partial remission; SD, stable disease; PD, progressive disease; KPS, Karnofsky performance status; HR, hazard ratio; CI, confidence interval.
Variables with significant statistical difference.
3.7. Survival results stratified by cytogenetic abnormalities
The Kaplan–Meier estimate for 3-year OS for patients harboring del(17p) vs. non del(17p) was 25% vs. 59% (p = 0.02) and 3-year PFS was 27% vs. 54% (p = 0.031) respectively. In patients harboring del(11q) vs. non del(11q), 3-year OS was 18% vs. 63% (p = 0.0001) and 3-year PFS was 0% vs. 66% (p = 0.001). Kaplan Meier curves and Log-rank tests for 17p and 11q deletions are shown (Fig. 2).
Fig. 2.
Overall survival, progression free survival, and non-relapse mortality stratified by the presence or absence of 17p deletion (A, C and E) or 11q deletion (B, D and F).
The presence of del(11q) was significantly associated with worse NRM (HR = 7.75; 95% CI: 2.65, 22.60; p = 0.0002). There was a trend for worse NRM in patients with del(17p) (HR = 1.92; 95% CI 0.75, 4.89; p = 0.17).
The median OS for sole Del 11q (n = 9), sole Del 17p (n = 9) and combined 11q and 17p deletions (n = 6) were 8.3, 9.3 and 5.1 months (p = 0.47) after HCT, compared to a median OS for those with neither 11q nor 17p deletions (n = 15) of 55 months (p < 0.0001 by log-rank test) (Supplemental Fig. 1).
Supplemental Fig. 1 related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.leukres.2014.04.006.
The median OS for patients with normal CLL panel FISH cytogenetics (n = 8), single FISH abnormality (n = 14) and 2 or more FISH abnormalities (n = 17) were 55, 29, and 6.8 months, respectively (p = 0.011).
4. Discussion
The effect of cytogenetic risk factors upon allogeneic transplant outcomes for CLL remains controversial. This series shows that del(17p), del(11q), and the presence of PD at transplant, confer a worse prognosis in patients undergoing allogeneic HCT for CLL. Our sample is important because of the number of patients having cytogenetic data available pre-transplant. Overall survival was 51% at 3 years which is comparable to published series [17,38–40], especially considering the higher proportion of CLL cases with adverse risk factors in our cohort. The large majority of our patients who were alive at the last follow-up remained in sustained CR (95%) which supports the beneficial role of allogeneic HCT as a curative approach in CLL.
In addition to del(11q) and del(17p), univariate analysis identified age >60, use of a MMUD, and progressive disease at the time of allografting to be associated with worse PFS and OS, consistent with findings reported by other studies [17,38,40]. In contrast, the presence of bulky adenopathy, number of prior therapies before allogeneic HCT, KPS, and percentage of BM involvement did not influence clinical outcomes in our series. While this disassociation between advanced disease characteristics and outcome is not unique, it does contrast with some published reports [38]. Moreover, we did not detect a statistically significant advantage of a particular regimen.
Published predictive models for transplantation outcomes in CLL patients suggest that less than a PR is associated with worse PFS and OS [41,42]. In contrast, in our cohort the presence of stable disease (SD) prior to transplantation did not result in worse outcomes when compared to patients with CR/PR (data not shown). However, patients with progressive disease (PD) had significantly worse PFS and OS in the univariate and multivariate analysis (Table 2). It is possible that patients with SD are able to maintain steady disease burden until a GVL effect is established, while those with PD are not afforded that chance.
We acknowledge limitations when interpreting our multivariate analysis. Although there are a large number of factors considered in relation to the number of events, this approach is justified because of the controversy regarding cytogenetic risk factors for CLL patients undergoing allogeneic transplant.
In our cohort of patients, the presence of 17p and 11q deletions were strong and independent negative predictors of worse PFS and OS in multivariate analysis. The median OS and PFS were less than a year in both groups of patients when calculated from the time of HCT. Published non-randomized data suggest that allogeneic HCT offers longer disease-free survival compared to conventional chemotherapy for patients with high risk disease factors [43]. Other data show that OS and PFS after HCT are worse for patients with 5 or more genetic abnormalities [44]. However, there is no consensus on the effect of specific cytogenetic aberrations on allogeneic HCT outcomes. The results presented here conflict with some published data, but our cohort has a high percentage of patients with available genetic information (91%), and has the highest percentage of patients with high-risk cytogenetic abnormalities (70%). A brief comparison of studies evaluating transplant outcomes, including cytogenetic features, in CLL patients is warranted.
Dreger et al. reported no association of poor risk genetic abnormalities and OS or PFS in the CLL3X trial, while fludarabine-refractory disease and the use of T cell depletion (TCD) predicted worse outcomes in a multivariate analysis [38]. The low numbers of patients with high risk cytogenetic features in the CLL3X study (13 patients with 17p deletion) prevent definitive conclusions regarding how the presence of del(17p) affects HCT outcomes. For the whole cohort, CLL3X reported higher 4 year OS (64%) and lower NRM (23%) than results in our series at 3 years for OS (51%) and NRM (35%). Direct comparison is complicated due to inherent differences in the patient populations and selection criteria. Cytogenetic information was only available for 80% of patients in the CLL3X series and only a few patients had an advanced HCT-CI ≥ 2 (10% in the CLL3X vs. 55% in our series). Furthermore our series included a much higher proportion of patients with 7/8 HLA MMUD donors or cord blood sources (23% vs. 2%). These key features likely contributed to the differences in outcomes, particularly NRM and overall survival between the two studies.
Brown et al. demonstrated a reduced 2-year PFS in CLL patients with del(17p) or del(11q) receiving an allogeneic HCT based on univariate analysis, but this did not hold up on multivariate analysis. 3 year OS and PFS for del(17p) patients were 30% and 11%, similar to the 3 year OS and PFS of 27 and 25% in our series [41]. In an expanded long term follow up series del(17p) and del(11q) did not significantly impact PFS or OS [42].
Toze et al., demonstrated worse OS for patients with del(17p) (OR: 6.9, p = 0.001); however, multivariate analysis did not confirm these results. The presence of del(17p) was also associated with a lower probability of achieving a remission after allogeneic HCT [45]. An important series from Sorror et al. reported NRM and OS at 5 years of 23% and 50%, however 17p and 11q deletions were underrepresented at only 14/82 (17%) of patients [17]. The largest series of transplanted del(17p) patients (n = 44) is an evaluation of EBMT patients revealing that long term disease free survival was achievable, with a 3 year OS and PFS 44% and 37% (n = 44). Direct comparison to patients without high risk cytogenetic features was not done [16].
Clearly larger series of prospective data are needed to fully elucidate the effect of these genetic abnormalities upon post allogeneic HCT outcomes. It also remains unclear how the availability of next generation sequencing will affect pre-transplant risk stratification and post-transplant disease response monitoring [46,47].
The data here demonstrate high NRM as compared to other published series. We have included death with SD as a NRM event, where alternative studies may have considered death with disease as a relapse event. Regardless of cytogenetic status, our patients were heavily pretreated and had high-risk features. The worse OS with del(11q) and del(17p) were not associated with higher progression as may be expected and Del 11q had a higher cumulative incidence of NRM. It is possible that the higher frequency of heavy pretreatment (80% of del(11q) patients received more than 3 chemoimmunotherapy regimens before proceeding with HCT) might have played a role. This finding argues in favor of considering an allogeneic transplant earlier in the disease course for patients with high risk cytogenetic features; however these data do not prove that transplanting patients with high risk cytogenetics earlier in the disease course would increase survival compared to delayed transplant at the cost of higher transplant related NRM. This is especially relevant as targeted therapies and antibodies with higher response rates for these patients become available.
It is important to note that 8 of 9 patients treated with alemtuzumab before HCT had 17p or 11q deletion and pre-HCT alemtuzumab therapy was associated with worse OS and PFS by univariate analysis. The series by Toze et al. showed worse OS when alemtuzumab was used either as part of the conditioning regimen to achieve T cell depletion or as a therapy for CLL prior to transplant. This finding was not reproduced in multivariate analysis [45]. Schetelig et al. demonstrated worse OS after HCT when alemtuzumab was administered during the conditioning regimen but not when it was used for CLL treatment. The multivariate analysis showed a worse PFS with the use of alemtuzumab during conditioning [16]. Since this was not a preselected variable it was not included in our multivariate analysis. Although alemtuzumab is known to deplete T cells, the therapy itself is unlikely responsible for the worse outcomes in del(17p) and del(11q) patients since the number of patients treated with alemtuzumab was small. Clinical trials have shown that relapse rates and TRM are not increased when alemtuzumab is included in HCT preparative regimens [48], however the impact of alemtuzumab on the outcomes after HCT for CLL remains unclear.
The prognostic importance of the HCT-CI for CLL patients undergoing HCT is established [49,50]. In our study there was a trend toward worse OS with higher HCT-CI scores, although this was not statistically significant. The median HCT-CI scores for patients carrying neither, either, or both del(11q) or del(17p) were 0, 1, and 3 respectively. Although HCT-CI was not a significant risk factor in the multivariate analysis for OS and PFS, it is possible that this is a confounding surrogate marker for a higher NRM in patients with del(11q) or del(17p). A larger case–control study with registry data might correct for HCT-CI and the number and nature of prior therapeutic regimens in the context of cytogenetic abnormalities.
Our results show that genetic risk factors predict for worse outcomes following allogeneic HCT for CLL. This might be mitigated by earlier referral and transplant for such patients, avoiding the increased toxicity and NRM that results from further delaying transplantation. Prospective studies should evaluate incorporating novel therapies such as ibrutinib, which has proven to induce responses irrespective of the presence of adverse genetic markers such as del(17p) or del(11q), in patients being considered for an allogeneic HCT.
Supplementary Material
Footnotes
Conflict of interest statement
None of the authors of this paper has a financial or personal relationship with other people or organisations that could inappropriately influence or bias the content of the paper.
References
- 1.Ries L, Melbert D, Krapcho M, Stinchcomb D, Howlader N, Horner M, et al. SEER cancer statistics review, 1975–2005. Bethesda, MD: National Cancer Institute; 2008. pp. 1975–2005. [Google Scholar]
- 2.Carballido E, Veliz M, Komrokji R, Pinilla-Ibarz J. Immunomodulatory drugs and active immunotherapy for chronic lymphocytic leukemia. Cancer Control. 2012;19:54–67. doi: 10.1177/107327481201900106. [DOI] [PubMed] [Google Scholar]
- 3.Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wierda W, O’Brien S, Wen S, Faderl S, Garcia-Manero G, Thomas D, et al. Chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab for relapsed and refractory chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4070–8. doi: 10.1200/JCO.2005.12.516. [DOI] [PubMed] [Google Scholar]
- 5.Keating MJ, O’Brien S, Albitar M, Lerner S, Plunkett W, Giles F, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4079–88. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 6.Byrd JC, Rai K, Peterson BL, Appelbaum FR, Morrison VA, Kolitz JE, et al. Addition of rituximab to fludarabine may prolong progression-free survival and overall survival in patients with previously untreated chronic lymphocytic leukemia: an updated retrospective comparative analysis of CALGB 9712 and CALGB 9011. Blood. 2005;105:49–53. doi: 10.1182/blood-2004-03-0796. [DOI] [PubMed] [Google Scholar]
- 7.Keating MJ, Cazin B, Coutre S, Birhiray R, Kovacsovics T, Langer W, et al. Campath-1H treatment of T-cell prolymphocytic leukemia in patients for whom at least one prior chemotherapy regimen has failed. J Clin Oncol. 2002;20:205–13. doi: 10.1200/JCO.2002.20.1.205. [DOI] [PubMed] [Google Scholar]
- 8.Kharfan-Dabaja MA, Pidala J, Kumar A, Terasawa T, Djulbegovic B. Comparing efficacy of reduced-toxicity allogeneic hematopoietic cell transplantation with conventional chemo-(immuno) therapy in patients with relapsed or refractory CLL: a Markov decision analysis. Bone Marrow Transplant. 2012;47:1164–70. doi: 10.1038/bmt.2012.71. [DOI] [PubMed] [Google Scholar]
- 9.Michallet M, Corront B, Hollard D, Gratwohl A, Milpied N, Dauriac C, et al. Allogeneic bone marrow transplantation in chronic lymphocytic leukemia: 17 cases. Report from the EBMTG. Bone Marrow Transplant. 1991;7:275–9. [PubMed] [Google Scholar]
- 10.deMagalhaes-Silverman M, Donnenberg A, Hammert L, Lister J, Myers D, Simpson J, et al. Induction of graft-versus-leukemia effect in a patient with chronic lymphocytic leukemia. Bone Marrow Transplant. 1997;20:175–7. doi: 10.1038/sj.bmt.1700850. [DOI] [PubMed] [Google Scholar]
- 11.Toze CL, Galal A, Barnett MJ, Shepherd JD, Conneally EA, Hogge DE, et al. Myeloablative allografting for chronic lymphocytic leukemia: evidence for a potent graft-versus-leukemia effect associated with graft-versus-host disease. Bone Marrow Transplant. 2005;36:825–30. doi: 10.1038/sj.bmt.1705130. [DOI] [PubMed] [Google Scholar]
- 12.Dreger P, Montserrat E. Autologous and allogeneic stem cell transplantation for chronic lymphocytic leukemia. Leukemia. 2002;16:985–92. doi: 10.1038/sj.leu.2402530. [DOI] [PubMed] [Google Scholar]
- 13.Gribben JG, Zahrieh D, Stephans K, Bartlett-Pandite L, Alyea EP, Fisher DC, et al. Autologous and allogeneic stem cell transplantations for poor-risk chronic lymphocytic leukemia. Blood. 2005;106:4389–96. doi: 10.1182/blood-2005-05-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Jurdi N, Reljic T, Kumar A, Pidala J, Bazarbachi A, Djulbegovic B, et al. Efficacy of adoptive immunotherapy with donor lymphocyte infusion in relapsed lymphoid malignancies. Immunotherapy. 2013;5:457–66. doi: 10.2217/imt.13.31. [DOI] [PubMed] [Google Scholar]
- 15.Khouri IF, Keating M, Korbling M, Przepiorka D, Anderlini P, O’Brien S, et al. Transplantlite: induction of graft-versus-malignancy using fludarabine-based nonablative chemotherapy and allogeneic blood progenitor-cell transplantation as treatment for lymphoid malignancies. J Clin Oncol. 1998;16:2817–24. doi: 10.1200/JCO.1998.16.8.2817. [DOI] [PubMed] [Google Scholar]
- 16.Schetelig J, van Biezen A, Brand R, Caballero D, Martino R, Itala M, et al. Allogeneic hematopoietic stem-cell transplantation for chronic lymphocytic leukemia with 17p deletion: a retrospective European Group for Blood and Marrow Transplantation analysis. J Clin Oncol. 2008;26:5094–100. doi: 10.1200/JCO.2008.16.2982. [DOI] [PubMed] [Google Scholar]
- 17.Sorror ML, Storer BE, Sandmaier BM, Maris M, Shizuru J, Maziarz R, et al. Five-year follow-up of patients with advanced chronic lymphocytic leukemia treated with allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. J Clin Oncol. 2008;26:4912–20. doi: 10.1200/JCO.2007.15.4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dreger P, Brand R, Milligan D, Corradini P, Finke J, Lambertenghi Deliliers G, et al. Reduced-intensity conditioning lowers treatment-related mortality of allogeneic stem cell transplantation for chronic lymphocytic leukemia: a population-matched analysis. Leukemia. 2005;19:1029–33. doi: 10.1038/sj.leu.2403745. [DOI] [PubMed] [Google Scholar]
- 19.Kharfan-Dabaja MA, Bazarbachi A. Hematopoietic stem cell allografting for chronic lymphocytic leukemia: a focus on reduced-intensity conditioning regimens. Cancer Control. 2012;19:68–75. doi: 10.1177/107327481201900107. [DOI] [PubMed] [Google Scholar]
- 20.Kharfan-Dabaja MA, Chavez JC, Khorfan KA, Pinilla-Ibarz J. Clinical and therapeutic implications of the mutational status of IgVH in patients with chronic lymphocytic leukemia. Cancer. 2008;113:897–906. doi: 10.1002/cncr.23671. [DOI] [PubMed] [Google Scholar]
- 21.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–56. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dreger P, Corradini P, Kimby E, Michallet M, Milligan D, Schetelig J, et al. Indications for allogeneic stem cell transplantation in chronic lymphocytic leukemia: the EBMT transplant consensus. Leukemia. 2007;21:12–7. doi: 10.1038/sj.leu.2404441. [DOI] [PubMed] [Google Scholar]
- 23.Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer J, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–74. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 24.Perkins J, Field T, Kim J, Kharfan-Dabaja MA, Fernandez H, Ayala E, et al. A randomized phase II trial comparing tacrolimus and mycophenolate mofetil to tacrolimus and methotrexate for acute graft-versus-host disease prophylaxis. Biol Blood Marrow Transplant. 2010;16:937–47. doi: 10.1016/j.bbmt.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 25.Pidala J, Kim J, Jim H, Kharfan-Dabaja MA, Nishihori T, Fernandez H, et al. A randomized phase II study to evaluate tacrolimus in combination with sirolimus or methotrexate after allogeneic hematopoietic cell transplantation. Haematologica. 2012 doi: 10.3324/haematol.2012.067140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dohner H, Stilgenbauer S, Benner A, Leupolt E, Krober A, Bullinger L, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:1910–6. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- 27.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–9. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–8. [PubMed] [Google Scholar]
- 29.Arora M, Nagaraj S, Witte J, DeFor TE, MacMillan M, Burns LJ, et al. New classification of chronic GVHD: added clarity from the consensus diagnoses. Bone Marrow Transplant. 2009;43:149–53. doi: 10.1038/bmt.2008.305. [DOI] [PubMed] [Google Scholar]
- 30.Kharfan-Dabaja MA, Anasetti C, Fernandez HF, Perkins J, Ochoa-Bayona JL, Pidala J, et al. Phase II study of CD4+-guided pentostatin lymphodepletion and pharmacokinetically targeted busulfan as conditioning for hematopoietic cell allografting. Biol Blood Marrow Transplant. 2013;19:1087–93. doi: 10.1016/j.bbmt.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 32.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–54. [Google Scholar]
- 33.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 34.Pidala J, Roman-Diaz J, Kim J, Nishihori T, Perkins J, Tate C, et al. Targeted IV busulfan and fludarabine followed by post-allogeneic hematopoietic cell transplantation rituximab demonstrate encouraging activity in CD20+ lymphoid malignancies without increased risk of infectious complications. Int J Hematol. 2011;93:206–12. doi: 10.1007/s12185-010-0747-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brunstein CG, Barker JN, Weisdorf DJ, DeFor TE, Miller JS, Blazar BR, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110:3064–70. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kharfan-Dabaja MA, Anasetti C, Fernandez HF, Perkins J, Ochoa-Bayona JL, Pidala J, et al. Phase II study of CD4+ guided pentostatin lymphodepletion and pharmacokinetically-targeted busulfan as conditioning for hematopoietic cell allografting. Biol Blood Marrow Transplant. 2013;19:1087–93. doi: 10.1016/j.bbmt.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thiede C, Florek M, Bornhauser M, Ritter M, Mohr B, Brendel C, et al. Rapid quantification of mixed chimerism using multiplex amplification of short tandem repeat markers and fluorescence detection. Bone Marrow Transplant. 1999;23:1055–60. doi: 10.1038/sj.bmt.1701779. [DOI] [PubMed] [Google Scholar]
- 38.Dreger P, Dohner H, Ritgen M, Bottcher S, Busch R, Dietrich S, et al. Allogeneic stem cell transplantation provides durable disease control in poor-risk chronic lymphocytic leukemia: long-term clinical and MRD results of the German CLL Study Group CLL3X trial. Blood. 2010;116:2438–47. doi: 10.1182/blood-2010-03-275420. [DOI] [PubMed] [Google Scholar]
- 39.Brown JR, Kim HT, Armand P, Cutler C, Fisher DC, Ho V, et al. Long-term follow-up of reduced-intensity allogeneic stem cell transplantation for chronic lymphocytic leukemia: prognostic model to predict outcome. Leukemia. 2013;27:362–9. doi: 10.1038/leu.2012.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khouri IF, Bassett R, Poindexter N, O’Brien S, Bueso-Ramos CE, Hsu Y, et al. Nonmyeloablative allogeneic stem cell transplantation in relapsed/refractory chronic lymphocytic leukemia: long-term follow-up, prognostic factors, and effect of human leukocyte histocompatibility antigen subtype on outcome. Cancer. 2011;117:4679–88. doi: 10.1002/cncr.26091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown JR, Kim HT, Li S, Stephans K, Fisher DC, Cutler C, et al. Predictors of improved progression-free survival after nonmyeloablative allogeneic stem cell transplantation for advanced chronic lymphocytic leukemia. Biol Blood Marrow Transplant. 2006;12:1056–64. doi: 10.1016/j.bbmt.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 42.Brown JR, Kim HT, Armand P, Cutler C, Fisher DC, Ho V, et al. Long-term follow-up of reduced-intensity allogeneic stem cell transplantation for chronic lymphocytic leukemia: prognostic model to predict outcome. Leukemia. 2013;27:362–9. doi: 10.1038/leu.2012.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caballero D, Garcia-Marco JA, Martino R, Mateos V, Ribera JM, Sarra J, et al. Allo-geneic transplant with reduced intensity conditioning regimens may overcome the poor prognosis of B-cell chronic lymphocytic leukemia with unmutated immunoglobulin variable heavy-chain gene and chromosomal abnormalities (11q- and 17p-) Clin Cancer Res. 2005;11:7757–63. doi: 10.1158/1078-0432.CCR-05-0941. [DOI] [PubMed] [Google Scholar]
- 44.Jaglowski SM, Ruppert AS, Heerema NA, Bingman A, Flynn JM, Grever MR, et al. Complex karyotype predicts for inferior outcomes following reduced-intensity conditioning allogeneic transplant for chronic lymphocytic leukaemia. Br J Haematol. 2012;159:82–7. doi: 10.1111/j.1365-2141.2012.09239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toze CL, Dalal CB, Nevill TJ, Gillan TL, Abou Mourad YR, Barnett MJ, et al. Allogeneic haematopoietic stem cell transplantation for chronic lymphocytic leukaemia: outcome in a 20-year cohort. Br J Haematol. 2012;158:174–85. doi: 10.1111/j.1365-2141.2012.09170.x. [DOI] [PubMed] [Google Scholar]
- 46.Puente XS, Pinyol M, Quesada V, Conde L, Ordonez GR, Villamor N, et al. Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature. 2011;475:101–5. doi: 10.1038/nature10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dreger P, Schnaiter A, Zenz T, Bottcher S, Rossi M, Paschka P, et al. TP53, SF3B1, and NOTCH1 mutations and outcome of allotransplantation for chronic lymphocytic leukemia: six-year follow-up of the GCLLSG CLL3X trial. Blood. 2013;121:3284–8. doi: 10.1182/blood-2012-11-469627. [DOI] [PubMed] [Google Scholar]
- 48.van Besien K, Kunavakkam R, Rondon G, De Lima M, Artz A, Oran B, et al. Fludarabine-melphalan conditioning for AML and MDS: alemtuzumab reduces acute and chronic GVHD without affecting long-term outcomes. Biol Blood Marrow Transplant. 2009;15:610–7. doi: 10.1016/j.bbmt.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sorror ML, Maris MB, Sandmaier BM, Storer BE, Stuart MJ, Hegenbart U, et al. Hematopoietic cell transplantation after nonmyeloablative conditioning for advanced chronic lymphocytic leukemia. J Clin Oncol. 2005;23:3819–29. doi: 10.1200/JCO.2005.04.569. [DOI] [PubMed] [Google Scholar]
- 50.Sorror ML, Storer BE, Maloney DG, Sandmaier BM, Martin PJ, Storb R. Outcomes after allogeneic hematopoietic cell transplantation with nonmyeloablative or myeloablative conditioning regimens for treatment of lymphoma and chronic lymphocytic leukemia. Blood. 2008;111:446–52. doi: 10.1182/blood-2007-07-098483. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.