Abstract
Purpose.
To evaluate a silk fibroin (SF) biomaterial as a substrate for corneal epithelial cell proliferation, differentiation, and stratification in vitro compared with denuded human amniotic membrane (AM).
Methods.
Primary human and rabbit corneal epithelial cells and immortalized human corneal limbal epithelial cells were cultured on the SF and denuded AM, respectively. The biological cell behavior, including the morphology, proliferation, differentiation, and stratification, on the two substrates was compared and analyzed.
Results.
Corneal epithelial cells can adhere and proliferate on the SF and denuded AM with a cobblestone appearance, abundant microvilli on the surface, and wide connection with the adjacent cells. MTT assay showed that cell proliferation on denuded AM was statistically higher than that on SF at 24 and 72 hours after plating (P = 0.001 and 0.0005, respectively). Expression of ΔNp63a and keratin 3/12 was detected in primary cell cultures on the two substrates with no statistical difference. When cultured at the air-liquid interface for 7 days, cells on SF could form a comparable stratified graft with a 2- to 3-cell layering, which compared similarly to AM cultures.
Conclusions.
SF, a novel biomaterial, could support corneal epithelial cells to proliferate, differentiate, and stratify, retaining the normal characteristic epithelium phenotype. Compared with AM, its unique features, including the transparency, ease of handling, and transfer, and inherent freedom from disease transmission, make it a promising substrate for corneal wound repair and tissue-engineering purposes.
Silk fibroin film could support corneal epithelial cells to proliferate, differentiate, and stratify, retaining the normal characteristic epithelium phenotype.
Introduction
With the substantial advancement of regenerative medicine and tissue-engineering techniques, stem cell therapy has been successfully practiced to treat refractory ocular surface disorders, such as limbal stem cell deficiency.1,2 To establish a cell sheet for transplantation, a suitable substrate carrier is required to transfer the cells from laboratory benchtop to bedside. A series of biomaterial-based substrates have been tested experimentally and/or clinically, such as human amniotic membrane (AM),3–5 fibrin glue,6,7 temperature-responsive polymers,8,9 and acellular porcine lamellar stroma.10,11 Among them, human AM is the clinical standard substrate for ocular surface repair owing to its biological properties that inhibit inflammation, tissue scarring, and angiogenesis.12
However, limitations regarding the use of AM exist. These include relatively poor mechanical strength, semitransparent appearance, difficulty of handling, and the potential risk of disease transmission, such as human immunodeficiency virus (HIV), hepatitis B virus, hepatitis C virus, and syphilis. To overcome these limitations, a novel biomaterial, silk fibroin (SF), the primary structural protein of Bombyx mori silkworm cocoons, has been explored and tested with remarkable progress. Previous studies have shown that SF could generate minimal immune and inflammatory responses when implanted within the body, and the material can be fully degraded by naturally occurring proteolytic enzymes.13,14 We have previously shown that thin films cast from SF could support the adherence, proliferation, and production of native matrix when human and rabbit fibroblasts were cultured in both two-dimensional and three-dimensional (3D) conditions, suggesting that these SF constructs can act as scaffolding for corneal stroma engineering purposes.15 This study sought to evaluate the application of SF as a carrier for corneal epithelial cell sheet generation by comparing the cell morphology, proliferation, differentiation, and stratification on SF with that of cultures on denuded human AM.
Materials and Methods
Preparation of Bombyx mori SF Films
Thin SF films were produced as described with minor modifications.16–19 The Bombyx mori silk cocoons were purchased from Tajima Shoji Co. (Yokohama, Japan), cut into small pieces, weighed, and boiled for 1 hour in distilled water containing 0.85 g of Na2CO3 for each gram of cocoon material. After boiling, the supernatant was discarded and the resulting fibrous material underwent three 20-minute washes in distilled water (dH2O) and then dried in a chemical fume hood for 12 hours. The dried cocoon material was dissolved in a concentrated solution of 9.3 M lithium bromide solution for 4 hours at 60°C. The silk solution was dialyzed against water for 48 hours with six total water changes using a dialysis cassette with a molecular weight cutoff of 3500 Da (Slide-A-Lyzer; Pierce Biotechnology, Rockford, IL). Following dialysis, 70 μL of the resulting 80 mg/mL silk solution was used directly to prepare films of fibroin by evaporation from a flat polydimethyl siloxane surface measuring 14 mm in diameter at room temperature within a biological clean bench.20 To produce water-insoluble films, the fibroin samples were water-annealed within a vacuum chamber pulled to 10 psi and filled with dH2O in the basin for 4 hours, and allowed to dry for more than 1 hour.21 Silk films were then sterilized by heating the films at 160°C for 2 hours in glass Petri dishes. The films were placed in the bottom of 24-well tissue culture plates (VWR, Radnor, PA). A similarly sterilized stainless steel o-ring (Superior Washer, Inc., Hauppauge, NY), measuring 15.4 mm in outer diameter and 11.6 mm in inner diameter, was placed on top of each film to stabilize the substrate and prevent film floating during culture. The film was hydrated in PBS (VWR) overnight at 4°C and, before use, PBS was aspirated.
Preparation of Denuded Human AM
Cryopreserved human AM was obtained from the Institute of Ophthalmology “Conde de Valenciana” (Mexico City, Mexico) and stored at −80°C. Before use, the AM was thawed by placing in a 37°C water bath for 20 minutes. The procedure for denuding AM was carried out as described previously.22 Briefly, in a sterile cell culture hood, native AM epithelium was removed by washing three times in PBS to remove storage medium and serum, and then incubating the AM in a 125 μg/mL thermolysin (Sigma-Aldrich, St. Louis, MO) solution at room temperature for 9 minutes. After treatment, the AM was washed in PBS three times for 15 minutes each on a shaker to get rid of the cellular debris. AM samples both before and after epithelial removal were fixed with 4% paraformaldehyde solution (PFA; Electron Microscopy Sciences, Hatfield, PA) and subjected to immunofluorescent staining of primary antipancytokeratin antibody (AE1/AE3; Abcam, Cambridge, MA). Additional pieces of AM were incubated in Karnovsky's fixative and underwent serial dehydration for scanning electron microscopic (SEM) examination. For use as a carrier for cell culture, denuded AM was transferred to 24-well plates with basement membrane side up using fine forceps. The firm attachment of AM to the plates was achieved by air-drying the AM in a cell culture hood for 30 minutes.
Preparation of Human and Rabbit Limbal Epithelial Cells
A human corneal limbal epithelial cell line (HCLE) was generously provided by Dr Ilene Gipson (Schepens Eye Research Institute, Harvard Medical School, Boston, MA) and stored in liquid nitrogen. Before seeding on the SF or AM substrates, cells were thawed and cultured for 24 to 48 hours in keratinocyte–serum-free medium (Gibco, Invitrogen Corporation, Grand Island, NY) supplemented with 0.2 ng/mL mouse epithelial growth factor (EGF; Invitrogen), 1% penicillin-streptomycin (P/S; VWR), bovine pituitary extract (Invitrogen), and 0.1% CaCl2.2H2O (Invitrogen). For preparation of primary cultures, either human limbal rings obtained from a regional eye bank (the Eye Bank for Sight Restoration, Inc., New York, NY) or rabbit limbal tissue harvested from New Zealand white rabbits (Charles River Laboratories International, Inc., Wilmington, MA), in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and with federal, state, and local regulations, were processed as described below.
After careful removal of the iris, residual conjunctiva, endothelial layer, and trabecular meshwork, the corneoscleral ring was washed in EpiLife medium (Invitrogen) containing 1% P/S (VWR) and 0.1% Fungizone (Invitrogen) for 20 minutes, divided into quadrants 1 × 1 mm in size and placed on a 6-well cell culture plate (VWR) precoated with 50 μg/mL collagen I (BD Biosciences, Bedford, MA) with epithelium side down and incubated at 37°C and 5% CO2 for 20 minutes. One drop of EpiLife medium supplemented with 1% human keratinocyte growth supplement (Invitrogen), with 1% P/S (VWR), 5% fetal bovine serum (Thermo Fisher Scientific Inc., Waltham, MA), 10 μg/mL mouse EGF (Invitrogen), and 10−10 M cholera toxin A (Sigma-Aldrich) was added to each explant and cultured overnight in the incubator. The next day, 1 mL of complete EpiLife culture medium was added and the medium was changed every 3 days until 80% confluence was achieved. For subculture, cells were washed with Dulbecco's PBS (VWR) twice and digested with TrypLE Express (Invitrogen) at 37°C for 12 minutes. A single cell suspension at a density of 2 × 104 cells/cm2 were seeded on SF and AM and cultured for 48 to 72 hours before they were collected and subjected to the following analysis.
Light Microscopy
Cell cultures were observed with a Carl Zeiss AxioVision Microscope (Carl Zeiss Microimaging GmbH, Jena, Germany) daily. Images were taken with an Observer Z1 fluorescent microscope (Carl Zeiss, AG) at ×10 (NA 0.45 air) objective lens using a 1.6 Optivar optic. An AxioCam HRm digital camera (Carl Zeiss) and AxioVision software (Carl Zeiss) were used to capture phase-contrast images of cultures.
Scanning Electron Microscopy
Denuded AM and cell sheets constructed on SF and AM were fixed in 400 μL of Karnovsky's fixative for 45 minutes at room temperature and washed in PBS three times. Afterwards the samples were dehydrated in a graded series of ethanol (50%, 80%, 90%, and 100%; VWR), for 7 minutes respectively. Samples were then further dried using hexamethyldisiloxane (HMDS; Sigma-Aldrich) solvent to remove residual water saturation for 2 minutes and then allowed to dry for 2 hours in a desiccator.23 After HMDS processing, samples were sputter coated with gold for 90 seconds, leaving an approximate 2-nm coating on the samples. Films were then imaged using a Quanta 600 environmental chamber SEM (FEI, Inc., Hillsboro, OR).
Immunofluorescent Staining
Both intact and denuded AM, cell cultures on SF and AM were fixed with 4% PFA (Electron Microscopy Sciences) for 15 minutes, whereas for p63 staining, samples were fixed with methanol (VWR) at −20°C for 10 minutes. After three washes in PBS for 5 minutes each, samples were blocked and permeabilized with solution containing 1% BSA (Sigma-Aldrich), 0.25% Triton X-100 (EMD Chemicals, Darmstadt, Germany), and 2.5% donkey serum (Abcam) in PBS for 50 minutes. Primary monoclonal antibodies recognizing keratin 3/12 (K3/12, 1:100; Abcam), ΔNp63a (1:200, Cell Signaling Technology, Inc. Danvers, MA) for human corneal epithelial cells (HCECs), and pancytokeratin (AE1/AE3, 1:100, Abcam) for intact AM samples were applied and incubated overnight at 4°C. After extensive washing, secondary antibodies were then incubated for 1 hour using appropriate isotype-matched nonspecific IgG as controls. Samples were mounted with VECTASHIELD mounting medium with 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA). Images were acquired using an Observer Z1 fluorescent microscope (Carl Zeiss, AG) with both ×10 (NA 0.45 air) and ×63 (NA 1.4 oil) objective lenses using a 1.6 Optivar optic. An AxioCam HRm digital camera (Carl Zeiss) and AxioVision 4.0 software (Carl Zeiss) was used to capture single and z-stack images (10–25-layer range) at 0.25-μm slices using DAPI, Green fluorescent protein (GFP) and Texas Red filter channels. The percentage of K3/12 and ΔNp63a expression in the primary cells on SF and denuded AM was compared and analyzed.
Cell Proliferation
Primary rabbit corneal epithelial cells (RCECs) were seeded on SF and denuded AM (n = 3) at a cell density of 2 × 104 cells/cm2 in a 24-well plate (VWR). The cultures were collected for MTT assay 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide at 24, 48, 72, and 144 hours after seeding, following manufacturer instructions. Briefly, 50 μL of MTT stock solution (5 mg/mL; Invitrogen) was added to the cultures containing 500 μL of fresh medium and incubated at 37°C for 4 hours in the dark. After the medium was aspirated, 200 μL of dimethyl sulfoxide (Sigma-Aldrich) was added and mixed thoroughly to release the formazan; 100 μL of the resultant solution was transferred into clear 96-well plates (VWR), and the absorbance was recorded at 540 nm using a Biomek plate reader (Beckman Coulter, Brea, CA). Wells containing SF and AM without cells culturing were set up as negative controls.
Cell Stratification
Primary RCECs were seeded at a density of 5 × 104 cells/cm2 on SF and denuded AM placed on the Transwell inserts fitted for 12-well plates (Corning, Lowell, MA) and cocultured with mitomycin C (Sigma-Aldrich)–treated NIH 3T3 cells at the bottom. After confluence, epithelial cells were cultured in an air-liquid interface for 1 week. Samples were collected and fixed with 4% PFA (Electron Microscopy Sciences) for 15 minutes. F-actin filaments were stained with Alexa Fluor 488 phalloidin (Invitrogen) and the cell nucleus with DAPI (Invitrogen), following the manufacturer instructions. Before imaging, slides were mounted with VECTASHIELD Mounting Medium with DAPI (Vector Laboratories).
Fluorescent images of f-actin formation were taken using an Observer Z1 fluorescent microscope (Carl Zeiss, AG) with a ×63 (NA 1.4 oil) objective lens. An AxioCam HRm digital camera (Carl Zeiss) and AxioVision software (Carl Zeiss) were used to capture z-stack images (20–60-layer range) at 0.200-μm slices using DAPI and Texas Red filter channels. Deconvolution was performed on each z-stack using 3D Huygens Deconvolution Software (Scientific Volume Imaging BV, Hilversum, The Netherlands). A total of 40 iterations were performed using the software's Classic Maximum Likelihood Estimation algorithm for each z-stack. All other settings were left at manufacturer default settings. Images were produced using both maximum intensity projection (MIP) and surface-rendering settings. MIP threshold levels were set at the default manufacturer settings, whereas surface-rendering threshold was set to match MIP image signal localization and intensity.
Statistics
All data were presented as sample set mean ± SD calculated for each group and compared using Student's unpaired t-test by Microsoft Excel (Microsoft Corporation, Redmond, WA). Test results were reported as two-tailed P values, where P less than 0.05 was considered statistically significant.
Results
Features of SF
Transparent circular SF films were made with 14-mm diameters and around a 40-μm axial thickness. After water-anneal processing was performed, the SF was nondissolvable in culture medium and PBS. The SF was sterilized by heating the samples at 160°C for 2 hours. The shape and physical properties remained stable after the annealing and sterilization processes. The thin transparent SF film was easy to handle when grabbed with fine forceps. The mechanical strength was enough for in vitro culture and transfer.
Characteristics of Denuded AM
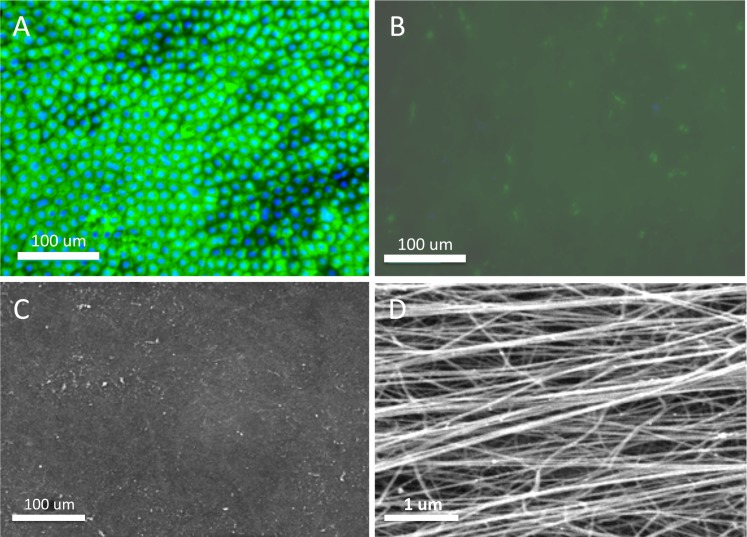
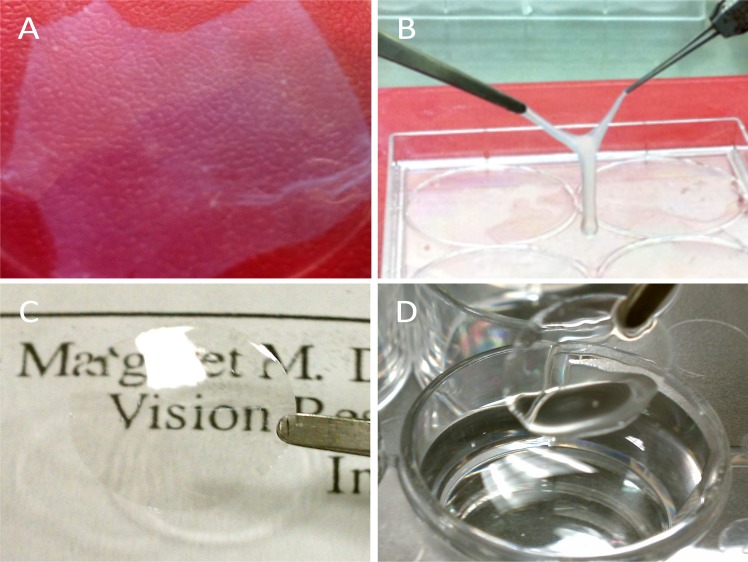
Before thermolysin treatment, uniform staining of pankeratin recognizing the cytoskeleton of cells was observed within the entire amniotic epithelial layer (Fig. 1A). In contrast, after treatment, only residual cellular debris on the basement membrane stained by pankeratin can be observed (Fig. 1B). SEM results confirmed the total removal of the epithelial cells with intact basement membranes (Figs. 1C, 1D), which is consistent with previous findings.22 Denuded AM showed a semitransparent appearance and the tendency of sticking together when lifted by two fine forceps (Figs. 2A, 2B). An Ultracell surgical sponge (Aspen Surgical, Caledonia, MI) was used to distinguish the orientation of the denuded AM, as the adherent side indicated the stroma presence. After air-drying, AM with basement membrane side up could firmly attach to either cell culture plate or Transwell insert; no detachment was observed during the subsequent cell culture. In contrast, silk film materials appeared transparent and also extremely handleable even after wetting (Figs. 2C, 2D).
Figure 1.
Denudation of cryopreserved amniotic membrane. AM with intact epithelium was illustrated by antipancytokeratin staining (green) (A) before thermolysin treatment and (B) after treatment. Cell nuclei were counterstained with DAPI (blue). SEM images of denuded AM showing the (C) denuded basement membrane and (D) a high-magnification image of the individual fibers on the stromal layer.
Figure 2.
Gross images showed that denuded AM spread on the tissue culture plates was (A) semitransparent, and (B) prone to fold and then adhere together when it was lifted by two forceps. Silk film was (C) highly transparent and (D) maintained shape when handled.
Morphology of Corneal Epithelial Cells on SF and AM
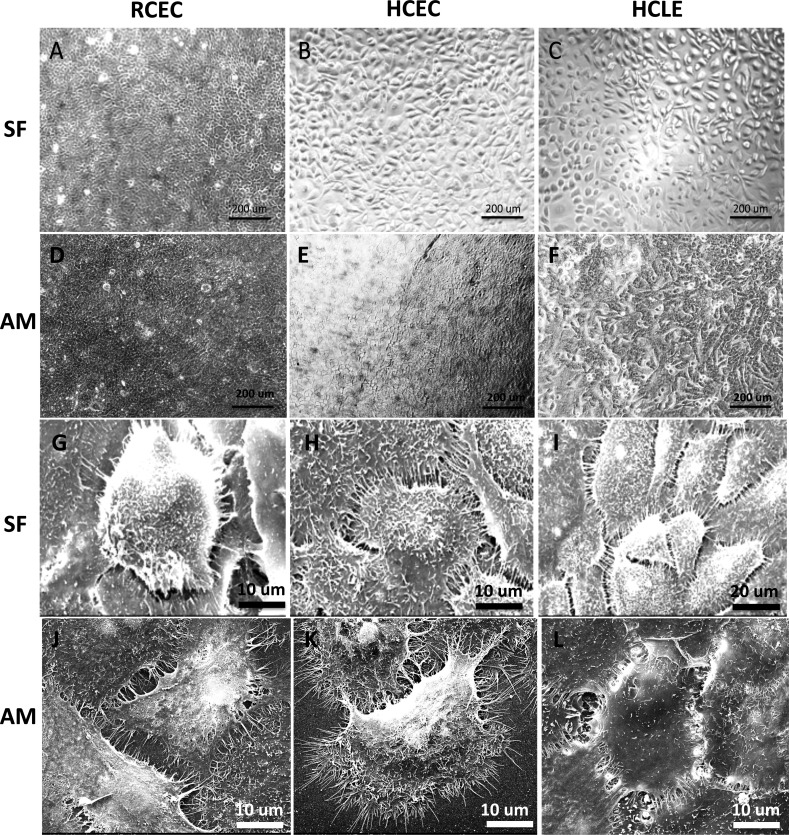
Corneal epithelial cell cultures from all three donor sources could adhere and proliferate on SF (Figs. 3A–C) and denuded AM (Figs. 3D–F) with a polygonal appearance under phase-contrast microscope. Abundant microvilli on the cell surface and wide junction with the neighbor cells can be viewed under SEM (Figs. 3G–L). Primary corneal epithelial cells tended to form clusters and small colonies when seeded on SF at a low cell density, whereas were randomly distributed on denuded AM. No significant difference of cell morphology was observed in HCLE cultures on SF and AM. No significant toxic response to cells grown on either SF or AM, such as cell shrinkage, intracellular vacuole, detachment from the substrate, and cell death were observed during the culture process.
Figure 3.
Phase-contrast images of corneal epithelial cells cultured on (A–C) SF and (D–F) AM showing comparable cell morphology; SEM images of corneal epithelial cells on (G–I) SF and (J–L) AM by using three different cell sources: (A, D, G, J) RCEC, (B, E, H, K) HCEC, and (C, F, I, L) HCLE demonstrating a high level of cell anchor point to the film surface, abundance of microvilli on the cell surface, and prevalent contacts between adjacent cells.
Cell Proliferation on SF and Denuded AM
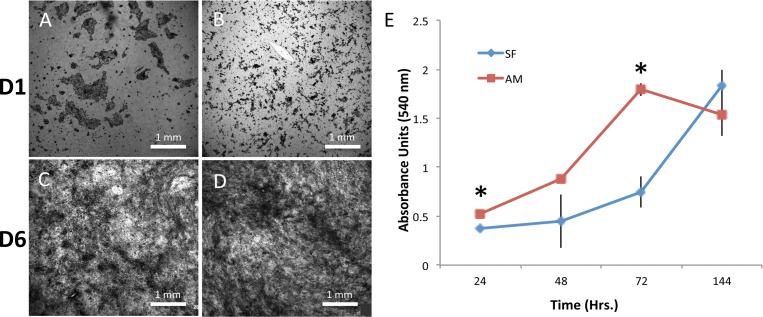
To expand cells ex vivo on AM, the epithelial cells must be removed, or denuded, from the culturing surface. This enhances cell adherence through the formation of strong integrin-based adhesion complexes with the basement membrane.22 In 2008, Hopkinson et al.22 first reported a protocol for denuding amniotic epithelial layer with thermolysin treatment, which was successfully used in this study. The vast majority of RCECs adhered to the two substrates after 8 hours post seeding, and cell density continued to increase 1 and 6 days after cell seeding on both SF (Figs. 4A, 4C) and AM (Figs. 4B, 4D). MTT assays showed that the values of light absorbance of cells grown showed that the number of cells on denuded AM at 24 and 72 hours post seeding were significantly higher (P = 0.001) than that of cells on SF (Fig. 4E). Logarithmic growth of cells on the AM and SF was observed at 48 (AM) and 72 (SF) hours after plating, respectively. No statistical difference was observed among the negative controls, suggesting the material itself did not affect MTT absorbance significantly.
Figure 4.
(A–D) MTT stained at days 1 and 6 in culture for RCEC cells grown on (A, C) SF and (B, D) AM. (E) At day 1 and day 3, cells cultured on AM had a statistically higher cell viability and proliferation as compared with SF (*P < 0.05, n = 4, error bars = SD).
Cell Differentiation on SF and Denuded AM
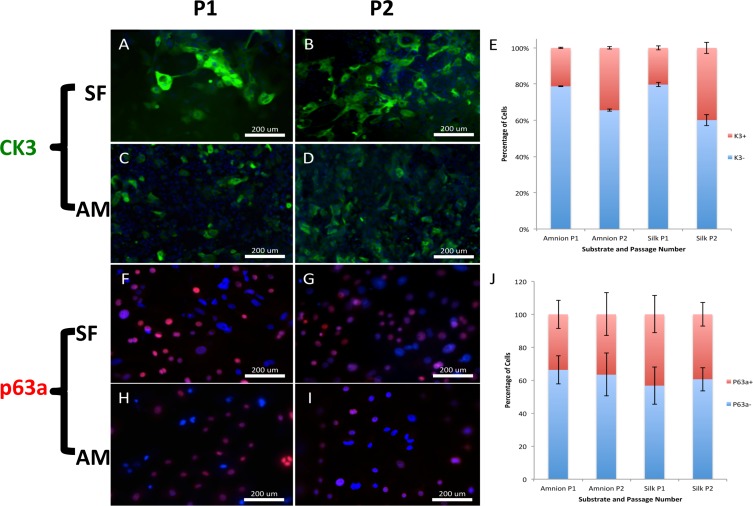
Immunofluorescence detection revealed that HCECs grown on SF and AM contained K3/12-positive (Figs. 5A–D) and ΔNp63a-positive (Figs. 5F–I) cells, indicating that there was a mixture of cell populations undergoing variable degrees of differentiation. There were slight changes of protein expression between P1 and P2 cells on the same substrate (Figs. 5E, 5J), reflecting the dynamics of cell proliferation and differentiation; however, no significant difference was found between SF and AM for the two markers.
Figure 5.
Immunofluorescent staining of HCECs cultured on (A, B) SF and (C, D) AM. Primary corneal epithelial cells underwent differentiation with subsequent passaging from (A, C) P1 and (B, D) P2, as demonstrated by CK3 expression (green). Immunofluorescent staining of HCECs cultured on (F, G) SF and (H, I) AM. Primary corneal epithelial cells underwent differentiation with subsequent passaging from (F, H) P1 and (G, I) P2, as demonstrated by p63a expression (red). No significant difference regarding the percentage expression of (E) K3 and (J) P63a proteins for cells grown on two substrates at passage 1 and 2 in culture was detected.
Cell Sheets Generated on SF and Denuded AM
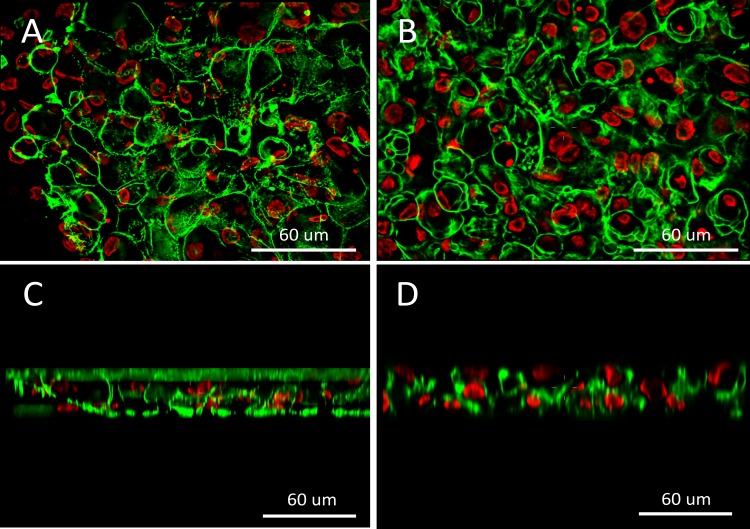
Stratified RCEC actin and nuclear structures were imaged on the both SF and AM substrates using spinning-disk confocal microscopy, and then reconstructed z-stacks were deconvolved to provide cross-sectional images of the stratified cells. The fluorescent images provided a 3D histological image of the multilayer stratified epithelial cell sheet structure, and represents the first known fluorescent images of their kind viewing epithelial stratification on silk film substrates (Fig. 6). Both substrates can support two to three layers of RCECs stratified and differentiated within 1 week of air-liquid interface culture. No significant stratified pattern was detected between cell sheets on SF and denuded AM. In addition, the natural autofluorescent signature exhibited by the silk film could be imaged, and demonstrated that the stratified cultures were well adhered to the substrate surface.
Figure 6.
Fluorescent confocal microscopy images of RCECs stratified on (A, C) silk film and (B, D) AM in both (A, B) en face and (C, D) cross-sectional views (red = nucleus; green = actin).
Discussion
In this study, SF films cast from Bombyx mori silkworm proteins were evaluated as a substrate for cell sheet generation and compared with the current clinical standard, denuded AM. Our results demonstrated that SF supports the growth of both primary and immortalized corneal epithelial cells. Cells grown on SF maintained corneal epithelial morphology, proliferative ability, and normal cell differentiation. Stratified epithelial cell sheets were generated on SF with similar structure as cultures on denuded AM. Our study confirmed that SF could serve as a promising alternative to AM for corneal regeneration and tissue engineering applications.
Increased investigation of novel biomaterials for regenerative medicine and tissue engineering in ophthalmology is required to enhance the physician's ability to address clinical problems not currently served by the current state of the art in biomaterial technology. Specifically, when referring to corneal regeneration, an ideal scaffold should have the following properties: (1) biocompatible material with nontoxic degradation byproducts; (2) free from disease transmission; (3) suitable for promoting cell growth and native tissue development; (4) transparent; (5) suitable mechanical properties; (6) modifiable for tissue growth in three dimensions; (7) modifiable shape and size to fit the corneal curvature or tissue defect; (8) available as a sterile product without compromising material properties; and (9) possess potential for the combination with a drug or gene delivery application.
Currently, human AM is the standard substrate for ex vivo expansion of corneal epithelial cells for transplantation and clinical applications in ocular surface reconstruction. AM has been used as a temporary bandage or a permanent basement membrane due to inherent anti-inflammatory, anti-angiogenic, and anti-scarring properties.12,24 Although AM meets the criteria of (1), (2), (7), and (8) above, the material has many limitations. AM has relatively poor mechanical properties, as it may tear during clinical procedure owing to material fragility.25 AM also has the tendency of curling and wrinkling when lifted with forceps or even tearing if it is not properly procured, which creates difficulty in handling and transferring the material during a clinical procedure. There is also the inclination of premature material melting or dissolution in eyes owing to extensive proteolytic activity with cases of severe inflammation.26,27 In addition, early detachment of the AM from the surgical site is problematic, along with the material's inherent opacity, which may interfere with the patient's vision. There is also the potential risk of disease transmission, and AM is required to be screened for potential infectious diseases, such as HIV, hepatitis B, and syphilis before implantation.
To circumvent these drawbacks, exploration of novel biomaterials is under way with promising progress. SF purified from Bombyx mori silkworm cocoons has been shown to be biocompatible and elicit a minimal inflammation response on implantation.14 The use of SF has been most intensely investigated in the fields of bone,19,28,29 cartilage,30–32 and connective tissue engineering.33,34
In this study, corneal epithelial cells were found to adhere to SF film and proliferate after plating without any detectable morphological difference when compared with denuded AM. No cytotoxic response or inhibition of cell growth was observed in the SF cultures, which indicates high biocompatibility of SF biomaterial to corneal epithelial cells. SF possesses reasonable tensile strength and was easy to handle and transfer. Furthermore, silk film with different curvatures can be manufactured and in vivo assessment of the fitness in rabbit eyes is ongoing in our study (data not shown). In addition, it is highly transparent and may be better suited for vision restoration than semitransparent AM. SF was easily sterilized using heat treatment and then stored at room temperature, which is also advantageous over the temperature-sensitive AM, which cannot be as easily sterilized and must be stored at −80°C.
Cell proliferation of RCECs on SF showed a delayed entry into growth phase when compared with AM, which may be because of the enhancement of cell proliferation through inherent growth factors known to be naturally present (i.e., EGF and keratocyte growth factor).12,35 In addition, previous studies have shown up to a 6-fold increase in the number of attached cells on denuded AM compared with SF film and tissue culture plates, indicating the superiority of AM in promoting cell adherence and proliferation.36 This may be due to the naturally occurring structural proteins, such as laminin and type VII collagen of the AM basement membrane, which may promote enhanced cell adhesion.12,37
Corneal epithelial cells retained the normal phenotype and differentiation properties when cultured on SF films, as demonstrated by the progenitor cell marker ΔNp63a38 and differentiation marker K3/12 expression.39 No statistical change in protein expression pattern was observed when cells on SF were passaged after reaching confluence. Although it has been reported that denuded AM can facilitate corneal epithelial cell differentiation compared with intact AM,40,41 the percentage of ΔNp63a-positive cells in both first and second and passage cultures were comparable in this study. When compared between SF and AM, the status of cell differentiation was parallel with no significant difference.
Stratified corneal epithelial cells sheets were successfully constructed on both SF and AM when cultured at an air-liquid interface for 1 week. The structure of cell sheets on two substrates was similar, with compact columnar cells on the basal layer with more squamous cells present on the apical layer. However, constructs on AM generally displayed more stratified layers (3–4 layers versus 2–3 layers on SF), which was consistent with what had been previously observed.36 However, robust stratification of rabbit corneal epithelial cells on fibroin was observed using porous silk film–coated chambers and a 3T3 feeder cell layer.42
In this study, the finer cytoskeletal architecture of the stratified epithelium was revealed through the use of fluorescent microscopy and image-processing software. This method proved useful in producing cross-sectional images that could provide greater insight into cellular components than what standard histology techniques will allow. In addition, the natural autofluorescent signature of the silk film allows for basal layer visualization and allows one to image cell attachment to the substrate surface. Future studies will continue using such imaging techniques to better observe cell structure–localized expression within the cell and at the attachment surface.
Conclusions
The feasibility of using Bombyx mori silk fibroin film as a substrate to support human and rabbit corneal epithelial cell proliferation, differentiation, and stratification was demonstrated. Further investigations into various modifications of silk film surface are under way to better understand how such alterations may affect cellular orientation, cell adherence, differentiation, and enhance soluble factor release or gene delivery for a given cell culture. Such insights will continue to lead to new understanding with the expectation of enhancing the ability to translate these naturally regenerative materials into clinical medicine.
Acknowledgments
Technical support for image acquisition and processing was supplied by Dr. Ryan Schreiner at the Weill Cornell Medical College.
Footnotes
Supported by National Institutes of Health Grants K08EY015829, R21EY019561, and R24EY015656; National Natural Science Foundation of China Grant 30801262; a Research to Prevent Blindness Career Development Award; and a Tri-Institutional Stem Cell Initiative.
Disclosure: J. Liu, None; B.D. Lawrence, None; A. Liu, None; I.R. Schwab, None; L.A. Oliveira, None; M.I. Rosenblatt, None
References
- 1. Pellegrini G, Traverso CE, Franzi AT, Zingirian M, Cancedda R, De Luca M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349:990–993. [DOI] [PubMed] [Google Scholar]
- 2. Tsai RJ, Li LM, Chen JK. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N Engl J Med. 2000;343:86–93. [DOI] [PubMed] [Google Scholar]
- 3. Grueterich M, Espana EM, Tseng SC. Ex vivo expansion of limbal epithelial stem cells: amniotic membrane serving as a stem cell niche. Surv Ophthalmol. 2003;48:631–646. [DOI] [PubMed] [Google Scholar]
- 4. Zakaria N, Koppen C, Van Tendeloo V, Berneman Z, Hopkinson A, Tassignon MJ. Standardized limbal epithelial stem cell graft generation and transplantation. Tissue Eng Part C Methods. 2009;16:921–927. [DOI] [PubMed] [Google Scholar]
- 5. Liu J, Sheha H, Fu Y, Giegengack M, Tseng SC. Oral mucosal graft with amniotic membrane transplantation for total limbal stem cell deficiency. Am J Ophthalmol. 2011;152:739–747.e1. [DOI] [PubMed] [Google Scholar]
- 6. Luengo Gimeno F, Lavigne V, Gatto S, Croxatto JO, Correa L, Gallo JE. Advances in corneal stem-cell transplantation in rabbits with severe ocular alkali burns. J Cataract Refract Surg. 2007;33:1958–1965. [DOI] [PubMed] [Google Scholar]
- 7. Higa K, Shimmura S, Kato N, et al. Proliferation and differentiation of transplantable rabbit epithelial sheets engineered with or without an amniotic membrane carrier. Invest Ophthalmol Vis Sci. 2007;48:597–604. [DOI] [PubMed] [Google Scholar]
- 8. Hayashida Y, Nishida K, Yamato M, et al. Ocular surface reconstruction using autologous rabbit oral mucosal epithelial sheets fabricated ex vivo on a temperature-responsive culture surface. Invest Ophthalmol Vis Sci. 2005;46:1632–1639. [DOI] [PubMed] [Google Scholar]
- 9. Nishida K, Yamato M, Hayashida Y, et al. Functional bioengineered corneal epithelial sheet grafts from corneal stem cells expanded ex vivo on a temperature-responsive cell culture surface. Transplantation. 2004;77:379–385. [DOI] [PubMed] [Google Scholar]
- 10. Pang K, Du L, Wu X. A rabbit anterior cornea replacement derived from acellular porcine cornea matrix, epithelial cells and keratocytes. Biomaterials. 2010;31:7257–7265. [DOI] [PubMed] [Google Scholar]
- 11. Fu Y, Fan X, Chen P, Shao C, Lu W. Reconstruction of a tissue-engineered cornea with porcine corneal acellular matrix as the scaffold. Cells Tissues Organs. 2009;191:193–202. [DOI] [PubMed] [Google Scholar]
- 12. Tseng SC, Espana EM, Kawakita T, et al. How does amniotic membrane work? Ocul Surf. 2004;2:177–187. [DOI] [PubMed] [Google Scholar]
- 13. Acharya C, Ghosh SK, Kundu SC. Silk fibroin protein from mulberry and non-mulberry silkworms: cytotoxicity, biocompatibility and kinetics of L929 murine fibroblast adhesion. J Mater Sci Mater Med. 2008;19:2827–2836. [DOI] [PubMed] [Google Scholar]
- 14. Wang Y, Rudym DD, Walsh A, et al. In vivo degradation of three-dimensional silk fibroin scaffolds. Biomaterials. 2008;29:3415–3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lawrence BD, Marchant JK, Pindrus MA, Omenetto FG, Kaplan DL. Silk film biomaterials for cornea tissue engineering. Biomaterials. 2009;30:1299–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang X, Kim HJ, Xu P, Matsumoto A, Kaplan DL. Biomaterial coatings by stepwise deposition of silk fibroin. Langmuir. 2005;21:11335–11341. [DOI] [PubMed] [Google Scholar]
- 17. Minoura N, Aiba S, Higuchi M, Gotoh Y, Tsukada M, Imai Y. Attachment and growth of fibroblast cells on silk fibroin. Biochem Biophys Res Commun. 1995;208:511–516. [DOI] [PubMed] [Google Scholar]
- 18. Nazarov R, Jin HJ, Kaplan DL. Porous 3-D scaffolds from regenerated silk fibroin. Biomacromolecules. 2004;5:718–726. [DOI] [PubMed] [Google Scholar]
- 19. Sofia S, McCarthy MB, Gronowicz G, Kaplan DL. Functionalized silk-based biomaterials for bone formation. J Biomed Mater Res. 2001;54:139–148. [DOI] [PubMed] [Google Scholar]
- 20. Lawrence BD, Cronin-Golomb M, Georgakoudi I, Kaplan DL, Omenetto FG. Bioactive silk protein biomaterial systems for optical devices. Biomacromolecules. 2008;9:1214–1220. [DOI] [PubMed] [Google Scholar]
- 21. Jin HJ, Park J, Karageorgiou V, et al. Water-stable silk films with reduced β-sheet content. Advanced Functional Materials. 2005;15:1241–1247. [Google Scholar]
- 22. Hopkinson A, Shanmuganathan VA, Gray T, et al. Optimization of amniotic membrane (AM) denuding for tissue engineering. Tissue Eng Part C Methods. 2008;14:371–381. [DOI] [PubMed] [Google Scholar]
- 23. Braet F, De Zanger R, Wisse E. Drying cells for SEM, AFM and TEM by hexamethyldisilazane: a study on hepatic endothelial cells. J Microsc. 1997;186:84–87. [DOI] [PubMed] [Google Scholar]
- 24. Liu J, Sheha H, Fu Y, Liang L, Tseng SC. Update on amniotic membrane transplantation. Expert Rev Ophthalmol. 2011;5:645–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Oyen ML, Cook RF, Calvin SE. Mechanical failure of human fetal membrane tissues. J Mater Sci Mater Med. 2004;15:651–658. [DOI] [PubMed] [Google Scholar]
- 26. Spoerl E, Wollensak G, Reber F, Pillunat L. Cross-linking of human amniotic membrane by glutaraldehyde. Ophthalmic Res. 2004;36:71–77. [DOI] [PubMed] [Google Scholar]
- 27. Ma DH, Wang SF, Su WY, Tsai RJ. Amniotic membrane graft for the management of scleral melting and corneal perforation in recalcitrant infectious scleral and corneoscleral ulcers. Cornea. 2002;21:275–283. [DOI] [PubMed] [Google Scholar]
- 28. Karageorgiou V, Meinel L, Hofmann S, Malhotra A, Volloch V, Kaplan D. Bone morphogenetic protein-2 decorated silk fibroin films induce osteogenic differentiation of human bone marrow stromal cells. J Biomed Mater Res A. 2004;71:528–537. [DOI] [PubMed] [Google Scholar]
- 29. Meinel L, Fajardo R, Hofmann S, et al. Silk implants for the healing of critical size bone defects. Bone. 2005;37:688–698. [DOI] [PubMed] [Google Scholar]
- 30. Morita Y, Tomita N, Aoki H, et al. Visco-elastic properties of cartilage tissue regenerated with fibroin sponge. Biomed Mater Eng. 2002;12:291–298. [PubMed] [Google Scholar]
- 31. Yan LP, Oliveira JM, Oliveira AL, Caridade SG, Mano JF, Reis RL. Macro/microporous silk fibroin scaffolds with potential for articular cartilage and meniscus tissue engineering applications. Acta Biomater. 2012;8:289–301. [DOI] [PubMed] [Google Scholar]
- 32. Talukdar S, Nguyen QT, Chen AC, Sah RL, Kundu SC. Effect of initial cell seeding density on 3D-engineered silk fibroin scaffolds for articular cartilage tissue engineering. Biomaterials. 2011;32:8927–8937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sahoo S, Toh SL, Goh JC. PLGA nanofiber-coated silk microfibrous scaffold for connective tissue engineering. J Biomed Mater Res B Appl Biomater. 2010;95:19–28. [DOI] [PubMed] [Google Scholar]
- 34. Etienne O, Schneider A, Kluge JA, et al. Soft tissue augmentation using silk gels: an in vitro and in vivo study. J Periodontol. 2009;80:1852–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Koizumi NJ, Inatomi TJ, Sotozono CJ, Fullwood NJ, Quantock AJ, Kinoshita S. Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res. 2000;20:173–177. [PubMed] [Google Scholar]
- 36. Bray LJ, George KA, Ainscough SL, Hutmacher DW, Chirila TV, Harkin DG. Human corneal epithelial equivalents constructed on Bombyx mori silk fibroin membranes. Biomaterials. 2011;32:5086–5091. [DOI] [PubMed] [Google Scholar]
- 37. Fukuda K, Chikama T, Nakamura M, Nishida T. Differential distribution of subchains of the basement membrane components type IV collagen and laminin among the amniotic membrane, cornea, and conjunctiva. Cornea. 1999;18:73–79. [PubMed] [Google Scholar]
- 38. Pellegrini G, Dellambra E, Golisano O, et al. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci U S A. 2001;98:3156–3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schermer A, Galvin S, Sun TT. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986;103:49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen B, Mi S, Wright B, Connon CJ. Differentiation status of limbal epithelial cells cultured on intact and denuded amniotic membrane before and after air-lifting. Tissue Eng Part A. 2010;16:2721–2729. [DOI] [PubMed] [Google Scholar]
- 41. Sudha B, Sitalakshmi G, Iyer GK, Krishnakumar S. Putative stem cell markers in limbal epithelial cells cultured on intact & denuded human amniotic membrane. Indian J Med Res. 2008;128:149–156. [PubMed] [Google Scholar]
- 42. Higa K, Takeshima N, Moro F, et al. Porous silk fibroin film as a transparent carrier for cultivated corneal epithelial sheets. [published online ahead of print November 19, 2010] J Biomater Sci Polym Ed. doi:10.1163/092050610X538218. [DOI] [PubMed] [Google Scholar]