Abstract
Purpose.
To study the effects of palmitoylethanolamide (PEA), a fatty acid ethanolamide, on aqueous humor outflow facility.
Methods.
The effects of PEA on outflow facility were measured using a porcine anterior segment–perfused organ culture model. The involvements of different receptors in PEA-induced changes were investigated using receptor antagonists and adenovirus delivered small hairpin RNAs (shRNAs). PEA-induced activation of p42/44 mitogen-activated protein kinase (MAPK) was determined by Western blot analysis using an antiphospho p42/44 MAPK antibody.
Results.
PEA caused a concentration-dependent enhancement of outflow facility, with the maximum effect (151.08 ± 11.12% of basal outflow facility) achieved at 30 nM of PEA. Pretreatment of anterior segments with 1 μM cannabinoid receptor 2 antagonist SR144528 and 1 μM PPARα antagonist GW6471, but not 1 μM cannabinoid receptor 1 antagonist SR141716A, produced a partial antagonism on the PEA-induced increase of outflow facility. Treatment of TM cells with PEA for 10 minutes activated phosphorylation of p42/44 MAPK, which was blocked by pretreatment with SR1444528 and GW6471, but not SR141716A. Knocking down the expression of either GPR55 or PPARα receptors with specific shRNAs for these receptors partially blocked PEA-induced increase in outflow facility and abolished PEA-induced phosphorylation of p42/44 MAPK. PD98059, an inhibitor of the p42/44 MAPK pathway, blocked both PEA-induced enhancement of aqueous humor outflow facility and PEA-induced phosphorylation of p42/44 MAPK.
Conclusions.
Our results demonstrate that PEA increases aqueous humor outflow through the TM pathway and these effects are mediated by GPR55 and PPARα receptors through activation of p42/44 MAPK.
This study describes the effects of palmitoylethanolamide (PEA), a fatty acid ethanolamide, on aqueous humor outflow facility, and the involvement of GPR55 and PPARα receptors and p42/44 mitogen-activated protein kinase in PEA-induced changes of aqueous humor outflow facility.
Introduction
Elevated intraocular pressure (IOP) is one of the major risk factors for glaucoma, a leading cause of human blindness.1–3 Marijuana smoking and administration of cannabinoids are known to reduce IOP.4,5 The maintenance of IOP depends on the dynamic balance between the secretion of aqueous humor by the ciliary body and the outflow of aqueous humor via the trabecular meshwork (TM) and uveoscleral routes.1,3 Both cannabinoid 1 (CB1) and cannabinoid 2 (CB2) cannabinoid receptors are known to be expressed in the TM, an important site for modulating the aqueous humor outflow.6–8 Our previous studies have demonstrated that the IOP-lowering effects of cannabinoids are at least partly mediated by increasing aqueous humor outflow via the TM pathway.7–10 In addition, previously we have demonstrated that both CB1 and CB2 receptors are involved in the aqueous humor outflow-enhancing effects of various synthetic and endogenous cannabinoids.7–10
Palmitoylethanolamide (PEA) was first discovered to be an endogenous ligand in mammalian tissues in the 1960s.11 It is a saturated fatty acid amide analog of the endocannabinoid arachidonylethanolamide (AEA), also named anandamide.12 PEA is synthesized from palmitic acid and ethanolamine in various tissues by actions of two enzymes, N-acyl-transferase and phospholipase D.13,14 It is degraded by fatty acid amide hydrolase (FAAH), the same enzyme that degrades AEA.14 PEA has broad-spectrum pharmacologic properties, producing antiinflammatory, analgesic, anticonvulsant, and antiproliferative effects.15 In human ocular tissues, a previous study has shown that there are lower levels of PEA in the choroid and ciliary body of glaucomatous eyes as compared with normal eyes.16 Recently, a clinical study has demonstrated that PEA was effective in reducing the increased IOP after the iridotomy procedure.17 In another clinical trial, PEA was found to significantly reduce IOP in patients diagnosed with primary open-angle glaucoma (POAG) or with ocular hypertension.18
For the current study, the first hypothesis to be tested is that PEA may affect aqueous humor outflow. The rationales behind this hypothesis are: (1) PEA has been reported to reduce IOP, but its mechanism of action is unknown17,18; (2) PEA is a saturated congener of AEA, which has been shown to increase aqueous humor outflow facility in a previous study of ours.10 To test this hypothesis, in this study we used the perfused porcine anterior segment organ culture model.
The second hypothesis to be tested in this study is that PEA may mediate its pharmacologic actions by acting on a SR144528-sensitive, non-CB1/CB2 receptor, G protein–coupled receptor 55 (GPR55), and peroxisome proliferator activated receptor-alpha (PPARα) in TM cells. Earlier studies have shown that some of the PEA effects in vivo can be blocked by the CB2 receptor antagonist SR144528.19,20 However, it has also been shown that PEA failed to bind CB2 receptor in vitro.15 Thus, PEA may produce its actions through a SR144528-sensitive, non-CB1/CB2 receptor. Another potential target for PEA is GPR55.21,22 It has been shown that PEA promotes the binding of GTPγS to GPR55.22 PEA is also known to activate the nuclear receptor PPARα.15,23 PEA can attenuate inflammation in wild-type mice but not in mice deficient in PPARα, indicating the crucial role of PPARα in the actions of PEA.23 To understand the involvement of potential receptor targets in the actions of PEA in TM tissues, in this study we used various receptor antagonists. Furthermore, to elucidate the roles of GPR55 and PPARα receptors in PEA-induced changes in aqueous humor outflow, we used adenovirus delivered short hairpin RNA (shRNA) to specifically knock down GPR55 and PPARα receptors in TM cells and tissues.
The third hypothesis to be tested in the current study is that PEA may cause changes in aqueous humor outflow through activation of the p42/44 mitogen-activated protein kinase (MAPK), also referred to as extracellular signal-related kinase 1/2 (ERK1/2) pathway in TM cells. PEA is known to activate the p42/44 MAPK pathway in a variety of tissues24,25 and the p42/44 MAPK pathway has been shown to play an important role in mediating functional changes of TM cells, including those induced by cannabinoids.7–9,26 Therefore, it is reasonable to postulate that the p42/44 MAPK pathway may be important for mediating the actions of PEA in the TM. To test this hypothesis, we used PD98059, an inhibitor of the p42/44 MAPK pathway.27 We also studied the PEA-induced phosphorylation of p42/44 MAPK in the TM cells and the involvements of several receptors in this process.
Methods
Materials
PEA and GW6471 were purchased from a commercial company (Tocris Cookson, Ellisville, MO), as was PD98059 (Sigma-Aldrich, St. Louis, MO), the cannabinoid receptor antagonists SR141716A and SR144528 (obtained from National Institute of Drug Abuse, Rockville, MD), and other commercial systems (BLOCK-iT Adenoviral RNAi Expression System and Lipofectamine 2000; Invitrogen Corp., Carlsbad, CA).
Porcine Anterior Segment Perfusion Model
A previously published protocol28 was followed for the anterior segment–perfused organ culture model. Fresh porcine eyes were obtained from a local slaughterhouse within 30 minutes following decapitation. Porcine anterior segment explants, comprising the intact cornea, the undisturbed trabecular meshwork, and a 2- to 5-mm rim of sclera with the ciliary body and iris gently removed, were mounted in a standard perfusion culture apparatus and perfused with Dulbecco's modified Eagle's medium (DMEM) for 1 day while outflow stabilized, using a constant perfusion head of 10 cm (approximately 7.35 mm Hg). Only those explants that stabilized between 1.5 and 8 μL/min at 7.35 mm Hg were used. Anterior eye cultures were maintained at 37°C with 5% CO2 and 95% air. It had been shown previously that in this model, outflow is through the trabecular meshwork, and flow rates are physiologic (approximately 2.75 μL/min).28 At the end of the perfusion study, the anterior segments were perfusion fixed at 7.35 mm Hg constant pressure with 4% paraformaldehyde for 1 hour. Anterior segments were then removed from the perfusion chamber, and 2- to 3-mm-wide wedges from each quadrant containing outflow tissues were cut and immersed in 10% formalin for 1 hour and then in 70% alcohol overnight. Subsequently, tissues were embedded in paraffin and stained with hematoxylin and eosin (HE). The viability of outflow pathway tissues was evaluated by light microscopy. Perfusion studies were regarded as invalid and data were discarded if more than one quadrant per eye had unacceptable morphologic findings, such as excessive trabecular meshwork cell loss and denudation of trabecular beams.
After establishing a baseline outflow, antagonists and/or enzyme inhibitors were applied to the perfusion medium 30 minutes prior to treatment with PEA and were present throughout the study. The anterior segments were then perfused continuously with drug-containing medium for 5 hours and the outflow facility was monitored every hour. Vehicle control was run in parallel. Ten eyes were used for each group of treatment.
Construction of Adenoviruses Expressing shRNas for PPARα and GPR55
Adenoviruses expressing shRNAs for PPARα and GPR55 were prepared according to a commercial protocol (BLOCK-iT Adenoviral RNAi Expression System; Invitrogen). Three shRNA sites were selected for the porcine tissue using a commercial system (BLOCK-iT RNAi Designer; rnaidesigner.invitrogen.com) and were BLAST (Basic Local Alignment Search Tool) analyzed (http://www.pubmed.gov) to rule out any homology with other genes. The selected sites for GPR55 sequence (XM_001,925,193.1) were as follows: GCC TCT ACT TCA TCA GCA TGT (296–316), GCA TCA TGG GTT TCT GTT CAT (584–604), and GCT ACT ACT TTG TCA TCA AAG (860–880). The selected sites for PPARα (NM_001,044,526) were as follows: GCC AAA CTG AAA GCA GAA ATC (585–505), GGA TTT ACG AGG CCT ACT TGA (667–687), and GGG ACA TGT ACT GAT CTA TCC (1435–1445). An adenovirus expressing shRNA for LacZ was constructed as a control adenovirus. The oligonucleotides were designed to have sense, loop, and antisense sequences and were cloned into pENTR/U6 entry vector, following which LR recombination reactions were performed between the pENTR/U6 entry vector containing the PPARα, GPR55, or LacZ shRNA and a destination vector (pAd/BLOCK-iT-DEST). The resulting plasmids were digested with PacI to expose the inverted terminal repeats (ITRs) and were transfected into the 293A producer cell line to generate adenovirus particles.
Plaque Assay
293A cells were seeded in six-well plates at 3 × 106 cells per well and incubated for 24 hours prior to transduction. Adenovirus particles were serial diluted and added to each well. After 3 hours, the transducing media was removed and replaced with 4 mL of agarose overlay consisting of equal volumes of a 1.2% low melting temperature agarose (SeaPlaque agarose; FMC, Rockland, ME) and 2× DMEM–10% fetal bovine serum. The assay was performed in triplicate. After 10 days of incubation the cells were stained with crystal violet and the plaques were counted. The number of plaques per well was determined, and the plaque-forming unit (PFU)/mL of virus inoculum was calculated according to the formula: PFU/mL = (average number of plaques per well)/[(0.2 mL) × (viral dilution factor)].
Culture of Porcine and Human Trabecular Meshwork Cells
The TM tissues were isolated from fresh porcine eyes or human cadaver eyes by blunt dissection. Culture of TM cells was performed according to previously published methods.29,30 The identity of trabecular meshwork cells was established by their morphology and their ability to take up acetylated low-density lipoprotein and to secrete tissue plasminogen activator.
Procedures for Transducing the TM Cells and Tissues with Adenoviruses
Transduction of TM cells was performed in the following manner. TM cells were trypsinized and plated in a six-well plate in 2 mL of DMEM with penicillin-streptomycin-fungizone and 10% FBS. Transduction was carried out by removing the regular medium and the addition of the DMEM medium containing 2% FBS and adenoviruses (8 × 106 plaque-forming unit [PFU]) to the cell monolayer. The cells were incubated at 37°C for 2 hours with the adenovirus-containing medium. Subsequently, the complete growth medium (DMEM plus 10% FBS) was added, and the cells were incubated at 37°C for 48 hours.
For transducing adenoviruses into the TM tissues, the perfused anterior segment of the porcine eye was given a bolus dose (8 × 106 PFU) of adenovirus particles and the anterior segments were perfused for 48 hours, following which the knockdown efficiency and outflow facility studies were conducted.
Western Blot Analysis of Receptor Proteins
The TM tissues were isolated from fresh porcine eyes by blunt dissection and the cultured TM cells were harvested in lysis buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, and 1 μg/mL leupeptin). Subsequently, TM tissues or cells were homogenized using a glass homogenizer in lysis buffer. Samples were centrifuged at 20,000g for 10 minutes, and the supernatant obtained was incubated with 4× Laemmli sample buffer at room temperature for 20 minutes. Subsequently, proteins were resolved on a 10% SDS–polyacrylamide gel using a minigel electrophoresis system (Invitrogen) and protein bands were transferred onto a nitrocellulose membrane. The nitrocellulose membranes were blocked with 5% nonfat dried milk in TBS-T buffer (10 mM Tris-HCl, pH 8.0, 150 mM NaCl, and 0.3% Tween 20) for 1 hour and then incubated overnight at 4°C with the anti-GPR55 antibody (Cayman Chemical, Ann Arbor, MI) or the anti-PPARα antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Subsequently, the membranes were washed twice for 5 minutes each time with TBS-T buffer and incubated with horseradish peroxidase–labeled secondary antibody for 1 hour at room temperature. The membranes were then washed three times with TBS-T buffer for 5 minutes each time and the antibody-recognized protein bands were visualized by an enhanced chemiluminescence detection kit (Thermo Scientific, Rockford, IL).
p42/44-MAPK Activity Assay
The TM cells were maintained in serum-free medium for 18 hours before various treatments. To activate the p42/44 MAPK, the cells were pretreated with URB597 for 15 minutes, followed by treatment with PEA plus URB597 for 10 minutes. To study the effects of various antagonists or inhibitors, the cells were pretreated with URB597 plus these antagonists or inhibitors (SR141716A, SR144528, GW6471, or PD98059) for 15 minutes, followed by PEA plus URB597 treatment for 10 minutes. At the end of the incubation period, 150 μL of ice-cold lysis buffer containing 50 mM β-glycerophosphate, 20 mM EGTA, 15 mM MgCl2, 1 mM NaVO4, 1 mM dithiothreitol (DTT), and 1 μg/mL of a protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN) were added. The cell lysates were incubated on ice for 5 minutes and then transferred to microcentrifuge tubes. The lysates were clarified by centrifugation at 14,000g for 10 minutes, the supernatants were collected, and protein concentrations were measured (Bradford protein assay reagent; Bio-Rad, Hercules, CA). After boiling with 2× Laemmli sample buffer for 5 minutes, 40 μL of cell lysate (containing 25 μg of protein) was run on a 10% SDS–polyacrylamide gel. Subsequently, the proteins were transferred onto a nitrocellulose membrane, and the total p42/44 MAP kinase bands were detected by Western blot analysis by using a rabbit polyclonal anti-p42/44 MAP kinase antibody (Cell Signaling Technology, Beverly, MA). The levels of phosphorylated p42/44 MAPK were determined using a mouse monoclonal antibody against phospho-p44/42 MAPK (Thr202/Tyr204; Cell Signaling Technology), according to procedures described by the manufacturer.
Data Analyses
For the anterior segment perfusion studies, outflow facility was calculated as the ratio of the rate of flow of perfusate (μL/min) to the steady state perfusion pressure (mm Hg). Drug effects were evaluated in each eye as the percentage change of outflow facility in drug-treated eyes over predrug baseline outflow facility. The data were presented as mean ± SEM, and plotted as change in outflow facility versus time (in minutes) using a commercial software system (Prism; GraphPad, San Diego, CA).
For the p42/44 MAPK phosphorylation assay, the bands on x-ray films were scanned (Personal Densitometer SI; Molecular Dynamics, Sunnyvale, CA) and were quantified using a commercial software system (ImageQuant; Molecular Dynamics). The results were expressed as mean ± SEM.
For both the anterior segment perfusion and p42/44 MAPK phosphorylation studies, t-test or one-way ANOVA with Newman–Keuls posttest was used to compare the data points of the different treatment groups. The level of significance was chosen as P < 0.05.
Results
Effects of PEA on Aqueous Humor Outflow Facility
Aqueous humor outflow facility studies were performed using the porcine anterior segment perfused organ culture model. As shown in Figure 1A, 30 nM PEA by itself produced a significant but transient increase in aqueous humor outflow facility. However, when the perfused organ culture was pretreated with 100 nM URB597, a FAAH inhibitor,31 for 30 minutes prior to the administration of 30 nM PEA, it enhanced the PEA-induced increase of outflow facility and prolonged the PEA-induced effects to at least 5 hours. In contrast, 100 nM URB597 alone had no effect on outflow facility. Furthermore, as shown in Figure 1B, this aqueous humor outflow-enhancing effect of PEA in the presence of URB597 is concentration and time dependent, with maximum effect (151.08 ± 11.12% of basal outflow facility) achieved at 2 hours after the administration of 30 nM of PEA. Since 30 nM PEA showed the highest effect on the outflow facility, this concentration was chosen for further studies.
Figure 1.
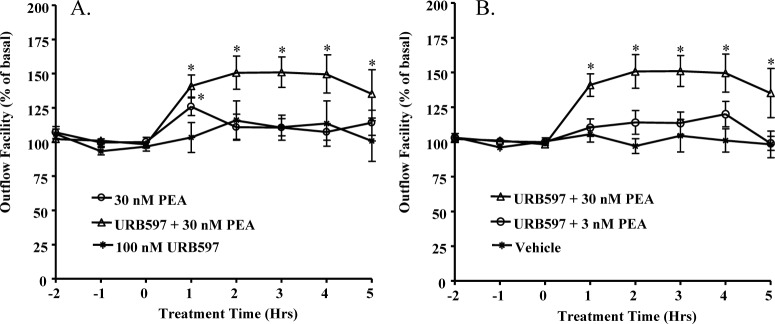
Effects of PEA on aqueous humor outflow facility. (A) The anterior segments of the porcine eyes were pretreated with vehicle or 100 nM URB597 for 30 minutes before the treatment with 30 nM PEA alone, 100 nM URB597 alone, or 30 nM PEA + 100 nM URB597 for 5 hours. (B) The anterior segments of the porcine eyes were pretreated with vehicle or 100 nM URB597 for 30 minutes before the treatment with vehicle, 3 nM PEA + 100 nM URB597, or 30 nM PEA + 100 nM URB597 for 5 hours. Results are expressed as mean ± SEM; n = 10. *Significant difference (A) between PEA + URB597 and URB597 alone, or (B) between PEA + URB597 and vehicle (P < 0.05, ANOVA with Newman–Keuls posttest).
Effects of CB1 and CB2 Antagonists on PEA-Induced Enhancement of Outflow Facility
The perfused organ culture model was pretreated with 1 μM of CB1 antagonist SR141716A32 or 1 μM of CB2 antagonist SR144528,33 respectively, for 30 minutes prior to the administration of 30 nM PEA plus 100 nM URB597. Interestingly, as shown in Figure 2B, CB2 receptor antagonist SR144528 partially blocked the aqueous humor outflow-enhancing effects induced by PEA. In contrast, CB1 receptor antagonist SR141716A had no effect on the PEA-induced increase in aqueous humor outflow (Fig. 2A).
Figure 2.
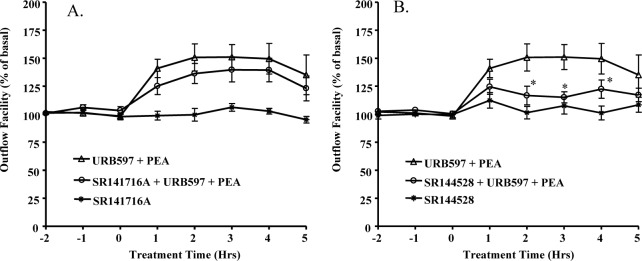
Effects of CB1 and CB2 antagonists on PEA-induced enhancement of outflow facility. The anterior segments were treated with either (A) 1 μM SR141716A or (B) 1 μM SRI45528 for 30 minutes before the treatment with (A) 30 nM PEA + 1 μM SR141716A or (B) 30 nM PEA + 1 μM SR145528 for 5 hours. Results are expressed as mean ± SEM; n = 10. *Significant differences between PEA + URB597 and PEA + URB597 + SR144528 (P < 0.05, ANOVA with Newman–Keuls posttest).
Effects of PPARα Antagonist on PEA-Induced Enhancement of Outflow Facility
To explore the functional role of PPARα receptor in TM outflow pathway, the perfused organ culture was pretreated with 1 μM of GW6471, an antagonist of PPARα,34 for 30 minutes prior to the administration of 30 nM PEA plus 100 nM URB597. As shown in Figure 3, GW6471 partially blocked the outflow-enhancing effects induced by PEA.
Figure 3.
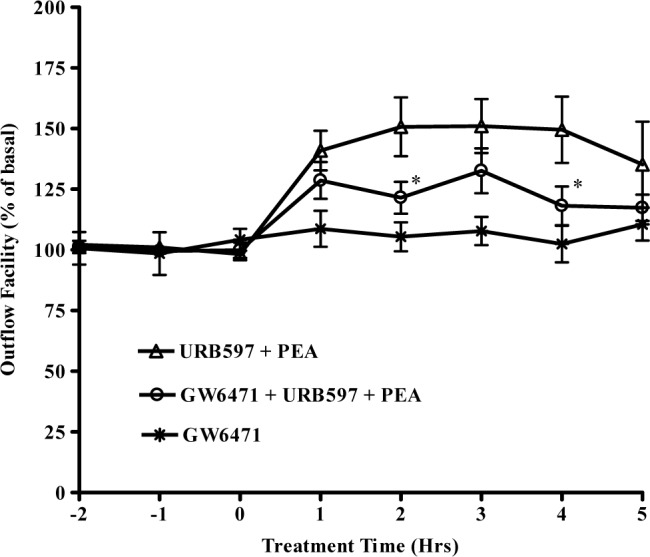
Effects of PPARα antagonist on PEA-induced enhancement of outflow facility. The anterior segments were treated with 1 μM GW6471 for 30 minutes before the addition of 30 nM PEA + 100 nM URB597 + 1 μM GW6471 for 5 hours. Results are expressed as mean ± SEM; n = 10. *Significant differences between PEA + URB597 and PEA + URB597 + GW6471 (P < 0.05, ANOVA with Newman–Keuls posttest).
Effects of GPR55 Knockdown on PEA-Induced Enhancement of Outflow Facility
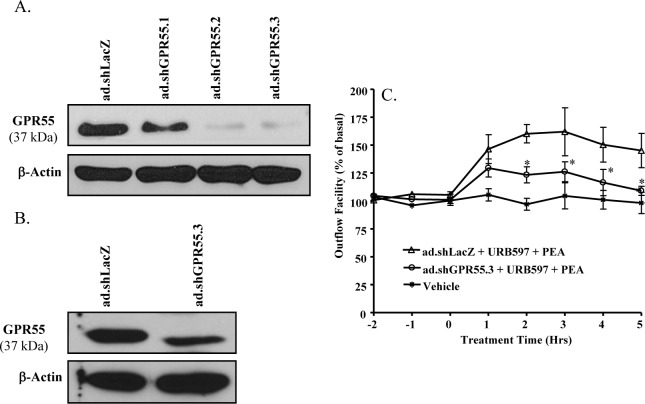
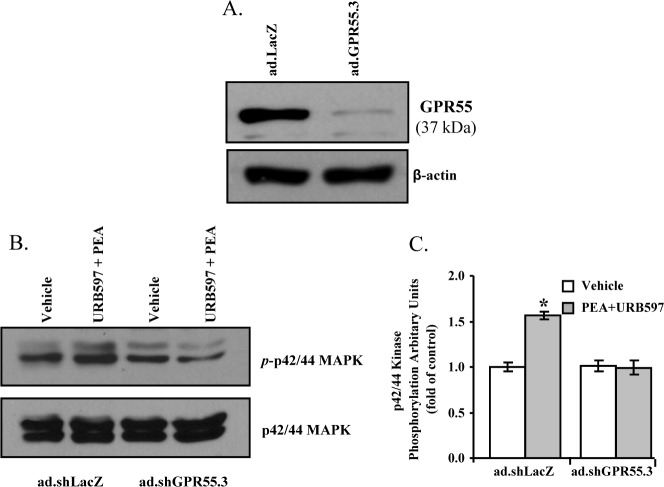
Short hairpin RNA (shRNA) is a highly effective tool to silence a specific gene.35 The knockdown efficiency of three different adenoviruses containing shRNAs for GPR55 (ad.sh GPR55.1-3) were studied in cultured TM cells. As shown in Figure 4A, after 48hour treatment with ad.sh GPR55.1-3, the expression level of GPR55 protein (37 kDa) in TM cells was reduced significantly when compared with the GPR55 expression level in control adenovirus (ad.shLacZ) transduced TM cells. Among the three adenoviruses, the ad.shGPR55.3 was determined to be the most efficient in knocking down the GPR55 expression in TM cells (Fig. 4A).
Figure 4.
Effects of GPR55 knockdown on PEA-induced enhancement of outflow facility. (A) Knockdown efficiency of GPR55 in porcine TM cells after being transduced with the adenovirus-expressing shRNA for GPR55 (ad.shGPR55.1-3) for 48 hours. (B) Knockdown efficiency of GPR55 in porcine TM tissue in the anterior chamber of the porcine eye after being transduced with ad.shGPR55.3 for 48 hours. (C) The anterior segments were transduced with ad.shGPR55.3 adenovirus for 48 hours, followed by perfusion with 30 nM PEA + 100 nM URB597 for 5 hours. Results are expressed as mean ± SEM; n = 10. *Significant differences between ad.shGPR55.3-treated and ad.shLacZ-treated eyes (P < 0.05, ANOVA with Newman–Keuls posttest).
Following confirmation of GPR55 knockdown in cultured TM cells, the perfused anterior segments of porcine eyes were treated with adenovirus particles containing shRNA against GPR55 (ad.shGPR55.3) for 48 hours and the TM tissues were dissected for Western blot analysis of GPR55 expression. As shown in Figure 4B, the GPR55 receptor protein expression in TM tissues was significantly knocked down by the ad.shGPR55.3 treatment. The ad.shGPR55.3-treated eyes were perfused with 100 nM URB597 for 30 minutes followed by perfusion with 30 nM PEA plus 100 nM URB597 for 5 hours. As shown in Figure 4C, GPR55 knockdown significantly blocked the increase in aqueous humor outflow induced by PEA.
Effects of PPARα Knockdown on PEA-Induced Enhancement of Outflow Facility
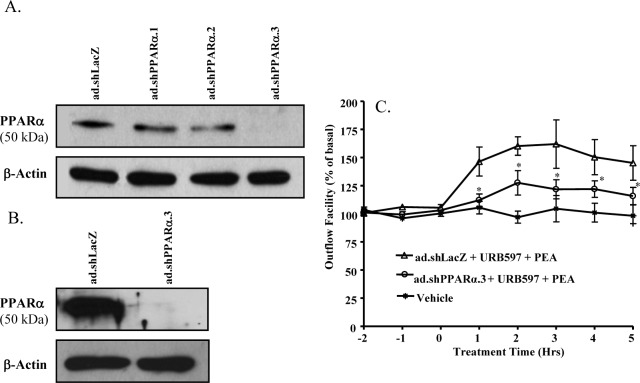
In this study, three different adenoviruses containing shRNAs for PPARα (ad.shPPARα.1-3) were first tested on cultured TM cells to study their knockdown efficiency. As shown in Figure 5A, after 48-hour treatment with ad.shPPARα.1-3, the expression level of PPARα protein (50 kDa) in TM cells was reduced significantly when compared with the PPARα expression level in control adenovirus, ad.shLacZ transduced TM cells. Among the three adenoviruses, ad.shPPARα.3 was determined to be the most efficient in knocking down the PPARα expression in TM cells (Fig. 5A).
Figure 5.
Effects of PPARα knockdown on PEA-induced enhancement of outflow facility. (A) Knockdown efficiency of PPARα in porcine TM cells after being transduced with the adenovirus-expressing shRNA for PPARα (ad.shPPARα.1-3) for 48 hours. (B) Knockdown efficiency of PPARα in porcine TM tissue in the anterior chamber of the porcine eye after being treated with ad.shPPARα.3 for 48 hours. (C) The anterior segments were transduced with ad.shPPARα.3 for 48 hours, followed by perfusion with 30 nM PEA + 100 nM URB597 for 5 hours. Results are expressed as mean ± SEM; n = 10. *Significant differences between ad.shPPARα.3-treated and ad.shLacZ-treated eyes (P < 0.05, ANOVA with Newman–Keuls posttest).
Following confirmation of knockdown of PPARα in cultured TM cells, the perfused anterior segments of porcine eyes were treated with ad.shPPARα.3 for 48 hours and the TM tissues were dissected for Western blot analysis of PPARα expression. As shown in Figure 5B, the PPARα receptor protein expression in TM tissues was significantly knocked down by the ad.shPPARα.3 treatment. The ad.shPPARα.3-treated eyes were perfused with 100 nM URB597 for 30 minutes followed by perfusion with 30 nM PEA plus 100 URB597. As shown in Figure 5C, PPARα knockdown significantly blocked the increase in aqueous humor outflow induced by PEA.
Effects of PEA on p42/44 MAPK Phosphorylation in Porcine Trabecular Meshwork Cells
As a first step to investigate the signaling pathway for PEA-induced enhancement of aqueous humor outflow facility, in the present study we explored the potential of PEA in activating the TM cell p42/44 MAPK pathway. As shown in Figure 6, treatment of TM cells with 30 nM PEA plus 100 nM URB597 for 10 minutes resulted in an increased phosphorylation of p42/44 MAPK compared with vehicle treatment.
Figure 6.
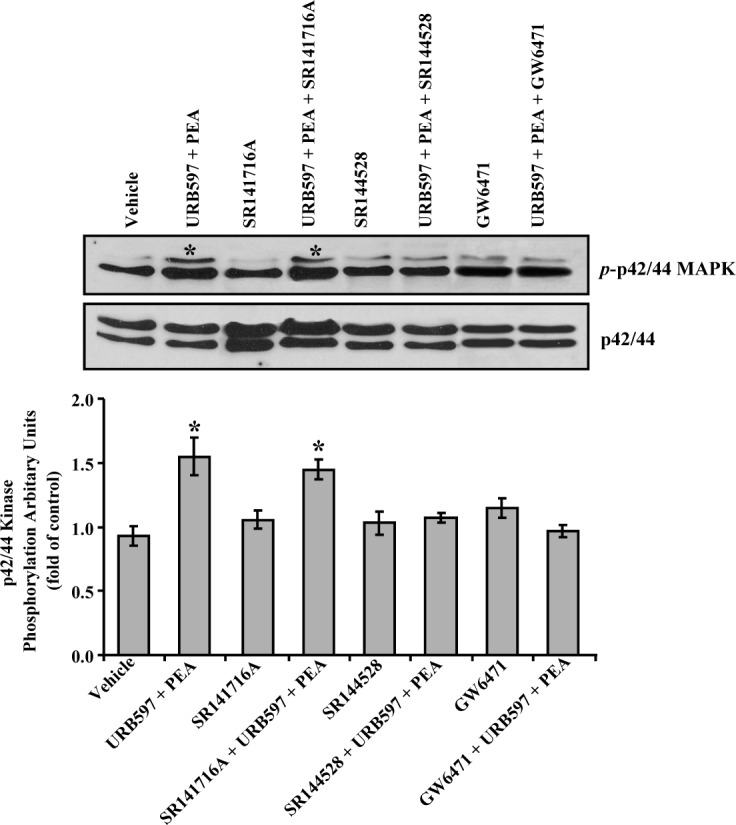
Effects of PEA on p42/44 MAP kinase phosphorylation in porcine TM cells. Porcine TM cells were serum-starved for 18 hours and pretreated with vehicle, 100 nM URB597, 100 nM URB597 + 1 μM SR141716A, 100 nM URB597 + 1 μM SR144528, or 100 nM URB597 + 1 μM GW6471 for 15 minutes, followed by stimulation with vehicle or 30 nM PEA + 100 nM URB597 for 10 minutes, and then the phosphorylation of p42/44 MAP kinase was measured. Top: Western blot representative of results obtained in three experiments. Bottom: Densitometry quantification of the phospho-p42/44 data from three experiments. Results are expressed as mean ± SEM, n = 3. *Significant difference from vehicle (P < 0.05, ANOVA with Newman–Keuls posttest).
To test the potential involvement of CB1 and CB2 cannabinoid receptors on PEA-stimulated phosphorylation of p42/44 MAPK, CB1 antagonist SR141716A and CB2 antagonist SR144528 were used. Pretreatment of trabecular meshwork (TM) cells for 30 minutes with 1 μM SR144528, but not 1 μM SR141716A, blocked PEA-induced p42/44 MAP kinase phosphorylation (Fig. 6). However, neither SR141716A nor SR144528 had any significant effect by itself on p42/44 MAPK phosphorylation.
To explore the functional role of PPARα receptor in PEA-induced phosphorylation of p42/44 MAPK, the TM cells were pretreated with 1 μM of GW6471, an antagonist of PPARα for 30 minutes prior to the addition of 30 nM PEA plus 100 nM URB597. As shown in Figure 6, GW6471 blocked the phosphorylation of p42/44 MAPK induced by PEA. However, GW6471 alone did not have any significant effect on p42/44 MAPK phosphorylation.
Effects of GPR55 or PPARα Knockdown on PEA-Induced Phosphorylation of p42/44 MAP Kinase in Porcine TM Cells
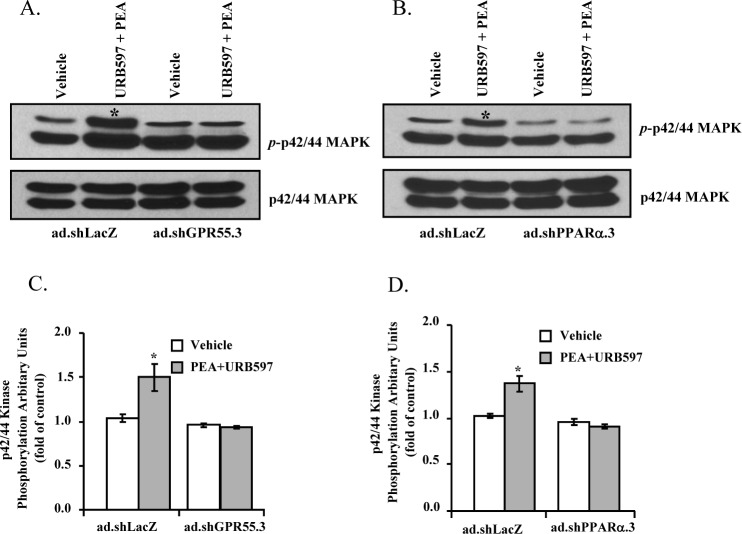
To elucidate the roles of GPR55 and PPARα in mediating PEA-induced phosphorylation of p42/44 MAPK, the adenoviruses containing shRNAs for GPR55 (ad.shGPR55.3) or PPARα (ad.shPPARα.3) were used to knock down the expression of GPR55 or PPARα, respectively, in TM cells. As shown in Figure 7, in control virus (ad.shLacZ)–treated porcine TM cells, the PEA induced an increase in the phosphorylation of p42/44 MAPK. However, in porcine TM cells treated with either ad.shGPR55.3 or ad.shPPARα.3, PEA failed to activate p42/44 MAPK phosphorylation (Fig. 7), indicating that knocking down of these receptors in porcine TM cells led to an inhibition of PEA-induced p42/44 MAPK phosphorylation.
Figure 7.
Effects of GPR55 or PPARα knockdown on PEA-induced phosphorylation of p42/44 MAP kinase in porcine TM cells. Porcine TM cells were transduced with adenoviruses expressing shRNAs for GPR55 (ad.shGPR55.3), PPARα (ad.shPPARα.3), or LacZ (ad.shLacZ) for 48 hours, and then stimulated with 30 nM PEA + 100 nM URB597 for 10 minutes. (A, B) Representative Western blot from three experiments showing the levels of phospho-p42/44 MAP kinase. (C, D) Densitometry quantification of the phospho-p42/44 data from three experiments. Results are expressed as mean ± SEM, n = 3. *Significant difference from vehicle (P < 0.05, t-test).
Effects of PD98059 on PEA-Induced Enhancement of Outflow Facility
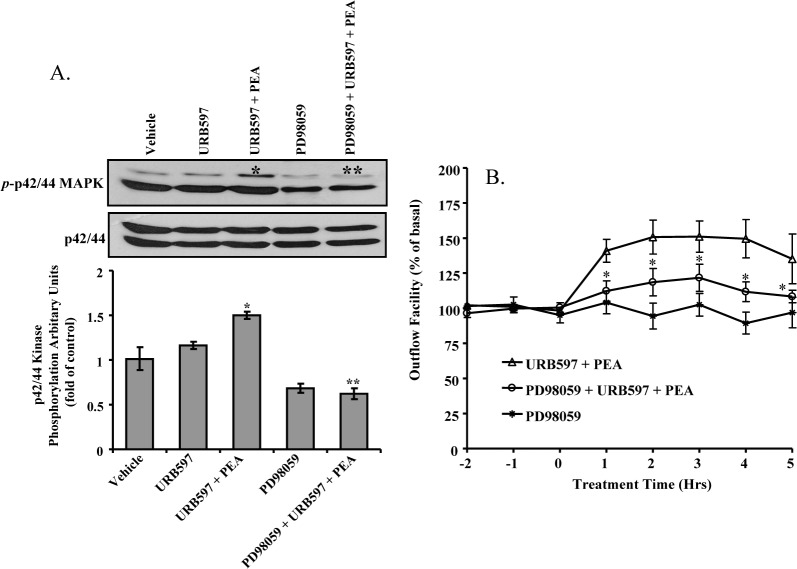
PD98059 is an inhibitor of the p42/44 MAPK pathway.27 As shown in Figure 8A, PEA-induced phosphorylation of p42/44 MAPK was completely blocked by pretreatment of TM cells with 30 μM of PD98059. To elucidate the role of p42/44 MAPK signaling pathway in the PEA-induced enhancement of outflow facility, 30 μM of PD98059 was administered to the perfused anterior segment prior to addition of PEA plus URB597. As shown in Figure 8B, PD98059 partially blocked outflow-enhancing effects of PEA. PD98059 by itself had no significant effect on outflow facility.
Figure 8.
Effects of PD98059 on PEA-induced enhancement of outflow facility. (A) Inhibition of PEA-induced phosphorylation of p42/44 MAP kinase by PD98059. Porcine TM cells were serum-starved for 18 hours before being pretreated with 30 μM PD98059 for 15 minutes, followed by treatment with vehicle or 30 nM of PEA + 100 nM URB597 for 10 minutes. Top: Representative Western blots of results obtained in three experiments. Bottom: densitometry quantification of p42/44 MAP kinase phosphorylation data from three experiments. Results are expressed as mean ± SEM, n = 3. *Significant difference from vehicle alone (P < 0.05, ANOVA with Newman–Keuls posttest). **Significant difference from PEA + URB597 (P < 0.05, ANOVA with Newman–Keuls posttest). (B) The anterior segments were treated with 100 nM URB597 + 30 μM PD98059 for 30 minutes before the addition of 30 nM PEA + 100 nM URB597 + 30 μM PD98059. Results are expressed as the mean ± SEM, n = 10. *Significant difference between PEA + URB597 and PEA + URB597 + PD98059 (P < 0.05, ANOVA with Newman–Keuls posttest).
Effects of GW6471 and PD98059 on PEA-Induced p42/44 MAPK Phosphorylation in Human TM Cells
To confirm that PPARα is involved in PEA-induced phosphorylation of p42/44 MAPK in human TM cells, we adopted a pharmacologic approach by using GW6471, an antagonist of PPARα. As shown in Figure 9, treatment of human TM cells with 1 μM of GW6471 significantly blocked the PEA-induced phosphorylation of p42/44 MAPK. GW6471 alone did not have any significant effect on p42/44MAPK phosphorylation in human TM cells (Fig. 9). Furthermore, PEA-induced phosphorylation of p42/44 MAPK was completely blocked by pretreatment of human TM cells with 30 μM of PD98059, an inhibitor of the p42/44 MAPK pathway (Fig. 9).
Figure 9.
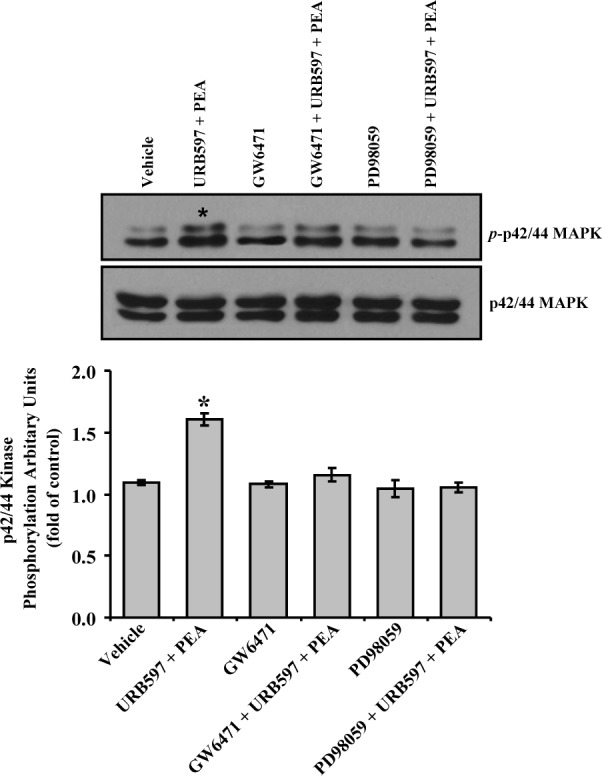
Effects of GW6471 and PD98059 on PEA-induced p42/44 MAPK phosphorylation in human trabecular meshwork cells. Human TM cells were serum-starved for 18 hours and pretreated with vehicle, 100 nM URB597, 100 nM URB597 + 1 μM GW6471, or 100 nM URB597 + 30 μM PD98059 for 15 minutes, followed by stimulation with vehicle or 30 nM PEA + 100 nM URB597 for 10 minutes, after which the phosphorylation of p42/44 MAP kinase was measured. Top: Western blot representative of results obtained in three experiments. Bottom: Densitometry quantification of the phospho-p42/44 data from three experiments. Results are expressed as mean ± SEM, n = 3. *Significant difference from vehicle (P < 0.05, ANOVA with Newman–Keuls posttest).
Effects of GPR55 Knockdown on PEA-Induced Phosphorylation of p42/44 MAP Kinase in Human TM Cells
To confirm the PEA signaling through GPR55 receptor in human TM cells, the adenoviruses containing shRNA for GPR55 (ad.shGPR55.3) were used to knock down the expression of GPR55 in human TM cells. The sequences encoding the shRNA showed 100% identity between porcine and human GPR55. When the human TM cells were transduced with ad.shGPR55.3, the expression level of GPR55 protein (37 kDa) was significantly reduced when compared with the GPR55 expression level in control adenovirus ad.shLacZ-treated human TM cells (Fig. 10A). As shown in Figures 10B and 10C, in control virus (ad.shLacZ)-treated human TM cells, the PEA induced an increase in the phosphorylation of p42/44 MAPK. However, in human TM cells treated with ad.shGPR55.3, PEA failed to activate p42/44 MAPK phosphorylation (Figs. 10B, 10C), indicating that knocking down of PPARα in human TM cells led to an inhibition of PEA-induced p42/44 MAPK phosphorylation.
Figure 10.
Effects of GPR55 knockdown on PEA-induced phosphorylation of p42/44 MAP kinase in human trabecular meshwork cells. Human TM cells were transduced with adenoviruses expressing shRNAs against GPR55 (ad.shGPR55.3) or LacZ (ad.shLacZ) for 48 hours, and then stimulated with 30 nM PEA + 100 nM URB597 for 10 minutes. (A) Representative Western blots from three experiments showing the GPR55 knockdown in human TM cells. (B) Representative Western blots from three experiments showing the levels of phospho-p42/44 MAPK. (C) Densitometry quantification of the phospho-p42/44 MAPK data from three experiments. Results are expressed as mean ± SEM, n = 3. *Significant difference from vehicle (P < 0.05, t-test).
Discussion
Cannabinoids have been shown to lower IOP long before the cannabinoid receptors were known.4,5,36 Regarding their mechanisms of actions, cannabinoids have been found to decrease aqueous humor production as well as increase aqueous humor outflow.4,36 In recent years, both CB1 and CB2 receptors have been detected in the anterior segments of the eye, including ciliary body and trabecular meshwork, two major sites of cannabinoid actions.6–8,37,38 In previous studies, we have demonstrated that the administration of cannabinoids increases aqueous humor outflow, and both CB1 and CB2 receptors are involved in the aqueous humor outflow-enhancing effects of cannabinoids.7–10 In addition, we have shown that AEA and 2-AG, two endocannbinoids, are able to increase aqueous humor outflow.9,10 Also, we have demonstrated the presence of fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MGL), two major degradation enzymes for endocannbinoids, in the trabecular meshwork.9,10 Furthermore, we have shown that inhibition of these degradation enzymes was able to prolong and enhance the effects AEA and 2-AG.9,10 PEA is a saturated congener of AEA.14,15 The present study provides evidence that PEA can regulate the aqueous humor outflow in the eye.
Recently, clinical trial studies have shown that the PEA can lower increased IOP in eyes that underwent the laser iridotomy procedure17 and in glaucomatous eyes.18 In the present study, we have demonstrated that administration of PEA leads to an increased aqueous humor outflow in perfused porcine anterior segments. Thus, our data on porcine eyes are consistent with the IOP-lowering effects of PEA reported on human eyes. To the best of our knowledge, this is the first time that PEA has been shown to enhance aqueous humor outflow facility.
In the current study, the effect of PEA was prolonged and enhanced by the administration of URB597, a FAAH inhibitor.31 These data suggest that the degradation of PEA was inhibited by URB597 in the perfused porcine anterior segments. In a previous study in rabbits, administration of PEA failed to exhibit any IOP-lowering effect.39 This lack of effects of PEA on IOP in rabbits may be due to the rapid hydrolysis of PEA in the absence of an FAAH inhibitor.
In this study we used SR141716A32 and SR144528,33 selective antagonists to CB1 and CB2 receptors, respectively, to explore the possible involvement of cannabinoid receptors in PEA-induced enhancement of aqueous humor outflow. Both the CB16,7,38,40 and CB2 receptors8,37 are known to be expressed in the TM tissues. Our results showed that the outflow-enhancing effect of PEA was partially blocked by CB2-selective antagonist SR144528 but not by CB1-selective antagonist SR141716A. Currently, it is not clear how SR144528 blocked the effects of PEA. One possibility is that PEA acts through the CB2 receptor, which is consistent with previous reports that the effects of PEA in vivo are blocked by SR144528.15,19,20 However, based on the facts that PEA does not bind to the CB2 receptor,15 it is unlikely that PEA acts through CB2. Another possibility is that PEA may act through a novel, non-CB1/CB2 receptor, which can be blocked by SR144528. A third possibility is that GPR55 and CB2 receptor may interact to form heterodimers; thus, the PEA-induced effects are sensitive to a CB2 antagonist.
PEA is known to activate GPR55, a G protein–coupled, putative novel cannabinoid receptor.21,22 It has been shown that PEA stimulates the binding of GTPγS to the GPR55 receptor.21,22 In the current study, knocking down GPR55 expression in TM tissues using adenovirus delivered shRNA specific for GPR55 partially blocked the PEA-induced enhancement of outflow facility. Clearly these experiments establish the involvements of GPR55 in mediating the PEA-induced increase of aqueous humor outflow through the TM pathway.
In this study, the aqueous humor outflow-enhancing effects of PEA were partially blocked by GW6471, a PPARα antagonist. PPARα is a ligand-activated nuclear transcription factor that regulates lipid metabolism and homeostasis.41 However, there is also evidence that PPARα agonist can work through nongenomic mechanisms.42 It is known that PEA directly activates PPARα,15,23 leading to antiinflammatory20 and antinoceceptive responses.43 In PPAR−/− mice, both the antiinflammatory and antinociceptive effects of PEA are ameliorated.23,43 Previously, PPARα has been shown to be expressed in rat ocular tissue.44 In the current study, the PPARα receptor has been detected in TM. Thus, PEA may bind to the PPARα receptor in TM cells to cause the changes in outflow facility. This hypothesis was further confirmed by our shRNA knockdown experiments, which demonstrated that knocking down the PPARα in the TM tissues resulted in a significant reduction of the outflow-enhancing effect of PEA.
A number of biochemical agents, such as growth factors, cytokines, and adenosines, have been shown to activate p42/44 MAPK in trabecular meshwork cells.26,45 It is known that p42/44 MAP kinase activation by these chemicals alters the functions of TM.26,45,46 Previously, we have shown that synthetic cannabinoids as well as endocannabinoids activate p42/44 MAPK in TM cells, and the p42/44 MAPK pathway is involved in the cannabinoid-induced changes in aqueous humor outflow facility.7–9 Consistent with these previous findings, in the current study, we have found the p42/44 MAPK pathway is activated by PEA in TM cells, and this activation of p42/44 MAPK is important for the PEA-induced enhancement of the aqueous humor outflow facility.
In the current study, the actions of PEA on outflow happen quickly within an hour, implying the involvement of nongenomic signaling mechanisms. Similar fast-acting PEA-induced antiinflammation and antinociceptive responses are noticed.19,23,42,43 Activation of GPR55 has also been shown to activate the p42/44 MAPK pathway. In addition, the nongenomic PPARα signaling has been shown to involve the Src family of tyrosine kinase and downstream phosphorylation of p42/44 MAPK.42 Although PEA is a ligand for nuclear transcription factor PPARα, our finding that PEA leads to increased phosphorylation of p42/44 MAPK in TM cells may explain the nongenomic nature of the signal transduction pathway that leads to PEA-induced increase in the aqueous humor outflow facility.
In the present study, we have found that PEA-induced activation of porcine TM cell p42/44 MAPK is significantly blocked by CB2 antagonist SR144528, shRNA for GPR55, as well as PPARα antagonist GW6471 and shRNA for PPARα. These data on TM p42/44 MAPK are consistent with our data on PEA-induced effects on aqueous humor outflow facility, further confirming the involvement of a non-CB1/CB2, SR144528-sensitive receptor, the GPR55 receptor, as well as the PPARα receptor in the actions of PEA in TM tissues. Furthermore, the involvements of GPR55 and PPARα in PEA-induced activation of p42/44 MAPK have been confirmed in human TM cells.
In conclusion, the current study demonstrates that the administration of PEA enhances aqueous humor outflow facility. This effect appears to be mediated at least partially by a SR144528-sensitive, non-CB1/CB2 receptor, the GPR55 receptor, and the PPARα receptor, and involves the p42/44 MAPK pathway. Taken together, our data provide a mechanistic basis for the potential of PEA as a new therapeutic agent for the treatment of elevated IOP.
Footnotes
Supported in part by National Institutes of Health Grants EY-13632 and DA-11551.
Disclosure: A. Kumar, None; Z. Qiao, None; P. Kumar, None; Z.-H. Song, None
References
- 1. Crowston JG, Weinreb RN. Glaucoma medication and aqueous humor dynamics. Curr Opin Ophthalmol. 2005; 16: 94– 100. [DOI] [PubMed] [Google Scholar]
- 2. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006; 90: 262– 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004; 363: 1711– 1720. [DOI] [PubMed] [Google Scholar]
- 4. Green K. Marijuana smoking vs cannabinoids for glaucoma therapy. Arch Ophthalmol. 1998; 116: 1433– 1437. [DOI] [PubMed] [Google Scholar]
- 5. Hepler RS, Frank IR. Marihuana smoking and intraocular pressure (Letter to the Editor). JAMA. 1971; 217: 1392. [PubMed] [Google Scholar]
- 6. Stamer WD, Golightly SF, Hosohata Y, et al. Cannabinoid CB(1) receptor expression, activation and detection of endogenous ligand in trabecular meshwork and ciliary process tissues. Eur J Pharmacol. 2001; 431: 277– 286. [DOI] [PubMed] [Google Scholar]
- 7. Njie YF, Kumar A, Qiao Z, Zhong L, Song ZH. Noladin ether acts on trabecular meshwork cannabinoid (CB1) receptors to enhance aqueous humor outflow facility. Invest Ophthalmol Vis Sci. 2006; 47: 1999– 2005. [DOI] [PubMed] [Google Scholar]
- 8. Zhong L, Geng L, Njie Y, Feng W, Song ZH. CB2 cannabinoid receptors in trabecular meshwork cells mediate JWH015-induced enhancement of aqueous humor outflow facility. Invest Ophthalmol Vis Sci. 2005; 46: 1988– 1992. [DOI] [PubMed] [Google Scholar]
- 9. Njie YF, He F, Qiao Z, Song ZH. Aqueous humor outflow effects of 2-arachidonylglycerol. Exp Eye Res. 2008; 87: 106– 114. [DOI] [PubMed] [Google Scholar]
- 10. Njie YF, Qiao Z, Xiao Z, Wang W, Song ZH. N-Arachidonylethanolamide-induced increase in aqueous humor outflow facility. Invest Ophthalmol Vis Sci. 2008; 49: 4528– 4534. [DOI] [PubMed] [Google Scholar]
- 11. Bachur NR, Masek K, Melmon KL, Udenfriend S. Fatty acid amides of ethanolamine in mammalian tissues. J Biol Chem. 1965; 240: 1019– 1024. [PubMed] [Google Scholar]
- 12. Devane WA, Hanus L, Breuer A, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992; 258: 1946– 1949. [DOI] [PubMed] [Google Scholar]
- 13. Bachur NR, Udenfriend S. Microsomal synthesis of fatty acid amides. J Biol Chem. 1966; 241: 1308– 1313. [PubMed] [Google Scholar]
- 14. Bisogno T, Maurelli S, Melck D, De Petrocellis L, Di Marzo V. Biosynthesis, uptake, and degradation of anandamide and palmitoylethanolamide in leukocytes. J Biol Chem. 1997; 272: 3315– 3323. [DOI] [PubMed] [Google Scholar]
- 15. LoVerme J, La Rana G, Russo R, Calignano A, Piomelli D. The search for the palmitoylethanolamide receptor. Life Sci. 2005; 77: 1685– 1698. [DOI] [PubMed] [Google Scholar]
- 16. Chen J, Matias I, Dinh T, et al. Finding of endocannabinoids in human eye tissues: implications for glaucoma. Biochem Biophys Res Commun. 2005; 330: 1062– 1067. [DOI] [PubMed] [Google Scholar]
- 17. Pescosolido N, Librando A, Puzzono M, Nebbioso M. Palmitoylethanolamide effects on intraocular pressure after Nd:YAG laser iridotomy: an experimental clinical study. J Ocul Pharmacol Ther. 2011; 27: 629– 635. [DOI] [PubMed] [Google Scholar]
- 18. Gagliano C, Ortisi E, Pulvirenti L, et al. Ocular hypotensive effect of oral palmitoyl-ethanolamide: a clinical trial. Invest Ophthalmol Vis Sci. 2011; 52: 6096– 6100. [DOI] [PubMed] [Google Scholar]
- 19. Conti S, Costa B, Colleoni M, Parolaro D, Giagnoni G. Antiinflammatory action of endocannabinoid palmitoylethanolamide and the synthetic cannabinoid nabilone in a model of acute inflammation in the rat. Br J Pharmacol. 2002; 135: 181– 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jaggar SI, Hasnie FS, Sellaturay S, Rice AS. The anti-hyperalgesic actions of the cannabinoid anandamide and the putative CB2 receptor agonist palmitoylethanolamide in visceral and somatic inflammatory pain. Pain. 1998; 76: 189– 199. [DOI] [PubMed] [Google Scholar]
- 21. Godlewski G, Offertaler L, Wagner JA, Kunos G. Receptors for acylethanolamides-GPR55 and GPR119. Prostag Other Lipid Mediat. 2009; 89: 105– 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ryberg E, Larsson N, Sjogren S, et al. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007; 152: 1092– 1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lo Verme J, Fu J, Astarita G, et al. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol Pharmacol. 2005; 67: 15– 19. [DOI] [PubMed] [Google Scholar]
- 24. Duncan RS, Chapman KD, Koulen P. The neuroprotective properties of palmitoylethanolamine against oxidative stress in a neuronal cell line. Mol Neurodegener. 2009; 4: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lepicier P, Bouchard JF, Lagneux C, Lamontagne D. Endocannabinoids protect the rat isolated heart against ischaemia. Br J Pharmacol. 2003; 139: 805– 815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shearer T, Crosson CE. Activation of extracellular signal-regulated kinase in trabecular meshwork cells. Exp Eye Res. 2001; 73: 25– 35. [DOI] [PubMed] [Google Scholar]
- 27. Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD. 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995; 270: 27489– 27494. [DOI] [PubMed] [Google Scholar]
- 28. Bradley JM, Vranka J, Colvis CM, et al. Effect of matrix metalloproteinases activity on outflow in perfused human organ culture. Invest Ophthalmol Vis Sci. 1998; 39: 2649– 2658. [PubMed] [Google Scholar]
- 29. Polansky JR, Weinreb RN, Baxter JD, Alvarado J. Human trabecular cells. I. Establishment in tissue culture and growth characteristics. Invest Ophthalmol Vis Sci. 1979; 18: 1043– 1049. [PubMed] [Google Scholar]
- 30. Tripathi RC, Tripathi BJ. Human trabecular endothelium, corneal endothelium, keratocytes, and scleral fibroblasts in primary cell culture. A comparative study of growth characteristics, morphology, and phagocytic activity by light and scanning electron microscopy. Exp Eye Res. 1982; 35: 611– 624. [DOI] [PubMed] [Google Scholar]
- 31. Piomelli D, Tarzia G, Duranti A, et al. Pharmacological profile of the selective FAAH inhibitor KDS-4103 (URB597). CNS Drug Rev. 2006; 12: 21– 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rinaldi-Carmona M, Barth F, Heaulme M, et al. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994; 350: 240– 244. [DOI] [PubMed] [Google Scholar]
- 33. Rinaldi-Carmona M, Barth F, Millan J, et al. SR 144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J Pharmacol Exp Ther. 1998; 284: 644– 650. [PubMed] [Google Scholar]
- 34. Xu HE, Stanley TB, Montana VG, et al. Structural basis for antagonist-mediated recruitment of nuclear co-repressors by PPARalpha. Nature. 2002; 415: 813– 817. [DOI] [PubMed] [Google Scholar]
- 35. Bantounas I, Phylactou LA, Uney JB. RNA interference and the use of small interfering RNA to study gene function in mammalian systems. J Mol Endocrinol. 2004; 33: 545– 557. [DOI] [PubMed] [Google Scholar]
- 36. Tomida I, Pertwee RG, Azuara-Blanco A. Cannabinoids and glaucoma. Br J Ophthalmol. 2004; 88: 708– 713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. He F, Song ZH. Molecular and cellular changes induced by the activation of CB2 cannabinoid receptors in trabecular meshwork cells. Mol Vis. 2007; 13: 1348– 1356. [PubMed] [Google Scholar]
- 38. Kumar A, Song ZH. CB1 cannabinoid receptor-mediated changes of trabecular meshwork cellular properties. Mol Vis. 2006; 12: 290– 297. [PubMed] [Google Scholar]
- 39. Mikawa Y, Matsuda S, Kanagawa T, Tajika T, Ueda N, Mimura Y. Ocular activity of topically administered anandamide in the rabbit. Jpn J Ophthalmol. 1997; 41: 217– 220. [DOI] [PubMed] [Google Scholar]
- 40. Straiker AJ, Maguire G, Mackie K, Lindsey J. Localization of cannabinoid CB1 receptors in the human anterior eye and retina. Invest Ophthalmol Vis Sci. 1999; 40: 2442– 2448. [PubMed] [Google Scholar]
- 41. Chinetti G, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors (PPARs): nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm Res. 2000; 49: 497– 505. [DOI] [PubMed] [Google Scholar]
- 42. Gardner OS, Dewar BJ, Graves LM. Activation of mitogen-activated protein kinases by peroxisome proliferator-activated receptor ligands: an example of nongenomic signaling. Mol Pharmacol. 2005; 68: 933– 941. [DOI] [PubMed] [Google Scholar]
- 43. LoVerme J, Russo R, La Rana G, et al. Rapid broad-spectrum analgesia through activation of peroxisome proliferator-activated receptor-alpha. J Pharmacol Exp Ther. 2006; 319: 1051– 1061. [DOI] [PubMed] [Google Scholar]
- 44. Rojas CV, Greiner RS, Fuenzalida LC, Martinez JI, Salem N, Jr, Uauy R. Long-term n-3 FA deficiency modifies peroxisome proliferator-activated receptor beta mRNA abundance in rat ocular tissues. Lipids. 2002; 37: 367– 374. [DOI] [PubMed] [Google Scholar]
- 45. Shearer TW, Crosson CE. Adenosine A1 receptor modulation of MMP-2 secretion by trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2002; 43: 3016– 3020. [PubMed] [Google Scholar]
- 46. Alexander JP, Acott TS. Involvement of the Erk-MAP kinase pathway in TNFalpha regulation of trabecular matrix metalloproteinases and TIMPs. Invest Ophthalmol Vis Sci. 2003; 44: 164– 169. [DOI] [PubMed] [Google Scholar]