Abstract
Zebrafish naturally form social groups called shoals. Previously, we have shown that submerging zebrafish eggs into low concentrations of alcohol (0.00, 0.25, 0.50, 0.75 and 1.00 vol/vol % external bath concentration) during development (24 hours post-fertilization) for two hours resulted in impaired shoaling response in seven month old young adult zebrafish. Here we investigate whether this embryonic alcohol exposure induced behavioural deficit persists to older age. Zebrafish embryos were exposed either to fresh system water (control) or to 1% alcohol for two hours, 24 hours after fertilization, and were raised in a high-density tank system. Social behaviour was tested by presenting the experimental fish with a computer animated group of zebrafish images, while automated tracking software measured their behaviour. Control fish were found to respond strongly to animated conspecific images by reducing their distanceand remaining close to the images during image presentation, embryonic alcohol treated fish did not. Our results suggest that the impaired shoaling response of the alcohol exposed fish was not due to altered motor function or visual perception, but likely to a Central Nervous System alteration affecting social behaviour itself. We found the effects of embryonic alcohol exposure on social behaviour not to diminish with age, a result that demonstrates the deleterious and potentially life-long consequences of exposure to even small amount of alcohol during embryonic development in vertebrates.
Keywords: ethanol, ethyl alcohol, fetal alcohol spectrum disorder, zebrafish, social behaviour, age
INTRODUCTION
Exposure of the developing fetus to alcohol (ethyl alcohol or ethanol) produces disorders collectively labeled under the umbrella term fetal alcohol spectrum disorder (FASD)[1-3]. Within FASD, fetal alcohol syndrome (FAS) is the most severe category, and is associated with heavy alcohol consumption and/or lengthy exposure during gestation. Furthermore, FAS is characterized by facial abnormalities, growth deficiencies and Central Nervous System deficits [3-5]. Alcohol related neurodevelopmental disorder (ARND) is believed to result from exposure to lower doses of alcohol compared to FAS, and is considered to be a milder disorder within FASD. Patients with ARND exhibit behavioural and cognitive abnormalities that are similar to the problems seen in individuals with FAS, but they lack the severe physical aberrations required for the FAS diagnosis [2,3]. Cognitive deficits, impaired academic performance, abnormal emotional functioning, and maladaptive social behaviour are common outcomes across the spectrum of FASD patients [6-8]. Approximately 1 in 100 children born in the Western world suffer from FASD, and the largest proportion of these children fall under the category of ARND [9,10]. Thus understanding the mechanisms underlying FASD, and particularly ARND, is of crucial importance.
The zebrafish is a powerful animal model [11] well suited for FASD research. A female zebrafish can produce 200 to 300 eggs every other day [12]. Because these eggs are small, a large number of them can be accurately exposed to alcohol at precise developmental stages and for specific durations, thereby reducing potential error variation among the exposed subjects [13]. Egg fertilization and development occur outside of the mother, and the fry grow without parental care [14].Thus potential confounds associated with the intra-uterine environment or with maternal care, characteristic of mammalian species, are absent in the zebrafish [15]. The zebrafish chorion is partially permeable to alcohol [16,17], which makes the alcohol exposure procedure extremely simple: eggs may be submerged in the desired alcohol concentration for the chosen length of time at the desired stage of development [1].
The powerful recombinant DNA-based techniques available for the zebrafish together with its biological features that are often very similar to those of mammals make this species a suitable animal model for FASD research. For example, several forward and reverse genetic tools have been developed for zebrafish [18] allowing efficient discovery and functional analysis of novel genes [14]. The sequencing of the entire zebrafish genome has been achieved [19]. High nucleotide sequence homology and functional similarities between the zebrafish and mammalian genes have also been demonstrated [19,see review by 20]. Last, zebrafish have a sophisticated vertebrate brain, sharing basic anatomical layout [21] and neurochemistry features [22] with those of higher order vertebrates including mammals. All these features of the zebrafish make this species an excellent candidate for the mechanistic exploration of human conditions, including FASD [1,13,23,24].
A devastating outcome of embryonic alcohol exposure is the life-long suffering caused by cognitive and behavioural deficits [Kodituwakku:2007dk; 25]. Clinical studies have shown that children with FASD have problems with social skills [7,8,26,27] and that these deficits continue into adolescence [28] and adulthood [25]. Recently, Hamilton et al. found impaired social behaviour in Long-Evans rats exposed to alcohol prenatally to persist into adulthood [29].
Zebrafish have a genetic predisposition to form groups, a behaviour called shoaling [30]. Furthermore, when a single zebrafish is presented with a live group of zebrafish or a computer generated animated group of images of zebrafish [4], the lone fish rapidly decreases the distance between itself and the group, and subsequently maintains this short distance as long as the shoaling stimulus is present, a behaviour we call the “shoaling response.” We use the shoaling response as a measure of social behaviour, where a strong response (large decrease between the single experimental fish and the group) is indicative of an appropriate social behaviour, and a weak response (small or no decrease in distance) is indicative of impaired social behaviour. In the past we have shown that embryonic alcohol exposure impaired social behaviour in adult zebrafish [1,31]. Specifically, we found a dose-dependent decrease in the shoaling response, i.e., control fish demonstrated a robust reduction in the distance towards the stimulus, while alcohol treated fish did not [1,31]. Notably, these fish were exposed to a mild alcohol dose only once during development (24 post fertilization) for a short duration (2 hours), while their social behaviour was tested at the age of seven month [1] or eight months [31].
The goal of the current study was to determine whether the social behaviour impairment seen in young adult zebrafish reported previously [1,23,31] remained observable in older fish, i.e. in twenty four-month-old experimental subjects. Zebrafish exhibit signs of age dependent changes similar to mammals [32], but it is not known whether the effect of embryonic alcohol exposure continues to manifest beyond the young adult stage (i.e. beyond seven or eight months). In the current study, zebrafish were exposed to 1.0% alcohol for two hours at the 24th hour post-fertilization stage. Using an automated experimental paradigm we induced and quantified social behaviour by measuring the distance of the experimental fish from a computer controlled animated group of five zebrafish (moving conspecific images, the animated shoal). Here, we report that twenty four-month-old zebrafish exposed to alcohol during their embryonic development exhibit a significantly diminished response to the animated shoal, while control (freshwater exposed) fish show a robust response to the animated shoal. We also report that the impairment observed in the alcohol treated fish is not accompanied by deficits in motor function nor it is likely caused by altered visual perception.
METHODS
Animals and Housing
Sexually mature adult zebrafish of the AB strain were bred at the University of Toronto, Mississauga Vivarium (Mississauga, Ontario, Canada) to obtain fertilized eggs. The progenitors of this population were obtained from the ZFIN Center (Eugene, Oregon, USA). AB is one of the most frequently studied zebrafish strains, which is often used in forward genetic (mutagenesis) studies [33]. Approximately 80 fertilized eggs were collected two hours post-fertilization (hpf) and washed with system water: deionized and sterile water supplemented with 60 mg/l Instant Ocean Sea Salt (Big Al's Pet Store, Mississauga, ON, Canada).
The low alcohol concentration and short exposure time were chosen to model the low levels and frequency of drinking more commonly seen during pregnancy that are associated with ARND. An equal number of eggs (approximately 40) were placed in containers with 100 ml of solution of the corresponding alcohol concentration (0 or 1.00 vol/vol %) for two hours. The eggs were subsequently washed with system water and maintained in 1.3 L tanks that were part of a nursery rack (Aquaneering Inc., San Diego, CA). Upon hatching, the fry were fed Larval AP 100 food (ZeiglerBros. Inc., Gardners, PA). After three weeks, zebrafish were moved to 2.8 L rearing tanks (Aquaneering Inc.) placed in a high-density rack system. The system had multistage filtration that contained a mechanical filter, a fluidized glass bed biological filter, an activated carbon filter, and a fluorescent UV light-sterilizing unit. Ten percent of the system water was replaced daily. While the fish were in the tanks of the high-density racks, they received a mixture of dried fish food (4 parts of Nelson Silver Cup, Aquaneering Inc. and powered spirulina, 1 part, Jehmco Inc., Lambertville, New Jersey). Fluorescent light tubes mounted on the ceiling provided illumination. Lights turned on at 08:00 hours and off at 21:00 hours. Zebrafish were housed in the tanks of the system rack in groups of 10 to 12 until they were 24-months-old. The sample sizes of treatment groups were as follows: 0.00% EtOH control, n = 26 and 1.00% EtOH n = 17. Over the course of two years we experienced attrition in sample size due to naturally occurring death within groups.
Behavioural Apparatus
The experimental set up consisted of a 37-l tank (50 · 25 · 30 cm, length × width × height) with a flat LCD computer screen (17 inch Samsung SyncMaster 732N monitor) placed on the left and right side of the tank along the width of the tank. Each monitor was connected to a Dell Vostro 1000 Laptop Running a custom made software application [see 2] that allowed the presentation of animated shoal. The experimental tank was illuminated by a 15 W fluorescent light-tube placed directly above it. The back side and the bottom of the tank were coated with white corrugated plastic sheets to increase the contrast and to reduce glare and reflections for video- tracking analysis. Two identical experimental set ups were used in parallel. The behaviour of the experimental fish was recorded onto the hard drive of a video camera (JVC GZ -MG37u and GZ-MG50) that was placed facing the tank and recorded a length view. The camera was placed six feet away from the experimental tank. The digital recordings were transferred to the hard drive of a desktop computer (Dell, Dimension 8400). These videos were later replayed and analyzed using the Ethovision Color Pro Videotracking software (Version 8.5 XT, Noldus Info Tech, Wageningen, the Netherlands).
Behavioural Test Procedure
Behavioural testing was conducted in a manner as described before [1]. To explore the potential effect of early developmental exposure to alcohol, we placed our adult experimental zebrafish into the test tank singly, and 30 seconds later we started a 23-minute long recording session. During the recording session, the subject was first presented with a 10-minute no stimulus (acclimation) period, followed by a 10-minute stimulus period during which, images of 5 zebrafish moving in a realistic manner with a speed similar to that of live zebrafish (ranging between 1.5 and 4 cm⁄s) were presented. Last, the recording session ended with a 3-minute no stimulus period. During each trial the stimuli were presented only on one side of the tank, but the side of presentation varied randomly across experimental subjects.
Quantification of Behaviour and Statistical Analysis
The digital video files (AVI format) were later replayed and analyzed by Ethovision as described in detail before [1]. Briefly, this tracking system allowed us to precisely quantify the swimming activity of the experimental fish (cumulative distance swam measured in centimeters (cm), a measure we call total distance moved) and the distance the fish maintained from the stimulus presentation computer screen adjacent to their experimental tank (average distance from stimulus in cm). The former measure may quantify alcohol-induced activity changes, while the latter may quantify the level of preference to conspecifics (group preference). In addition, we also quantified mean absolute angular velocity (the amount of turning).
Data obtained from the aforementioned measures were used to calculate the “reduction in distance to the stimulus” (RDTS), “immediate stimulus response” (ISR) and the “immediate change in turning” (ICT). RDTS was calculated by subtracting the average distance from the stimulus during the acclimation period (10-minute no-stimulus) from the average distance from the stimulus during the stimulus period (10-minute conspecific presentation). We subtracted the distance from the stimulus measured during the last acclimation minute (9 to 10) from the distance measured during the first minute of stimulus presentation (10-11) to obtain the immediate stimulus response (ISR). The method of calculating ISR was also employed to calculate the immediate change in turning (ICT) using the absolute turn angle as a measure.
Data were analyzed using SPSS (version 14.1) for the PC. We used a mixed analysis of variance (ANOVA) to investigate the effect of time intervals (twenty three 1-minute intervals, the repeated measure factor), the effect of alcohol treatment (0.0% or 1.0%, between subject factor), the effect of sex (between subject factor) and the interaction between these factors. In addition, to compare embryonic alcohol exposed and non-exposed fish, we conducted independent t-tests for the secondary calculated behavioural measures including RDTS, ISR and ICT. Last, we also investigated whether fish reduced their distance to the stimulus significantly below random chance using one-sample, one-tailed t-tests with Bonferroni correction.
Results
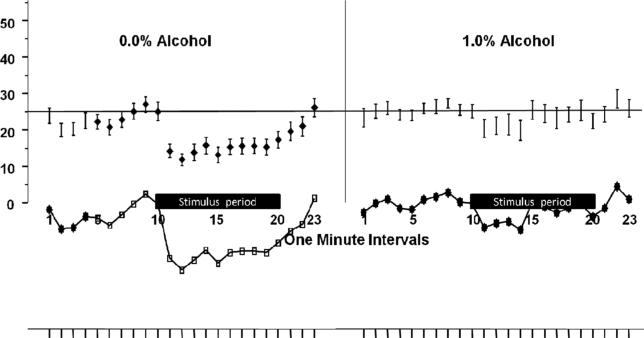
The sight of a group of conspecifics, whether live or computer generated, has been found to induce a shoaling response [4], i.e., upon stimulus presentation experimental zebrafish decreased the distance towards the stimulus (conspecific shoal), and remained within close proximity of the stimulus [2]. Figure 1 shows that two-year-old control zebrafish that were not exposed to alcohol during development perform this behaviour well (large decrease in distance to the stimulus). The figure also demonstrates that alcohol treated fish exhibit a blunted shoaling response, i.e. even two years after their exposure the impairment is robust in these fish. To analyze this apparent alcohol effect and other potential behavioural differences between control and alcohol treated fish, we first examined whether sex of the fish played any role. The effect of sex and the sex interaction terms were found non-significant. Thus, for subsequent data analyses we pooled data for the sexes. Our statistical analysis found a significant main effect of Interval [F (22, 858) = 4.40, p < 0.0001], main effect of alcohol treatment [F (1, 39) = 9.15, p = 0.004], and also a significant Interval X Alcohol treatment interaction [F (22,858) = 1.66, p < 0.029], indicating that embryonic alcohol exposure significantly affected the distance from the stimulus in an interval dependent manner in the two year old fish.
Figure 1.
The distance between the adult experimental zebrafish and the stimulus presentation computer screen is significantly reduced upon presentation of conspecific images in control fish (0.0% EtOH) but not in fish exposed to alcohol during their embryonic development (1.0%). The graph shows the average distance plotted for 1-minute intervals of the 23 -minute behavioural recording session. Mean ± SEM are shown. Sample sizes are given in the Methods section. The alcohol concentration to which fish were exposed during their embryonic development (one exposure at 2 hours at the 24th hour post fertilization) is indicated above the graphs. The black horizontal bar along the x axis denotes the time during which the stimulus was present.
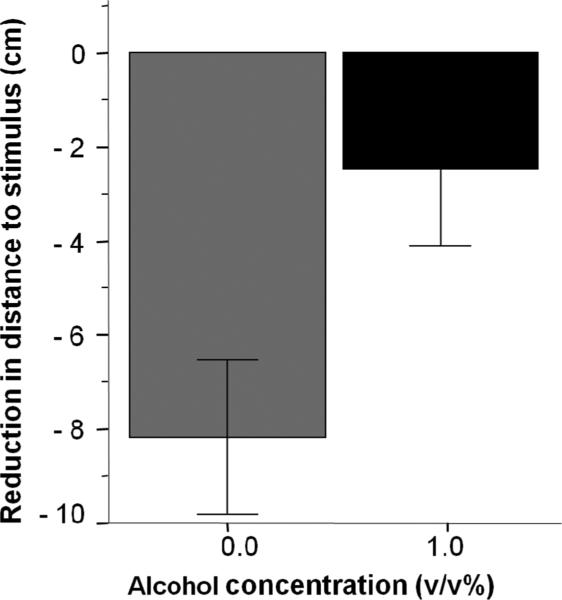
To further explore the significant Interval X Alcohol treatment interaction, we calculated the reduction of distance to stimulus (RDTS) (Figure 2). We employed an independent t-test to examine whether the RDTS differed between the embryonic alcohol exposed and non-exposed fish, and found the difference significant [t (41) = −2.350, p = 0.024]. Next, using a one-sample t – test, with Bonferroni correction, we examined whether the reduction in distance to stimulus was statistically different from chance, i.e. zero-cm change. We found that control fish significantly reduced their distance to the stimulus screen, but fish previously exposed to alcohol during development did not (controls t (25) = −4.98, p < 0.0001; 1.0% EtOH t (16) = −1.51, p = 0.151).
Figure 2.
Reduction of distance between experimental fish and the stimulus computer screen in response to conspecific stimulus presentation. Mean ± SEM are shown. Sample sizes are given in the Methods section. Values represent the difference between the distance fish were from the stimulus presentation screen before and during conspecific stimulus presentation. Larger negative values represent larger reduction of distance, i.e., stronger response to the conspecific images. The concentration of alcohol into which fish were placed for 2 hours at 24th hour post fertilization is indicated on the x-axis. Note the robust and significant reduction of distance in control (0% alcohol) fish. Also note that fish exposed to alcohol (1.0% group) did not reduce their distance in response to conspecific stimulus presentation significantly below the 0 cm chance level.
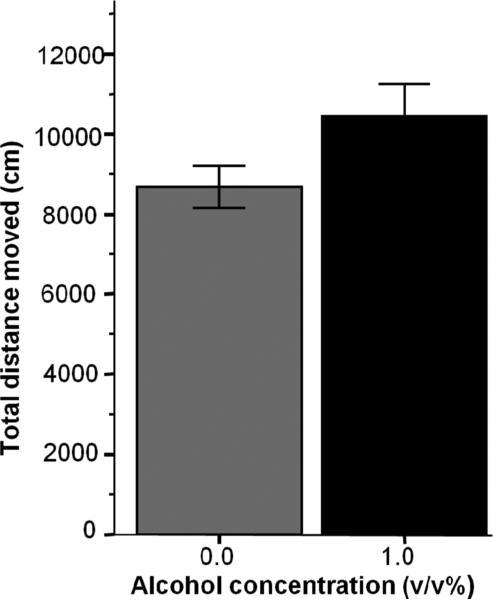
Embryonic alcohol exposure may impair motor function, which may manifest as impaired shoaling. Reduced ability to move may manifest as impaired shoaling. Our results indicated, however, that alcohol treated fish were apparently able to traverse not shorter but longer total distances during the 23-minute recording session as compared to control fish (Figure 3), an apparent difference that nevertheless did not reach significance [t (41) = −1.929, p = 0.061].
Figure 3.
Total distance swam by the adult experimental zebrafish during the 23-minute recording session does not significantly differ between the control (0.0%) and alcohol exposed (1.0%) fish. Mean ± SEM are shown. Sample sizes are given in the Methods section. The concentration of alcohol treatment (one exposure for 2 hours, 24th hour post fertilization) is shown under the x-axis.
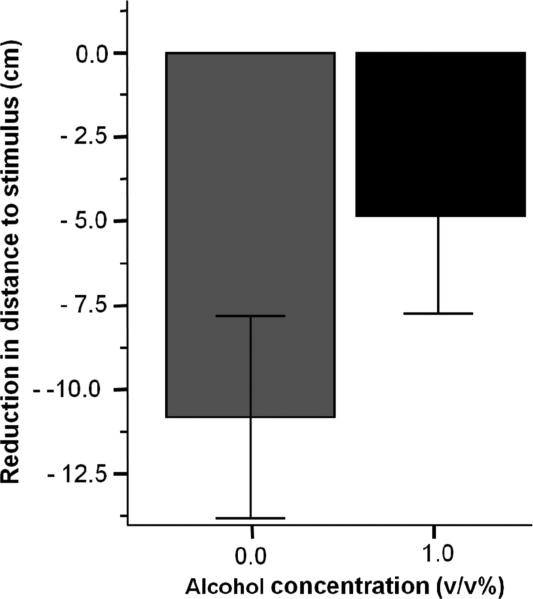
Finally, the only perceptual modality that fish could use in the current experiment was vision. Impaired vision may translate to impaired shoaling responses. We did not have an independent measure of visual acuity but used an alternative behavioural measure to investigate this possibility. We quantified the immediate stimulus response (ISR) and the immediate change in turning (ICT) to assess how/whether zebrafish responded to the appearance of visual stimuli (the animated shoal), Zebrafish have been shown to approach images that do not resemble zebrafish with a brief reduction in the distance towards the stimulus [34], that is, when fish were presented with novel moving images, they briefly explored these images. Notably, this exploration was different from the shoaling response in that the reduction of distance to these images was not maintained beyond the first minute. Figure 4 shows the ISR. Our statistical analysis demonstrated a lack of significant difference [t (41) = −1.359, p > 0.05] in the decrease in the distance to stimulus between alcohol treated and control fish during the first stimulus presentation.
Figure 4.
Reduction of the distance to the stimulus during the first 1 minute presentation of the conspecific images does not significantly differ between the control (0.0%) and alcohol exposed (1.0%) fish. Mean ± SEM are shown. Sample sizes are given in the Methods section. The treatment condition (dose of single alcohol exposure employed for 2 hours, 24th hour post fertilization) is shown under the X-axis.
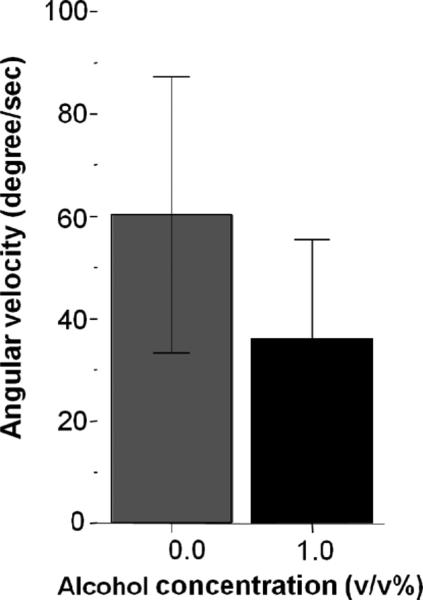
The mean absolute angular velocity may also be utilized to quantify stimulus-induced changes in behaviour. We expected an increase in the amount of turning during the first minute of stimulus presentation. If the experimental fish could see the stimuli, they were expected to change the direction of their swimming to explore the horizontally moving stimulus images, which should translate to increased angular velocity when quantified using the frontal view of the test tank. Figure 5 shows the ICT, demonstrating that there was an increase in the amount of turning in alcohol treated and control fish during the first minute of stimulus presentation. Statistical analysis revealed that there was no difference between alcohol-treated and untreated fish in the ICT response [t (41) = 0.653, p > 0.05].
Figure 5.
The increase of absolute angular velocity induced during the first minute of presentation of the conspecific images does not significantly differ between the control (0.0%) and alcohol exposed (1.0%) fish. Mean ± SEM are shown. Sample sizes are given in the Methods section. The concentration of alcohol treatment (one exposure at 2 hours at the 24th hour post fertilization) is shown under the x-axis. Note that both groups showed a significant increase in angular velocity in response to the stimulus presentation.
Discussion
Children, adolescents and adults with FASD suffer from deficits in social skills[8]. We found embryonic alcohol exposed zebrafish to also exhibit impaired behavioural responses to social stimuli during their adult stage of development [1,31]. Recently, Hamilton et al. demonstrated a similar phenomenon in rats. These authors found that social behaviour was impaired in adult Long-Evans rats prenatally exposed to alcohol [29]. To our knowledge no reports have evaluated whether embryonic alcohol exposure induced behavioural impairments in social behaviour persist beyond the young adult stage in zebrafish. Our study provides the first piece of evidence giving an affirmative answer to this question. Given the inherent bias for novel findings and against negative results in peer reviewed publications, the importance of replication of data has been emphasized [35,36]. Our findings not only extend the life-stage through which the embryonic alcohol induced abnormalities persist, but also provide important replication of previous results.
To quantify social behaviour of zebrafish, we measured their response to animated conspecific images. When a single zebrafish is presented with a group of conspecifics, the single fish reduces its distance towards the group (exploratory response), and maintains a close proximity to the group regardless of whether the group is a live shoal or a computer animated shoal [1,4,34]. We have labeled this behaviour the shoaling response [e.g. 37]. We found the untreated fish to robustly decrease their distance towards the stimulus, while fish treated with 1% alcohol during their embryonic development did not, results that corroborate our past findings [1,31]. Furthermore, analysis of the reduction of distance showed that while control fish were significantly below zero (chance), alcohol treated fish were not, results that demonstrate abolishment of the shoaling response in the alcohol treated fish. Thus, we conclude that the effect of embryonic alcohol exposure is long lasting and persists at least up to two years of age in zebrafish, a species whose life span is on average about 3 years. At two years of age, zebrafish are considered old, but not geriatric [32]. Our finding of the long-lasting effect of embryonic alcohol exposure in zebrafish is in line with both clinical data [25] and results obtained for non-human mammalian species as well [29].
At first glance the alcohol concentration used in the present study (1% vol/vol) may appear high. However, previous studies have shown that only a fraction of the alcohol employed in the external bath reaches the inside of the egg because of the protective nature of the intact chorion [1,17,24,38]. For example, we have previously reported the internal alcohol concentration to be approximately 1/25th to 1/30th of the external concentration across a range of external doses (from 0.25% to 1.00%). According to these findings, zebrafish eggs exposed to an external dose of 1% alcohol received no more than 0.04 %, i.e. half of the legal blood alcohol limit in Canada and several States in the USA. Although multiple studies confirmed the dramatic reduction of alcohol inside the egg, not all findings agree numerically. Ali et al [38] exposed zebrafish embryos to a very high concentration of alcohol (10%) for 1 hour, and found the alcohol concentration inside the embryos to be 0.86%, a more than 1/10th reduction. Lovely et al. examined the amount of alcohol present in tissue of zebrafish embryos exposed to 1% alcohol during early exposure, with embryos being exposed either from 6 to 24 hpf (for 18 hours) or from 24 to 48 hpf (24 hours) [17] and found the tissue concentration to be about 1/3rd of the external concentration [17]. Notably, in each of these experiments different procedures and alcohol quantification methodology was utilized. For example, in our studies we used the AM1 Alcohol Analyser (Analox Instruments, London, UK), a highly sensitive device specifically designed to measure alcohol from tissue homogenates or liquid based upon an enzymatic reaction [1]. We examined the amount of alcohol present in a solution that was comprised of the supernatant produced from sonicated eggs that were prenatally exposed to alcohol [1]. Also notably, our handling procedure involved a rigorous washing step to quickly and efficiently remove any external alcohol from the surface of the egg before homogenization and quantification. Lovely et al. used headspace gas chromatography while Ali et al. used high-resolution magic-angle spinning proton magnetic resonance spectroscopy to determine alcohol concentrations. [17,38]. Irrespective of the exact methods employed, however, all three studies showed that the alcohol concentration inside the egg was significantly lower than the external bath concentration.
Prenatal alcohol exposure has been reported to cause motor impairments in the clinical population as well as in primate and rodent animal models of FASD, but the effects appear consistent only in case of prolonged alcohol exposure and/or exposure to higher alcohol concentrations [39]. It is notable that our immersion procedure involved only a single two hour-long alcohol exposure to a low alcohol concentration (0.040%). Our previous studies [1,23] found no signs of motor impairment and in the current study too, we did not observe or detect any changes in the behaviour of the alcohol treated fish that would suggest impaired ability to move. For example, the analysis of angular velocity (turning ability) during the first stimulus presentation revealed no significant difference between alcohol exposed and control fish. Furthermore, the lack of significant difference between alcohol-exposed and control fish in the total distance moved also suggested unimpaired motor function in the alcohol-treated fish. Thus we conclude that the impaired response to the conspecific images was not due to gross motor dysfunction in the alcohol treated fish.
Embryonic alcohol exposure has been found to cause visual impairment in the clinical population [40], and has also been shown to lead to alterations of eye development in zebrafish [41-43]. Notably, however, the alcohol concentration we employed was lower in comparison to those employed by others [41,42], and the length of exposure period was also substantially shorter in our study compared to those of others [25,41-43]. Fetal alcohol syndrome (FAS) is diagnosed based on three criteria: (1) facial abnormalities; (2) growth retardation and (3) CNS dysfunction and/or impairment [5]. The goal of the present study was to use an alcohol concentration that produced behavioral impairments in the absence of any obvious physical abnormalities in order to recapitulate the alcohol related neurodevelopment (ARND) phenotype in zebrafish. We examined our experimental subjects for physical malformations and failed to find any observable gross structural abnormalities of the eyes (or of any other organs) in adult fish that were treated with alcohol during their development. Furthermore, recent work by Bailey et al. showed that embryonic alcohol exposure to 1 % ethanol at 24 hpf for 3 hours (one hour longer exposure duration than what we employed) also did not result in any morphological abnormality, for example did not affect eye development as quantified at 48 hpf [44], findings that corroborate our own. It is also notable that Buske and Gerlai observed impaired shoaling in freely moving shoals of embryonic alcohol exposed fish too [23]. In these freely moving shoals, visual impairment may not translate to impaired shoaling. For example, blind cave tetras or blinded experimental shoaling fish exhibit normal levels of shoaling as they can detect the proximity of their shoal mates using their lateral line, a system that works on the basis of echolocation (for experimental examples see [45]. Nevertheless, it is still possible that our alcohol exposed fish suffered from modest visual impairment.
To determine whether vision was impaired by embryonic alcohol exposure we analyzed the response of experimental fish to the appearance of the stimulus shoal, i.e. during the very first minute of stimulus presentation. Saif et al. have shown that zebrafish approach novel objects, even if these objects did not resemble zebrafish [34]. The appearance of novel objects were found to induce a transient reduction in the distance to the stimulus [34]. We found both control and the alcohol exposed fish to decrease their distance to the stimulus during the first minute of stimulus presentation, and our statistical analysis confirmed no significant differences between these groups. It is notable that the conspecific images also induced increased angular velocity during the first minute of stimulus presentation and that this increase was statistically indistinguishable between the alcohol-treated and control fish. Our current behavioural data thus suggest that the alcohol-exposed fish perceived the conspecific images presented on the computer screen and responded to them just as well as control fish during the first minute of their presentation, but the alcohol exposed fish did not maintain their close proximity to the images while the control fish did. These results imply that alcohol treated fish could see the images and exhibited exploratory responses but they did not perform shoaling.
Finding that alcohol exposed fish explored the stimulus during the first minute as much as controls is notable for another reason too. It suggests that embryonic alcohol exposed zebrafish were not indifferent to the shown images. If the alcohol exposed fish could see and respond to the conspecific images, why did they not reduce their distance to these images, i.e. why did they fail to exhibit the shoaling response always observed in control zebrafish?
One speculative answer to this question is that the alcohol treated zebrafish perhaps could not recognize the images as those of conspecifics. It is also possible that although the alcohol treated fish may be able to recognize their conspecifics as such, they may not be motivated to shoal due to impaired functioning of their motivational system, working hypotheses whose validity will be examined in the future Although we do not have direct (behavioral) evidence for altered motivation induced by the 24 hpf alcohol exposure, this treatment has been shown to significantly retard the development of the dopaminergic neurotransmitter system in zebrafish [24]. Furthermore, the amount of dopamine and of its metabolite, DOPAC, has been shown to be significantly reduced in the brain of adult zebrafish that were exposed to alcohol during their embryonic development [23,31]. Notably, the sight of conspecifics has been shown to be rewarding [46]. Last, conspecific images have been shown to induce a rapid elevation of dopamine and DOPAC levels in the brain of zebrafish [31,34] but not in zebrafish that were exposed to alcohol during their embryonic development [31]. Conversely, a dopamine D1-receptor antagonist has been found to disrupt the shoaling response to conspecific images in zebrafish [47]. Although the above reviewed findings make a compelling case for the dopaminergic system as a potentially relevant mechanistic reason why embryonic alcohol exposed fish do not shoal, it is clearly too early to say how alcohol may modify the development of the brain of zebrafish, and what biological mechanisms may underlie the observed impairment of the shoaling response. The persistent long-lasting and robust behavioural alteration we report in the current study, however, may facilitate the discovery of such mechanisms, and ultimately may lead to the development of effective treatment and also to the discovery of biomarkers that may be utilized in early diagnosis of even the milder forms of FASD.
Highlights.
Zebrafish embryos were exposed to 1% bath ethanol at 24 hpf for 2h.
Shoaling response to animated conspecific images was quantified
Embryonic exposure impaired shoaling response in 2 year old fish
Exposure of embryos to even low dose of ethanol has long lasting negative effects
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fernandes Y, Gerlai R. Long-term behavioral changes in response to early developmental exposure to ethanol in zebrafish. Alcohol Clin Exp Res. 2009;33:601–9. doi: 10.1111/j.1530-0277.2008.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saverino C, Gerlai R. The social zebrafish: Behavioral responses to conspecific, heterospecific, and computer animated fish. Behav Brain Res. 2008;191:77–87. doi: 10.1016/j.bbr.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abel EL. Fetal Alcohol Syndrome: From Mechanism to Prevention. CRC Press; Boca Raton, FL: 1996. [Google Scholar]
- 4.Qin M, Wong A, Seguin D, Gerlai R. Induction of Social Behavior in Zebrafish: Live Versus Computer Animated Fish as Stimuli. Zebrafish. 2014;11:185–97. doi: 10.1089/zeb.2013.0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stratton KR, Howe CJ, Battaglia FC, editors. Fetal alcohol syndrome: diagnosis, epidemiology, prevention, and treatment. National Academy Press; Washington, D.C: 1996. [Google Scholar]
- 6.Kodituwakku PW. Defining the behavioral phenotype in children with fetal alcohol spectrum disorders: A review. Neuroscience & Biobehavioral Reviews. 2007;31:192–201. doi: 10.1016/j.neubiorev.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 7.Rasmussen C, Becker M, McLennan J, Urichuk L, Andrew G. An evaluation of social skills in children with and without prenatal alcohol exposure. Child Care Health Dev. 2011;37:711–8. doi: 10.1111/j.1365-2214.2010.01152.x. [DOI] [PubMed] [Google Scholar]
- 8.Kully-Martens K, Denys K, Treit S, Tamana S, Rasmussen C. A Review of Social Skills Deficits in Individuals with Fetal Alcohol Spectrum Disorders and Prenatal Alcohol Exposure: Profiles, Mechanisms, and Interventions. Alcohol Clin Exp Res. 2011;36:568–76. doi: 10.1111/j.1530-0277.2011.01661.x. [DOI] [PubMed] [Google Scholar]
- 9.Sampson PD, Streissguth AP, Bookstein FL, Little RE, Clarren SK, Dehaene P, et al. Incidence of fetal alcohol syndrome and prevalence of alcohol-related neurodevelopmental disorder. Teratology. 1997;56:317–26. doi: 10.1002/(SICI)1096-9926(199711)56:5<317::AID-TERA5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 10.May PA, Gossage JP. Estimating the prevalence of fetal alcohol syndrome. A summary. Alcohol Res Health. 2001;25:159–67. [PMC free article] [PubMed] [Google Scholar]
- 11.Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nature Reviews Genetics. 2007;8:353–67. doi: 10.1038/nrg2091. [DOI] [PubMed] [Google Scholar]
- 12.Spence R, Gerlach G, Lawrence C, Smith C. The behaviour and ecology of the zebrafish, Danio rerio. Biological Reviews. 2007;83:13–34. doi: 10.1111/j.1469-185X.2007.00030.x. [DOI] [PubMed] [Google Scholar]
- 13.Fernandes Y, Tran S, Abraham E, Gerlai R. Embryonic alcohol exposure impairs associative learning performance in adult zebrafish. Behav Brain Res. 2014;265:181–7. doi: 10.1016/j.bbr.2014.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nüsslein-Volhard C. The zebrafish issue of Development. Development. 2012;139:4099–103. doi: 10.1242/dev.085217. [DOI] [PubMed] [Google Scholar]
- 15.Bilotta J, Barnett JA, Hancock L, Saszik S. Ethanol exposure alters zebrafish development: A novel model of fetal alcohol syndrome. Neurotoxicol Teratol. 2004;26:737–43. doi: 10.1016/j.ntt.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Loucks E, Ahlgren S. Assessing Teratogenic Changes in a Zebrafish Model of Fetal Alcohol Exposure. JoVE. 2012 doi: 10.3791/3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lovely CB, Nobles RD, Eberhart JK. Developmental age strengthens barriers to ethanol accumulation in zebra. Alcohol. 2014;48:595–602. doi: 10.1016/j.alcohol.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grunwald DJD, Eisen JSJ. Headwaters of the zebrafish emergence of a new model vertebrate. Nature Reviews Genetics. 2002;3:717–24. doi: 10.1038/nrg892. [DOI] [PubMed] [Google Scholar]
- 19.Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496:498–507. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roest Crollius H, Weissenbach J. Fish genomics and biology. Genome Res. 2005;15:1675–82. doi: 10.1101/gr.3735805. [DOI] [PubMed] [Google Scholar]
- 21.Tropepe V, Sive HL. Can zebrafish be used as a model to study the neurodevelopmental causes of autism? Genes Brain Behav. 2003;2:268–81. doi: 10.1034/j.1601-183x.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- 22.Chatterjee D, Gerlai R. High precision liquid chromatography analysis of dopaminergic and serotoninergic responses to acute alcohol exposure in zebrafish. Behav Brain Res. 2009;200:208–13. doi: 10.1016/j.bbr.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buske C, Gerlai R. Early embryonic ethanol exposure impairs shoaling and the dopaminergic and serotoninergic systems in adult zebrafish. Neurotoxicology and Teratology. 2011;33:698–707. doi: 10.1016/j.ntt.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahabir S, Chatterjee D, Gerlai R. Strain dependent neurochemical changes induced by embryonic alcohol exposure in zebrafish . Neurotoxicology and Teratology. 2014;41:1–7. doi: 10.1016/j.ntt.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Streissguth AP, Aase JM, Clarren SK, Randels SP, LaDue RA, Smith DF. Fetal alcohol syndrome in adolescents and adults. JAMA: the Journal of the American Medical Association. 1991;265:1961–7. [PubMed] [Google Scholar]
- 26.Thomas SE, Kelly SJ, Mattson SN, Riley EP. Comparison of social abilities of children with fetal alcohol syndrome to those of children with similar IQ scores and normal controls. Alcohol Clin Exp Res. 1998;22:528–33. [PubMed] [Google Scholar]
- 27.O'Connor MJ, Frankel F, Paley B, Schonfeld AM, Carpenter E, Laugeson EA, et al. A controlled social skills training for children with fetal alcohol spectrum disorders. Journal of Consulting and Clinical Psychology. 2006;74:639–48. doi: 10.1037/0022-006X.74.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olson HC, Feldman JJ, Streissguth AP, Sampson PD, Bookstein FL. Neuropsychological deficits in adolescents with fetal alcohol syndrome: clinical findings. Alcohol Clin Exp Res. 1998;22:1998–2012. [PubMed] [Google Scholar]
- 29.Hamilton DA, Barto D, Rodriguez CI, Magcalas CM, Fink BC, Rice JP, et al. Effects of moderate prenatal ethanol exposure and age on social behavior, spatial response perseveration errors and motor behavior. Behav Brain Res. 2014;269:44–54. doi: 10.1016/j.bbr.2014.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller NY, Gerlai R. Shoaling in zebrafish: what we don’t know. Reviews in the Neurosciences. 2011;22:17–25. doi: 10.1515/RNS.2011.004. [DOI] [PubMed] [Google Scholar]
- 31.Fernandes Y, Rampersad M, Gerlai R. Embryonic Alcohol Exposure Impairs the Dopaminergic System and Social Behavioral Responses in Adult Zebrafish. Int J Neuropsychopharmacol. 2015;18:1–8. doi: 10.1093/ijnp/pyu089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keller ET, Murtha JM. The use of mature zebrafish (Danio rerio) as a model for human aging and disease. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology. 2004;138:335–41. doi: 10.1016/j.cca.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Lockwood B, Bjerke S, Kobayashi K, Guo S. Acute effects of alcohol on larval zebrafish: a genetic system for large-scale screening. Pharmacology Biochemistry and Behavior. 2004;77:647–54. doi: 10.1016/j.pbb.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Saif M, Chatterjee D, Buske C, Gerlai R. Sight of conspecific images induces changes in neurochemistry in zebrafish. Behav Brain Res. 2013;243:294–9. doi: 10.1016/j.bbr.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benjamini Y, Hechtlinger Y. Discussion: An estimate of the science-wise false discovery rate and applications to top medical journals by Jager and Leek. Biostatistics. 2014;15:13–6–discussion39–45. doi: 10.1093/biostatistics/kxt032. [DOI] [PubMed] [Google Scholar]
- 36.Fonio E, Golani I, Benjamini Y. Measuring behavior of animal models: faults and remedies. Nat Meth. 2012;9:1167–70. doi: 10.1038/nmeth.2252. [DOI] [PubMed] [Google Scholar]
- 37.Jia J, Fernandes Y, Gerlai R. Short-term memory in zebrafish (Danio rerio). Behav Brain Res. 2014;270:29–36. doi: 10.1016/j.bbr.2014.04.046. [DOI] [PubMed] [Google Scholar]
- 38.Ali S, Champagne DL, Alia A, Richardson MK. Large-Scale Analysis of Acute Ethanol Exposure in Zebrafish Development: A Critical Time Window and Resilience. PLoS ONE. 2011;6:e20037. doi: 10.1371/journal.pone.0020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schneider ML, Moore CF, Adkins MM. The Effects of Prenatal Alcohol Exposure on Behavior: Rodent and Primate Studies. Neuropsychol Rev. 2011;21:186–203. doi: 10.1007/s11065-011-9168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strömland K. Visual impairment and ocular abnormalities in children with fetal alcohol syndrome. Addiction Biology. 2004;9:153–7. doi: 10.1080/13556210410001717024. [DOI] [PubMed] [Google Scholar]
- 41.Matsui JI, Egana AL, Sponholtz TR, Adolph AR, Dowling JE. Effects of ethanol on photoreceptors and visual function in developing zebrafish. Invest Ophthalmol Vis Sci. 2006;47:4589–97. doi: 10.1167/iovs.05-0971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arenzana FJ, Carvan MJ, III, Aijón J, Sánchez-González R, Arévalo R, Porteros A. Teratogenic effects of ethanol exposure on zebrafish visual system development. Neurotoxicology and Teratology. 2006;28:342–8. doi: 10.1016/j.ntt.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Zhang C, Turton QM, Mackinnon S, Sulik KK, Cole GJ. Agrin function associated with ocular development is a target of ethanol exposure in embryonic zebrafish. Birth Defects Research Part a: Clinical and Molecular Teratology. 2011;91:129–41. doi: 10.1002/bdra.20766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bailey JM, Oliveri AN, Zhang C, Frazier JM, Mackinnon S, Cole GJ, et al. Neurotoxicology and Teratology. Neurotoxicology and Teratology. 2015;48:1–8. doi: 10.1016/j.ntt.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerlai R. Social behavior of zebrafish: From synthetic images to biological mechanisms of shoaling. Journal of Neuroscience Methods. 2014;234:59–65. doi: 10.1016/j.jneumeth.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 46.Al-Imari L, Gerlai R. Sight of conspecifics as reward in associative learning in zebrafish (Danio rerio). Behav Brain Res. 2008;189:216–9. doi: 10.1016/j.bbr.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 47.Scerbina T, Chatterjee D, Gerlai R. Dopamine receptor antagonism disrupts social preference in zebrafish: a strain comparison study. Amino Acids. 2012;43:2059–72. doi: 10.1007/s00726-012-1284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]