Abstract
Objectives
Although prison employees share the same tuberculosis (TB) risk environment with prisoners, the magnitude of TB problems among prison employees is unknown in most resource-limited prisons. This survey was conducted to investigate the prevalence and correlates of tuberculin skin test (TST) positivity among employees in Malaysia’s largest prison.
Methods
Consented, full-time prison employees were interviewed using a structured questionnaire that included sociodemographic data, history of working in the correctional system and TB-related risk. TST was placed intradermally and read after 48–72 h. Induration size of ≥10 mm was considered positive. Logistic regression analyses were conducted to explore associations with TST positivity.
Results
Of the 445 recruited prison employees, 420 (94.4%) had complete data. Most were young (median=30.0 years) men (88.8%) who had only worked at this prison (76.4%) for a median total employment period of 60 months (IQR 34.5–132.0). The majority were correctional officers, while civilian employees represented only 7.6% of the sample. Only 26 (6.2%) reported having ever been screened for TB since employment. Prevalence of TST positivity was 81% and was independently associated with longer (≥12 months) prison employment (AOR 4.9; 95% CI 1.5 to 15.9) and current tobacco smoking (AOR=1.9, 95% CI 1.2 to 3.2).
Conclusions
Latent TB prevalence was high in this sample, approximating that of prisoners in this setting, perhaps suggesting within prison TB transmission in this facility. Formal TB control programmes for personnel and prisoners alike are urgently needed within the Malaysian correctional system.
INTRODUCTION
Tuberculosis (TB) remains a significant occupational hazard, affecting personnel working in environments with high TB prevalence.1 Settings such as healthcare institutions, drug treatment centres, mines, prisons and other congregate settings, particularly in low and middle income countries (LMICs), facilitate TB transmission among residents and employees alike.1–5 Delayed diagnosis of infectious cases, poor implementation of TB control measures and concentration of individuals at high risk for TB are major contributory factors to the high burden of TB in congregate settings, where healthcare resources are generally suboptimal.6–8 Owing to poor deployment of infection control measures in these facilities, reinfection, in addition to reactivation of latent TB infection (LTBI), plays a major role in the development of active TB disease, and hence persistence of TB among individuals in these settings.9,10 TB transmission is further fuelled in prisons where individuals with HIV are also concentrated, primarily due to criminalisation of people who inject drugs, thereby placing large numbers of individuals with suppressed immune systems who are not only at increased risk of acquiring TB, but also at risk of progressing to active TB disease.11 Eventually, through frequent movement of their residents, these ‘pockets of high TB transmission’ amplify TB, including the more dangerous drug-resistant form, in the general population and sustain TB epidemics in these settings, where resources are limited.10,12,13 The elevated risk of TB infection and active disease among healthcare workers (HCWs) in LMICs is well established, with overall high LTBI prevalence (54%, range 33–79%) and annual TB disease incidence (69–5780 per 100 000 population).3 Another occupationally exposed risk group (staff of a homeless shelter) showed similarly high (47.1%) prevalence of LTBI in a low TB burden country, Italy, where the prevalence of LTBI in the general population remains very low.4
Prisons, documented reservoirs for TB, facilitate TB transmission within their walls and to the general population given the porous nature of these settings, with many inmates and prison employees entering and leaving these facilities daily.6,14 Consequently, TB prevalence in prisons is generally high among inmates and is estimated to be up to 100 times higher than that in the general population.6 Moreover, prisons with limited implementation of TB control measures contribute to transmission of multidrug resistant TB (MDR-TB) to their respective communities.6,12,13 In some regions, especially countries of the former Soviet Union, incarceration stands alone as a major contributory factor to the growing epidemics of TB and MDR-TB in the free community.15 Prison employees become at particularly high risk for TB due to prolonged exposure to inmates in poorly ventilated and overcrowded housing units, in transportation vehicles and in healthcare facilities.16 In addition to their security duties, employees in some understaffed LMIC prisons also deliver health education and promotion to prisoners,17 increasing their likelihood of contact with infectious cases. Such exposures to personnel, however, would be markedly reduced if TB were to be adequately controlled among prisoners themselves. Despite being a high risk group for TB infection, TB screening programmes targeting prison employees are inconsistently implemented worldwide, irrespective of the country’s economic status.16,18 A 2003 European Union survey revealed that only half of participating prisons implement routine (annual) screening for active TB or LTBI in their facilities.14 Limited data exist on LTBI among prison personnel from LMIC correctional settings and in this study, we sought to provide empiric data on LTBI prevalence and correlates of tuberculin skin test (TST) positivity among full-time prison employees in Malaysia’s largest prison, where routine screening for TB is not available.
METHODS
The study is a cross-sectional convenience TST assessment that was conducted among full-time employees of Kajang Prison, the largest prison in Malaysia, from January to February 2011. Malaysia is a middle-income country with an intermediate TB (incidence=82 per 100 000 population) burden19 and an incarceration rate of 138/100 000 population,20 one of the highest in the Asia-Pacific region. The high incarceration rate is partially explained by the harsh criminalisation of drug possession as part of national antidrug policies. No formal TB screening policies or treatment of LTBI exist for either prisoners or for correctional staff within Malaysia’s prison system. Chest radiograph is the only diagnostic tool used to screen for active TB when new prison staff are employed. No regular TB assessments are implemented subsequently in the Malaysian prison system. On the other hand, symptomatic prisoners are referred to the prison’s medical staff for evaluation (passive case detection). Owing to the unavailability of diagnostic tools in the prison, most of the ill inmates are referred to a Ministry of Health hospital for further assessment. Kajang prison is a high security prison located near the capital and was designed for 3500 inmates, yet census exceeds 4000 inmates, operating at 119% capacity. At the time of the survey, there were around 1000 full-time prison employees, including correctional officers, healthcare personnel and administrative staff. Of these, more than half (N=550) worked in the first shift.
Based on prison personnel census being about 1000 and estimating that the prevalence of TST positivity among this population is 50% (unknown, but based on HCWs data in Malaysia) with a 5% margin of error, the sample size needed was 399 subjects (StatCalc, EpiInfo, CDC, USA). Owing to differences in work schedule, the recruitment was conducted by convenience for staff working during the first shift. With the assistance of the prison management, we delivered frequent reminders to each prison department to invite employee participation in the study.
Information sessions that covered the study content, risks and benefits were delivered to employees who presented at the screening site. Consented personnel were interviewed using a structured questionnaire, which included detailed sociodemographic information, prison work history, current rank and working department, previous TB screening, history of active TB disease, current tobacco smoking or alcohol use and history of chronic illnesses (diabetes mellitus, cancer or chronic renal impairment); HIV risk was not assessed. The WHO clinical scoring for TB screening in correctional facilities identified an individual’s recommendation for further assessment for active TB disease.6 The scoring system includes cough ≥2 weeks, expectoration (two points for each), fever, weight loss and chest pain (one point for each). Participants who scored 5 or more points were referred to a community hospital for further assessment (through chest radiograph) for active TB disease and did not undergo TST, until active TB was excluded. TST was administered by a single investigator (HAAA) through intradermal injection of two tuberculin units of Purified Protein Derivative (PPD) RT-23 (Staten Institut, Denmark) and induration was measured after 48–72 h by the same investigator. Induration size of 10 mm or more was considered positive.
The study protocol was reviewed and approved by the University of Malaya Medical Centre’s Medical Ethics Committee. Participation was voluntary and prison employees provided written informed consent. Individuals who refused to participate were not in any way disadvantaged nor was their participation reported to superior officers. All participants with positive TST results were counselled about the importance of taking a LTBI treatment regimen. The Malaysian Ministry of Health, however, does not currently recommend treatment for LTBI except for people living with HIV/AIDS or children with direct contact to an active TB case; no staff members were ultimately prescribed treatment.
Statistical analysis
Data were analysed using SPSS V.19 (IBM, Sunnyvale, USA). The primary outcome was TST positivity. Categorical variables were presented as frequencies, while continuous variables were presented as mean or median and SD or IQR depending on variables’ normality. Bivariate logistic regression model was investigated and variables significant at p<0.10 were entered in the final multivariate regression model and controlled for potential confounding variables.
RESULTS
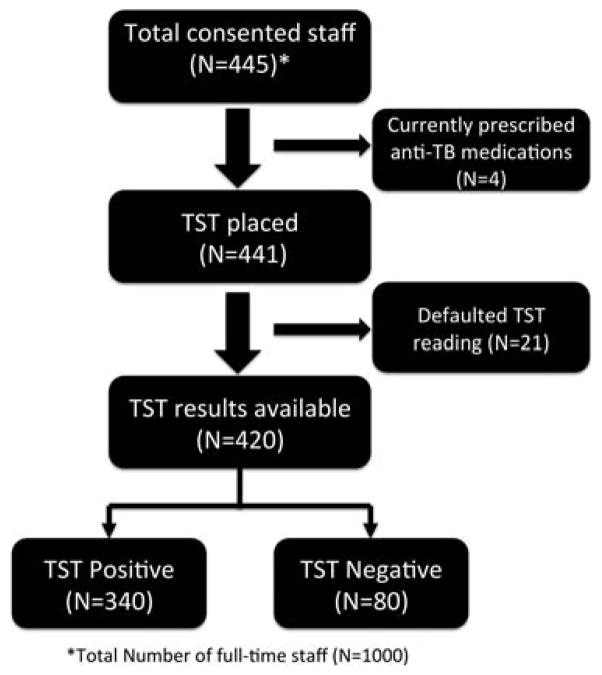
Overall, 445 prison employees attended the information sessions and subsequently provided written informed consent. Four prison personnel had been previously diagnosed with active TB disease and were receiving anti-TB medications at the time of the survey and were excluded from TST placement. Out of the 441 prison personnel tested, 21 subjects defaulted on TST reading within 72 h, leaving 420 with complete data for final analysis (figure 1).
Figure 1.
Disposition of the study participants.
Table 1 summarises the participants’ background data. The majority of participants were men (88.8%), aged between 21 and 64 (median 30 years, IQR 26–42) and living in housing units provided by the prison department (71%). Most of the participants were lower ranked (wardens, corporals and sergeants) correctional officers (64.8%) and the majority was working as prisoner cell guards (71.7%). Most had not worked anywhere other than this prison before (76.4%). Most had been employed by the criminal justice system for an average duration of 60 months (IQR 34.5–132.0). The majority (78.6%) of participants reported close contact with a prisoner diagnosed with active TB disease inside the prison at some point, but only 61 (14.5%) of participants reported close contact with a known active TB case outside the prison.
Table 1.
Logistic regression analysis of factors associated with tuberculin positivity among prison personnel (N=420)
| Variables | N (%) | OR (95% CI) | p Value | Adjusted OR (95% CI) | p Value |
|---|---|---|---|---|---|
| Gender | |||||
| Female | 47 (11.2) | Referent | |||
| Male | 373 (88.8) | 1.75 (0.87 to 3.49) | 0.11 | ||
| Age* | |||||
| <50 years | 378 (90.00) | Referent | |||
| ≥50 years | 42 (10.00) | 0.55 (0.27 to 1.13) | 0.10 | 0.57 (0.27 to 1.21) | 0.14 |
| Education | |||||
| University level | 109 (26.00) | Referent | |||
| Secondary level | 311 (74.00) | 0.86 (0.49 to 1.52) | 0.62 | ||
| Residence | |||||
| Private | 123 (29) | Referent | |||
| Prison facility | 297 (71) | 0.89 (0.52 to 1.54) | 0.69 | ||
| Total work duration in the correctional system* | |||||
| <12 months | 13 (3.10) | Referent | |||
| ≥12 months | 407 (96.90) | 5.34 (1.74 to 16.35) | 0.003 | 4.95 (1.54 to 15.93) | 0.007 |
| Rank | |||||
| Civilian employees | 32 (7.62) | Referent | |||
| Correctional officers | 388 (92.38) | 1.21 (0.50 to 2.90) | 0.67 | ||
| Current department/post* | |||||
| Administration | 71 (16.90) | Referent | |||
| Other working positions (guards, clinical staff) | 349 (83.10) | 1.72 (0.95 to 3.12) | 0.07 | 1.47 (0.79 to 2.74) | 0.22 |
| History of working in other prisons | |||||
| No | 321 (76.40) | Referent | |||
| Yes | 99 (23.60) | 0.77 (0.44 to 1.34) | 0.36 | ||
| Reported history of contact with a person with active TB inside prison | |||||
| No | 90 (21.40) | Referent | |||
| Yes | 330 (78.60) | 0.99 (0.54 to 1.79) | 0.96 | ||
| Known contact with a person with active TB outside prison | |||||
| No | 359 (85.50) | Referent | |||
| Yes | 61 (14.50) | 1.66 (0.75 to 3.65) | 0.21 | ||
| Current tobacco smoking* | |||||
| No | 183 (43.60) | Referent | |||
| Yes | 237 (56.40) | 2.00 (1.22 to 3.28) | 0.006 | 1.94 (1.17 to 3.22) | 0.01 |
| Current alcohol use* | |||||
| No | 375 (89.30) | Referent | |||
| Yes | 45 (10.70) | 2.61 (0.91 to 7.50) | 0.07 | 2.32 (0.76 to 6.54) | 0.14 |
| Diabetes mellitus | |||||
| No | 395 (94.00) | Referent | |||
| Yes | 25 (6.00) | 1.25 (0.42 to 3.75) | 0.69 | ||
These variables were included in the multivariate logistic regression model.
Bold numbers represent significance at multivariate regression model.
TB, tuberculosis.
Despite being at increased risk of TB infection, only 26 (6.2%) reported having been screened for active TB disease after joining the service, mostly due to presentation with symptoms suggestive of active TB disease. Five (1.2%) reported completing a previous course of active TB disease treatment, but no documentation was provided. The majority of officers had been previously vaccinated with Bacillus Calmette–Guérin (BCG, 98.1%) in accordance with previous Malaysian Ministry of Health TB vaccination policy (at infancy and during primary schooling). Nine (2.1%) participants had WHO clinical scores of 5 or more, but active TB disease was excluded in all after consultations at the local civilian hospital, and they were subsequently enrolled in the study. The exclusion of active TB disease was based on normal chest radiograph. Over half (56.4%) of participants were current tobacco smokers, 45 (10.7%) drank alcohol and 25 (6.0%) reported having diabetes mellitus.
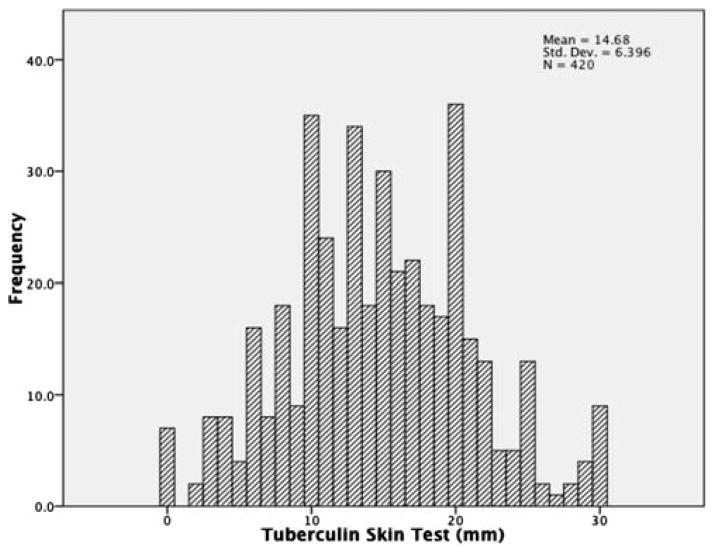
The prevalence of TST positivity in this sample of prison employees was 81%. Mean induration size was 14.7 mm (SD=6.4). Figure 2 shows the distribution of TST indurations in mm in this sample. In the multivariate analysis, TST positivity was independently associated with having worked in the correctional system for 12 months or more (AOR 4.9, 95% CI 1.5 to 15.9) and smoking tobacco (AOR=1.9, 95% CI 1.2 to 3.2), after adjusting for other potential variables. These results remain unchanged when those with close contact with active TB cases in the community were excluded from the analysis, but the association of work duration with positive TST was insignificant (AOR 2.8, 95% CI 0.2 to 31.8) when the analysis involved only those who reported no contact with an active TB case inside the prison, indicating the possibility of the presence of intense TB transmission inside the prison, particularly with high prevalence of undiagnosed active TB among prisoners.
Figure 2.
Distribution of tuberculin induration size in the sample.
DISCUSSION
To our knowledge, this is the first structured tuberculin survey among full-time personnel of the Malaysian correctional system. The survey revealed an extraordinary high (81%) prevalence of TST positivity in this sample, which was associated with longer prison employment and highlights the potential occupational risk in this overcrowded setting that has no routine TB screening programme. The study also underpins the detrimental contribution of tobacco smoking on increasing risk of TB infection, particularly in congregate settings.
Important to these findings is that the TST positivity among prison personnel parallels recent reports of TST positivity among prisoners within this prison21 and also from a remote prison in North-eastern Malaysia.22 TST positivity is markedly higher than TST findings not only from the general population (36%) within this region,23 but also among HCWs (52.1%) within the same state in Malaysia.24 Together, these findings confirm that Malaysian prisons are amplifiers of TB, solely by virtue of entering these facilities, making them a health risk for prisoners, many of whom have HIV, and an occupational hazard for prison personnel.
Although limited data exist, surveys conducted in other correctional settings, albeit primarily performed in low TB burden countries, confirmed the elevated risk of TB exposure among employees of criminal justice systems. A tuberculin survey among full-time employees of a Canadian prison revealed a high (32%) prevalence of TST positivity that was independently associated with longer duration of prison service, travelling to a TB endemic area and BCG vaccination.25 Of note, the majority (46%) of those prison workers with a positive TST had no other recognised TB risk factor; highlighting the possibility of unrecognised TB transmission from inmates with undiagnosed active TB disease. TB screening in the correctional system of the USA showed generally lower, but variable prevalence of TST positivity. A tuberculin screening involving 1323 prison employees in a New Mexico State prison system reported a prevalence of TST positivity of 10.5% (range 6.7–14.5%).26 In another TST screening among yet another high-risk group, health workers in multiple correctional institutions in the USA showed high (17.7%) prevalence of LTBI, but a place of birth from a TB endemic region rather than occupational risk factor was independently associated with TST positivity.27 In Australia, another low TB incidence country, deployment of TB control measures, including improved prisoner housing conditions, was associated with a lower overall prevalence of TST positivity among prison staff compared to inmates (6% and 11%, respectively)28 and the relatively low LTBI prevalence (6%) was similar to that in the Australian general population (4–6%). A recent nationwide TB surveillance assessment in Kazakhstan identified prison employees as one of the major risk groups for TB,29 suggesting that prison employees may represent a conduit for TB transmission from the prison setting into their respective communities.
The higher prevalence of TST positivity reported in this survey might be attributed to several factors. Correctional settings with limited implementation of evidence-based TB control programmes render these settings as reservoirs augmenting transmission of TB among inmates and staff members alike.30 This was largely attributed to the missed diagnosis of active TB cases among prisoners and hence the facilitation of on-going TB transmission.25,31 Our recent study of an intensified TB case finding revealed a very high (12.0%) prevalence of undiagnosed active TB among HIV-infected inmates in the same Malaysian prison,11 which may have contributed to the extraordinarily high reported prevalence of TST positivity among prisoners (88.8%) and prison officers (81.0%) in this prison.21
Longer duration of working in congregate settings with limited implementation of infection control measures, implying cumulative TB exposure, increases the risk of infection and TST conversion. HCWs working in LMIC healthcare facilities for more than 1 year have a 1.5–2.4 risk of being infected with TB compared to those with employment duration of less than 1 year.3 Our study shows similar result where prison employees working for more than 12 months in the correctional system were fivefold more likely to be TST positive compared to those working less than 12 months, implying that transmission occurs shortly after reporting to service. Moreover, the magnitude of increased risk among prison personnel is markedly higher than among HCWs. Similarly, TST conversion among police officers in short-term detention centres in Connecticut, USA, was associated with longer duration of tenure.32
Tobacco smoking is common, and especially increasing in LMICs, and an underestimated risk factor for TB poses a major hindrance to global TB control efforts.33 Through its direct damage to the lung structure and disruption of the respiratory immunological and cellular functions, tobacco increases the risk of LTBI and progression to active TB disease by about twofold, and worsens TB prognosis.34,35 In this study, more than half of participants (56.4%) reported current tobacco smoking, and the association with LTBI in our sample was similar to these previous reports and confirms them. Evidence-based treatments and structural interventions to reduce tobacco consumption may save lives by decreasing the risk of TB and related mortality, and should be integrated into TB control programmes,34,35 particularly in high-transmission settings such as prisons.
Prison staff members play an important role in bridging TB from prisons to the community, but such an association has not been adequately addressed. To properly control TB among this high-risk population, routine screening of prison personnel on entry into service and regularly thereafter needs to be implemented in the criminal justice systems. Unlike many hospitals that proactively address occupational risks, prisons often lack the resources and political will to conduct effective TB screening for their personnel and prisoners.36 Consequently, TB screening programmes of prison employees are inconsistently implemented in correctional settings globally.18 In the case of occupational risk for prison personnel, effective screening of prisoners is central to TB control. Although almost all (90.9%) European Union prisons reported screening of inmates on prison entry, only half implement annual TB screening programmes for their staff; mostly through chest radiography and TST.14 Similarly, only half of the jails in the USA reported regular annual TB screening of their staff, in accordance with national recommendations.16 Given the increased risk of LTBI among prison personnel, invigorated efforts are urgently needed to protect them through better screening and treatment of LTBI among prisoners and personnel alike.
Parallel control programmes need to be implemented to address TB control among prisoners, utilising passive detection and intensified TB case finding.11 To properly address TB control, the WHO recommends the implementation of the ‘Three Is’ (intensified case finding, isoniazid preventive therapy (IPT) and infection control), particularly among people living with HIV, with particular attention to correctional settings.37 Active case findings in prisoners and in prison personnel need to be prioritised together with regular monitoring of TST conversion and symptom development in this high risk group.1 Infection control measures, including administrative, engineering and personal protection measures, are of utmost importance in preventing transmission in congregate settings such as prisons.1,7,8 Personal protection equipment (PPE) use should also be enforced during transportation and admission to other facilities.1 IPT, for at least 6 months, is an effective intervention in preventing TB,38 but its utility in a high-transmission setting needs further substantiation.39 A mass provision of IPT for gold miners in South Africa showed waning efficacy shortly after the end of 9 months of IPT, further confirming the intense TB transmission in such closed settings.40 The implementation of these measures remains limited in most LMIC prisons.30,41
Although this study is limited by the unavailability of comparative data in the general Malaysian community, the reported prevalence of TST positivity in this study is higher than that in the general population in the Western Pacific region (36%)23 and that among HCWs in Malaysia (52.1%).24 The majority of participants were vaccinated with BCG as part of the Malaysian vaccination policy and though BCG vaccination may impair the specificity of TST, its effect is minimal after adolescence.42 No association was found between BCG vaccination and TST positivity from previous TST surveys in Malaysia,21,22 and there was no association with younger age and TST, making BCG contribution to TST reactivity unlikely. Interferon Gamma Release Assays (IGRAs) offer a suitable alternative to TST in countries where repeated BCG vaccination is still implemented, but its advantage over TST differs with the TB burden of the country, being of less superiority in high-burden settings.43 The Centers for Disease Control and Prevention recommend interpretation of TST irrespective of BCG vaccination history.44
CONCLUSIONS
The prevalence of TST positivity among the prison employees in this survey is high (81%) and documents the high likelihood of attributable occupational TB exposure by working in this high-transmission setting. In most settings worldwide, this occupational workforce is seldom provided adequate TB screening and there is an urgent need to prioritise TB control protocols for prison employees, particularly in LMICs. Central to these efforts are TB control efforts for prisoners themselves. Such TB control efforts should minimally include: (1) effective TB screening programme on entry to prison (inmates) or service (employees) and regularly thereafter; (2) isolation and timely access to treatment for infectious pulmonary TB cases; (3) deployment of infection control measures, including PPE; (4) IPT for prison personnel with LTBI; and (5) staff educational programmes to increase awareness about transmission risk of TB and prevention measures.12,45 Additional measures might also include addressing tobacco smoking cessation programmes, which may be integrated into occupational TB control programmes in prisons, and to improve personnel health.
What this paper adds.
Prisons house large numbers of people with or at risk for tuberculosis (TB), thereby potentially increasing occupational TB exposure to prison personnel.
Surveys from two prisons in Malaysia showed high prevalence of latent TB infection (LTBI, >80%) and previously undiagnosed active TB disease (12%) among prisoners, irrespective of HIV status.
Limited data exist about the prevalence of LTBI among prison personnel in low-income and middle-income countries, including Malaysia.
The study revealed extraordinarily high (81%) prevalence of LTBI among full-time personnel of Malaysia’s largest prison, and this was correlated with longer employment duration and current tobacco smoking.
TB control programmes targeting prisoners and prison personnel are urgently needed to reduce TB incidence and ongoing transmission in the prison.
Acknowledgments
The authors would like to thank the Malaysian Prison Department for allowing us to use their facilities to conduct the survey. The authors extend their appreciation to the officers who agreed to participate in the survey.
Funding This research was funded by the University of Malaya (Grant RG052 and High Impact Research Grant HIRGA E000001-20001) for HAAA and AK and the US National Institute on Drug Abuse for research (R01 DA025943, FLA) and career development (K24 DA017072, FLA).
Footnotes
Contributors HAA, FLA, AK were involved in the conceptualisation of the study. HAA, CT were involved in the data collection. HAA was involved in the data analysis. HAA was involved in the manuscript preparation. HAA, CT, FLA, AK were involved in the revision and approval of content.
Competing interests None.
Patient consent Obtained.
Ethics approval University of Malaya Medical Centre Medical Ethics Committee.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Bayarri MJR, Martín FMS. Pulmonary tuberculosis as an occupational disease. Arch Bronconeumol. 2004;40:463–72. doi: 10.1016/s1579-2129(06)60358-3. [DOI] [PubMed] [Google Scholar]
- 2.Lönnroth K, Jaramillo E, Williams BG, et al. Drivers of tuberculosis epidemics: the role of risk factors and social determinants. Soc Sci Med. 2009;68:2240–6. doi: 10.1016/j.socscimed.2009.03.041. [DOI] [PubMed] [Google Scholar]
- 3.Joshi R, Reingold AL, Menzies D, et al. Tuberculosis among health-care workers in low- and middle-income countries: a systematic review. PLoS Med. 2006;3:e494. doi: 10.1371/journal.pmed.0030494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Renzi S, Tomao P, Martini A, et al. Screening for tuberculosis among homeless shelter staff. Am J Infect Control. 2012;40:459–61. doi: 10.1016/j.ajic.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Harris TG, Meissner SJ, Proops D. Delay in diagnosis leading to nosocomial transmission of tuberculosis at a New York City health care facility. Am J Infect Control. 2013;41:155–60. doi: 10.1016/j.ajic.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization (WHO) Tuberculosis control in prisons: a manual for programme managers. Geneva: 2000. [Google Scholar]
- 7.Cocchiarella LA, Cohen RAC, Conroy L, et al. Positive tuberculin skin test reactions among house staff at a public hospital in the era of resurgent tuberculosis. Am J Infec Control. 1996;24:7–12. doi: 10.1016/s0196-6553(96)90047-4. [DOI] [PubMed] [Google Scholar]
- 8.Humphreys H. Control and prevention of healthcare-associated tuberculosis: the role of respiratory isolation and personal respiratory protection. J Hosp Infect. 2007;66:1–5. doi: 10.1016/j.jhin.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Charalambous S, Grant AD, Moloi V, et al. Contribution of reinfection to recurrent tuberculosis in South African gold miners. Int J Tuberc Lung Dis. 2008;12:942–8. [PubMed] [Google Scholar]
- 10.Basu S, Stuckler D, McKee M. Addressing institutional amplifiers in the dynamics and control of tuberculosis epidemics. Am J Trop Med Hyg. 2011;84:30–7. doi: 10.4269/ajtmh.2011.10-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Darraji HAA, Abd Razak H, Ng KP, et al. The diagnostic performance of a single GeneXpert MTB/RIF assay in an intensified tuberculosis case finding survey among HIV-infected prisoners in Malaysia. PLoS ONE. 2013;8:e73717. doi: 10.1371/journal.pone.0073717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruddy MC, Davies AP, Yates MD, et al. Outbreak of isoniazid resistant tuberculosis in north London. Thorax. 2004;59:279–86. doi: 10.1136/thx.2003.010405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eliseev PI, Maryandyshev AO, Nikishova EI, et al. Epidemiological analyses of tuberculosis in Archangelsk, Russia and implementation of a rapid assay for detection of resistance in this high burden setting. Int J Mycobacteriol. 2013;2:103–8. doi: 10.1016/j.ijmyco.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Aerts A, Hauer B, Wanlin M, et al. Tuberculosis and tuberculosis control in European prisons. Int J Tuberc Lung Dis. 2006;10:1215–23. [PubMed] [Google Scholar]
- 15.Stuckler D, Basu S, McKee M, et al. Mass incarceration can explain population increases in TB and multidrug-resistant TB in European and central Asian countries. Proc Natl Acad Sci USA. 2008;105:13280–5. doi: 10.1073/pnas.0801200105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Binswanger IA, Brien KO, Benton K, et al. Tuberculosis testing in correctional officers: a national random survey of jails in the United States. Int J Tuberc Lung Dis. 2010;14:464–70. [PubMed] [Google Scholar]
- 17.Waisbord S. Participatory communication for tuberculosis control in prisons in Bolivia, Ecuador, and Paraguay. Rev Panam Salud Publica. 2010;27:168–74. doi: 10.1590/s1020-49892010000300003. [DOI] [PubMed] [Google Scholar]
- 18.Rutz HJ, Bur S, Lobato MN, et al. Tuberculosis control in a large urban jail: discordance between policy and reality. J Public Health Manag Pract. 2008;14:442–7. doi: 10.1097/01.PHH.0000333878.55572.ed. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization (WHO) Global Tuberculosis Report 2012. WHO Press; Geneva: 2012. [Google Scholar]
- 20.Walmsley R. World Prison Population List. 9. 2011. [Google Scholar]
- 21.Al-Darraji HAA, Kamarulzaman A, Altice FL. Latent tuberculosis infection in a Malaysian prison: implications for a comprehensive integrated control program in prisons. BMC Public Health. 2014;14:22. doi: 10.1186/1471-2458-14-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Margolis B, Al-Darraji HAA, Wickersham JA, et al. Prevalence of tuberculosis symptoms and latent tuberculous infection among prisoners in northeastern Malaysia. Int J Tuberc lung Dis. 2013;17:1538–44. doi: 10.5588/ijtld.13.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dye C, Scheele S, Dolin P, et al. Global burden of tuberculosis—estimated incidence, prevalence, and mortality by country. JAMA. 1999;282:677–86. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 24.Tan L-H, Kamarulzaman A, Liam C-K, et al. Tuberculin skin testing among healthcare workers in the University of Malaya Medical Centre, Kuala Lumpur, Malaysia. Infect Control Hosp Epidemiol. 2002;23:584–90. doi: 10.1086/501975. [DOI] [PubMed] [Google Scholar]
- 25.Jochem K, Tannenbaum TN, Menzies D. Prevalence of tuberculin skin test reactions among prison workers. Can J Pub Heal. 1997;88:202–6. doi: 10.1007/BF03403888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spencer SS, Morton AR. Tuberculosis surveillance in a state prison system. Am J Public Health. 1989;79:507–9. doi: 10.2105/ajph.79.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitchell CS, Gershon RRM, Lears MK, et al. Risk of tuberculosis in correctional healthcare workers. J Occup Env Med. 2005;47:580–6. doi: 10.1097/01.jom.0000161738.88347.e4. [DOI] [PubMed] [Google Scholar]
- 28.MacIntyre CR, Craine J, Randall M. Risk of transmission of tuberculosis among inmates of an Australian prison. Epidemiol Infec. 1999;123:445–50. doi: 10.1017/s095026889900312x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terlikbayeva A, Hermosilla S, Galea S, et al. Tuberculosis in Kazakhstan: analysis of risk determinants in national surveillance data. BMC Infect Dis. 2012;12:262. doi: 10.1186/1471-2334-12-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vinkeles Melchers NVS, van Elsland SL, Lange JMa, et al. State of affairs of tuberculosis in prison facilities: a systematic review of screening practices and recommendations for best TB control. PLoS ONE. 2013;8:e53644. doi: 10.1371/journal.pone.0053644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pelletier AR, Diferdinando GT, Greenberg AJ, et al. Tuberculosis in a correctional facility. Arch Intern Med. 1993;153:2692–5. [PubMed] [Google Scholar]
- 32.Cooper-Arnold K, Morse T, Hodgson M, et al. Occupational tuberculosis among deputy sheriffs in Connecticut: a risk model of transmission. Appl Occup Environ Hyg. 1999;14:768–76. doi: 10.1080/104732299302198. [DOI] [PubMed] [Google Scholar]
- 33.Basu S, Stuckler D, Bitton A, et al. Projected effects of tobacco smoking on worldwide tuberculosis control: mathematical modelling analysis. BMJ. 2011;343:1–11. doi: 10.1136/bmj.d5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bates MN, Kalakdina A, Pai M, et al. Risk of tuberculosis from exposure to tobacco smoke. Arch Intern Med. 2007;167:335–42. doi: 10.1001/archinte.167.4.335. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Shen H. Review of cigarette smoking and tuberculosis in China: intervention is needed for smoking cessation among tuberculosis patients. BMC Public Health. 2009;9:292. doi: 10.1186/1471-2458-9-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sridhar M, Ross-Plummer R. The prevention of tuberculosis in prison staff. Occup Med. 2000;50:614–15. doi: 10.1093/occmed/50.8.614. [DOI] [PubMed] [Google Scholar]
- 37.World Health Organization (WHO) Who three I’s meeting: Intensified Case Finding (ICF), Isoniazid Preventive Therapy (IPT) and TB Infection Control (IC) for people living with HIV. Geneva: 2008. [Google Scholar]
- 38.Smieja M, Marchetti C, Cook D, et al. Isoniazid for preventing tuberculosis in non-HIV infected persons (Review) Cochrane Database Syst Rev. 1999;(1):CD001363. doi: 10.1002/14651858.CD001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drobniewski F, Balabanova Y, Zakamova E, et al. Rates of latent tuberculosis in health care staff in Russia. PLoS Med. 2007;4:e55. doi: 10.1371/journal.pmed.0040055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Churchyard GJ, Fielding KL, Lewis JJ, et al. A trial of mass isoniazid preventive therapy for tuberculosis control. N Engl J Med. 2014;370:301–10. doi: 10.1056/NEJMoa1214289. [DOI] [PubMed] [Google Scholar]
- 41.Al-Darraji HAA, Kamarulzaman A, Altice FL. Isoniazid preventive therapy in correctional facilities: a systematic review. Int J Tuberc Lung Dis. 2012;16:871–9. doi: 10.5588/ijtld.11.0447. [DOI] [PubMed] [Google Scholar]
- 42.Farhat M, Greenaway C, Pai M, et al. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? Int J Tuberc Lung Dis. 2006;10:1192–204. [PubMed] [Google Scholar]
- 43.Pai M, Banaei N. Occupational screening of health care workers for tuberculosis infection: tuberculin skin testing or interferon-γ release assays? Occup Med (Lond) 2013;63:458–60. doi: 10.1093/occmed/kqt105. [DOI] [PubMed] [Google Scholar]
- 44.Centre for Disease Control and Prevention (CDC), American Thoracic Society (ATS) Targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med. 2000;161:S221–47. doi: 10.1164/ajrccm.161.supplement_3.ats600. [DOI] [PubMed] [Google Scholar]
- 45.Campbell R, Sneller V-P, Khoury N, et al. Probable transmission of multidrug-resistant tuberculosis in a correctional facility—California. MMWR. 1993;42:48–50. [PubMed] [Google Scholar]