Abstract
We assessed the impact of lymphoedema (defined as >10% limb volume change) on quality of life (QOL), ability to perform activities of daily living (ADLs), and coping in 277 melanoma patients. Limb volume was measured prospectively, preoperatively, and every 3–6 months for 18 months postoperatively using a perometer. Three questionnaires were administered to measure quality of life, coping and impact on activities of daily living. Statistical analyses were conducted using longitudinal logistic regression models. At 18 months, 31% of patients with upper-extremity nodal basin treatment and 40% of lower extremity nodal basin treatment patients had lymphoedema. Patients with lower-extremity lymphoedema reported lower QOL scores than those with upper-extremity lymphoedema. Over 18 months, both groups with mild and moderate lymphoedema showed improvement in coping (odds ratio [OR]: 7.2, 95% confidence interval [CI]: 3.5–14.5) and performance of ADLs (OR: 6.8, CI: 3.2–14.3). Over the course of 18 months, males were found to have poorer coping scores than females (OR: 2.4, CI: 1.2–5.1). Lymphoedema was associated with improvement in coping over time (p=0.08) and a higher reported interference with ADLs (OR: 2.5, CI: 1.3–5.0), primarily household tasks and sleep. Patient education about lymphoedema at the time of surgical consent may improve self-efficacy and coping ability. Effective management of lymphoedema may improve patient QOL and reduce interference with ADLs.
Keywords: melanoma, quality of life, patient outcome assessment, lymphatic disorders, coping skills, self-efficacy
1. Introduction
The worldwide incidence of melanoma was estimated to be 232,130 in 2012 (GLOBOCAN, 2012). Fortunately, more than 90% of melanomas are diagnosed when they are surgically resectable (Burnet et al., 2005). Five-year overall survival for patients with surgically treatable melanoma are favourable at early stages, with significant variation based on stage at diagnosis: 97% with stage I, 53% with stage II, and 39% with stage III (Balch et al., 2009). Considering the large number of melanoma survivors, examining the long-term morbidity associated with melanoma treatment is important (DeSantis et al., 2014).
Lymphoedema continues to be one of the most common patient-reported physical issues in melanoma survivors (Beckjord et al., 2013). Surgical treatment of melanoma typically includes a wide local excision of the primary tumour and sentinel lymph node biopsy (SLNB) for the pathologic assessment of regional nodal basins,(NCCN, 2010) with or without subsequent therapeutic lymph node dissection (TLND). Both TLND and SLNB put patients at risk for lymphoedema (Hyngstrom et al., 2013). A systematic review of studies that reported the incidence of lymphoedema following lymph node surgery for melanoma found that this incidence ranged from 1% to 39% following axillary lymph node surgery and from 6% to 66% following pelvic and/or inguinal lymph node surgery; due to variability in the criterion for diagnosis of lymphoedema and lack of prospective data a specific prevalence estimate is unknown (Cormier et al., 2010).
A number of psychological outcomes are known to impact compliance with respect to self-care for lymphoedema prevention and/or management: coping style, perceived social support, and anxiety (Cohen et al., 2001). Among breast cancer survivors, self-efficacy—a construct of social cognitive theory that represents a person’s belief in their ability to influence and change perceived situations (Bandura, 1997)—is linked to compliance with lymphoedema risk reduction strategies (Sherman and Koelmeyer, 2012). Similarly, in melanoma patients, increased self-efficacy has been directly tied to increased compliance with recommended sun-protection and skin self-examination behaviours (Manne et al., 2004, Mujumdar et al., 2009). Thus, teaching patients to perform lymphoedema self-care and self-maintenance through lymphoedema risk reduction strategies could be best accomplished by improving self-efficacy, despite the negative psychological effects of treatment.
To maximize self-efficacy, patients need to return to as normal a life as possible after definitive surgical treatment for melanoma. It has been found that the appearance of surgical scars is often bothersome to patients, and increased swelling of the treated limb may reduce confidence in one’s appearance and comfort level in social settings (Cassileth et al., 1983, Fu et al., 2013). Furthermore, lymphoedema has been widely shown to negatively impact self-efficacy and coping abilities in cancer survivors (Tobin et al., 1993, Pyszel et al., 2006), in particular, the ability of breast cancer survivors to complete activities of daily living (ADLs) and self-care (Dunberger et al., 2013, Ridner et al., 2012). To date, the effects of lymphoedema on self-efficacy in patients who have been treated for melanoma has not been evaluated. Self-efficacy in the oncology setting is influenced by patient age, performance status, and sex; however, a gap in the knowledge related to self-efficacy and management of lymphoedema among melanoma patients still exists (Mystakidou et al., 2010). The purpose of the current study was to evaluate lymphoedema coping efficacy, the ability to adapt and adjust to the diagnosis of lymphoedema, the impact of lymphoedema on daily activities, and the overall quality of life (QOL) of melanoma patients before and after lymph node surgery.
2. Methods
2.1. Patient cohort
Patients were approached for participation in this prospective, longitudinal study prior to definitive surgical intervention that included SLNB and/or TLND at the ilio-inguinal node basin or the axillary node basin for pathologically confirmed stage I to III cutaneous melanoma (Balch et al., 2009). We excluded patients who had distant metastases (stage IV), were younger than 18 years, had previously undergone lymph node surgery in the affected nodal basin, had previously been diagnosed with lymphoedema, had implanted medical devices (i.e., a replacement joint or pacemaker), were currently undergoing treatment for another malignancy, or did not speak English. All patients underwent surgical treatment for their melanoma at The University of Texas MD Anderson Cancer Center between 2006 and 2012 and agreed to return for regular follow-up visits after treatment at 3–6 month intervals for a total of 4 potential visits. Baseline demographic and clinical data were collected using patients’ medical records and limb volume, coping efficacy, impact of lymphoedema on ADLs, and QOL were collected at the time of consent and at each follow-up appointment. All patients enrolled in the study underwent SLNB and/or TLND within 30 days of the baseline visit. All assessments were administered again at each follow-up visit. The study protocol was approved by the MD Anderson Cancer Center Institutional Review Board, and enrolled patients provided written informed consent to participate in the study.
2.2. Limb volume assessment
Limb volume was measured in the Melanoma and Skin Center at MD Anderson in a climate-controlled room with a standard air temperature of 22° Celsius using an upright perometer (Perometer Type 1000 M, Per-System, Wuppertal, Germany) which is an optoelectronic volumetry device that uses infrared light to calculate limb volume (Tierney et al., 1996). All patients underwent limb volume measurement at baseline and at least one of a total of four subsequent measurements at 3- to 6-month intervals for the first 18 months following surgery. The affected limb and the contralateral unaffected limb were measured at each time point to allow for comparisons accounting for changes in body weight over time. Patients were asked to refrain from excessive alcohol consumption or strenuous exercise within 24 hours prior to the measurement appointments. For upper-extremity measurements, patients were positioned with the arm to be measured at a 45° laterally from the long axis of the body while holding a fixed hand block; arm volume was measured from the styloid process of the ulna to the limiting point of the axilla. Lower-extremity measurements were taken with the leg placed on a fixed marker on the base of the perometer and the patient standing upright; the perometer frame was raised up the limb from the lateral malleolus to the highest point possible within the perineum. Patients who reported symptoms of lymphoedema and showed a limb volume change (LVC) of at least 10% were referred for evaluation by a lymphoedema therapist.
LVC was calculated as a percentage change from baseline, taking the affected and contralateral limbs into account, using the following formula (Ancukiewicz et al., 2011):
A indicates the affected limb, and C indicates the contralateral unaffected limb. For the purposes of this analysis, patients who had lymph node surgery in bilateral nodal basins were excluded. Patients who missed more than 2 of the 4 measurement time periods were also excluded from this analysis. Patients were stratified into three groups with LVCs of 5% or less (no lymphoedema), 5% to less than 10% (at risk for lymphoedema), and 10% or more (lymphoedema) (Cormier et al., 2009, Hyngstrom et al., 2013).
2.3. Coping efficacy instrument
Coping Efficacy with Lymphoedema is a 13-item instrument that is scored on a Likert scale of 1 to 6, with 1 indicating “strongly agree” and 6 indicating “strongly disagree,” and a maximum total score of 78 (Heppner et al., 2001). This instrument is designed to assess self-reported problem-solving strategies and problem-solving efficacy in patients with lymphoedema. The instrument has been validated in the breast cancer–related lymphoedema population.
For modelling purposes, coping was categorized as a dichotomous variable, with outcomes defined as either above or below the overall median for all patients in the cohort with measurements at that time point. These groups were classified as “coping well” or “coping poorly,” respectively, with respect to lymphoedema for the purposes of this study.
2.4. Assessment of impact on ADLs
Effects of Lymphoedema on Activities of Daily Living is a 12-item tool with a maximum total score of 120 that captures the impact of lymphoedema on personal factors such as mood, body image, and relationships and on functional factors including ability to work, sleep, complete housework, drive, and perform self-care tasks. Each item is scored on a scale from 0 (“does not interfere”) to 10 (“completely interferes”). This instrument was developed on the basis of qualitative assessments of patients with secondary lymphoedema due to breast cancer treatment (Armer et al., 2008). To our knowledge, this is the first time this tool has been used in a melanoma population.
2.5. QOL assessment
QOL was assessed using the Functional Assessment of Cancer Therapy–Melanoma (FACT-M).(FACIT, 2010) The FACT-M is a 50-item instrument with a maximum total score of 172 that was validated for use in patients with all stages of melanoma (Askew et al., 2011, Askew et al., 2009, Swartz et al., 2012). The FACT-M was found to have strong internal consistency (Cronbach α=0.85) and test-retest reliability (r=0.81). The melanoma subsection of the instrument includes surgery-specific questions and several items that address lymphoedema-related concerns, such as swelling and range of motion.
2.6. Statistical analysis
All statistical analyses were completed using longitudinal mixed model regression, controlling for body mass index (kg/m2), age, sex, and treatment factors. Coping efficacy and ADL interference scores were converted to binary outcomes using the median score as the cut-off point. Bivariate general linear mixed models were created to examine the association of patient factors (including body mass index, age, and sex), treatment factors (extremity and surgery type), and LVC group. All outcomes were evaluated over time using longitudinal logistic regression models. Missing outcomes were assumed to be missing at random. Longitudinal mixed model evaluation takes these missing and unbalanced results into account when building models. Reported p-values are two-sided, and significance was defined by a p-value of ≤0.05. All analyses were conducted using SAS version 9.1.3 statistical software (SAS Institute, Cary, North Carolina).
3. Results
3.1. Patient cohort
A total of 277 patients were enrolled. A total of 142 (51.3%) of the participants were female, the majority were white (n=266, 96.0%), 168 (61.3%) were of normal weight (body mass index ≤30), and 171 (61.7%) were at least 50 years old (range, 19–85 years) (Table 1). One hundred twenty-five (45.1%) patients underwent an SLNB as their most invasive lymph node surgery, and the other 152 (54.9%) underwent a TLND either independently or following an SLNB that had revealed node-positive disease. Twenty-four patients were treated with adjuvant radiation to the nodal basin following TLND, and five patients were treated with radiation to the primary tumour bed (recurrence or satellite disease) following wide local excision. A total of 244 patients had follow up data at 3 to 6 months, 197 patients at 9 to 12 months, and 126 patients at 15 to 18 months.
Table 1.
Characteristics of patients in cohort (n=277).
| Characteristic | Lower extremity | Upper extremity | Total | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Sex | ||||||
| Male | 48 | 37.5 | 87 | 58.4 | 135 | 48.7 |
| Female | 80 | 62.5 | 62 | 41.6 | 142 | 51.3 |
| Age | ||||||
| Under 50 years | 50 | 39.1 | 56 | 37.6 | 106 | 38.3 |
| 50 years or older | 78 | 60.9 | 93 | 62.4 | 171 | 61.7 |
| Race | ||||||
| White | 121 | 94.5 | 145 | 97.3 | 266 | 96.0 |
| Hispanic | 4 | 3.1 | 1 | 0.7 | 5 | 1.8 |
| African American | 3 | 2.3 | 2 | 1.3 | 5 | 1.8 |
| Other | 0 | 0.0 | 1 | 0.7 | 1 | 0.4 |
| Education | ||||||
| High school or less | 40 | 31.3 | 28 | 18.8 | 68 | 24.6 |
| Some college | 35 | 27.3 | 41 | 27.5 | 76 | 27.4 |
| College graduate | 35 | 27.3 | 54 | 36.2 | 89 | 32.1 |
| Graduate school or more | 18 | 14.1 | 21 | 14.1 | 39 | 14.1 |
| Unknown | 0 | 0.0 | 5 | 3.4 | 5 | 1.8 |
| Surgery type | ||||||
| SLNB | 52 | 40.6 | 73 | 49.0 | 125 | 45.1 |
| TLND | 76 | 59.4 | 76 | 51.0 | 152 | 54.9 |
| Body mass index | ||||||
| Under 30 | 75 | 58.6 | 93 | 63.7 | 168 | 61.3 |
| 30 or over | 53 | 41.4 | 53 | 36.3 | 106 | 38.7 |
| Surgery side | ||||||
| Right | 65 | 50.8 | 64 | 43.0 | 129 | 46.6 |
| Left | 61 | 47.7 | 75 | 50.3 | 136 | 49.1 |
SLNB: sentinel lymph node biopsy, TLND: therapeutic lymph node dissection
3.2. Lymphoedema
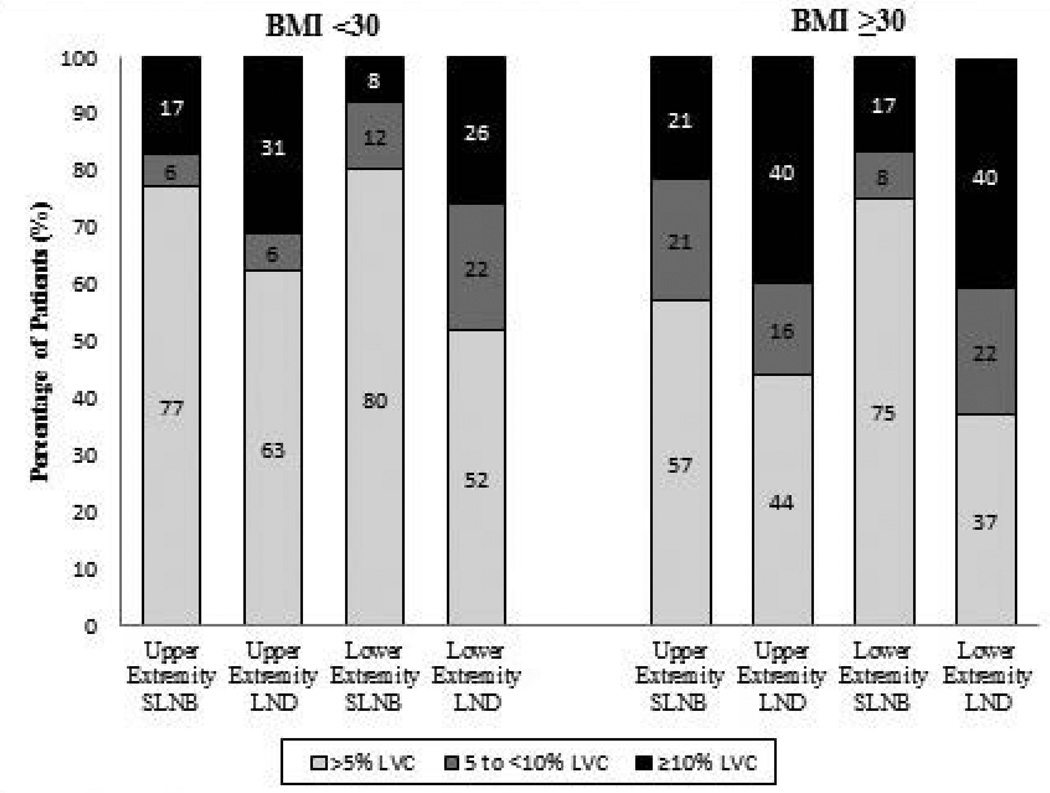
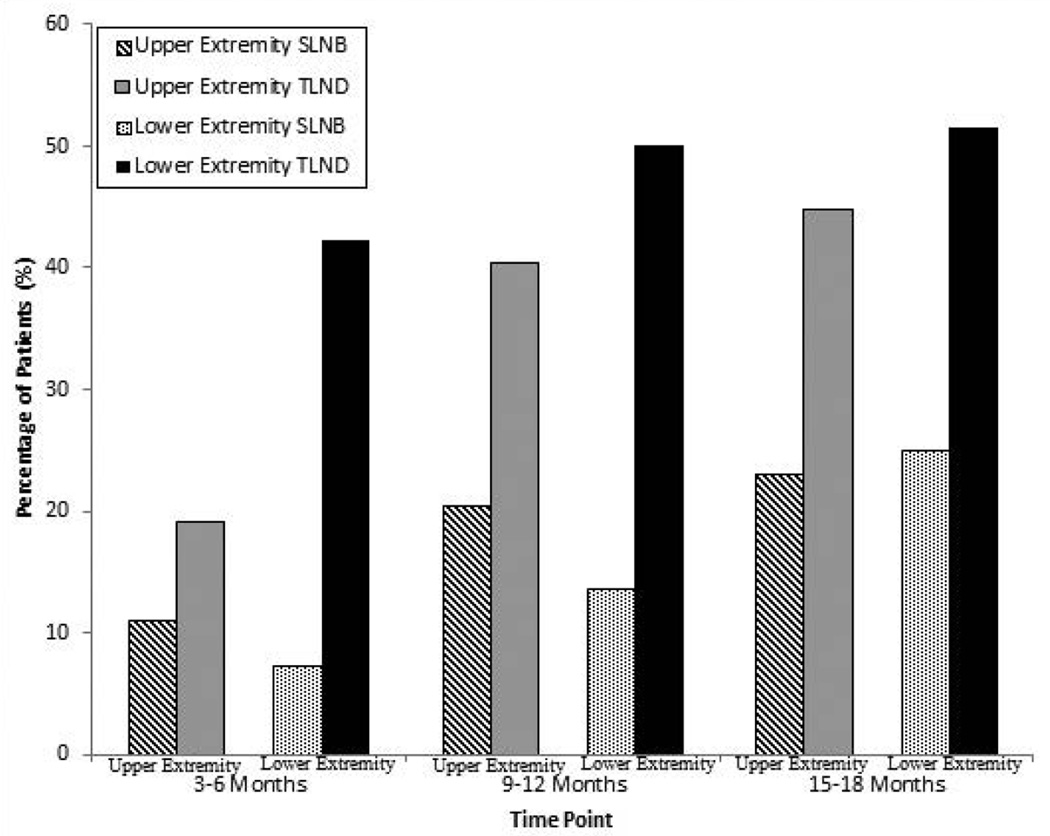
Limb volume data were available for 245 patients at baseline, 197 patients at 3–6 months, and 126 patients at 15–18 months (Table 2). At 15–18 months, an LVC greater than 10% was observed in 36.8% of patients who had upper-extremity TLND, 38.7% of those who had lower-extremity TLND, 10% of those who had upper-extremity SLNB, and 25.0% of those who had lower-extremity SLNB. The cumulative incidences of lymphoedema, which is the incidence of all patients who had LVC over 10% at any single time point, at 15–18 months stratified by type of surgery and extremity were 44.7% for upper-extremity TLND, 51.6% for lower-extremity TLND, 15.2% for upper-extremity SLNB, and 25.0% for lower-extremity SLNB (Fig. 1). When stratified by body mass index, 35.4% of patients with a body mass index of at least 30 had lymphoedema whereas only 18.7% of patients with a BMI less than 30 had lymphoedema (Fig. 2). The mixed models showed that the factors most strongly associated with lymphoedema were a body mass index of ≥30 (odds ratio [OR]: 2.19, 95% confidence interval [CI]: 1.37–3.52) and TLND (OR: 1.65, 95% CI: 1.65–4.41). Sex, age, and extremity where node surgery was done (upper vs. lower) were not found to be associated with lymphoedema.
Table 2.
Limb volume change over time stratified by extremity, surgery type, and body mass index
| LVC at 3–6 months (n=244) | LVC at 9–12 months (n=197) | LVC at 15–18 months (n=126) < | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <5% (n=153) |
5–10% (n=38) | >10% (n=53) | <5% (n=119) | 5–10% (n=27) |
>10% (n=51) | <5% (n=73) | 5–10% (n=17) | >10% (n=36) | |||||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | ||
| Upper Extremity | 89 | 67.4 | 23 | 17.4 | 20 | 15.2 | 66 | 62.3 | 11 | 10.4 | 29 | 27.4 | 45 | 63.4 | 8 | 11.3 | 18 | 25.4 | |
| SLNB | 45 | 70.3 | 12 | 18.8 | 7 | 10.9 | 35 | 71.4 | 5 | 10.2 | 9 | 18.4 | 26 | 78.8 | 3 | 9.1 | 4 | 12.1 | |
| TLND | 44 | 64.7 | 11 | 16.2 | 13 | 19.1 | 31 | 54.4 | 6 | 10.5 | 20 | 35.1 | 19 | 50.0 | 5 | 13.2 | 14 | 36.8 | |
| Lower Extremity | 64 | 57.1 | 15 | 13.4 | 33 | 29.5 | 53 | 58.2 | 16 | 17.6 | 22 | 24.2 | 28 | 50.9 | 9 | 16.4 | 18 | 32.7 | |
| SLNB | 33 | 80.5 | 5 | 12.2 | 3 | 7.3 | 29 | 78.4 | 4 | 10.8 | 4 | 10.8 | 18 | 75.0 | 0 | 0.0 | 6 | 25.0 | |
| TLND | 31 | 43.7 | 10 | 14.1 | 30 | 42.3 | 24 | 44.4 | 12 | 22.2 | 18 | 47.4 | 10 | 32.3 | 9 | 29.0 | 12 | 38.7 | |
| Body mass index | |||||||||||||||||||
| < 30 | 103 | 69.1 | 25 | 16.8 | 21 | 14.1 | 81 | 68.1 | 13 | 10.9 | 25 | 21.0 | 49 | 62.8 | 10 | 12.8 | 19 | 24.4 | |
| ≥ 30 | 50 | 52.6 | 13 | 13.7 | 32 | 33.7 | 38 | 48.7 | 14 | 18.0 | 26 | 33.3 | 24 | 50.0 | 7 | 14.6 | 17 | 35.4 | |
| Total | 153 | 62.7 | 38 | 15.6 | 53 | 21.7 | 119 | 60.4 | 27 | 13.7 | 51 | 25.9 | 73 | 57.9 | 17 | 13.5 | 36 | 28.6 | |
LVC: limb volume change; SLNB: sentinel lymph node biopsy; TLND: therapeutic lymph node dissection
Figure 1.
Figure 2.
3.3. Coping efficacy
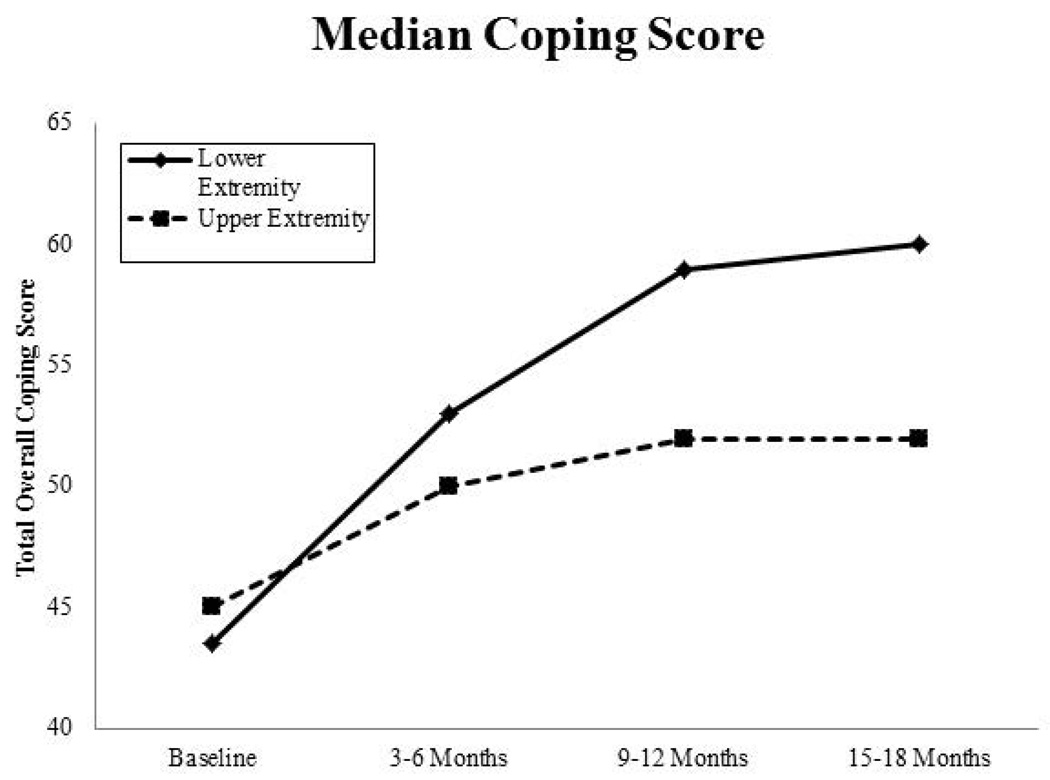
In general, scores for coping efficacy improved minimally over time. Patients who had lower-extremity surgery reported a greater improvement in coping scores from baseline to 15–18 months than patients who had upper-extremity surgery (Fig. 3). At 9–12 months, those at risk for developing lymphoedema (LVC of 5% to <10%) reported a higher median coping efficacy score than those with a LVC of <5% or those with lymphoedema; however, none of these differences were statistically significant at any time point. Female sex was associated with coping well (above the median score) (OR: 2.91, 95% CI: 1.35–6.27). Time since baseline was also associated with coping well (OR: 8.36, 95% CI: 4.01–17.42) and coping scores at 15–18 months were more likely to be above the median than scores at baseline (OR: 6.67, 95% CI: 3.3–3.47). Other covariates that were included, but not found to be significant in the model, included extremity, surgery type, and QOL scores.
Figure 3.
3.4. Impact of lymphoedema on ADLs
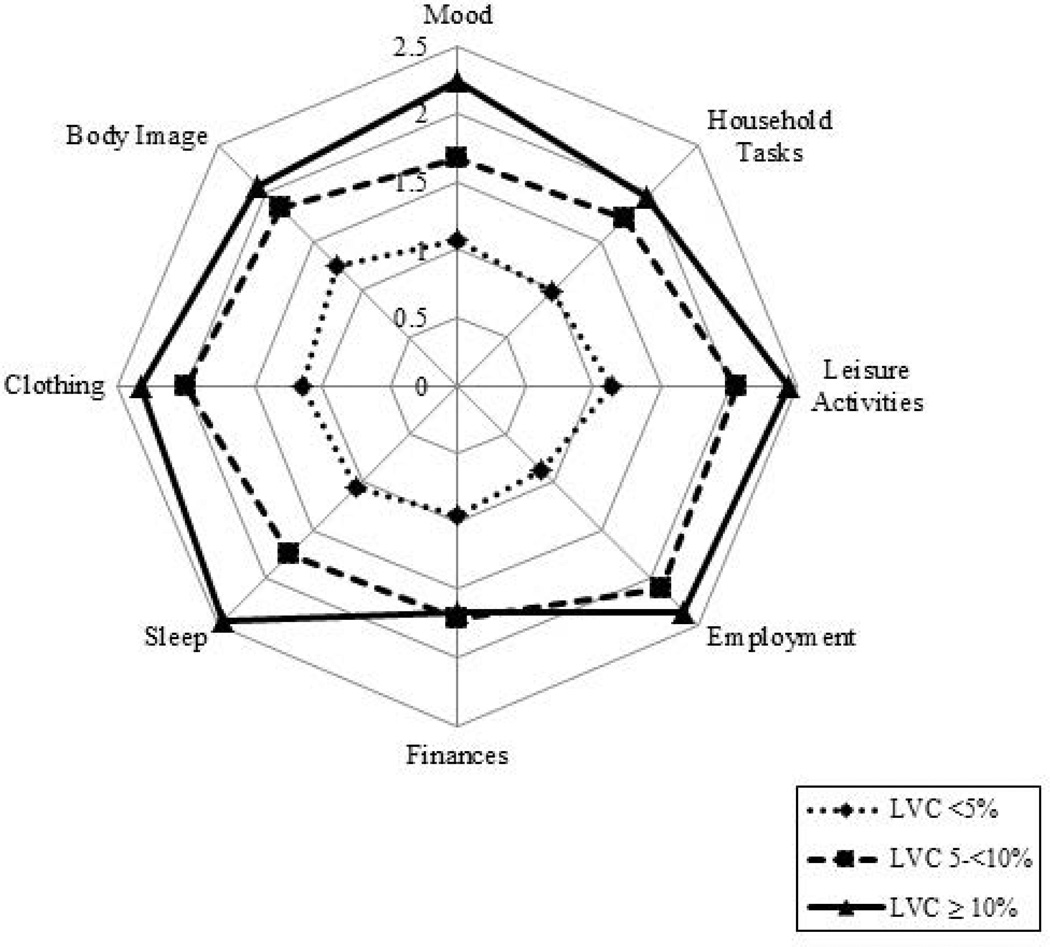
After 9–12 months, those at risk for lymphoedema reported a higher median score for impact of lymphoedema on ADLs than those in other LVC groups, but all scores remained very low. Significant variability was noted for each of the 12 items of this instrument, with the range for each item encompassing the full scale, from 0 (no interference) to 10 (complete interference). An evaluation of means showed that those with lymphoedema reported limited but more interference compared to those at risk or without lymphoedema in most items, with the most variation in sleep and choice of clothing (Fig. 4).
Figure 4.
Since these scores varied so widely, a model was structured to define the outcomes as either above or at 0 for any item. Interference with ADLs to any LE outcome was significantly associated with lower-extremity lymph node surgery (vs. upper–extremity surgery) (OR: 2.32, 95% CI: 1.23–4.38), TLND (vs. SLNB) (OR: 3.99, 95% CI: 1.23–4.38), and lymphoedema (vs. no lymphoedema) (OR: 2.53, 95% CI: 1.29–4.97). ADL scores using the binary model indicated an increase in interference over time using a longitudinal model compared with the baseline (OR: 9.28, 95% CI: 4.24–20.26). Sex was not associated with ADL interference and was controlled for in the model.
3.5 QOL
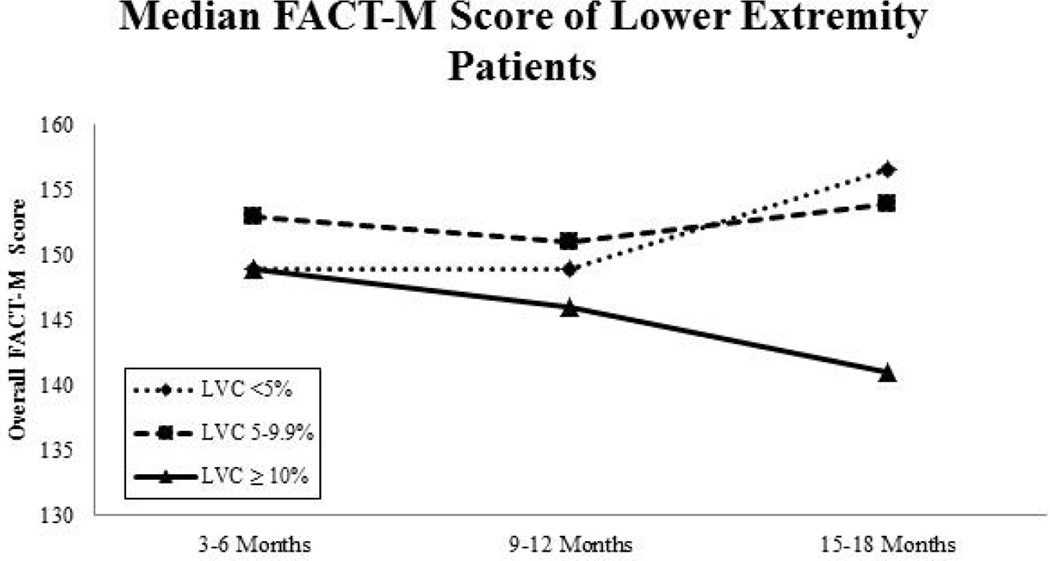
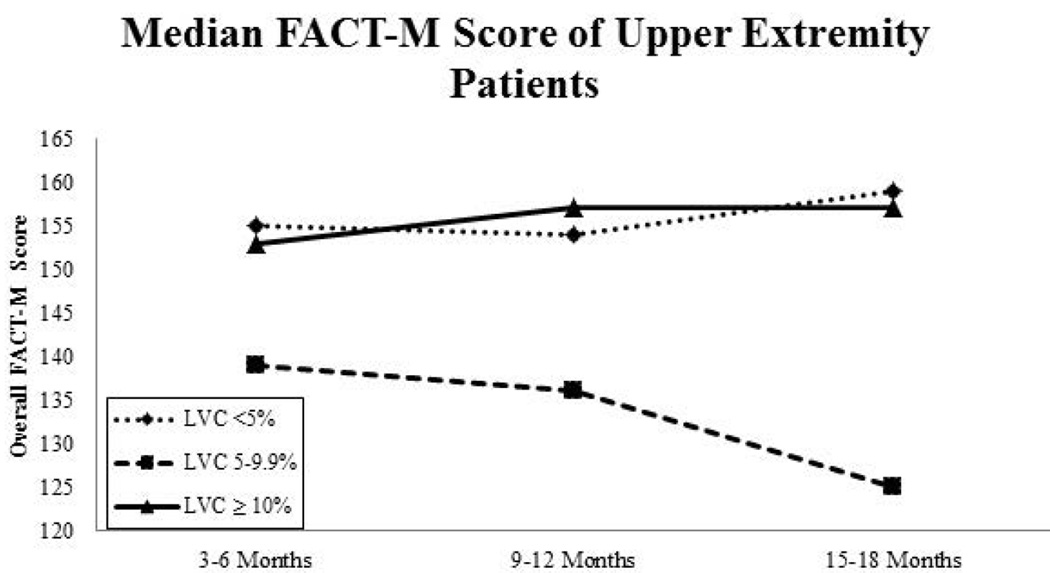
QOL (FACT-M) scores were fairly stable throughout the duration of the study, but baseline scores and later scores did differ significantly in some patient subsets stratified by extremity and LVC. Lower-extremity patients with LVC <10% showed improvements in median FACT-M scores over time, whereas the FACT-M scores of lower-extremity patients with lymphoedema (LVC >10%) declined from baseline (Fig. 5A). Interestingly, in upper-extremity patients, median FACT-M scores declined over time for those considered at risk for lymphoedema but increased slightly for those with an LVC ≥10% (Fig. 5B). In the mixed model, patients who had lower-extremity surgery (vs. upper-extremity) (OR: 2.43, 95% CI: 1.34–4.35, p<0.01) or TLND (vs. SLNB) (OR: 3.11, 95% CI: 1.63–5.93, p<0.01) had lower FACT-M scores. In general, FACT-M scores improved over time, but remained relatively stable (minimal changes over time) with patients reporting significantly higher scores than at baseline (OR: 6.75, 95% CI: 3.19–14.29, p<0.01). Sex and lymphoedema were controlled for, but not found to be a significant predictor of overall FACT-M scores.
Figure 5.
4. Discussion
Lymphoedema developed in a substantial proportion of patients with melanoma who underwent definitive surgical treatment with lymph node surgery, including those who received SLNB alone. The incidences of lymphoedema (LVC ≥10%) following SLNB were 25% after lower-extremity surgery and 12% after upper-extremity surgery. These changes in limb volume were accompanied by negative effects on ADLs and coping efficacy. For instance, patients who developed minimal swelling (LVC of 5% to <10%) had increased interference with ADLs. This result confirms our previous report that a minimal LVC can have a profound and lasting impact on patients (Cormier et al., 2009). In addition, patients with lymphoedema experienced more interference with ADLs than those without lymphoedema (OR: 2.53, 95% CI: 1.29–4.97). Similarly, previous studies in gynaecologic cancer survivors have reported that patients with lower-limb lymphoedema are more likely to take extended sick leave or file for disability than patients who do not develop lymphoedema in the post-treatment period (Dunberger et al., 2013). This post-treatment morbidity can increase the burden of cancer and can decrease the social well-being, functional well-being, and financial security of a patient and his or her family (Mrazek and Chao, 2014).
Furthermore, coping efficacy improved slightly over time for all patients regardless of lymphoedema status. This is in contrast to a recent qualitative study of 19 Stage III melanoma survivors who were between 6 months and 3 years post-treatment and their caregivers which found patients and caregivers feel that they did not receive appropriate information related to the risk for lymphoedema following surgical treatment (Tan et al., 2014). In the study by Tan and colleagues, at least one patient specifically stated he had no understanding of lymphoedema or how to apply compression. The results of this study mirror our findings in that all patients over time reported that they were able to better cope with their melanoma and associated long-term side effects of treatment, including lymphoedema. Therefore, education about lymphoedema, the early identification of lymphoedema signs and symptoms, and lymphoedema risk reduction practices (National Lymphedema Network, 2014) should be a component of pre-operative education in patients undergoing lymph node surgery.
Despite its strengths, this study had a few limitations. The first is the limited number of patients within each group at later time points, which makes subgroup analysis difficult. Also, only a small number of patients in this cohort were treated with radiation therapy (24 had radiation to the nodal basin, and five had radiation to the primary tumour site), which limited any analysis of radiation therapy as a risk factor. Radiation therapy has been shown to predict lymphoedema in breast and cervical cancer patients (Kim et al., 2012, Shah et al., 2012). Finally, missing data due to failure to follow-up or obtain measurements is a limitation within this prospectively collected data.
Patient education about the risks of developing lymphoedema and the early signs and symptoms of lymphoedema is increasingly being recognized as a critical component of medical care for patients undergoing lymph node surgery and may help increase self-efficacy. It is important to ensure that the increasing number of melanoma survivors feel capable of managing post-surgical morbidities such as lymphoedema. Self-efficacy has been shown to significantly predict compliance with management strategies for chronic illnesses, such as diabetes,(Basak Cinar and Schou, 2014) chronic obstructive pulmonary disease, (Jackson et al., 2014) and hepatitis C (Bonner et al., 2013). Similarly, in this study patients who develop lymphoedema report better coping over time which would indicate increased self-efficacy and ability to cope with any prescribed lymphoedema treatment. However, due to the large number of patients who travel for treatment at the institution, compliance was not studied in this cohort. In a study of people with filarial lymphoedema in India, an intervention was designed to improve self-efficacy through education about skin care and simple exercises that can be done to control the swelling; not only self-efficacy related to lymphoedema care, but also overall QOL, improved after the intervention was delivered (Narahari et al., 2013). Although the oncologic setting is very different from that of filarial lymphoedema, it is reasonable to believe that proper education may help improve QOL and self-efficacy, particularly when managing lymphoedema in the post-operative period.
Our results demonstrate that lymphoedema is a significant risk after either SLNB or TLND and confirm that lymphoedema can be accompanied by decreased self-efficacy and increased interference with ADLs. To our knowledge, this is the first study to prospectively measure lymphoedema after lymph node surgery and its impact on these psychological outcomes in patients with melanoma. Future research should be designed to improve education about lymphoedema and the identification and awareness of lymphoedema in high-risk groups.
Lymphoedema impacts quality of life
Lower extremity lymphoedema patients cope less effectively with lymphoedema but improve over time
Household chores and sleep are most impacted by lymphoedema
Acknowledgments
This research was supported in part by the National Institutes of Health through The University of Texas MD Anderson Cancer Center’s Cancer Center Support Grant, CA016672.
Footnotes
Conflict of interest statement: The authors have no conflicts of interest to disclose.
References
- Ancukiewicz M, Russell TA, Otoole J, Specht M, Singer M, Kelada A, Murphy CD, Pogachar J, Gioioso V, Patel M, Skolny M, Smith BL, Taghian AG. Standardized method for quantification of developing lymphedema in patients treated for breast cancer. Int J Radiat Oncol Biol Phys. 2011;79:1436–1443. doi: 10.1016/j.ijrobp.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armer JM, Henggeler MH, Brooks CW, Zagar EA, Homan S, Stewart BR. The Health Deviation of Post-Breast Cancer Lymphedema: Symptom Assessment and Impact on Self-Care Agency. Self Care Depend Care Nurs. 2008;16:14–21. [PMC free article] [PubMed] [Google Scholar]
- Askew RL, Swartz RJ, Xing Y, Cantor SB, Ross MI, Gershenwald JE, Palmer JL, Lee JE, Cormier JN. Mapping FACT-melanoma quality-of-life scores to EQ-5D health utility weights. Value Health. 2011;14:900–906. doi: 10.1016/j.jval.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Askew RL, Xing Y, Palmer JL, Cella D, Moye LA, Cormier JN. Evaluating minimal important differences for the FACT-Melanoma quality of life questionnaire. Value Health. 2009;12:1144–1150. doi: 10.1111/j.1524-4733.2009.00570.x. [DOI] [PubMed] [Google Scholar]
- Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S, Eggermont AM, Flaherty KT, Gimotty PA, Kirkwood JM, Mcmasters KM, Mihm MC, Jr, Morton DL, Ross MI, Sober AJ, Sondak VK. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandura A. Self-efficacy: The exercise of control. Macmillan; 1997. [Google Scholar]
- Basak cinar A, Schou L. The role of self-efficacy in health coaching and health education for patients with type 2 diabetes. Int Dent J. 2014 doi: 10.1111/idj.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckjord EB, Reynolds KA, Van londen G, Burns R, Singh R, Arvey SR, Nutt SA, Rechis R. Population-Level Trends in Post-Treatment Cancer Survivors’ Concerns and Associated Receipt of Care: Results from the 2006 and 2010 LIVESTRONG Surveys. Journal of psychosocial oncology. 2013:125–151. doi: 10.1080/07347332.2013.874004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner JE, Esserman DA, Golin CE, Evon DM. Self-Efficacy and Adherence to Antiviral Treatment for Chronic Hepatitis C. J Clin Gastroenterol. 2013 doi: 10.1097/MCG.0000000000000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnet NG, Jefferies SJ, Benson RJ, Hunt DP, Treasure FP. Years of life lost (YLL) from cancer is an important measure of population burden--and should be considered when allocating research funds. Br J Cancer. 2005;92:241–245. doi: 10.1038/sj.bjc.6602321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassileth BR, Lusk EJ, Tenaglia AN. Patients' perceptions of the cosmetic impact of melanoma resection. Plast Reconstr Surg. 1983;71:73–75. doi: 10.1097/00006534-198301000-00016. [DOI] [PubMed] [Google Scholar]
- Cohen SR, Payne DK, Tunkel RS. Lymphedema: strategies for management. Cancer. 2001;92:980–987. doi: 10.1002/1097-0142(20010815)92:4+<980::aid-cncr1410>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Cormier JN, Askew RL, Mungovan KS, Xing Y, Ross MI, Armer JM. Lymphedema beyond breast cancer: a systematic review and meta-analysis of cancer-related secondary lymphedema. Cancer. 2010;116:5138–5149. doi: 10.1002/cncr.25458. [DOI] [PubMed] [Google Scholar]
- Cormier JN, Xing Y, Zaniletti I, Askew RL, Stewart BR, Armer JM. Minimal limb volume change has a significant impact on breast cancer survivors. Lymphology. 2009;42:161–175. [PMC free article] [PubMed] [Google Scholar]
- Desantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- Dunberger G, Lindquist H, Waldenstrom AC, Nyberg T, Steineck G, Avall-lundqvist E. Lower limb lymphedema in gynecological cancer survivors--effect on daily life functioning. Support Care Cancer. 2013;21:3063–3070. doi: 10.1007/s00520-013-1879-3. [DOI] [PubMed] [Google Scholar]
- Facit. [Accessed 6 June 2014];Functional Assesment of Chronic Illness Therapy. 2010 [Online]. Available: http://www.facit.org/FACITOrg. [Google Scholar]
- Fu MR, Ridner SH, Hu SH, Stewart BR, Cormier JN, Armer JM. Psychosocial impact of lymphedema: a systematic review of literature from 2004 to 2011. Psychooncology. 2013;22:1466–1484. doi: 10.1002/pon.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Globocan. [Accessed 13 March 2014];GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. 2012 [Online]. Available: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx. [Google Scholar]
- Heppner PP, Cooper C, Mulholland A, Wei M. A brief, multidimensional, problem-solving psychotherapy outcome measure. Journal of Counseling Psychology. 2001;48:330. [Google Scholar]
- Hyngstrom JR, Chiang YJ, Cromwell KD, Ross MI, Xing Y, Mungovan KS, Lee JE, Gershenwald JE, Royal RE, Lucci A, Armer JM, Cormier JN. Prospective assessment of lymphedema incidence and lymphedema-associated symptoms following lymph node surgery for melanoma. Melanoma Res. 2013;23:290–297. doi: 10.1097/CMR.0b013e3283632c83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson BE, Coultas DB, Ashmore J, Russo R, Peoples J, Uhm M, Singh KP, Bae S. Domain-Specific Self-Efficacy Is Associated with Measures of Functional Capacity and Quality of Life among Moderate to Severe COPD Patients. Ann Am Thorac Soc. 2014 doi: 10.1513/AnnalsATS.201308-273BC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Choi JH, Ki EY, Lee SJ, Yoon JH, Lee KH, Park TC, Park JS, Bae SN, Hur SY. Incidence and risk factors of lower-extremity lymphedema after radical surgery with or without adjuvant radiotherapy in patients with FIGO stage I to stage IIA cervical cancer. Int J Gynecol Cancer. 2012;22:686–691. doi: 10.1097/IGC.0b013e3182466950. [DOI] [PubMed] [Google Scholar]
- Manne S, Fasanella N, Connors J, Floyd B, Wang H, Lessin S. Sun protection and skin surveillance practices among relatives of patients with malignant melanoma: prevalence and predictors. Prev Med. 2004;39:36–47. doi: 10.1016/j.ypmed.2004.02.028. [DOI] [PubMed] [Google Scholar]
- Mrazek AA, Chao C. Surviving cutaneous melanoma: a clinical review of follow-up practices, surveillance, and management of recurrence. Surg Clin North Am. 2014;94:989–1002. vii–viii. doi: 10.1016/j.suc.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujumdar UJ, Hay JL, Monroe-hinds YC, Hummer AJ, Begg CB, Wilcox HB, Oliveria SA, Berwick M. Sun protection and skin self-examination in melanoma survivors. Psychooncology. 2009;18:1106–1115. doi: 10.1002/pon.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mystakidou K, Tsilika E, Parpa E, Gogou P, Theodorakis P, Vlahos L. Self-efficacy beliefs and levels of anxiety in advanced cancer patients. Eur J Cancer Care (Engl) 2010;19:205–211. doi: 10.1111/j.1365-2354.2008.01039.x. [DOI] [PubMed] [Google Scholar]
- Narahari SR, Bose KS, Aggithaya MG, Swamy GK, Ryan TJ, Unnikrishnan B, Washington RG, Rao BP, Rajagopala S, Manjula K, Vandana U, Sreemol TA, Rojith M, Salimani SY, Shefuvan M. Community level morbidity control of lymphoedema using self care and integrative treatment in two lymphatic filariasis endemic districts of South India: a non randomized interventional study. Trans R Soc Trop Med Hyg. 2013;107:566–577. doi: 10.1093/trstmh/trt054. [DOI] [PubMed] [Google Scholar]
- Nccn. [August 22, 2011];NCCN clinical practice guidelines in oncology: melanoma. 2010 [Online]. Available: http://www.nccn.org/professionals/physician_gls/PDF/melanoma.pdf. [Google Scholar]
- Pyszel A, Malyszczak K, Pyszel K, Andrzejak R, Szuba A. Disability, psychological distress and quality of life in breast cancer survivors with arm lymphedema. Lymphology. 2006;39:185–192. [PubMed] [Google Scholar]
- Ridner SH, Bonner CM, Deng J, Sinclair VG. Voices from the shadows: living with lymphedema. Cancer Nurs. 2012;35:E18–E26. doi: 10.1097/NCC.0b013e31821404c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah C, Arthur D, Riutta J, Whitworth P, Vicini FA. Breast-cancer related lymphedema: a review of procedure-specific incidence rates, clinical assessment AIDS, treatment paradigms, and risk reduction. Breast J. 2012;18:357–361. doi: 10.1111/j.1524-4741.2012.01252.x. [DOI] [PubMed] [Google Scholar]
- Sherman KA, Koelmeyer L. Psychosocial predictors of adherence to lymphedema risk minimization guidelines among women with breast cancer. Psychooncology. 2012 doi: 10.1002/pon.3111. [DOI] [PubMed] [Google Scholar]
- Swartz RJ, Baum GP, Askew RL, Palmer JL, Ross MI, Cormier JN. Reducing patient burden to the FACT-Melanoma quality-of-life questionnaire. Melanoma Res. 2012;22:158–163. doi: 10.1097/CMR.0b013e3283511dbf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JD, Butow PN, Boyle FM, Saw RP, O'reilly AJ. A qualitative assessment of psychosocial impact, coping and adjustment in high-risk melanoma patients and caregivers. Melanoma Res. 2014;24:252–260. doi: 10.1097/CMR.0000000000000059. [DOI] [PubMed] [Google Scholar]
- Tierney S, Aslam M, Rennie K, Grace P. Infrared optoelectronic volumetry, the ideal way to measure limb volume. Eur J Vasc Endovasc Surg. 1996;12:412–417. doi: 10.1016/s1078-5884(96)80005-0. [DOI] [PubMed] [Google Scholar]
- Tobin MB, Lacey HJ, Meyer L, Mortimer PS. The psychological morbidity of breast cancer-related arm swelling. Psychological morbidity of lymphoedema. Cancer. 1993;72:3248–3252. doi: 10.1002/1097-0142(19931201)72:11<3248::aid-cncr2820721119>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]