The envelope protein of an MS-associated retrovirus activates brain endothelium
Keywords: auto-immune disease, blood–brain barrier, leukocyte adhesion, retroviral envelope
Abstract
The MSRV (multiple sclerosis-associated retrovirus) belongs to the human endogenous retrovirus HERV-W family. The envelope protein originating from the MSRV has been found in most patients with multiple sclerosis (MS). This protein (Env-ms) has pro-inflammatory properties for several types of immune cells and could therefore play a role in MS pathogenesis by promoting the leukocyte diapedesis observed in the central nervous system of patients. Our study aims to analyze the effects of Env-ms on the blood–brain barrier (BBB) at a molecular and functional level. We demonstrate that the recombinant MSRV envelope is able to stimulate several inflammatory parameters in a human BBB in vitro model, the HCMEC/D3 brain endothelial cell line. Indeed, Env-ms induces over-expression of ICAM-1, a major mediator of leukocyte adhesion to endothelial cells, in a dose-dependent manner as well as a strong dose-dependent production of the pro-inflammatory cytokines IL-6 and IL-8. Furthermore, using a silencing approach with siRNAs, we show that Env-ms is recognized via the Toll-like receptor 4 receptor, a pattern recognition receptor of innate immunity present on endothelial cells. We also show, using functional assays, that treatment of brain endothelial cells with Env-ms significantly stimulated the adhesion and the transmigration of activated immune cells through a monolayer of endothelial cells. These findings support the hypothesis that MSRV could be involved in the pathogenesis of MS disease or at least in maintenance of inflammatory conditions, thus fueling the auto-immune disorder. MSRV could also play a role in other chronic inflammatory diseases.
Introduction
Multiple sclerosis-associated retrovirus (MSRV) is an enveloped retrovirus initially isolated from cell cultures from patients with multiple sclerosis (MS) (1). It is the first identified member of the human endogenous retrovirus W (HERV-W) family (2) which has infected the germline and then has been transmitted to the offspring of their hosts in the course of primate evolution (3). Most of the integrated HERV-W display deletions or mutations in their open reading frames encoding functional proteins (4), thus preventing a complete expression. However, genetic elements of endogenous HERV are involved in genetic rearrangements (5) that can contribute to their genetic diversification and their occasional expression (3) which was reported in several diseases [see, for review, (6)]. To date, MSRV is the unique example of HERV-W expressed as viral particles. Pathological consequences of the exposure to MSRV were demonstrated in humanized SCID mice that responded by strong pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α, leading to death by brain hemorrhages (7). Interestingly, pro-inflammatory and immunopathogenic effects of MSRV are achieved by its envelope protein (Env-ms) through the activation of Toll-like receptor (TLR) 4 and its CD14 co-receptor (8, 9). Of note, another member of the HERV-W family, integrated in the chromosome 7, possesses an expressed Env, called syncytin (Env-syn). However, Env-syn is found only in placenta playing an important role in the physiology of syncytiotrophoblast formation (2, 10), whereas several studies demonstrate the presence of Env-ms in macrophages or microglial cells in active plaques of all MS brains (11, 12). Moreover, Env-ms is found circulating in plasma from 73% of patients with MS and not in control donors (11). Interestingly, blood circulating Env-ms is also detected among some schizophrenic patients in correlation with their level of blood C-reactive protein showing a link between Env-ms and the systemic inflammation status of the patients (13). The precise action of MSRV in the pathogenesis of MS remains still unclear. For instance, the relative chronology of the expression of Env-ms, i.e. in activated monocytes/macrophages of the brain versus in the circulating blood, is unknown and difficult, even impossible, to assess. Several lines of evidence suggest that the Env expression can originate from the trans-activation of endogenous sequences belonging to the HERV-W family by infectious co-factors such as Epstein–Barr virus which is often found in MS patients (11, 14).
MS is an inflammatory demyelinating disease in which infiltrating lymphocytes and macrophages are observed in the perivascular space of central nervous system (CNS). The recruitment of immune cells to areas of demyelination is largely conditioned by blood–brain barrier (BBB) integrity and by the inflammatory environment [see (15–18) for reviews]. In the present study, we measured the pro-inflammatory properties of two different envelope proteins originating either from MSRV (Env-ms) or its endogenous counterpart HERV-W element stably inserted into the chromosome 7 (syncytin Env-syn). We assessed envelope protein effects on human brain endothelial cells (HCMEC/D3 cell line) reconstituting an in vitro BBB model (19), and on primary human umbilical vein endothelial cells (HUVECs). Furthermore, we investigated the mechanisms by which each envelope protein interacts with endothelial cells. We report that the recombinant MSRV envelope protein is able to trigger the secretion of several pro-inflammatory cytokines and the over-expression of ICAM-1, an adhesion molecule involved in key steps of leukocyte transendothelial migration, on both HCMEC/D3 and HUVECs. We also show that the pattern recognition receptor TLR4 is implicated in Env-ms recognition by HCMEC/D3 cells.
Methods
Recombinant proteins
The recombinant Env-ms and Env-syn proteins were obtained as previously described (8). Monoclonal antibodies GN-mAb01 and GN-mAb03, detecting both Env-ms and Env-syn proteins and GN-mAb12, detecting gag protein, were obtained from GeNeuro (Geneva, Switzerland).
Cells and culture conditions
HCMEC/D3 cells were obtained from P. O. Couraud (Institut Cochin, Paris, France) and cultured as previously described (19). Briefly, cells were seeded onto collagen type 1 (Sigma-Aldrich, St Louis, MO, USA) coated flasks in supplemented EBM-2 medium (Lonza ‘bullet kit’, Basel, Switzerland) containing 2.5% fetal bovine serum (FBS) and growth factors, bFGF, VEGF, IGF, EGF at a final concentration 4× lower than recommended by the furnisher. Cells were obtained at passage 26 and cultured up to passage 36.
Primary HUVECs were cultured in M199 medium containing 20% heat-inactivated FBS, ECGS (Endothelial Cells Growth Supplement, 50 µg ml−1), heparin (100 µg ml−1) and antibiotics. Cells were cultured up to passage 5 onto collagen type 1 (Sigma) coated flasks.
HL-60 cells, a human promyelocytic leukemia cell line, was cultured in RPMI 1640 containing 10% FBS.
Cell stimulation
HCMEC/D3 or HUVECs were seeded onto collagen type 1 coated 24-well plates until reaching confluency. Media were then replaced with media containing recombinant proteins or stimulating cytokines for 16h. All conditions were tested in triplicate. After stimulation, cells were trypsinized and analyzed by flow cytometry for the expression of ICAM-1 and supernatants were collected and frozen at −80°C before analysis by ELISA for the production of cytokines (IL-6, IL-8 and TNF-α detection kits purchased from PromoKine/Promcell, Heidelberg, Germany).
Adhesion and transmigration assays
For adhesion assays, HL-60 cells were cultured at a concentration of 2.105 cells ml−1 and activated with vitamin D3 (18.7 µg ml−1) and indomethacin (10−7 M; Sigma-Aldrich) for 72h. HL-60 cells were detached with a scraper and labeled with calcein AM (Invitrogen Molecular Probes, Carlsbad, CA, USA) just before the experiment. Then 1.106 cells were left to adhere for 35min onto a confluent HCMEC/D3 monolayer representing a surface of ~3.8cm2. After three washing steps with PBS, the remaining adherent cells were lysed with 1 % SDS. For each condition, cell lysate is plotted in triplicate on a 96-well plate before fluorescence measurement, with a Victor 3 spectrophotometer (1420 multilabel counter; Perkin Elmer).
For transmigration assays, HCMEC/D3 (2×104 per well) were grown to confluency onto collagen-coated (100 µg ml−1) porous polycarbonate membrane (Transwell, 8-µm pore size, 6.5mm diameter; Costar, Cambridge, MA, USA) for 3–4 days at 37°C and stimulated with TNF-α (100U ml−1) or 2 µg ml−1 of Env-ms for 18h prior to the assay. For inhibition experiments, Env-ms (2 µg ml−1) was pre-incubated with monoclonal antibodies specific for Env-ms or gag protein (30 µg ml−1, 30min 4°C). Activated HL-60 cells (105 cells per well) were added to the upper compartment, A concentration of 2×10−8 M fMLP (Calbiochem-Novabiochem) was added in the lower compartment, to create a chemotactic gradient for peripheral mono nuclear cells. After 1h at 37°C, migrated HL-60 cells were recovered from the bottom well, centrifuged and quantified using a CyQUANT assay kit (Molecular Probes, Eugene, OR, USA).
Immunostaining for flow cytometry
After trypsinization, living cells were incubated for 1h at 4°C with anti-ICAM-1 primary antibody (clone 2D5 produced locally as previously described) (20). After centrifugation, pellets were re-suspended in 50 µl of RPMI 1640 containing the secondary antibody coupled with FITC (goat anti-mouse-FITC purchased from Jackson ImmunoResearch, Philadelphia, PA, USA) and left to incubate for 30min at 4°C. After centrifugation, cells were re-suspended in 500 µl of RPMI 1640 + 10% FBS and fluorescence was measured with a FACSCAN flow cytometer (Becton Dickinson, Le Pont-De-Claix, France). Results were analyzed using WinMDI software.
Immunostaining for fluorescence microscopy
Cells were seeded onto LabTek chambers (Nunc, Rochester, NY, USA) until confluence and were then stimulated for 16h in the chambers. Cells were then washed twice with pre-warmed (37°C) PBS containing Ca++ and Mg++ and then fixed for 30min at room temperature with PBS Ca-Mg containing 2% PFA and 2% sucrose. After fixation, we performed two quenching steps with PBS containing glycine 0.1M and NaN3 0.01% for 10min and a permeabilization step using PBS containing 0.2% saponin, 2% BSA, for 20min at room temperature. Staining was performed using the anti-ICAM-1 primary antibody clone 2D5 with a secondary goat anti-mouse antibody coupled to TRITC (Jackson ImmunoResearch); anti-TLR4 (Santa Cruz, Santa Cruz, CA, USA) with a secondary goat anti-rabbit antibody coupled to Alexa 488; or the anti-golgin 97 (Invitrogen) with a secondary goat anti-mouse antibody coupled with Alexa 555. Nuclei were labeled with Hoechst 33342. Cells were observed with an epi-fluorescence microscope (Carl Zeiss AxioImager) with the pseudo-confocal module APOTOME. Images were analyzed with ImageJ software (ImageJ, NIH, USA). For co-localization experiments, the Pearson’s correlation coefficient (Rr) and the Mander’s overlap coefficient (R) were calculated using the ImageJ co-localization plugin after background subtraction.
Western blotting
After reaching confluence, HCMEC/D3 cells were trypsinized and lysed for 30min on ice with a Tris–HCl 50mM, pH 7.5, NaCl 150mM buffer containing 1% NP40 or 0.1% SDS (as mentioned in each case) and a protease inhibitor cocktail (Sigma-Aldrich). Whole cell extracts were submitted to SDS–PAGE (10% acrylamide gels), blotted onto polyvinylidene difluoride membranes and stained with a rabbit polyclonal primary antibody raised against TLR4 (Santa Cruz) or actin (Sigma-Aldrich) and a secondary goat anti-rabbit antibody coupled with peroxidase (Invitrogen). Bands were quantified with BioRad Image Lab software.
Treatment with peptide N-glycosidase
Before analysis by western blotting, some extracts were treated by peptide N-glycosidase (PNGase) using a deglycosylation kit (Sigma, GlycoProfile II, Enzymatic In-solution N-Deglycosylation kit). Extracts were treated according to the furnisher’s recommendations but omitting the denaturation step before adding the PNGase to avoid precipitation of the proteins. Briefly, 50 µg of extracts were treated with 2.5 units of PNGase or water as a negative control for 2h at 37°C, then immediately analyzed by western blotting.
siRNA transfections
siRNA targeting TLR4 were transfected according to the manufacturer’s recommendations (Invitrogen). Briefly, we transfected the cells with a set of three siRNAs targeting TLR4 or with a negative control at a final concentration of 20nM (Stealth RNAi all provided by Invitrogen) using Lipofectamine RNAiMAX (Invitrogen) when cells reached 30% confluency. Cells were re-transfected 48h later under the same conditions. Forty-eight hours later, the transfected and control cells were treated with Env-ms or LPS and then analyzed.
Statistics
All results are expressed as mean ± SE. Data were analyzed using R statistical software; two-sided Student t-test was used. Differences between conditions were considered significant at P <0.05.
Results
Effects of Env-ms on HCMEC/D3 brain endothelial cells
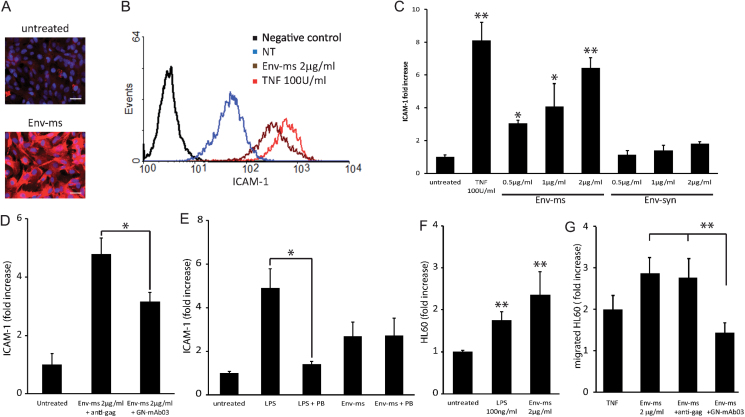
First, we analyzed the effect of Env-ms on confluent monolayers of HCMEC/D3 cells. We observed by fluorescence microscopy an increase in expression of ICAM-1 adhesion molecules (Fig. 1A). In order to quantify ICAM-1 expression, cells were analyzed by flow cytometry for surface expression of ICAM-1 after incubation with TNF-α, which is known to activate endothelial cells, or with Env-ms; both treatments induced a strong ICAM-1 expression (Fig. 1B). The induction of ICAM-1 expression was specific for Env-ms, since treatment with Env-syn had no significant effect and was dose dependent on the ENv-ms concentration (Fig. 1C). To further confirm the specificity of Env-ms pro-inflammatory properties, we pre-incubated Env-ms with monoclonal antibodies recognizing either Env-ms, or the gag protein of HERV as a negative control, before treating HCMEC/D3 cells. After overnight incubation with the treated Env-ms, ICAM-1 surface expression by HCMED/D3 cells was measured by flow cytometry as detailed above. Anti-Env-ms antibodies blocked ~40% of Env-ms-induced ICAM-1 expression as compared to the irrelevant anti-gag antibodies (Fig. 1D). We performed a complementary control to ensure that there is no LPS contamination in the recombinant envelope protein. For that purpose, we used polymyxin B (PB) antibiotic that can neutralize LPS activity. LPS or Env-ms were pretreated or not with PB and then incubated overnight with HCMEC/D3 cells. The pre-treatment with PB almost completely abolished the LPS-induced ICAM-1 increased expression but had no effect on Env-ms-induced ICAM-1 expression, indicating that Env-ms pro-inflammatory activity was not caused by LPS contamination (Fig. 1E).
Fig. 1.
Env-ms specifically induces over-expression of ICAM-1 on HCMEC/D3 cells but not Env-syn. (A) Indirect immunostaining of ICAM-1 (red) on untreated HCMEC/D3 cells or after overnight treatment with Env-ms at 2 µg ml−1. Nuclei are stained in blue. Scale bar: 20 µm. (B and C). Flow cytometry analysis of ICAM-1 expression after overnight treatment of HCMEC/D3 cells (B) with TNF-α (100U ml−1) or Env-ms (2 µg ml−1) (NT: not treated) and (C) with TNF-α (100U ml−1), Env-ms or Env-syn. Values are means ± SE of fluorescence intensity in two independent experiments each performed in duplicate. Negative control: an irrelevant antibody of the same isotype (IgG1) as the monoclonal antibody specific for ICAM-1. (D) Inhibition using monoclonal antibodies. HCMEC/D3 cells were stimulated overnight with Env-ms (2 µg ml−1) pre-incubated for 40min at 4°C with mAbs (30 µg ml−1) specific for Env-ms (GN-mAb03) or gag (GN-mAb12) proteins. ICAM-1 expression was then measured by flow cytometry. Results represent the mean of the fold of increase (ratio between mean of fluorescence of the activated cells and untreated cells) ± SE of three independent experiments. (E) PB inhibition assay: HCMEC/D3 cells were treated overnight with Env-ms (2 µg ml−1) or LPS (0.1 µg ml−1), pre-incubated or not for 30min at 37°C with PB (25 µg ml−1). ICAM-1 expression was measured by flow cytometry (see above). Results represent the mean ± SE of two independent experiments. (F) Env-ms stimulates the adhesion between immune cells and the endothelial monolayer. Results represent means of fold of increase compared to untreated condition ± SE of two independent experiments each performed in triplicate. (G) Env-ms (2 µg ml−1) stimulates the transmigration of HL-60 cells through the endothelial monolayer. TNF (100U ml−1) was used as a positive control for transmigration. Results represent means of fold of increase compared to untreated condition ± SE of three independent experiments each performed in duplicate. *P ≤ 0.05; **P ≤ 0.01.
We investigated the effects of Env-ms at a functional level by measuring the adhesion between activated immune cells and a monolayer of HCMEC/D3 cells. For that purpose, we used activated calcein-labeled HL-60 cells adhering to the HCMEC/D3 monolayer. After several washing steps and lysis of all remaining adherent cells, we quantitated adherence of HL-60 cells by fluorescence readings. We observed an increase of adherent HL-60 cells when HCMEC D/3 cells had previously been treated with Env-ms (Fig. 1F). This increase was even stronger than the positive control with LPS treatment, but this was not statistically significant. These results are consistent with our observations (Fig. 1A and B) of increased expression of ICAM-1 following Env-ms treatment, since ICAM-1 is a major mediator of the adhesion between activated immune cells and endothelial cells [(20, 21), see (22) for review].
Adhesion of leukocytes to the endothelium can be followed by leukocyte transendothelial migration. As shown in Fig. 1 (G), Env-ms was able to stimulate transendothelial migration of HL-60 cells; this increased transmigration was inhibited (by 80%) when Env-ms was pre-incubated with a specific monoclonal antibody (GN-mAb03), while an antibody specific for Gag protein was devoid of any inhibitory effect.
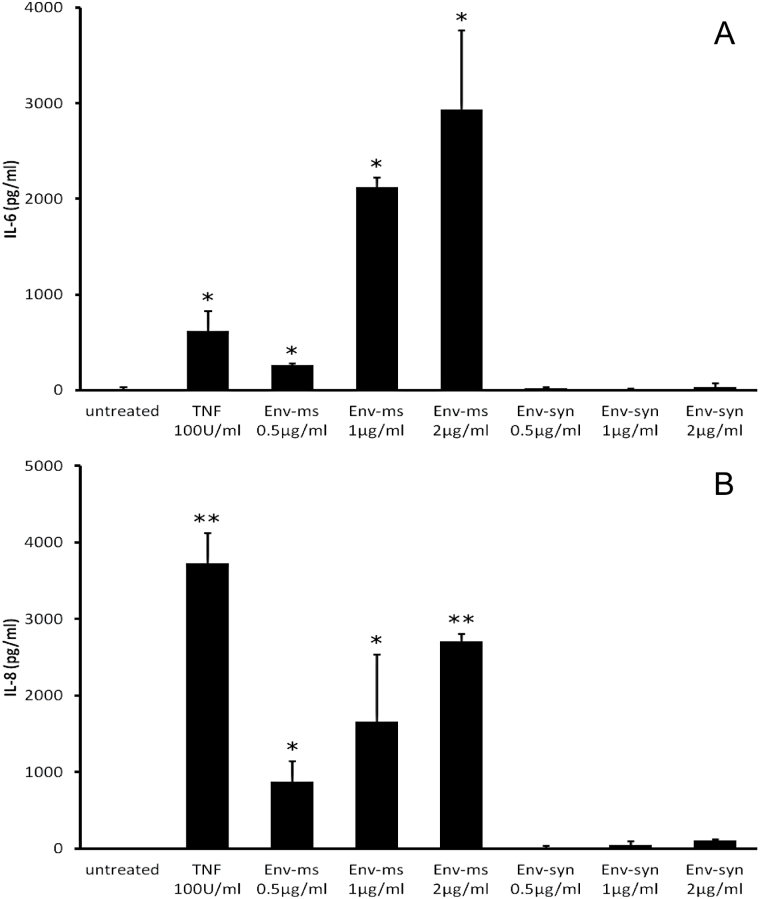
Recombinant Env-ms induced a strong and dose-dependent production of IL-6 (Fig. 2A) and IL-8 (Fig. 2B) but did not induce significant production of TNF-α (data not shown). The induction of these pro-inflammatory cytokines is specific of Env-ms since Env-syn had no effect.
Fig. 2.
Env-ms, but not Env-syn, induces a dose-dependant production of IL-6 and IL-8. (A) Analysis of IL-6 production by HCMEC/D3 cells. Cells were treated overnight with TNF-α, Env-ms or Env-syn, then IL-6 was measured in the supernatants of the culture by ELISA. Results represent the mean ± SE of two independent experiments each performed in duplicate. (B) Analysis of IL-8 production by HCMEC/D3 cells. Same conditions as for IL-6 measurements. *P ≤ 0.05; **P ≤ 0.01.
Taken together, these results indicate that Env-ms exerts a specific pro-inflammatory activity on brain endothelial cells.
Effects of Env-ms on primary endothelial cells
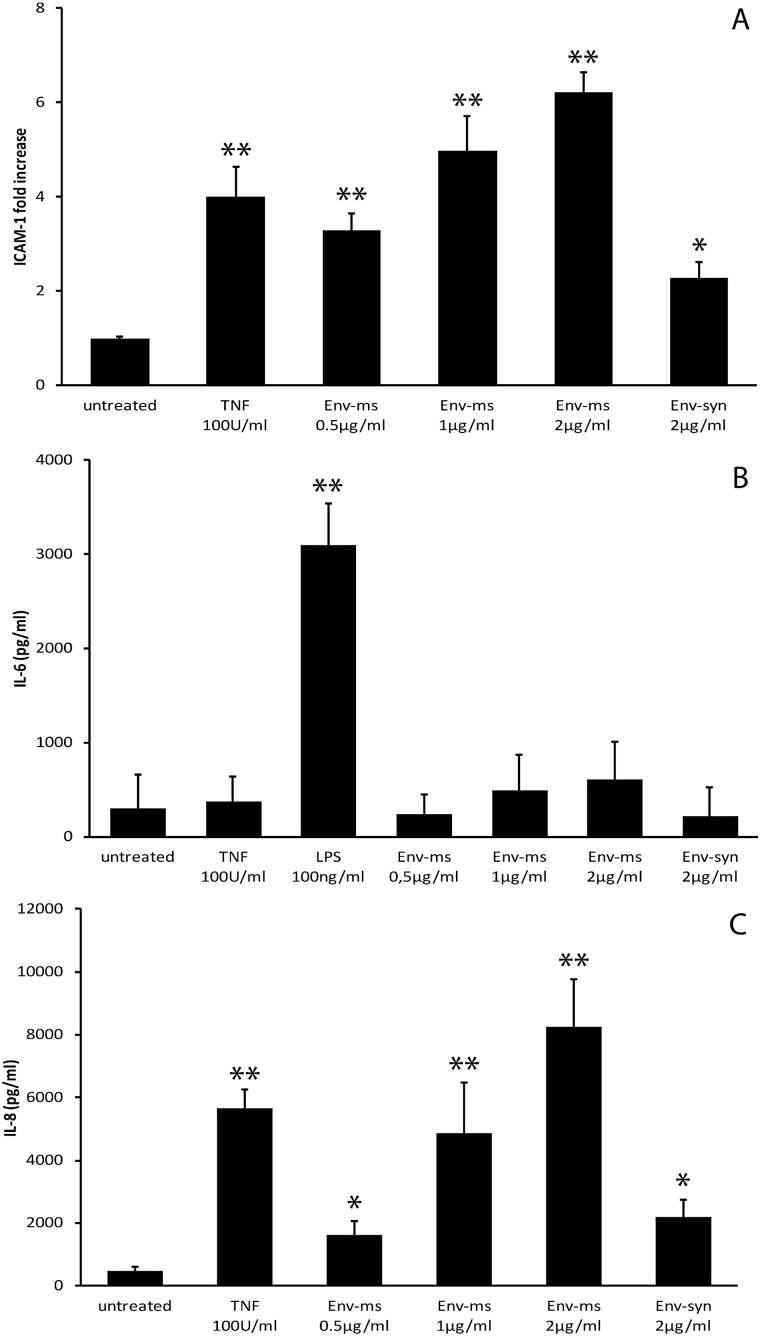
The effects of Env-ms were further studied on primary HUVEC. Recombinant Env-ms induced an increased expression of ICAM-1 (Fig. 3A) similar to that observed previously with HCMEC/D3 cells. Furthermore, HUVECs responded to Env-ms by a strong dose-dependent production of IL-8 (Fig. 3C). In contrast, no significant production of IL-6 was obtained after incubation of HUVECs with either Env-ms or TNF-α (Fig. 3B). However, primary HUVECs are able to produce IL-6 in response to LPS stimulation (Fig. 3B). Taken together, these results indicate that Env-ms also induces a pro-inflammatory response of HUVECs but with a slightly different cytokine profile than that of HCMEC/D3 cells.
Fig. 3.
Env-ms has significant, but different effects on HUVECs, compared to HCMEC/D3 cells. (A) Flow cytometry analysis of ICAM-1 expression after overnight treatment of HUVECs with TNF-α, Env-ms or Env-syn. (B) Analysis of IL-6 production by HUVECs. Cells were treated overnight with TNF-α, Env-ms or Env-syn, IL-6 was measured with ELISA. (C) Analysis of IL-8 production by HUVECs. Same conditions as for IL-6 measurements. Results represent the mean ± SE of 2–4 independent experiments each performed in triplicate. *P ≤ 0.05; **P ≤ 0.001.
Implication of TLR4 in Env-ms recognition by endothelial cells
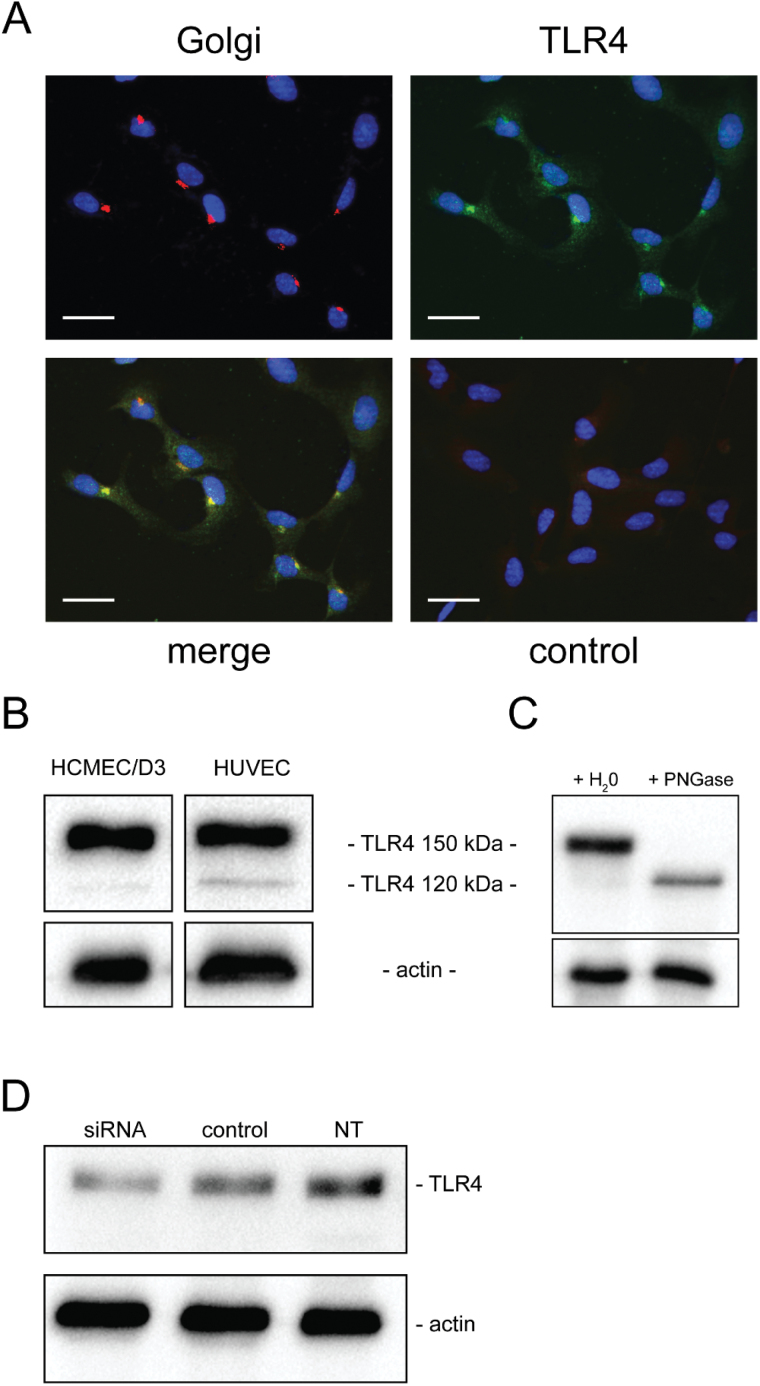
TLR4 is expressed by HCMEC/D3.
It has been previously shown that Env-ms interacts with the receptor of innate immunity TLR4/CD14, on monocytes and dendritic cells (8); therefore, we investigated whether this pathway may be used by endothelial cells to respond to ENV-ms. TLR4 expression was analyzed in permeabilized HCMEC/D3 cells by fluorescent microscopy which showed that TLR4 mostly co-localized with the Golgi apparatus (Fig. 4A) as has been previously described in other cell types (23, 24). Analysis by western blotting demonstrated that in both HCMEC/D3 and HUVECs, TLR4 was expressed as a 150kDa protein, with a minor band at 120kDa (Fig. 4B). It has been reported in the literature that the heavy form is the fully processed, glycosylated and active form which can be expressed at the cell membrane, while the light form remains intracellular (25). These observations suggest that a fully glycosylated TLR4 form could be expressed by the HCMEC/D3 cells. Treatment of the whole cell extracts with PNGase resulted in the disappearance of the heavy form in favor of the light form (Fig. 4C), showing that the heavy form is glycosylated.
Fig. 4.
TLR4 expression in endothelial cells. (A) TLR4 co-localizes with the Golgi apparatus. Fluorescent staining of Golgi apparatus (red), TLR4 (green) and nuclei (blue) on HCMEC/D3 cells (not confluent) after fixation with PFA 2% and permeabilization with saponin 0.2%. Negative control is obtained after staining with the secondary antibodies only. Pearson’s correlation coefficient Rr = 0.67 and Mander’s overlap coefficient R = 0.75. Scale bar: 20 µm. (B) TLR4 is expressed on HCMEC/D3 and on HUVECs. Cells were lysed with the Tris–HCl 50mM, pH 7.5, NaCl 150mM buffer containing 1% NP40. The presence of TLR4 in the whole extracts is then analyzed by western blotting. (C) TLR4 heavy form (150kDa) leads to a light form (120kDa) after treatment with PNGase. Whole extracts, prepared with a lysis buffer containing only NP40, were treated with PNGase, or water as a negative control, for 2h and then analyzed by western blotting. (D) TLR4 expression on HCMEC/D3 cells after double transfection with siRNAs targeting TLR4 or control siRNAs; cells were lysed with the Tris–HCl 50mM, pH 7.5, NaCl 150mM buffer containing 1% NP40. The presence of TLR4 in the whole extracts was then analyzed by western blotting and quantified with ImageJ software. NT: not transfected.
TLR4 knock down abolishes HCMEC/D3 response to Env-ms.
Subsequently, in order to verify that TLR4 is the receptor implicated in Env-ms recognition, we transfected HCMEC/D3 cells with TLR4-targeting siRNAS and assessed the efficiency of TLR4 knock down by western blotting (Fig. 4D). After quantification with ImageJ, we measured a 44% decrease of TLR4 expression after two rounds of transfection with TLR4-targeting siRNA when compared to control siRNA-transfected cells and a 59% decrease compared to untransfected cells.
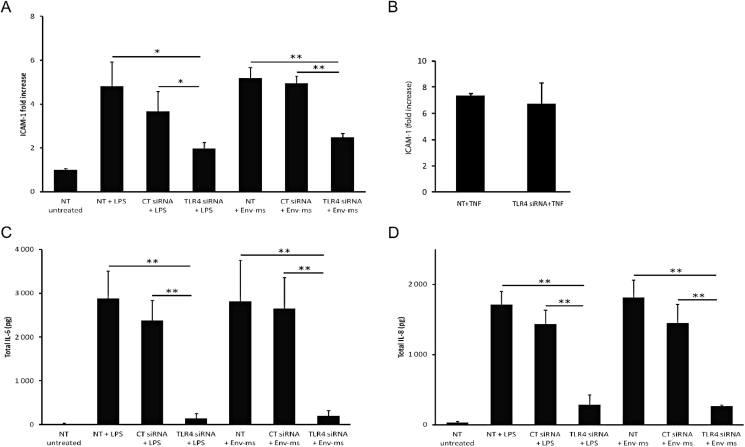
We then analyzed the effect of reduced TLR4 expression on the induction of ICAM-1 expression and on the production of pro-inflammatory cytokines by LPS, a well-known TLR4 ligand, or by Env-ms (Fig. 5). The results showed that ICAM-1 expression, induced by either LPS or Env-ms, was significantly reduced (by at least half) in knocked down cells compared to the cells transfected with a control siRNA (Fig. 5A). The specificity of TLR4 silencing by these siRNAs is shown in Fig. 5(B), which shows that TNF-α is still able to induce the expression of ICAM-1 after transfection with these siRNAs. Furthermore, the production of IL-6 and IL-8 cytokines induced by either LPS or Env-ms was dramatically decreased (by ~90%) after TLR4 knock down as compared to control transfected cells (Fig. 5C and D). Taken together, these results support the hypothesis that TLR4 is implicated in Env-ms recognition by HCMEC/D3 cells.
Fig. 5.
Effects of Env-ms are greatly impaired after knock down of TLR4 receptor. (A) siRNA-transfected HCMEC/D3 cells were stimulated overnight with LPS or Env-ms. ICAM-1 expression was measured by flow cytometry. Cells were either not transfected with siRNA (NT) and not stimulated with LPS or Env-ms (untreated), or transfected with control siRNA (CT siRNA) or TLR4 siRNA and stimulated with Env-me (2µg ml−1) or LPS (0.1 µg ml−1). Results represent the mean ± SE of three independent experiments each performed in triplicate. (B) Same as in (A): untransfected cells (NT) or cells transfected with TLR4 siRNA were stimulated with TNFα (100U ml−1, 18h). (C, D) HCMEC/D3 cells were transfected and then stimulated overnight with LPS or Env-ms. Production of IL-6 and IL-8 was measured by ELISA assays. Results represent the mean ± SE of two independent experiments each performed in triplicate. NT: not transfected, CT: control. *P ≤ 0.01; **P ≤ 0.001.
Discussion
MS is a chronic neurological disease, whose etiology remains poorly defined. However, it is well established that inflammatory process in the brain yields to the destruction of oligodendrocytes which build myelin sheaths around axons in the brain and spinal cord (26). Under inflammatory conditions, vascular endothelial cells respond by the secretion of factors and the increase of adhesion molecules that lead to an enhancement of leukocyte adhesiveness and to their migration into tissues (27). The recruitment of activated leukocytes across BBB endothelial cells is a critical step in triggering inflammation and CNS tissue injury in the course of MS (18). Therefore, the initiation of the inflammatory process, along with its outcome on the neuroimmunological context, remains a central point of interest in the understanding of MS.
The discovery of the retroviral element MSRV, isolated from the cerebrospinal fluid of a patient with MS (1), has opened new issues about a putative microbial contribution in MS physiopathology, reviewed in (6). Several lines of evidence indicate that MSRV could participate to the activation of some immune cells. MSRV and its envelope protein, Env-ms, trigger T-cell receptor Vβ (28) and TLR4 (8), similarly to superantigens such as those encoded by mouse mammary tumor virus (29, 30).
Since Env-ms could activate innate immunity through the TLR4/CD14 pathway, we further sought pro-inflammatory activity on endothelial cells which are known to express TLR and respond to TLR signals (27, 31). Indeed, we demonstrate that Env-ms is able to activate the vascular endothelium including the brain endothelium in a manner consistent with the fact that MS is an inflammatory disease of the CNS. Regarding the activation pathways, our results show that the inhibition of Env-ms induced pro-inflammatory effects is incomplete after TLR4 knock down. This most probably arises from the fact that TLR4 expression is not completely abolished after transfection with siRNAs as observed on our western blotting experiment (Fig. 5D). Nevertheless, we cannot exclude the implication of TLR4 co-receptor CD14 (32). Indeed, although CD14 is not detected in HCMED/D3 cells by western blotting, it is known to be present in serum (33, 34). In our model, it would be difficult to perform experiments with serum depletion since HCMEC/D3 cells are very sensitive to any modification of culture medium, therefore no definitive conclusion concerning the role of CD14 can be drawn.
One interesting observation of our study concerns the different effects of Env-ms in endothelial cells of different origins. The envelope protein seems to exert a slightly higher pro-inflammatory activity on HCMEC/D3 cells that originate from the brain than on HUVECs that originate from umbilical vein. Indeed, Env-ms triggers the over-expression of ICAM-1 and the production of IL-6 and IL-8 by HCMEC/D3 while only ICAM-1 expression and IL-8 production are strongly stimulated on HUVEC. This is not due to differences in the levels of TLR4 expression between HCMEC and HUVECs since we verified by western blotting that TLR4 was expressed at a similar level in both cell types (Fig. 4B). Concerning the cytokine production, HUVECs are known to produce a much lower amount of IL-6 compared to IL-8 in response to LPS and their response to TNF-α is controversial and depends on culture conditions as already discussed by Makó et al. (35). That could explain the absence of IL-6 production after treatment with Env-ms and TNF-α. However, in our conditions, HUVECs are able to produce significant amounts of IL-6 in response to LPS but not in response to Env-ms (Fig. 3C). These variations observed between LPS and Env-ms effects on HUVECs could be explained by a lower affinity of the latter with TLR4 possibly due to structural differences. Finally, in spite of these variations, we show that Env-ms exerts its pro-inflammatory activity on both types of vascular endothelial cells.
The presence of Env-ms protein was detected in the peripheral blood of most of MS patients as a soluble protein in the serum and as a membrane-associated protein at the surface of circulating monocytes and to a lesser extend on B and NK cells (14). Histology of brain tissues of some postmortem MS patients revealed that perivascular lesions contained a large number of macrophages and microglial cells which expressed Env protein as well as HLA-DR (11). Whatever the initial origin of Env-ms, either at the periphery or in the CNS, its presence in both compartments may contribute establishing conditions for neuro-inflammatory damage. This is further strengthened by the fact that human oligodendrocyte differentiation is impaired after exposure to Env-ms leading to the reduction in expression of myelin protein (36). It is striking to note that again in this latter case, Env-ms triggers TLR4 pathway in oligodendroglial precursor cells which respond by the production of pro-inflammatory cytokines and reactive oxygen species. Interestingly, TLR4 is expressed by other cells contributing to the BBB formation. Firstly, astrocytes have TLR4 but their capacity to respond to its ligands has been a subject of debates. Recently, it has been shown that astrocyte response to LPS stimulation requires CD14 expression (37). Secondly, pericytes, which surround endothelial cells to build capillaries, possess a functional TLR4 that responds to LPS stimulation by the production of pro-inflammatory cytokines and up-regulation of ICAM-1 (38). Although the reactivity of pericytes and astrocytes to an Env-ms signal remains to be firmly established, one may consider that the convergence in the BBB of cells expressing TLR4 may favor the particular sensitivity of the CNS to the exposure of TLR4 ligands inducing synergic paracrine signals.
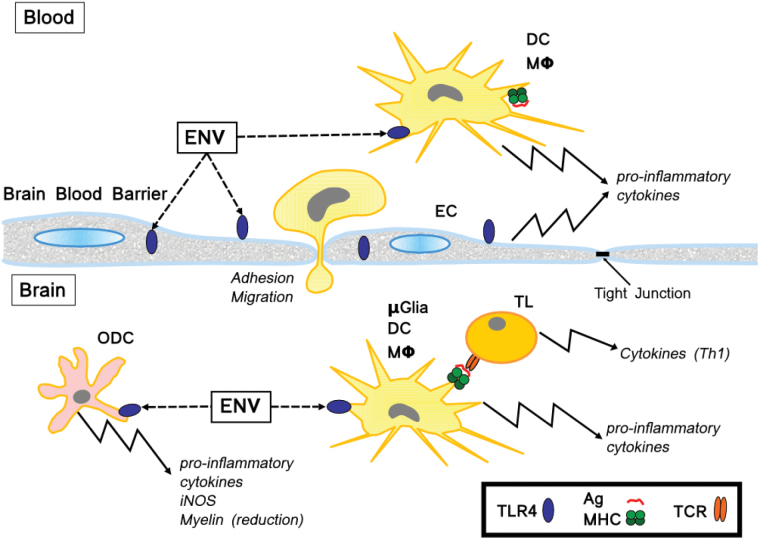
Altogether, these data highlight the importance of the Env-ms/TLR4 pathway in the demyelination and in the inflammation associated with MS (Fig. 6). In the blood, Env-ms can activate on the one hand circulating monocytes, dendritic cells and macrophages and on the other hand vascular endothelial cells leading to the production of pro-inflammatory cytokines, the enhancement of the adhesion of circulating cells to endothelium and then their migration through the BBB into the brain. Env-ms in the brain provokes activation of macrophages, microglial and dendritic cells which orient the Th1 response of T lymphocytes. In parallel, Env-ms interferes with oligodendrocyte cells in activating their precursors to produce pro-inflammatory cytokines and in reducing their capacity to build myelin sheaths. Simultaneous inflammatory conditions together with a myelinization defect may favor the rise of myelin-specific Th1 T lymphocytes leading to auto-immunity. Obviously, interfering with the Env-ms/TLR4 pathway represents a promising therapeutic approach which is currently progressing by the mean of specific anti-Env-ms neutralizing antibodies (39).
Fig. 6.
Schematic representation of the effects of Env-ms. DC = dendritic cell; EC = endothelial cell; ENV = envelope protein of HERV-W MSRV; MΦ = macrophage; ODC = oligodendrocyte cell; µGlia = microglial cell; TL = T lymphocyte.
In conclusion, our study brings new evidence about the pro-inflammatory properties of MSRV envelope protein, defines the mechanism by which Env-ms stimulates endothelial cells and reinforces the proposal that MSRV may play a key role in MS pathogenesis and probably in other neurodegenerative disorders, such as schizophrenia (13, 40).
Funding
Institutional grants from Institut National de la Santé et de la Recherche Médicale (INSERM); Fondation pour l’Aide a la Recherche sur la Sclérose En Plaques (France); and Association Française contre les Myopathies, France.
Acknowledgements
Mrs M. Pezet is acknowledged for expertise in flow cytometry and Zuzana Macek-Jilkova for help with artwork.
Conflict of interest statement: P.N.M. and H.P. are authors of the patent ‘Composition for Treating Pathology Associated With MSRV/HERV-W’ (WO2005080437) for the development in clinics of therapeutic antibody to treat Multiple Sclerosis: ongoing clinical trial (NCT01639300) by GeNeuro (H.P.).
References
- 1. Perron H., Geny C., Laurent A., et al. 1989. Leptomeningeal cell line from multiple sclerosis with reverse transcriptase activity and viral particles. Res. Virol. 140:551. [DOI] [PubMed] [Google Scholar]
- 2. Blond J. L., Lavillette D., Cheynet V., et al. 2000. An envelope glycoprotein of the human endogenous retrovirus HERV-W is expressed in the human placenta and fuses cells expressing the type D mammalian retrovirus receptor. J. Virol. 74:3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Belshaw R., Pereira V., Katzourakis A., et al. 2004. Long-term reinfection of the human genome by endogenous retroviruses. Proc. Natl Acad. Sci. USA 101:4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Löwer R. Löwer J. and Kurth R. 1996. The viruses in all of us: characteristics and biological significance of human endogenous retrovirus sequences. Proc. Natl Acad. Sci. USA 93:5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Feschotte C. and Gilbert C. 2012. Endogenous viruses: insights into viral evolution and impact on host biology. Nat. Rev. Genet. 13:283. [DOI] [PubMed] [Google Scholar]
- 6. Dolei A. and Perron H. 2009. The multiple sclerosis-associated retrovirus and its HERV-W endogenous family: a biological interface between virology, genetics, and immunology in human physiology and disease. J. Neurovirol. 15:4. [DOI] [PubMed] [Google Scholar]
- 7. Firouzi R., Rolland A., Michel M., et al. 2003. Multiple sclerosis-associated retrovirus particles cause T lymphocyte-dependent death with brain hemorrhage in humanized SCID mice model. J. Neurovirol. 9:79. [DOI] [PubMed] [Google Scholar]
- 8. Rolland A. Jouvin-Marche E. Viret C. Faure M. Perron H. and Marche P. N. 2006. The envelope protein of a human endogenous retrovirus-W family activates innate immunity through CD14/TLR4 and promotes Th1-like responses. J. Immunol. 176:7636. [DOI] [PubMed] [Google Scholar]
- 9. Perron H., Dougier-Reynaud H. L., Lomparski C., et al. 2013. Human endogenous retrovirus protein activates innate immunity and promotes experimental allergic encephalomyelitis in mice. PLoS One 8:e80128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mi S., Lee X., Li X., et al. 2000. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature 403:785. [DOI] [PubMed] [Google Scholar]
- 11. Perron H., Germi R., Bernard C., et al. 2012. Human endogenous retrovirus type W envelope expression in blood and brain cells provides new insights into multiple sclerosis disease. Mult. Scler. 18:1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Perron H., Lazarini F., Ruprecht K., et al. 2005. Human endogenous retrovirus (HERV)-W ENV and GAG proteins: physiological expression in human brain and pathophysiological modulation in multiple sclerosis lesions. J. Neurovirol. 11:23. [DOI] [PubMed] [Google Scholar]
- 13. Leboyer M. Tamouza R. Charron D. Faucard R. and Perron H. 2013. Human endogenous retrovirus type W (HERV-W) in schizophrenia: a new avenue of research at the gene-environment interface. World J. Biol. Psychiatry 14:80. [DOI] [PubMed] [Google Scholar]
- 14. Mameli G., Poddighe L., Mei A., et al. 2012. Expression and activation by Epstein Barr virus of human endogenous retroviruses-W in blood cells and astrocytes: inference for multiple sclerosis. PLoS One 7:e44991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Holman D. W. Klein R. S. and Ransohoff R. M. 2011. The blood-brain barrier, chemokines and multiple sclerosis. Biochim. Biophys. Acta 1812:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alexander J. S. Zivadinov R. Maghzi A. H. Ganta V. C. Harris M. K. and Minagar A. 2011. Multiple sclerosis and cerebral endothelial dysfunction: Mechanisms. Pathophysiology 18:3. [DOI] [PubMed] [Google Scholar]
- 17. McFarland H. F. and Martin R. 2007. Multiple sclerosis: a complicated picture of autoimmunity. Nat. Immunol. 8:913. [DOI] [PubMed] [Google Scholar]
- 18. Correale J. and Villa A. 2007. The blood-brain-barrier in multiple sclerosis: functional roles and therapeutic targeting. Autoimmunity 40:148. [DOI] [PubMed] [Google Scholar]
- 19. Weksler B. B., Subileau E. A., Perrière N., et al. 2005. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 19:1872. [DOI] [PubMed] [Google Scholar]
- 20. Duperray A., Languino L. R., Plescia J., et al. 1997. Molecular identification of a novel fibrinogen binding site on the first domain of ICAM-1 regulating leukocyte-endothelium bridging. J. Biol. Chem. 272:435. [DOI] [PubMed] [Google Scholar]
- 21. Gorina R. Lyck R. Vestweber D. and Engelhardt B. 2014. β2 integrin-mediated crawling on endothelial ICAM-1 and ICAM-2 is a prerequisite for transcellular neutrophil diapedesis across the inflamed blood-brain barrier. J. Immunol. 192:324. [DOI] [PubMed] [Google Scholar]
- 22. Ley K. Laudanna C. Cybulsky M. I. and Nourshargh S. 2007. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7:678. [DOI] [PubMed] [Google Scholar]
- 23. Espevik T. Latz E. Lien E. Monks B. and Golenbock D. T. 2003. Cell distributions and functions of Toll-like receptor 4 studied by fluorescent gene constructs. Scand. J. Infect. Dis. 35:660. [DOI] [PubMed] [Google Scholar]
- 24. Hornef M. W. Frisan T. Vandewalle A. Normark S. and Richter-Dahlfors A. 2002. Toll-like receptor 4 resides in the Golgi apparatus and colocalizes with internalized lipopolysaccharide in intestinal epithelial cells. J. Exp. Med. 195:559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ohnishi T. Muroi M. and Tanamoto K. 2003. MD-2 is necessary for the toll-like receptor 4 protein to undergo glycosylation essential for its translocation to the cell surface. Clin. Diagn. Lab. Immunol. 10:405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kremer D. Aktas O. Hartung H. P. and Küry P. 2011. The complex world of oligodendroglial differentiation inhibitors. Ann. Neurol. 69:602. [DOI] [PubMed] [Google Scholar]
- 27. Danese S. Dejana E. and Fiocchi C. 2007. Immune regulation by microvascular endothelial cells: directing innate and adaptive immunity, coagulation, and inflammation. J. Immunol. 178:6017. [DOI] [PubMed] [Google Scholar]
- 28. Perron H., Jouvin-Marche E., Michel M., et al. 2001. Multiple sclerosis retrovirus particles and recombinant envelope trigger an abnormal immune response in vitro, by inducing polyclonal Vbeta16 T-lymphocyte activation. Virology 287:321. [DOI] [PubMed] [Google Scholar]
- 29. Cazenave P. A., Marche P. N., Jouvin-Marche E., et al. 1990. V beta 17 gene polymorphism in wild-derived mouse strains: two amino acid substitutions in the V beta 17 region greatly alter T cell receptor specificity. Cell 63:717. [DOI] [PubMed] [Google Scholar]
- 30. Rassa J. C. Meyers J. L. Zhang Y. Kudaravalli R. and Ross S. R. 2002. Murine retroviruses activate B cells via interaction with toll-like receptor 4. Proc. Natl Acad. Sci. USA 99:2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pegu A. Qin S. Fallert Junecko B. A. Nisato R. E. Pepper M. S. and Reinhart T. A. 2008. Human lymphatic endothelial cells express multiple functional TLRs. J. Immunol. 180:3399. [DOI] [PubMed] [Google Scholar]
- 32. da Silva Correia J. Soldau K. Christen U. Tobias P. S. and Ulevitch R. J. 2001. Lipopolysaccharide is in close proximity to each of the proteins in its membrane receptor complex. transfer from CD14 to TLR4 and MD-2. J. Biol. Chem. 276:21129. [DOI] [PubMed] [Google Scholar]
- 33. Frey E. A., Miller D. S., Jahr T. G., et al. 1992. Soluble CD14 participates in the response of cells to lipopolysaccharide. J. Exp. Med. 176:1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Verhasselt V. Buelens C. Willems F. De Groote D. Haeffner-Cavaillon N. and Goldman M. 1997. Bacterial lipopolysaccharide stimulates the production of cytokines and the expression of costimulatory molecules by human peripheral blood dendritic cells: evidence for a soluble CD14-dependent pathway. J. Immunol. 158:2919. [PubMed] [Google Scholar]
- 35. Makó V., Czúcz J., Weiszhár Z., et al. 2010. Proinflammatory activation pattern of human umbilical vein endothelial cells induced by IL-1β, TNF-α, and LPS. Cytometry A 77:962. [DOI] [PubMed] [Google Scholar]
- 36. Kremer D., Schichel T., Förster M., et al. 2013. Human endogenous retrovirus type W envelope protein inhibits oligodendroglial precursor cell differentiation. Ann. Neurol. 74:721. [DOI] [PubMed] [Google Scholar]
- 37. Tarassishin L. Suh H. S. and Lee S. C. 2014. LPS and IL-1 differentially activate mouse and human astrocytes: role of CD14. Glia 62:999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Guijarro-Muñoz I. Compte M. Álvarez-Cienfuegos A. Álvarez-Vallina L. and Sanz L. 2014. Lipopolysaccharide activates Toll-like receptor 4 (TLR4)-mediated NF-κB signaling pathway and proinflammatory response in human pericytes. J. Biol. Chem. 289:2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Curtin F., Lang A. B., Perron H., et al. 2012. GNbAC1, a humanized monoclonal antibody against the envelope protein of multiple sclerosis-associated endogenous retrovirus: a first-in-humans randomized clinical study. Clin. Ther. 34:2268. [DOI] [PubMed] [Google Scholar]
- 40. Perron H. Mekaoui L. Bernard C. Veas F. Stefas I. and Leboyer M. 2008. Endogenous retrovirus type W GAG and envelope protein antigenemia in serum of schizophrenic patients. Biol. Psychiatry 64:1019. [DOI] [PubMed] [Google Scholar]