Abstract
Despite maximal surgical and medical therapy, the treatment of glioblastoma remains a seriously vexing problem, with median survival well under 2 years and few long-term survivors. Targeted therapy has yet to produce significant advances in treatment of these lesions in spite of advanced molecular characterization of glioblastoma and glioblastoma cancer stem cells. Recently, immunotherapy has emerged as a promising mode for some of the hardest to treat tumors, including metastatic melanoma. Although immunotherapy has been evaluated in glioblastoma in the past with limited success, better understanding of the failures of these therapies could lead to more successful treatments in the future. Furthermore, there is a persistent challenge for the use of immune therapy to treat glioblastoma secondary to the existence of redundant mechanisms of tumor-mediated immune suppression. Here we will address these mechanisms of immunosuppression in glioblastoma and therapeutic approaches.
Keywords: glioblastoma, immunosuppression, immunotherapy
Regulation of the Immune System
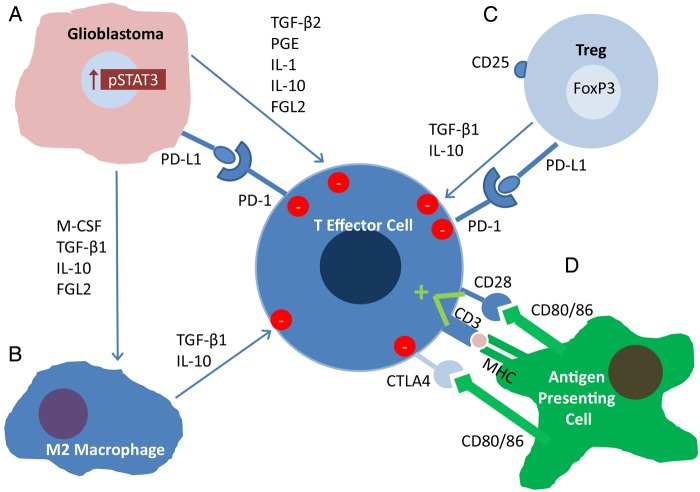
A variety of immune suppressive mechanisms are utilized in the setting of glioblastoma to prevent their immune detection and eradication (Fig. 1). For example, regulatory T cells (Tregs) can either originate in the thymus, and are referred to as naturally occurring Tregs (nTregs), or can be induced (iTregs) upon exposure to antigens in a tolerogenic environment. Both types of Tregs could potentially contribute to glioblastoma-mediated immune suppression, but nTregs may predominate within the glioblastoma microenvironment.1 Tregs suppress immune responses by secreting cytokines, such as transforming growth factor beta (TGF-β) and interleukin (IL)-10, and by cell-to-cell mediated contact, and in this way can prevent autoimmune reactions.2 Cytotoxic T-lymphocyte–associated protein 4 (CTLA-4) is a surface receptor that is present on activated T effector cells. CD28, which normally binds with B7 on an antigen-presenting cell to provide the costimulatory signal necessary for T cell activation, can bind this cell surface receptor and induce T cell anergy.3 On Tregs, CTLA-4 can be constitutively active, which contributes to their ability to suppress the immune system.4 CTLA-4 and programmed death 1 (PD-1) are often referred to as immune checkpoints, as these 2 help form a system of checks and balances that keep immune proliferation and activation regulated. The ligand for PD-1, programmed death ligand 1 (PD-L1), engages with PD-1 to provide another signal to suppress activated CD4+ and CD8+ cells.5 Studies of tumor microenvironments have revealed that engagement of this system is capable of inducing T cell apoptosis.6
Fig. 1.
Immunosuppression in the glioblastoma microenvironment. (A) Partially driven by increased STAT3 expression, glioblastoma cells secrete immunosuppressive factors such as TGFβ-2, PGE, IL-1, IL-10 and FGL2, all of which suppress the activity of effector cells. PD-L1 expressed on its surface also engages PD-1 to suppress effector activity. M-CSF, TGFβ-1 and IL-10 skew macrophages to the immunosuppressive M2 phenotype. (B) M2 Macrophages secrete TGFβ-1 and IL-10, suppressing effector cells further. (C) Regulatory T cells secrete TGFβ-1 and IL-10 as well, further suppressing immune reactivity, while also expressing PD-L1. (C) M-CSF, TGFβ-1 and IL-10, secreted by the glioblastoma, skew tumor associated macrophages to the immunosuppressive M2 phenotype. (D) Antigen is presented to T cells by antigen presenting cells within an MHC molecule, but a costimulatory signal from CD80/86 to CD28 is required for activation. CD80/86 can also suppress activity by engaging the CTLA4 receptor on the activated T cell.
Secreted Immunosuppressive Factors
By examining tumor cyst fluid, it was discovered that lymphocyte activity could be suppressed using factors secreted by tumors.7 These secreted factors were later identified as IL-1 and TGF-β.8–11 This discovery led to the development of trabedersen, an antisense oligonucleotide that inhibited TGF-β2; but despite initial promise, treatment by convection-enhanced delivery ultimately did not lead to a viable treatment strategy.12 Inhibitors of TGF-β receptor kinase activity are currently in clinical testing. Although both clinical tolerability and therapeutic activity have been limited so far,13 preclinical studies support their use.14 Subsequently, many other secreted factors in the glioblastoma microenvironment have been discovered, all of which have some varying level of effect on the immune response to glioblastoma. For example, colony stimulating factor 1 (CSF-1), produced by gliomas,15 has been shown to polarize the macrophage to a glioma-supportive M2 phenotype, which enhances glioma progression.16 Furthermore, vascular endothelial growth factor not only increases glioma tumorigenicity and growth17–20 but can also inhibit the maturation and function of dendritic cells.21 Other cytokines include IL-10, prostaglandin E, nitric oxide, regeneration and tolerance factor, and arginase 1.22–25 Regeneration and tolerance factor was found to inhibit the ability of natural killer (NK) cells to lyse target cells.26 Serum arginase I levels have been found to be associated with neutrophil degranulation and immunosuppression in glioblastoma patients.27 These secreted factors may be upregulated after gliomas undergo conventional therapy.28 Of note, some glioma-secreted cytokines may have both immune stimulatory and inhibitory roles depending on the context. Granulocyte macrophage CSF, which functions as a cytokine and growth factor for granulocytes and monocytes, can induce myeloid-derived suppressor cells (MDSCs).29
Glioma Cell Surface Immunosuppressive Factors
Glioma cells can express CD95 (Fas/apoptosis antigen 1) ligand on their surface, which can induce apoptosis and thus reduce the number of infiltrating T cells in the tumor microenvironment.30,31 Similarly, CD70, by direct cell-to-cell mediated contact, may have an apoptotic effect on immune cells in vitro.32,33 Yet, the biological effects of CD70 are context dependent, and immune stimulatory effects may dominate in vivo, at least in murine glioma models.34,35 Lectin-like transcript 1 has been shown to be a glioblastoma-expressed inhibitory ligand for NK cells.36 The most prototypical example is, of course, PD-L1, the ligand for PD-1, which is upregulated in the glioblastoma microenvironment37 and may be associated with the mesenchymal glioblastoma subtype.38 PD-L1 is expressed on immune cells and gliomas39–42 and has been shown to inhibit T cell activation and induce T cell apoptosis.6,43 This upregulation in glioblastoma may be due to loss of the tumor suppressor gene phosphatase and tensin homolog.39
Enthusiasm for immune checkpoint therapeutics is based on the observed improvement in survival time of melanoma patients in phase III clinical trials,44,45 which ultimately led to FDA approval of ipilimumab. Preclinical efficacy of anti-CTLA approaches in murine models of glioma46 has provided support for their implementation in glioblastoma patients. Several anti–PD-1 antibodies (nivolimumab, pembrolizumab) have also been clinically tested in melanoma patients,47–49 with FDA approval of nivolimumab and pembrolizumab in 2014.
The use of immune checkpoint inhibitors in glioblastoma patients has preclinical justification. For example, CTLA-4 blockade with coadministration of IL-12 led to tumor clearance in a murine model system, with an associated increased immune effector response and reduction in Tregs.50 Furthermore, anti–PD-1 blockade has been shown to improve survival in murine glioma model systems in combination with radiotherapy.51 Additionally, in a murine model of intracerebral glioma, combinatorial therapy of indoleamine 2,3-dioxygenase (IDO), CTLA-4, and PD-L1 was curative in a marked number of mice.52 An alternative/supplementary approach for inhibiting Tregs is the use of the anti-IL2Rα antibody, daclizumab, which has been shown to decrease Treg numbers and increase the ratio of effector T cells to Tregs in patients undergoing standard-of-care treatment with temozolomide.53 Cumulatively, these data provide a sufficient rationale for the use of immune checkpoint inhibitors, and a variety of clinical trials are currently under way using these strategies in glioblastoma patients.
Immunosuppressive Cells in the Glioma Microenvironment
Glioblastoma patients can be profoundly immunosuppressed. One key feature is a general paucity of CD4-positive T cells in peripheral blood with an increased proportion of Tregs.54 Chemokine C-C ligand 2, produced by glioblastoma cells, and other soluble factors55 can trigger the trafficking of Tregs to the tumor microenvironment.56 However, not all glioblastomas show a significant infiltration of Tregs,57 suggesting a heterogeneity of immune suppressive mechanisms. The functional activity of the Tregs in the tumor microenvironment may be further enhanced by the activity of IDO, an intracellular enzyme overexpressed in many cancers, including gliomas, which suppresses T cell activation and proliferation by creating a tryptophan shortage.58
Microglia and macrophages, the latter derived from monocytes, can constitute up to 12% of the tumor mass within gliomas.59 Macrophages are believed to be activated either by the classical pathway, involving bacterial products and interferon-γ (ie, M1), or by alternative activation with T-helper 2–type cytokines such as IL-4, IL-10, IL-13, and/or macrophage CSF.60 The M2 macrophages generally express CD163 and CD204 and display an immune suppressive phenotype. Glioblastomas have the highest frequency of infiltration by tumor-associated macrophages, which tend to be of the M2 lineage.61 Factors produced by glioblastoma cancer stem cells may be responsible for the skew to the M2 phenotype in the glioblastoma microenvironment.62 In a study of an immune modulating microRNA, miR-142-3p, it was discovered that M2 macrophages are dependent on the stimulation of the TGF-β receptor pathway, unlike M1 macrophages.63 Moreover, tumor-associated macrophages have been shown to enhance the invasiveness of glioma stem cells (GSCs) via the TGF-β1 signaling pathway.64 MDSCs also contribute to glioblastoma-mediated immune suppression.65,66 Normal monocytes exposed to glioma cells acquire properties akin to those of MDSCs.67 Both galectin-1 and arginase have been tied to MDSC suppressor function.68 Whereas both CD14-positive and CD15-positive MDSC subtypes have been found in the blood of glioma patients, it is the CD15 subtype that was found to be upregulated in the glioblastoma microenvironment.69 Based on data from other types of malignancies,70 it is likely that NK cells also play a key role in glioblastoma-mediated immune suppression, and this is an area of active investigation. An emerging area of therapeutic development has been directed toward targeting innate immunity.16
Hubs of Tumor-Mediated Immunosuppression
Given the heterogeneity and redundancy of immune suppressive mechanisms in glioblastoma, identifying key hubs that mediate many of these functions is theoretically appealing. Signal transducer and activator of transcription 3 (STAT3) is a transcription factor that has been found to be ubiquitously expressed in glioblastoma cells.71 IL-10 activation of STAT3 in macrophages inhibits their proliferation.72 Blocking STAT3 activation in the glioma microenvironment leads to an upregulation of immune-stimulatory cytokines IL-2, IL-4, IL-12, and IL-15 and of costimulatory molecules CD80 and CD86.72 GSCs are known to be resistant to conventional treatment methods and are likely to be capable of restoring all of the cells within a glioblastoma after other portions of the tumor are effectively treated.73 GSCs also cause immunosuppression in the glioma microenvironment by activating the STAT3 pathway and by increasing the number of Tregs.74 Hypoxia further induces STAT3 and its transcriptionally regulated downstream pathways, those of hypoxia inducible factor 1 α and vascular endothelial growth factor.75 A variety of approaches targeting STAT3 are currently in preclinical development.75–86
A second hub of glioblastoma-mediated immune suppression has recently been described. Fibrinogen-like protein 2 (FGL2) is a secreted factor that has been implicated by many different investigators as immunosuppressive. A recent study has shown that FGL2 is overexpressed in glioblastoma relative to low-grade glioma samples.87 An examination of data from The Cancer Genome Atlas showed that high levels of FGL2 mRNA portended worse survival for patients than did low expression. Furthermore, higher protein levels of FGL2 were found in high-grade gliomas than in low-grade tumors. FGL2 augmented glioma immunosuppression by increasing the expression levels of PD-1, expanding the frequency of tumor-supportive M2 macrophages, and enhancing the number of MDSCs and Tregs, especially within the glioma microenvironment. Anti-FGL2 antibody treatment not only increased survival time in a murine model of intracerebral gliomas but also reduced the number of Tregs, M2 immunosuppressive macrophages, and MDSCs, and decreased the expression of PD-1. Given these data, FGL2 may be an important immunosuppressive factor that merits further targeting, specifically in glioblastoma.
Placing Immune Suppressive Targeting into Therapeutic Context
Although there is currently great enthusiasm for the use of modulators to inhibit immune suppression, one needs to bear in mind that multiple steps are necessary for the development of an optimal antitumor immune response. First, there must be an immunological target (ie, antigen) present for the immune system to be directed toward. Examples of glioblastoma-specific targets and approaches include: epidermal growth factor receptor variant III, isocitrate dehydrogenase 1 mutation, and the IMA-950 glioblastoma profile panel. In the setting of minimal antigen or target expression, alternative immunotherapeutic approaches such as NK chimeric antigen receptor immunotherapy could be utilized. Second, the immune system must be activated. There are a variety of ways to do this, including triggering/engaging a costimulatory molecule such as 4-1BB or OX40; providing proinflammatory cytokines such as granulocyte macrophage CSF, interferon-γ, IL-12, and IL-15; engaging innate immunity with toll-like receptor agonists such as cytosine-phosphate-guanine or poly–inosinic:cytidylic acid; providing activated antigen-presenting cells by adoptive transfer of dendritic cells; or using stimulator of interferon gene (“Sting”) agonists. Thereafter, there needs to be sufficient trafficking and infiltration into the tumor site. This may depend on tumor expression of chemokines such as CX3CR1. Finally, there must be maintenance of the immune effector function that is blocked and inhibited by tumor-mediated immune suppression. Strategies that can be employed for this function include, but are not limited to, inhibition of immunosuppression cytokines such as TGF-β and IL-10; targeting of key hubs of multimodality immune suppression such as STAT3 and FGL2; and, the most successful thus far, use of immune checkpoint inhibitors.
As we move into the next generation of immunotherapeutic strategies, it is evident that combinatorial approaches that address each step in this continuum will be necessary to maximally impact outcomes. One needs to bear in mind that there is a great deal of plasticity within glioblastoma, and multiple operational mechanisms of immune suppression exist. When one mechanism of immune suppression is inhibited, other mechanisms can assume a dominant role. Thus, there has been an increasing interest in directing therapeutics to targets that control multiple mechanisms of tumor-mediated immune suppression. Until optimal combinatorial approaches are implemented, it is likely that glioblastoma patients will be stratified and selected for various immune therapeutic strategies based on immune biomarkers, similar to the strategies based on genomic profiling for targeted therapeutics.
Funding
The Dr. Marnie Rose Foundation and the National Institutes of Health CA1208113, P50 CA127001 and P30 CA016672 (A.B.H.).
Conflict of interest statement. M.W. has received research grants from Acceleron, Actelion, Alpinia Institute, Bayer, Isarna, MSD, Merck & Co, Novocure, PIQUR, and Roche and honoraria for lectures, advisory board participation, or consulting from Celldex, Immunocellular, Isarna, Magforce, MSD, Merck & Co, Northwest Biotherapeutics, Novocure, Pfizer, Roche and Teva. A.B.H. has received research grants from Merck, has been a paid consultant for Bristol Myers Squibb, serves on the Scientific Advisory Board of Caris Life Sciences, holds equity in Caris Life Sciences, and receives licensing and royalty fees from Celldex Therapeutics. E.N. has no conflicts of interest to report.
References
- 1.Wainwright DA, Sengupta S, Han Y, et al. Thymus-derived rather than tumor-induced regulatory T cells predominate in brain tumors. Neuro Oncol. 2011;13(12):1308–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nature immunology. 2010;11(1):7–13. [DOI] [PubMed] [Google Scholar]
- 3.Thompson CB, Allison JP. The emerging role of CTLA-4 as an immune attenuator. Immun. 1997;7(4):445–450. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192(2):303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wintterle S, Schreiner B, Mitsdoerffer M, et al. Expression of the B7-related molecule B7-H1 by glioma cells: a potential mechanism of immune paralysis. Cancer Res. 2003;63(21):7462–7467. [PubMed] [Google Scholar]
- 6.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. [DOI] [PubMed] [Google Scholar]
- 7.Kikuchi K, Neuwelt EA. Presence of immunosuppressive factors in brain-tumor cyst fluid. J Neurosurg. 1983;59(5):790–799. [DOI] [PubMed] [Google Scholar]
- 8.de Martin R, Haendler B, Hofer-Warbinek R, et al. Complementary DNA for human glioblastoma-derived T cell suppressor factor, a novel member of the transforming growth factor-beta gene family. EMBO J. 1987;6(12):3673–3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fontana A, Hengartner H, de Tribolet N, et al. Glioblastoma cells release interleukin 1 and factors inhibiting interleukin 2-mediated effects. J Immunol. 1984;132(4):1837–1844. [PubMed] [Google Scholar]
- 10.Wrann M, Bodmer S, de Martin R, et al. T cell suppressor factor from human glioblastoma cells is a 12.5-kd protein closely related to transforming growth factor-beta. EMBO J. 1987;6(6):1633–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frei K, Gramatzki D, Tritschler I, et al. Transforming growth factor-beta pathway activity in glioblastoma. Oncotarget. 2015;6(8):5963–5977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bogdahn U, Hau P, Stockhammer G, et al. Targeted therapy for high-grade glioma with the TGF-beta2 inhibitor trabedersen: results of a randomized and controlled phase IIb study. Neuro Oncol. 2011;13(1):132–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodon J, Carducci MA, Sepulveda-Sanchez JM, et al. First-in-human dose study of the novel transforming growth factor-beta receptor I kinase inhibitor LY2157299 monohydrate in patients with advanced cancer and glioma. Clin Cancer Res. 2015;21(3):553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ueda R, Fujita M, Zhu X, et al. Systemic inhibition of transforming growth factor-beta in glioma-bearing mice improves the therapeutic efficacy of glioma-associated antigen peptide vaccines. Clin Cancer Res. 2009;15(21):6551–6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alterman RL, Stanley ER. Colony stimulating factor-1 expression in human glioma. Mol Chem Neuropathol. 1994;21(2–3):177–188. [DOI] [PubMed] [Google Scholar]
- 16.Pyonteck SM, Akkari L, Schuhmacher AJ, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19(10):1264–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deryugina EI, Soroceanu L, Strongin AY. Up-regulation of vascular endothelial growth factor by membrane-type 1 matrix metalloproteinase stimulates human glioma xenograft growth and angiogenesis. Cancer Res. 2002;62(2):580–588. [PubMed] [Google Scholar]
- 18.Goldman CK, Kim J, Wong WL, et al. Epidermal growth factor stimulates vascular endothelial growth factor production by human malignant glioma cells: a model of glioblastoma multiforme pathophysiology. Mol Biol Cell. 1993;4(1):121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ke LD, Shi YX, Yung WK. VEGF(121), VEGF(165) overexpression enhances tumorigenicity in U251 MG but not in NG-1 glioma cells. Cancer Res. 2002;62(6):1854–1861. [PubMed] [Google Scholar]
- 20.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359(6398):843–845. [DOI] [PubMed] [Google Scholar]
- 21.Gabrilovich DI, Chen HL, Girgis KR, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2(10):1096–1103. [Erratum appears in Nat Med 1996 Nov;2(11):1267] [DOI] [PubMed] [Google Scholar]
- 22.Hishii M, Nitta T, Ishida H, et al. Human glioma-derived interleukin-10 inhibits antitumor immune responses in vitro. Neurosurg. 1995;37(6):1160–1166; discussion 1166–1167. [DOI] [PubMed] [Google Scholar]
- 23.Nitta T, Hishii M, Sato K, Okumura K. Selective expression of interleukin-10 gene within glioblastoma multiforme. Brain Res. 1994;649(1-2):122–128. [DOI] [PubMed] [Google Scholar]
- 24.Kuppner MC, Sawamura Y, Hamou MF, et al. Influence of PGE2- and cAMP-modulating agents on human glioblastoma cell killing by interleukin-2-activated lymphocytes. J Neurosurg. 1990;72(4):619–625. [DOI] [PubMed] [Google Scholar]
- 25.Hegardt P, Widegren B, Sjögren HO. Nitric-oxide–dependent systemic immunosuppression in animals with progressively growing malignant gliomas. Cell Immunol. 2000;200(2):116–127. [DOI] [PubMed] [Google Scholar]
- 26.Roth P, Aulwurm S, Gekel I, et al. Regeneration and tolerance factor: a novel mediator of glioblastoma-associated immunosuppression. Cancer Res. 2006;66(7):3852–3858. [DOI] [PubMed] [Google Scholar]
- 27.Sippel TR, White J, Nag K, et al. Neutrophil degranulation and immunosuppression in patients with GBM: restoration of cellular immune function by targeting arginase I. Clin Cancer Res. 2011;17(22):6992–7002. [DOI] [PubMed] [Google Scholar]
- 28.Authier A, Farrand KJ, Broadley KW, et al. Enhanced immunosuppression by therapy-exposed glioblastoma multiforme tumor cells. Int J Cancer. 2015;136(11):2566–2578. [DOI] [PubMed] [Google Scholar]
- 29.Kohanbash G, McKaveney K, Sakaki M, et al. GM-CSF promotes the immunosuppressive activity of glioma-infiltrating myeloid cells through interleukin-4 receptor-alpha. Cancer Res. 2013;73(21):6413–6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Badie B, Schartner J, Prabakaran S, et al. Expression of Fas ligand by microglia: possible role in glioma immune evasion. J Neuroimmunol. 2001;120(1-2):19–24. [DOI] [PubMed] [Google Scholar]
- 31.Weller M, Weinstock C, Will C, et al. CD95-dependent T cell killing by glioma cells expressing CD95 ligand: more on tumor immune escape, the CD95 counterattack, and the immune privilege of the brain. Cell Physiol Biochem. 1997;7:282–288. [Google Scholar]
- 32.Wischhusen J, Jung G, Radovanovic I, et al. Identification of CD70-mediated apoptosis of immune effector cells as a novel immune escape pathway of human glioblastoma. Cancer Res. 2002;62(9):2592–2599. [PubMed] [Google Scholar]
- 33.Chahlavi A, Rayman P, Richmond AL, et al. Glioblastomas induce T-lymphocyte death by two distinct pathways involving gangliosides and CD70. Cancer Res. 2005;65(12):5428–5438. [DOI] [PubMed] [Google Scholar]
- 34.Aulwurm S, Wischhusen J, Friese M, et al. Immune stimulatory effects of CD70 override CD70-mediated immune cell apoptosis in rodent glioma models and confer long-lasting antiglioma immunity in vivo. Int J Cancer. 2006;118(7):1728–1735. [DOI] [PubMed] [Google Scholar]
- 35.Miller J, Eisele G, Tabatabai G, et al. Soluble CD70: a novel immunotherapeutic agent for experimental glioblastoma. J Neurosurg. 2010;113(2):280–285. [DOI] [PubMed] [Google Scholar]
- 36.Roth P, Mittelbronn M, Wick W, et al. Malignant glioma cells counteract antitumor immune responses through expression of lectin-like transcript-1. Cancer Res. 2007;67(8):3540–3544. [DOI] [PubMed] [Google Scholar]
- 37.Berghoff AS, Kiesel B, Widhalm G, et al. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro Oncol. 2015;178:1064–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doucette TA, Rao G, Rao A, et al. Immune heterogeneity of glioblastoma subtypes: extrapolation from The Cancer Genome Atlas. Cancer Immunol Res. 2013;1(2):112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parsa AT, Waldron JS, Panner A, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13(1):84–88. [DOI] [PubMed] [Google Scholar]
- 40.Avril T, Saikali S, Vauleon E, et al. Distinct effects of human glioblastoma immunoregulatory molecules programmed cell death ligand-1 (PDL-1) and indoleamine 2,3-dioxygenase (IDO) on tumour-specific T cell functions. J Neuroimmunol. 2010;225(1–2):22–33. [DOI] [PubMed] [Google Scholar]
- 41.Bloch O, Crane CA, Kaur R, et al. Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clin Cancer Res. 2013;19(12):3165–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y, Carlsson R, Ambjorn M, et al. PD-L1 Expression by neurons nearby tumors indicates better prognosis in glioblastoma patients. J Neurosci. 2013;33(35):14231–14245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Butte MJ, Keir ME, Phamduy TB, et al. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27(1):111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–2526. [DOI] [PubMed] [Google Scholar]
- 46.Fecci PE, Ochiai H, Mitchell DA, et al. Systemic CTLA-4 blockade ameliorates glioma-induced changes to the CD4+ T cell compartment without affecting regulatory T-cell function. Clin Cancer Res. 2007;13(7):2158–2167. [DOI] [PubMed] [Google Scholar]
- 47.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32(10):1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384(9948):1109–1117. [DOI] [PubMed] [Google Scholar]
- 50.Vom Berg J, Vrohlings M, Haller S, et al. Intratumoral IL-12 combined with CTLA-4 blockade elicits T cell-mediated glioma rejection. J Exp Med. 2013;210(13):2803–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeng J, See AP, Phallen J, et al. Anti–PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys. 2013;86(2):343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wainwright DA, Chang AL, Dey M, et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4 and PD-L1 in mice with brain tumors. Clin Cancer Res. 2014;20(20):5290–5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sampson JH, Schmittling RJ, Archer GE, et al. A pilot study of IL-2Ralpha blockade during lymphopenia depletes regulatory T-cells and correlates with enhanced immunity in patients with glioblastoma. PLoS One. 2012;7(2):e31046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fecci PE, Mitchell DA, Whitesides JF, et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66(6):3294–3302. [DOI] [PubMed] [Google Scholar]
- 55.Crane CA, Ahn BJ, Han SJ, et al. Soluble factors secreted by glioblastoma cell lines facilitate recruitment, survival, and expansion of regulatory T cells: implications for immunotherapy. Neuro Oncol. 2012;14(5):584–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jordan JT, Sun W, Hussain SF, et al. Preferential migration of regulatory T cells mediated by glioma-secreted chemokines can be blocked with chemotherapy. Cancer Immunol Immunother. 2008;57(1):123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heimberger AB, Abou-Ghazal M, Reina-Ortiz C, et al. Incidence and prognostic impact of FoxP3+ regulatory T cells in human gliomas. Clin Cancer Res. 2008;14(16):5166–5172. [DOI] [PubMed] [Google Scholar]
- 58.Uyttenhove C, Pilotte L, Theate I, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9(10):1269–1274. [DOI] [PubMed] [Google Scholar]
- 59.Badie B, Schartner JM. Flow cytometric characterization of tumor-associated macrophages in experimental gliomas. Neurosurg. 2000;46(4):957–961; discussion 961–962. [DOI] [PubMed] [Google Scholar]
- 60.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3(1):23–35. [DOI] [PubMed] [Google Scholar]
- 61.Komohara Y, Ohnishi K, Kuratsu J, et al. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J Pathol. 2008;216(1):15–24. [DOI] [PubMed] [Google Scholar]
- 62.Wu A, Wei J, Kong LY, et al. Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro Oncol. 2010;12(11):1113–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu S, Wei J, Wang F, et al. Effect of miR-142–3p on the M2 macrophage and therapeutic efficacy against murine glioblastoma. J Natl Cancer Inst. 2014;106(8); doi:10.1093/jnci/dju162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ye XZ, Xu SL, Xin YH, et al. Tumor-associated microglia/macrophages enhance the invasion of glioma stem-like cells via TGF-beta1 signaling pathway. J Immunol. 2012;189(1):444–453. [DOI] [PubMed] [Google Scholar]
- 65.Almand B, Clark JI, Nikitina E, et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166(1):678–689. [DOI] [PubMed] [Google Scholar]
- 66.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181(8):5791–5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rodrigues JC, Gonzalez GC, Zhang L, et al. Normal human monocytes exposed to glioma cells acquire myeloid-derived suppressor cell-like properties. Neuro Oncol. 2010;12(4):351–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Verschuere T, Toelen J, Maes W, et al. Glioma-derived galectin-1 regulates innate and adaptive antitumor immunity. Int J Cancer. 2014;134(4):873–884. [DOI] [PubMed] [Google Scholar]
- 69.Gielen PR, Schulte BM, Kers-Rebel ED, et al. Increase in both CD14-positive and CD15-positive myeloid-derived suppressor cell subpopulations in the blood of patients with glioma but predominance of CD15-positive myeloid-derived suppressor cells in glioma tissue. J Neuropathol Exp Neurol. 2015;74(5):390–400. [DOI] [PubMed] [Google Scholar]
- 70.O'Sullivan T, Saddawi-Konefka R, Vermi W, et al. Cancer immunoediting by the innate immune system in the absence of adaptive immunity. J Exp Med. 2012;209(10):1869–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abou-Ghazal M, Yang DS, Qiao W, et al. The incidence, correlation with tumor-infiltrating inflammation, and prognosis of phosphorylated STAT3 expression in human gliomas. Clin Cancer Res. 2008;14(24):8228–8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O'Farrell AM, Liu Y, Moore KW, et al. IL-10 inhibits macrophage activation and proliferation by distinct signaling mechanisms: evidence for Stat3-dependent and -independent pathways. EMBO J. 1998;17(4):1006–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nduom EK, Hadjipanayis CG, Van Meir EG. Glioblastoma cancer stem-like cells: implications for pathogenesis and treatment. Cancer J. 2012;18(1):100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wei J, Barr J, Kong LY, et al. Glioblastoma cancer-initiating cells inhibit T-cell proliferation and effector responses by the signal transducers and activators of transcription 3 pathway. Mol Cancer Ther. 2010;9(1):67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wei J, Wu A, Kong LY, et al. Hypoxia potentiates glioma-mediated immunosuppression. PLoS One. 2011;6(1):e16195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hussain SF, Kong LY, Jordan J, et al. A novel small molecule inhibitor of signal transducers and activators of transcription 3 reverses immune tolerance in malignant glioma patients. Cancer Res. 2007;67(20):9630–9636. [DOI] [PubMed] [Google Scholar]
- 77.Ball S, Li C, Li PK, et al. The small molecule, LLL12, inhibits STAT3 phosphorylation and induces apoptosis in medulloblastoma and glioblastoma cells. PloS One. 2011;6(4):e18820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sai K, Wang S, Balasubramaniyan V, et al. Induction of cell-cycle arrest and apoptosis in glioblastoma stem-like cells by WP1193, a novel small molecule inhibitor of the JAK2/STAT3 pathway. J Neurooncol. 2012;107(3):487–501. [DOI] [PubMed] [Google Scholar]
- 79.Kohsaka S, Wang L, Yachi K, et al. STAT3 inhibition overcomes temozolomide resistance in glioblastoma by downregulating MGMT expression. Mol Cancer Ther. 2012;11(6):1289–1299. [DOI] [PubMed] [Google Scholar]
- 80.de Groot J, Liang J, Kong LY, et al. Modulating antiangiogenic resistance by inhibiting the signal transducer and activator of transcription 3 pathway in glioblastoma. Oncotarget. 2012;3(9):1036–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McFarland BC, Gray GK, Nozell SE, et al. Activation of the NF-kappaB pathway by the STAT3 inhibitor JSI-124 in human glioblastoma cells. Mol Cancer Res. 2013;11(5):494–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wei J, Wang F, Kong LY, et al. MiR-124 inhibits STAT3 signaling to enhance T cell–mediated immune clearance of glioma. Cancer Res. 2013;73(13):3913–3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ashizawa T, Akiyama Y, Miyata H, et al. Effect of the STAT3 inhibitor STX-0119 on the proliferation of a temozolomide-resistant glioblastoma cell line. Int J Oncol. 2014;45(1):411–418. [DOI] [PubMed] [Google Scholar]
- 84.Zheng Q, Han L, Dong Y, et al. JAK2/STAT3 targeted therapy suppresses tumor invasion via disruption of the EGFRvIII/JAK2/STAT3 axis and associated focal adhesion in EGFRvIII-expressing glioblastoma. Neuro Oncol. 2014;169:1229–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liang Q, Ma C, Zhao Y, et al. Inhibition of STAT3 reduces astrocytoma cell invasion and constitutive activation of STAT3 predicts poor prognosis in human astrocytoma. PloS One. 2013;8(12):e84723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fujita M, Zhu X, Sasaki K, et al. Inhibition of STAT3 promotes the efficacy of adoptive transfer therapy using type-1 CTLs by modulation of the immunological microenvironment in a murine intracranial glioma. J Immunol. 2008;180(4):2089–2098. [DOI] [PubMed] [Google Scholar]
- 87.Yan J, Kong LY, Hu J, et al. FGL2 as a multimodality regulator of tumor-mediated immune suppression and therapeutic target in gliomas. J Natl Cancer Inst. 2015;107(8); doi:10.1371/journal.pone.0018820. [DOI] [PMC free article] [PubMed] [Google Scholar]