Abstract
Cancer immunotherapy represents the biggest change in the cancer treatment landscape in the last several years. Indeed, the clinical successes in several cancer types have generated widespread enthusiasm that immune-based treatments may influence the management of patients with malignant brain tumors as well. A number of promising clinical trials in this area are currently ongoing in neuro-oncology, and a wave of additional efforts are sure to follow. However, the basic immunology underlying immunotherapy—and the nuances unique to the immunobiology in the central nervous system—is often not in the daily lexicon of the practicing neuro-oncologist and neurosurgeon. To this end, here we provide a timely and working overview of key principles of fundamental immunology as a pragmatic context for understanding where therapeutic efforts may act in the cellular dynamics of the immune response. Moreover, we review the issues of lymphatic drainage, antigen presentation, and the blood–brain barrier as considerations that are germane to thinking about immunity to tumors arising in the brain. Together, these topics will provide a foundation for the exciting efforts in immune-based treatments that will hopefully provide real benefit to brain tumor patients.
Keywords: adaptive immunity, antigen presentation, blood-brain barrier, immunology, immunotherapy, innate immunity
There has been a rather general feeling that the host has only a limited capacity at best to rid itself of naturally arising cancer cells. … However, until we know how to direct the full force of specific immunity against tumor cells, the true magnitude of this potential will remain unknown. … With the advances that have been made and the powerful new tools that are available, the cancer immunologist's long search for specificity may finally be rewarded.”
Lloyd J. Old, “Cancer Immunology: The Search for Specificity.” G.H.A. Clowes Memorial Award.1
This prescient quote from one of the scions of cancer immunology, the late Lloyd Old, reflects the powerful and growing influence of immunity in oncology today. In fact, the introduction of therapeutics that augment the immune system's ability to fight cancer represents one of the—if not the—most exciting areas in oncology today.2 Drugs targeting molecules such as cytotoxic T-lymphocyte-associated protein 4 (CTLA4) and the programmed cell death protein 1/programmed death-ligand 1 (PD1/PDL1) axis have shown dramatic clinical responses across a range of cancer types,3–5 generating tremendous enthusiasm for cancer immunotherapy and a sense that we are only at the tip of the iceberg with these approaches. For career cancer immunotherapists, this recent evolution represents a stunning redemption for a field that has been moribund more often than not over the last 4 decades (reviewed by Dunn et al6). Thus, it is clear that understanding the immune system and its influence in cancer is no longer the sole domain of the basic scientist but also of the practicing clinician treating patients. From peptide-based cancer vaccines such as rindopepimut7 to cell-based therapies such as DCVax,8 immunology is influencing neuro-oncology today.
Herein, we review several key general principles in immunology and comment on perceived nuances that may be more relevant in the brain than in other anatomic sites. Specifically, we will review the role of the immune system, the cellular compartments that compose it, how immune cells recognize antigens, the basics of lymphocyte signaling, and finally the unique features of central nervous system immunobiology. Although a comprehensive overview of basic immunology is beyond the scope of this review, the concepts we emphasize are directed at framing a timely working understanding of brain tumor immunology and immunotherapy as neuro-oncologists begin to integrate immune-based treatments into their practices and open exciting clinical trials at their institutions worldwide.
Cellular Compartments of the Immune System: “Innate” and “Adaptive”
The immune system exists in humans not only to protect individuals from infection but also, more broadly, to recognize “danger” or other deviations from normal physiologic homeostasis.9 This view of the immune system is inclusive and helpful in order to understand how immune cells could recognize “self” and eliminate tumor cells that are “nonviral” in etiology, for instance. The many cell types that make up a functional immune system are often grouped into “innate” and “adaptive” categories. While there is some utility to this compartmentalization, it is important to remember that immunity is highly integrated, and effective immune responses reflect a complex orchestration among many cell types.
Innate Immunity
A critical distinction between innate and adaptive immune cells is the molecular basis of antigen recognition, which, in turn, reflects the evolutionary role that each cell type likely plays in response to infections, tumors, or cell death. Innate cells can be considered the first line of defense in the immune response and include macrophages, natural killer (NK) cells, basophils, eosinophils, neutrophils, and dendritic cells (DCs). A common misconception is that innate recognition is “nonspecific” in nature. In contrast, innate cells such as macrophages and DCs express receptors, termed “pattern recognition receptors,” that recognize conserved structures, termed “pathogen associated molecular patterns” (PAMPs) on microbes.10 For example, one of the ligands for Toll-like receptor 4 is lipopolysaccharide, a moiety found in bacterial cell membranes.11 Additional classes of receptors recognize “danger associated molecular patterns” (DAMPs), which include heat shock proteins, uric acid, high-mobility group box 1 protein (HMGB1), and other structures available during tissue damage and cell death.12 Tumor cell DNA may also activate innate immunity via the stimulator of interferon genes (STING) pathway.13,14 Additionally, NK cells express an array of activating and inhibitory receptors that are influenced by the expression of major histocompatibility complex (MHC) molecules on target cells.15 Perhaps the most canonical CNS innate cells are the microglia, a population similar to tissue-resident macrophages which performs a broad range of protective functions, including danger-signal recognition, phagocytosis, and immunoregulatory cytokine secretion in addition to dynamic roles in neuronal health and survival.16 Importantly, innate cell receptors are encoded in the germline and are heritable, in contrast to antigen receptors in adaptive immunity. Thus, innate immune cells are early responders to the presence of infection or tissue damage which initiates a functional immune response.
Adaptive Immunity
The adaptive compartment of the immune system comprises T and B lymphocytes. Whereas innate immune cells act early in the initiation of the immune response, there is a delay of several days before naïve T and B cells are able to exert their effector functions. The power of the adaptive immune system lies in the incredible diversity and specificity of its recognition as well as the memory of antecedent antigen encounter that can be manifested in a recall response later in life. Although innate immune cells express germline-encoded receptors, the molecular structure of antigen receptors in mature T and B cells is completely distinct. Specifically, T and B cell antigen receptors are generated by a process called somatic recombination—ie, gene segments are recombined imperfectly after birth in a stochastic mechanism that generates a diversity repertoire that exceeds 2 × 107 distinct types of T cells in humans.17 It is statistically impossible for any 2 individuals to harbor identical adaptive lymphocyte specificities—even identical twins. T cell receptors are heterodimers of alpha and beta chains, whereas B cells express immunoglobulin antibody receptors generated in a similar recombination process. The extraordinary diversity and specificity of the adaptive immune system is central to the many ongoing efforts to exploit the immune system to treat cancer.
How T Cells Recognize Antigens: Antigen Presentation and Signaling
T cells recognize peptide fragments of distinct lengths only when they are bound and “presented” to them in specific MHC molecules. MHC class I molecules present short peptides of 8–10 amino acids in length to CD8+ T cells, whereas MHC class II molecules present longer peptides to CD4+ T cells. Whereas all nucleated cells express MHC class I molecules (human leukocyte antigen [HLA]–A, –B, and –C in humans), expression of MHC class II (HLA-DP, -DQ, and -DR) is typically restricted to macrophages, DCs, and some epithelial and endothelial subsets. In both cases, the heterodimeric T cell receptor must contact residues in both the presented antigen and the MHC molecule, which is the basis of the concept of “MHC restriction.”18 In cancer immunology, T cells may recognize a number of distinct antigens present in tumor cells but not normal cells, including the protein products of expressed somatic mutations, termed “neoantigens.”19,20 MHC molecules are highly polymorphic, and therefore antigens capable of presentation by one individual may not be well presented by another individual harboring a distinct set of MHC alleles, or haplotype. T cells become activated when 2 criteria are met: (a) their T cell receptors engage cognate antigen presented by MHC and (b) costimulation occurs by interaction of T cell CD28 with molecule B7.1 or B7.2. Negative costimulatory signals, or “checkpoints,” attenuate costimulation and impair T cell responses. These checkpoints include CTLA4 and the PD1/PDL1 axis and can be thought of as a type of immune system brake.
Afferent and Efferent Pathways in an Integrated Immune Response: Where Therapies Converge
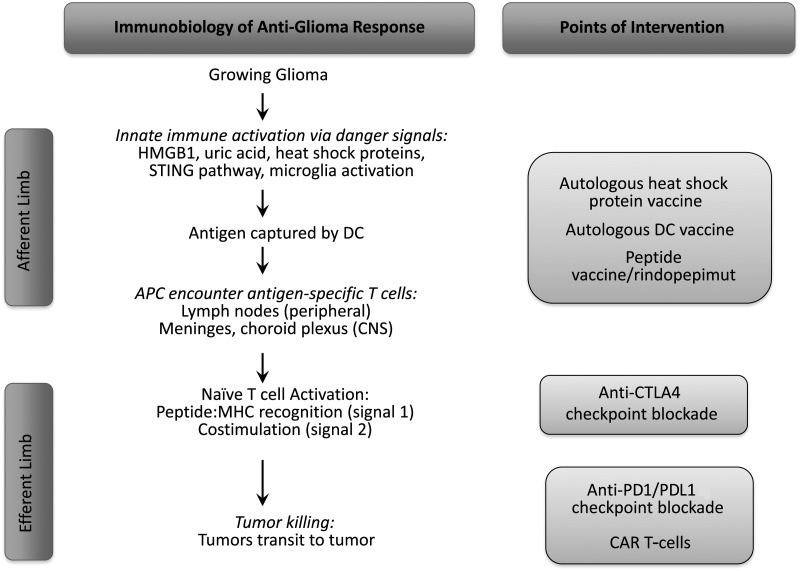
During the development of an integrated immune response to tumors, the innate and adaptive compartments work together to stimulate immunity that is protective, specific, and potentially long-lasting (schematized in Fig. 1). Importantly, emerging immunotherapies are designed to augment responses at several crucial steps, highlighting the complexity of this process and the manifold ways it can be modulated. The CNS-specific nuances of this process will be discussed below. First, antigen presenting cells (APCs) of the innate immune system, such as DCs, ingest tumor-specific antigens following activation by by-products of altered tissue or cell death such as DAMPs. Heat shock proteins released from tumor cells may be particularly effective chaperones for tumor-specific peptide antigens and may both activate DCs and serve as antigen couriers; the administration of autologous heat shock complexes is the basis for an ongoing phase II clinical trial (NCT01814813).21 Similarly, microglia can be activated by these components and exhibit a greater effector role than antigen presenting role relative to DCs. Subsequently, activated DCs migrate to draining lymph nodes, which are secondary lymphoid tissues that serve as hubs where naïve T cells sample antigens presented by immigrant DCs. The capture of antigen and its successful presentation to T cells is the critical step in the afferent pathway of the immune response, and several clinical trial approaches are based on the hypothesis that this pathway is impaired in glioblastoma patients. For instance, DCs pulsed with autologous tumor cells are adoptively transferred in the DCVax trial in an effort to augment antigen presentation. Moreover, vaccine-based trials, such as the rindopepimut trials, attempt to provide a higher level of tumor-specific antigen to fuel the afferent limb of the immune response.
Fig. 1.
Schematic diagram of the afferent and efferent limbs of the anti-glioma immune response and where distinct immunotherapeutic modalities may act. CAR, chimeric antigen receptor.
When naïve tumor-specific T cells recognize their cognate antigen on MHC class I and II molecules, both CD4+ helper T cells and CD8+ cytotoxic T cells become activated, which is the last phase of the afferent pathway of immunity that can be abrogated by checkpoint blockade antibodies that inhibit CTLA4, a negative regulator of this step.22 Subsequently, the efferent limb of adaptive immunity comprises activated T cells homing to the tumor location and attempting to eradicate their targets. Checkpoint blockade antibodies inhibiting the PD1/PDL1 axis are thought to act at this efferent step.22 Thus, the development of immunity to growing tumors is an integrated and dynamic process, and understanding the salient sequence of events is critical to understanding efforts to augment its efficacy in a number of distinct steps during its evolution.
Nuances of Central Nervous System Immunobiology
In contrast to long-standing dogma, we do not view the CNS as “immunologically privileged.”23 This terminology represents a conceptual albatross that has likely attenuated enthusiasm for CNS immunotherapies over decades. However, a number of clinical scenarios—such as infectious encephalitis and autoimmune demyelinating disease—demonstrate clearly that the CNS is not immunologically quiescent. However, there are features of CNS immunobiology that remain poorly understood due to the anatomic differences between this system and others and render the CNS immunologically specialized rather than inert. Specifically, we will review 3 of these areas below—(i) lymphatic drainage, (ii) antigen presentation, and (iii) the blood–brain barrier. An improved understanding of these areas will likely be translationally relevant.
Are There Draining Lymph Nodes from the Brain?
The lack of obvious lymphoid tissue in the brain is a clear distinction between the CNS and most other anatomic sites in the body. However, several studies in animals and humans have raised the possibility that antigens may drain to the cervical lymph node chain, thereby providing a plausible mechanism by which the immune system samples the CNS.24 Specifically, radiolabeled antigen injected into a range of intracerebral sites can be recovered in the cervical or retropharyngeal lymph nodes.25,26 Moreover, in models of autoimmune encephalitis, immunizing antigens were recovered within cellular populations in the cervical lymph nodes.27 At least experimentally, intracranially injected antigen appears to track through the subarachnoid space to the cribriform plate of the ethmoid bone in the anterior skull base,24,28,29 where it transits to the nasal mucosa through perivascular spaces to enter the lymphatic basins. In humans, a recent study identified similar populations of B cells in both the cervical lymph nodes and the brains of multiple sclerosis patients, suggesting that pathogenic B cells reside in the lymph node terminus as part of the demyelinating disease course.30 Taken together, these observations support a model in which antigens—such as those from tumors or infectious etiologies—are made available to the peripheral immune system by a CSF-to-lymphatics pathway across the skull base, thereby representing a possible key conduit for an effective afferent immune response. Strikingly, 2 remarkable recent studies showed evidence that a true lymphatic drainage system exists in the meninges of the dural venous sinuses in mice,31,32 strongly corroborating this model and raising the intriguing possibility that a similar system may exist in humans. The accessibility of CNS antigen to secondary lymphoid tissue may be via soluble draining antigen or via antigen-loaded APCs emigrating from the brain. This model is appealing to brain tumor immunotherapists and immunologists because it adheres to conventional thinking that antigen must be presented in secondary lymphoid structures such as lymph nodes in order for an effective immune response to be initiated. Nevertheless, further work is needed to determine the physiologic relevance of the role of select lymph node sites to brain tumor immunity and whether other described areas of possible antigen presentation—such as the meninges and choroid plexus33,34—also play a role or are actually more critical to this process than previously appreciated. Ultimately, it is clear that clarifying the anatomic basis for antigen presentation may significantly influence how we vaccinate in the therapeutic setting.
Antigen Presentation in the CNS
Ongoing work has focused on identifying the cellular basis for the development of immune responses in the CNS. Many cell types have been implicated as possible CNS APCs, including endothelial cells, astrocytes, microglia, perivascular macrophages, choroid plexus epithelial cells, and DCs.35,36 It has been challenging to determine which of these subsets is the physiologically relevant APC. However, recent work provides strong evidence that the DC may play a key role in CNS antigen presentation.37 DCs carrying a model antigen drain to the cervical lymph nodes when injected intracerebrally and lead to the development of a systemic immune response.38 These findings were corroborated by a similar observation that DCs injected into tumors in murine glioma models transited to the cervical lymph nodes and stimulated an increase in intratumoral T cells.39–42 Moreover, DCs were sufficient to induce the development of autoimmunity in a preclinical model of multiple sclerosis, and candidate DCs were identified in autopsy human tissue.43
Two recent studies shed additional light on where antigen-presenting DCs are found in the steady state of normal mouse brains. Specifically, conventional DCs were identified in the choroid plexus and in the meninges and were able to present antigen to T cells.34 Moreover, DCs were identified in the rostral migrating stream in transit to the cervical lymph nodes.44 Together, these findings suggest that DCs may represent the pivotal cell that presents antigen to T cells. Ultimately, additional work in physiologically relevant brain tumor models will be necessary to determine the requirement for this cell type in anti-glioma immunity.
The Blood–Brain Barrier
Next to immune privilege, the blood–brain barrier is another concept that is frequently used to dismiss the potential utility of immunotherapy in the treatment of brain tumors. However, the more we understand the biology of this structure, the less of an obstacle it appears to be in brain tumor immunobiology. The blood–brain barrier refers to an anatomic structure that limits the promiscuous transit of molecules between the intraluminal space of CNS capillaries and the parenchyma of the brain. Importantly, this barrier is not a single entity but rather comprises several features. First, tight junctions, which are apposed multiprotein adhesion complexes,45 link brain capillary endothelial cells together much more closely than what is observed in the systemic circulation. Much of the surface area of the CNS capillary system is also ensheathed by the basement membranes of pericytes and the foot processes of astrocytes that make up the glia limitans (reviewed by Abbott et al46). Select regions of the brain lack a functional blood–brain barrier; these would be the circumventricular organs: neurohypophysis, median eminence, vascular organ of the lamina terminalis, subforniceal organ, pineal gland, subcommisural organ, choroid plexus, and the area postrema. Within brain tumors, the blood–brain barrier is often substantially altered and dysregulated. For instance, endothelial cells often appear dysmorphic, and close apposition of capillary endothelium can be lost.47,48 Moreover, recent work suggests that brain tumor–initiating cells may contribute to capillary endothelial composition within malignant gliomas,49 and gliomas may actually disrupt the encircling astrocytic foot processes and perturb the normal homeostatic regulation of local cerebral vasculature.50 While the intact blood–brain barrier may restrict the porous ingress of solutes and molecules from the circulation, lymphocytes are able to traverse this structure via chemokine axes and multistep adhesion processes,51 which has been directly demonstrated in preclinical models of autoimmunity using stunning intravital techniques.52 In the glioblastoma setting, transport across the barrier may be altered; although paracellular transport may be less restricted, the possibility exists that dysregulated endothelium may impair the active trafficking of lymphocytes into brain tissue.53 Further studies will be pivotal to clarify the influence of the blood–brain barrier on immunotherapeutic approaches. Importantly, this structure should not be viewed as a static and absolutely hermetic seal to immune-based treatments for brain tumors, however.
Conclusions
The application of immunology to the treatment of cancer represents an incredibly exciting development in oncology, and the rise of immunotherapy will continue to be visible, from the portfolios of pharmaceutical giants to the offices of clinicians around the world. There is no question that immunotherapy will have a large footprint on the clinical trial efforts in neuro-oncology over the next several years as well,54 especially as we understand further the degree of immunologic compromise often seen in glioma patients.55 In this review, we have revisited the basic principles of immunology and highlighted several areas that are unique to our thinking about immune response in the CNS. As additional brain tumor immunotherapy efforts evolve, we will continue to learn new insights into how spontaneous immune responses to brain tumors develop, how best to stimulate therapeutic immunity in our patients, how stimulated immunity fails, and which patients are best suited to immune-based treatments. Ultimately, our commitment to rigorous science in the study of CNS immunobiology and our ability to exploit our discoveries in translational settings will give us the best chance of improving the lives of patients with malignant brain tumors.
Funding
None declared.
Conflict of interest statement. None declared.
References
- 1.Old LJ. Cancer immunology: the search for specificity—G.H.A. Clowes Memorial Lecture. Cancer Res. 1981;41(2):361–375. [PubMed] [Google Scholar]
- 2.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. [DOI] [PubMed] [Google Scholar]
- 3.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991–998. [DOI] [PubMed] [Google Scholar]
- 7.Sampson JH, Archer GE, Mitchell DA, et al. An epidermal growth factor receptor variant III–targeted vaccine is safe and immunogenic in patients with glioblastoma multiforme. Mol Cancer Ther. 2009;8(10):2773–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wheeler CJ, Black KL. DCVax-Brain and DC vaccines in the treatment of GBM. Exp Opin Invest Drugs. 2009;18(4):509–519. [DOI] [PubMed] [Google Scholar]
- 9.Matzinger P. Tolerance, danger, and the extended family. Ann Rev Immunol. 1994;12:991–1045. [DOI] [PubMed] [Google Scholar]
- 10.Janeway CA., Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):1–13. [DOI] [PubMed] [Google Scholar]
- 11.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388(6640):394–397. [DOI] [PubMed] [Google Scholar]
- 12.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8(4):279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woo SR, Fuertes MB, Corrales L, et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 2014;41(5):830–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohkuri T, Ghosh A, Kosaka A, et al. STING contributes to antiglioma immunity via triggering type I IFN signals in the tumor microenvironment. Cancer Immunol Res. 2014;2(12):1199–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yokoyama WM. Natural killer cell immune responses. Immunol Res. 2005;32(1–3):317–325. [DOI] [PubMed] [Google Scholar]
- 16.Nayak D, Roth TL, McGavern DB. Microglia development and function. Ann Rev Immunol. 2014;32:367–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nikolich-Zugich J, Slifka MK, Messaoudi I. The many important facets of T-cell repertoire diversity. Nat Rev. Immunol. 2004;4(2):123–132. [DOI] [PubMed] [Google Scholar]
- 18.Zinkernagel RM, Doherty PC. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature. 1974;248(5450):701–702. [DOI] [PubMed] [Google Scholar]
- 19.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69–74. [DOI] [PubMed] [Google Scholar]
- 20.Hacohen N, Fritsch EF, Carter TA, et al. Getting personal with neoantigen-based therapeutic cancer vaccines. Cancer Immunol Res. 2013;1(1):11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crane CA, Han SJ, Ahn B, et al. Individual patient-specific immunity against high-grade glioma after vaccination with autologous tumor derived peptides bound to the 96 KD chaperone protein. Clin Cancer Res. 2013;19(1):205–214. [DOI] [PubMed] [Google Scholar]
- 22.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Medawar PB. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol. 1948;29(1):58–69. [PMC free article] [PubMed] [Google Scholar]
- 24.Laman JD, Weller RO. Drainage of cells and soluble antigen from the CNS to regional lymph nodes. J Neuroimmune Pharmacol. 2013;8(4):840–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bradbury MW, Cserr HF, Westrop RJ. Drainage of cerebral interstitial fluid into deep cervical lymph of the rabbit. Am J Physiol. 1981;240(4):F329–F336. [DOI] [PubMed] [Google Scholar]
- 26.Szentistvanyi I, Patlak CS, Ellis RA, Cserr HF. Drainage of interstitial fluid from different regions of rat brain. Am J Physiol. 1984;246(6 Pt 2):F835–F844. [DOI] [PubMed] [Google Scholar]
- 27.de Vos AF, van Meurs M, Brok HP, et al. Transfer of central nervous system autoantigens and presentation in secondary lymphoid organs. J Immunol. 2002;169(10):5415–5423. [DOI] [PubMed] [Google Scholar]
- 28.Goldmann J, Kwidzinski E, Brandt C, et al. T cells traffic from brain to cervical lymph nodes via the cribroid plate and the nasal mucosa. J Leukocyte Biol. 2006;80(4):797–801. [DOI] [PubMed] [Google Scholar]
- 29.Kaminski M, Bechmann I, Pohland M, et al. Migration of monocytes after intracerebral injection at entorhinal cortex lesion site. J Leukocyte Biol. 2012;92(1):31–39. [DOI] [PubMed] [Google Scholar]
- 30.Stern JN, Yaari G, Vander Heiden JA, et al. B cells populating the multiple sclerosis brain mature in the draining cervical lymph nodes. Sci Transl Med. 2014;6(248):248ra107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Louveau A, Smirnov I, Keyes TJ, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523(7560):337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aspelund A, Antila S, Proulx ST, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212(7):991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bartholomaus I, Kawakami N, Odoardi F, et al. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature. 2009;462(7269):94–98. [DOI] [PubMed] [Google Scholar]
- 34.Anandasabapathy N, Victora GD, Meredith M, et al. Flt3L controls the development of radiosensitive dendritic cells in the meninges and choroid plexus of the steady-state mouse brain. J Exp Med. 2011;208(8):1695–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sedgwick JD, Hickey WF. Antigen presentation in the central nervous system. In: Keane RW, Hickey WF, eds. Immunology of the Nervous System. New York: Oxford University Press; 1997:364–418. [Google Scholar]
- 36.Becher B, Bechmann I, Greter M. Antigen presentation in autoimmunity and CNS inflammation: how T lymphocytes recognize the brain. J Mol Med. 2006;84(7):532–543. [DOI] [PubMed] [Google Scholar]
- 37.McMahon EJ, Bailey SL, Miller SD. CNS dendritic cells: critical participants in CNS inflammation? Neurochem Int. 2006;49(2):195–203. [DOI] [PubMed] [Google Scholar]
- 38.Karman J, Ling C, Sandor M, Fabry Z. Initiation of immune responses in brain is promoted by local dendritic cells. J Immunol. 2004;173(4):2353–2361. [DOI] [PubMed] [Google Scholar]
- 39.Ehtesham M, Kabos P, Gutierrez MA, et al. Intratumoral dendritic cell vaccination elicits potent tumoricidal immunity against malignant glioma in rats. J Immunother. 2003;26(2):107–116. [DOI] [PubMed] [Google Scholar]
- 40.Fujita M, Zhu X, Ueda R, et al. Effective immunotherapy against murine gliomas using type 1 polarizing dendritic cells—significant roles of CXCL10. Cancer Res. 2009;69(4):1587–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuwashima N, Nishimura F, Eguchi J, et al. Delivery of dendritic cells engineered to secrete IFN-{alpha} into central nervous system tumors enhances the efficacy of peripheral tumor cell vaccines: dependence on apoptotic pathways. J Immunol. 2005;175(4):2730–2740. [DOI] [PubMed] [Google Scholar]
- 42.Okada H, Tsugawa T, Sato H, et al. Delivery of interferon-alpha transfected dendritic cells into central nervous system tumors enhances the antitumor efficacy of peripheral peptide-based vaccines. Cancer Res. 2004;64(16):5830–5838. [DOI] [PubMed] [Google Scholar]
- 43.Greter M, Heppner FL, Lemos MP, et al. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med. 2005;11(3):328–334. [DOI] [PubMed] [Google Scholar]
- 44.Mogensen KE, Vignaux F, Gresser I. Enhanced expression of cellular receptors for human interferon alpha on peripheral lymphocytes from patients with Down's syndrome. FEBS Lett. 1982;140(2):285–287. [DOI] [PubMed] [Google Scholar]
- 45.Huber JD, Egleton RD, Davis TP. Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends Neurosci. 2001;24(12):719–725. [DOI] [PubMed] [Google Scholar]
- 46.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7(1):41–53. [DOI] [PubMed] [Google Scholar]
- 47.Long DM. Capillary ultrastructure and the blood-brain barrier in human malignant brain tumors. J Neurosurg. 1970;32(2):127–144. [DOI] [PubMed] [Google Scholar]
- 48.Vajkoczy P, Menger MD. Vascular microenvironment in gliomas. J Neurooncol. 2000;50(1–2):99–108. [DOI] [PubMed] [Google Scholar]
- 49.Wang R, Chadalavada K, Wilshire J, et al. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010;468(7325):829–833. [DOI] [PubMed] [Google Scholar]
- 50.Watkins S, Robel S, Kimbrough IF, Robert SM, Ellis-Davies G, Sontheimer H. Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nature Communications. 2014;5:4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holman DW, Klein RS, Ransohoff RM. The blood-brain barrier, chemokines and multiple sclerosis. Biochim Biophys Acta. 2011;1812(2):220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lodygin D, Odoardi F, Schlager C, et al. A combination of fluorescent NFAT and H2B sensors uncovers dynamics of T cell activation in real time during CNS autoimmunity. Nat Med. 2013;19(6):784–790. [DOI] [PubMed] [Google Scholar]
- 53.Ransohoff RM, Kivisakk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol. 2003;3(7):569–581. [DOI] [PubMed] [Google Scholar]
- 54.Reardon DA, Freeman G, Wu C, et al. Immunotherapy advances for glioblastoma. Neuro Oncol. 2014;16(11):1441–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dunn GP, Fecci PE, Curry WT. Cancer immunoediting in malignant glioma. Neurosurgery. 2012;71(2):201–222. discussion 222–223. [DOI] [PubMed] [Google Scholar]