Abstract
Objectives
To test the hypothesis that men and women with both low bone mineral density and sarcopenia have a higher risk of fracture than those with only one or neither conditions.
Design
The Osteoporotic Fractures in Men study and the Study of Osteoporotic Fractures in women are prospective observational studies with a mean follow up of 9 years (2000–2012) and 8years (1997–2009) respectively.
Setting
US clinical centers
Participants
5,544 men (mean age=73.7 years) and 1,114 women (mean age=77.6 years); all ≥age 65; able to walk without assistance, and without bilateral hip replacement.
Measurements
Sarcopenia was defined as low appendicular lean mass plus either slowness or weakness; and low bone mineral density, by the World Health Organization definition of T-score<−1.0. Participants were classified as normal bone mineral density and no sarcopenia (N=3367 men, 308 women); sarcopenic only (N=79 men; 48 women); low bone mineral density only (N=1986 men; 626 women), and low bone mineral density and sarcopenic (N=112 men; 132 women).
Results
Compared to men with normal bone mineral density and no sarcopenia, the Hazard ratio [HR] for fracture was 3.79 (95% confidence interval [CI], 2.65–5.41) among men with low bone mineral density and sarcopenia, 1.67 (95% CI, 1.45–1.93) among men with low bone mineral density only, and 1.14 (95% CI, 0.62–2.09) among men with sarcopenia only. Women with low bone mineral density and sarcopenia (HR, 2.27; 95% CI, 1.37–3.76), and women with low bone mineral density alone (HR, 2.62; 95% CI, 1.74–3.95), but not women with only sarcopenia had increased risk of fracture compared to normal women.
Conclusion
Men with both low bone mineral density and sarcopenia are at especially high risk of fracture.
Keywords: EPIDEMIOLOGY, OSTEOPOROSIS, SARCOPENIA, BONE-MUSCLE INTERACTIONS, FRACTURE RISK ASSESSMENT
INTRODUCTION
Age-related deterioration in both bone and muscle manifested as low bone mineral density (BMD) and sarcopenia may contribute to fractures. It is well established that individuals with low BMD have an increased risk of fracture (1).
Falls and functional impairments, which are known to be associated with fractures, have been previously linked to sarcopenia(2) (3). Furthermore, myosteatosis, which results in reduced muscle strength and function, has been associated with fractures (4) (5). Hence, sarcopenia may increase risk of fractures.
Sarcopenia was initially defined as the loss of muscle mass (6). However, more recent definitions of sarcopenia add components of muscle strength and/or physical performance, because the loss of muscle mass is not sufficient to characterize the sarcopenic syndrome (7). Inclusion of these additional measures in the operational definition of sarcopenia may improve the prediction of clinical outcomes, such as fractures.
In 2009, the term “sarco-osteopenia” was coined to emphasize that both weak bones and weak muscles may contribute to fractures in the elderly (8). To our knowledge, the combined effect of both sarcopenia, defined as low muscle mass and strength, and low BMD on fracture risk has not yet been studied.
The purpose of the current study was to compare the incidence of non-vertebral fractures among men and women based on both low BMD and sarcopenia. We hypothesized that individuals with both sarcopenia and low BMD will have the greatest risk of fracture compared to individuals with only one or neither condition.
METHODS
Study population
We examined data of 5,544 white and black men (mean age=73.7years) from the Osteoporotic Fractures in Men (MrOS) study and 1,114 white and black women (mean age=77.6 years) from the Study of Osteoporotic Fractures (SOF). The MrOS and SOF studies are both multicenter prospective cohort studies designed to identify risk factors for osteoporosis and osteoporotic fracture. In MrOS, 5994 older men were recruited from six sites (Birmingham, AL; Minneapolis, MN; Palo Alto, CA; Pittsburgh, PA; Portland, OR; and San Diego, CA) across the United States from March 2000 to April 2002(9, 10). In SOF, 9,704 women were recruited from four US sites (Baltimore, MD; Minneapolis, MN; Pittsburgh, PA; Portland, OR) between 1986–1988. The original SOF cohort was enhanced by the addition of 662 African American women recruited between 1997–98. To be eligible, both men and women needed to be age 65 years or older, be able to walk without assistance from another person, and have reported no bilateral hip replacement. Human subjects approval was obtained at all sites with written informed consent obtained from all participants.
Body composition by whole body Dual Energy X-ray Absorptiometry (DXA) was available for the 5,544 white and black MrOS men at baseline and a subset of 1,114 white and black SOF women (when recruited for the year 10 exam). Women without whole body DXA were not included since sarcopenia cannot be assessed without appendicular lean mass.
Bone Mineral Density (BMD) measurement
In men, total hip BMD (g/cm2) and femoral neck BMD (g/cm2) were measured using DXA Hologic QDR 4500(Bedford, MA). In women, BMD was measured by DXA using Hologic 1000 and 2000 scanners. Details of the measurement and densitometry procedures have been published elsewhere (11) (12). Briefly, certified technicians performed the DXA scans following a strict protocol. To assess longitudinal performance of the scanners, an anthropometric spine phantom was scanned daily and a hip phantom weekly at each clinical center. In both genders, the right hip was scanned unless there was a fracture, implant, hardware, or other problem, in which case the left hip was scanned. Individuals were classified as having low BMD according to the 1994 World Health Organization (WHO) recommendations if their femoral neck T-score was < −1 (13). Subjects were considered to have normal BMD if their T-score was >= −1. The T-score was calculated using the National Health and Nutrition Examination Survey III reference database (14). Young Caucasian women were used as the reference population in both men and women as recommended by the International Society for Clinical Densitometry (ISCD) (15).
Sarcopenia assessment
The definition of sarcopenia was based on the European Working Group on Sarcopenia in Older persons (EWSOP) (7). Participants were classified as sarcopenic if they had low lean mass plus either slowness (classified by gait speed) or weakness (assessed by grip strength). Low lean mass was defined using the approach of Newman et al (16) to correct appendicular lean mass for height and fat mass. Linear regression was used to model the relationship between appendicular lean mass on height (meters) and fat mass (kg). The 20th percentile of the distribution of residuals was used as the cutpoint for low muscle mass. Separate models were fit for men and women. We concentrated on the residual method because in the Framingham study, the residuals method was associated with mobility limitations in both men and women but other definitions of sarcopenia were not (17). Walking speed was calculated as the average two usual walking pace attempts over 6 meters and expressed as m/s. Slowness was defined as gait speed slower than 0.8 m/s. Grip strength was measured using a Jamar dynamometer (Jackson, MI, USA) in men and a handheld dynamometer (Sparks Instruments and Academics, Coralville, Iowa) in women. The maximum grip strength from all attempts was used in our analysis. Weakness was assessed by grip strength and characterized as less than 30 kg for men, or less than 20 kg for women. For each participant, height was measured on a Harpenden stadiometer (DyFed, UK). Lean mass of extremities and total body fat were obtained using the Hologic QDR 4500 and 2000 for men and women, respectively. Appendicular lean mass was calculated as the sum of lean mass in the arms and legs. Bone mineral content was removed from the lean mass calculation.
Subjects’ classification
Men and women were classified into four groups based on their bone mass and sarcopenia status: 1) Individuals with normal BMD and no sarcopenia (N=3367, 61% men; 308, 28% women), 2) individuals with normal BMD and sarcopenia (N=79 men, 1% men; 48, 4% women), 3) individuals with low BMD and no sarcopenia (N=1986 men, 36% men; 626, 56% women), 4) and individuals with low BMD and sarcopenia (N= 112 men, 2% men; 132, 12% women).
Other Measurements
Covariates were assessed at baseline in men and at year 10 in women at the time of the whole body DXA. Participants completed questionnaires and interviews that collected information on demographics, lifestyle, medical history and a medication inventory. Participants were asked to bring all prescription and over-the-counter medications to the clinic for verification of use (18). Smoking status was categorized as current or not (former, none) and alcohol consumption was assessed by the average number of drinks per week. Participants were asked if they walked as a form of exercise. Self-rated health was categorized as excellent/good vs fair, poor or very poor. Information on history of falls in the past year and previous fractures was obtained. Functional status was assessed by asking about difficulty with five instrumental activity of daily living (IADL) (“walking 2 or 3 blocks outside on level ground”, “climbing up 10 steps without resting”, “preparing meals”, “doing heavy housework”, and “shopping for groceries or clothes”). Weight was measured on balance beam scales (except for one of the MrOS site which used a digital scale). Body mass index (BMI) was calculated as the weight in kilograms divided by the square of height in meters.
Fracture Ascertainment
All non-spine fractures were identified through our mailed questionnaire follow-ups which were mailed every 4 months to ask if the participants had sustained a new fracture; these contacts were > 95% complete. Participants who reported a fracture were asked about the circumstances of the fracture. The degree of trauma was categorized into: “fall from a standing height or less”;“fall on stairs, steps or curb”, “fall from more than standing height”, and traumatic. Traumatic fractures (minimal, moderate, and severe) were included since they have been previously associated with low BMD (19). Pathological fractures were excluded. All fractures were confirmed by radiographic report and adjudicated centrally over a mean of 9 years for men and 8 years for women. These analyses included fractures that occurred between 2000 and 2012 in men, and between 1997 and 2009 in women. The follow-up time ended at the date of the first fracture, date of death, date of last contact or database lock. In sensitivity analyses, we excluded traumatic fractures.
Statistical analysis
Baseline characteristics were compared across the groups using ANOVA for continuous variables and Chi Square for categorical ones. Pairwise comparisons of the baseline characteristics were calculated and p-values were included in Table 1.
Table 1.
| a. Baseline characteristics of older men by bone and body composition
| |||||
|---|---|---|---|---|---|
| Characteristics | Normal BMD and no sarcopenia (N=3367) |
Sarcopenia alone (N=79) |
Low BMD alone (N= 1986) |
Low BMD and Sarcopenia (N=112) |
P value |
| Race | |||||
| White, n(%) | 3173(94) | 76(96) | 1943(98) | 111(99) | ac |
| Age (yr) | 72.8±5.5 | 80.5±6.0 | 74.6±6.0 | 79.6±6.3 | abcde |
| Body mass index, kg/m2 | 28.3±3.8 | 26.4±3.5 | 26.2±3.4 | 24.5±2.7 | abcef |
| Appendicular skeletal Mass (kg) | 25.3±3.4 | 20.3±2.3 | 23.4±3.0 | 19.4±2.0 | abcde |
| Current smoker, n (%) | 114 (3.4) | 1(1.3) | 68(3.4) | 8(7.1) | |
| Alcohol use (drinks/week) | 4.7±7.3 | 5.7±10.9 | 3.8±5.8 | 3.0±5.0 | acdf |
| Previous fracture, n(%) | 1764(52.4) | 43(54.4) | 1228(61.9) | 64(57.1) | a |
| Rheumatoid arthritis, n(%) | 174(5.2) | 9(11.4) | 89(4.5) | 9(8.0) | bd |
| Current oral and/or inhaled steroid user, n(%) | 235(7.3) | 12(16.9) | 196(10.2) | 267(24.8) | abce |
| Walks for exercise, n(%) | 1660(49.3) | 32(40.5) | 1033(52.0) | 52(46.4) | d |
| Excellent/Good Health Status, n(%) | 2917(86.7) | 61(77.2) | 1718(86.6) | 77(68.8) | bcde |
| Gait speed (m/s) | 1.2±0.2 | 0.9±0.2 | 1.2±0.2 | 0.9±0.3 | bcde |
| Grip strength (kg) | 39.8.1±7.9 | 26.3±5.8 | 37.9±7.5 | 25.4±7.0 | abcde |
| Functional status | |||||
| # of IADL impairments | 0.3±0.8 | 1.2±1.4 | 0.3±0.8 | 1.3±1.6 | bcde |
| Any falls last 12 months, n(%) | 693 (20.6) | 37(46.8) | 420(21.2) | 32(28.6) | bcdf |
| 2 or more falls last 12 months, n(%) | 306(9.1) | 21(26.6) | 165(8.3) | 18(16.1) | bcde |
| Total Hip BMD (g/cm2) | 1.03±0.11 | 0.97±0.09 | 0.84±0.09 | 0.79±0.10 | abcdef |
| Femoral neck BMD (g/cm2) | 0.86±0.10 | 0.82±0.07 | 0.66±0.06 | 0.64±0.06 | abcdef |
| b. Baseline characteristics of older women by bone and body composition
| |||||
|---|---|---|---|---|---|
| Characteristics | Normal BMD and no sarcopenia (N=308) | Sarcopenia alone (N=48) | Low BMD alone (N=626) | Low BMD and Sarcopenia (N= 132) | P value |
| Race | |||||
| White, n(%) | 65(21.1) | 27(56.3) | 393(62.8) | 103(78.0) | abcef |
| Age (yr) | 75.6±4.2 | 77.0±3.5 | 78.3±4.3 | 79.1±4.0 | abcdf |
| Body mass index, kg/m2 | 30.8±4.7 | 28.4±4.5 | 26.9±4.5 | 27.3±4.7 | abcd |
| Appendicular skeletal Mass (kg) | 17.0±2.7 | 13.3±1.9 | 15.0±2.3 | 12.6±1.5 | abcde |
| Current smoker, n (%) | 21(6.8) | 4(8.3) | 40(6.4) | 11(8.3) | |
| Alcohol use (drinks/week) | 0.7±1.8 | 1.5±3.6 | 1.1±2.8 | 1.0±2.9 | ab |
| Previous fracture, n(%) | 69(22.5) | 9(18.8) | 136(21.8) | 24(18.2) | |
| Rheumatoid arthritis, n(%) | 33(10.8) | 5(10.4) | 59(9.4) | 6(4.6) | c |
| Current oral and/or inhaled steroid user, n(%) | 15(4.9) | 5(10.4) | 28(4.5) | 13(9.9) | ce |
| Walks for exercise, n(%) | 119(38.8) | 15(31.3) | 272(43.7) | 51(38.6) | d |
| Excellent/Good Health Status, n(%) | 238(77.3) | 37(77.1) | 493(78.8) | 104(78.8) | |
| Gait speed (m/s) | 0.86±0.21 | 0.86±0.19 | 0.90±0.22 | 0.88±0.20 | a |
| Grip strength (kg) | 19.7±4.9 | 15.5±3.8 | 18.5±4.8 | 15.6±3.5 | abcde |
| Functional status | |||||
| # of IADL impairments | 1.1±1.3 | 1.0±1.2 | 0.8±1.3 | 0.8±1.1 | a |
| Any falls last 12 months, n(%) | 91(29.6) | 12(25.0) | 189(30.2) | 44(33.3) | |
| 2 or more falls last 12 months, n(%) | 31(10.1) | 5(10.4) | 79(12.6) | 13(9.9) | |
| Total Hip BMD (g/cm2) | 0.93±0.13 | 0.91±0.12 | 0.70±0.11 | 0.71±0.09 | acdf |
| Femoral neck BMD (g/cm2) | 0.85±0.11 | 0.83±0.09 | 0.60±0.08 | 0.61±0.08 | acdf |
Significance (p<0.05): a normal vs low BMD, b normal vs sarcopenic, c normal vs low BMD and sarcopenia, d low BMD vs sarcopenic, e low BMD vs low BMD and sarcopenia, f sarcopenia vs low BMD and sarcopenia
For the primary outcome, we initially adjusted for age. The incidence rates of non-spine fractures for each of the four groups were estimated using a Poisson distribution. Using Cox Proportional Hazards Models, the age and multivariable adjusted Hazard ratios (HR) and 95% Confidence Intervals were calculated. Participants with normal BMD and no sarcopenia formed the referent group. The multivariable-adjusted model included established risk factors for fracture: age, race, fall history, previous fracture history, current smoking, glucocorticoids, rheumatoid arthritis, alcohol consumption, IADL impairments, and physical activity. We used backward elimination to drop all variables that did not reach a statistically significant level of p<0.1. The interaction term between low BMD and sarcopenia on fracture risk was assessed. We also studied the association between low BMD and fracture risk adjusting for sarcopenia, and the association between sarcopenia and fracture risk adjusting for low BMD. In participants who experienced a non-spine fracture, pairwise comparisons were done to compare the circumstances of the fracture across the four groups.
Separate analyses were done for men and women using SAS 9.3 (SAS Institute, Inc., Cary, NC).
RESULTS
The majority of men were white with little difference in race across the groups. Sarcopenic men with or without low BMD were older than the other groups but there was little variability in smoking and alcohol consumption among the groups. A higher percentage of women with low BMD with or without sarcopenia were white and these women tended to be older. Total hip and femoral neck BMD were the lowest in the low BMD and sarcopenia group in men, and in the low BMD with or without sarcopenia groups in women. Unlike sarcopenic women, sarcopenic men had a higher number of IADL impairments, and a higher percentage of falls.
Men
A total of 870 (16%) men experienced a non-spine fracture: 402 (12%, normal); 11 (14%, sarcopenic); 421 (21%, low BMD), and 36 (32%, both low BMD and sarcopenia). The age-adjusted incidence of non-spine fracture was similar in normal men (13.2 per 1,000) and those with sarcopenia alone (15.1 per 1,000), but was much higher in men with both low BMD and sarcopenia (46.5 per 1,000) (Figure 1). Men with low BMD and sarcopenia had a 4-fold increased risk of fracture in comparison to normal men, HR= 3.75(2.64 to 5.32), Table 2. Men with sarcopenia alone did not have a statistically significant higher risk of fractures HR=1.19(0.65 to 2.17), however, those with low BMD alone had an intermediary risk of fracture HR=1.79(1.56 to 2.05) in between normal men and men with both conditions. These associations remained significant after adjusting for important covariates (Table 2). The interaction term between sarcopenia and low BMD was borderline significant (p=0.06). Low BMD was associated with fracture risk after adjusting for sarcopenia (HR, 1.97; 95% CI, 1.72–2.25). Similarly, sarcopenia was associated with fracture risk after adjusting for low BMD (HR, 2.25; 95% CI, 1.68–3.03). Exclusion of traumatic fractures showed somewhat similar results (Table 2b, p-interaction=0.11).
Figure 1. Age-adjusted incidence rate (per 1,000) of non-spine fractures by BMD and body composition.
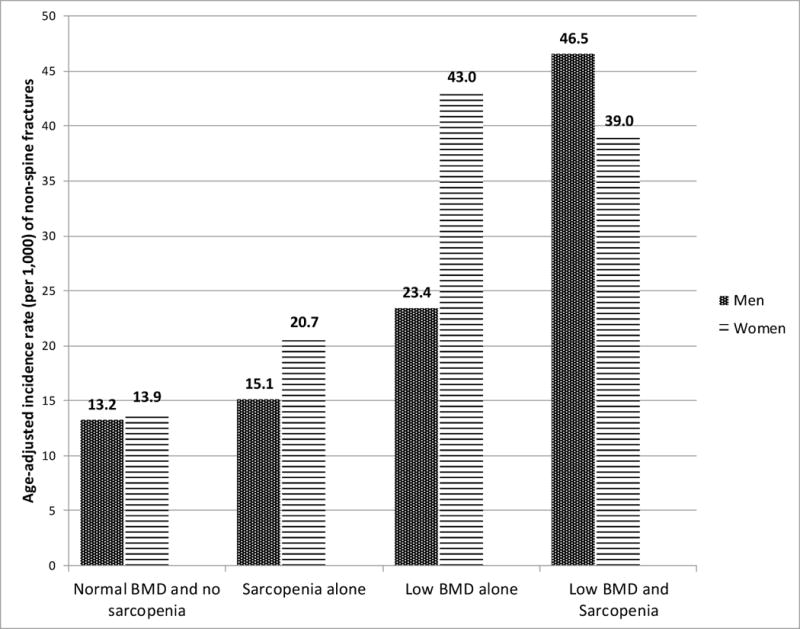
The incidence rates of non-spine fractures per 1,000 person years are shown on this graph. In men, the incidence rate was the highest for participants with both low BMD and sarcopenia. Men with only one or neither conditions had lower incidence rates. On the other hand, the incidence rates in women with low BMD, with or without sarcopenia, were similar. Women with neither conditions and with sarcopenia alone had lower rates of non-spine fractures.
Table 2.
Hazard ratio (95% confidence intervals) for non-spine fractures by sarcopenia, osteopenia/osteoporosis, and sarco-osteopenia/sarco-osteoporosis
| a) All fractures
| ||
|---|---|---|
| Variable (unit) | Age adjusted HR (95% CI) |
MVa adjusted HR(95% CI) |
| Men | ||
| Normal BMD and lean mass | 1.00 (Ref) | 1.00 |
| Sarcopenia alone | 1.19(0.65,2.17) | 1.14(0.62,2.09) |
| Low BMD alone | 1.79(1.56,2.05) | 1.67(1.45,1.93) |
| Low BMD and sarcopenia | 3.75(2.64,5.32) | 3.79(2.65,5.41) |
| Women | ||
| Normal BMD and lean mass | 1.00 (Ref) | 1.00 |
| Sarcopenia alone | 1.50(0.66,3.42) | 1.26(0.55,2.90) |
| Low BMD alone | 3.09(2.08,4.59) | 2.62(1.74,3.95) |
| Low BMD and sarcopenia | 2.80(1.72,4.58) | 2.27(1.37,3.76) |
| b) Traumatic fractures excluded (N=5,377 in men, N=1,087 in women)
| ||
|---|---|---|
| Variable (unit) | Age adjusted HR (95% CI) |
MVa adjusted HR(95% CI) |
| Men | ||
| Normal BMD and lean mass | 1.00 (Ref) | 1.00 |
| Sarcopenia alone | 1.26(0.67,2.38) | 1.20(0.64,2.28) |
| Low BMD alone | 1.88(1.61,2.20) | 1.82(1.55,2.13) |
| Low BMD and sarcopenia | 4.16(2.87,6.01) | 4.08(2.79,5.96) |
| Women | ||
| Normal BMD and lean mass | 1.00 (Ref) | 1.00 |
| Sarcopenia alone | 1.55(0.68, 3.55) | 1.27(0.55,2.92) |
| Low BMD alone | 2.95(1.97,4.42) | 2.42(1.59,3.68) |
| Low BMD and sarcopenia | 2.74(1.66,4.52) | 2.14(1.27,3.58) |
multivariable model (MV): adjustment included age, race, fall history, previous fracture, current smoking, steroids, rheumatoid arthritis, alcohol consumption, IADL impairments, and physical activity
Women
Overall, 272 (25%) women experienced a non-spine fracture: 31 (10%, normal); 7 (15%, sarcopenic); 194 (33%, low BMD), and 40 (32%, both low BMD and sarcopenia). The age-adjusted incidence of fracture ranged from 13.9 per 1,000 in normal women to about 40 per 1,000 in women with low BMD or both low BMD and sarcopenia (Figure 1). Of interest, there was little gender difference in fracture incidence rates in subjects with low BMD and sarcopenia (Figure 1). Women with low BMD with or without sarcopenia had an approximate 3-fold increased risk of fracture compared to normal women, HR= 2.80(1.72 to 4.58) and 3.09 (2.08 to 4.59) respectively, Table 2. The effect size decreased to 2.5 in both groups after adjusting for important covariates but remained statistically significant. Women with sarcopenia alone had a similar fracture rate as normal women. The interaction term between sarcopenia and low BMD was not statistically significant (p=0.37). Low BMD was associated with fracture risk after adjusting for sarcopenia (HR, 3.48; 95% CI, 2.47–4.90). However, sarcopenia was not associated with fracture risk after adjusting for low BMD (HR, 1.09; 95% CI, 0.79–1.49). Exclusion of traumatic fractures revealed similar results (Table 2b, p-interaction=0.33).
Circumstances of the fracture
Overall 80% of fractures in men and 90% in women followed a fall. In men with both low BMD and sarcopenia, 75% of their non-spine fractures followed a fall from a standing height or less. In comparison, fewer fractures in the other groups followed a fall from a standing height, between 56% and 64% (Figure 2a.). Pairwise comparisons showed that differences were statistically significant between men with both low BMD and sarcopenia and men with low BMD alone, and between men with both low BMD and sarcopenia and men without both conditions.
Figure 2. Proportion of fractures that were due to a fall from a standing height or less by low BMD and sarcopenia in older individuals.
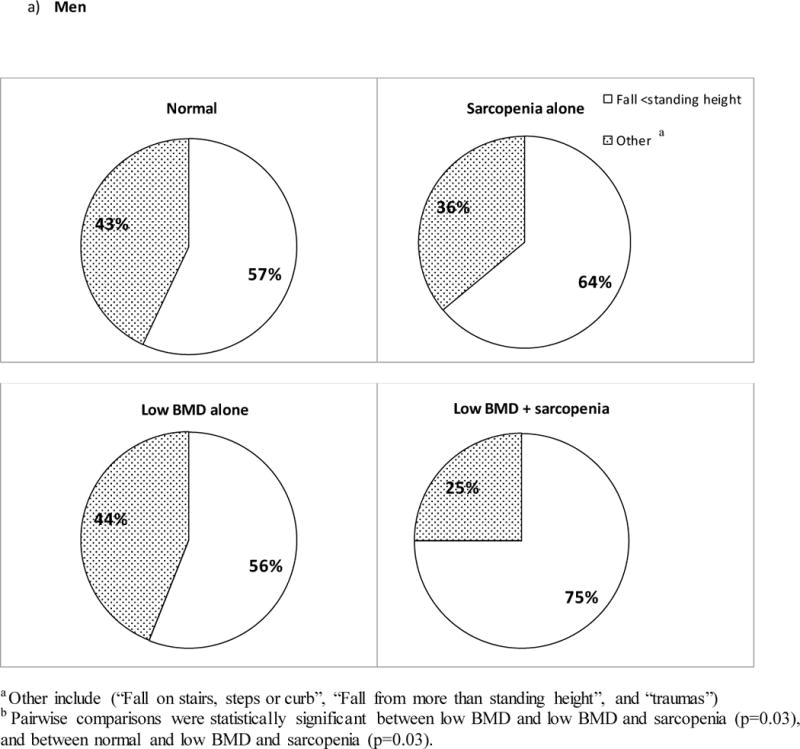
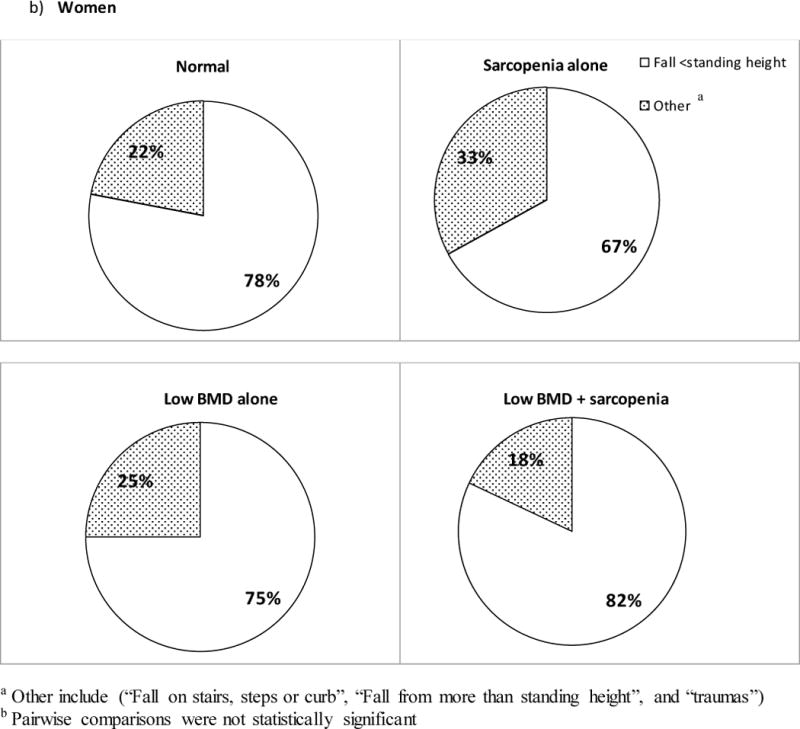
a) Results show that the proportion of men with the least degree of trauma was the highest in those with both low BMD and sarcopenia. A statistically significant difference was observed for all pairwise comparisons except between the sarcopenic alone and the low BMD and sarcopenia combined groups. b) In women, although the proportion of fractures with the least degree of trauma was the highest in the low BMD and sarcopenia combined group, pairwise comparisons were not statistically significant.
Similarly, in women, a higher proportion of fractures in subjects with both low BMD and sarcopenia were due to a fall from < standing height (82%) compared to women with low BMD alone (75%), sarcopenia alone (67%), and normal women (78%). However, these differences were not statistically significant (Figure 2b).
DISCUSSION
The findings of this study show that men with both low BMD and sarcopenia have a 4-fold higher risk for non-spine fractures compared to men with normal BMD and no sarcopenia. In men, the borderline significance of the interaction term suggests that the effect of sarcopenia and low BMD on fracture risk may depend on each other. The risk of fracture was about 2.5-fold higher in women with both sarcopenia and low BMD as well as in women with low BMD alone. Sarcopenia alone was not an independent risk factor for fractures in men and women. Our findings illuminate a previously unrecognized and potentially strong role of sarcopenia in the risk of fractures among older men.
The coexistence of low BMD and sarcopenia in older men resulted in a much higher risk of fractures. Since, physical activity, IADL impairments, history of falls, and other mobility disorder risk factors were adjusted for in our analyses, this suggest that the increased risk of non-spine fractures in men with both low BMD and sarcopenia could be attributed to the crosstalk between muscles and bones. Mechanical stimuli, pleiotropy, and hormones are known to play major roles in this crosstalk possibly affecting bone strength (20). Indeed, circumstances of fractures showed that these men had a higher proportion of fractures due to a lower degree of trauma compared to men with low BMD alone and normal men. Low muscle mass and strength have been associated with low BMD(21) (22) (23) (24) (25) (26) and poorer quality of bones (27) (28) which may be explained by the mechanical stimuli exerted by muscles. Muscles and bones share common genetic factors and are believed to be under the influence of pleiotropic genes responsible for the synchronized deterioration of both tissues with age (29). Muscles and bones also act as endocrine target organs, which are under the influence of similar hormones such as testosterone and estrogen. Estradiol regulates bone resorption, and may also enhance muscle contractile forces on bone (30) (31). Androgens affect muscle mass and strength and trabecular bone formation (32).
Unlike our findings among men, the risk of non-spine fractures in women with low BMD alone and women with both low BMD and sarcopenia was similar suggesting that low BMD may be the driving force for non-spine fractures in women. Although the proportion of fractures due to a lower degree of trauma was higher in women with low BMD and sarcopenia, statistical significance was not met. Gender differences in fracture risk could be explained by the fact that muscle strength decline is generally two times greater in men compared to women (33). In addition, low testosterone levels have been associated with a decrease in muscle mass and strength (34). Since men lose more testosterone with age compared to women, this decline could play a role in the onset and severity of sarcopenia in older men (35). Another possible explanation is the inadequate power to detect the risk of non-spine fractures in sarcopenic women due to their small sample size.
One of the strengths of this study is that the data were obtained from two very well established cohorts: MrOS and SOF, designed to understand the risk of fractures in older subjects. Another strength is that we adopted a unique approach in assessing the risk of non-spine fractures by classifying participants based on their bone and body composition. Additionally, the use of the residuals method to assess appendicular lean mass has been shown to be a good predictor of mobility limitations. Other appendicular lean mass assessment methods, such as the appendicular skeletal muscle index, do not account for total body fat (17) (16).
One main limitation of this study is that the definitions and algorithms of sarcopenia are still controversial (36). For instance, the “International working group on sarcopenia” includes only the gait speed and the ratio of appendicular lean mass over height squared (ALM/height2) in its algorithm without assessing muscle strength (37). On the other hand, the Foundation for the National Institutes of Health (FNIH) uses the ratio of appendicular lean mass over body mass index for muscle mass assessment as well as different muscle strength cutoffs (with or without physical performance assessment). The same analysis was repeated for the EWSOP (using ALM/ height2 instead of the residuals method), the international working group, and the FNIH operational definitions. Although not shown here, the results were roughly the same for older men across all three definitions. In women, results were similar expect for the FNIH definition which showed that participants with both low BMD and sarcopenia were not at a higher risk for non-spine fractures.
To conclude, men with both low BMD and sarcopenia had a much higher risk of fractures compared to men with only one or neither condition. This finding was not apparent in women suggesting gender differences in the role of sarcopenia on osteoporotic fractures. If our results are confirmed, assessment of sarcopenia status concomitantly with low bone mass status may assist in identifying men at the highest risk of future fracture. Development of treatments for sarcopenia management could potentially prevent fractures, especially in older men with both low BMD and sarcopenia.
Acknowledgments
Corresponding author has listed everyone who contributed significantly and has obtained written consent from all contributors who are not authors.
Eric S. Orwoll consults for and has received research support from Amgen, Lilly, and Merck and serves on the advisory board of Wright Medical Tech. Kristine E. Ensrud serves as a consultant on a Data Monitoring Committee for Merck Sharpe & Dohme. Peggy M. Cawthon has received research support from GSK, Lilly, IMS Health, and Merck and serves as a consultant for Lilly.
Grant supporters: The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128.
The Study of Osteoporotic Fractures (SOF) is supported by National Institutes of Health funding. The National Institute on Aging (NIA) provides support under the following grant numbers: R01 AG005407, R01 AR35582, R01 AR35583, R01 AR35584, R01 AG005394, R01 AG027574, and R01 AG027576.
Footnotes
| Elements of Financial/Personal Conflicts | Peggy M. Cawthon | Kristine E. Ensrud | Eric S. Orwoll | All Remaining authors | ||||
|
| ||||||||
| Yes | No | Yes | No | Yes | No | Yes | No | |
|
| ||||||||
| Employment or Affiliation | ||||||||
|
| ||||||||
| Grants/Funds | ||||||||
|
| ||||||||
| GSK | XXX | |||||||
| Lilly | XXX | XXX | ||||||
| IMS Health | XXX | XXX | ||||||
| Amgen | XXX | |||||||
| Merck | ||||||||
|
| ||||||||
| Honoraria | ||||||||
|
| ||||||||
| Speaker Forum | ||||||||
|
| ||||||||
| Consultant | ||||||||
|
| ||||||||
| Lilly | XXX | |||||||
| Merck Sharpe & Dohme | XXX | |||||||
|
| ||||||||
| Stocks | ||||||||
|
| ||||||||
| Royalties | ||||||||
|
| ||||||||
| Expert Testimony | ||||||||
|
| ||||||||
| Board Member | ||||||||
|
| ||||||||
| Wright Medical Tech | XXX | |||||||
|
| ||||||||
| Patents | ||||||||
|
| ||||||||
| Personal Relationship | ||||||||
|
| ||||||||
Other authors do not have conflicts of interests or disclosures to make.
Author Contributions:
Didier Chalhoub (dic14@pitt.edu) conducted the data analysis. He had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
All authors 1) made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; 2) participated in drafting the manuscript or revising it critically for important intellectual content; 3) approved the final version of the submitted manuscript, and 4) agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved
Biostatisticians Stephanie Harrison, MPH (SLitwack@psg.ucsf.edu, San Francisco Coordinating Center, California Pacific Medical Center, San Francisco, CA, USA) and Lily Lui (LLui@psg.ucsf.edu, San Francisco Coordinating Center, California Pacific Medical Center, San Francisco, CA, USA) were involved in adding new variables to existing datasets, helping with the SAS coding, and double checking the analysis of the manuscript.
Maria Aguiluz-Abunto MD (mba31@pitt.edu, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA) helped with the interpretation of results.
Sponsor’s Role: “none”
Contributor Information
Didier Chalhoub, Email: dic14@pitt.edu.
Peggy M. Cawthon, Email: pcawthon@sfcc-cpmc.net.
Kristine E. Ensrud, Email: ensru001@umn.edu.
Marcia L. Stefanick, Email: stefanick@stanford.edu.
Deborah M. Kado, Email: dkado@ucsd.edu.
Robert Boudreau, Email: BoudreauR@edc.pitt.edu.
Susan Greenspan, Email: greenspn@pitt.edu.
Anne Newman, Email: NewmanA@edc.pitt.edu.
Joseph Zmuda, Email: zmudaj@edc.pitt.edu.
Eric S. Orwoll, Email: orwoll@ohsu.edu.
References
- 1.Ensrud KE. Epidemiology of fracture risk with advancing age. The journals of gerontology Series A, Biological sciences and medical sciences. 2013;68(10):1236–42. doi: 10.1093/gerona/glt092. [DOI] [PubMed] [Google Scholar]
- 2.Landi F, Liperoti R, Russo A, et al. Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clinical nutrition. 2012;31(5):652–8. doi: 10.1016/j.clnu.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. Journal of the American Geriatrics Society. 2002;50(5):889–96. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 4.Lang T, Cauley JA, Tylavsky F, et al. Computed tomographic measurements of thigh muscle cross-sectional area and attenuation coefficient predict hip fracture: the health, aging, and body composition study. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2010;25(3):513–9. doi: 10.1359/jbmr.090807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gielen E, Verschueren S, O’Neill TW, et al. Musculoskeletal frailty: a geriatric syndrome at the core of fracture occurrence in older age. Calcified tissue international. 2012;91(3):161–77. doi: 10.1007/s00223-012-9622-5. [DOI] [PubMed] [Google Scholar]
- 6.Epidemiologic and methodologic problems in determining nutritional status of older persons. The American journal of clinical nutrition; Proceedings of a conference; Albuquerque, New Mexico. October 19–21, 1988; 1989. pp. 1121–235. [PubMed] [Google Scholar]
- 7.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age and ageing. 2010;39(4):412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Binkley N, Buehring B. Beyond FRAX: it’s time to consider “sarco-osteopenia”. Journal of clinical densitometry: the official journal of the International Society for Clinical Densitometry. 2009;12(4):413–6. doi: 10.1016/j.jocd.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study–a large observational study of the determinants of fracture in older men. Contemporary clinical trials. 2005;26(5):569–85. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemporary clinical trials. 2005;26(5):557–68. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Ensrud KE, Palermo L, Black DM, et al. Hip and calcaneal bone loss increase with advancing age: longitudinal results from the study of osteoporotic fractures. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 1995;10(11):1778–87. doi: 10.1002/jbmr.5650101122. [DOI] [PubMed] [Google Scholar]
- 12.Lewis CE, Ewing SK, Taylor BC, et al. Predictors of non-spine fracture in elderly men: the MrOS study. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2007;22(2):211–9. doi: 10.1359/jbmr.061017. [DOI] [PubMed] [Google Scholar]
- 13.Kanis JA, Melton LJ, 3rd, Christiansen C, et al. The diagnosis of osteoporosis. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 1994;9(8):1137–41. doi: 10.1002/jbmr.5650090802. [DOI] [PubMed] [Google Scholar]
- 14.Looker AC, Wahner HW, Dunn WL, et al. Updated data on proximal femur bone mineral levels of US adults. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 1998;8(5):468–89. doi: 10.1007/s001980050093. [DOI] [PubMed] [Google Scholar]
- 15.Bianchi ML, Baim S, Bishop NJ, et al. Official positions of the International Society for Clinical Densitometry (ISCD) on DXA evaluation in children and adolescents. Pediatric nephrology. 2010;25(1):37–47. doi: 10.1007/s00467-009-1249-z. [DOI] [PubMed] [Google Scholar]
- 16.Newman AB, Kupelian V, Visser M, et al. Sarcopenia: alternative definitions and associations with lower extremity function. Journal of the American Geriatrics Society. 2003;51(11):1602–9. doi: 10.1046/j.1532-5415.2003.51534.x. [DOI] [PubMed] [Google Scholar]
- 17.Dufour AB, Hannan MT, Murabito JM, et al. Sarcopenia definitions considering body size and fat mass are associated with mobility limitations: the Framingham Study. The journals of gerontology Series A, Biological sciences and medical sciences. 2013;68(2):168–74. doi: 10.1093/gerona/gls109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pahor M, Chrischilles EA, Guralnik JM, et al. Drug data coding and analysis in epidemiologic studies. European journal of epidemiology. 1994;10(4):405–11. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 19.Mackey DC, Lui LY, Cawthon PM, et al. High-trauma fractures and low bone mineral density in older women and men. JAMA: the journal of the American Medical Association. 2007;298(20):2381–8. doi: 10.1001/jama.298.20.2381. [DOI] [PubMed] [Google Scholar]
- 20.Bonewald LF, Kiel DP, Clemens TL, et al. Forum on bone and skeletal muscle interactions: summary of the proceedings of an ASBMR workshop. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2013;28(9):1857–65. doi: 10.1002/jbmr.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verschueren S, Gielen E, O’Neill TW, et al. Sarcopenia and its relationship with bone mineral density in middle-aged and elderly European men. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2013;24(1):87–98. doi: 10.1007/s00198-012-2057-z. [DOI] [PubMed] [Google Scholar]
- 22.Taaffe DR, Cauley JA, Danielson M, et al. Race and sex effects on the association between muscle strength, soft tissue, and bone mineral density in healthy elders: the Health, Aging, and Body Composition Study. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2001;16(7):1343–52. doi: 10.1359/jbmr.2001.16.7.1343. [DOI] [PubMed] [Google Scholar]
- 23.Park JH, Song YM, Sung J, et al. The association between fat and lean mass and bone mineral density: the Healthy Twin Study. Bone. 2012;50(4):1006–11. doi: 10.1016/j.bone.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 24.Bijlsma AY, Meskers MC, Molendijk M, et al. Diagnostic measures for sarcopenia and bone mineral density. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2013;24(10):2681–91. doi: 10.1007/s00198-013-2376-8. [DOI] [PubMed] [Google Scholar]
- 25.Lima RM, Bezerra LM, Rabelo HT, et al. Fat-free mass, strength, and sarcopenia are related to bone mineral density in older women. Journal of clinical densitometry: the official journal of the International Society for Clinical Densitometry. 2009;12(1):35–41. doi: 10.1016/j.jocd.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Marin RV, Pedrosa MA, Moreira-Pfrimer LD, et al. Association between lean mass and handgrip strength with bone mineral density in physically active postmenopausal women. Journal of clinical densitometry: the official journal of the International Society for Clinical Densitometry. 2010;13(1):96–101. doi: 10.1016/j.jocd.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Lebrasseur NK, Achenbach SJ, Melton LJ, 3rd, et al. Skeletal muscle mass is associated with bone geometry and microstructure and serum insulin-like growth factor binding protein-2 levels in adult women and men. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2012;27(10):2159–69. doi: 10.1002/jbmr.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szulc P, Blaizot S, Boutroy S, et al. Impaired bone microarchitecture at the distal radius in older men with low muscle mass and grip strength: the STRAMBO study. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2013;28(1):169–78. doi: 10.1002/jbmr.1726. [DOI] [PubMed] [Google Scholar]
- 29.Karasik D, Kiel DP. Evidence for pleiotropic factors in genetics of the musculoskeletal system. Bone. 2010;46(5):1226–37. doi: 10.1016/j.bone.2010.01.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khosla S, Oursler MJ, Monroe DG. Estrogen and the skeleton. Trends in endocrinology and metabolism: TEM. 2012;23(11):576–81. doi: 10.1016/j.tem.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lowe DA, Baltgalvis KA, Greising SM. Mechanisms behind estrogen’s beneficial effect on muscle strength in females. Exercise and sport sciences reviews. 2010;38(2):61–7. doi: 10.1097/JES.0b013e3181d496bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lang TF. The bone-muscle relationship in men and women. Journal of osteoporosis. 2011;2011:702735. doi: 10.4061/2011/702735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. The journals of gerontology Series A, Biological sciences and medical sciences. 2006;61(10):1059–64. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 34.LeBlanc ES, Wang PY, Lee CG, et al. Higher testosterone levels are associated with less loss of lean body mass in older men. The Journal of clinical endocrinology and metabolism. 2011;96(12):3855–63. doi: 10.1210/jc.2011-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. The Journal of clinical endocrinology and metabolism. 2002;87(2):589–98. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- 36.Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. The journals of gerontology Series A, Biological sciences and medical sciences. 2014;69(5):547–58. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. Journal of the American Medical Directors Association. 2011;12(4):249–56. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


