Abstract
Objective
Masked hypertension (MH) refers to non-elevated office blood pressure (BP) with elevated out-of-office BP, but its reproducibility has not been conclusively established. We examined one-week reproducibility of MH by home BP monitoring (HBPM) and ambulatory BP monitoring (ABPM).
Methods
We recruited 420 adults not on BP-lowering medication with recent clinic BP between 120/80 and 149/95 mm Hg. For main comparisons, participants with office average <140/90 mm Hg were considered to have MH if awake ABPM average was ≥135/85 mm Hg; they were considered to have MH by HBPM if the average was ≥135/85 mm Hg. Percent agreements were quantified using kappa. We also examined prevalence of MH defined as office average <140/90 mm Hg with 24-hour ABPM average ≥130/80 mm Hg. We conducted sensitivity analyses using different threshold BP levels for ABPM-office pairings and HBPM-office pairings for defining MH.
Results
Prevalence rates of MH based on office-awake ABPM pairings were 44% and 43%, with agreement of 71% (kappa=0.40; 95% CI 0.31–0.49). MH was less prevalent (15% and 17%) using HBPM-office pairings, with agreement of 82% (kappa=0.30; 95% CI 0.16–0.44), and more prevalent when considering 24-hour average (50% and 48%). MH was also less prevalent when more stringent diagnostic criteria were applied. Office-HBPM pairings and office-awake ABPM pairings had fair agreement on MH classification on both occasions, with kappas of 0.36 and 0.30.
Conclusions
MH has fair short-term reproducibility, providing further evidence that for some people, out-of-office BP is systematically higher than when measured in the office setting.
Keywords: masked hypertension, reproducibility, home blood pressure monitoring, ambulatory blood pressure monitoring
Introduction
Masked hypertension (MH), defined as non-elevated office blood pressure (BP) with elevated average out-of-office BP, conveys cardiovascular disease (CVD) risk approaching that of sustained hypertension (BP elevated in office and out-of-office) [1–5]. Detection of MH requires either ambulatory BP monitoring or home BP monitoring to acquire data for calculating out-of-office BP average. Given that either method requires resources including equipment and time, it would be valuable to have a strategy for targeting which patients with a non-elevated office BP ought to have systematically performed out-of-office BP measurements in order to detect MH. In order to be able to predict which patients with non-elevated office BP are more likely to have MH, it is first necessary to demonstrate that the MH classification is reproducible and not merely due to random BP fluctuations [4].
To date, there are extremely limited data on the reproducibility of MH [6–8]. Both ambulatory BP monitoring and home BP monitoring have been used as out-of-office measurement techniques to classify MH, but they may not be interchangeable for this purpose [8–10].
We previously examined the short-term reproducibility of masked hypertension among a sample of 50 adults with a “borderline” office BP measurement [8]. Among this sample, prevalence rates of MH based on office-awake ABPM pairings were 54% and 53%, with agreement of 73% (kappa=0.47; 95% CI 0.21–0.72). MH was less prevalent (43% and 35%) using HBPM-office pairings, with agreement of 69% (kappa=0.34; 95% CI 0.06–0.62). Office-HBPM pairings and office-awake ABPM pairings had poor agreement on MH classification on both occasions, with kappas of −0.06 and 0.10. Given the uncertainty in our prior estimates, we undertook a large-scale study in order to quantify the reproducibility of MH and the agreement between home and ambulatory BP monitoring methods.
Methods
Study Recruitment and Setting
Our sample size of 420 adults was based on power to rule out kappa values less than or equal to 0.35 if the true magnitude of kappa in the target population is approximately 0.50 or greater, taking into account the possible loss of 20 participants. We posted signs in seven primary care clinics inviting people with a recent office (clinic) BP measurement that was “borderline” or “a little high” to participate. Individuals interested in participating contacted a study coordinator to confirm eligibility and schedule their study visits. Study coordinators also recruited potentially eligible participants via electronic medical records review of vital signs documented during their most recent primary care clinic visit. To be eligible, a person had to be 30 years of age or older, have a primary care clinician, and be on no BP-lowering medications. Most recent primary care clinic visit BP had to be between 120–149 mm Hg systolic or 80–95 mm Hg diastolic with neither greater than 149/95 mm Hg. Exclusion criteria included pregnancy, dementia, any condition that would preclude wearing an ambulatory BP monitor, and persistent atrial fibrillation or other arrhythmia. We also excluded potential enrollees if research visit 1 initial office BP was ≥160/100 mm Hg or <110/70 mm Hg as such participants would be more likely to have sustained hypertension or sustained normotension, respectively. All study procedures took place in a clinical research center.
Office Blood Pressure
Following check-in procedures at visits 1 through 4, participants were placed in an exam room within the clinical research center. After at least a 5-minute rest, same arm BP was measured three times by a validated [11] office-type oscillometric device (Welch Allyn Vital Signs) according to recommended timing and positioning and using the appropriate BP cuff size. The second and third measurements were averaged to determine the participant’s office BP measurement for the visit.
We included patients with initial office BPs ≥140/90 mm Hg in order to be able to account for the possibility that some people classified as having sustained hypertension or white-coat hypertension at one time may be classified as having masked hypertension or another hypertension variant at a different time [6]. Only by allowing people with an elevated office BP to participate could that possibility be examined.
Ambulatory Blood Pressure Monitoring
Participants underwent two 24-hour ambulatory blood pressure monitoring sessions one week apart using the Oscar 2 oscillometric monitor (Suntech Medical, Morrisville, NC). The Oscar 2 has been validated for use in adults by both the British Hypertension Society protocol and the International Protocol for the validation of BP measuring devices [12,13]. The monitors were programmed to measure BP at 30 minute intervals from 6am to 10pm and at 1 hour intervals from 10pm to 6am. For the first 143 participants, we defined the awake period as 10am to 10pm and sleep period as midnight to 6am. For participants 144 through 420, we incorporated a participant diary and used it to define sleep and awake periods. Maximum BP measurement time was limited to less than 140 seconds, and the monitors were set for a maximum pressure of 220 mm Hg. Participants were given verbal instructions on wearing the monitor, including that that they should try to leave the cuff on during the entire monitoring period, that they should try to hold their cuffed arm as still as possible during a reading to ensure that the monitor would get an accurate reading, that cuff inflation would cause a tight feeling around the arm, and that faulty readings would trigger a repeat measurement. The minimum number of readings we accepted as an adequate ABPM session was 14 for awake and 6 for sleep.
Home Blood Pressure Monitoring
At the second study visit, participants were instructed on how to correctly perform home BP measurements using an Omron 705 CP home BP monitor. The Omron 705 CP monitor has been previously validated [14]. Between the second and third visits and between the fourth and fifth visits, such a home monitor was loaned to participants, and they were asked to conduct home BP measurements on 5 consecutive days with three measurements taken in the morning and three measurements taken in the evening. Participants were instructed that these measurements were to be performed in the sitting position after a 5-minute rest with 1 minute between measurements. Participants were asked to record dates, times, and BP measurements on a pre-printed form. Home BP monitoring average was calculated by discarding the first 2 days of measurements and the first measurement of each triplicate set of measurements and averaging the remaining 12 measurements [15]. Only participants with all 5 days’ measurements were included in home BP analyses.
Other Variables
Height and weight were measured at the first study visit and used to calculate body mass index (BMI). Arm circumference at mid-biceps was measured at the first study visit and used to guide BP cuff size. Demographics and medical history items were collected by self-administered questionnaire.
Analysis
MH based on awake ABPM was defined as a preceding visit office BP average <140/90 mm Hg with either a mean awake ABPM systolic BP ≥135 mm Hg or mean awake ABPM diastolic BP ≥85 mm Hg [16]. MH based on home BP monitoring was defined as a preceding office BP average <140/90 mm Hg with either a mean home systolic BP ≥135 mm Hg or mean home diastolic BP ≥85 mm Hg [16]. The home BP prevalence and reproducibility analyses were repeated using a lower home BP systolic BP threshold of ≥130 mm Hg [17]. MH based on 24-hour BP monitoring was defined as a preceding office BP average <140/90 mm Hg with either a mean 24-hour ambulatory systolic BP ≥130 mm Hg or mean 24-hour diastolic BP ≥80 mm Hg [16]. We calculated the percent agreement for the classification of MH based on ABPM-office pairings between the first and second sets of measurements. We calculated the percent agreement for the classification of MH based on home BP-office BP pairings between the first and second sets of measurements. We also calculated the agreement of different out-of-office measurement methods at each time period. Agreement was quantified using the kappa statistic, its 95% confidence interval (CI) and p-value [18]. We defined a comparison “gold standard” of MH as MH present by 24-hour ABPM-office BP pairings at both sessions and calculated the prevalence of MH based on this definition. We performed sensitivity analyses by repeating the above analyses using an office average of 130/85 mm Hg (rather than 140/90 mm Hg) as the cutoff for elevated office BP. This analysis also serves to address the issue of whether classification of MH is largely due to measurements that vary only slightly around the cutoffs (which would be more likely due to random variation as opposed to systematic variation).
We repeated the prevalence calculations using each participant’s eligibility BP (the measurement performed in their actual clinical setting) paired with the first ABPM session. We also repeated the prevalence and reproducibility calculations after eliminating the possible “white-coat” period (first two hours) of the ABPM sessions. Finally, we compared the agreements of MH among participants for whom we used a sleep diary compared to those for whom we used time-based sleep periods. Analyses were conducted using SAS 9.3 (SAS Institute Inc., Cary, NC).
Study Approval
This study was approved by the Office of Human Research Ethics at the University of North Carolina at Chapel Hill.
Results
Participant Characteristics
The mean ± standard deviation (±SD) age of the 420 participants was 48 (±12) years. Most participants were between 45 and 64 years (46%) or between 30 and 44 years (43%) (Table 1). Slightly more than half were female. Approximately 21% were Black. Only 9 participants did not have sufficient awake ambulatory BP monitor readings at the first session, and 13 did not have sufficient awake ambulatory BP monitor readings at the second session. Eighty-one participants did not conduct sufficient home BP measurements during the first interval, and 86 participants did not conduct sufficient home BP measurements during the second interval.
Table 1.
Participant Characteristics (N=420)
| Characteristic | n | Percent |
|---|---|---|
| Age group (years) | ||
| 30–44 | 181 | 43 |
| 45–64 | 194 | 46 |
| >65 | 44 | 11 |
| Female sex | 237 | 56 |
| Race | ||
| Black | 90 | 21 |
| White | 314 | 75 |
| Other | 16 | 4 |
| Hispanic ethnicity | 17 | 4 |
| Education level | ||
| Some high school | 6 | 2 |
| High school graduate | 25 | 6 |
| Some college | 81 | 19 |
| College graduate | 307 | 73 |
| Insurance status | ||
| Private | 302 | 72 |
| Public | 51 | 12 |
| Both | 36 | 9 |
| Uninsured | 28 | 7 |
| Self-reported health | ||
| Excellent/very good | 284 | 68 |
| Good | 112 | 27 |
| Fair or poor | 23 | 5 |
| Nonsmoker | 389 | 93 |
| Drink alcohol | 299 | 71 |
| BMI | ||
| Normal (<25 kg/m2) | 116 | 28 |
| Overweight (25–29 kg/m2) | 145 | 34 |
| Obese (≥30 kg/m2) | 159 | 38 |
| Married or living with partner | 270 | 64 |
BMI, body mass index
Blood Pressures
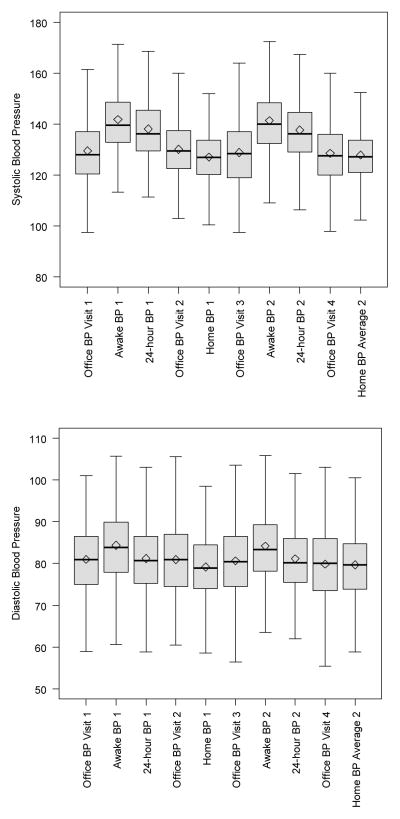
The mean eligibility BP of participants was 134/79 ±11/9 mm Hg. Research visit office BP average was ≥140 mm Hg systolic or ≥ 90 mm Hg diastolic for 28% of participants at visit 1, 29% at visit 2, 27% at visit 3, and 25% at visit 4. The mean ±SD office BP average of participants at the first office visit was 130/81 ±13/9 mm Hg (Figure 1). The first sets of average awake ambulatory BPs ranged from 113/61 mm Hg to 197/118 mm Hg with a mean of 142/84 ±14/9 mm Hg. Home BP averages from the first set of sessions ranged from 100/58 mm Hg to 165/108 mm Hg with a mean of 127/79 ±11/8 mm Hg. The mean ±SD office BP average of participants at the third measurement session was 129/81 ±13/9 mm Hg. The second sets of average awake ambulatory BPs ranged from 109/60 mm Hg to 189/115 mm Hg with a mean of 141/84 ±13/9 mm Hg. Home BP averages from the second set of sessions ranged from 102/56 mm Hg to 169/106 mm Hg with a mean of 128/80 ±11/8 mm Hg.
Figure 1.
Blood Pressure (mm Hg) Distributions from Each Set of Measurements
Prevalence of Masked Hypertension in the Study Sample
Using the office BP average paired with the immediately following awake ABPM average, the prevalence of MH based on the first sets of measurements was 44% (95% CI 39%–49%), and the prevalence based on the second sets of measurements was 43% (95% CI 38%–48%) (Table 2). When considering the entire 24-hours of ABPM measurements, the prevalence of MH was 50% (95% CI 45%–55%) based on the first sets of measurements and 48% based on the second sets of measurements (95% CI 43%–53%). The prevalence of MH based on two “positive” 24-hour ABPM- office BP average pairings was 33% (95% CI 28%–37%). Among those with a “normal” (<140/90 mm Hg) preceding office BP, based on the first 24h-hour ABPM, 69.4% (95% CI 64.1%–74.7%) had MH, and based on the second 24-hour ABPM, 65.9% (95% CI 60.5%–71.3%) had MH.
Table 2.
Prevalence of Masked Hypertension Using Different Out-of-Office Measurement Methods at Two Time Periods
| 1st set of measurements | 2nd set of measurements | |||
|---|---|---|---|---|
| n/N (%) | 95% CI | n/N (%) | 95% CI | |
| Awake ABPM (≥135/85 mm Hg) | 180/408 (44.1%) | 39.3%–48.9% | 173/406 (42.6%) | 37.8%–47.4% |
| 24-hour ABPM (≥130/80 mm Hg) | 204/408 (50.0%) | 45.2%–54.9% | 195/408 (47.8%) | 43.0%–52.6% |
| Home BP monitoring (≥135/85 mm Hg) | 49/337 (14.5%) | 10.8%–18.3% | 56/333 (16.8%) | 12.8%–20.8% |
| Home BP monitoring (≥130/85 mm Hg) | 81/337 (24.0%) | 19.5%–28.6% | 85/333 (25.5%) | 20.8%–30.2% |
Using a home BP threshold of >135/85 mm Hg, the prevalence of MH based on the series of home BP measurements paired with the preceding visit office BP average was 15% (95% CI 11%–19%) by the first set of measurements and 17% (95% CI 13%–21%) by the second set of measurements. Using the lower home systolic BP threshold of >130 mm Hg, the prevalence of MH was 24.0% (95% CI 19.5%–28.6%) by the first set of measurements and 25.5% (95% CI 20.8%–30.2%) by the second set.
Reproducibility of Masked Hypertension Classification
When performed on two different occasions and paired with corresponding office BP average, awake ABPM average was concordant in classifying participants 71% of the time with a kappa of 0.40 (95% CI 0.31–0.49) (Table 3 and Figure 2). MH classification based on pairings of home BP monitoring average with preceding visit office BP average was in agreement 82% of the time with a kappa of 0.30 (95% CI 0.16–0.44). During both time periods, home BP monitoring-office pairings had fair agreement with awake ABPM-office pairings in classifying participants (k=0.36 and k=0.30). Awake ABPM was highly concordant with 24-hour ABPM in classifying participants at both time periods (92% and 94%).
Table 3.
Agreement on Classification of Masked Hypertension
| Percent agreement | Kappa* | 95% CI | P-value | |
|---|---|---|---|---|
| Awake ABPM average, two occasions one week apart | 70.5 | 0.40 | 0.31–0.49 | <0.0001 |
| 24-hour ABPM average, two occasions, one week apart | 67.9 | 0.36 | 0.27–0.45 | <0.0001 |
| Home BP monitoring (135/85 mm Hg threshold), two series of measurements one week apart | 82.0 | 0.30 | 0.16–0.44 | <0.0001 |
| Home BP monitoring (130/85 mm Hg threshold), two series of measurements one week apart | 78.7 | 0.41 | 0.29–0.53 | <0.0001 |
| Home BP (135/85 mm Hg threshold) and awake ABPM using the first set of measurements | 72.1 | 0.36 | 0.27–0.45 | <0.0001 |
| Home BP (135/85 mm Hg threshold) and awake ABPM using the second set of measurements | 68.8 | 0.30 | 0.21–0.39 | <0.0001 |
| Awake ABPM and 24-hour ABPM using the first set of measurements | 91.9 | 0.84 | 0.79–0.89 | <0.0001 |
| Awake ABPM and 24-hour ABPM using the second set of measurements | 93.6 | 0.87 | 0.82–0.92 | <0.0001 |
A kappa of 0 indicates agreement no better than expected by chance; a kappa of 1 indicates perfect agreement.
Figure 2. Prevalence of Blood Pressure Classifications at Baseline and One Week Later.
Based on the first 24-hour ambulatory blood pressure (BP) monitoring session and its preceding office visit BP average, the prevalence of masked hypertension was 49.8%. Among these participants, 66% had masked hypertension at repeat monitoring one week later.
ABPM, ambulatory blood pressure monitoring
Sensitivity Analysis
Using an office average <130/85 mm Hg paired with awake ABPM average ≥135/85, 98 of 408 participants (24%) still had MH at the first session, and 82 of 406 (20%) had MH at the second session. The agreement between the two sessions using this office threshold was 74% (k=0.25; 95% CI 0.14–0.35). Using the lower office threshold paired with home BP averages, the prevalence rates of MH were 5% and 7% with 91% agreement (k=0.23; 95% CI −0.04–0.43). Office-home pairing and office awake-ABPM pairing were 83% concordant (k=0.29; 95% CI −0.17–0.41) the first time and 83% concordant (k=0.31; 95% CI −0.18–0.43) the second time.
Among participants who we classified based on time-periods alone, the agreement of MH using 24-hour ABPM average was 65.2% with a kappa of 0.30 (95% CI 0.14–0.46). Among those for whom we had a sleep diary, the agreement of MH was 69.3% with a kappa of 0.39 (95% CI 0.28–0.50). When defining ABPM based on awake ABPM average, the agreement was 67.4% (k=0.33; 95% CI 0.17–0.49) among those without a diary and 72.1% (k=0.44; 95% CI 0.33–0.55) with a diary.
Out-of-Office Reproducibility Following Single Clinical Office BP Measurement
Based on the eligibility visit office BP (before any measurements taken in the research setting), 263 participants had non-elevated BP (<140/90 mm Hg). The first awake ABPM session revealed elevated BP in 156 of these participants (59%), and the second ABPM session revealed elevated BP in 161 of them (61%), with agreement of 82% (k=0.61, 95% CI 0.51–0.71) The first home BP monitoring series of measurements revealed elevated BP in 41 of these participants with a “normal” office BP, and the second first home BP monitoring series revealed elevated BP in 49 of them. The agreement of home measurements among these participants was 84% (k=0.50, 95% CI 0.35–0.66).
Eliminating the White-coat Period from ABPM
After eliminating the first two hours from each participant’s ABPM sessions, 176 of 408 (43%) still had MH at the first session, and 172 of 406 (42%) had MH at the second session. The agreement between the two sessions was 72% (k=0.43; 95% CI 0.34–0.52). Using these averages, the agreement between MH based on awake ABPM and home BP monitoring were 72% at first sessions (k=0.35) and 69% at second sessions (k=0.30).
Discussion
We previously reported based on a sample of 50 adults that the short-term reproducibility of MH was fair to moderate [8]. The current study affirms our findings that the MH classification is reproducible. We again found fair agreement in the short-term reproducibility of MH when measured by office BP and ambulatory BP average pairings and when measured by office BP and home BP monitoring average pairings.
There are few other published studies on the reproducibility of MH in adults. One study examined this issue using a database of 196 patients who had undergone repeated ABPM for clinical indications (e.g., suspected white-coat hypertension) [6]. At the first ABPM session, 25 (13%) were classified as having MH. Upon re-monitoring, 11 of the 25 still had MH while 7 had sustained hypertension, 5 had true normotension, and 2 had white-coat hypertension. One person with white-coat hypertension at the first monitoring session had MH on re-monitoring. Overall kappa was reported as 0.26. Of note, most of the patients (80%) were already receiving antihypertensive treatment. Antihypertensive medications could also have been added or adjusted between monitoring sessions. Also, the average time between the two monitoring sessions was 1.5±1.5 years. Patients’ office and/or mean ambulatory BPs could change substantially over that length of time, influenced not only by factors such as medication changes but also, as the authors themselves pointed out, physical activity and weight changes.
Another study reported the reproducibility of MH among a group of 503 Japanese workers who were not under treatment for hypertension [7]. The reproducibility of MH was 59% (kappa=0.58) over a six month period using morning HBPM for the out-of-office measurements. However, in addition to the six month time period, this study had other notable limitations. The office BP measurement used could have been taken as long as one month before or after the out-of-office monitoring. Further, because the office measurements were taken by physicians, the white-coat effect may be greater than if measurements were taken by a nurse or medical assistant using an automatic oscillometric monitor.
In our study, the higher prevalence of MH detected by ABPM compared to HBPM may be due to factors that contribute to elevated out-of-office BP that occur outside of home life. Assuming that ABPM is the “gold standard” technique for diagnosing MH, an elevated BP average on home monitoring may be sufficient to rule in MH (adequate specificity), but a non-elevated average on HBPM does not rule it out (poor sensitivity). Others have reached similar conclusions [19].
In sensitivity analyses using a 10/5 mm Hg lower office BP cutoff paired with elevated out-of-office measurements to define MH, the prevalence rates of MH were lower (as expected), but the agreement levels were similar. These analyses highlight that MH is not merely a classification ascribed to small fluctuations in BP around borderline threshold levels. For some people, out-of-office BP average is substantially (e.g., 20 mm Hg systolic) higher than office BP. Such differences may equal a doubling of CVD risk [20].
We also found that agreement is improved when awake and sleep periods are defined by a diary rather than time periods. Although several studies in the literature [21,22] use defined time periods, this finding suggests that the diary approach is preferred.
We also examined the prevalence of MH using the participants’ most recent clinic BP rather than their research office visit BPs. Again, the prevalence of MH was high. Therefore, the high prevalence of MH and the agreement levels that we found were not merely reflections of slightly lower BPs attained when measured in research office settings [23].
Our prevalence estimates should not be construed to represent prevalence of MH in adults in the general population or in the primary care population. Rather, they demonstrate the high pre-test probability of MH among patients seen in the office with BPs in the prehypertension range. Our findings are similar to findings of other investigators. Shimbo and colleagues found a prevalence of MH of 34% among a community sample of adults with prehypertension [24]. Among the subset with BP in the 130–139/85–89 mm Hg range, prevalence of MH was 52%, providing additional evidence of the high pretest probability of MH among people with BP in the upper prehypertension range.
Interestingly, we found a low prevalence of white-coat hypertension in our study sample, reflecting the higher averages from ambulatory BP monitoring than office BP measurements as shown in Figure 1. With an average awake ambulatory BP that is 15 mm Hg higher than office BP average, there will be few patients amongst those with office systolic BPs 140–150 mm Hg who have awake ambulatory BPs < 135/85 mm Hg.
Our study population was generally healthy, and therefore might not be representative of the general population. With regard to the home BP monitoring, we note limitations as well. We did not use the same device for office measurements and home BP measurements. We also relied on participants’ written reports of their home BPs, which may have been inaccurate. We did perform checks of reports against the home BP device memory, however.
Our consistent findings that MH has fair short-term reproducibility—which is comparable in our data to the reproducibility of the other classifications— supports the hypothesis that there are factors other than random BP variability that explain the MH phenotype. On one hand, there may be factors that cause people’s BP to be higher outside the office setting. For example, work stress, home strain, trait-anger, and high stress in general may all lead to BP elevations that are diminished when a person is sitting in a health care provider’s office [24–28]. Other factors that transiently raise BP, such as smoking, may contribute to an elevated out-of-office BP average that is not detected in a clinical setting due to the time refraining from them before and during a clinic visit [4,29,30]. On the other hand, it is also possible that there are factors specifically attributable to being in the office setting that lead to a measured BP that is lower than the person’s “average” BP. ABPM measures BP in people’s natural environments. It is possible that the long-standing paradigm of measuring someone’s BP only when they are at rest and in a comfortable setting does not accurately reflect the BP that the target organs see every day. This difference between office BP and ambulatory BP may explain ABPM’s better predictive ability [31].
Conclusions
Masked hypertension represents a common and reproducible BP phenotype. Future research should seek to identify factors consistently associated with MH with a goal of helping clinicians decide which patients with a non-elevated office BP should be considered for out-of-office BP monitoring. Further study also is needed to determine whether treatment of MH, which would have to be guided by out-of-office BP measurements, leads to reduced CVD events.
Acknowledgments
Sources of Funding: This study was funded by grant R01 HL098604 from the National Heart Lung and Blood Institute with additional support provided by UL1 RR025747 from the National Institutes of Health.
Footnotes
Prior Presentation: An abstract of this work was presented as a poster session at the Society of Clinical Research Associates Annual Meeting, September 2013.
Disclosures: Dr. Viera serves on the Medical Advisory Board of Suntech Medical, manufacturer of the Oscar 2 ambulatory BP monitor.
References
- 1.Liu JE, Roman MJ, Pini R, Schwartz JE, Pickering TG, Devereux RB. Cardiac and arterial target organ damage in adults with elevated ambulatory and normal office blood pressure. Ann Intern Med. 1999;131(8):564–572. doi: 10.7326/0003-4819-131-8-199910190-00003. [DOI] [PubMed] [Google Scholar]
- 2.Sega R, Trocino G, Lanzarotti A, Carugo S, Cesana G, Schiavina R, et al. Alterations of cardiac structure in patients with isolated office, ambulatory, or home hypertension: Data from the general population (Pressione Arteriose Monitorate E Loro Associazioni [PAMELA] Study) Circulation. 2001;104(12):1385–1392. doi: 10.1161/hc3701.096100. [DOI] [PubMed] [Google Scholar]
- 3.Fagard RH, Cornelissen VA. Incidence of cardiovascular events in white-coat, masked and sustained hypertension versus true normotension: a meta-analysis. J Hypertens. 2007;25:2193–2198. doi: 10.1097/HJH.0b013e3282ef6185. [DOI] [PubMed] [Google Scholar]
- 4.Verberk WJ, Kessesl AG, de Leeuw PW. Prevalence, causes, and consequences of masked hypertension: a meta-analysis. Am J Hypertens. 2008;21:969–975. doi: 10.1038/ajh.2008.221. [DOI] [PubMed] [Google Scholar]
- 5.Pierdomenico SD, Pannarle G, Rabbia F, Lapenna D, Licitra R, Zito M, et al. Prognostic relevance of masked hypertension in subjects with prehypertension. Am J Hypertens. 2008;21:879–883. doi: 10.1038/ajh.2008.196. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Dov IZ, Ben-Arie L, Mekler J, Bursztyn M. Reproducibility of white-coat and masked hypertension in ambulatory BP monitoring. Int J Cardiol. 2007;117(3):355–9. doi: 10.1016/j.ijcard.2006.04.088. [DOI] [PubMed] [Google Scholar]
- 7.Kawabe H, Saito I. Reproducibility of masked hypertension determined from morning and evening home blood pressure measurements over a 6-month period. Hypertens Res. 2007;30:845–851. doi: 10.1291/hypres.30.845. [DOI] [PubMed] [Google Scholar]
- 8.Viera AJ, Hinderliter AL, Kshirsagar AV, Fine J, Dominik R. Reproducibility of masked hypertension in adults with untreated borderline office blood pressure: comparison of ambulatory and home monitoring. Am J Hypertens. 2010;23(11):1190–1197. doi: 10.1038/ajh.2010.158. [DOI] [PubMed] [Google Scholar]
- 9.Stergiou GS, Salgami EV, Tzamouranis DG, Roussias LG. Masked hypertension assessed by ambulatory versus home blood pressure monitoring: is it the same phenomenon? Am J Hypertens. 2005;18:772–778. doi: 10.1016/j.amjhyper.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Zhuo S, Wen W, Li-Yuan M, Shu-Yu W, Yi-Xin W. Home blood pressure measurement in prehypertension and untreated hypertension: comparison with ambulatory blood pressure monitoring and office blood pressure. Blood Press Monit. 2009;14:245–250. doi: 10.1097/MBP.0b013e328332fd25. [DOI] [PubMed] [Google Scholar]
- 11.Jones CR, Taylor K, Poston L, Shennan AH. Validation of the Welch Allyn ‘Vital Signs’ oscillometric blood pressure monitor. J Hum Hypertens. 2001;15:191–195. doi: 10.1038/sj.jhh.1001141. [DOI] [PubMed] [Google Scholar]
- 12.Jones SC, Bilous M, Winship S, Finn P, Goodwin J. Validation of the Oscar 2 oscillometric 24-hour ambulatory blood pressure monitor according to the International Protocol for the validation of blood pressure measuring devices. Blood Press Monit. 2004;9:219–223. doi: 10.1097/00126097-200408000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Goodwin J, Bilous M, Winship S, Finn P, Jones SC. Validation of the Oscar 2 oscillometric 24-hour ambulatory blood pressure monitor according to British Hypertension Society protocol. Blood Press Monit. 2007;12:113–117. doi: 10.1097/MBP.0b013e3280acab1b. [DOI] [PubMed] [Google Scholar]
- 14.O’Brien E, Mee F, Atkins N, Thomas M. Evaluation of three devices for self-measurement of blood pressure according to the revised British Hypertension Society Protocol: the Omron HEM-705CP, Philips HP5332, and Nissei DS-175. Blood Press Monit. 1996;1(1):55–61. [PubMed] [Google Scholar]
- 15.Verberk WJ, Kroon AA, Kessels AG, Lenders JW, Thien T, van Montfrans GA, et al. The optimal scheme of self blood pressure measurement as determined from ambulatory blood pressure recordings. J Hypertens. 2006;24(8):1541–1548. doi: 10.1097/01.hjh.0000239289.87141.b6. [DOI] [PubMed] [Google Scholar]
- 16.O’Brien E, Parati G, Stergiou G. Ambulatory blood pressure measurement: what is the international consensus? Hypertension. 2013;62:00–00. doi: 10.1161/HYPERTENSIONAHA.113.02148. [DOI] [PubMed] [Google Scholar]
- 17.Niiranen TJ, Asayama K, Thijs L, Johansson JK, Ohkubo T, Kikuya M, et al. International Database of Home blood pressure in relation to Cardiovascular Outcome Investigators. Outcome-driven thresholds for home blood pressure measurement: international database of home blood pressure in relation to cardiovascular outcome. Hypertension. 2013;61(1):27–34. doi: 10.1161/HYPERTENSIONAHA.111.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005;37(5):360–363. [PubMed] [Google Scholar]
- 19.Hanninen MR, Niiranen TJ, Puukka PJ, Jula AM. Comparison of home and ambulatory blood pressure measurement in the diagnosis of masked hypertension. J Hypertens. 2010;28:709–714. doi: 10.1097/HJH.0b013e3283369faa. [DOI] [PubMed] [Google Scholar]
- 20.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 21.Viera AJ, Zhu S, Hinderliter AL, Shimbo D, Person SD, Jacobs DR., Jr Diurnal blood pressure pattern and development of prehypertension or hypertension in young adults: the CARDIA study. J Am Soc Hypertens. 2011;5:48–55. doi: 10.1016/j.jash.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viera AJ, Lin FC, Hinderliter AL, Shimbo D, Person SD, Pletcher MJ, Jacobs DR., Jr Nighttime blood pressure dipping in young adults and coronary artery calcium 10–15 years later: the coronary artery risk development in young adults study. Hypertension. 2012;59:1157–63. doi: 10.1161/HYPERTENSIONAHA.112.191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Myers MG, Godwin M, Dawes M, Kiss A, Tobe SW, Kaczorowski J. Measurement of blood pressure in the office: recognizing the problem and proposing the solution. Hypertension. 2010;55:195–200. doi: 10.1161/HYPERTENSIONAHA.109.141879. [DOI] [PubMed] [Google Scholar]
- 24.Shimbo D, Newman JD, Schwartz J. Masked hypertension and prehypertension: diagnostic overlap and interrelationships with left ventricular mass: the masked hypertension study. Am J Hypertens. 2012;25:664–671. doi: 10.1038/ajh.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindquist TL, Beilin LJ, Knuiman MW. Influence of lifestyle, coping, and job stress on blood pressure in men and women. Hypertension. 1997;29(1Pt1):1–7. doi: 10.1161/01.hyp.29.1.1. [DOI] [PubMed] [Google Scholar]
- 26.Suls J, Wan CK, Costa PT., Jr Relationship of trait anger to resting blood pressure: a meta-analysis. Health Psychol. 1995;14(5):444–456. doi: 10.1037//0278-6133.14.5.444. [DOI] [PubMed] [Google Scholar]
- 27.Schum JL, Jorgensen RS, Verhaeghen P, Sauro M, Thibodeau R. Trait anger, anger expression, and ambulatory blood pressure: a meta-analytic review. J Behav Med. 2003;26(5):395–415. doi: 10.1023/a:1025767900757. [DOI] [PubMed] [Google Scholar]
- 28.James GD, Yee LS, Harshfield GA, Blank SG, Pickering TG. The influence of happiness, anger, and anxiety on the blood pressure of borderline hypertensives. Psychosom Med. 1986;48:502–508. doi: 10.1097/00006842-198609000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Omvik P. How smoking affects blood pressure. Blood Pressure. 1996;5:71–77. doi: 10.3109/08037059609062111. [DOI] [PubMed] [Google Scholar]
- 30.Mann SJ, James GD, Wang RS, Pickering TG. Elevation of ambulatory systolic blood pressure in hypertensive smokers. A case-control study. JAMA. 1991;265:2226–2228. [PubMed] [Google Scholar]
- 31.Verdecchia P, Angeli F, Cavallini C. Ambulatory blood pressure for cardiovascular risk stratification. Circulation. 2007;115:2091–2093. doi: 10.1161/CIRCULATIONAHA.107.697086. [DOI] [PubMed] [Google Scholar]