Abstract
Ig class switch DNA recombination (CSR) in B cells is crucial to the maturation of antibody responses. It requires IgH germline IH-CH transcription and expression of AID, both of which are induced by engagement of CD40 or dual engagement of a Toll-like receptor (TLR) and B cell receptor (BCR). Here, we have addressed cross-regulation between two different TLRs or between a TLR and CD40 in CSR induction by using a B cell stimulation system involving lipopolysaccharides (LPS). LPS mediated long-term primary class-switched antibody responses and memory-like antibody responses in vivo and induced generation of class-switched B cells and plasma cells in vitro. Consistent with the requirement for dual TLR and BCR engagement in CSR induction, LPS, which engages TLR4 through its lipid A moiety, triggered cytosolic Ca2+ flux in B cells through its BCR-engaging polysaccharidic moiety. In the presence of BCR crosslinking, LPS synergized with a TLR1/2 ligand (Pam3CSK4) in CSR induction, but much less efficiently with a TLR7 (R-848) or TLR9 (CpG) ligand. In the absence of BCR crosslinking, R-848 and CpG, which per se induced marginal CSR, virtually abrogated CSR to IgG1, IgG2a, IgG2b, IgG3 and/or IgA, as induced by LPS or CD154 (CD40 ligand) plus IL-4, IFN-γ or TGF-β, and reduced secretion of class-switched Igs, without affecting B cell proliferation or IgM expression. The CSR inhibition by TLR9 was associated with the reduction in AID expression and/or IgH germline IH-S-CH transcription, and required co-stimulation of B cells by CpG with LPS or CD154. Unexpectedly, B cells also failed to undergo CSR or plasma cell differentiation when co-stimulated by LPS and CD154. Overall, by addressing the interaction of TLR1/2, TLR4, TLR7 and TLR9 in the induction of CSR and modulation of TLR-dependent CSR by BCR and CD40, our study suggests the complexity of how different stimuli cross-regulate an important B cell differentiation process and an important role of TLRs in inducing effective T-independent antibody responses to microbial pathogens, allergens and vaccines.
Keywords: antibody, AID; B cells; B cell receptor (BCR); CpG; class switch DNA recombination (CSR); cytokine; germinal center; immunoglobulin; lipopolysaccharides (LPS); T-independent antibody response; Toll-like Receptor (TLR)
Introduction
Immunoglobulin (Ig) class switch DNA recombination (CSR) substitutes an Ig heavy chain (IgH) constant region (e.g. Cμ in IgM) with a downstream region (e.g. Cγ, Cα or Cε of IgG, IgA or IgE), thereby altering the effector function(s) of an antibody without changing its antigen specificity [1]. Pentameric/hexameric IgMs, as produced by non-switched B cells, do not enter the extra-vascular space; class-switched monomeric IgGs and IgEs and monomeric/dimeric IgAs can distribute systemically. IgAs in the intestinal, respiratory and reproductive mucosae control commensal and environmental microbes. CSR, together with somatic hypermutation (SHM) and subsequent positive selection for high-affinity antibody mutants, are essential for the maturation of an effective antibody response during an infection or after vaccination [2].
Induction of CSR in B lymphocytes entails germline IH-S-CH transcription in the IgH locus, as driven by IH promoters and encompassing upstream (donor) and downstream (acceptor) switch (S) regions, where recombination occur. It also requires expression of activation induced cytidine deaminase (AID) in B cells [1, 3]. Both IgH germline IH-S-CH transcription and Aicda (encoding AID) transcripts are induced in B cells activated by primary CSR-inducing stimuli, e.g., T-dependent CD40 signals and T-independent dual Toll-like receptor (TLR)/B cell receptor (BCR) signals [1]. In T-independent antibody responses, B cells are induced to express AID and undergo CSR upon dual engagement of their TLRs and BCR by microbe-associated molecular patterns (MAMPs) and repetitive antigenic ligands, respectively [4, 5]. Dual TLR/BCR engagement also plays an important role in CSR induction in T-dependent antibody responses, before the emergence of specific T helper (TH) cells, by directly activating B cells for CSR induction or by priming B cells for CD40 engagement by trimeric CD154 expressed on TH cells for CSR induction. T-dependent and T-independent primary CSR-inducing stimuli also enable secondary stimuli, i.e., cytokine IL-4 and TGF-β (as well as IFN-γ in the mouse), to induce IgH germline IH-S-CH transcription and histone modifications in the donor and acceptor S regions [6, 7], thereby directing CSR to specific Ig isotypes. IL-4 induces activation of STAT6, which is then recruited to the Iγ1 and Iε promoters to induce Iγ1-Sγ1-Cγ1 and Iε-Sε-Cε germline transcription, and directs CSR to IgG1 and IgE. Likewise, IFN-γ induces germline Iγ2a-Sγ2a-Cγ2a transcription for CSR to IgG2a through Stat1/2, whereas TGF-β induces germline Iγ2b-Sγ2b-Cγ2b and Iα-Sα-Cα transcription through transcription factors Smad and Runx for CSR to IgG2b and IgA, respectively [3]. Targeting of AID to the donor and acceptor S regions is mediated by 14-3-3 adaptor proteins, which simultaneously bind 5’-AGCT-3’ repeats, as frequently occurring in all S regions, and H3K9acS10ph, as specifically induced in the S regions set to recombine [8-10].
As a mature B cell expresses relatively high levels of different TLRs, e.g., TLR1/2, TLR4, TLR7 and TLR9 in the mouse [11-13], it would activate multiple TLRs when exposed to pathogens that contain different MAMPs, such as TLR1/2 ligand triacyl lipopeptides, TLR4 ligand lipid A, and TLR9 ligand bacterial unmethylated DNA, raising the possibility that signals from different TLRs synergize to induce CSR. In addition, B cell-intrinsic TLR signals contributed to class-switched T-dependent antibody responses against protein antigens and viruses [14-16], suggesting a functional interaction of TLRs and CD40 in sustaining and shaping the processes of antibody affinity maturation [17], likely through modulation of B cell differentiation, including CSR. Signals emanating from innate and/or adaptive immune receptors, e.g., those from T-independent TLRs and/or T-dependent CD40, can be integrated in the same B cell [18-21]. Integration of such signals can lead to enhanced or suppressed B cell activation and differentiation, depending on the context. For instance, human naive B cells require co-stimulation of an agonistic anti-CD40 Ab, a TLR ligand, such as the TLR9 ligand CpG oligodeoxynucleotide (CpG), and BCR crosslinking for robust proliferation and induction of AID expression and CSR [22]. By contrast, stimulation of mouse B cells with CpG could suppress CD40-induced IgG1 and IgE secretion [23]. Despite these findings, how different TLRs or TLRs and CD40 regulate each other in CSR induction remains poorly understood, in part due to the previous lack of a robust in vitro B cell stimulation system that employs efficient TLR ligands and CD154.
Here, we have addressed the cross-regulation between TLRs and between a TLR and CD40 in CSR by establishing a B cell stimulation system involving LPS, a widely used TLR4 ligand in activation of macrophages and B cells, other TLR ligands (including TLR1/2 ligand Pam3CSK4, TLR7 ligand R-848 and TLR9 ligand CpG), BCR crosslinking anti–Igδ mAb/dex, and CD154 [5]. We have addressed the role of LPS in the potentiation of long-term T-independent class-switched specific antibody responses in vivo as well as in triggering Ca2+ flux, a cardinal feature of BCR signals, and inducing efficient generation of class-switched B cells and plasma cells in vitro. We have also analyzed the impact of Pam3CSK4, R-848 and CpG on LPS-induced CSR and B cell differentiation into plasma cells. We have further addressed the inhibitory effect of CpG on CD154-induced CSR and the timing requirement for such inhibitory effect. Finally, we have shown the unexpected impact of B cell co-stimulation by LPS and CD154 on CSR and plasma cell differentiation.
Methods
Mice and Immunization
C57BL/6 mice were maintained in pathogen-free vivaria, and were used for experiments at 8-12 weeks of age and without any apparent infection or disease. For immunization, mice were injected intraperitoneally (i.p.) with 25 μg or 10 μg of NP-LPS (average 0.2 molecule of 4-hydroxy-3-nitrophenyl acetyl coupled to 1 molecule of LPS; Biosearch Technologies) in 100 μl of PBS. All protocols were in accordance with the rules and regulations of the Institutional Animal Care and Use Committee (IACUC) of the University of Texas Health Science Center at San Antonio and those of IACUC of the University of California, Irvine.
B cells and CSR
Single B cell suspensions were prepared from spleens or pooled axillary, inguinal and cervical lymph nodes using a 70-μm cell strainer. Spleen B cells were resuspended in ACK Lysis Buffer (Lonza) to lyse red blood cells and, after quenching with RPMI-1640 medium (Invitrogen) supplemented with FBS (10% v/v, Hyclone), penicillin-streptomycin (1% v/v, Invitrogen) and amphotericin B (1% v/v, Invitrogen) (RPMI-FBS), were resuspended in RPMI-FBS before purification of B cells. Lymph node cells were directly resuspended in RPMI-FBS before purification. B cells were purified by depletion of cells expressing CD43, CD4, CD8, CD11b, CD 49b, CD90.2, Gr-1 or Ter-119 using the EasySep™ Mouse B cell Isolation kit (StemCell Technologies) following the manufacturer’s protocol, resulting in a preparation of more than 99% B220+Igδhi B cells. For labeling with carboxyfluorescein diacetate succinimidyl ester (CFSE, Invitrogen), B cells were resuspended in PBS at 107 cell/ml and incubated with 4 μM CFSE at 37°C for 5 m and immediately quenched with RPMI-FBS. After pelleting, B cells were resuspended and cultured at 3 × 105 cell/ml in RPMI-FBS supplemented with 50 μM β-mercaptoethanol.
For CSR induction, B cells were stimulated with different combinations of LPS (3 μg/ml or as indicated, from E. coli, serotype 055:B5, deproteinized by chloroform extraction, Sigma-Aldrich), TLR1/2 ligand Pam3CSK4 (100 ng/ml, Invivogen), TLR7 ligand R-848 (30 ng/ml, Invivogen), TLR9 ligand CpG ODN1826 with a phosphorothioate backbone (CpG, 1 μM, sequence 5’–TCCATGACGTTCCTGACGTT–3’; Operon) and CD154 (3 U/ml, mouse CD154-containing membrane fragments of baculovirus-infected Sf21 insect cells). Anti–Igδ mAb (clone 11-26c) conjugated to dextran (anti–δ mAb/dex; Fina Biosolutions) was added, as indicated, to crosslink BCRs. Recombinant IL-4 (3 ng/ml) was added for CSR to IgG1 and IgE, IFN-γ (50 ng/ml) for CSR to IgG2a, and TGF-β (2 ng/ml) for CSR to IgG2b (all from R&D Systems). After stimulation, B cells were harvested, stained with fluorochrome-conjugated mAbs (Table 1) in Hank’s Buffered Salt Solution (HBSS) for 15 m and analyzed by flow cytometry for Igγ3, Igγ1, Igγ2b, Igγ2a and Igα expression (CSR to IgG3, IgG1, IgG2b, IgG2a and IgA) and other B cell surface molecules. For intracellular Igε analysis, B cells (2 × 106) stimulated by CD154 or LPS plus IL-4 were harvested and treated with 200 μl of trypsin for 2 m to cleave off FcεRI, as described [10, 24], followed by quenching with 1 ml RMPI-FBS. Cells were then fixed in 200 μl of formaldehyde (3.7% v/v) at 25°C for 10 m and, after quenching with 22 μl 1 M glycine (pH 7.0), were permeabilized in 500 μl 90% cold methanol on ice for 30 m before staining with fluorochrome-conjugated Abs and flow cytometry analysis. FACS data were analyzed by the FlowJo® software (Tree Star).
Table 1.
Antibodies.
| Antibody to |
Type |
Company |
Cat. No. |
Applications |
|---|---|---|---|---|
| Mouse B220, PE-conjugated | Rat mAb | eBioscience | 25-0452-83 | FCM |
| Mouse B220, APC-conjugated | Rat mAb | BD Pharmingen | 553092 | FCM |
| Mouse CD138, biotin-conjugated | Rat mAb | BD Pharmingen | 553713 | FCM |
| Mouse Ig | Goat pAb | Southern Biotech | 1012-01 | ELISA |
| Mouse IgG | Goat pAb | Southern Biotech | 1030-01 | ELISA |
| Mouse IgM, FITC-conjugated | Rat mAb | BD Pharmingen | 553437 | FCM |
| Mouse IgG1, FITC-conjugated | Rat mAb | BD Pharmingen | 553443 | FCM |
| Mouse IgG1, APC-conjugated | Rat mAb | BD Pharmingen | 550874 | FCM |
| Mouse IgG1, biotin-conjugated | Goat pAb | Southern Biotech | 1070-08 | ELISA |
| Mouse IgG2a, FITC-conjugated | Rat mAb | BD Pharmingen | 553390 | FCM |
| Mouse IgG2b, biotin-conjugated | Goat pAb | Southern Biotech | 1090-08 | FCM |
| Mouse IgG3, FITC-conjugated | Rat mAb | BD Biosciences | 553403 | FCM |
| Mouse IgG3, biotin-conjugated | Goat pAb | Southern Biotech | 1100-08 | ELISA |
| Mouse IgA, FITC-conjugated | Rat mAb | BD Pharmingen | 559354 | FCM |
| Mouse IgA, biotin-conjugated | Goat pAb | Southern Biotech | 1040-08 | FCM, ELISA |
| Mouse IgE, PE-conjugated | Rat mAb | eBioscience | Dec-92 | FCM |
Abbreviations: mAb, monoclonal antibody; pAb, polyclonal antibody; FCM, flow cytometry; ELISA, enzyme-linked immunosorbent assay.
B cell proliferation
For analysis of B cell proliferation in vitro, purified CFSE-labeled naïve B cells were cultured for 96 h in the presence of appropriate stimuli and then analyzed by flow cytometry for CFSE intensity (CFSE distributes equally into the two daughter cells when a cell divides). The number of B cell divisions was determined by the “Proliferation Platform” of the FlowJo® software. CSR as a function of the cell division number was the ratio of class-switched B cells in each division over the total number of B cells in that division.
Real-time single B cell ratiometric Ca2+ imaging and quantification
B (B220+) cells from C57/BL6 mice were immobilized on poly-L-lysine-coated chamber slides, loaded with 1 μM Fura-2/AM, washed and incubated in Ringer’s solution containing Ca2+ (2 mM; when thapsigargin was used for stimulation, Ca2+ was not added until after cells were stimulated for 2 m). B cells were stimulated with LPS, lipid A, anti-δ-mAb/dex or thapsigargin for up to 20 m and up to 40 cells were imaged simultaneously in each imaging chamber. Real-time fluorescence intensities at 340 nm and 380 nm of each B cell were measured by a custom-built Ca2+ imaging microscope using the MetaFluor® Fluorescence Ratio Imaging software [25]. The ratio of fluorescence intensity at 340 nm over that at 380 nm was plotted as a measurement of the free cytosolic Ca2+ level, of up to 10 cells (representative of 40 cells analyzed), with the threshold set as greater than 220% that of background.
RNA isolation and qRT-PCR analysis of transcripts
Total RNA was extracted from 5 × 106 B cells using the RNeasy Mini Kit (Qiagen). First-strand cDNA was synthesized from 2 μg RNAs using the SuperScript™ III System (Invitrogen) and analyzed by qPCR using SYBR Green (Dynamo HS kit; New England Biolabs) and appropriate primers (Table 2). PCR was performed in a MyiQ Real-Time PCR System (Bio-Rad Laboratories) according to the following protocol: 95°C for 30 s, 40 cycles of 95°C for 10 s, 60°C for 30 s, 72°C for 30 s. Melting curve analysis was performed at 72°C–95°C. The ΔΔCt method was used to analyze levels of transcripts and data were normalized to the level of Cd79b, which encodes the BCR Igβ chain constitutively expressed in B cells.
Table 2.
Oligonucleotide sequences for RT-PCR analysis.
| Transcript |
Forward primer |
Reverse primer |
|---|---|---|
| Aicda | 5′- AGAAA GTCAC GCTGG AGACC -3′ | 5′- CTCCTCTTCACCACGTAGCA -3′ |
| Iγ1-Cγ1 | 5′- TCGAGAAGCCTGAGGAATGTG -3′ | 5′- GGATCCAGAGTTCCAGGTCACT -3′ |
| Iμ-Cγ1 | 5′- CCAGGCACCGCAAATGCC -3′ | 5′- GGACAGTCACTGAGCTGCTC -3′ |
| Iγ2a-Cγ2a | 5′- GCTGATGTACCTACCTGAGAGA -3′ | 5′- GCTGGGCCAGGTGCTCGAGGTT-3′ |
| Iμ-Cγ2a | 5′- ACCTGGGAATGTATGGTTGTGGCTT -3′ | 5′- GCTGGGCCAGGTGTTCGAGGTT -3′ |
| Iγ3-Cγ3 | 5′- GAGGTGGCCAGAGGAGCAAGAT -3′ | 5′- AGCCAGGGACCAAGGGATAGAC -3′ |
| Iμ-Cγ3 | 5′- ACCTGGGAATGTATGGTTGTGGCTT -3′ | 5′- AGCCAGGGACCAAGGGATAGAC -3′ |
| Iα-Cα | 5′- CAAGAAGGAGAAGGTGATTCAG -3′ | 5′- GAGCTGGTGGGAGTGTCAGTG -3′ |
| Iμ-Cα | 5′- ACCTGGGAATGTATGGTTGTGGCTT -3′ | 5′- GAGCTGGTGGGAGTGTCAGTG -3′ |
| CD79b | 5′- TGTTGGAATCTGCAAATGGA -3′ | 5′- TAGGCTTTGGGTGATCCTTG -3′ |
Secreted Ig
To determine titers of total IgG1, IgG3 or IgA, sera and culture supernatants were first diluted 4 – 100-fold and 10-fold, respectively, with PBS (pH 7.4) plus 0.05% (v/v) Tween-20 (PBST). Two-fold serially diluted samples and standards for each Ig isotypes were incubated in 96-well plates coated with pre-adsorbed goat anti–IgG (to capture IgG1 and IgG3) or anti–IgA Abs (all 1 mg/ml; Table 1). After washing with PBST, captured Igs were detected with biotinylated anti–IgG1, –IgG3 or –IgA (Table 1), followed by reaction with horseradish peroxidase (HRP)-labeled streptavidin (Sigma-Aldrich), development with o-phenylenediamine and measurement of absorbance at 492 nm. Ig concentrations were determined using Prism® (GraphPad Software) or Excel® (Microsoft) software.
To analyze titers of high-affinity NP-specific Abs, sera were diluted 1,000-fold in PBST. Two-fold serially diluted samples were incubated in a 96-well plate pre-blocked with BSA and coated with NP3-BSA (average 3 NP molecules on one BSA molecule). Captured Igs were detected with biotinylated Ab to IgG2b or IgG3. Data are expressed as relative values based on end-point dilution factors.
Results
LPS promotes long-term class-switched antibody response in vivo and induces class switching in vitro
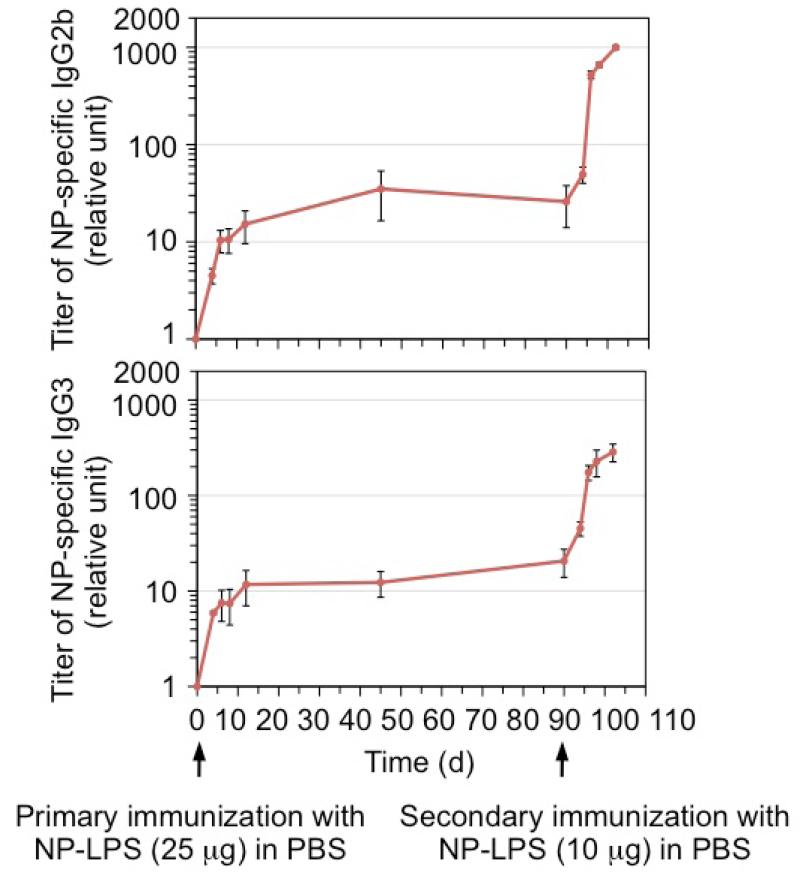
We have previously shown that immunization of C57BL/6 mice with NP-LPS conjugate (which crosslinks the BCR of NP-specific B cells and engages their TLR4), but not NP26-Ficoll (which only crosslinks BCR) or LPS, elicited class-switched NP-specific IgG2b and IgG3, two IgG subclasses characteristically induced by LPS, nine days after injection [5]. We tracked the serum levels of these antibodies in NP-LPS-immunized mice for 90 days. Unlike levels of NP-specific IgG1 in mice immunized with the T-dependent antigen NP-CGG that peaked at d 14 – d 20 and then gradually declined (data not shown), NP-specific IgG2b and IgG3 persisted at the initial peak levels (Figure 1). Upon secondary injection with a lower dose of NP-LPS, mice mounted a strong antibody response, increasing NP-specific IgG2b and IgG3 levels by 50- and 15-fold, respectively, 12 days after the injection (Figure 1).
Figure 1.
NP-LPS induces long-term and secondary antibody response in vivo. Three C57BL/6 mice (8-12 week old) were injected intraperitoneally with NP-LPS (25 μg) in PBS at d 0 and re-injected with NP-LPS (10 μg) in PBS at d 90 (second arrow). Sera were collected prior to the first injection and at different days, and analyzed for titers of NP3-binding IgG2b and IgG3 (depicted as relative units). Titers at d 0 were beyond the detection limit and set as 1 for the graph purpose.
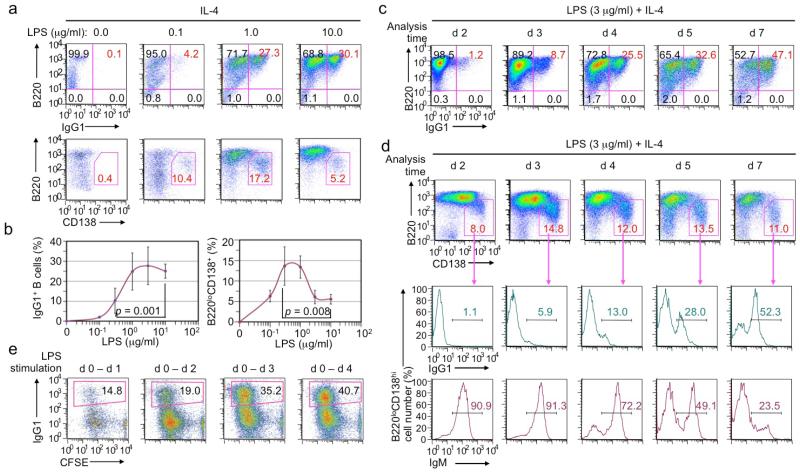
As levels of class-switched antibodies depend on B cell class switching and differentiation into antibody-secreting plasma cells, we next analyzed LPS induction of CSR and plasma cell differentiation in vitro. In the presence of IL-4, LPS induced CSR to IgG1 occurred in a dose-dependent manner, with an optimal dose range of 1-10 μg/ml, while B220loCD138hi plasma cells formed at highest frequencies when B cells were stimulated with intermediate doses of LPS (Figure 2a, 2b). It also occurred in a stimulation time-dependent manner, with IgG1+ B cells kept accumulating, up to seven days of stimulation (Figure 2c). By contrast, the proportion of B220loCD138hi plasma cells did not change, possibly reflecting a continuous increase of newly generated IgG1+ plasma cells and loss of IgM+ plasma cells that formed within the first 2-3 days of LPS stimulation and were likely short-lived (Figure 2d). Interestingly, even a brief 24 h-stimulation of LPS was sufficient to drive a substantial proportion (14.8%) of B cells to complete multiple cell divisions and class switching by d 4, and B cells stimulated by LPS for 3 d underwent CSR at levels comparable to those stimulated for 4 d (Figure 2e).
Figure 2.
LPS induces B cells to undergo CSR and plasma cell differentiation in vitro in a dose- and time-dependent manner (flow cytometry analysis). (a, b) CSR to IgG1 in B cells stimulated with LPS at the indicated concentrations plus IL-4 for 4 d (a, top panels), and formation of B220loCD138hi plasma cells in the same cell culture (a, bottom panels). Mean and s.d. of data from four independent experiments were depicted in (b). p values calculated by paired student t test. (c) CSR to IgG1 in B cells stimulated with LPS plus IL-4, starting at d 0, and harvested at d 2, d 3, d 4, d 5 and d 7, as indicated. (d) Formation of B220loCD138hi plasma cells (top panels) as well as the proportion of IgG1+ (middle panels) and IgM+ (bottom panels) within those plasma cells in cultures of B cells stimulated with LPS plus IL-4, starting at d 0, and harvested at d 2, d 3, d 4, d 5 and d 7, as indicated. (e) CSR to IgG1 in B cells stimulated with LPS plus IL-4 for the period indicated, pelleted to remove LPS, and then cultured in RPMI-FBS only until being harvested at d 4. Data in (a, c, d, e) are representative of three independent experiments.
These results show that LPS induces long-term memory-like class-switched antibody responses in vivo as well as B cell class switching and differentiation into class-switched plasma cells in vitro.
LPS induces Ca2+ flux in B cells
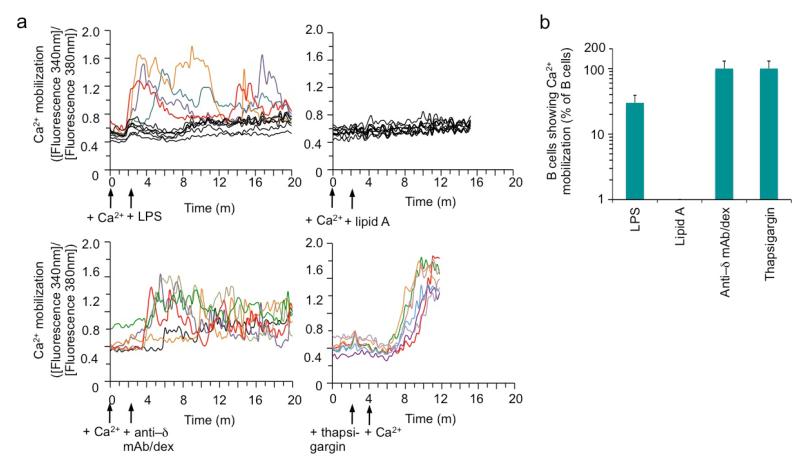
Our previous findings that dual engagement of a TLR and BCR can induce efficient CSR [5] prompted us to analyze the difference of LPS, which consists of a TLR4-engaging lipid A moiety and a polysaccharidic moiety, and monophosphoryl lipid A in triggering BCR signaling. We adapted a quantitative real-time microscopic approach to quantify Ca2+ flux, a hallmark of the BCR signaling, in B cells at the single cell level. Within seconds of LPS stimulation, a proportion of B cells started to release into the cytosol free Ca2+, as detected by its binding to Fura-2, in a fluctuation manner over the course of 20 m (Figure 3a). Overall, more than 25% of B cells showed Ca2+ flux upon LPS stimulation, as compared to virtually all B cells that did so upon stimulation by anti–Igδ mAb/dex and none upon stimulation by lipid A (Figure 3b). Thus, stimulation of B cells with LPS triggers not only TLR4 signaling through its lipid A moiety, but also BCR signaling, likely through its polysaccharidic moiety, thereby satisfying the requirement of dual TLR/BCR signaling for the induction of high levels of CSR in B cells.
Figure 3.
LPS induces Ca2+ mobilization in a proportion of B lymphocytes in the normal B cell repertoire. (a) Real-time ratiometric imaging of cytosolic Ca2+ levels in B cells pre-loaded with Fura, washed and incubated in Ringer’s solution containing Ca2+ (2 mM), and then stimulated with LPS, lipid A, anti–δ mAb/dex or thapsigargin (as indicated by arrow) for up to 20 m (when thapsigargin was used for stimulation, Ca2+ was not added until after cells were stimulated for 2 m). Depicted is the trace of ratio of fluorescence intensity at 340 nm over that at 380 nm, as a measurement of the free cytosolic Ca2+ level, of up to 10 cells (representative of at least 80 cells analyzed). Each colored trace depicted Ca2+ mobilization (above the threshold set as greater than 220% that of background) in an individual cells and black traces depicted cells with no Ca2+ mobilization. Data are representative of four independent experiments. (b) Quantification of B cells showing Ca2+ mobilization upon stimulation by with LPS, lipid A, anti-δ-mAb/dex or thapsigargin (which blocks Ca2+ transport back to ER, thereby raising cytosolic Ca2+ levels in all cells). Data are mean and s.d. from triplicates.
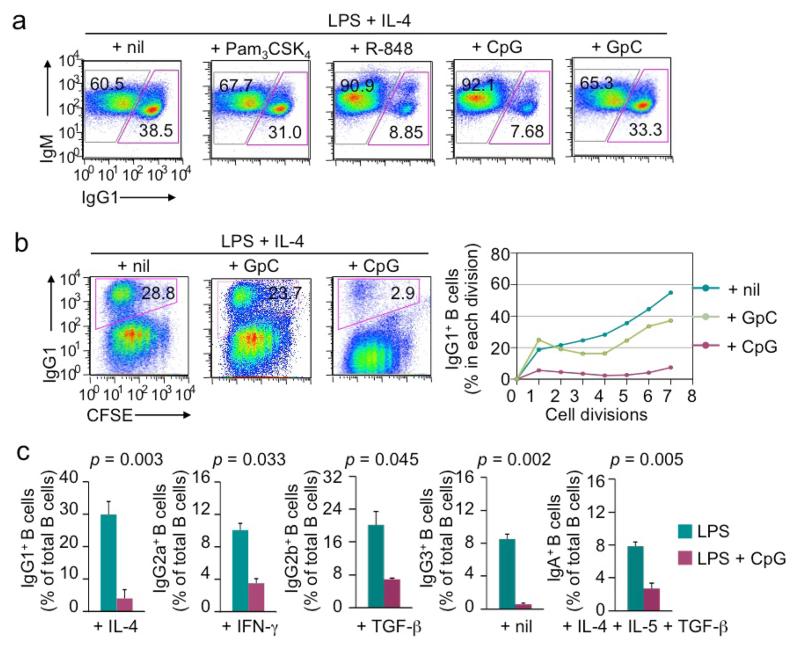
LPS-induced CSR is enhanced by Pam3CSK4 and CpG in the presence of BCR crosslinking
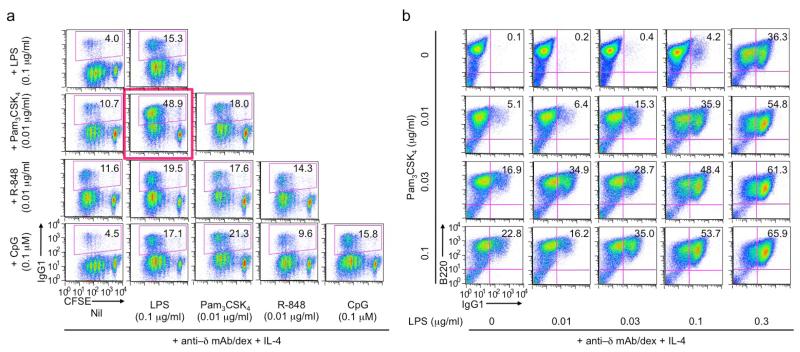
Prompted by the consideration that a B cell would encounter more than one MAMP molecule (TLR ligand) during a natural infection, we next analyzed the modulation of LPS-induced CSR by the ligand of other TLRs (TLR1/2, TLR7 and TLR9) in the presence of anti–Igδ mAb/dex, which triggers BCR signaling, as required for TLR ligands to induce efficient CSR [5]. At suboptimal doses, LPS (0.1 μg/ml), Pam3CSK4 (0.01 μg/ml), R-848 (0.01 μg/ml) and CpG (0.1 μM) all induced marginal levels of CSR to IgG1 in the presence of IL-4 (panels within the first column, Figure 4a) – CSR was only slightly increased, to 15%-18%, when the dose doubled (panels on the slope, Figure 4a). Pam3CSK4 and CpG, but not R-848, enhanced LPS induction of CSR. In particular, Pam3CSK4 displayed a synergizing activity with LPS for CSR induction (Figure 4a, red box) and did so in a dose-dependent fashion, leading to class switching in more than 65% of B cells (Figure 4b). The pairwise combination between Pam3CSK4, R-848 and CpG displayed additive, but not synergistic, effect in CSR induction (Figure 4a). Notably, in the absence of anti–Igδ mAb/dex, CSR frequencies were in general below 5%, irrespective of different pair-wise combinations (data not shown).
Figure 4.
LPS-induced CSR to IgG1 is enhanced by Pam3CSK4 and CpG in the presence of BCR signaling (flow cytometry analysis). (a) CSR to IgG1 in CFSE-labeled B cells stimulated with suboptimal doses of LPS, Pam3CSK4, R-848 and CpG (as indicated), alone or in pair-wise combination, all in the presence of anti–δ mAb/dex (100 ng/ml) and IL-4 for 4 d. (b) CSR to IgG1 in B cells stimulated with different concentrations of Pam3CSK4 and LPS in the presence of anti–δ mAb/dex (100 ng/ml) and IL-4 for 4 d.
LPS-induced CSR is inhibited by R-848 and CpG in the absence of BCR crosslinking
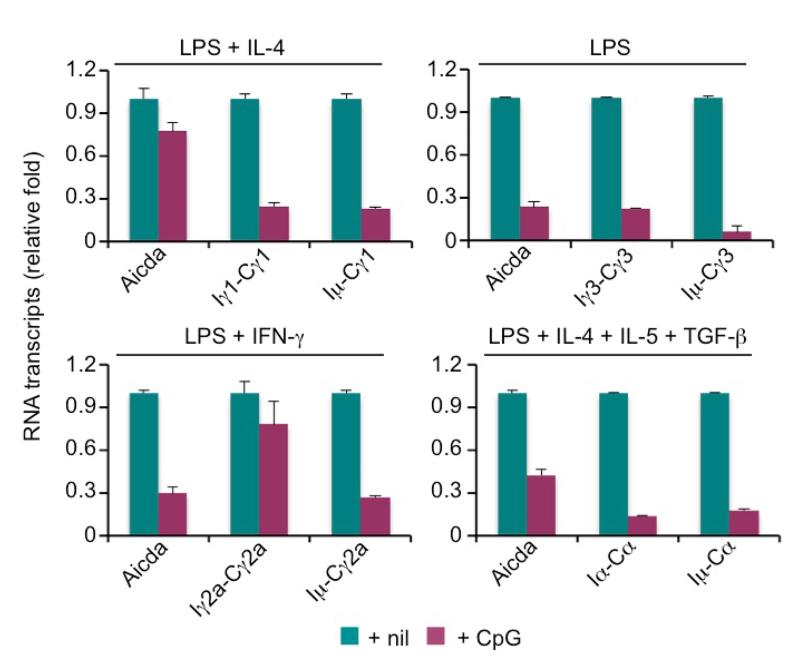
In the presence of BCR crosslinking by anti–Igδ mAb/dex, endosomal TLR9 ligand CpG and TLR7 ligand R-848 could induce CSR at a high level, as we have previously shown [5], and moderately enhance LPS-induced CSR. Surprisingly, in the absence of BCR crosslinking, CpG and R-848 reduced CSR to IgG1 induced by LPS plus IL-4 by over 90% at a wide range of concentrations (Figure 5a and data not shown). By contrast, the control GpC ODN, which did not engage TLR9, or TLR1/2 ligand Pam3CSK4 did not inhibit LPS-induced CSR. CpG-mediated inhibition was independent of any alterations in B cell division and was not associated with any changes in the surface expression of IgM (Figure 5a, 5b). In addition, CpG inhibited LPS induced CSR to IgG3, other IgG isotypes and IgA, as specified by cytokines, such as IFN-γ (for CSR to IgG2a), TGF-β (for CSR to IgG2b) or IL-4, IL-5 plus TGF-β (for CSR to IgA), as assessed by surface Ig expression (Figure 5c) and generation of post-recombination Iμ-Cγ1, Iμ-Cγ2a, Iμ-Cγ3 and Iμ-Cα transcripts (Figure 6). We further addressed the molecular mechanisms underlying CpG inhibition of CSR by analyzing induction of AID expression and germline IH-S-CH transcription. CpG reduced Aicda transcript levels in B cells treated with LPS, LPS plus IFN-γ or LPS plus IL-4, IL-5 and TGF-β, as well as germline Iγ1-Cγ1, Iγ3-Cγ3 and Iα-Cα transcripts in B cells treated with LPS plus IL-4, LPS and LPS plus IL-4, IL-5 and TGF-β, respectively (Figure 6).
Figure 5.
LPS-induced CSR is inhibited by CpG and R-848 in the absence of BCR crosslinking (flow cytometry analysis). (a) The proportion of switched IgG1+IgM− B cells and IgM+IgG1− unswitched B cells in B cells after stimulation with LPS (3 μg/ml) plus IL-4 in the presence of nil, Pam3CSK4, R-848, CpG or GpC for 4 d. (b) CSR to IgG1 in CFSE-labeled B cells stimulated with LPS (3 μg/ml) plus IL-4 in the absence or presence of GpC (0.3 μM) or CpG (0.3 μM; left panels) for 4 d and depiction of the proportion of switched IgG1+ cells in B cells that had completed each cell division (right panels). (c) CSR to different IgG isotypes or IgA in B cells stimulated with LPS (3 μg/ml) plus appropriate cytokines (as indicated) in the absence or presence of CpG (0.3 μM) (mean and s.d. of data from three independent experiments; p values calculated by paired student t test). Data in (a) and (b) are representative of three independent experiments.
Figure 6.
LPS-induced Aicda expression and IgH germline IH-S-CH transcription are inhibited by CpG (real-time qRT-PCR analysis). Levels of mRNA transcripts, as indicated, in B cells stimulated with LPS plus IL-4, nil, IFN-γ or IL-4, IL-5 and TGF-β, as indicated, in the absence or presence of CpG for 48 h (mean and s.d. of data from triplicate samples). Data are representative of three independent experiments.
Overall, our data show that LPS-induced CSR to all IgG isotypes and IgA is inhibited by TLR7 or TLR9, likely due to the downregulation of Aicda transcription and/or germline IH-S-CH transcription.
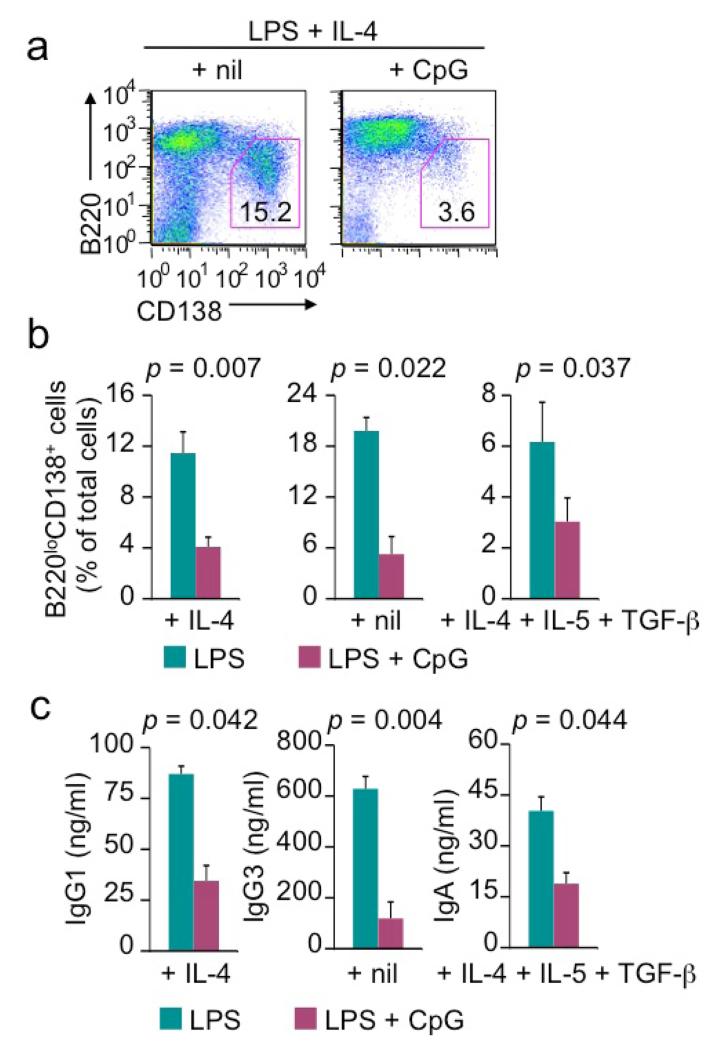
LPS-induced plasma cell differentiation and secretion of class-switched Igs are inhibited by CpG
Like CSR, LPS-induced B cell differentiation into plasma cells was inhibited by CpG, as shown by 50-80% reduction of the formation of B220loCD138hi plasma cells in B cells stimulated with LPS plus IL-4, nil or IL-4, IL-5 and TGF-β (Figure 7a, 7b). This decrease was observed for both IgM+ plasma cells within 4 d of stimulation and for class-switched IgG1+, IgG3+ and IgA+ plasma cells after 7 d of stimulation (data not shown). Consistent with its suppression of plasma cell formation, CpG reduced the titers of secreted IgG1, IgG3 and IgA in B cell cultures upon stimulation with LPS plus IL-4, nil and IL-4/IL-5/TGF-β, respectively for 7 d, without affecting titers of secreted IgM (Figure 7c and data not shown). Thus, LPS-induced B cell class switching and plasma cell differentiation are inhibited by CpG in the absence of BCR crosslinking, leading to impairment in the secretion of class-switched IgG isotypes and IgA.
Figure 7.
LPS-induced plasma cell differentiation and secretion of class-switched Ig are inhibited by CpG in the absence of BCR crosslinking. (a) Formation of B220loCD138hi plasma cells in cultures of B cells stimulated with LPS (1 μg/ml) plus IL-4 in the absence or presence of CpG (0.3 μM) for 4 d (flow cytometry analysis). (b) Formation of B220loCD138hi plasma cells in cultures of B cells stimulated with LPS plus IL-4, nil or IL-4, IL-5 and TGF-β, as indicated, in the absence or presence of CpG for 4 d (flow cytometry analysis). (c) ELISA of IgG1, IgG3 or IgA secreted into the supernatant of B cell cultures after stimulation with LPS plus IL-4, nil or IL-4, IL-5 and TGF-β, as indicated, in the absence or presence of CpG for 4 d. Data are mean and s.d. from three independent experiments (p values calculated by paired student t test).
CD154-induced CSR and B cell differentiation are inhibited by CpG in the absence of BCR crosslinking
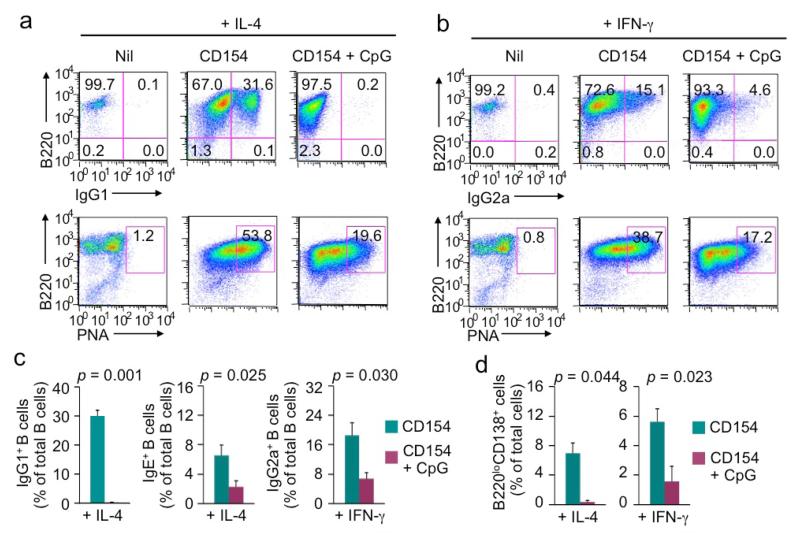
We next addressed the impact of CpG on CSR and B cell differentiation induced by CD154, the T-dependent primary B cell stimulus. Like LPS, CD154, together with IL-4 or IFN-γ, induced CSR to IgG1 or IgG2a in a time- and dose-dependent manner (Figure 8 and data not shown) – CD154 plus IL-4 also induced CSR to IgE and did so more efficiently as compared to LPS plus IL-4 [10, 24]. In addition, upon CD154 stimulation, B cells expressed high levels of lectins that were bound by PNA (PNAhi, which is a hallmark of germinal center B cells in vivo) and differentiate into B220loCD138hi plasma cells. In the absence of BCR crosslinking, CpG virtually abolished CD154-induced generation of IgG1+ B cells and impaired generation of IgE+ and IgG2a+ B cells, without affecting expression of IgM on B cells (Figure 8a-8c and data not shown). It also reduced the expression levels of PNA-binding lectins on B cells and hampered the generation of B220loCD138hi plasma cells without affecting B cell proliferation or viability (Figure 8d and data not shown).
Figure 8.
CD154-induced B cell class switching and differentiation into PNAhi B cells and plasma cells are inhibited by CpG in the absence of BCR crosslinking (flow cytometry analysis). (a) CSR to IgG1 (top panels) and expression of PNA-binding lectins (bottom panels) in B cells stimulated with CD154 (3 unit/ml) plus IL-4 in the absence or presence of CpG (0.3 μM) for 4 d. (b) CSR to IgG1 (top panels) and expression of PNA-binding lectins (bottom panels) in B cells stimulated with CD154 (3 unit/ml) plus IFN-γ in the absence or presence of CpG for 4 d. (c) CSR to IgG1, IgE or IgG2a in B cells stimulated with CD154 plus IL-4 or IFN-γ in the absence or presence of CpG for 4 d. Data are mean and s.d. from three independent experiments (p values calculated by paired student t test). (d) Formation of B220loCD138hi plasma cells in cultures of B cells stimulated with LPS (1 μg/ml) plus IL-4 or IFN-γ in the absence or presence of CpG (0.3 μM) for 4 d. Data are mean and s.d. from three independent experiments (p values calculated by paired student t test).
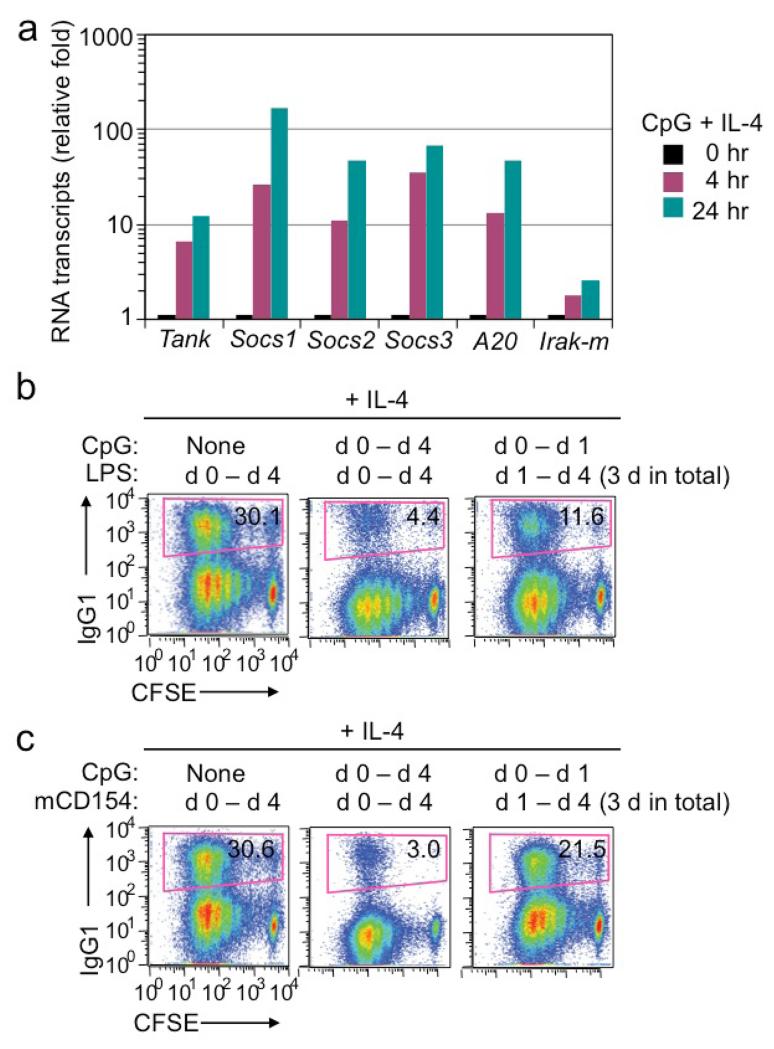
B cells quickly upregulated expression of several putative negative regulators of NF-κB activation, such as TANK, SOCS1, SOCS2, SOCS3 and A20, upon stimulation by CpG plus IL-4, up to 30-fold (Socs3) within 4 h of stimulation and 170-fold (Socs1) within 24 h (Figure 9a). To address whether CpG mediated CSR inhibition by first upregulating expression of these and other putative negative regulators of B cell activation/differentiation, we stimulated B cells with CpG plus IL-4 for 24 h and then washed B cells extensively to remove CpG before re-stimulating B cells with LPS or CD154 plus IL-4. Like freshly isolated naïve B cells (Figure 1c and data not shown), B cells pre-treated with CpG could still undergo CSR to IgG1 at frequencies of 11% and 21% after 3 d (d 1 to d 4) of stimulation by LPS or CD154, respectively, plus IL-4 (Figure 9b, 9c).
Figure 9.
CpG-mediated inhibition of LPS- or CD154-induced CSR requires co-stimulation of B cells by CpG with LPS or CD154. (a) Levels of transcripts, as indicated, in B cells stimulated with CpG plus IL-4 for 0, 4 or 24 h (real-time qRT-PCR analysis). (b, c) CSR to IgG1 in CFSE-labeled B cells stimulated with LPS (3 μg/ml, b) or CD154 (3 unit/ml, c) plus CpG (0.3 mM) for the period as indicated plus IL4 (flow cytometry analysis). Data are representative of three independent experiments.
Overall, out data show that both T-independent and T-dependent CSR as well as plasma cell differentiation, as induced by LPS and CD154, respectively, are inhibited by CpG, in a manner that requires co-stimulation of B cells by LPS or CD154 with CpG.
Co-stimulation of B cells by LPS and CD154 results in inhibition of CSR and plasma cell differentiation
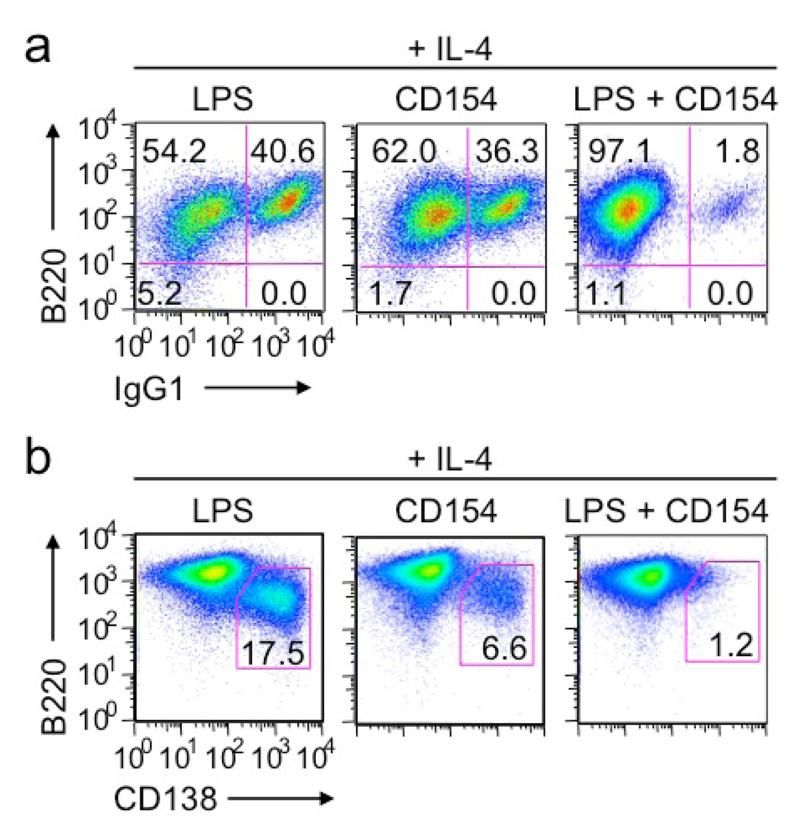
The CSR inhibition mediated by co-stimulation of LPS or CD154 with CpG prompted us to address the impact of co-stimulation of B cells by LPS and CD154 on CSR. While LPS and CD154 each induced CSR at high levels (up to 40% of B cells were IgG1+ when stimulated in the presence of IL-4), they together failed to induce CSR in most B cells without causing any reduction in the number of live B cells (Figure 10a and data not show). Likewise, plasma cell differentiation was induced by LPS or CD154 plus IL-4, but was virtually abolished in co-stimulated B cells (Figure 10b). Thus, two primary CSR-inducing stimuli, LPS and CD154, synergize to inhibit B cell class switching and plasma cell differentiation.
Figure 10.
Co-stimulation of B cells by LPS and CD154 hampers CSR and plasma cell differentiation (flow cytometry analysis). (a, b) CSR to IgG1 (a) and formation of B220loCD138hi plasma cells (b) in B cell cultures after stimulation with LPS (3 μg/ml), CD154 (3 unit/ml) or both plus IL-4 for 4 d. Data are representative of three independent experiments.
Discussion
Here we have shown that LPS efficiently induces CSR and plasma cell differentiation. Such induction likely underpins the adjuvant effect of LPS in a primary class-switched specific antibody response, as we have previously shown [5] and further confirmed here. The antibody response to NP-LPS, which was free of proteins and was administered in PBS, would be largely independent of T cells, despite the suggestion that E. coli LPS could skew TH1 cell response [26] and T cell TLRs could directly modulate certain T cell functions [27]. It would unfold in “germinal center”-like structures, as B cells stimulated in vitro by LPS express high levels of peanut agglutinin (PNA)-binding lectins [5]. These molecules are characteristically induced by CD154 and are hallmarks of germinal center B cells in mice responding to a T-dependent antigen, such as NP-CGG. The generation of high-affinity specific antibodies evoked by NP-LPS would entail T-independent SHM and positive selection in germinal center-like structures. Further, the NP-LPS-induced antibody response was sustained for over 90 days, suggesting that either the contraction phase of germinal center-like structures is protracted or plasma cells derived from class-switched B cells remain circulating after exiting secondary lymphoid organs and actively secrete antibodies. By contrast, levels of specific antibodies elicited by NP-CGG taper off (Ref. [28] and our own unpublished data), in part due to plasma cells’ homing to the bone marrow, in which they are perhaps less active in secreting antibodies.
LPS is an adjuvant for the generation of memory antibody responses, as challenging of NP-LPS-immunized mice with the same conjugated hapten at a lower dose elicited a rapid and markedly increased response. Our findings differ from previous data showing that NP-LPS could induce an anamnestic IgG1 response when used to challenge mice previously immunized with NP-CGG [28], as they suggest that LPS per se promotes the generation of memory or memory-like B cells. These B cells might also contribute to the maintenance of the serological memory when subjected to reactivation by bystander T cells or TLR ligands to differentiate into plasma cells, as it occurs in human memory B cells [29]. The adjuvant effect of LPS to induce an effective T-independent antibody response also contrasts the role of TLR ligands to boost the T-dependent antibody responses [15, 16, 30], and may represent a new strategy of vaccines that can target subjects with impairment in T cell functions.
Efficient CSR induction by LPS is underpinned by the ability of LPS to trigger not only TLR4 signaling (as lipid A does), but also BCR signaling, as indicated by the increased cytosolic Ca2+ flux, as confirmed by both flow cytometry [5] and quantitative imaging (this study) – LPS can also induce Ca2+ flux in dendritic cells, perhaps through engagement of CD14 [31]. While BCR signaling promotes TLR-induced class switching of IgM+IgD+ B cells, it does hamper the differentiation of these B cells into plasma cells, thereby explaining the seemingly mutually exclusive generation of IgG1+ cells and CD138hi cells during early stimulation (e.g., within 72 h). The polyclonal nature of early antibody responses supports a role of TLRs, but not BCR, as the driving force in the generation of short-lived extrafollicular plasmablasts. Nevertheless, BCR signaling would promote the differentiation of class-switched B cells into plasma cells in vivo, as suggested by the important role of BCR affinities towards the antigen in plasma cell differentiation in germinal centers [32] and our data showing increased IgG1+CD138hi cells from d 4 to d 7 of stimulation.
BCR signaling also dictates the outcome of the regulation of LPS-induced CSR by other TLRs and cross-regulation of different TLRs in CSR induction. In the presence of BCR crosslinking, TLR ligands showed synergizing or additive effect, with the strongest synergy occurring between two surface TLRs (TLR1/2 and TLR4). This suggests that class-switched T-independent antibody responses would be particularly important in host immunity to Gram-negative bacteria, which possess both lipoproteins (TLR1/2 ligands) and LPS. TLR4 and TLR7 also synergized in vivo to induce class-switched specific antibody responses [15]. In the absence of BCR crosslinking, however, intracellular TLR7 and TLR9 inhibited LPS-induced CSR (as well as plasma cell differentiation), suggesting that non-antigen-specific B cells (which do not have activated BCR signaling) are tolerant for these irreversible differentiation processes upon co-stimulation by different TLRs. By contrast, autoreactive B cells activated by dual engagement BCR and endosomal TLRs by autoantigen and endogenous TLR ligands, respectively, could be hyper-activated by TLR4 ligands, thereby explaining, at least in part, the exacerbation of autoimmune symptoms by bacterial infection [33, 34]. The B cell-intrinsic role of TLRs, particularly TLR7, and TLR signaling, e.g., that mediated by MyD88, in autoimmunity has been shown in several mouse models [35, 36], emphasizing the relevance of our findings on regulation of TLR-induced CSR in autoimmune diseases.
TLR7 and TLR9 inhibition of LPS-induced CSR occurred in the presence of virtually normal B cell proliferation, survival and IgM expression. The underlying molecular mechanisms would entail modulation of specific gene expression central to CSR, as indicated by CpG-mediated reduction of germline IH-S-CH transcription and/or Aicda expression in LPS-stimulated B cells. The lack of downregulation of germline Iγ2a-Cγ2a transcripts reflects CpG upregulation of T-bet (ref. [23] and our unpublished data), a transcription factor that promotes germline Iγ2a-Sγ2a-Cγ2a transcription and CSR to IgG2a. In fact, despite its inhibition of CSR to IgG2a induced by LPS or CD40 together with IFN-γ, CpG could functionally replace, at least to certain extent, IFN-γ for the induction of CSR to IgG2a by CD40 [23]. As we have shown [5, 24, 37, 38], induction of Aicda expression and CSR depends on activation of both the canonical and non-canonical NF-κB pathways and upregulation of the homeodomain transcription factor HoxC4, and is mediated by the Rab7 small GTPase, which is upregulated in activated B cells and promotes canonical NF-κB activation. TLR7- and TLR9-signaling could modulate one or more of these molecules and pathways. Alternatively, but not mutually exclusively, they may selectively upregulate microRNAs that target Aicda 3’-UTR, thereby downregulating Aicda transcript levels and/or inhibiting their translation to reduce AID expression, in a manner similar to what histone deacetylase (HDAC) inhibitors (HDIs) do to dampen CSR as well as antibody and autoantibody responses [39, 40]. Like HDIs, TLR9 inhibited LPS-induced plasma cell differentiation, perhaps also through a microRNA-dependent pathway.
The cross-inhibition of LPS, CpG and CD154 in the induction of CSR (and plasma cell differentiation) is unexpected, since all three are primary CSR-inducing stimuli that can individually drive CSR, albeit at different degrees [1]. Cross-inhibition of engaged receptors has also been documented in other cell types, e.g., macrophages, in which selected immunoreceptor tyrosine-based activation motif (ITAM)-bearing receptors upon engagement could negatively regulate TLR signaling in macrophages [20, 41]. As shown by our data, co-stimulation was necessary for the strongest inhibition to take place, suggesting that these stimuli synergistically upregulate (a) putative negative regulator(s) of CSR. Interestingly, it has recently been shown that LPS and an agonistic anti–CD40 Ab induced B cells to express IL-35, but not IL-10; IL-35 played a B cell-intrinsic role in downregulating antibody responses, but not IL-10 [42]. An alternative possibility is that certain signal adaptors important for all three pathways[43-45], such as TRAF6, are “sequestered” due to hyper-activation, leading to the dampening of signaling.
During host immune responses to microbial pathogens and allergens, a B cell is exposed to several innate stimuli (e.g., TLR ligands) and adaptive stimuli (e.g., those from cognate T cells) [4, 46] and has to integrate different signals of different strengths for effective and focused specific antibody responses. Our findings have provided an example of how different stimuli cross-regulate an important B cell differentiation process in a context-dependent manner, namely the nature and timing of stimuli. They also raise the possibility that potent TLR ligands may not always elicit optimal antibody responses, a point further emphasized by the reduced class switched antibody response in vivo by repeated treatment of CpG [47]. Rather, a fine-tuned control of the kinetics (timing and sequence) of different stimuli would be critical for the generation of sustained antibody responses. Finally, identification of the negative regulators of CSR would be highly relevant of our understanding of mechanisms underlying autoimmune diseases and inflammation.
Acknowledgments
We thank Dr. Andrew Lees (Fina Biosolutions) for anti–δ mAb/dextran. We thank Mr. Adam Idica for help. This work was supported by NIH grants AI 105813 and AI 079705 to P.C. P.C. was also supported by the Zachry Foundation Distinguished Chair and the Alliance for Lupus Research Target Identification in Lupus Grant ALR 295955. T. L. was supported by NIH T32 training grant AI 060573.
Abbreviations
- AID
activation-induced cytidine deaminase
- BCR
B cell receptor
- CpG
CpG oligodeoxynucleotide
- CSR
class switch DNA recombination
- Ig
immunoglobulin
- LPS
lipopolysaccharides
- NP
4-hydroxy-3-nitrophenyl acetyl
- NP-LPS
NP-conjugated LPS
- PNA
peanut agglutinin
- T-dependent
thymus-dependent
- T-independent
thymus-independent
- TLR
Toll-like receptor
Footnotes
Declaration of Interest
The authors have no financial conflicts of interest.
References
- 1.Xu Z, Zan H, Pone EJ, Mai T, Casali P. Immunoglobulin class-switch DNA recombination: induction, targeting and beyond. Nat. Rev. Immunol. 2012;12:517–531. doi: 10.1038/nri3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casali P. Somatic recombination and hypermutation in the immune system. In: Krebs JE, Goldstein ES, Kilpatrick ST, editors. Lewin’s Genes XI. Jones & Bartlett; Sudbury, MA: 2014. pp. 459–507. [Google Scholar]
- 3.Stavnezer J, Guikema JE, Schrader CE. Mechanism and regulation of class switch recombination. Annu. Rev. Immunol. 2008;26:261–292. doi: 10.1146/annurev.immunol.26.021607.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cerutti A, Puga I, Cols M. Innate control of B cell responses. Trends Immunol. 2011;32:202–211. doi: 10.1016/j.it.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pone EJ, Zhang J, Mai T, White CA, Li G, Sakakura JK, Patel PJ, Al-Qahtani A, Zan H, Xu Z, Casali P. BCR-signalling synergizes with TLR-signalling for induction of AID and immunoglobulin class-switching through the non-canonical NF-κB pathway. Nat. Commun. 2012;3(767):1–12. doi: 10.1038/ncomms1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li G, Zan H, Xu Z, Casali P. Epigenetics of the antibody response. Trends Immunol. 2013;34:460–470. doi: 10.1016/j.it.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaidyanathan B, Yen WF, Pucella JN, Chaudhuri J. AIDing chromatin and transcription-coupled orchestration of immunoglobulin class-switch recombination. Front. Immunol. 2014;5:120. doi: 10.3389/fimmu.2014.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu Z, Fulop Z, Wu G, Pone EJ, Zhang J, Mai T, Thomas LM, Al-Qahtani A, White CA, Park SR, Steinacker P, Li Z, Yates J, 3rd, Herron B, Otto M, Zan H, Fu H, Casali P. 14-3-3 adaptor proteins recruit AID to 5’-AGCT-3’-rich switch regions for class switch recombination. Nat. Struct. Mol. Biol. 2010;17:1124–1135. doi: 10.1038/nsmb.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lam T, Thomas LM, White CA, Li G, Pone EJ, Xu Z, Casali P. Scaffold functions of 14-3-3 adaptors in B cell immunoglobulin class switch DNA recombination. PLoS One. 2013;8:e80414. doi: 10.1371/journal.pone.0080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li G, White CA, Lam T, Pone EJ, Tran DC, Hayama KL, Zan H, Xu Z, Casali P. Combinatorial H3K9acS10ph histone modification in IgH locus S regions targets 14-3-3 adaptors and AID to specify antibody class-switch DNA recombination. Cell Rep. 2013;5:702–714. doi: 10.1016/j.celrep.2013.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gururajan M, Jacob J, Pulendran B. Toll-like receptor expression and responsiveness of distinct murine splenic and mucosal B-cell subsets. PLoS One. 2007;2:e863. doi: 10.1371/journal.pone.0000863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bekeredjian-Ding I, Jego G. Toll-like receptors--sentries in the B-cell response. Immunology. 2009;128:311–323. doi: 10.1111/j.1365-2567.2009.03173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shay T, Kang J. Immunological Genome Project and systems immunology. Trends Immunol. 2013;34:602–609. doi: 10.1016/j.it.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438:364–368. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 15.Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, Ravindran R, Stewart S, Alam M, Kwissa M, Villinger F, Murthy N, Steel J, Jacob J, Hogan RJ, Garcia-Sastre A, Compans R, Pulendran B. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011;470:543–547. doi: 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hou B, Saudan P, Ott G, Wheeler ML, Ji M, Kuzmich L, Lee LM, Coffman RL, Bachmann MF, DeFranco AL. Selective utilization of Toll-like receptor and MyD88 signaling in B cells for enhancement of the antiviral germinal center response. Immunity. 2011;34:375–384. doi: 10.1016/j.immuni.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang IY, Park C, Harrison K, Kehrl JH. TLR4 signaling augments B lymphocyte migration and overcomes the restriction that limits access to germinal center dark zones. J. Exp. Med. 2009;206:2641–2657. doi: 10.1084/jem.20091982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee MS, Kim YJ. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu. Rev. Biochem. 2007;76:447–480. doi: 10.1146/annurev.biochem.76.060605.122847. [DOI] [PubMed] [Google Scholar]
- 19.Bezbradica JS, Medzhitov R. Integration of cytokine and heterologous receptor signaling pathways. Nat. Immunol. 2009;10:333–339. doi: 10.1038/ni.1713. [DOI] [PubMed] [Google Scholar]
- 20.Ivashkiv LB. Cross-regulation of signaling by ITAM-associated receptors. Nat. Immunol. 2009;10:340–347. doi: 10.1038/ni.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pone EJ, Zan H, Zhang J, Al-Qahtani A, Xu Z, Casali P. Toll-like receptors and B-cell receptors synergize to induce immunoglobulin class-switch DNA recombination: relevance to microbial antibody responses. Crit. Rev. Immunol. 2010;30:1–29. doi: 10.1615/critrevimmunol.v30.i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruprecht CR, Lanzavecchia A. Toll-like receptor stimulation as a third signal required for activation of human naive B cells. Eur. J. Immunol. 2006;36:810–816. doi: 10.1002/eji.200535744. [DOI] [PubMed] [Google Scholar]
- 23.Liu N, Ohnishi N, Ni L, Akira S, Bacon KB. CpG directly induces T-bet expression and inhibits IgG1 and IgE switching in B cells. Nat. Immunol. 2003;4:687–693. doi: 10.1038/ni941. [DOI] [PubMed] [Google Scholar]
- 24.Pone EJ, Lam T, Edinger AL, Xu Z, Casali P. B Cell Rab7 mediates induction of AID expression and class-switching in T-dependent and T-independent antibody responses. J. Immunol. 2014 doi: 10.4049/jimmunol.1401896. in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lioudyno MI, Kozak JA, Penna A, Safrina O, Zhang SL, Sen D, Roos J, Stauderman KA, Cahalan MD. Orai1 and STIM1 move to the immunological synapse and are up-regulated during T cell activation. Proc. Nat. Acad. Sci. U.S.A. 2008;105:2011–2016. doi: 10.1073/pnas.0706122105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pulendran B, Kumar P, Cutler CW, Mohamadzadeh M, Van Dyke T, Banchereau J. Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. J. Immunol. 2001;167:5067–5076. doi: 10.4049/jimmunol.167.9.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reynolds JM, Dong C. Toll-like receptor regulation of effector T lymphocyte function. Trends Immunol. 2013;34:511–519. doi: 10.1016/j.it.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Haniuda K, Nojima T, Ohyama K, Kitamura D. Tolerance induction of IgG+ memory B cells by T cell-independent type II antigens. J. Immunol. 2011;186:5620–5628. doi: 10.4049/jimmunol.1100213. [DOI] [PubMed] [Google Scholar]
- 29.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199–2202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed SS, Plotkin SA, Black S, Coffman RL. Assessing the safety of adjuvanted vaccines. Sci. Transl. Med. 2011;3:93rv92. doi: 10.1126/scitranslmed.3002302. [DOI] [PubMed] [Google Scholar]
- 31.Zanoni I, Ostuni R, Capuano G, Collini M, Caccia M, Ronchi AE, Rocchetti M, Mingozzi F, Foti M, Chirico G, Costa B, Zaza A, Ricciardi-Castagnoli P, Granucci F. CD14 regulates the dendritic cell life cycle after LPS exposure through NFAT activation. Nature. 2009;460:264–268. doi: 10.1038/nature08118. [DOI] [PubMed] [Google Scholar]
- 32.Phan TG, Paus D, Chan TD, Turner ML, Nutt SL, Basten A, Brink R. High affinity germinal center B cells are actively selected into the plasma cell compartment. J. Exp. Med. 2006;203:2419–2424. doi: 10.1084/jem.20061254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tomita H, Yamada M, Sekigawa I, Yoshiike T, Iida N, Hashimoto H. Systemic lupus erythematosus-like autoimmune abnormalities induced by bacterial infection. Clin. Exp. Rheum. 2003;21:497–499. [PubMed] [Google Scholar]
- 34.Doria A, Canova M, Tonon M, Zen M, Rampudda E, Bassi N, Atzeni F, Zampieri S, Ghirardello A. Infections as triggers and complications of systemic lupus erythematosus. Autoimmunity Rev. 2008;8:24–28. doi: 10.1016/j.autrev.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 35.Walsh ER, Pisitkun P, Voynova E, Deane JA, Scott BL, Caspi RR, Bolland S. Dual signaling by innate and adaptive immune receptors is required for TLR7-induced B-cell-mediated autoimmunity. Proc. Nat. Acad. Sci. U.S.A. 2012;109:16276–16281. doi: 10.1073/pnas.1209372109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Groom JR, Fletcher CA, Walters SN, Grey ST, Watt SV, Sweet MJ, Smyth MJ, Mackay CR, Mackay F. BAFF and MyD88 signals promote a lupuslike disease independent of T cells. J. Exp. Med. 2007;204:1959–1971. doi: 10.1084/jem.20062567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park SR, Zan H, Pal Z, Zhang J, Al-Qahtani A, Pone EJ, Xu Z, Mai T, Casali P. HoxC4 binds to the promoter of the cytidine deaminase AID gene to induce AID expression, class-switch DNA recombination and somatic hypermutation. Nat. Immunol. 2009;10:540–550. doi: 10.1038/ni.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White CA, Seth Hawkins J, Pone EJ, Yu ES, Al-Qahtani A, Mai T, Zan H, Casali P. AID dysregulation in lupus-prone MRL/Faslpr/lpr mice increases class switch DNA recombination and promotes interchromosomal c-Myc/IgH loci translocations: modulation by HoxC4. Autoimmunity. 2011;44:585–598. doi: 10.3109/08916934.2011.577128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White CA, Pone EJ, Lam TS, Tat C, Hayama KL, Li G, Zan H, Casali P. HDAC inhibitors upregulate B cell microRNAs that silence AID and Blimp-1 expression for epigenetic modulation of antibody and autoantibody response. J. Immunol. 2014;193 doi: 10.4049/jimmunol.1401702. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zan H, Tat C, Casali P. MicroRNAs in lupus. Autoimmunity. 2014;47:272–285. doi: 10.3109/08916934.2014.915955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ivashkiv LB. A signal-switch hypothesis for cross-regulation of cytokine and TLR signalling pathways. Nat. Rev. Immunol. 2008;8:816–822. doi: 10.1038/nri2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen P, Roch T, Lampropoulou V, O’Connor RA, Stervbo U, Hilgenberg E, Ries S, Dang VD, Jaimes Y, Daridon C, Li R, Jouneau L, Boudinot P, Wilantri S, Sakwa I, Miyazaki Y, Leech MD, McPherson RC, Wirtz S, Neurath M, Hoehlig K, Meinl E, Grutzkau A, Grun JR, Horn K, Kuhl AA, Dorner T, Bar-Or A, Kaufmann SH, Anderton SM, Fillatreau S. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature. 2014;507:366–370. doi: 10.1038/nature12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.West AP, Koblansky AA, Ghosh S. Recognition and signaling by toll-like receptors. Annu. Rev. Cell. Dev. Biol. 2006;22:409–437. doi: 10.1146/annurev.cellbio.21.122303.115827. [DOI] [PubMed] [Google Scholar]
- 44.Verstrepen L, Bekaert T, Chau TL, Tavernier J, Chariot A, Beyaert R. TLR-4, IL-1R and TNF-R signaling to NF-κB: variations on a common theme. Cell. Mol. Life Sci. 2008;65:2964–2978. doi: 10.1007/s00018-008-8064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Graham JP, Arcipowski KM, Bishop GA. Differential B-lymphocyte regulation by CD40 and its viral mimic, latent membrane protein 1. Immunol. Rev. 2010;237:226–248. doi: 10.1111/j.1600-065X.2010.00932.x. [DOI] [PubMed] [Google Scholar]
- 46.Vinuesa CG, Chang PP. Innate B cell helpers reveal novel types of antibody responses. Nat. Immunol. 2013;14:119–126. doi: 10.1038/ni.2511. [DOI] [PubMed] [Google Scholar]
- 47.Heikenwalder M, Polymenidou M, Junt T, Sigurdson C, Wagner H, Akira S, Zinkernagel R, Aguzzi A. Lymphoid follicle destruction and immunosuppression after repeated CpG oligodeoxynucleotide administration. Nat. Med. 2004;10:187–192. doi: 10.1038/nm987. [DOI] [PubMed] [Google Scholar]