Abstract
Circulating levels of the placental glycoprotein hormone human chorionic gonadotropin (hCG) are higher in women carrying female v. male fetuses; yet, the significance of this difference with respect to maternal factors, environmental exposures and neonatal outcomes is unknown. As a first step in evaluating the biologic and clinical significance of sex differences in hCG, we conducted a population-level analysis to assess its stability across subgroups. Subjects were women carrying singleton pregnancies who participated in prenatal and newborn screening programs in CA from 2009 to 2012 (1.1 million serum samples). hCG was measured in the first and second trimesters and fetal sex was determined from the neonatal record. Multivariate linear models were used to estimate hCG means in women carrying female and male fetuses. We report fluctuations in the ratios of female to male hCG by maternal factors and by gestational age. hCG was higher in the case of a female fetus by 11 and 8% in the first and second trimesters, respectively (P <0.0001). There were small (1–5%) fluctuations in the sex difference by maternal race, weight and age. The female-to-male ratio in hCG decreased from 17 to 2% in the first trimester, and then increased from 2 to 19% in the second trimester (P <0.0001). We demonstrate within a well enumerated, diverse US population that the sex difference in hCG overall is stable. Small fluctuations within population subgroups may be relevant to environmental and physiologic effects on the placenta and can be probed further using these types of data.
Keywords: fetal sex difference, hCG, placental hormones, pregnancy, sexual dimorphism
Introduction
A longstanding mystery surrounds the epidemiologic observation that human chorionic gonadotropin (hCG) – a placental glycoprotein hormone – is higher in maternal circulation in the case of a female than a male fetus.1–5 This has been proposed as an explanation as to why women carrying a female fetus are more likely to suffer from hyperemesis gravidarum (i.e. nausea) than those carrying a male fetus;6 but otherwise has not been elucidated in terms of clinical significance. The stability of placental sex differences within and across populations is also unknown. We present here a population-level analysis of sexual dimorphic expression of hCG.
Placental hormones have been identified as relevant to sex-specific fetal development. hCG can bind the luteinizing hormone/hCG receptor (LHCGR) and stimulate steroidogenesis in the fetal testis in the first trimester of pregnancy.2,7 This interaction is essential to normal male development in that defects in hCG-LHCGR binding, due to mutations in the LHCGR gene, are associated with hypogonadism and pseudohermaphroditism.8 It is theorized that a male-specific negative feedback mechanism between the fetus and the placenta explains lower circulating levels in the male v. the female.2 There is little known on the relationship of placental hCG and female-specific development. In a comparison study of hCG in fetal tissues, the highest concentrations were detected in the fetal ovary – ~five-fold higher than in the testes.9 Hyperglycosylated hCG can bind and activate the TGF-β receptor.10 TGF-β plays a role in fetal ovary development and possibly in fetal origins of polycystic ovarian syndrome.11
The hCG observation poses an important epidemiologic question, as to whether fetal sex differences in placental function are primarily a genetic/epigenetic phenomenon (i.e. the presence/absence of the Y-chromosome and X-inactivation), or might female/male differences in hCG and other proteins also be indicators of non-genetic influences on the fetal environment such as nutrition, maternal health, maternal stress or chemical exposures. Circulating hCG levels can change in response to chemical exposures,12–14 maternal stress,15 maternal nutrition,16 exogenous estrogen exposure17 and other factors that can modulate the endocrine milieu of pregnancy.
hCG was put into widespread use over three decades based on its predictive power in estimating risk of fetal defects such as Down’s syndrome.18 It later was observed that it can be useful in predictions of non-genetic, adverse pregnancy outcomes such as preterm labor,19 preeclampsia20 and placental abruption.21 Lower second trimester hCG was correlated with increased risk of cryptorchidism in male infants as compared with normal controls.22 The ability of hCG to predict non-genetic fetal disorders has become a new possibility given that it is measured at the population level, and data has been collected for more than a decade. First trimester hCGβ first went into widespread use as a prenatal screening analyte in the 1990s.
The aim of the current analysis was to robustly characterize sex differences in hCG within a well enumerated, racially/ethnically and economically diverse US population. The second aim was to explore the stability of the hCG sex difference within subgroups of the population that might represent genetic as well as non-genetic differences related to social disadvantage, lifestyle, maternal stress, health, nutrition and chemical exposures.
Method
Genetic Disease Screening Program (GDSP)
Study subjects were residents of the State of California who: (a) underwent routine prenatal serum screening in the first and/or second trimesters of pregnancy from April 1, 2009 through December 2012, (b) were carrying singleton pregnancies, (c) delivered their babies in California and (d) participated in the California Newborn Screening Program. For this analysis, the sex of the baby was abstracted from the newborn screening database and merged with the prenatal screening database. The first trimester blood draw was between 10 and 13 weeks 6 days gestation (median time of prenatal screen was 12.0 weeks) and the second trimester blood draw was between 15 and 20 weeks gestation (median time of prenatal screen was 17.0 weeks). Blood samples were shipped to one of the six regional laboratories, where they were analyzed for a panel of proteins using automated methods. The Genetic Disease Laboratory reviewed results before transmission to the GDSP database. Total hCG was measured by the AutoDELFIA® time-resolved fluoroimmunoassay (PerkinElmer, Waltham, MA, USA). All data were entered directly into a centralized database along with information collected on the sample requisition form related to maternal demographics, maternal weight and health. Analyte-specific multiple of the medians (MoM) are calculated centrally, and referred to here as the clinical MoM. The Committee for the Protection of Human Subjects of the California Department of Public Health approved the protocol for this study. Data were abstracted in October 2013. All data were de-identified by the California Genetic Disease Screening Program before transfer to the collaborating institution.
Gestational age multiple of the median (ga-MoM)
The clinical MoM’s are normalized values of the serum analytes that were designed for the calculation of risks of Down’s Syndrome and other types of birth defects.23,24 Epidemiologic analyses have used the clinical MoM more than the analyte values. The concept behind the MoM is to compare each person’s lab value to the expected value for that individual for that gestational day. Information used in the normalization process includes dating method (nuchal translucency, ultrasound, last menstrual period, physical examination), maternal race/ethnicity, weight of the mother, insulin-dependent diabetes, smoking and ovum donor status. This information is collected through a one-page requisition form that the physician completes at the time of the screening. The result is a unitless ratio with a median of 1.0. The database is monitored periodically for analytical drift in the assays and shifts over time in gestational age and other subgroup medians. As needed, the medians are revised in order to maintain the population MoM median value at 1. For the purposes of this analysis, we calculated a ga-MoM that is only adjusted for gestational age and not the other factors. This allows us to model the effects of the other factors on the MoM without the risk of overadjustment. To calculate the ga-MoM, we fit a model of the log median analyte, calculated for each day of gestation, regressed on the day of gestation using a smoothing function. For first trimester hCG, a linear term for gestational day was included in the model. For second trimester hCG, linear and quadratic terms for gestational age were included. An exponential decay to a non-zero constant was tried for hCG but the quadratic model fit the data better. We output the predicted smoothed gestational age medians for the 3-year time period covered in our dataset. Each person’s analyte value was divided by the smoothed gestational age median to generate the ga-MoM.
Statistical analysis
The associations of maternal and fetal factors (fetal sex, maternal race/ethnicity, weight, age, smoking, diabetes and ovum donor status) with log analyte and ga-MoM values were calculated using linear regression models. All estimates were adjusted for dating method, fetal sex, maternal race/ethnicity, maternal age, the inverse of maternal weight in pounds, year of estimated due date, month of screening, smoking, diabetes and ovum donor status. The goal of the modeling approach was to describe, using the information available, the variability in the analyte and in the ga-MoM values. Race groups were generated according to the criteria used by the GDSP. Maternal age categories are according to clinical cut-offs. Maternal weight categories are based on quartiles calculated in this population. We output predicted means for each subgroup and predicted values for each subject on the log scale and then back transformed them to the linear scale. We used the predicted values per subject in order to calculate standard errors in the original units. We calculated fold differences for each category from the referent group on the linear scale. To test the interaction of these factors with fetal sex, we used the same strategy as above and included in the model an interaction term for fetal sex by maternal factor. We output predicted means for females and males, and calculated the female/male ratio for each subgroup. P-values are reported for the main effects as well as for the interactions. Mixed effects models were used to estimate the intraclass correlation coefficient (ICC) for first and second trimester hCG. To visually inspect the change in the fetal sex ratio over time in gestation, we calculated a ratio of median female to median male analyte values for each day of prenatal screening. All analyses were carried out in SAS 9.2 (Cary, NC, USA) and plots were generated in R (R Foundation, www.r-project.org).
Results
The sample presented here was restricted to those pregnancies that resulted in a livebirth; yet, the clinical MoM’s are based on all pregnancies in California. The median values for the MoM’s were equal to 1.00, indicating that our sample was not significantly different from the underlying population, even though it excluded pregnancies that ended in miscarriage, termination or otherwise were not captured in the neonatal screening program. Subject characteristics were highly consistent within the two screening intervals (Table 1). More women had second than first trimester screening, and 38% of women had screening in both trimesters. The ICC for the hCG ga-MoM was 0.74 (95% CI 0.74, 0.74), indicating fairly high reproducibility of hCG within a pregnant woman over time.
Table 1.
Sample characteristics of participants in Genetic Disease Screening Program of the California Department of Public Health
| First trimester | Second trimester | |
|---|---|---|
| n | 429,087 | 659,581 |
| Year [n (%)] | ||
| 2009 | 3,040 (0.7) | 80,360 (12) |
| 2010 | 147,522 (34) | 211,803 (32) |
| 2011 | 171,606 (40) | 228,423 (35) |
| 2012 | 106,895 (25) | 138,968 (21) |
| Fetal sex [n (%)] | ||
| Female | 209,520 (49) | 321,744 (49) |
| Male | 217,778 (51) | 334,907 (51) |
| Race/ethnicity [n (%)] | ||
| Asian | 56,632 (13) | 77,794 (12) |
| Black | 18,889 (4.4) | 32,315 (4.9) |
| Hispanic | 195,274 (46) | 322,041 (49) |
| White | 139,176 (32) | 198,836 (30) |
| Other | 19,114 (4.5) | 28,595 (4.3) |
| Diabetic [n (%)] | 4,554 (1.1) | 5,969 (0.9) |
| Current smoker [n (%)] | 3,462 (0.8) | 7,702 (1.2) |
| Ovum donor [n (%)] | 1,493 (0.4) | 1,794 (0.3) |
| Maternal age (years) [n (%)] | ||
| <20 | 18,716 (4.4) | 38,056 (6) |
| 20–34 | 310,576 (72.4) | 484,248 (73) |
| >34 | 99,763 (23.3) | 137,242 (21) |
| Maternal weight (kg) [n (%)] | ||
| ≤59 | 110,808 (26) | 166,857 (25) |
| 59–79 | 213,929 (50) | 326,001 (50) |
| 79< | 104,342 (24) | 166,715 (25) |
| Dating method [n (%)] | ||
| Last menstrual period | 12,264 (3) | 39,627 (6) |
| Nuchal translucency | 282,062 (66) | 299,373 (45) |
| Ultrasound | 134,761 (31) | 317,187 (48) |
| Physical exam | 3,394 (1) | |
In the first row for first and second trimesters in Table 2, we present the estimate for the overall sex ratio for each analyte. This ratio represented the overall, or genetically dominant, fetal sex difference. The ratios below it are the subgroup fluctuations from the genetic baseline. Associations were essentially the same when calculated using the analyte values adjusted for gestational age (Table 2) and the ga-MoM values (data not shown). Demographic differences by race, weight, smoking, insulin-dependent diabetes and ovum donor status were consistent with what has been reported previously.24–28
Table 2.
Predicted mean values of hCG (IU/l), by maternal factors, predicted mean values by placental-fetal sex, and female/male ratios in hCG levels and their fluctuations across subgroups
| All | Female | Male | Female/male ratio | Interaction with sex P-value | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Predicted mean (S.E.) | Relative to referent ratioa | Predicted mean (S.E.) | ||||
| First trimester hCG | ||||||
| Sex | 67.0 (0.03) | 60.4 (0.03) | 1.11 | |||
| Race | <0.0001 | |||||
| Hispanic | 58.4 (0.03) | Ref. | 61.3 (0.04) | 55.7 (0.04) | 1.10 | |
| White | 65.0 (0.04) | 1.11 | 69.1 (0.05) | 61.3 (0.04) | 1.13 | |
| Black | 67.3 (0.11) | 1.15 | 71.3 (0.16) | 63.5 (0.14) | 1.12 | |
| Asian | 79.1 (0.06) | 1.35 | 82.9 (0.09) | 75.8 (0.08) | 1.09 | |
| Age (years) | 0.0005 | |||||
| <20 | 65.1 (0.11) | Ref. | 68.0 (0.16) | 62.4 (0.14) | 1.09 | |
| 20–34 | 62.8 (0.03) | 0.96 | 66.1 (0.04) | 59.7 (0.03) | 1.11 | |
| 34< | 65.7 (0.05) | 1.01 | 69.5 (0.07) | 62.3 (0.06) | 1.12 | |
| Weight (kgs) | 0.0229 | |||||
| ≤59 | 77.3 (0.04) | Ref. | 81.5 (0.06) | 73.5 (0.05) | 1.11 | |
| 59–79 | 63.7 (0.02) | 0.82 | 67.3 (0.03) | 60.5 (0.03) | 1.11 | |
| 79< | 51.2 (0.03) | 0.66 | 53.8 (0.04) | 48.8 (0.03) | 1.10 | |
| Non-smoker | 63.7 (0.02) | Ref. | 67.1 (0.03) | 60.5 (0.03) | 1.11 | |
| Smoker | 48.0 (0.19) | 0.75 | 50.8 (0.28) | 45.4 (0.24) | 1.12 | 0.99 |
| Non-diabetic | 63.7 (0.02) | Ref. | 67.1 (0.03) | 60.5 (0.03) | 1.11 | |
| Diabetic | 53.4 (0.19) | 0.84 | 55.7 (0.28) | 51.4 (0.25) | 1.08 | 0.14 |
| Non-ovum donor | 63.5 (0.02) | Ref. | 66.9 (0.03) | 60.4 (0.03) | 1.11 | |
| Ovum donor | 77.9 (0.43) | 1.23 | 83.5 (0.61) | 73.1 (0.55) | 1.14 | 0.08 |
| Second trimester hCG | ||||||
| Sex | 20.9 (0.01) | 19.4 (0.01) | 1.08 | |||
| Race | <0.0001 | |||||
| Hispanic | 18.8 (0.01) | Ref. | 19.5 (0.01) | 18.1 (0.01) | 1.08 | |
| White | 20.5 (0.01) | 1.09 | 21.3 (0.02) | 19.7 (0.02) | 1.08 | |
| Black | 20.6 (0.03) | 1.10 | 21.6 (0.05) | 19.6 (0.04) | 1.10 | |
| Asian | 25.1 (0.02) | 1.34 | 25.5 (0.03) | 24.7 (0.03) | 1.03 | |
| Age (years) | 0.0129 | |||||
| <20 | 20.8 (0.03) | Ref. | 21.5 (0.04) | 20.2 (0.04) | 1.06 | |
| 20–34 | 19.8 (0.01) | 0.95 | 20.5 (0.01) | 19.0 (0.01) | 1.08 | |
| 34< | 21.2 (0.02) | 1.02 | 21.9 (0.02) | 20.5 (0.02) | 1.07 | |
| Weight (kgs) | <0.0001 | |||||
| ≤59 | 24.4 (0.01) | Ref. | 25.0 (0.02) | 23.8 (0.02) | 1.05 | |
| 59–79 | 20.1 (0.01) | 0.82 | 20.9 (0.01) | 19.4 (0.01) | 1.08 | |
| 79< | 16.4 (0.01) | 0.67 | 17.1 (0.01) | 15.7 (0.01) | 1.09 | |
| Non-smoker | 20.2 (0.01) | Ref. | 20.9 (0.01) | 19.5 (0.01) | 1.07 | |
| Smoker | 15.4 (0.05) | 0.76 | 16.1 (0.07) | 14.7 (0.07) | 1.10 | 0.11 |
| Non-diabetic | 20.1 (0.01) | Ref. | 20.9 (0.01) | 19.4 (0.01) | 1.08 | |
| Diabetic | 17.8 (0.06) | 0.89 | 18.4 (0.09) | 17.2 (0.08) | 1.07 | 0.88 |
| Non-ovum donor | 20.1 (0.01) | Ref. | 20.8 (0.01) | 19.4 (0.01) | 1.07 | |
| Ovum donor | 27.9 (0.16) | 1.39 | 28.6 (0.23) | 27.3 (0.22) | 1.05 | 0.33 |
hCG, human chorionic gonadotropin.
All P-values for differences with referent group are <0.0001.
Fluctuations in female/male ratios by hormone
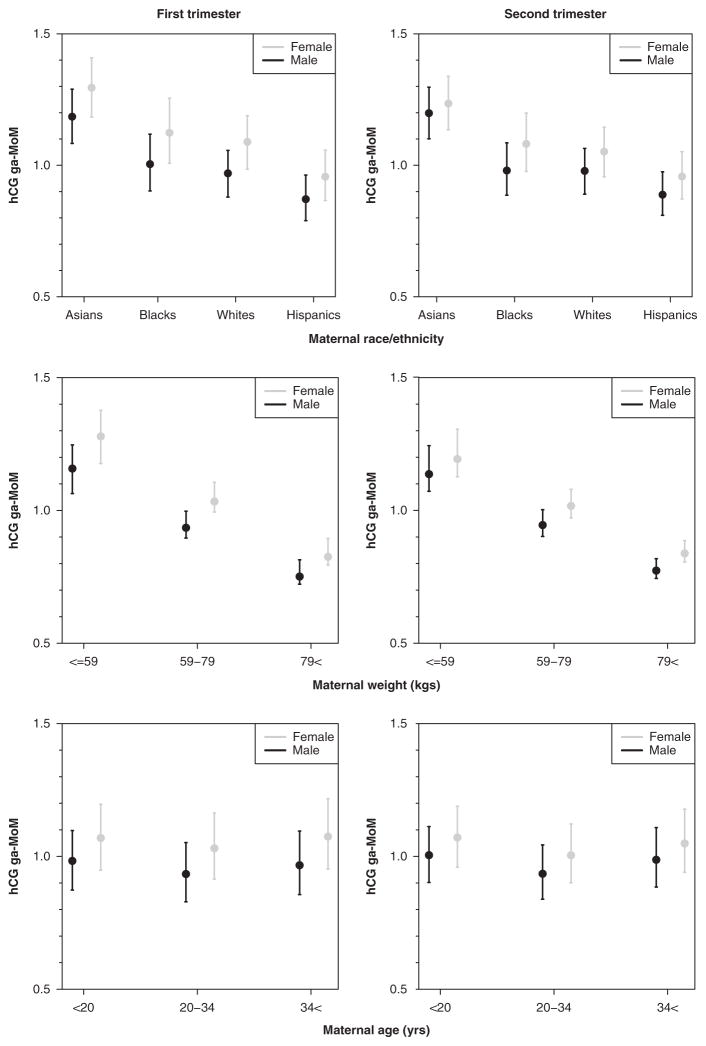
hCG was 11% higher in female pregnancies in the first trimester and 8% higher in the second trimester (Table 2, Fig. 1). In first trimester, the greatest female/male ratio was in White women (13%) v. Asian women (9%). In second trimester, the female/male ratio was highest in Black women (10%) and lowest in Asian women (3%) (Fig. 2). All estimates were adjusted for gestational age at the time of the blood draw, maternal weight, age, year and month of blood draw, diabetes and smoking status. The female/male ratio increased with increasing maternal age in the first trimester (9–12%), but not in the second trimester (Fig. 2). Conversely, maternal weight had a stronger effect on the ratio in the second trimester (5–9%) as compared with the first (Fig. 2). We did not detect significant fluctuations in the ratio by smoking, diabetes or ovum donor even though the overall effects of these exposures on circulating hCG were large, ranging from 16% lower for first trimester insulin-dependent diabetes to 39% higher for second trimester ovum donor status.
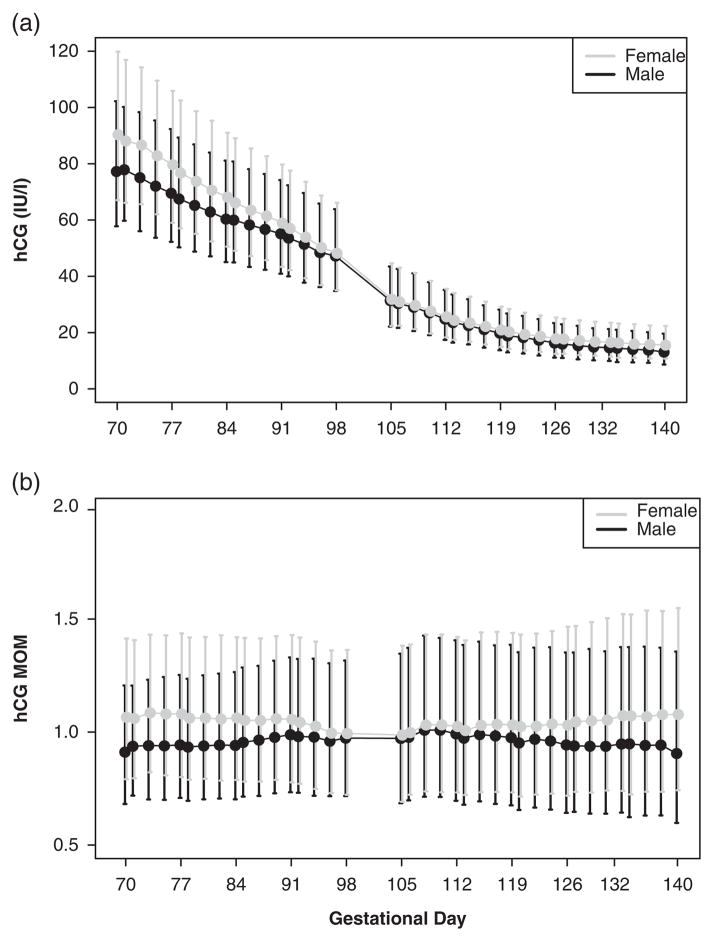
Fig. 1.
Placental-fetal sex differences in circulating hCG in first and second trimester pregnancies. The dots and lines are median and 25th/75th percentile values for each gestational day of prenatal screening. Female values are denoted in gray, and male values are denoted in black. hCG, human chorionic gonadotropin; MoM, multiple of the medians.
Fig. 2.
Predicted means (± standard errors) in female and male placental hCG multiples of the median, normalized for ga-MoM, across categories of maternal race, weight and age in the first and second trimesters. All of these plots represent significant fluctuations in the female-to-male ratio across categories (P <0.01). hCG, human chorionic gonadotropin; ga-MoM, gestational age multiple of the median.
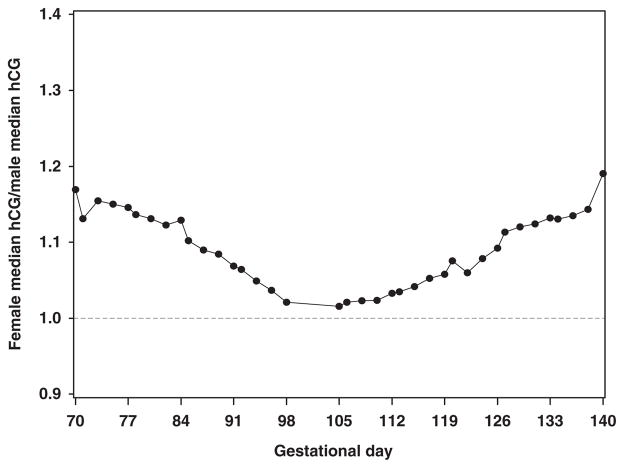
To assess the change in the female/male ratio over time in pregnancy, we fit a model to estimate the effect of gestational age on the female/male ratio in analyte values adjusting for other factors that may also be changing over time. The female/male ratio in hCG differed significantly with gestational day. The hCG ratio decreased from 17 to 2% in the first trimester, and increased from 2 to 19% in the second trimester (P <0.0001, Fig. 3).
Fig. 3.
Variability in female/male ratio in hCG by gestational age. Black dots represent the ratio calculated for each gestational day for the median female hCG to the median male hCG. hCG, human chorionic gonadotropin.
Discussion
We report fetal sex differences in hCG measured in early pregnancy, and the degree to which those differences fluctuate by maternal factors and by gestational age. The female/male ratio in hCG was the most variable across racial subgroups, with the smallest differences in Asian women and women younger than 20 years, and the maximum differences in African-American women. We did not detect significant fluctuations in female/male ratios by smoking, ovum donor status or insulin-dependent diabetes, which may indicate a lack of susceptibility of sex-specific hCG regulation to these types of adversity. It may also be due to the extremely low prevalence of these three exposures in the dataset (≤1%). These types of clinical data are widely available and offer a novel framework to study maternal–placental–fetal interactions that may be responsive to genetic, physiologic and environmental influences and relevant to obstetric complications and fetal outcomes.
Approximately 75–80% of pregnancies in California during this time period are represented in our dataset, giving us confidence that we are reporting unconfounded differences that have a basis in sexually dimorphic placental function. The female/male ratios were similar with or without adjustment for maternal factors; however, they did change considerably when we calculated group-level means v. individual-level predicted values, which were then summarized as means. Proper adjustment for individual-level gestational age is critical in making valid inferences on dynamic relationships such as these in early pregnancy. It is possible that previous studies did not detect sex differences in hCG in early pregnancy because they did not incorporate individual-level information on gestational age, but instead conducted tests of group means.4
Race was a major source of variability in hCG as well as in the fluctuations in the female/male ratios. This may relate to polymorphic genes that encode synthesis and/or metabolism of hCG that may also cluster by race or ethnic groups; although none have been identified to our knowledge. In the context of CA, we can speculate on explanations for higher mean levels of hCG in Asian women v. Hispanic women, after controlling for other factors, that might include: (a) higher proportions of farm workers among Hispanic women or members of families of farm workers with higher than average pesticide exposure; and (b) higher proportions of Asians living in coastal cities and higher fish consumption as compared with Hispanics. Hispanic women had 45% higher odds of neural tube defects (NTDs) than White women, which may also indicate different experiences in pregnancy with respect to environmental factors and their effects on placental–fetal hormones.29 Risk of NTDs, anencephaly in particular, is higher for female fetuses.30 Similar to US trends, African-American women in CA have the highest rates of preterm birth and infant mortality which are two outcomes that also may have origins in early maternal–placental–fetal interactions and are known to have a sex bias.31–33 Another difference that could contribute to differences in hCG regulation by race might be maternal and/or paternal nativity or time since initial migration into California.34–36 This cannot be directly studied in this database, but should be considered in future analyses.
Maternal weight is a known contributor to the endocrine milieu of pregnancy,16 and maternal age is tightly correlated with ovarian function.37 Both weight and age were correlated with fluctuations in the hCG sex difference. This could be a function of cumulative environmental exposures, chemical body burden, physiologic changes or a combination of all three. Information on body mass index and gestational weight gain was not available.
In order to alter circulating hCG levels consistently, the non-genetic or genetic factor would need to work at the level of methylation, transcription of the genes [chorionic gonadotropin α (CGA), chorionic gonadotropin β (CGB, CGB1, CGB2, CGB5, CGB7)] that encode the protein, the translation of the mRNA into protein, the packaging and secretion of the protein, glycosylation (addition of carbohydrate structures), the association or coupling of the α and β subunits of the protein, and/or the metabolism and excretion of the protein from maternal circulation.38 It is likely that the types of variability that we report here reflect changes in different combinations of these mechanisms, some of which are sexually dimorphic, under the control of polymorphic genes that vary by race, and also susceptible to factors in the maternal environment.
We show here that the fetal sex differences persist in the clinical MoM after normalization for other factors. This difference has been reported across time and populations, but has never incited clinical efforts to determine and correct for fetal sex in screening protocols largely because of the difficulty in obtaining an accurate fetal sex prediction at these early time points. On the other hand, lack of adjustment for fetal sex in epidemiologic analyses may result in bias or possibly missing a sex-specific association or dose–response relationship.
The gestational age trends in the female/male ratio may tell us something about the mechanism driving the difference. The downward trend in the hCG fetal sex ratio followed by the upward trend in the second trimester suggests that there may be a switch to a different placental secretion pattern or hormonal function in the second trimester. Given that placental growth, invasion and expansion are the dominant forces in the second trimester, and male placentas are generally larger at birth than female placentas, we would predict that male hCG would be higher in the second trimester. However, that is not the case in these data at 20 weeks, and may offer support for the presence of a negative feedback mechanism in the male, or a role for hCG in ‘non-canonical’ placental functions.2
The clinical currency in these data is the MoM. This is the value recorded in prenatal charts and used by the obstetrician to identify and properly manage a high-risk pregnancy. It is useful clinically because it ‘removes’ the subclinical sources of variability from the analyte value and yields a normalized value that can more easily be interpreted at the level of the individual. However, for the purposes of epidemiologic analyses in which we would like to study the effects of maternal race/ethnicity, weight or body mass index, smoking, and health, or other variables correlated with these factors, the clinical MoM is not useful. We propose here to use the ga-MoM, which removes the gestational age effects on the analyte yet, preserves the types of variability that we would otherwise study. In this dataset with extremely stable group mean values, the analyte and the ga-MoM essentially yielded the same results. However, in a smaller dataset the differences between the two would be greater. For the sake of capturing important gestational age variability, the analyte values would be preferable over the MoM. Precaution has to be taken in correctly modeling the gestational age dependence as it is rarely linear and mis-specification can bias estimation of associations.
In conclusion, we demonstrate that fetal sex differences in circulating hCG in early pregnancy were associated with maternal race, weight, age which may be reflective of environmental and physiologic differences in the fetal environment. These types of analyses at the population level potentially allow us to understand non-canonical placental functions, including more direct observations of fetal origins of health and disease. In the case of this analysis, changes in the size of the expected female/male ratio were characterized as potential indicators of normal v. abnormal placental regulation of sex-specific fetal development. We are making a recommendation to researchers in the field of fetal origins to consider using these data to understand placental responses to maternal environmental factors and placental contributions to sex-specific fetal outcomes.
Acknowledgments
The authors acknowledge the mentorship of Ilpo Huhtaniemi and Ulf-Håkan Stenman on hCG and Susan Fisher’s mentor-ship in placental biology. The authors thank Nerissa Wu for her contributions.
Financial Support
This work has been supported by the National Institute of Environmental Health Sciences (J.J.A., grant numbers 5K99ES017780-02, R00ES017780-06); and the Mt. Zion Health Fund of the Jewish Community Endowment Fund granted through the UCSF National Center of Excellence in Women’s Health (J.J.A.).
Footnotes
Conflicts of Interest.
None.
Ethnical Standards
The authors assert that all procedures contributing to this work comply with the ethnical standards of the Helsinki Declaration of 1975, as revised in 2008, and have been approved by institutional committees (Committee for the Protection of Human Subjects, State of California; University of Pittsburgh Institutional Review Board).
References
- 1.Bremme K, Lagerström M, Andersson O, Johansson S, Eneroth P. Influences of maternal smoking and fetal sex on maternal serum oestriol, prolactin, hCG, and hPI levels. Arch Gynecol Obstet. 1990;247:95–103. doi: 10.1007/BF02390666. [DOI] [PubMed] [Google Scholar]
- 2.Clements JA, Reyes FI, Winter JS, Faiman C. Studies on human sexual development. III. Fetal pituitary and serum, and amniotic fluid concentrations of LH, CG, and FSH. J Clin Endocrinol Metab. 1976;42:9–19. doi: 10.1210/jcem-42-1-9. [DOI] [PubMed] [Google Scholar]
- 3.Cowans NJ, Stamatopoulou A, Maiz N, Spencer K, Nicolaides KH. The impact of fetal gender on first trimester nuchal translucency and maternal serum free beta-hCG and PAPP-A MoM in normal and trisomy 21 pregnancies. Prenat Diagn. 2009;29:578–581. doi: 10.1002/pd.2246. [DOI] [PubMed] [Google Scholar]
- 4.Steier JA, Myking OL, Bergsjo PB. Correlation between fetal sex and human chorionic gonadotropin in peripheral maternal blood and amniotic fluid in second and third trimester normal pregnancies. Acta obstetricia et gynecologica Scandinavica. 1999;78:367–371. [PubMed] [Google Scholar]
- 5.Yaron Y, Lehavi O, Orr-Urtreger A, et al. Maternal serum HCG is higher in the presence of a female fetus as early as week 3 post-fertilization. Hum Reprod. 2002;17:485–489. doi: 10.1093/humrep/17.2.485. [DOI] [PubMed] [Google Scholar]
- 6.Kauppila A, Huhtaniemi I, Ylikorkala O. Raised serum human chorionic gonadotrophin concentrations in hyperemesis gravidarum. Br Med J. 1979;1:1670–1671. doi: 10.1136/bmj.1.6179.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huhtaniemi IT, Korenbrot CC, Jaffe RB. HCG binding and stimulation of testosterone biosynthesis in the human fetal testis. J Clin Endocrinol Metab. 1977;44:963–967. doi: 10.1210/jcem-44-5-963. [DOI] [PubMed] [Google Scholar]
- 8.Scott HM, Mason JI, Sharpe RM. Steroidogenesis in the fetal testis and its susceptibility to disruption by exogenous compounds. Endocr Rev. 2009;30:883–925. doi: 10.1210/er.2009-0016. [DOI] [PubMed] [Google Scholar]
- 9.Huhtaniemi IT, Korenbrot CC, Jaffe RB. Content of chorionic gonadotropin in human fetal tissues. J Clin Endocrinol Metab. 1978;46:994–997. doi: 10.1210/jcem-46-6-994. [DOI] [PubMed] [Google Scholar]
- 10.Berndt S, Blacher S, Munaut C, et al. Hyperglycosylated human chorionic gonadotropin stimulates angiogenesis through TGF-β receptor activation. FASEB J. 2013;27:1309–1321. doi: 10.1096/fj.12-213686. [DOI] [PubMed] [Google Scholar]
- 11.Hatzirodos N, Bayne RA, Irving-Rodgers HF, et al. Linkage of regulators of TGF-β activity in the fetal ovary to polycystic ovary syndrome. FASEB J. 2011;25:2256–2265. doi: 10.1096/fj.11-181099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ridano ME, Racca AC, Flores-Martín J, et al. Chlorpyrifos modifies the expression of genes involved in human placental function. Reprod Toxicol. 2012;33:331–338. doi: 10.1016/j.reprotox.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Honkisz E, Zieba-Przybylska D, Wojtowicz AK. The effect of triclosan on hormone secretion and viability of human choriocarcinoma JEG-3 cells. Reprod Toxicol. 2012;34:385–392. doi: 10.1016/j.reprotox.2012.05.094. [DOI] [PubMed] [Google Scholar]
- 14.Fowler PA, Bhattacharya S, Gromoll J, Monteiro A, O’Shaughnessy PJ. Maternal smoking and developmental changes in luteinizing hormone (LH) and the LH receptor in the fetal testis. J Clin Endocrinol Metab. 2009;94:4688–4695. doi: 10.1210/jc.2009-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Catalano RA, Saxton KB, Bruckner TA, et al. Hormonal evidence supports the theory of selection in utero. Am J Hum Biol. 2012;24:526–532. doi: 10.1002/ajhb.22265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis RM, Demmelmair H, Gaillard R, et al. The placental exposome: placental determinants of fetal adiposity and postnatal body composition. Ann Nutr Metab. 2013;63:208–215. doi: 10.1159/000355222. [DOI] [PubMed] [Google Scholar]
- 17.Jukic AMZ, Weinberg CR, Baird DD, Wilcox AJ. The association of maternal factors with delayed implantation and the initial rise of urinary human chorionic gonadotrophin. Hum Reprod. 2011;26:920–926. doi: 10.1093/humrep/der009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brock DJ, Sutcliffe RG. Alpha-fetoprotein in the antenatal diagnosis of anencephaly and spina bifida. Lancet. 1972;2:197–199. doi: 10.1016/s0140-6736(72)91634-0. [DOI] [PubMed] [Google Scholar]
- 19.Jelliffe-Pawlowski LL, Shaw GM, Currier RJ, et al. Association of early-preterm birth with abnormal levels of routinely collected first- and second-trimester biomarkers. YMOB. 2013;208:492.e491–492.e411. doi: 10.1016/j.ajog.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taché V, Baer RJ, Currier RJ, et al. Population-based biomarker screening and the development of severe preeclampsia in California. Am J Obstet Gynecol. 2014;211:377.e1–8. doi: 10.1016/j.ajog.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blumenfeld YJ, Baer RJ, Druzin ML, et al. Association between maternal characteristics, abnormal serum aneuploidy analytes, and placental abruption. Am J Obstet Gynecol. 2014;211:144.e1–9. doi: 10.1016/j.ajog.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 22.Chedane C, Puissant H, Weil D, Rouleau S, Coutant R. Association between altered placental human chorionic gonadotrophin (hCG) production and the occurrence of cryptorchidism: a retrospective study. BMC Pediatr. 2014;14:191. doi: 10.1186/1471-2431-14-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wald NJ, Cuckle H, Brock JH, et al. Maternal serum-alpha-fetoprotein measurement in antenatal screening for anencephaly and spina bifida in early pregnancy. Report of U.K. collaborative study on alpha-fetoprotein in relation to neural-tube defects. Lancet. 1977;1:1323–1332. [PubMed] [Google Scholar]
- 24.Wald NJ, Rodeck C, Hackshaw AK, et al. First and second trimester antenatal screening for Down’s syndrome: the results of the Serum, Urine and Ultrasound Screening Study (SURUSS) J Med Screen. 2003;10:56–104. doi: 10.1258/096914103321824133. [DOI] [PubMed] [Google Scholar]
- 25.Miron P, Côté YP, Lambert J. Effect of maternal smoking on prenatal screening for Down syndrome and trisomy 18 in the first trimester of pregnancy. Prenat Diagn. 2008;28:180–185. doi: 10.1002/pd.1930. [DOI] [PubMed] [Google Scholar]
- 26.Spencer K, Cicero S, Atzei A, Otigbah C, Nicolaides KH. The influence of maternal insulin-dependent diabetes on fetal nuchal translucency thickness and first-trimester maternal serum biochemical markers of aneuploidy. Prenat Diagn. 2005;25:927–929. doi: 10.1002/pd.1229. [DOI] [PubMed] [Google Scholar]
- 27.Spencer K, Heath V, El-Sheikhah A, Ong CY, Nicolaides KH. Ethnicity and the need for correction of biochemical and ultrasound markers of chromosomal anomalies in the first trimester: a study of Oriental, Asian and Afro-Caribbean populations. Prenat Diagn. 2005;25:365–369. doi: 10.1002/pd.1153. [DOI] [PubMed] [Google Scholar]
- 28.Lambert-Messerlian G, Dugoff L, Vidaver J, et al. First- and second-trimester Down syndrome screening markers in pregnancies achieved through assisted reproductive technologies (ART): a FASTER trial study. Prenat Diagn. 2006;26:672–678. doi: 10.1002/pd.1469. [DOI] [PubMed] [Google Scholar]
- 29.Feuchtbaum L, Carter J, Dowray S, Currier RJ, Lorey F. Birth prevalence of disorders detectable through newborn screening by race/ethnicity. Genet Med. 2012;14:937–945. doi: 10.1038/gim.2012.76. [DOI] [PubMed] [Google Scholar]
- 30.Stumpf DA, Cranford RE, Elias S, et al. The infant with anencephaly. The Medical Task Force on Anencephaly. N Engl J Med. 1990;322:669–674. doi: 10.1056/NEJM199003083221006. [DOI] [PubMed] [Google Scholar]
- 31.Sabol BA, de Sam Lazaro S, Salati J, et al. Racial and ethnic differences in pregnancy outcomes in women with chronic hypertension. Obstet Gynecol. 2014;123(Suppl 1):168S–169S. [Google Scholar]
- 32.Carlsen F, Grytten J, Eskild A. Changes in fetal and neonatal mortality during 40 years by offspring sex: a national registry-based study in Norway. BMC Pregnancy Childbirth. 2013;13:101. doi: 10.1186/1471-2393-13-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooperstock M, Campbell J. Excess males in preterm birth: interactions with gestational age, race, and multiple birth. Obst Gynecol. 1996;88:189–193. doi: 10.1016/0029-7844(96)00106-8. [DOI] [PubMed] [Google Scholar]
- 34.Becerra TA, von Ehrenstein OS, Heck JE, et al. Autism spectrum disorders and race, ethnicity, and nativity: a population-based study. Pediatrics. 2014;134:e63–e71. doi: 10.1542/peds.2013-3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lorusso L, Bacchini F. A reconsideration of the role of self-identified races in epidemiology and biomedical research. Stud Hist Philos Biol Biomed Sci. 2015;52:1–9. doi: 10.1016/j.shpsc.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 36.Ahmed AT, Quinn VP, Caan B, et al. Generational status and duration of residence predict diabetes prevalence among Latinos: the California Men’s Health Study. BMC Public Health. 2009;9:392. doi: 10.1186/1471-2458-9-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Donnenfeld AE, Icke KV, Pargas C, Dowman C. Biochemical screening for aneuploidy in ovum donor pregnancies. Am J Obstet Gynecol. 2002;187:1222–1225. doi: 10.1067/mob.2002.126986. [DOI] [PubMed] [Google Scholar]
- 38.Hussa RO. Biosynthesis of human chorionic gonadotropin. Endocr Rev. 1980;1:268–294. doi: 10.1210/edrv-1-3-268. [DOI] [PubMed] [Google Scholar]