Abstract
Background
Nutrients related to one-carbon metabolism were previously shown to be significantly associated with the risk of cancer. The aim of this meta-analysis was to evaluate potential relationships between one-carbon metabolic factors and renal cell cancer (RCC) risk.
Methods
PubMed, EMBASE, and Cochrane Library databases were searched through March 2015 for observational studies of quantitative RCC risk estimates in relation to one-carbon metabolic factors. The relative risks (RRs) with 95% confidence intervals (CIs) measured the relationship between one-carbon metabolic factors and RCC risk using a random-effects model.
Results
Of the 463 citations and abstracts identified by database search, seven cohorts from five observational studies reported data on 133,995 individuals, and included 2,441 RCC cases. Comparing the highest with the lowest category, the pooled RRs of RCC were 0.72 (95%CI: 0.52–1.00; P = 0.048) for vitamin B12. In addition, an increase in folic acid supplementation of 100 μg/day was associated with a 3% lower risk of RCC (RR, 0.97; 95%CI: 0.93–1.00; P = 0.048). Similarly, an increase of 5 nmol/L of vitamin B2 was associated with a reduced risk of RCC 0.94 (95%CI: 0.89–1.00; P = 0.045). Sensitivity analyses suggested that a higher serum vitamin B6 might contribute to a reduced risk of RCC (RR, 0.83; 95%CI: 0.77–0.89; P < 0.001).
Conclusions
Higher levels of serum vitamin B2, B6, B12, and folic acid supplementation lowered the risk of RCC among the study participants.
Introduction
Renal cell cancer (RCC) is diagnosed in more than 120,000 patients in the USA and Europe, annually, resulting in nearly 60,000 deaths [1]. A third of patients with RCC are diagnosed in stage IV, with a 5-year survival rate of 15% approximately [2, 3]. Therefore, more effective preventive strategies to reduce the risk of RCC are needed. Recent studies have shown that several lifestyle factors such as high physical activity, alcohol, and intake of fruits and vegetables are associated with a lower incidence of RCC [4–11]. B vitamins are the main coenzyme precursors involved in the transfer of one-carbon groups and are essential for DNA methylation and DNA repair mechanisms [12]. Therefore, B vitamins have been linked with the risk of cancer [13]. Several meta-analyses [14–18] have evaluated the relationship between one-carbon metabolism and multiple cancers, but the relationship between one-carbon metabolic factors and the risk of RCC is not established.
Previous meta-analysis [9] indicated that protein or fat intake including red meat, poultry, and seafood might not be associated with the risk of RCC. Further, the dietary intake of fruits and vegetables has been closely related to the risk of gastric [19], prostate [20], colorectal [21], ovarian [22], and breast cancer [23]. Finally, another important study [24] suggested that consumption of cruciferous vegetables may be associated with reduced RCC risk. Among the supplemental nutrient subtypes, one-carbon metabolic factors may inhibit carcinogenesis and reduce the risk of RCC. However, data correlating one-carbon metabolism and subsequent incidence of RCC is limited.
Although a series of studies have evaluated the association between one-carbon metabolic factors and RCC risk, the results are controversial or inconclusive. Results of the present meta-analysis elucidate the relationship between one-carbon metabolism and the risk of RCC.
Methods
Data Sources, Search Strategy, and Selection Criteria
PubMed, EMBASE, and the Cochrane library were searched for articles published up to March 2015, using the search terms "renal cell carcinoma" OR "renal cell cancer" and "one-carbon metabolism biomarkers" or "folate" or “folic acid” or "vitamin B6" or “pyridoxine” or “cobalamin” or "vitamin B12" or "cysteine" or "riboflavin" or “thiamine” or "homocysteine". The search was limited to articles that were published in English. We also manually searched reference lists from all the relevant original research and review articles to identify additional potentially eligible studies. The literature search was performed in duplicate by two independent reviewers.
Inclusion criteria were: (1) observational studies investigating the relationship between one-carbon metabolism and the risk of RCC; and (2) those specifying the number of participants in each category of one-carbon metabolic factors. For studies without adequate data, we contacted the authors or searched the articles that reported a similar database. Studies without the necessary data were excluded.
Data Collection and Quality Assessment
Data extraction and assessment were conducted independently by two authors. Publication information (i.e., first author’s name, and publication year), characteristics of the studies (i.e., country, study design, study quality, and adjusted factors), characteristics of participants (i.e., sample size, mean age, gender, educational background, body mass index [BMI], smoking, alcohol consumption, and history of hypertension), and the number of cases and participants in each category were extracted. Disagreement was resolved by consensus with a third reviewer.
Two reviewers independently evaluated the quality of the studies using the Newcastle—Ottawa Scale (NOS) (S1 Table) [25]. The NOS assessment is based on essential points of an observational study, i.e., selection (4 scores), comparability (2 scores), and outcome (3 scores). The three-point questionnaire produced a total score that ranged from 0 (the worst) to 9 (the best). In cases of a disagreement, a consensus was reached after a group discussion.
Statistical Analysis
Effect estimate (RR, OR, or HR) and its 95% confidence interval (CI) were used to examine the relationship between one-carbon metabolic factors and the risk of RCC. Further, the risk estimates with maximal adjustment for potential confounders were used. Risk ratios (RRs) combined with the random-effects model were used as the summary statistic [26, 27].
The RRs were significant when the 95% CI did not include 1.00. First, the random-effects model was used to calculate summary RRs and 95% CIs for the high versus low one-carbon metabolic factors [27]. Second, category-specific risk estimates were transformed into estimates of the risk ratio (RR), which were associated with an increase in the level of one-carbon metabolic factors using the generalized least-squares method for trend estimation [28, 29]. The summary RRs for an increase in the level of one-carbon metabolic factors were calculated using random-effects meta-analysis [27]. Statistical heterogeneity among studies was evaluated using Q and I-square statistics, and P values < 0.10 indicated significant heterogeneity [30, 31]. Sensitivity analysis was used to explore potential sources of heterogeneity and to evaluate the influence of the included individual studies in our meta-analysis [32]. In the plan stage, subgroup analyses were used to explore the relationship between one-carbon metabolic factors, and the incidence of RCC risk in specific sub-populations. However, subgroup analyses were not conducted under conditions involving small number of trials.
In the planning stage, potential publication bias was evaluated by Egger [33] and Begg [34] tests. However, few studies reported the relationship between several one-carbon metabolic factors and the risk of RCC. All P values were two-sided and alpha values of P < 0.05 were considered statistically significant for all included studies. Statistical analyses were performed with STATA software (v. 12.0; Stata Corporation, College Station, TX, USA).
Results
Literature Search
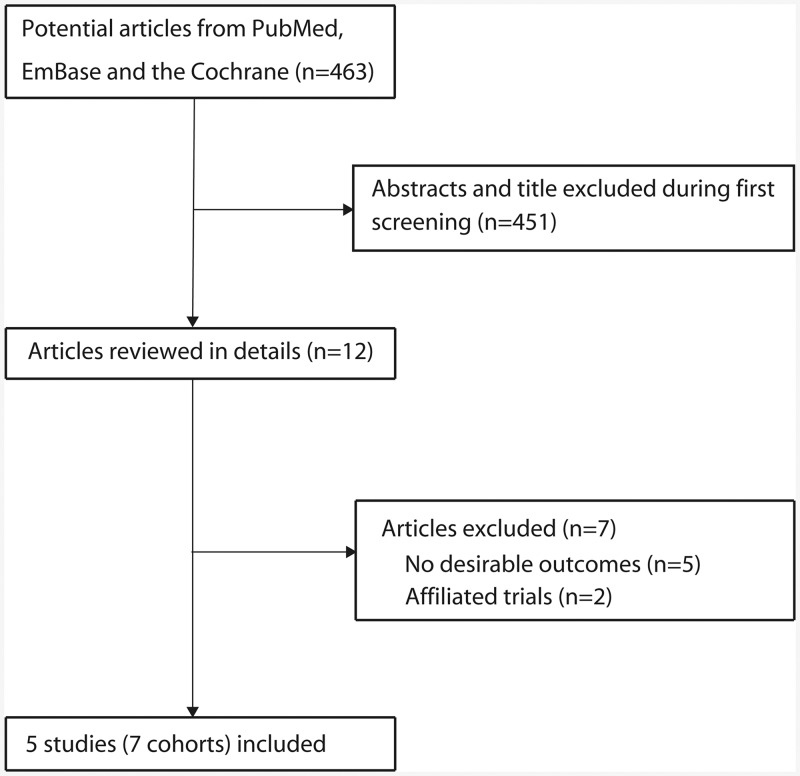
The primary search produced 463 records. After scanning titles and abstracts, 451 irrelevant articles were excluded. Twelve full-text articles were reviewed, and finally five studies [35–39] with seven cohorts were included in this meta-analysis (Fig 1). A manual search of the reference lists within these studies did not yield any new eligible studies. The general characteristics of the included studies and participants are presented in Table 1.
Fig 1. Flow diagram outlining the literature search and study selection process.
Table 1. Baseline characteristic of studies included in the systematic review and meta-analysis.
| Variable | IMRCC [35] | NLCS [36] | EPIC [37] | ATBC [38] | NHS [39] | HPFS [39] | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Non-cases | Cases | Non-cases | Cases | Non-cases | Cases | Non-cases | Cases | Non-cases | Cases | Non-cases | |
| Country | Italy | Netherlands | 10 countries in Europe | Finland | USA | USA | ||||||
| Study design | Case control | Case cohort | Case control | Nested case control | Cohort | Cohort | ||||||
| Assessment of exposure | FFQ | FFQ | Plasma samples | Plasma samples | FFQ | FFQ | ||||||
| Study quality | 7 | 8 | 9 | 8 | 8 | 8 | ||||||
| Sample size | 767 | 1534 | 314 | 4438 | 556 | 556 | 224 | 224 | 225 | 76983 | 211 | 47675 |
| Mean age (years) | 62.0 | 62.0 | 61.9 | 61.4 | 56.9 | 56.9 | 57.0 | 57.0 | 61.0 | 61.0 | 62.0 | 62.0 |
| Sex (percentage male) | 64.4 | 64.4 | 65.9 | 49.4 | 56.0 | 56.0 | 100 | 100 | 0 | 0 | 100 | 100 |
| Education (< 7 years) | 48.5 | 55.3 | - | - | 41.0 | 37.0 | 79.5 | 78.6 | - | - | - | - |
| Education (7–11 years) | 27.6 | 29.8 | - | - | 22.0 | 25.0 | - | - | - | - | - | - |
| Education (> 11 years) | 23.9 | 14.9 | - | - | 37.0 | 39.0 | - | - | - | - | - | - |
| BMI (< 25) | 36.8 | 36.7 | Mean: 25.5 | Mean: 25.0 | 32.0 | 40.0 | Mean: 26.7 | Mean: 25.9 | - | - | - | - |
| BMI (25–30) | 45.4 | 49.1 | 45.0 | 43.0 | - | - | - | - | ||||
| BMI (> 30) | 17.8 | 14.2 | 23.0 | 16.0 | - | - | - | - | ||||
| Smoking (never) | 41.1 | 41.7 | 24.8 | 35.8 | 41.0 | 44.0 | - | - | - | - | - | - |
| Smoking (current) | 30.8 | 30.4 | 36.3 | 28.3 | 30.0 | 23.0 | - | - | - | - | - | - |
| Smoking (ex-smokers) | 28.1 | 27.9 | 38.9 | 35.9 | 29.0 | 32.0 | - | - | - | - | - | - |
| Alcohol (never) | 17.1 | 15.1 | - | - | 7.0 | 4.0 | Mean: 7.4 | Mean: 11.2 | Mean: 6.0 | Mean: 6.0 | Mean: 11.0 | Mean: 11.0 |
| Alcohol (current) | 74.7 | 77.5 | - | - | 92.0 | 95.0 | ||||||
| Alcohol (ex-drinkers) | 8.2 | 7.4 | - | - | 2.0 | 1.0 | ||||||
| History of hypertension (yes) | 35.0 | 25.0 | - | - | 37.0 | 37.0 | 32.0 | 32.0 | ||||
| History of hypertension (no) | 50.0 | 59.0 | - | - | 63.0 | 63.0 | 68.0 | 68.0 | ||||
| Adjusted factors | Period of interview, education, BMI, smoking, alcohol intake and family history of kidney cancer | Age, sex, smoking, BMI and history of hypertension | Waist-to-hip ratio, hypertension, educational attainment, smoking status, plasma cotinine, alcohol intake at recruitment and alcohol intake. | Age, BMI and smoking; folate additionally adjusted for protein and fat; vitamin B6, riboflavin and homocysteine additionally adjusted for serum folate; vitamin B12 additionally adjusted for protein, leisure-time physical activity and serum folate. | Age, smoking status, BMI, history of hypertension, history of diabetes, physical activity, fruit and vegetable intake, alcohol intake, and parity | Age, smoking status, BMI, history of hypertension, history of diabetes, physical activity, fruit and vegetable intake, and alcohol intake | ||||||
Study Characteristics
Three of the included studies were case studies [35, 37], two were cohorts [39], one was a nested case control study [38], and the remaining study was a case cohort [36]. These studies were published between 2006 and 2014, which comprised 133,995 individuals, and contained 2,441 RCC cases. Four cohorts were conducted in Europe [35–38], two were performed in the U.S. [39], and the remaining one was carried out in Australia [37]. IMRCC [35] and EPIC cohorts [37] reported education, BMI, and alcohol intake status. Similarly, three cohorts reported a history of smoking [35–37] and hypertension status [37, 39]. The quality of a study was evaluated using NOS, and one cohort scoring 9 [37], four cohorts scoring 8 [36, 38, 39], and the remaining two cohorts scoring 7 [35, 37].
High versus Low One-Carbon Metabolic Factors
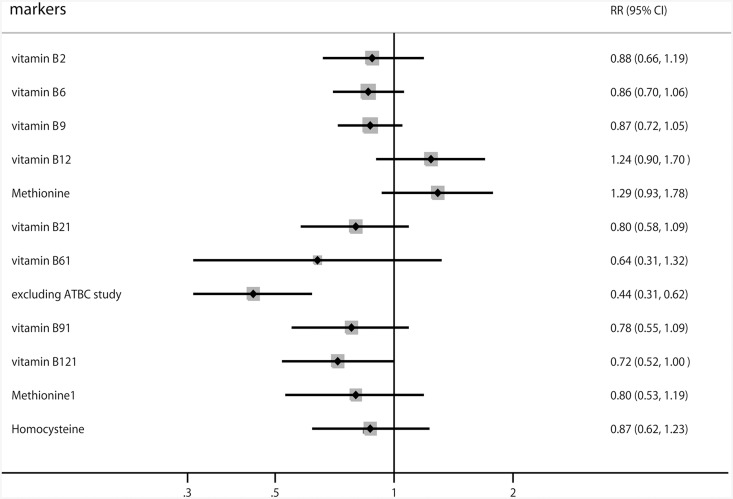
Fig 2 shows the RRs within the meta-analyses according to high versus low one-carbon metabolic factor levels. The summary RRs were 0.88 (95% CI: 0.66–1.19; P = 0.395) for vitamin B2 supplementation, 0.86 (95% CI: 0.70–1.06; P = 0.167) for vitamin B6 supplementation, 0.87 (95% CI: 0.72–1.05; P = 0.143) for folic acid supplementation, 1.24 (95% CI: 0.90–1.70; P = 0.194) for vitamin B12 supplementation, and 1.29 (95% CI: 0.93–1.78; P = 0.123) for methionine. Similarly, no significant associations were seen among plasma vitamin B2, plasma vitamin B6, plasma folate, plasma methionine, and plasma homocysteine levels. Further, compared with the lowest plasma category of vitamin B12, the pooled RR for RCC was 0.72 (95% CI: 0.52–1.00; P = 0.048). Finally, according to a sensitivity analysis, the highest category of plasma vitamin B6 was associated with a reduced risk of RCC (RR, 0.44; 95% CI: 0.31–0.62; P < 0.001) when excluding the ATBC study [38], which specifically included male participants and were aligned to a nested case control design.
Fig 2. Summary of the calculated relative risks associated with high versus low one-carbon metabolic factors and renal cell cancer.
Dose-Response Analysis
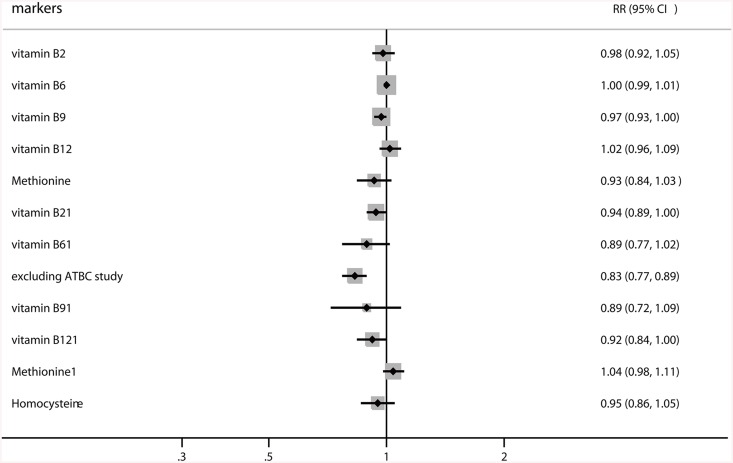
The findings of the dose-response meta-analysis suggested a significant association between increase (100 μg/day) in folic acid supplementation and the risk of RCC (RR, 0.97; 95% CI: 0.93–1.00; P = 0.048). Furthermore, the summary RR of RCC for an increase in plasma vitamin B2 levels per 5 nmol/L was 0.94 (95% CI: 0.89–1.00; P = 0.045). In addition, the sensitivity analysis indicated that an increase in vitamin B6 by 15 nmol/L was associated with a reduced risk of RCC (RR, 0.83; 95% CI: 0.77–0.89; P<0.001) [38]. Finally, no significant associations were found between one-carbon metabolic factor increments and the risk of RCC (Fig 3).
Fig 3. Summary of the dose-response meta-analysis of studies correlating increased one-carbon metabolism with subsequent risk of renal cell cancer.
Discussion
Previous observational studies [35–39] correlating one-carbon metabolic factors with the risk of RCC have been inconclusive. EPIC and MCSS studies [37] found a decreased risk of RCC with high plasma vitamin B6 or vitamin B12. Several other studies failed to find any significant association with RCC, although most of the observed RRs were below unity [35, 36, 38, 39]. Further, the cut-off value of each category for one-carbon metabolism differed among studies. Finally, the incidence of RCC was lower than the expected value in individual studies, and always required broad confidence intervals, i.e., values exhibited no statistically significant differences. We therefore performed a comprehensive, quantitative meta-analysis to evaluate any potential relationship between one-carbon metabolic factors and the incidence of RCC risk.
This meta-analysis including published observational studies explored the potential correlations between one-carbon metabolic factors and the incidence of RCC. We found a statistically significant inverse association between folic acid supplementation, plasma vitamin B2 levels and the risk of RCC. Further, analyses of high versus low one-carbon metabolic factors indicated that plasma vitamin B12 was associated with a reduced risk of RCC. Finally, according to sensitivity analysis, vitamin B6 might play an important role in the risk of RCC.
Most of our findings were consistent with a recently published case control study that was conducted in ten European countries [37]. This study included 556 cases and 556 control subjects, which suggested that participants with higher plasma concentrations of vitamin B6 were associated with a lower risk of RCC. However, on the contrary, the other plasma biomarkers did not display any significant association with the incidence of RCC. In addition, a replication study that was conducted in Australia [37] suggested that the high plasma vitamin B6 levels were associated with a reduced risk of RCC (OR, 0.47; 95% CI: 0.23–0.99). Further, we used the generalized least-squares method for trend estimation and found that an increase in plasma vitamin B6 levels per 15 nmol/L, might be a protective factor for RCC. Finally, an ATBC study [38] indicated that participants with the lowest serum folate levels (≤ 6.64 nmol/L) had a 68% increase in the risk of RCC (OR, 1.68; 95% CI: 1.06–2.65) and a 22% increase in the risk of RCC per 100 μg, which was comparable to those with higher serum folate levels. Variables including study design, gender and source populations might play an important role in these associations. The ATBC study [38] included participants within a homogeneous population for randomization; however, EPIC and MCCS [37] were population-based observational studies. Furthermore, the biochemical measurements of vitamin B6 were performed with different methodologies. For example, the ATBC study [38] used tyrosine decarboxylase assay, and the EPIC and MCCS studies used chromatography/tandem mass spectrometry. Finally, we also conducted a sensitivity analysis excluding the ATBC study, which concluded that higher serum vitamin B6 was associated with a lower RCC risk.
Our current study also indicated that an inverse association remained statistically significant for folic acid supplementation (RR, 0.97; 95% CI: 0.93–1.00; P = 0.048). This result was consistent with IMRCC [35], which suggested that an increase in folic acid supplementation by 100 μg per day was associated with a reduced risk of RCC (RR, 0.94; 95% CI: 0.88–1.00). The other nutrients related to one-carbon metabolic supplementation were not associated with RCC. Studies on folic acid supplementation found that a higher intake was related to a reduced risk of oral, pharyngeal [40], breast [15], bladder [16], esophageal and pancreatic cancer [41]. However, the relationship between folic acid supplementation and the risk of RCC was unknown. Due to limited evidence supporting this association, and multiple nutrients related to one-carbon metabolism as demonstrated in this analysis, we conclude that the inverse association with folic acid, might be due to chance.
Our current meta-analysis has several strengths. First, the large sample size allowed us to quantitatively assess the association between one-carbon metabolic factors and the risk of RCC. Thus, our findings were potentially more robust than those of any individual study. Second, the dose-response analysis included a wide range of one-carbon metabolic factors, which allowed an accurate assessment of the relationship between per unit increments of one-carbon metabolic factors and the risk of RCC.
The study limitations were as follows: (1) the adjusted models differed across the included studies, with variable factors playing an important role in the development of RCC; (2) we could not differentiate the effects of one-carbon metabolic factors from confounding factors including BMI, smoking, alcohol status, and history of hypertension due to limited evidence; (3) publication bias and restricted cubic splines cannot available due to few studies reported the relationship between one-carbon metabolic factors and the risk of RCC; (4) publication bias; and finally (5) pooled data, which restricted a more detailed and comprehensive analysis.
The findings of our study indicated that serum vitamin B2, vitamin B6, vitamin B12, and folic acid supplementation were inversely associated with the risk of RCC. Large prospective cohort studies are needed to verify these associations.
Supporting Information
(DOC)
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1. Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18: 581–592. [DOI] [PubMed] [Google Scholar]
- 2. DeVita V, Hellman S, Roseberg S (2001) Cancer: Principles & practice of oncology, 6th edn Philadelphia, Lippincott Williams & Wilkins. [Google Scholar]
- 3. Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. N Engl J Med. 1996;335: 865–875. [DOI] [PubMed] [Google Scholar]
- 4. Mahabir S, Leitzmann MF, Pietinen P, Albanes D, Virtamo J, Taylor PR. Physical activity and renal cell cancer risk in a cohort of male smokers. Int J Cancer. 2004;108: 600–605. [DOI] [PubMed] [Google Scholar]
- 5. Tavani A, Zucchetto A, Dal Maso L, Montella M, Ramazzotti V, Talamini R, et al. Lifetime physical activity and the risk of renal cell cancer. Int J Cancer. 2007;120: 1977–1980. [DOI] [PubMed] [Google Scholar]
- 6. Moore SC, Chow WH, Schatzkin A, Adams KF, Park Y, Ballard-Barbash R, et al. Physical activity during adulthood and adolescence in relation to renal cell cancer. Am J Epidemiol. 2008;168: 149–157. 10.1093/aje/kwn102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Song DY, Song S, Song Y, Lee JE. Alcohol intake and renal cell cancer risk: a meta-analysis. Br J Cancer. 2012;106: 1881–1890. 10.1038/bjc.2012.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bellocco R, Pasquali E, Rota M, Bagnardi V, Tramacere I, Scotti L, et al. Alcohol drinking and risk of renal cell carcinoma: results of a meta-analysis. Ann Oncol. 2012;23: 2235–2244. 10.1093/annonc/mds022 [DOI] [PubMed] [Google Scholar]
- 9. Lee JE, Spiegelman D, Hunter DJ, Albanes D, Bernstein L, van den Brandt PA, et al. Fat, protein, and meat consumption and renal cell cancer risk: a pooled analysis of 13 prospective studies. J Natl Cancer Inst. 2008;100: 1695–1706. 10.1093/jnci/djn386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee JE, Giovannucci E, Smith-Warner SA, Spiegelman D, Willett WC, Curhan GC. Intakes of fruits, vegetables, vitamins A, C, and E, and carotenoids and risk of renal cell cancer. Cancer Epidemiol Biomarkers Prev. 2006;15: 2445–2452. [DOI] [PubMed] [Google Scholar]
- 11. Weikert S, Boeing H, Pischon T, Olsen A, Tjonneland A, Overvad K, et al. Fruits and vegetables and renal cell carcinoma: findings from the European prospective investigation into cancer and nutrition (EPIC). Int J Cancer. 2006;118: 3133–3139. [DOI] [PubMed] [Google Scholar]
- 12. Lee JE, Mannisto S, Spiegelman D, Hunter DJ, Bernstein L, van den Brandt PA, et al. Intakes of fruit, vegetables, and carotenoids and renal cell cancer risk: a pooled analysis of 13 prospective studies. Cancer Epidemiol Biomarkers Prev. 2009;18: 1730–1739. 10.1158/1055-9965.EPI-09-0045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim YI. Folate and colorectal cancer: an evidence-based critical review. Mol Nutr Food Res. 2007;51: 267–292. [DOI] [PubMed] [Google Scholar]
- 14. Zhang YF, Zhou L, Zhang HW, Hou AJ, Gao HF, Zhou YH. Association between folate intake and the risk of lung cancer: a dose-response meta-analysis of prospective studies. PLoS One. 2014;9: e93465 10.1371/journal.pone.0093465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen P, Li C, Li X, Li J, Chu R, Wang H. Higher dietary folate intake reduces the breast cancer risk: a systematic review and meta-analysis. Br J Cancer. 2014;110: 2327–2338. 10.1038/bjc.2014.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. He H, Shui B. Folate intake and risk of bladder cancer: a meta-analysis of epidemiological studies. Int J Food Sci Nutr. 2014;65: 286–292. 10.3109/09637486.2013.866641 [DOI] [PubMed] [Google Scholar]
- 17. Wu W, Kang S, Zhang D. Association of vitamin B6, vitamin B12 and methionine with risk of breast cancer: a dose-response meta-analysis. Br J Cancer. 2013;109: 1926–1944. 10.1038/bjc.2013.438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Collin SM, Metcalfe C, Refsum H, Lewis SJ, Zuccolo L, Smith GD, et al. Circulating folate, vitamin B12, homocysteine, vitamin B12 transport proteins, and risk of prostate cancer: a case-control study, systematic review, and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2010;19: 1632–1642. 10.1158/1055-9965.EPI-10-0180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bertuccio P, Rosato V, Andreano A, Ferraroni M, Decarli A, Edefonti V, et al. Dietary patterns and gastric cancer risk: a systematic review and meta-analysis. Ann Oncol. 2013;24: 1450–1458. 10.1093/annonc/mdt108 [DOI] [PubMed] [Google Scholar]
- 20. Meng H, Hu W, Chen Z, Shen Y. Fruit and vegetable intake and prostate cancer risk: a meta-analysis. Asia Pac J Clin Oncol. 2014;10: 133–140. 10.1111/ajco.12067 [DOI] [PubMed] [Google Scholar]
- 21. Aune D, Lau R, Chan DS, Vieira R, Greenwood DC, Kampman E, et al. Nonlinear reduction in risk for colorectal cancer by fruit and vegetable intake based on meta-analysis of prospective studies. Gastroenterology. 2011;141: 106–118. 10.1053/j.gastro.2011.04.013 [DOI] [PubMed] [Google Scholar]
- 22. Hu J, Hu Y, Hu Y, Zheng S. Intake of cruciferous vegetables is associated with reduced risk of ovarian cancer: a meta-analysis. Asia Pac J Clin Nutr. 2015;24: 101–109. 10.6133/apjcn.2015.24.1.22 [DOI] [PubMed] [Google Scholar]
- 23. Jung S, Spiegelman D, Baglietto L, Bernstein L, Boggs DA, van den Brandt PA, et al. Fruit and vegetable intake and risk of breast cancer by hormone receptor status. J Natl Cancer Inst. 2013;105: 219–236. 10.1093/jnci/djs635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brock KE, Ke L, Gridley G, Chiu BC, Ershow AG, Lynch CF, et al. Fruit, vegetables, fibre and micronutrients and risk of US renal cell carcinoma. Br J Nutr. 2012;108: 1077–1085. 10.1017/S0007114511006489 [DOI] [PubMed] [Google Scholar]
- 25. Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses.[WWW document]. Available: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed 29 January 2013. 2001. [Google Scholar]
- 26. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7: 177–188. [DOI] [PubMed] [Google Scholar]
- 27. Ades AE, Lu G, Higgins JP. The interpretation of random-effects meta-analysis in decision models. Med Decis Making. 2005;25: 646–654. [DOI] [PubMed] [Google Scholar]
- 28. Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata Journal. 2006;6: 40. [Google Scholar]
- 29. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135: 1301–1309. [DOI] [PubMed] [Google Scholar]
- 30. Deeks JJ, Higgins J, Altman DG. Analysing Data and Undertaking Meta‐Analyses. Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series. 2008: 243–296. [Google Scholar]
- 31. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ: British Medical Journal. 2003;327: 557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tobias A. Assessing the influence of a single study in the meta-anyalysis estimate. Stata Technical Bulletin. 1999;8. [Google Scholar]
- 33. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994: 1088–1101. [PubMed] [Google Scholar]
- 35. Bosetti C, Scotti L, Maso LD, Talamini R, Montella M, Negri E, et al. Micronutrients and the risk of renal cell cancer: a case-control study from Italy. Int J Cancer. 2007;120: 892–896. [DOI] [PubMed] [Google Scholar]
- 36. van Dijk BA, Schouten LJ, Oosterwijk E, Hulsbergen-van de Kaa CA, Kiemeney LA, Goldbohm RA, et al. Carotenoid and vitamin intake, von Hippel-Lindau gene mutations and sporadic renal cell carcinoma. Cancer Causes Control. 2008;19: 125–134. [DOI] [PubMed] [Google Scholar]
- 37. Johansson M, Fanidi A, Muller DC, Bassett JK, Midttun O, Vollset SE, et al. Circulating biomarkers of one-carbon metabolism in relation to renal cell carcinoma incidence and survival. J Natl Cancer Inst. 2014;106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gibson TM, Weinstein SJ, Mayne ST, Pfeiffer RM, Selhub J, Taylor PR, et al. A prospective study of one-carbon metabolism biomarkers and risk of renal cell carcinoma. Cancer Causes Control. 2010;21: 1061–1069. 10.1007/s10552-010-9534-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cho E, Giovannucci EL, Joh HK. Nutrients related to one-carbon metabolism and risk of renal cell cancer. Cancer Causes Control. 2013;24: 373–382. 10.1007/s10552-012-0123-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Galeone C, Edefonti V, Parpinel M, Leoncini E, Matsuo K, Talamini R, et al. Folate intake and the risk of oral cavity and pharyngeal cancer: a pooled analysis within the International Head and Neck Cancer Epidemiology Consortium. Int J Cancer. 2015;136: 904–914. 10.1002/ijc.29044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Larsson SC, Giovannucci E, Wolk A. Folate intake, MTHFR polymorphisms, and risk of esophageal, gastric, and pancreatic cancer: a meta-analysis. Gastroenterology. 2006;131: 1271–1283. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.