Abstract
Establishment of the dorsal-ventral body axis during fly oogenesis depends on pushing forces provided by polymerizing microtubules.
In multicellular animals, the localization of specific molecules to some but not other cells during development defines three body axes: anterior-posterior (AP; head to tail), dorsal-ventral (DV; back to belly), and left-right. On page 999 of this issue, Zhao et al. (1) describe an elegant application of live-cell imaging to investigate molecular polarity and body axis establishment in the fruit fly Drosophila melanogaster. Their insights debunk a long-standing linkage between the mechanisms that establish the AP and DV body axes, and illuminate a new role for microtubule polymerization pushing forces.
The fruit fly AP and DV body axes are established during oogenesis, through polarization of an oocyte (2, 3). The effector molecules and mechanisms that specify these axes are well understood, but how the molecules are initially localized in the oocyte has not been clear. Oogenesis occurs within ovarioles, with ~18 of these egg production tubes forming each of the two ovaries (2, 3). Ovarian and follicular stem cells at the anterior end of each ovariole produce oocyte and somatic follicle cell precursors. An oocyte precursor undergoes four cellular divisions, generating an egg chamber with 16 cells connected by ring canals: one cell becomes the oocyte while the others become nurse cells. Nurse cell contents are transported through the ring canals into the oocyte, which enlarges during maturation (see the figure). Proliferating follicle cells produced by the follicular stem cells surround each egg chamber.
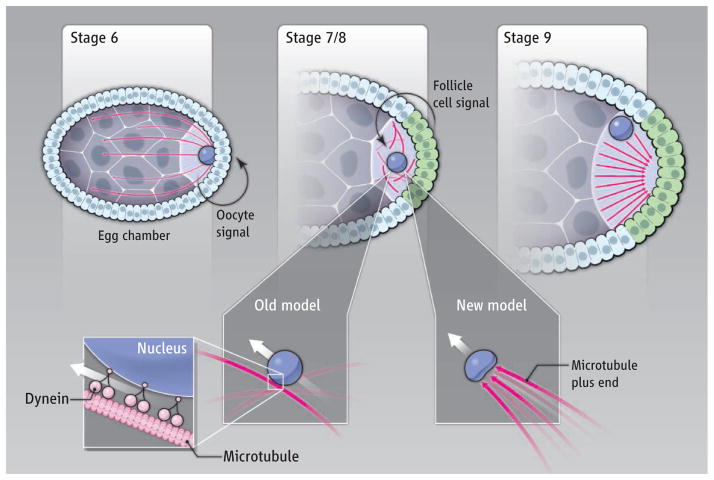
Figure 1. Pushing forces.
(Top) At stage 6 of Drosophila oogenesis, a signal from the oocyte specifies the follicle cells to adopt a posterior fate (green). A signal from the posterior follicle cells to the oocyte (stages 7 and 8) induces reorganization of the oocyte microtubule cytoskeleton such that microtubule polarity is reversed by stage 9. (Bottom) One model shows oocyte nuclear migration mediated by the microtubule motor protein dynein. In the model of Zhao et al., forces are provided by microtubule polymerization posterior to the oocyte nucleus.
The origins of the AP and DV body axes during Drosophila oogenesis require a relay of signals. One early signal from the oocyte instructs adjacent follicle cells to adopt a posterior fate. Subsequently, these posterior follicle cells signal back to the oocyte to reorganize the microtubule cytoskeleton (2, 3). Microtubules are polarized polymers in which one end (minus end) is stable and does not grow, while the other (plus end) is dynamic and grows or shortens through the addition or subtraction of tubulin subunits. Before generation of the posterior follicle cell signal, oocyte microtubules are organized with their minus ends abutting the posterior surface (cortex) of the oocyte, and their growing plus ends extend through ring canals into the nurse cells. This early network of polarized microtubules facilitates the transport of macromolecules from the nurse cells into the oocytes, with force and cargo delivery provided by the microtubule motor protein dynein. In response to the posterior follicle cell signal, microtubule minus ends disappear from the posterior cortex and eventually reappear at the anterior and lateral oocyte cortex, while new plus ends become enriched at the posterior oocyte cortex. Plus end– and minus end–directed motor proteins then transport two key messenger RNAs (mRNAs), one to each pole of the oocyte. After fertilization, the proteins encoded by these two mRNAs establish anterior and posterior cell fates and hence the AP axis (4).
Reorganization of microtubules triggers another key event in axis formation: migration of the oocyte nucleus to the peripheral anterior cortex. Subsequently, the nucleus generates a signal that specifies dorsal follicle cell fate (5, 6). Because inactivation of dynein results in mispositioning of the oocyte nucleus, the dogma has been that dynein motors, through their association with the nuclear envelope, pull the oocyte nucleus to an anterodorsal site as they move along polarized microtubules (7, 8). In this view, establishing the AP and DV axes depends on the same polarized microtubules that are oriented by the posterior follicle cell signal. However, mislocalization of the oocyte nucleus in dynein mutants was observed in late-stage oocytes, and nuclear position at the time of migration had not previously been reported.
Zhao et al. conducted live-cell examination of oocyte nuclear migration and microtubule dynamics using spinning-disk confocal microscopy and transgenic fly strains that express fluorescent protein fusions. They observed that in an oocyte, a microtubule organizing center (MTOC), which nucleates microtubule polymerization, is positioned posterior to the oocyte nucleus before its migration. Microtubule plus ends grow out from the MTOC and push on the nucleus. These pushing forces produce substantial “dents” in the oocyte nucleus that cannot be explained by microtubule minus end–directed dynein pulling forces acting from the oocyte nuclear envelope. Indeed, the dent positions correlate precisely with the MTOC’s position and the direction of nuclear movement, and laser ablation of the MTOC eliminates both the dents and movement.
Presumably, MTOC-nucleated microtubules that reach the posterior cortex contribute to the pushing forces and maintain MTOC proximity to the moving nucleus. The precise path taken by the nucleus in an oocyte is somewhat random, but the geometry of the oocyte ensures that these pushing forces ultimately move the nucleus to a peripheral site at the anterior oocyte cortex. Zhao et al. also found that nuclear migration is normal in mutant oocytes lacking dynein function. Mislocalization of the oocyte nucleus in these mutants later in oogenesis indicates that dynein anchors the nucleus after it arrives, but does not do the moving.
These results of Zhao et al. show that establishment of the DV and AP axes is not linked through a common dependence on the same polarized microtubules. Indeed, as first suggested by an earlier study (9), the MTOC that organizes the pushing forces may mediate the reversal of microtubule polarity that occurs in response to the posterior follicle cell signal and is required for proper localization of the anterior and posterior determinant mRNAs. Thus, DV may even precede AP axis formation. These results also provide an intriguing example of how microtubule polymerization can generate functionally important pushing forces within a cell.
The identity of the follicle cell signal that induces oocyte nuclear movement and microtubule reorganization remains unknown. Also not clear is the dynamic organization of microtubules within the developing oocyte. The further application of live-cell imaging—in particular, the tracking of microtubule plus end growth—may provide clarity. Seeing is believing, especially when done over time in a living tissue.
References
- 1.Zhao T, et al. Science. 2012;336:999. doi: 10.1126/science.1219147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roth S, Lynch JA. Cold Spring Harb Perspect Biol. 2009;1:a001891. doi: 10.1101/cshperspect.a001891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinhauer J, Kalderon D. Dev Dyn. 2006;235:1455. doi: 10.1002/dvdy.20770. [DOI] [PubMed] [Google Scholar]
- 4.St Johnston D, Nüsslein-Volhard C. Cell. 1992;68:201. doi: 10.1016/0092-8674(92)90466-p. [DOI] [PubMed] [Google Scholar]
- 5.González-Reyes A, et al. Nature. 1995;375:654. doi: 10.1038/375654a0. [DOI] [PubMed] [Google Scholar]
- 6.Roth S, et al. Cell. 1995;81:967. doi: 10.1016/0092-8674(95)90016-0. [DOI] [PubMed] [Google Scholar]
- 7.Duncan JE, Warrior R. Curr Biol. 2002;12:1982. doi: 10.1016/s0960-9822(02)01303-9. [DOI] [PubMed] [Google Scholar]
- 8.Januschke J, et al. Curr Biol. 2002;12:1971. doi: 10.1016/s0960-9822(02)01302-7. [DOI] [PubMed] [Google Scholar]
- 9.Januschke J, et al. Development. 2006;133:129. doi: 10.1126/science.1223141. [DOI] [PubMed] [Google Scholar]