Viscum album extract (European mistletoe), containing immune-active compounds with dose-dependent cytotoxic activity, is being used as an adjuvant cancer treatment in Europe. Few studies have been done with high-dose, fever-inducing Viscum album treatment. The authors retrospectively analyzed the case notes of patients with resectable bladder cancer who underwent initiation of high-dose Viscum album treatment at their clinic between January 2006 to December 2012. High-dose Viscum album showed a possible beneficial effect in 5 of 8 patients. No tumor progression was observed. Treatment was generally well tolerated and no patient stopped treatment because of side effects.
Abstract
Introduction:
Viscum album extract (European mistletoe), containing immuno-active compounds with dose-dependent cytotoxic activity, is being used as an adjuvant cancer treatment in Europe. Few studies have yet been done with high-dose, fever-inducing Viscum album treatment.
Objective:
To explore whether subcutaneous injections of high-dose Viscum album have a preventive effect on risk of recurrence of bladder cancer.
Methods:
We retrospectively analyzed the case records of patients with resectable bladder cancer who underwent initiation of high-dose Viscum album treatment at our clinic between January 2006 and December 2012.
Main Outcome Measures:
We calculated tumor recurrence and progression risk and explored case records to assess whether treatment had a likely, possible, or unlikely beneficial effect.
Results:
Eight patients were identified, 7 of whom had nonmuscle-invasive bladder cancer and 1 with muscle-invasive cancer. Four patients had frequently recurring tumors before treatment. Among the 8 patients, 28 episodes of recurrence were observed. Median tumor-free follow-up duration was 48.5 months. High-dose Viscum album showed a possible beneficial effect in 5 of 8 patients, could not be assessed in 2 patients, and had an uncertain effect in 1 patient. No tumor progression was observed. Treatment was generally well tolerated and no patient stopped treatment because of side effects.
Conclusion:
High-dose Viscum album treatment may have interrupted frequently recurring tumors in individual patients with recurrent bladder cancer. Prospective studies are needed to assess whether this treatment offers an additional, bladder-sparing preventive option for patients with intermediate- to high-risk nonmuscleinvasive bladder cancer.
INTRODUCTION
There are an estimated 386,000 new cases of bladder cancer reported globally each year, with 150,000 deaths.1 Approximately 70% of patients with bladder cancer present with nonmuscleinvasive cancer, with recurrence in 50% to 70% of cases and progression to muscle-invasive cancer in 10% to 20% of cases.2 Active and passive tobacco exposure is the main risk factor for bladder cancer, followed by occupational exposure to benzene derivatives and arylamines.3
Radical cystectomy with neoadjuvant chemotherapy is the standard therapy for muscle-invasive bladder cancer.3 Treatment of nonmuscle-invasive bladder cancer, which includes pathology Stages Ta, T1, and Tis, is transurethral resection. Intravesical bacillus Calmette-Guérin immunotherapy is used to reduce recurrence and progression risk in these patients.4 Intravesical treatment with mitomycin C has also been shown to reduce tumor recurrences and is used in the immediate postoperative period after resection of nonmuscleinvasive cancer.5 Cystectomy should be considered for patients at high risk of recurrence and is indicated when intravesical bacillus Calmette-Guérin immunotherapy fails.5
Whole plant extract of Viscum album (European mistletoe) contains a variety of immunoactive compounds with dose-dependent cytotoxic activity and is used as adjuvant cancer therapy in Europe.6,7 The immunoactive compounds include mistletoe lectins, viscotoxins, and other low-molecular-weight proteins, including VisalbCBA (Viscum album chitin-binding agglutinin), oligosaccharides and polysaccharides, flavonoids, and triterpene acids. The whole plant extract and several of its compounds on their own are cytotoxic, and mistletoe lectins in particular have strong apoptosis-inducing effects. The antitumor activity of mistletoe lectins, including a prophylactic effect, has also been linked to their immunostimulatory effect, including in vitro and in vivo activation of monocytes/macrophages, granulocytes, natural killer cells, T cells, dendritic cells, and the induction of a variety of cytokines.6,8,9–11 Furthermore, Viscum album extracts appear to interfere with tumoral angiogenesis.6 A recent trial showed survival benefit in patients with advanced pancreatic cancer who were receiving Viscum album extracts,12,13 and durable tumor regression has been documented in case reports.14
Most studies to date have tested the effects of low doses of Viscum album extract.6,7 However, more recently, clinicians have explored the use of high doses of the extract in light of its strongly dose-dependent cytotoxic activity6,7 and with the aim of increasing the immunostimulatory and fever-inducing effect of this treatment at initial doses.8 Careful patient monitoring with high-dose treatment is important. Manufacturers recommend starting with the lowest strength, titrating upward until a mild fever and/or local inflammatory reaction occurs. Anaphylactic reactions to Viscum album extracts have been reported but are rare.15 High-dose fever-inducing Viscum album extract treatment had a tumor-reducing effect in patients with advanced hepatocellular carcinoma.16 Fever or hyperthermia has direct cytotoxic effects in human beings, activates antitumor immune mechanisms, and results in improved drug delivery to tumor sites through vasodilatation.17 Tumor remissions in the context of febrile infections have been well documented,18 which has led to the development of hyperthermia treatment for cancer. Combining intravesical hyperthermia treatment with intravesical mitomycin C treatment has been shown to reduce bladder cancer recurrence by 59%.19 In a recent pilot study, hyperthermia treatment alone, when used before transurethral resection in patients with intermediate- to high-risk non-muscle-invasive bladder cancer, resulted in 53% of patients showing complete tumor remission.20 New targets for immunotherapy, beyond classic intravesical bacillus Calmette-Guérin immunotherapy, are also showing promising results.21
However, to our knowledge, no studies have yet been done to explore the effect of high-dose, fever-inducing Viscum album extract treatment on bladder cancer recurrence. Therefore we did a retrospective analysis of the case notes of a series of patients with bladder cancer undergoing treatment with subcutaneous injections of high-dose Viscum album (Salicis, grown on willow trees) extract at one hospital outpatient clinic, to explore the effect of high-dose treatment on tumor recurrence.
METHODS
We reviewed the case notes of all patients with bladder cancer being treated at the outpatient clinic of the Alexander von Humboldt Klinik, Bad Steben, Germany. This hospital is a specialty center for high-dose Viscum album extract treatment.22 Inclusion criteria were patients with confirmed, resectable (nonmuscle-invasive or muscle-invasive), urothelial bladder cancer who had undergone treatment with at least three injections of high-dose Viscum album (Salicis) extract between January 2006 and December 2012. We excluded patients who had had a cystectomy or those with advanced, inoperable bladder cancer. We reviewed patient data until December 31, 2013. Patients were contacted to verify their personal details, and we acquired written informed consent. We conducted brief interviews, which included exploration and documentation of patients’ experiences during treatment with high-dose Viscum album extract treatment. Formal ethics approval was not required to carry out this case series. All patients were sent copies of our final analysis.
We calculated recurrence and progression risk in these patients using the European Organisation for Research and Treatment of Cancer (EORTC) risk tables.23 In cases where patients had not responded to intravesical bacillus Calmette-Guérin immunotherapy, defined as recurrence of a high-grade tumor at 3 months after treatment, or recurrence after intravesical bacillus Calmette-Guérin immunotherapy, we used the progression risk of more than 25% after 5 years as reported by Davis et al.24
We assessed the pattern of bladder cancer recurrence in included cases and categorized patients into 1 of 4 groups: likely beneficial effect, possible beneficial effect, unlikely beneficial effect, and not possible to assess. We considered high-dose Viscum album extract to have a likely beneficial effect when recurrence and progression risk was close to 100% and there was no more than 1 tumor recurrence after treatment. We considered there to be a possible beneficial effect when patients had no further recurrences 1 month after the start of treatment, tumor-free follow-up more than 30 months after treatment, and recurrence risk at time of treatment initiation between 62% and 78% (based on an EORTC score). We considered treatment to have an unlikely beneficial effect when patients had more than 1 recurrence after treatment. Patients with other circumstances explaining tumor nonrecurrence—for example, patients already tumor-free after another treatment or when tumor-free follow-up was less than 30 months—were categorized as not possible to assess.
For all included patients in this analysis, the treatment used was Iscucin Salicis (Wala Heilmittel GmbH, Bad Boll, Germany), an aqueous extract of Viscum album (European mistletoe) grown on willow trees (Salicis). Preparation involves extraction from freeze-dried whole plant with isotonic solution over 14 days without fermentation or heating, in accordance with methods reported in the German Homeopathic Pharmacopoeia (Rule 38).25 One ampoule (1 mL) of a 1:20 concentration (5%, Strength H) contains 50 mg of the “mother extract” (including approximately 5.9 μg/mL of lectin, a key active ingredient)26; concentration 1:400 (0.25%, Strength G) contains 2.5 mg; concentration 1:8000 (0.0125%, Strength F) contains 0.125 mg; and concentration 1:160,000 (0.000625%, Strength E) contains 0.00625 mg. Strengths F, G, and H fulfilled our criteria for high dose. Iscucin Salicis is licensed for the German market by the German Federal Institute for Drugs and Medical Devices.
For included patients in this series, the schedules of Viscum album extract treatment were individualized but followed general principles. All injections were given as 1-mL aqueous solutions subcutaneously in the lower aspect of the abdomen or upper part of the thigh. An initial dose of Iscucin Salicis at the highest strength, H (except Strength F in Case 2), was given in the outpatient clinic to observe reaction. Patients who responded with high fever and inflammatory reactions at the injection site received further injections, usually once weekly for 3 to 8 weeks. If no or little reaction occurred, injections were continued daily over 3 to 4 days to achieve the required inflammatory reaction. In patients with little or no reaction even after daily administration, Strength H was followed by weekly or twice-weekly injections of Iscucin Salicis Potency series II (a set containing Strengths D, E, F, and G) given in the order of 2×G, 2×F, 3×E, and 3×D and repeated over several months. One patient (Case 8) also received other types of Viscum album extract preparations.
Because the Alexander von Humboldt Klinik does not provide specialized urology services, all patients were followed up simultaneously by a private-practicing urologist or at a urology center.
CASE SUMMARIES
We identified eight patients who met our inclusion criteria: seven with nonmuscle invasive bladder cancer (pTa and pT1) and one with muscle-invasive bladder cancer (pT2a) who had refused cystectomy. Three cases are reported here in detail.
Case 1
A 59-year-old woman presented in February 2006 with 2 months of right-sided, colicky abdominal pain. Her medical history highlighted the removal of a uterine myoma and right-knee arthrosis with prosthetic replacement. She was a smoker (20 cigarettes per day since early adulthood). A urothelial carcinoma of the right renal pelvis, Stage pT3G1, was diagnosed by computed tomographic (CT) scan and renal biopsy. Nephroureterectomy was performed in February 2006. During cystoscopy in April 2006, 4 superficial bladder tumors were removed. (Upper urinary tract urothelial cancer has a 20% to 50% risk of recurrence, as does bladder cancer.2) She received 6 rounds of intravesical bacillus Calmette-Guérin immunotherapy (BCG Medac, Hamburg, Germany) at weekly intervals beginning in May 2006. Two new tumors—1 was high-grade (World Health Organization Grade 3)—were removed in November 2006. Cystectomy was not recommended to her (although 2013 European guidelines recommend cystectomy for failure to respond to bacillus Calmette-Guérin immunotherapy5). In November 2006, the patient was treated with high-dose Viscum album extract treatment, which was provided as follows:
Month 1: Iscucin Salicis Strength H was given in twice-weekly injections, with the first injection at the clinic, then self-administered at home. The patient’s temperature reached up to 40° C, and she experienced redness and 2 to 3 cm of swelling at the injection site within hours after each injection.
Months 2 to 4: Iscucin Salicis Potency Series II was given in twice-weekly injections. No fever or local reaction occurred.
Cotreatment: Aurum muriaticum D12, a homeopathic remedy, was given orally to address depressed mood. This treatment started simultaneously with Viscum album extract treatment and was given during Months 1 to 4.
There was no further recurrence of bladder cancer tumors after the initiation of Viscum album extract treatment at follow-up with annual cystoscopy/ureteroendoscopy and CT scan in 2007, and with magnetic resonance imaging in 2009 and 2012. This patient took early retirement in 2008 unrelated to her medical condition, feels well today, and remains socially active. She has reduced smoking to 7 or 8 cigarettes a day.
The patient told us: “The mistletoe treatment was intense, like I imagine chemotherapy [would be] but without nausea and vomiting. Shortly after starting mistletoe treatment I began feeling better and felt my strength return. Without the regular encouragement by Dr W, I would not have been able to tolerate the fever reactions. I think mistletoe stopped my tumors from returning.”
Case 2
A 62-year-old woman presented with hematuria and received a diagnosis of superficial bladder cancer in 1991 (pathology records were missing). Her medical history was remarkable for longstanding essential hypertension and for hysterectomy for treatment of multiple uterine myomas at age 40 years. She smoked 7 to 10 cigarettes per day.
In 1997 and 2001, she had bladder cancer recurrences, Stage pTaG2 (data obtained from medical records; pathology reports were missing). After 2 further recurrences in May and June 2005, Stage pTaG1 and pTis, respectively, she received intravesical instillation of mitomycin C and a single course of bacillus Calmette-Guérin immunotherapy consisting of 6 intravesical treatments. Intravesical bacillus Calmette-Guérin immunotherapy was not continued because it induced cystitis. In June 2008, she had a multifocal pTaG2 and pTisG3 recurrence, causing right ureteral ostium stenosis with hydronephrosis, which was alleviated by placement of a ureteral stent. In July 2008, clear cell adenocarcinoma of the left kidney, a cancer of different cellular origin than urothelial cancer, was diagnosed (pT1aG2L0M0), and a partial nephrectomy was performed. In August 2008, a multifocal bladder tumor (Stage pTisG3) was found. Cystectomy was recommended, but the patient declined treatment.
She looked for additional treatment options and began Viscum album extract treatment in November 2008. Strength F rather than Strength H was selected because she appeared too fragile to tolerate a high fever. The treatment schedule was as follows:
Months 1 to 3: Iscucin Salicis Strength F was given in once-weekly injections. No fever or local reaction occurred.
Months 4 to 59 (end of study): Iscucin Salicis Potency Series II was administered in once-weekly injections. No fever or local reaction developed at any time. Treatment was continued long term to compensate for the lack of initial inflammatory reaction.
Cotreatment: Argentum nitricum compositum and Arsenicum album as supportive homeopathic remedies were given at different times.
No further recurrence was observed at annual cystoscopy assessments. Her quality of life, however, was affected by frequent bladder infections with dysuria, probably because of the ureteral stent. Antibiotic treatments provided limited, temporary relief. Dysuria stopped with placement of a new stent in November 2013 (1 month before study completion). Because of the last transurethral resection she has incontinence, as she is unaware when her bladder is full.
The patient told us: “Since the mistletoe treatment I no longer live in fear of the cancer returning. However, I have been in constant discomfort and pain because of my urinary [tract] infections.”
Case 8
After hematuria developed in a 64-year-old woman, a high-grade, muscle-invasive urothelial bladder cancer (Stage pT2aG3) was diagnosed in May 2007. The tumor was resected transurethrally. No local tissue infiltration and no metastases were found after a CT scan and bone scintigraphy. The patient had worked for 15 years in a polyvinyl chloride factory, a known risk factor for liver cancer, but not bladder cancer. She had never smoked. Her medical history was unremarkable. She was offered a cystectomy but felt overwhelmed by the cancer diagnosis and refused any invasive procedures.
She contacted different physicians for alternative treatment options and began Viscum album extract treatment in May 2007. She received no mitomycin C instillations, no chemotherapy, and no radiotherapy. Viscum album extract treatment was provided as follows:
Months 1 to 4: Iscucin Salicis Strength H was given daily on Days 1 and 2, then weekly. The reaction was a temperature of 40° C and local redness and itching on Day 2.
Month 5 to 10: Iscucin Salicis was given weekly in varying strengths: Strength F in Month 5, Strength E in Months 6 to 8, and Strength D in Months 9 to 10. No fever or local reaction developed at any time.
Months 12 to 13: After a 7-week break in treatment, Iscucin Salicis Potency Series II was given weekly for 7 weeks. No fever or local reaction occurred.
Months 16 to 36: After a treatment break from Months 13 to 15, the patient received weekly injections of abnobaVISCUM Betulae (Abnoba Heilmittel GmbH, Pforzheim, Germany; Viscum album from a birch tree). There was a reaction of local redness, itching, nausea, and headache only after the first 2 injections. After the following injections, she reported an increase in energy.
Months 43 to 45: After a 6-month treatment break, Iscucin Pini (Viscum album from a pine tree) Potency Series II was given weekly.
Cotreatment: Argentum D30/Echinacea D6 aa (Weleda, Schwäbisch Gmünd, Germany), a homeopathic remedy designed to improve temperature distribution and control, was injected with each Iscucin injection during Months 1 to 8. Other supportive homeopathic medications were given orally at differing times (Thuja e summatibus D12, Argentum nitricum compositum, Staphisagria LM, Equisetum arvense Silicea cultum D3, Senecio compositum, Tendo/Allium cepa compositum).
In September 2008, 16 months after the initial diagnosis, she had recurrence (Stages pTaG2 and pTisG3 tumors), for which she underwent transurethral resection. Treatment with Viscum album extract was continued as mentioned earlier, but she also received intravesical bacillus Calmette-Guérin immunotherapy in November 2008 (Month 12), which caused gross hematuria. Intravesical bacillus Calmette-Guérin immunotherapy was restarted in April 2009, again causing gross hematuria, and she received 1 instillation every 3 months until the end of 2012. The patient meticulously kept urologist appointments, initially with quarterly (and since 2010 approximately once every 6 months) cystoscopies and abdominal ultrasound examinations. She has been tumor-free for more than 5 years’ follow-up.
The patient told us: “When I had the initial mistletoe injections it felt like my abdomen was cooking and as if it was an anthill. Today I feel even better and stronger than before my bladder cancer diagnosis.”
RESULTS
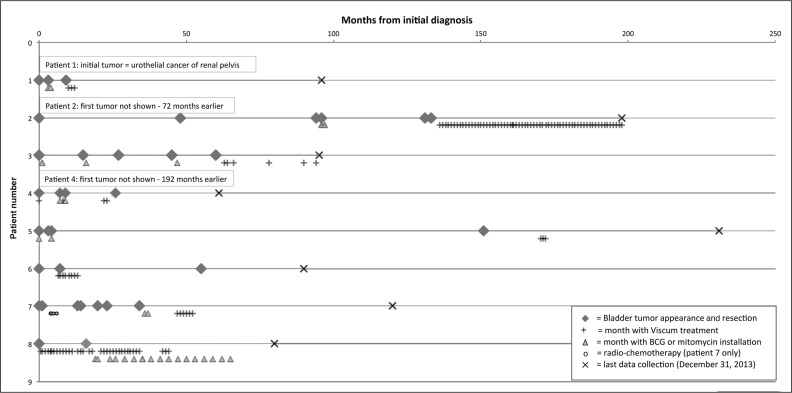
Tables 1a and 1b highlight the number of tumor recurrences and the outcomes for each patient. Reported tumors were removed by transurethral resection in all cases. Figure 1 shows a timeline of tumor recurrences and treatment for each patient.
Table 1a.
Patient overview
| Case No. | Sex | Age at diagnosis, years | Cancer description and stage | Cystectomy | Chemotherapy/radiotherapy (excluding mitomycin C) | Number of recurrence episodes | |||
|---|---|---|---|---|---|---|---|---|---|
| Total recurrences from diagnosis | Recurrences after BCG | Recurrences after mitomycin C | Recurrences after Viscum album | ||||||
| 1a | F | 59 | Renal urothelial cancer, pT3G1; bladder recurrence, pTaG3 | No | No | 2 | 1 | Not received | 0 |
| 2a | F | 62 | pTaG2 and Tis | No | No | 6 | 2 | 2 | 0 |
| 3a | M | 68 | pTaG2 | No | No | 4 | Not received | 4 | 0 |
| 4a | M | 53 | pTaG2 | No, refused | No | 4 | Not received | 2 | 3 |
| 5a | M | 59 | pT1aG1 | No | No | 3 | Not received | 3 | 0 |
| 6a | M | 74 | pT1G1 | No | No | 2 | Not received | Not received | 1 |
| 7a | M | 50 | pT1G3 | No | Radiochemotherapy | 6 | 0 | Not received | 0 |
| 8b | F | 63 | pT2aG3 and Tis | No, refused | No, refused | 1 | 0 | Not received | 1 |
Table 1b.
Patient overview
| Case No. | 5-year risk at Viscum album initiationc | Outcomed | |
|---|---|---|---|
| Recurrence risk, % | Progression risk, % | ||
| 1a | 62 | 25 | 7 years tumor-free since V album treatment |
| 2a | 78 | 17 | 5 years tumor-free since V album treatment |
| 3a | 62 | 6 | 32 months tumor-free since V album treatment |
| 4a | 62 | 6 | Recurrences after V album treatment; now 35 months tumor-free |
| 5a | 62 | 6 | 5 years tumor-free; was already 18 months tumor-free before V album treatment |
| 6a | 62 | 45 | Stage Tis 4 years after V album treatment; now 30 months tumor-free |
| 7a | 78 | 45 | Recurrences after radiochemotherapy; tumor-free since BCG and V album treatment (6 years since V album) |
| 8b | Very high risk | Very high risk | 5 years tumor-free since V album treatment and BCG despite muscle-invasive cancer |
Figure 1.
Treatment timeline.
BCG = bacillus Calmette-Guérin immunotherapy.
Among the 8 patients, 28 episodes of recurrence were observed from diagnosis until December 2013 (median = 3.5 recurrences per patient; range = 1–6 per patient). After intravesical bacillus Calmette-Guérin immunotherapy or mitomycin C therapy (received by 7 patients), 12 episodes of recurrence occurred over 694 months of follow-up (median = 1.5 recurrences per patient; range = 0–4 recurrences; 5-year cumulative incidence = 1.0). Following initiation of treatment with high-dose Viscum album extract, 5 recurrences occurred over 523 months of follow-up (median = 0 recurrences per patient; range = 0–3 recurrences; 5-year cumulative incidence = 0.55). These outcomes were not comparable, however, because the interventions overlapped considerably. Among the 4 patients who received intravesical bacillus Calmette-Guérin immunotherapy, 2 patients had further recurrences but stopped having recurrences once Viscum album extract treatment was initiated (see Figure 1, Patients 1 and 2). The tumor-free follow-up from initiation of Viscum album extract treatment until December 31, 2013 was 421 patient months (median = 48.5 tumor-free months per patient; range = 32–86 tumor-free months). Final cystoscopy controls (all negative for recurrences) were performed between November 2013 and April 2014 for all patients except Case 5, in which the last cystoscopy occurred in November 2011. (For this patient, tumor-free follow-up was counted only until November 2011.) None of the patients was identified with cancer progression.
The outcomes for each patient are described by category of effect.
Possible Beneficial Effect
Case 1: The patient is 7 years tumor-free since the start of Viscum album extract treatment, after failure of intravesical bacillus Calmette-Guérin immunotherapy. This is a positive outcome for a patient for whom cystectomy was indicated.
Case 2: The patient is 5 years tumor-free since the start of Viscum album extract treatment, despite frequent prior recurrences (including after intravesical bacillus Calmette-Guérin immunotherapy and mitomycin treatment) and a 78% recurrence risk. We did not define her recurrences as a failure of intravesical bacillus Calmette-Guérin immunotherapy because she had not received maintenance treatment.
Case 3: The patient had frequent tumor recurrence despite mitomycin C treatment and is now 32 months tumor-free after Viscum album extract treatment.
Case 6: This patient was at high risk of progression because of initial pT1 pathology. A Stage T1G1 tumor recurrence was detected within less than 1 month of initiation of Viscum album extract treatment; this recurrence could have been preexisting when treatment was started and therefore was not included in our analysis of recurrences. This patient went on to have a Stage pTis (carcinoma in situ) recurrence but is now 30 months tumor-free.
Case 8: This patient had a poor prognosis because of a diagnosis of muscle-invasive disease and refusal to have cystectomy and chemotherapy. A 12-week delay in cystectomy can reduce 3-year cancer survival to 35%.27 The fact that she had only 1 nonmuscle-invasive recurrence and 5 years of tumor-free survival was therefore very unexpected, and we considered that Viscum album extract treatment together with intravesical bacillus Calmette-Guérin immunotherapy could have influenced the outcome in this patient. The effect of Viscum album extract treatment therefore seems likely, because her risk of recurrence and progression without cystectomy and chemotherapy was almost 100%. However, given the difficulty in attributing the effect to either intravesical bacillus Calmette-Guérin immunotherapy or Viscum album extract treatment, we conservatively rate the effect as a possible beneficial effect.
Unlikely or Uncertain Beneficial Effect
Case 4: This patient had an unlikely beneficial effect because of 2 recurrences despite Viscum album extract treatment. The patient now has 30 months of tumor-free follow-up.
Case 5: The effect could not be assessed in this patient. He had 6 recurrences and is now 5 years tumor-free. He was already 18 months tumor-free before receiving Viscum album extract treatment, and therefore assessment was not possible.
Case 7: Assessment was not possible. This patient had a high-risk Stage pT1G3 tumor and five Ta recurrences, one of which was high-grade, despite radiochemotherapy. He then had seven years of tumor-free survival after two courses of intravesical bacillus Calmette-Guérin immunotherapy and Viscum album extract treatment, which we consider a very satisfactory outcome. We were unable to distinguish between the beneficial effect of intravesical bacillus Calmette-Guérin immunotherapy and Viscum album extract treatment because these treatments were given in relatively short succession after the last tumor recurrence.
In summary, an effect from Viscum album extract treatment appeared to be a possibility in five patients (Cases 1, 2, 3, 6, and 8), could not be assessed in two cases (Cases 5 and 7), and was unlikely in one patient (Case 4).
Tolerability
Most patients experienced fever up to 40° C and local redness at the injection site (less than 5 cm in diameter in all cases) as part of the intended immune reaction. One patient had nausea and headache after using a particular additional Viscum album extract preparation, from a birch tree (Case 8). No patient needed to stop treatment because of side effects.
DISCUSSION
We present retrospective data from a series of eight patients with recurrent bladder cancer who had been treated with high-dose Viscum album extract treatment. Seven patients had nonmuscle-invasive cancers with frequently recurring tumors, one of whom had not responded to intravesical bacillus Calmette-Guérin immunotherapy; therefore, they were a difficult patient group to manage. Patients had either intermediate-risk or high-risk tumors, as defined by European guidelines.5 One patient with muscle-invasive cancer had refused standard therapy, and so tumor recurrence and progression was almost certain. However, our analysis shows substantial and consistent decrease of recurrences in this series of patients. Despite unfavorable prognoses, we observed mainly positive outcomes after Viscum album extract treatment. Recurrences occurred in only three patients, and only one of those patients had three recurrences, and the patients had consistent, long, tumor-free periods subsequent to these recurrences, with no patient progressing. We therefore consider individual variation a less likely explanation, and a beneficial effect of Viscum album extract treatment as a possible explanation.
The analysis was not designed to compare recurrence incidence between intravesical bacillus Calmette-Guérin immunotherapy/mitomycin C treatment and Viscum album extract treatment. It also is not our intent to suggest management of muscle-invasive cancer without cystectomy. High-dose subcutaneous injections with Viscum album (Salicis) extract may, however, have a preventive role in frequent recurrences of bladder cancer.
Few studies exist regarding the effect of Viscum album extract treatment in recurrence of bladder cancer. In one small study, intravesical treatment with lectin-standardized Viscum album extract seemed to have preventive effect at 12-month follow-up, similar to intravesical bacillus Calmette-Guérin immunotherapy-treated historical controls.28 A small, prospective, randomized study in which low-dose, subcutaneous injections of lectin-standardized Viscum album extract were compared with no prophylactic intervention showed no benefit.29 A randomized trial in 60 patients, comparing intravesical Viscum fraxini-2 with intravesical bacillus Calmette-Guérin immunotherapy found a recurrence rate of 73% vs 30%; muscle-invasive bladder cancer developed in 5 patients in each group.30 A Phase 1b/2a dose-escalation study of intravesical treatment with Viscum album extract showed promising results31 and is currently being followed by a Phase 3 study.32 The treatment approach used in the patients in our case series differed from the approaches used in these other studies; the physicians at our clinic used subcutaneous application (more convenient than intravesical) and Viscum album extract grown on the willow tree (Salicis), which was selected on the basis of positive clinical experience.33
A potential mechanism of action for Viscum album extract in preventing recurrence of bladder cancer is its known antitumor and immune-modulating activity and the potential beneficial effect of fever in cancer treatment as described earlier.8,15 We note, however, that Viscum album extract induced fever after the first 2 to 3 doses only and thus may not be considered fever therapy.
A recurring theme reported by patients in this case series was that the initially exhausting fever reaction was followed by a gain in energy and strength. This is consistent with systematic reviews on the beneficial effect on quality of life and cancer-related fatigue of Viscum album extract treatment.6,7 Some patients experienced the treatment as a turning point in their treatment course, after which they felt more confident that they had overcome the cancer.
This report has several limitations. First, it is retrospective, and there are considerable differences among patients in terms of follow-up and treatment. Second, defining patients into certain categories after data mining is subject to researcher bias. Rather than a cohort, we present a series of individual cases, each assessed individually. The strengths of this report are that we provide direct observation from clinical practice, provide a qualitative judgment of each individual patient history, and have not merely provided an analysis of the best cases.
CONCLUSION
A prospective study is now needed to assess whether high-dose subcutaneous Viscum album (Salicis) extract can be an additional, bladder-sparing preventive option for patients with medium- to high-risk nonmuscle-invasive bladder cancer.
Acknowledgments
We thank Sally Hargreaves, MD, (Imperial College) for helpful input into the paper.
Kathleen Louden, ELS, of Louden Health Communications provided editorial assistance.
Footnotes
Disclosure Statement
Dr Kienle and Dr Kiene report that their institute has received restricted research funding from the companies Wala and Weleda in the past five years, unrelated to this report.
Dr von Schoen-Angerer is supported by a grant from the Mahle Stiftung, Stuttgart, Germany, for the writing of case series and case reports. The funder had no role at any stage of the analysis or manuscript preparation.
Authors’ Contributions
Johannes Wilkens, MD, treated patients with Viscum album extract, provided patient information, and reviewed the manuscript. Tido von Schoen-Angerer, MD, MPH, conceptualized the report, wrote the manuscript, and prepared Figure 1. Gunver S Kienle, MD; Helmut Kiene, MD; Jan Vagedes, MD; and Johannes Wilkens, MD, critically reviewed the manuscript. All authors read and approved the final manuscript.
Apothecary
The garden is the poor man’s apothecary.
— German proverb
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011 Mar-Apr;61(2):69–90. doi: 10.3322/caac.20107. DOI: http://dx.doi.org/10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet. 2009 Jul 18;374(9685):239–49. doi: 10.1016/S0140-6736(09)60491-8. DOI: http://dx.doi.org/10.1016/S0140-6736(09)60491-8. [DOI] [PubMed] [Google Scholar]
- 3.Witjes JA, Compérat E, Cowan NC, et al. European Association of Urology EAU guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2013 guidelines. Eur Urol. 2014 Apr;65(4):778–92. doi: 10.1016/j.eururo.2013.11.046. DOI: http://dx.doi.org/10.1016/j.eururo.2013.11.046. [DOI] [PubMed] [Google Scholar]
- 4.Han RF, Pan JG. Can intravesical bacillus Calmette-Guérin reduce recurrence in patients with superficial bladder cancer? A meta-analysis of randomized trials. Urology. 2006 Jun;67(6):1216–23. doi: 10.1016/j.urology.2005.12.014. DOI: http://dx.doi.org/10.1016/j.urology.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 5.Babjuk M, Burger M, Zigeuner R, et al. European Association of Urology EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol. 2013 Oct;64(4):639–53. doi: 10.1016/j.eururo.2013.06.003. DOI: http://dx.doi.org/10.1016/j.eururo.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Kienle GS, Kiene H. Review article: influence of Viscum album L (European mistletoe) extracts on quality of life in cancer patients: a systematic review of controlled clinical studies. Integr Cancer Ther. 2010 Jun;9(2):142–57. doi: 10.1177/1534735410369673. DOI: http://dx.doi.org/10.1177/1534735410369673. [DOI] [PubMed] [Google Scholar]
- 7.Horneber MA, Bueschel G, Huber R, Linde K, Rostock M. Mistletoe therapy in oncology. Cochrane Database Syst Rev. 2008 Apr 16;(2):CD003297. doi: 10.1002/14651858.CD003297.pub2. DOI: http://dx.doi.org/10.1002/14651858.CD003297.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kienle GS, Kiene H. Die Mistel in der Onkologie: Fakten und konzeptionelle Grundlagen. New York, NY: Schattauer; 2003. [Google Scholar]
- 9.Büssing A, editor. Mistletoe: the genus Viscum (medicinal and aromatic plants—industrial profiles) 1st ed. Amsterdam, The Netherlands: CRC Press; 2000. [Google Scholar]
- 10.Yoon TJ, Yoo YC, Kang TB, et al. Antitumor activity of the Korean mistletoe lectin is attributed to activation of macrophages and NK cells. Arch Pharm Res. 2003 Oct;26(10):861–7. doi: 10.1007/BF02980033. DOI: http://dx.doi.org/10.1007/BF02980033. [DOI] [PubMed] [Google Scholar]
- 11.Choi SH, Lyu SY, Park WB. Mistletoe lectin induces apoptosis and telomerase inhibition in human A253 cancer cells through dephosphorylation of Akt. Arch Pharm Res. 2004 Jan;27(1):68–76. doi: 10.1007/BF02980049. DOI: http://dx.doi.org/10.1007/BF02980049. [DOI] [PubMed] [Google Scholar]
- 12.Tröger W, Galun D, Reif M, Schumann A, Stanković N, Milićević M. Viscum album [L] extract therapy in patients with locally advanced or metastatic pancreatic cancer: a randomised clinical trial on overall survival. Eur J Cancer. 2013 Dec;49(18):3788–97. doi: 10.1016/j.ejca.2013.06.043. DOI: http://dx.doi.org/10.1016/j.ejca.2013.06.043. [DOI] [PubMed] [Google Scholar]
- 13.Tröger W, Galun D, Reif M, Schumann A, Stanković N, Milićević M. Quality of life of patients with advanced pancreatic cancer during treatment with mistletoe: a randomized controlled trial. Dtsch Arztebl Int. 2014 Jul 21;111(29–30):493–502. doi: 10.3238/arztebl.2014.0493. DOI: http://dx.doi.org/10.3238/arztebl.2014.0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orange M, Lace A, Fonseca MP, von Laue BH, Geider S, Kienle GS. Durable regression of primary cutaneous B-cell lymphoma following fever-inducing mistletoe treatment: two case reports. Glob Adv Health Med. 2012 Mar;1(1):18–25. doi: 10.7453/gahmj.2012.1.1.006. DOI: http://dx.doi.org/10.7453/gahmj.2012.1.1.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kienle GS, Grugel R, Kiene H. Safety of higher dosages of Viscum album L in animals and humans—systematic review of immune changes and safety parameters. BMC Complement Altern Med. 2011 Aug 28;11:72. doi: 10.1186/1472-6882-11-72. DOI: http://dx.doi.org/10.1186/1472-6882-11-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mabed M, El-Helw L, Shamaa S. Phase II study of viscum fraxini-2 in patients with advanced hepatocellular carcinoma. Br J Cancer. 2004 Jan 12;90(1):65–9. doi: 10.1038/sj.bjc.6601463. DOI: http://dx.doi.org/10.1038/sj.bjc.6601463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Owusu RA, Abern MR, Inman BA. Hyperthermia as adjunct to intravesical chemotherapy for bladder cancer. BioMed Res Int. 2013;2013:262313. doi: 10.1155/2013/262313. DOI: http://dx.doi.org/10.1155/2013/262313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kienle GS. Fever in cancer treatment: Coley’s therapy and epidemiologic observations. Glob Adv Health Med. 2012 Mar;1(1):92–100. doi: 10.7453/gahmj.2012.1.1.016. DOI: http://dx.doi.org/10.7453/gahmj.2012.1.1.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lammers RJ, Witjes JA, Inman BA, et al. The role of a combined regimen with intravesical chemotherapy and hyperthermia in the management of non-muscle-invasive bladder cancer: a systematic review. Eur Urol. 2011 Jul;60(1):81–93. doi: 10.1016/j.eururo.2011.04.023. DOI: http://dx.doi.org/10.1016/j.eururo.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 20.Sousa A, Inman BA, Piñeiro I, et al. A clinical trial of neoadjuvant hyperthermic intravesical chemotherapy (HIVEC) for treating intermediate and high-risk non-muscle invasive bladder cancer. Int J Hyperthermia. 2014 May;30(3):166–70. doi: 10.3109/02656736.2014.900194. DOI: http://dx.doi.org/10.3109/02656736.2014.900194. [DOI] [PubMed] [Google Scholar]
- 21.Sverrisson EF, Espiritu PN, Spiess PE. New therapeutic targets in the management of urothelial carcinoma of the bladder. Res Rep Urol. 2013 Mar 1;5:53–65. doi: 10.2147/RRU.S29131. DOI: http://dx.doi.org/10.2147/RRU.S29131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kienle GS, Albonico HU, Baars E, Hamre HJ, Zimmermann P, Kiene H. Anthroposophic medicine: an integrative medical system originating in Europe. Glob Adv Health Med. 2013 Nov;2(6):20–31. doi: 10.7453/gahmj.2012.087. DOI: http://dx.doi.org/10.7453/gahmj.2012.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006 Mar;49(3):466–77. doi: 10.1016/j.eururo.2005.12.031. DOI: http://dx.doi.org/10.1016/j.eururo.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 24.Davis JW, Sheth SI, Doviak MJ, Schellhammer PF. Superficial bladder carcinoma treated with bacillus Calmette-Guerin: progression-free and disease specific survival with minimum 10-year followup. J Urol. 2002 Feb;167(2 Pt 1):494–500. doi: 10.1016/S0022-5347(01)69072-4. DOI: http://dx.doi.org/10.1016/S0022-5347(01)69072-4. [DOI] [PubMed] [Google Scholar]
- 25.German homoeopathic pharmacopoeia: GHP 2012. Stuttgart, Germany: Medpharm Scientific Publishers; 2012. [Google Scholar]
- 26.Zuzak T, Rist L, Viviani A, et al. [Das Mistelpräparat Iscucin®—Herstellung, Analytik, Wirkung in vitro]. [Article in German] Der Merkurstab. 2004 Nov-Dec;57(6):467–73. [Google Scholar]
- 27.Sánchez-Ortiz RF, Huang WC, Mick R, Van Arsdalen KN, Wein AJ, Malkowicz SB. An interval longer than 12 weeks between the diagnosis of muscle invasion and cystectomy is associated with worse outcome in bladder carcinoma. J Urol. 2003 Jan;169(1):110–5. doi: 10.1016/S0022-5347(05)64047-5. DOI: http://dx.doi.org/10.1016/S0022-5347(05)64047-5. [DOI] [PubMed] [Google Scholar]
- 28.Elsässer-Beile U, Leiber C, Wetterauer U, et al. Adjuvant intravesical treatment with a standardized mistletoe extract to prevent recurrence of superficial urinary bladder cancer. Anticancer Res. 2005 Nov-Dec;25(6C):4733–6. [PubMed] [Google Scholar]
- 29.Goebell PJ, Otto T, Suhr J, Rübben H. Evaluation of an unconventional treatment modality with mistletoe lectin to prevent recurrence of superficial bladder cancer: a randomized phase II trial. J Urol. 2002 Jul;168(1):72–5. DOI: http://dx.doi.org/10.1016/S0022-5347(05)64834-3. [PubMed] [Google Scholar]
- 30.Hekal IA, Samer T, Ibrahim EI. Viscum Fraxini 2, as an adjuvant therapy after resection of superficial bladder cancer: prospective clinical randomized study. Proceedings of the 43rd Annual Congress of The Egyptian Urological Association in conjunction with The European Association of Urology; 2008 Nov 10–14; Hurghada, Egypt. p. 8. [abstract], 120. [Google Scholar]
- 31.Eisenbraun J. Dose-escalation-study with a mistletoe extract from the ash tree as intravesical instillation in patients with superficial bladder cancer: an ICH/GCP phase Ib/IIa study. Phytomedicine. 2011 Oct 15;18(Suppl 1):S15. DOI: http://dx.doi.org/10.1016/j.phymed.2011.09.036. [Google Scholar]
- 32.Ruebben H. Intravesical mistletoe extract in superficial bladder cancer: a phase III efficacy study [clinical trial study record] [Internet] Bethesda, MD: US National Institutes of Health; 2014. Apr, [updated 2015 Mar; cited 2015 Apr 24]. Available from: https://clinicaltrials.gov/ct2/show/NCT02106572. [Google Scholar]
- 33.Wilkens J. [Die Weidenmistel beim Blasenkarzinom]. [Article in German] Der Merkurstab. 2007 Sep-Oct;60(5):446–9. [Google Scholar]