Abstract
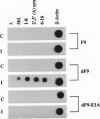
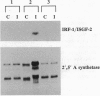
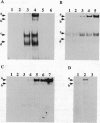
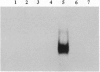
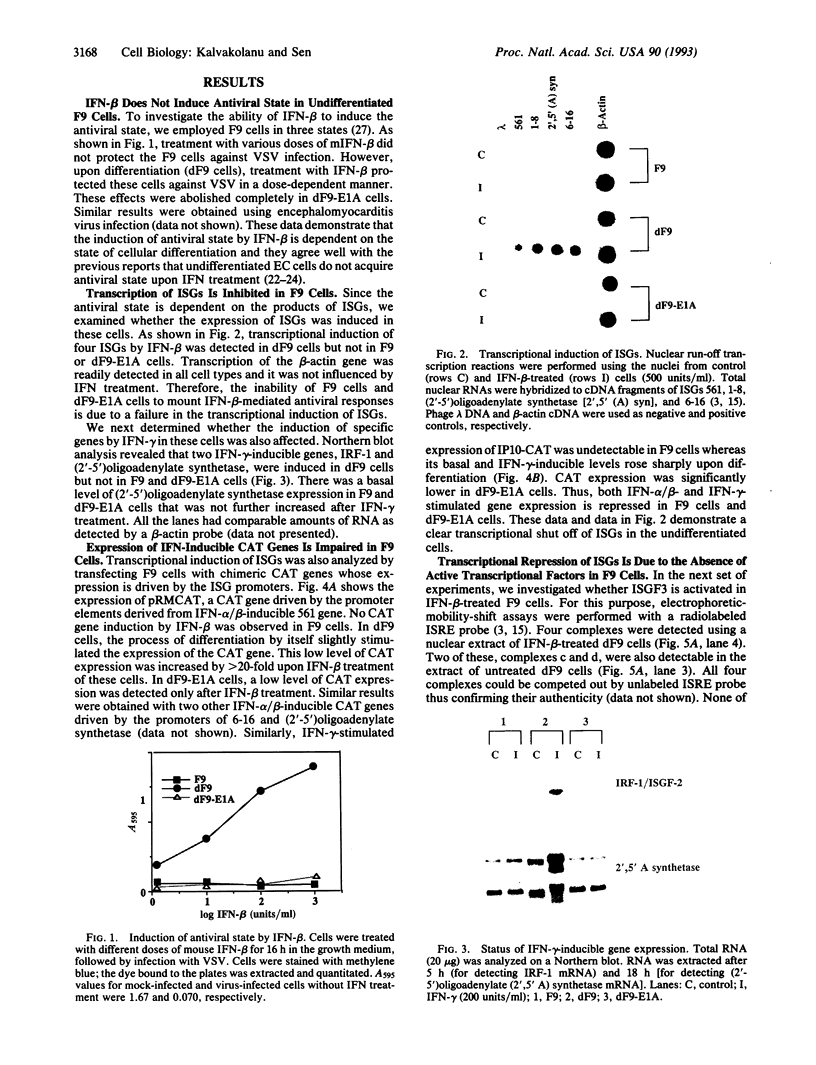
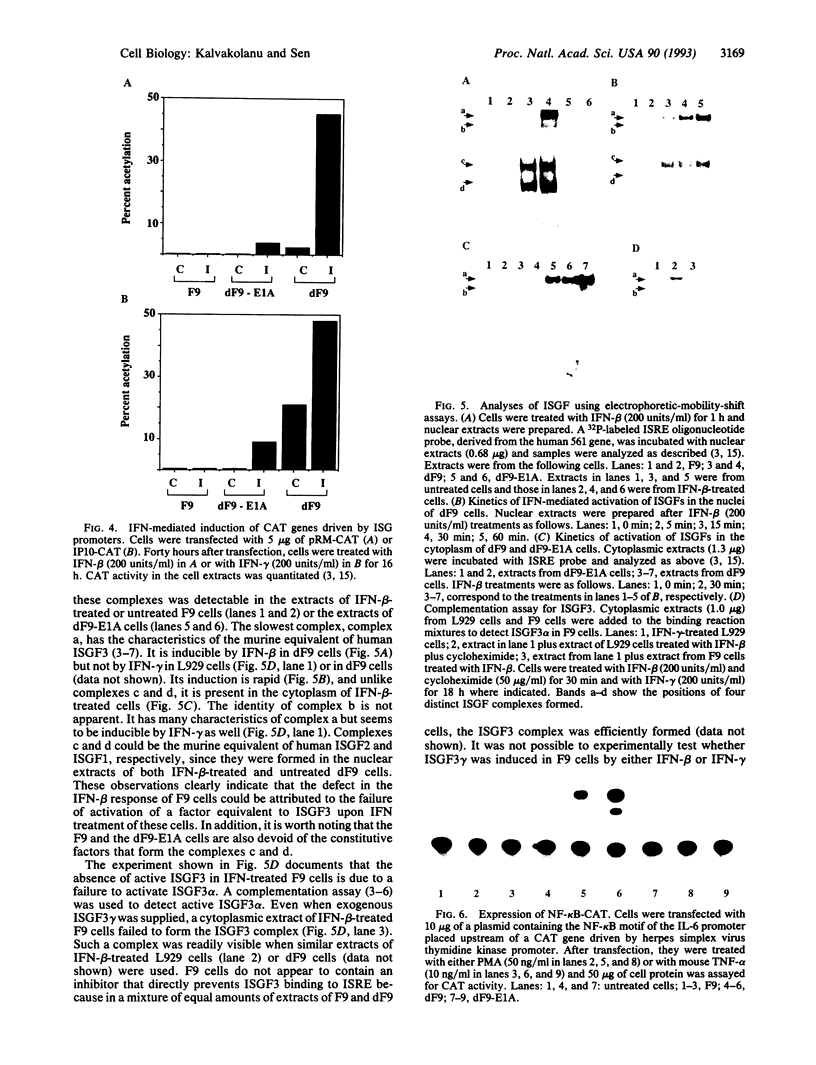
We have recently shown that the adenovirus E1A gene products block interferon-alpha-induced signal transduction and transcription factor NF-kappa B-mediated gene induction. Here we report that the same responses are also blocked in undifferentiated F9 teratocarcinoma cells. The block was removed upon cellular differentiation and regained upon the introduction of viral E1A into the differentiated cells. In undifferentiated cells, interferon-beta failed to induce the transcription of interferon-responsive genes because of a lack of activation of the cognate trans-acting factors. As a result, in these cells, virus replication was not inhibited by interferon. Similarly, in undifferentiated but not in differentiated F9 cells, tumor necrosis factor alpha failed to stimulate NF-kappa B-mediated transcription of a reporter gene because of a failure in the activation of NF-kappa B trans-acting factor. These results suggest that a cellular E1A-like activity, present in undifferentiated F9 cells, and adenoviral E1A use similar mechanisms for repressing the expression of specific cellular genes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackrill A. M., Foster G. R., Laxton C. D., Flavell D. M., Stark G. R., Kerr I. M. Inhibition of the cellular response to interferons by products of the adenovirus type 5 E1A oncogene. Nucleic Acids Res. 1991 Aug 25;19(16):4387–4393. doi: 10.1093/nar/19.16.4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguet M., Gresser I., Hovanessian A. G., Bandu M. T., Blanchard B., Blangy D. Specific high-affinity binding of 125I-labeled mouse interferon to interfevon resistant embryonal carcinoma cells in vitro. Virology. 1981 Oct 30;114(2):585–588. doi: 10.1016/0042-6822(81)90241-5. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay S. K., Kalvakolanu D. V., Sen G. C. Gene induction by interferons: functional complementation between trans-acting factors induced by alpha interferon and gamma interferon. Mol Cell Biol. 1990 Oct;10(10):5055–5063. doi: 10.1128/mcb.10.10.5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay S. K., Sen G. C. Role of protein phosphorylation in activation of interferon-stimulated gene factors. J Biol Chem. 1992 Mar 25;267(9):6389–6395. [PubMed] [Google Scholar]
- Barlow D. P., Randle B. J., Burke D. C. Interferon synthesis in the early post-implantation mouse embryo. Differentiation. 1984;27(3):229–235. doi: 10.1111/j.1432-0436.1984.tb01433.x. [DOI] [PubMed] [Google Scholar]
- Burke D. C., Graham C. F., Lehman J. M. Appearance of interferon inducibility and sensitivity during differentiation of murine teratocarcinoma cells in vitro. Cell. 1978 Feb;13(2):243–248. doi: 10.1016/0092-8674(78)90193-9. [DOI] [PubMed] [Google Scholar]
- Dale T. C., Imam A. M., Kerr I. M., Stark G. R. Rapid activation by interferon alpha of a latent DNA-binding protein present in the cytoplasm of untreated cells. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1203–1207. doi: 10.1073/pnas.86.4.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis M. K., Lehman J. M. Control of beta-interferon expression in murine embryonal carcinoma F9 cells. Mol Cell Biol. 1989 Aug;9(8):3553–3556. doi: 10.1128/mcb.9.8.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X. Y. A transcription factor with SH2 and SH3 domains is directly activated by an interferon alpha-induced cytoplasmic protein tyrosine kinase(s). Cell. 1992 Jul 24;70(2):323–335. doi: 10.1016/0092-8674(92)90106-m. [DOI] [PubMed] [Google Scholar]
- Gutch M. J., Reich N. C. Repression of the interferon signal transduction pathway by the adenovirus E1A oncogene. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):7913–7917. doi: 10.1073/pnas.88.18.7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada H., Willison K., Sakakibara J., Miyamoto M., Fujita T., Taniguchi T. Absence of the type I IFN system in EC cells: transcriptional activator (IRF-1) and repressor (IRF-2) genes are developmentally regulated. Cell. 1990 Oct 19;63(2):303–312. doi: 10.1016/0092-8674(90)90163-9. [DOI] [PubMed] [Google Scholar]
- Janaswami P. M., Kalvakolanu D. V., Zhang Y., Sen G. C. Transcriptional repression of interleukin-6 gene by adenoviral E1A proteins. J Biol Chem. 1992 Dec 5;267(34):24886–24891. [PubMed] [Google Scholar]
- Kalvakolanu D. V., Bandyopadhyay S. K., Harter M. L., Sen G. C. Inhibition of interferon-inducible gene expression by adenovirus E1A proteins: block in transcriptional complex formation. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7459–7463. doi: 10.1073/pnas.88.17.7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler D. S., Levy D. E. Protein kinase activity required for an early step in interferon-alpha signaling. J Biol Chem. 1991 Dec 5;266(34):23471–23476. [PubMed] [Google Scholar]
- Krause D., Silverman R. H., Jacobsen H., Leisy S. A., Dieffenbach C. W., Friedman R. M. Regulation of ppp(A2'p)nA-dependent RNase levels during interferon treatment and cell differentiation. Eur J Biochem. 1985 Feb 1;146(3):611–618. doi: 10.1111/j.1432-1033.1985.tb08695.x. [DOI] [PubMed] [Google Scholar]
- La Thangue N. B., Thimmappaya B., Rigby P. W. The embryonal carcinoma stem cell Ela-like activity involves a differentiation-regulated transcription factor. Nucleic Acids Res. 1990 May 25;18(10):2929–2938. doi: 10.1093/nar/18.10.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenardo M. J., Fan C. M., Maniatis T., Baltimore D. The involvement of NF-kappa B in beta-interferon gene regulation reveals its role as widely inducible mediator of signal transduction. Cell. 1989 Apr 21;57(2):287–294. doi: 10.1016/0092-8674(89)90966-5. [DOI] [PubMed] [Google Scholar]
- Levy D. E., Kessler D. S., Pine R., Darnell J. E., Jr Cytoplasmic activation of ISGF3, the positive regulator of interferon-alpha-stimulated transcription, reconstituted in vitro. Genes Dev. 1989 Sep;3(9):1362–1371. doi: 10.1101/gad.3.9.1362. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. Mechanisms of viral-mediated trans-activation of transcription. Adv Virus Res. 1989;37:35–83. doi: 10.1016/s0065-3527(08)60832-5. [DOI] [PubMed] [Google Scholar]
- Nilsen T. W., Wood D. L., Baglioni C. Virus-specific effects of interferon in embryonal carcinoma cells. Nature. 1980 Jul 10;286(5769):178–180. doi: 10.1038/286178a0. [DOI] [PubMed] [Google Scholar]
- Porter A. C., Chernajovsky Y., Dale T. C., Gilbert C. S., Stark G. R., Kerr I. M. Interferon response element of the human gene 6-16. EMBO J. 1988 Jan;7(1):85–92. doi: 10.1002/j.1460-2075.1988.tb02786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich N. C., Pfeffer L. M. Evidence for involvement of protein kinase C in the cellular response to interferon alpha. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8761–8765. doi: 10.1073/pnas.87.22.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford M. N., Hannigan G. E., Williams B. R. Interferon-induced binding of nuclear factors to promoter elements of the 2-5A synthetase gene. EMBO J. 1988 Mar;7(3):751–759. doi: 10.1002/j.1460-2075.1988.tb02872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler C., Shuai K., Prezioso V. R., Darnell J. E., Jr Interferon-dependent tyrosine phosphorylation of a latent cytoplasmic transcription factor. Science. 1992 Aug 7;257(5071):809–813. doi: 10.1126/science.1496401. [DOI] [PubMed] [Google Scholar]
- Sen G. C., Lengyel P. The interferon system. A bird's eye view of its biochemistry. J Biol Chem. 1992 Mar 15;267(8):5017–5020. [PubMed] [Google Scholar]
- Shenk T., Flint J. Transcriptional and transforming activities of the adenovirus E1A proteins. Adv Cancer Res. 1991;57:47–85. doi: 10.1016/s0065-230x(08)60995-1. [DOI] [PubMed] [Google Scholar]
- Suemori H., Hashimoto S., Nakatsuji N. Presence of the adenovirus E1A-like activity in preimplantation stage mouse embryos. Mol Cell Biol. 1988 Aug;8(8):3553–3555. doi: 10.1128/mcb.8.8.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veals S. A., Schindler C., Leonard D., Fu X. Y., Aebersold R., Darnell J. E., Jr, Levy D. E. Subunit of an alpha-interferon-responsive transcription factor is related to interferon regulatory factor and Myb families of DNA-binding proteins. Mol Cell Biol. 1992 Aug;12(8):3315–3324. doi: 10.1128/mcb.12.8.3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velazquez L., Fellous M., Stark G. R., Pellegrini S. A protein tyrosine kinase in the interferon alpha/beta signaling pathway. Cell. 1992 Jul 24;70(2):313–322. doi: 10.1016/0092-8674(92)90105-l. [DOI] [PubMed] [Google Scholar]
- Weigel R. J., Devoto S. H., Nevins J. R. Adenovirus 12S E1A gene represses differentiation of F9 teratocarcinoma cells. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9878–9882. doi: 10.1073/pnas.87.24.9878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel R. J., Nevins J. R. Adenovirus infection of differentiated F9 cells results in a global shut-off of differentiation-induced gene expression. Nucleic Acids Res. 1990 Oct 25;18(20):6107–6112. doi: 10.1093/nar/18.20.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. N., Hovanessian A. G. Interferon enhances 2-5A synthetase in embryonal carcinoma cells. Nature. 1979 Nov 1;282(5734):74–76. doi: 10.1038/282074a0. [DOI] [PubMed] [Google Scholar]