Abstract
Methyl methacrylate used in bone cements has drawbacks of toxicity, high exotherm, and considerable shrinkage. A new resin, based on silorane/oxirane chemistry, has been shown to have little toxicity, low exotherm, and low shrinkage. We hypothesized that silorane-based resins may also be useful as components of bone cements as well as other bone applications and began testing on bone cell function in vitro and in vivo. MLO-A5, late osteoblast cells, were exposed to polymerized silorane (SilMix) resin (and a standard polymerized bisGMA/TEGDMA methacrylate (BT) resin and compared to culture wells without resins as control. A significant cytotoxic effect was observed with the BT resin resulting in no cell growth, whereas in contrast, SilMix resin had no toxic effects on MLO-A5 cell proliferation, differentiation, nor mineralization. The cells cultured with SilMix produced increasing amounts of alkaline phosphatase (1.8-fold) compared to control cultures. Compared to control cultures, an actual enhancement of mineralization was observed in the silorane resin-containing cultures at days 10 and 11 as determined by von Kossa (1.8–2.0 fold increase) and Alizarin red staining (1.8-fold increase). A normal bone calcium/phosphate atomic ratio was observed by elemental analysis along with normal collagen formation. When used in vivo to stabilize osteotomies, no inflammatory response was observed, and the bone continued to heal. In conclusion, the silorane resin, SilMix, was shown to not only be non cytototoxic, but actually supported bone cell function. Therefore, this resin has significant potential for the development of a nontoxic bone cement or bone stabilizer.
Keywords: siloranes, bone stabilization, mineralization, MLO-A5 cell line, osteotomy
INTRODUCTION
Bone cements have been used for decades in the fixation of prosthetic devices. Polymethyl methacrylate (PMMA)-based cement is a well-recognized conventional bone cement that provides reasonably good clinical results; however, severe problems are still associated with its use, such as, cytotoxicity, thermal injury, respiratory and cardiovascular complications in addition to polymerization shrinkage, which can affect the stability of the implant.1–5 The interaction between resin and bone causes internal stress that can lead to gap formation between the PMMA and the bone.6
Silorane-based resins have been developed by 3M-ESPE7 for the production of dental composite materials. These resins have proved to have superior characteristics to bisGMA/TEGDMA (bisphenol A glycidyl methacrylate and triethylene glycol dimethacrylate), the two usual monomer components of dental composites. The term “silorane” was introduced to represent hybrid monomer systems that contain both siloxane and oxirane structural moieties. Siloranes contain a cyclosiloxane backbone, which imparts hydrophobicity 8; in addition, they contain cycloaliphatic oxirane sites that have high reactivity and present less shrinkage during polymerization than methacrylates.9,10 Some cyclosiloxanes have been reported to undergo cationic ring-opening polymerization with volume expansion.11 These resins exhibit excellent biocompatibility. Cytotoxicity ratings are as good as or better than those for typical methacrylate dental monomers, such as bisGMA-based polymer. They also are nonmutagenic.12–14 Marginal integrity and microleakage of silorane-based restorative systems are reported to be superior to methacrylate-based systems.15 Shear bond strength and other mechanical properties have also been studied and found to be better than the methacrylate resins.16–20 It was also shown that a silorane-based dental composite can effectively bond to bone.21
In order to begin to develop better bone cements, we analyzed the effect of silorane-based resins on bone cell function in vitro and in vivo. One aim of the present study was to analyze the effect of silorane-based resin on bone cell proliferation, differentiation, and mineralization. MLO-A5 cells were used as an in-vitro model for bone formation. MLO-A5 cells are a post-osteoblast/pre-osteocyte-like cell line established from the long bones of 14-day-old mice expressing the large T-antigen driven by the osteocalcin promoter.22 These cells express extremely high levels of alkaline phosphatase and osteocalcin, as well as, osteopontin, periostin, bone sialoprotein, and PTH type 1 receptor compared to primary osteoblasts and osteoblast cell lines.22 Previously, we had shown that the MLO-A5 cells mineralize in culture, forming sheets not nodules, and that this mineralized matrix contains a ratio of calcium to phosphorus similar to bone.23 These cells will mineralize in the absence of beta glycerolphosphate (bGP) in 6–7 days, but this process is accelerated by the addition of an external source of phosphate. Spectra obtained by Fourier transform infrared spectroscopy, FTIR, of these cultures were shown to be very similar to normal bone.22,23 The MLO-A5 cells appear to be a good model for in vitro lamellar bone formation. These cells were used for the present study in order to obtain insight into the potential mechanisms by which bone would form in the presence of silorane-based resins in comparison to the effect of a methacrylate composite, bisGMA/TEGDMA (BT). A second aim of the study was to examine the effects of silorane resin on bone cell function in vivo and to determine if the resin elicited an inflammatory response. We chose to use the standard femoral osteotomy approach in mice. The silorane resin was used to stabilize the osteotomy was up to one month for radiographic and histological analysis. Overall, silorane resin had little or no negative effects on bone cell function.
MATERIALS AND METHODS
Preparation of resins
The resins were prepared as described previously.17,24,25 Briefly, the silorane-based resin SilMix, is a 1/1 wt/wt mixture of two silicon-containing oxiranes, bis[2-(3{7oxabicy-clo[4.1.0]heptyl})-ethyl]methylphenyl silane (PHEPSI)26 and 2,4,6,8-tetrakis(2-(7-oxabicyclo[4.1.0]heptan-3-yl)ethyl)-2,4,6,8-tetramethyl-1,3,5,7,2,4,6,8-tetraoxatetrasilocane (CYGEP)27 (see Figure 1). A conventional methacrylate-based matrix resin bisGMA (BisGMA/TEDGMA 50/50) used in dental composites was used as a control. For the drop method, the silorane (SilMix) and methacrylate (Z250: bisGMA/TEGDMA) resins were obtained from 3M-ESPE (St. Paul, MN, and Seefeld, Germany). For the rest of the samples, the methacrylate monomer system (BT) was a 1:1 mixture by weight of two methacrylates, bisGMA (purity: 93%, Esstech, Inc.) and TEGDMA (purity: 97%, Sartomer). With the exception of the resin drop samples, the silorane monomers (SilMix) were synthesized using an adapted procedure for PHEPSI26 and CYGEP27 resulting in a >95.8% purity as determined by 1H NMR spectroscopy. All resin samples were prepared at room temperature (~ 20°C) and under yellow light in order to prevent premature polymerization. The photoinitiator system (see Figure 1) used for all the resins consisted of phenyl[p-2-hydroxytetradecyloxypheny-l]iodonium hexafluoroantimonate (PI, Gelest, Inc., Tullytown, PA); camphorquinone (CQ, Aldrich, Milwaukee, WI), and ethyl-4-dimethylaminobenzoate (EDMAB, Fisher Scientific / ACROS, Pittsburgh, PA). The photoinitiator and monomer systems were combined using a speed mixer and mixed for periods of 5–15 minutes depending on the amount of material. The final mass composition was 0.15% EDMAB, 1.0% CQ, 3.0% PI, and 95.85% SilMix and the BT composition was 0.15% EDMAB, 1.0% CQ, 3.0% PI, and 95.85% BisGMA/TEGDMA. Resins were prepared the same day and used within a 2-h period after preparation.
FIGURE 1.
Chemical structure of siloranes and photoinitiator system used for the resins. The silorane resin used for the in vitro bone cell assays and in vivo is composed of SilMix a 1/1 wt/wt ofPHEPSI/CYGEP.
Resin polymer characterization
To ensure that resin polymerization was complete, the degree of conversion (DC) of the SilMix and BT resins was analyzed using FTIR spectroscopy (Perkin–Elmer Spectrum One, ATR sampling mode) analysis. A Delrin mold was fixed on the FTIR instrument; the resin (80 mg) was added to the mold, and the resin polymerized via light cure with a 3M curing lamp, (3M XL3000, St. Paul, MN, 450 mW/cm2 light intensity) for three 40-sec intervals. The solid discs (n = 6) were detached from the mold, and half were subjected to 2 h sterilization below laminar hood UV light (1 h per side). The other half were allowed to dark cure. FTIR spectra were collected from the unpolymerized resin at 2 min after light cure and at 4 h after light cure (polymer with dark cure) (n = 3). The FTIR spectra were baseline corrected, and the DC was calculated for each polymer sample using a polymerization dependent peak [BT: 1638 cm−1 (C =C), and SilMix: 883 cm−1 (oxirane ring opening)], which was compared to an internal standard [BT-1608 cm−1(phenyl), and SilMix 1258 cm−1(C—O in ring)]. The DC was calculated as the difference in the peak ratios from the unpolymerized resin assuming that the unpolymerized resin spectra represented no (0%) polymerization.
Culture of MLO-A5 cells with resin
Approximately 50 μL of the resin was dropped into the center of a NUNC brand Thermanox coverslip (Electron Microscopy Science Hatfield, PA) and polymerized with a 3M curing lamp (450 mW/cm2 light intensity) for three 40-sec intervals. Thermanox coverslips with polymerized resin drops of 5-mm diameter or without resin for control were used in triplicate. The coverslips were placed in 24-well plates, and MLO-A5 cells were plated at a density of 3.5 × 104 cells/cm2 in α-MEM containing 5% fetal bovine serum (FBS) and 5% calf serum (CS). Cells were cultured for 24, 48, and 72 h, then washed with PBS, and harvested with trypsin-EDTA. The cell number was measured using a Coulter Counter (Z1 Coulter particle counter, Beckman Coulter Fullerton, CA). In these experiments, it was observed that cells did not attach well to the resin drop surfaces; therefore a second experimental design generated discs of SilMix and BT polymer of 9 mm diameter by 0.7 mm thickness. Discs were prepared by placing 80 mg of the freshly mixed resin into Delrin ring molds (McMaster-Carr, Elmhurst, IL), which were fixed on glass slides. The resin was light cured (3M curing lamp, 450 mW/cm2 light intensity) for three 40-sec intervals at a distance of 1 mm from the top of the sample. Solid polymer discs were detached from the molds and sterilized under laminar hood UV light for 1 h on each side. The discs, which covered the entire bottom surface of 48-well culture plates, were placed in the wells prior to addition of MLO-A5 cells at a density of 3.5 × 104 cells/cm2. After 24 and 48 h of incubation, cell attachment and proliferation were assessed by measuring the cell number using the Coulter counter assay and the Trypan blue dye exclusion (TBE) assay.
The cell monolayer was washed with 0.5 mL of PBS, and then pooled with the respective supernatants. Trypsin/EDTA (0.2 mL) was added to the cell layer and incubated for 2–3 min at 37°C/5% CO2. Meanwhile, the cells in the supernatants were pelleted and treated with 0.05 mL Trypsin/EDTA at 37° C/5% CO2. Trypsinized cell suspensions were pooled (1.25 mL), then centrifuged for 2 min (5000 rpm). The obtained cell pellet was re-suspended in 100 μL of PBS. In each microcentrifuge tube, 20 μL of 0.4% trypan blue dye was added, mixed thoroughly, and allowed to stand for 3–5 min at room temperature. A hemacytometer was loaded with 10 μL cell suspension, and cells were counted under a microscope. Similar procedures were performed with cells grown on the polystyrene control wells.
Cell viability in response to polymer extracts
To study the effect of leachables on cell viability, sterilized discs were inserted into 48-well plates and washed with 0.5 mL of culture media for 1 h at 37°C/5% CO2. The used media was discarded, and fresh media (0.5 mL) was added to the polymer discs as well as the control wells (n = 4) and incubated for 48 h at 37°C/5% CO2. In parallel and in the same plate, the 1 h pre-incubated wells were seeded with 0.5 mL of 3.5 × 104 cells/mL and grown for 48 h. Then, the media in these wells containing cells were removed and replaced with 0.5 mL of conditioned media exposed to the discs (assumed to contain leachables from the polymer discs). After incubation for 24 h at 37°C/5% CO2, cell viability was measured using the MTT assay. For the MTT assay, 50 μL of 5 mg/mL of MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide, Sigma M5655] in phosphate buffered saline (PBS) were added to the culture plates and returned to the incubator. After 4 h of incubation time, the supernatants with unreacted MTT were discarded, and the purple formazan crystals in the cells were dissolved by adding dimethyl sulfoxide (DMSO, 0.5 mL). Formazan/DMSO aliquots were read at 550 nm in a 96-well plate reader (Molecular Devices Corp., Menlo Park, CA).
Culture of cells for mineralization
Due to low attachment of cells on resin surfaces, mineralizaton analysis was carried out using resin drops. Approximately the resin (50 μL) was dropped into the center of a NUNC brand Thermanox coverslip (Electron Microscopy Science Hatfield, PA) and polymerized with a 3M curing lamp, (450 mW/cm2 light intensity) for three 40-sec intervals. To rule out any effects of cells potentially settling in the middle of the well and being displaced by the resin for successive experiments, resin (20–30 μL) was placed either in the center of the coverslip or toward the side, but not touching the edge of the coverslip. After polymerization, the sample and Thermanox discs were washed with PBS and placed in 24-well plates for sterilization under laminar hood ultraviolet light for ~ 1–2 h, before use.
Thermanox coverslips with polymerized resin drops or without resin for control were used in triplicate. The coverslips were placed in 24-well plates, and MLO-A5 cells were cultured as described previously.22 MLO-A5 cells were plated at a density of 3.5 × 104 cells/cm2 in α-MEM containing 5% fetal bovine serum (FBS) and 5% calf serum (CS). Upon confluency, designated day 0, media was removed, and the cells were incubated in mineralizing media, α-MEM with 10% FBS, 4 mM of β-glycerolphosphate, bGP, and 100 μg/mL of ascorbic acid. Media were changed every two days through 11 days.23
Alkaline phosphatase assay
MLO-A5 cells were cultured on cover slips for 6 days under mineralization conditions as described above and analyzed for alkaline phosphatase enzyme activity. Briefly, cells were fixed with 10% buffered formalin for 10 min and washed with PBS two times. Fresh solution containing 0.033% NBT (nitro blue tetrazolium) and 0.017% BCIP (5-bromo-4-chloro-3-indolyl phosphate) in ALP buffer (100 mM sodium chloride, 5 mM magnesium chloride, 100 mM Tris-HCl, pH 9.5) was added to the cultures and incubated at 37°C for 20 min. The purple stained area was measured using a semiautomated imaging system as described previously.22,23
Immunohistochemical staining for collagen type 1
MLO-A5 cells were plated on coverslips and cultured as described above. After 6 days in culture, the cells were washed with PBS (two times), then fixed with 95% ethanol for 5 min and washed with PBS (three times). The cultures were then incubated with blocking solution, (PBS + 1% horse serum + 0.05% NaN3) for 2 h at room temperature, followed by incubation with polyclonal antibody to type I collagen, LF-67, that recognizes the C-telopeptide of collagen type 1 (the antibody was kindly provided by Dr. Larry W. Fisher, National Institutes of Health, Bethesda, MD, USA). A 1:400 dilution in PBS + horse serum was added for 1 h at room temperature, followed by incubation with Cy-3 conjugated donkey anti-rabbit IgG in blocking solution, 1:250 for 1 h and followed by washing with PBS (six times). The cells were then examined, and photos were taken using fluorescence microscopy (Nikon eclipse E800 microscope).23
Scanning electron microscopy (SEM)
MLO-A5 cells were cultured on coverslips for 24 and 48 h as well as 6 and 10 days. At the end of the culture, the cells were gently washed with PBS, and fixed with 10% formalin for 20 min, washed again with PBS and dehydrated in a graded series of ethanol, and dried using hexamethyl disilazone (HMDS) for 5 min. After dehydration, the coverslips were attached to SEM stubs and sputter-coated with gold-palladium. The gold-palladium-coated cultures were examined using a FEI/Philips XL30 field emission environmental scanning electron microscope. An accelerated voltage in a range of 15 to 25 KeV was used for the secondary and backscatter electron imaging. For X-ray microanalysis EDS, the cultures were carbon-coated and examined with 15 KeV accelerating voltage. X-ray spectra and maps for calcium and phosphorus distribution were acquired.23
Von Kossa staining for phosphate quantification
The MLO-A5 cultured cells were washed with PBS and fixed with 10% buffered formalin for 10 min The samples were washed with water several times before a 2% silver nitrate solution was added and the plates exposed to UV light for 20 min and followed by rinsing with water. Five percent sodium thiosulfate was added for 3 min before rinsing. A modified van Gieson stain was then used as a counterstain following the von Kossa stain. This stain consisted of five parts 1% acid fuchsin and 95 parts picric acid, which was added for 5 min followed by washing with 95% ETOH (two times), 100% ETOH (two times), and then air drying before analysis. The mineralized area and total area were measured using a semiautomated imaging system as described previously.22,23 Briefly, the area of von Kossa-stained matrix was quantified by automated image analysis using a video analysis program (Jandel Scientific, San Rafael, CA) linked to a video screen camera (CCD/RGB; Sony Corp., Park Ridge, NJ) and microscope (model BH2; Olympus Corp., Precision Instruments Division, Lake Success, NY) equipped with metallurgical lenses.
Alizarin red staining for calcium quantification
The MLO-A5 cells were cultured for mineralization and fixed in formalin as mentioned previously above. Fixed cultures were washed three times with TBS (Tris-buffered saline) and then stained with 4 nM alizarin red S dye (AR-S) for 5 min. Cultures were then rinsed with water followed by a 15-min wash with TBS to reduce nonspecific AR-S stain. The mineralized areas were measured using a semiautomated imaging system as described previously.23
Stabilization of osteotomized murine femuri with SilMix
Surgical procedures
All animal experimental procedures were approved by the Institutional Animal Care and Use Committee of authors’ institution. Eight 12-week old C57black6 mice were housed in the animal care facility under a 12-h/12-h light/dark cycle. The mice were anesthetized with 3.5% isoflurane and maintained with ketamine/dexdormitor (75/0.25 mg/kg body weight, intraperitoneally). The skin over the right hind limb was shaved, swabbed with betadine, and draped under aseptic conditions. Using the sterile instruments, a 1.5-cm skin incision was made on lateral aspect of thigh extending from the vastus lateralis muscle to the patellar ligament insertion, preserving the patellar ligament. The patella was retracted medially with the knee extended. The knee was slowly flexed to expose the intercondylar notch. The intramuscular septum between the vastus lateralis and hamstring muscles was separated using blunt dissection, and the periosteum was incised to expose the femur. A transverse fracture of the femur was created using an electrical round saw. A 0.7-mm K-wire was gently inserted into the intercondylar entry point, through the fractured femur, to the appropriate depth (approximately the level of lesser trochanter), which served as intramedullary fixation for the fracture to prevent angulations or displacement. SilMix resin (50–70 ll) was applied around the fracture site and cured using a dental curing lamp for 20 sec (three times. After polymerization of SilMix resin, the stability of the fixed femur was evaluated, then the K-wire was removed. The capsule and skin were sutured with 4–0 nylon. The animal was allowed to fully recover in a separate cage on a warming pad and was allowed activity ad libitum. An analgesic (buprenorphine hydrochloride, 0.05 mg/kg) was administered subcutaneously twice per day during three postoperative days.
Radiographic evaluation
At days 0, 7, 14, 21, and 28 post-surgery, the animals were anesthetized with ketamine/dexdormitor (75/0.25 mg/kg body weight, intraperitoneally). Radiographs of the femora were obtained using a Faxitron MX-20 (Faxitron X-Ray LLC, Lincolnshire, IL) at 26 KV and 10 sec. The fracture healing, angulation, or displacement of SilMixresin stabilized osteotomized femora was evaluated.
Histological assessment
The animals were sacrificed at days 7 (n = 4) or 28 (n = 4) postsurgery. The SilMix resin stabilized femora with surrounding tissues were harvested, fixed in 10% buffered formaldehyde for 2 days, decalcified in 14% EDTA for 3 weeks, and then incubated with 15% and 30% sucrose, serially, for 2 days. The samples were embedded for frozen sections allowing retention of both bone and resin, and the sections were cut longitudinally at 12 lm. The serial sections were stained with hematoxylin and eosin. The newly formed bone at the fracture site was evaluated.
Statistical Analysis
Statistical significance was determined either using the one-way ANOVA and Tukey post test or in some cases the three-way ANOVA for significance at the p < 0.05 level. Experiments were repeated a minimum of two times with similar results.
RESULTS
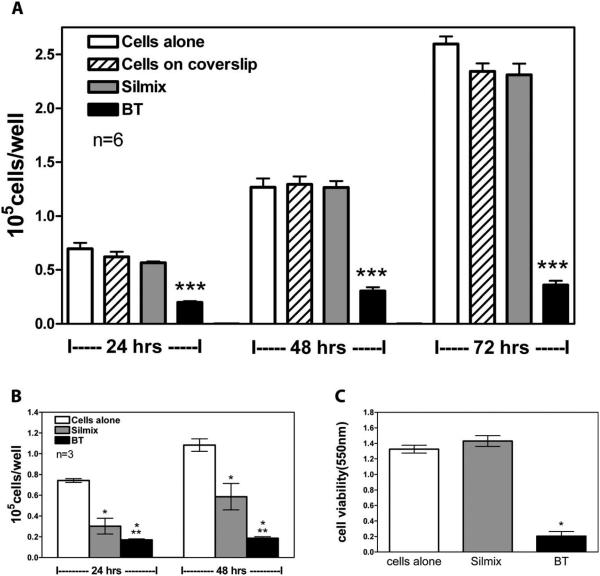
The chemical structure of the silorane that was used in this study is shown in Figure 1. The degree of polymerization of the SilMix and the BT resins can be found in Figure 2. Peak ratios of a spectral peak associated with polymerization (883 cm−1 representing ring-opening in siloranes) with an unchanging peak (1257 cm−1 in curing siloranes) were calculated for each material. For the BT specimens, degree of polymerization was calculated, based on the 1637 cm−1 (C =C) associated with polymerization with respect to 1714 cm−1 (C =O). For the first set of in vitro experiments, resin drops were placed either in the center or off-center in culture wells. No significant differences were observed depending on drop placement. The cell number was higher in the wells containing silorane resin drops at 24, 48, and 72 h as compared to a greater than 50% reduction in methacrylate BT containing wells [Figure 3(A)]. In contrast to the resin drop cultures, there were pronounced decreases in cell numbers for cells grown on the polymer disc surfaces [Figure 3(B)]. In order to test if this effect was due to toxic leachables, extraction of the disc resins was performed. The amount of formazan produced by cells in the presence of SilMix disc extracts was similar to levels of formazan produced by control cells (tissue culture grade polystyrene conditioned media); however, BT resin extracts generated considerable toxicity [Figure 3(C)]. This shows that no toxic component was released by the SilMix resin in contrast to the BT resin.
FIGURE 2.
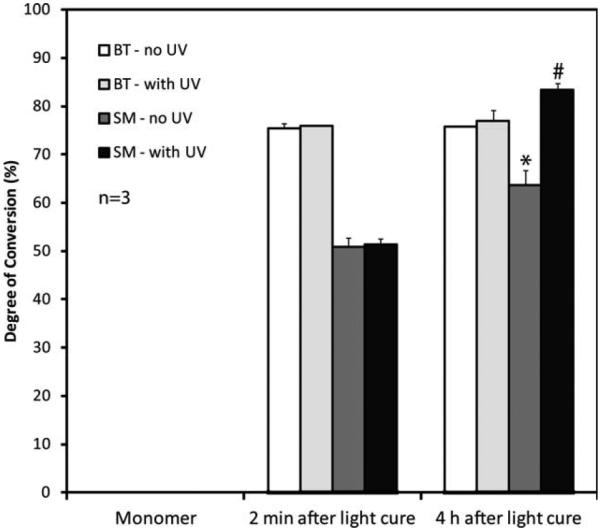
Overall degree of conversion for bisGMA/TEGDMA (BT) without ultraviolet light, UV (open bars) or with UV sterilization treat ment (dotted bars) and SilMix (SM) polymers without UV (hatched bars) and with UV (gray bars). The DC of 4 h after light cure and UV sterilized polymers are representative of the DC of discs used for the cell proliferation tests (n = 3). *Significant change (p < 0.05) in the DC of the SM without and with UV light treatment at 4 h, as well as the DC of the SM from 2 min to 4 h. #Significant increase (p < 0.05) in the DC of SM with UV relative to BT with UV using three way ANOVA.
FIGURE 3.
Effects of silorane and BT resins and resin leachables on MLO A5 cell proliferation. Effects on cell number using resin drops (A) and resin discs (B). Silorane and BT disc media conditioned for 48 h added to MLO A5 cells (C). ***Significantly different from SilMix (p < 0.001); *significantly different from cells alone (p < 0.05; n = 3); **significantly different from SilMix (p < 0.05) using one way ANOVA and Tukey post test (n = 3).
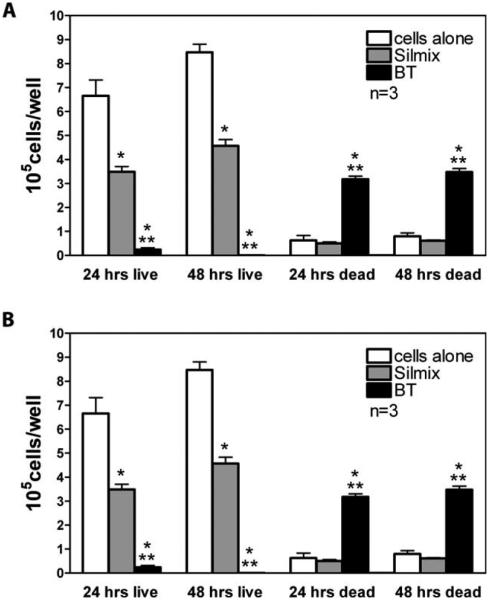
Using the trypan blue dye exclusion method, the number of live cells on SilMix surfaces were significantly lower (p < 0.05) than the controls [Figure 4(A)]. The number of dead cells were also lower but not significantly different from the number of dead cells in the controls. Upon calculation of the percent of live and dead cells, the percentage of live cells obtained with SilMix was similar to the controls [Figure 4(B)]. However, the number and percent of live cells for BT was very low at 24 h with most cells dead at 48 h.
FIGURE 4.
Effects of silorane and bisGMA/TEGDMA (BT) resins on MLO A5 cell proliferation and attachment to polymer disc surfaces. (A) Effects of SilMix and BT on live and dead cell number. (B) In com parison to BT, most of the cells in wells with SilMix were viable with a percentage of live cells greater than 87% and similar to the controls. Compared to the respective time, *significantly different from cells alone (p < 0.05); **significantly different from SilMix (p < 0.05) using one way ANOVA and Tukey post test.
Because the cells did not adhere well to the resin discs, the resin drop approach was used to examine osteoblast differentiation and function. Collagen type 1 is essential for the normal mineralization of bone.28 Immunofluorescent staining for type 1 collagen was performed on MLO-A5 cells at day 6 of culture (Figure 5). The pattern is clearly fibrillar at 6 days and of greater intensity in the silorane containing culture (right panel) compared to control (left panel) [Figure 5(A)]. Alkaline phosphatase activity at 6 days was significantly higher in culture wells with silorane than in the control [Figure 5(B)].
FIGURE 5.

Effects of silorane on collagen matrix formation and alkaline phosphatase. Immunohistochemical staining of collagen type 1 fibers in the control (left) and SilMix (right) revealed an intact collagen network that appeared thicker in the SilMix resin drop cultures (A). (×10 magnification). No negative effects were observed on collagen matrix formation at 6 days of culture. At 6 days, significantly elevated alkaline phospha tase was observed in the SilMix containing cultures compared to the control. No alkaline phosphatase was detected in the BT cultures at 6 days (B). *Significantly different from cells alone (p < 0.05) and from BT (p < 0.001) using one way ANOVA and Tukey post test. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
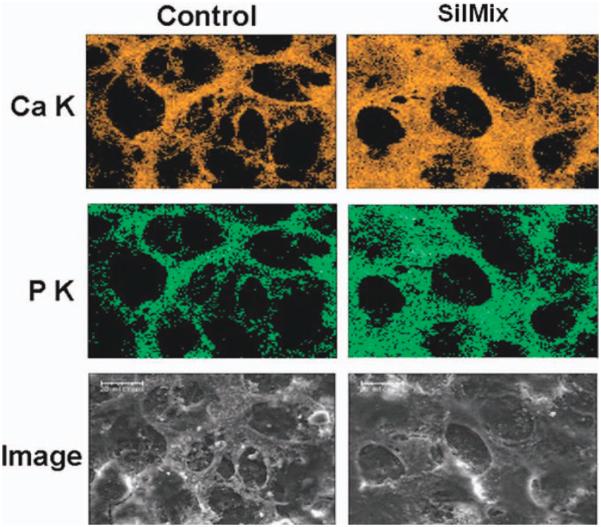
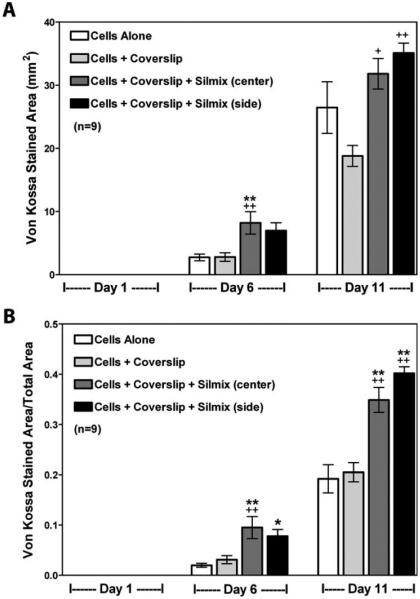
Analysis of the ultrastructure of the cultures using SEM was also performed [Figure (6)]. The top row shows the cells after 24 h in culture. In contrast to the cells cultured in the BT-containing wells, which were dying at 24 h and absent by 48 h, the control cultures (left panel), as well as silorane containing cultures(right panel), showed high cell confluency. After 6 days under mineralization conditions, the cells in the silorane containing cultures appeared similar to controls, with a well-formed honeycomb-like matrix. These cells cultured up to 10 days showed the formation of a mineralized matrix covering the entire well. The mineralized honeycomb-like matrix formed by the MLO-A5 cells in the presence of silorane was analyzed for mineral content using energy dispersive spectroscopy (EDS) to obtain calcium and phosphorus distribution maps (Figure 7). Calcium (A) and phosphorus (B) were co-localized within the mineralized structures of the matrix (C-SEM). Quantification of mineralization was performed using both von Kossa (Figure 8) and Alizarin Red staining (Figure 9). Von Kossa detects phosphate, whereas alizarin red detects calcium.29 As can be seen in Figure 8, von Kossa staining increased with extended time in culture in the control wells and the wells containing silorane. There was a complete absence of staining in the wells containing BT.
FIGURE 6.
Secondary electron micrographs of MLO A5 cells cultured for 24 and 48 h, and 6 and 10 days on cover slips with resin drops. At 24 h, the morphology of cells exposed to the SilMix appears normal, but with some membrane ruffling. The BT exposed cells appear necrotic. By 48 h, no cells are visible in the BT cultures. In contrast, the SilMix cultures show normal cell growth and matrix formation and appear healthy at 10 days of culture (scale bars = 100 μm except for 6 days at 20 lm).
FIGURE 7.
Elemental analysis (EDS) of the mineralized honeycomb like matrix formed by the MLO A5 cells at 11 days of culture on cover slips with resin drops. The calcium component pattern colorized as orange (Ca K) completely matches or overlays with the phosphate component pattern colorized as green (P K) and is consistent with the micrograph image (Image). Again, by this approach the cell matrix formed in the Sil Mix containing wells appears more mineralized (scale bars = 20 μm). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
FIGURE 8.
Quantitation of mineral formed in cultures on cover slips with resin drops using von Kossa staining. A good correlation is observed between each stain for phosphate in (A) and per total area measured in (B). +Significantly different from cells + coverslip (p < 0.05); *significantly different from cells alone (p < 0.05); ++significantly different from cells + coverslip (p < 0.01); **significantly different from cells alone (p < 0.01) using one way ANOVA and Tukey post test.
FIGURE 9.

Quantitation of mineral formed in cultures using Alizarin red staining for calcium at 6 and 10 days on cover slips with resin drops. Whereas the BT treated cultures had no detectable mineral, surprisingly, the SilMix cultures had greater staining for mineral than control cultures. Neither of the cover slips with just the resins, exhib ited any background staining. *Significantly different from cells alone (p < 0.01); **Significantly different from BT + cells (p < 0.001) using one way ANOVA and Tukey post test.
In these cultures in which the resin was centered in the well, the mineralized matrix appeared to build up around the silorane resin drop. Next, mineralization assays were performed on cells grown on Thermanox discs with the silorane drop placed in the center as compared to the sides of the disc. No significant effects were observed on mineralization whether quantified using the total stained area or the total stained area divided by the total area in the well which included the resin drop (Figures 8 and 9). No significant difference was found whether the drop was placed in the center or at the side of the coverslip. Therefore, the position of the resin had no effect on cellular differentiation and mineralization.
Radiographic assessment of osteotomies of femora (transverse straight-line fracture) was performed by X-ray at days 0, 7, 14, 21, and 28 post-operation. Radiographs postsurgery showed that there was no sign of displacement of the femoral fracture at 28 days (Figure 10). The fracture gap became opaque, and no external callus was observed at 28 days postsurgery (other time points are not shown). The osteotomized femurs were encased by SilMix resin, and there was no displacement of the stabilized fractured bones. Furthermore, no inflammatory response was observed around the fracture sites (Figure 11). One to two layers of cells were observed between the bone and material at day 7 postsurgery (Figure 11). The granulation tissue containing fibroblasts and interspersed small blood vessels were seen between the muscle tissue and biomaterial. Histological evaluation showed that there was no sign of displacement of the femoral fracture at 28 days postsurgery (Figure 12). SilMix resin residue was observed around the fracture site. The fracture gap was filled by newly formed woven bone. Blood vessels were present in the newly formed woven bone area. No inflammatory reaction or external callus was observed at the fracture site.
FIGURE 10.
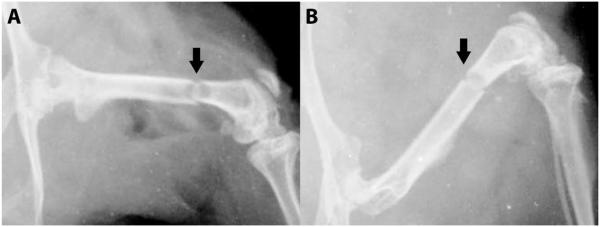
Radiographs of the SilMix resin stabilized murine femori at day 0 (A) and 28 (B) postsurgery. There was no sign of displacement of the femoral fracture (arrow). The fracture gap was coalescing at 28 days (magnification = ×2).
FIGURE 11.
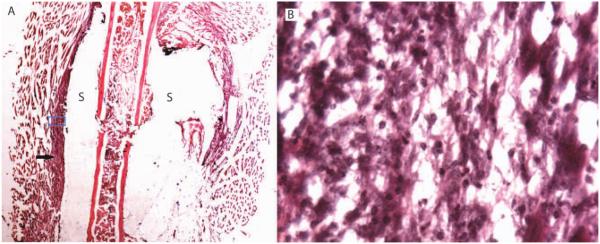
Histological section showing SilMix resin (S) encasing the murine osteotomized femur. The arrow shows granulation tissue between muscle tissue and biomaterial and filling the fracture gap (A). No inflammatory response was observed at 7 days postsurgery. The blue box is the area of magnification as reflected in B (original magnification: A, ×1; B, ×40).
FIGURE 12.
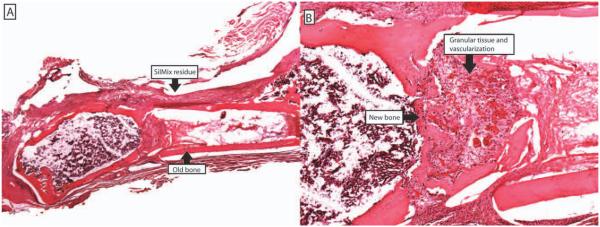
Histological section showing SilMix resin residue around the fracture site, newly formed woven bone filling the fracture gap, many blood vessels present in the newly formed woven bone area at 28 days. No inflammatory reaction was observed (original magnification: A, ×1; B, ×4).
DISCUSSION
We have previously developed silorane-based resins superior to methacrylate-based resins based on enhanced biocompatibility and significantly less polymerization stress without an associated proportional reduction in mechanical properties.7,8 Siloranes are now being used as matrix resins to produce low stress/shrinkage dental composites with reduced cytotoxicity and genotoxicity.12 Clearly, the siloranes are superior with respect to a lower exothermic temperature, less shrinkage, and less toxicity, compared to methacrylates representing a major step forward for use beyond dental composites. Therefore, we hypothesized that siloranes may be an improvement and serve as replacements of methacrylates used in orthopedic applications, such as bone cements, and performed in vitro and in vivo experiments to begin to test this hypothesis.
In this study, we demonstrated that silorane-based resins are nontoxic to bone cells and support parameters of bone formation both in vitro and in vivo. The mineralized, formed matrix was composed of collagen type I in a honey-comb-shaped structure, and the mineral component contained calcium and phosphate in a normal ratio compared to controls and normal bone. Surprisingly, our experiments showed that regardless of placement of silorane in culture wells the bone cells mineralized similarly, if not to a greater extent than control wells. This result may be due to the fact that the resin drop itself displaced surface area and increased cell number per area. Whereas this could explain the increase in mineralized matrix, it could not explain the increase in alkaline phosphatase. Further experiments are required to validate this observation.
Surfaces on prosthetic devices and bone cements can have either beneficial or detrimental effects on bone cells.30 Many materials have ideal structural properties to function as implants, cements, or scaffolds but do not have the necessary biocompatibility or bioactivity. Conversely, many materials have neutral or enhancing biological properties but lack the necessary mechanical properties. Materials can be toxic, neutral, or can even support bone growth, especially with the inclusion of growth factors.31 In this study, low bone cell attachment to silorane surfaces was observed. We have shown that surfaces can modulate the differentiation of osteoblasts,32 and Boyan and coworkers have shown that surface roughness and microtopography plays a role in bone cell function and mineralization.33,34 Therefore, biocompatibility and induction of bone growth become important issues. Even though low bone cell attachment was observed, this property could be improved using approaches such as surface etching.
Also, in this study, we demonstrated that when the silorane resin was placed around a bone defect, a femoral osteotomy, no inflammation was observed. Surprisingly, at 28 days, the bone began to heal in the absence of callus. This result raises the question as to whether this approach could be used in patients to stabilize bone. Pediatric orthopedic surgeons are often faced with fracture situations where the bone is either too small to support plate stabilization, or too close to the physis such that fracture stability cannot be achieved without jeopardizing the integrity of the physis. An inert stabilizer that is not toxic to physeal cartilage could be ideal in this setting. Other fracture applications might include patients with severely osteoporotic bone, where screws may not achieve good integration, or in patients with significant contractures (as in cerebral palsy or stroke) that do not allow for standard nail or plate insertion without creating further injury. Other uses include battlefield situations or natural disasters, where fractures could be stabilized before transport for more permanent treatment. This material would be easy to remove from the fracture site for definitive treatment. Therefore, in addition to being a substitute for methyl methacrylate in bone cement, silorane resin could function as a bone stabilizer. These concepts are undergoing further testing in our laboratory.
CONCLUSION
In conclusion, bisGMA/TEGDMA was toxic and totally inhibited bone cell growth while the low toxicity of silorane resin supported bone cell differentiation, matrix formation, and mineralization. In addition, all of the experimental methods, such as von Kossa and Alizarin red staining, and the SEM and EDS analyses, were in agreement and complementary with regard to quantitation and mineral composition. These studies clearly show that the silorane is superior to the bisGMA/TEGDMA with regard to support of bone cell function and has the potential to be used as a component of bone cement or as a bone-stabilizing material.
ACKNOWLEDGMENTS
We thank Dr. James Hamilton at the School of Medicine and Dr. Donna Pacicca at Children’s Mercy Hospital, Kansas City for their insight and useful discussions.
Contract grant sponsor: National Institute of Dental and Craniofacial Research; contract grant numbers: P03DE09696, DE07294, DOD W81XWH-07-1-0696, MoLSRB 13234
REFERENCES
- 1.Lewis G. Alternative acrylic bone cement formulations for cemented arthroplasties: present status, key issues, and future prospects. J Biomed Mater Res B Appl Biomater. 2008;84:301–319. doi: 10.1002/jbm.b.30873. [DOI] [PubMed] [Google Scholar]
- 2.Ritter MA, Gioe TJ, Sieber JM. Systemic effects of polymethylmethacrylate: increased serum levels of gamma-glutamyltranspeptidase following arthroplasty. Acta Orthop Scand. 1984;55:411–413. doi: 10.3109/17453678408992385. [DOI] [PubMed] [Google Scholar]
- 3.Peebles DJ, Ellis RH, Stride SD, Simpson BR. Cardiovascular effects of methylmethacrylate cement. Br Med J. 1972;1:349–351. doi: 10.1136/bmj.1.5796.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donaldson AJ, Thomson HE, Harper NJ, Kenny NW. Bone cement implantation syndrome. Br J Anaesth. 2009;102:12–22. doi: 10.1093/bja/aen328. [DOI] [PubMed] [Google Scholar]
- 5.Peszkowski J. Intraoperative complications connected with the use of bone cement. Anaesth Resusc Intensive Ther. 1974;2:71–76. [PubMed] [Google Scholar]
- 6.Vallo CI, Schroeder WF. Properties of acrylic bone cements formulated with Bis-GMA. J Biomed Mater Res B Appl Biomater. 2005;74:676–685. doi: 10.1002/jbm.b.30211. [DOI] [PubMed] [Google Scholar]
- 7.Guggenberger R, Weinmann W. Exploring beyond methacrylates. Am J Dent. 2000;13:82D–84D. [PubMed] [Google Scholar]
- 8.Eick JD, Smith RE, Pinzino CS, Kostoryz EL. Stability of silorane dental monomers in aqueous systems. J Dent. 2006;34:405–410. doi: 10.1016/j.jdent.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Braga RR, Ferracane JL. Alternatives in polymerization contraction stress management. Crit Ev Oral Biol Med. 2004;15:176–184. doi: 10.1177/154411130401500306. [DOI] [PubMed] [Google Scholar]
- 10.Weinmann W, Thalacker C, Guggenberger R. Siloranes in dental composites. Dent Mater. 2005;21:68–74. doi: 10.1016/j.dental.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Belfield KD, Zhang G. Photoinitiated cationic ring-operated polymerization of a cyclosiloxane. Polym Bull. 1997;38:165–168. [Google Scholar]
- 12.Kostoryz EL, Wetmore LA, Brockmann WG, Yourtee DM, Eick JD. Genotoxicity assessment of oxirane-based dental monomers in mammalian cells. J Biomed Mater Res A. 2004;68:660–667. doi: 10.1002/jbm.a.20077. [DOI] [PubMed] [Google Scholar]
- 13.Schweikl H, Schmalz G, Weinmann W. Mutagenic activity of structurally related oxiranes and siloranes in Salmonella typhimurium. Mut Res Gen Toxicol Environ Mut. 2000;521:19–27. doi: 10.1016/s1383-5718(02)00207-3. [DOI] [PubMed] [Google Scholar]
- 14.Schweikl H, Schmalz G, Weinmann W. The induction of gene mutations and micronuclei by oxiranes and siloranes in mammalian cells in vitro. J Dent Res. 2004;83:17–21. doi: 10.1177/154405910408300104. [DOI] [PubMed] [Google Scholar]
- 15.Palin WM, Fleming GJ, Nathwani H, Burke FJ, Randall RC. In vitro cuspal deflection and microleakage of maxillary premolars restored with novel low-shrink dental composites. Dent Mater. 2005;21:324–335. doi: 10.1016/j.dental.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Alster D, Feilzer AJ, de Gee AJ, Davidson CL. Polymerization contraction stress in thin resin composite layers as a function of layer thickness. Dent Mater. 1997;13:146–150. doi: 10.1016/S0109-5641(97)80115-7. [DOI] [PubMed] [Google Scholar]
- 17.Feilzer AJ, de Gee AJ, Davidson CL. Quantitative determination of stress reduction by flow in composite restorations. Dent Mater. 1990;6:167–171. doi: 10.1016/0109-5641(90)90023-8. [DOI] [PubMed] [Google Scholar]
- 18.Holder AJ, Kilway KV, Code JE, Giese GJ, Travis DM, Fleckenstein JE, Marzulf KR, Clevenger RC, Vastlik HL, Eick JD, Chappelow CC. Toward a cohesive theory of polymerization volume change 1: general requirements and oxiranes. Macromol Theo Sim. 2005;14:117–124. [Google Scholar]
- 19.Rokicki G. Aliphatic cyclic carbonates and spiroorthocarbonates as monomers. Prof Polym Sci. 2000;25:259–342. [Google Scholar]
- 20.Sadhir RK, Luck RM. Expanding monomers: synthesis, characterization, and applications. In: Sadhir RK, Luck RM, editors. Expanding Monomers: Synthesis, Characterization, and Applications. CVC Press; Boca Raton, FL: 1992. [Google Scholar]
- 21.Wu X, Watts DC. Bonding of a silorane-based composite system to bone. Adv Eng Mater. 2009;11:B204–B208. [Google Scholar]
- 22.Kato Y, Boskey A, Spevak L, Dallas M, Hori M, Bonewald LF. Establishment of an osteoid preosteocyte-like cell MLO-A5 that spontaneously mineralizes in culture. J Bone Miner Res. 2001;16:1622–1633. doi: 10.1359/jbmr.2001.16.9.1622. [DOI] [PubMed] [Google Scholar]
- 23.Barragan Adjemian C, Nicolella D, Dusevich V, Dallas MR, Eick JD, Bonewald LF. Mechanism by which MLO-A5 late osteoblasts/early osteocytes mineralize in culture: similarities with mineralization of lamellar bone. Calcif Tissue Int. 2006;79:340–353. doi: 10.1007/s00223-006-0107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chappelow CC, Pinzino CS, Holder AJ, Chen SS, Eick JD. Expandable monomer silicon analogs and siloranes I: formulation and photopolymerization. J Dent Res. 2005;84:1466. [Google Scholar]
- 25.Eick JD, Kotha SP, Chappelow CC, Holder AJ, Kilway KV, Pinzino CS. Expandable monomer silicon analogs and siloranes II: physical properties testing. J Dent Res. 2005;84:1467. [Google Scholar]
- 26.Crivello JV, Bi D. The synthesis and cationic polymerization of multifunctional silicon-containing epoxy monomers and oligomers. J Polym Sci Part A: Polym Chem. 1994;32:683–697. [Google Scholar]
- 27.Aoki S. Preparation of Epoxy Group-Bearing Organopolysiloxane or Organosilane. 2001. US Patent6,255,428.
- 28.Landis WJ. An overview of vertebrate mineralization with emphasis on collagen-mineral interaction. Gravit Space Biol Bull. 1999;12:15–26. [PubMed] [Google Scholar]
- 29.Bonewald LF, Harris SE, Rosser J, Dallas MR, Dallas SL, Camacho NP, Boyan B, Boskey A. von Kossa staining alone is not sufficient to confirm that mineralization in vitro represents bone formation. Calcif Tissue Int. 2003;72:537–547. doi: 10.1007/s00223-002-1057-y. [DOI] [PubMed] [Google Scholar]
- 30.Luk AS, Winet H, Bao JY. Effect of polymethylmethacrylate particles on mature bone in the optical bone chamber. J Biomed Mater Res. 2001;55:177–184. doi: 10.1002/1097-4636(200105)55:2<177::aid-jbm1004>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 31.Braddock M, Houston P, Campbell C, Ashcroft P. Born again bone: tissue engineering for bone repair. News Physiol Sci. 2001;16:208–213. doi: 10.1152/physiologyonline.2001.16.5.208. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz Z, Lohmann CH, Oefinger J, Bonewald LF, Dean DD, Boyan BD. Implant surface characteristics modulate differentiation behavior of cells in the osteoblastic lineage. Adv Dent Res. 1999;13:38–48. doi: 10.1177/08959374990130011301. [DOI] [PubMed] [Google Scholar]
- 33.Boyan BD, Sylvia VL, Liu Y, Sagun R, Cochran DL, Lohmann CH, Dean DD, Schwartz Z. Surface roughness mediates its effects on osteoblasts via protein kinase A and phospholipase A2. Biomaterials. 1999;20:2305–2310. doi: 10.1016/s0142-9612(99)00159-3. [DOI] [PubMed] [Google Scholar]
- 34.Lohmann CH, Sagun R, Jr, Sylvia VL, Cochran DL, Dean DD, Boyan BD, Schwartz Z. Surface roughness modulates the response of MG63 osteoblast-like cells to 1,25-(OH)(2)D(3) through regulation of phospholipase A(2) activity and activation of protein kinase A. J Biomed Mater Res. 1999;47:139–151. doi: 10.1002/(sici)1097-4636(199911)47:2<139::aid-jbm4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]