Abstract
Objective
Sodium retention occurs commonly in cardiac and liver disease, requiring the administration of diuretics to restore fluid balance. Whether obesity is associated with sodium retention has not been fully evaluated.
Methods
In a large single-center cohort of critically ill patients, we evaluated whether admission body mass index was associated with the administration of either oral or intravenous diuretics during the intensive care unit (ICU) stay.
Main results
Of 7724 critically ill patients, 3946 (51.1%) were prescribed diuretics during the ICU stay. Overweight, class I obesity, and class II/III obesity were associated with a 1.35 (95% confidence interval [CI], 1.20–1.53; P < .001), 1.56 (95% CI, 1.35–1.80; P < .001), and 1.91 (95% CI, 1.61–2.26; P < .001) adjusted risk of receiving diuretics within the ICU, respectively. In adjusted analysis, a 5-kg/m2 increment of body mass index was associated with a 1.19 (95% CI, 1.14–1.23; P < .001) increased adjusted risk of within-ICU diuretics. Among those patients receiving loop diuretics, obese patients received significantly larger daily diuretic doses.
Conclusion
Critically ill obese patients are more likely to receive diuretics during their stay in the ICU and to receive higher dosages of diuretics. Our data suggest that obesity is an independent risk factor for sodium retention.
Keywords: Sodium retention, Fluid balance, Loop diuretics BMI
1. Introduction
Sodium retention commonly occurs in patients with cardiac, renal, and liver disease, primarily through activation of the renin-angiotensin-aldosterone system, and is associated with hypertension, pulmonary congestion, and mortality [1–6]. Obesity is associated with physiologic mechanisms that lead to sodium retention, including overactivation of the sympathetic nervous system and the renin-angiotensin-aldosterone axis, leading to renal tubular sodium reabsorption [7–13]. However, whether obesity is clinically associated with sodium retention has not been well described.
Diuretics are the mainstay therapy in patient populations to prevent the complications of sodium retention. Diuretics, by blocking renal sodium reabsorption, can restore normal body fluid volume but have also been associated with kidney injury [14]. Given the widespread use of intravenous fluids during critical illness, we hypothesized that obese patients would be at greater risk for sodium retention, thereby requiring diuretic administration within the intensive care unit (ICU). Because of unquantifiable fluid gains and losses during critical illness, including respiration, gastrointestinal loss, and oral intake, we chose to use the administration of diuretics as the primary end point and secondarily analyzed peak fluid balance and total ICU fluid balance as additional outcomes. Using a large single-center conception cohort of critically ill patients, we evaluated whether admission body mass index (BMI) was associated with the administration of diuretics during the ICU stay, controlling for demographics, measures of severity of illness, and medical comorbidities.
2. Methods
2.1. Study population
We used the publicly available Multiparameter Intelligent Monitoring in Intensive Care II research database managed by the Laboratory for Computational Physiology at Massachusetts Institute of Technology (MIT) and at the Beth Israel Deaconess Medical Center (BIDMC) [15]. BIDMC is a 700-bed urban academic medical center located in Boston, MA, USA, with 77 adult ICU beds. The database contains information obtained from clinical documentation such as laboratory results, electronic records, and bedside monitor trends and waveforms for all individuals admitted to a BIDMC ICU between 2001 and 2008. The Institutional Review Boards of BIDMC and MIT have approved the use of the Multiparameter Intelligent Monitoring in Intensive Care II database for research.
During the period between 2001 and 2008, 23 455 patients were admitted to the ICU at BIDMC. Of these, 8491 had a recorded BMI. We excluded 767 individuals who lacked information on baseline demographics, comorbidities, severity of illness, and fluid balance, leaving a final sample size of 7724 ICU patients.
2.2. Outcome
The primary outcome was the administration of either oral or intravenous diuretics at any time after ICU admission, as indicated by the electronic provider order entry system. As a secondary outcome, we evaluated other markers of fluid retention, including fluid balance at time of ICU discharge and highest positive fluid balance volume that occurred at any point during the ICU stay. Information on fluid balance was recorded by nursing staff into the bedside electronic flow sheet.
2.3. Exposure
The primary exposure was BMI as recorded on admission, categorized according to the World Health Organization classification [16]—less than 18.5 (underweight), 18.5 to 24.9 (normal), 25.0 to 29.9 (overweight), 30.0 to 34.9 (obesity class I), and greater than 35 (obesity classes II and III were combined into 1 group)—and as a continuous variable per 5 units of BMI (in kilograms per square meter). This variable is automatically calculated by the bedside electronic record using weight and height that were recorded on the day of admission.
Because weight at the time of critical illness might not reflect usual body weight, we performed a validation study among 150 randomly selected subjects within the data set to determine body weight measurements obtained during noncritical illness. Of the 150 individuals, 86 had documented weights during noncritical illness in the electronic medical record. The Spearman correlation coefficient between ICU and non-ICU body weight measurements was 0.93 (P < .001), with a median difference of −0.03 kg (interquartile range, −2.8 to 2.9).
2.4. Covariates
Demographic information included age, sex, and race, coded as White, African American, Asian, Hispanic, other, or unknown. Medical comorbidities were determined by Elixhauser discharge coding except for “obesity” [17]. Intensive care unit types included cardiac, surgical, cardiothoracic, and medical units. Predictors of illness severity included admission Simplified Acute Physiology Score (SAPS II) [18].
2.5. Statistical analysis
Baseline characteristics are presented stratified by BMI category, with group differences assessed by analyses of variances. Exposure and outcome measures (BMI, daily urine output, discharge fluid balance, and peak fluid balance) were winsorized at the 0.5 and 99.5 percentiles to limit the effect of outliers.
We used logistic regression to assess the relationship between BMI category and ICU diuretics use, using the normal BMI category as reference. We also examined BMI as a continuous variable, per 5-kg/m2 increment. We adjusted for age, sex, and race (model 1) and added Elixhauser comorbidities, ICU type, and SAPS score as covariates in model 2. Race and ICU type were included as multicategory variables. Age and SAPS score were included as continuous variables. In secondary analyses, we examined whether BMI was associated with discharge fluid balance and peak fluid balance.
To determine whether preadmission diuretic use affected the association between obesity and ICU diuretic administration, we performed a sensitivity analysis of those patients with an identifiable prehospitalization medication record (n = 6055). Using natural language processing to identify admission medication sections of discharge summaries [19], we examined whether the association between BMI and within ICU diuretic use was independent of preadmission diuretic use.
In addition, among patients who received loop diuretics (n = 3874), we examined whether BMI was associated with cumulative loop diuretic dosage throughout the ICU stay [20]. Loop diuretic dosage was winsorized at the 0.5 and 99.5 percentiles to limit the effect of outliers.
In addition, to determine whether BMI was associated with urinary sodium avidity, we performed a sensitivity analysis of those patients (n = 402) with measured admission urine electrolytes. We examined whether BMI was associated with a fractional excretion of sodium (FENA) less than 1%, in keeping with current definitions of renal sodium avidity, in unadjusted and adjusted (using all covariates from model 2) analyses.
Finally, because more aggressive fluid resuscitation occurs with treatment of sepsis, we examined whether the association between BMI and diuretic use was modified by an admission diagnosis of sepsis by entering an interaction term between sepsis and BMI into our adjusted model.
All analyses were performed using JMP Pro (SAS Institute, Cary, NC).
3. Results
Of the 7724 critically ill patients, 188 (2.4%) were underweight, 2328 (30.1%) were normal weight, 2737 (35.4%) were overweight, 1479 (19.1%) had class I obesity, and 992 (12.8%) had at least class II obesity. Obesity was associated with a higher prevalence of hypertension and diabetes than those with normal BMIs but without differences in admission blood pressures (Table 1). As seen in Table 1, patients in the highest obesity category tended to be younger and have lower SAPS scores; but their length of stay was similar.
Table 1.
Baseline characteristics stratified by BMI
| 1. BMI < 18.5
|
2. BMI 18.5–24.99
|
3. BMI 25.0–29.99
|
4. BMI 30.0–34.99
|
5. BMI ≥ 35.0
|
P | |
|---|---|---|---|---|---|---|
| n = 188 | n = 2328 | n = 2737 | n = 1479 | n = 992 | ||
| Demographics | ||||||
| Age, mean (SD), y | 68.3 (16.5) | 66.3 (17.3) | 65.1 (15.3) | 63.4 (13.5) | 60.2 (13.2) | <.001 |
| Female, n (%) | 131 (69.7) | 987 (42.4) | 832 (30.4) | 475 (32.1) | 440 (44.4) | <.001 |
| Race, n (%) | ||||||
| Asian | 18 (9.6) | 78 (3.4) | 36 (1.3) | 5 (0.3) | 3 (0.3) | <.001 |
| Black/African | 8 (4.3) | 111 (4.8) | 136 (5.0) | 79 (5.3) | 69 (7.0) | |
| Hispanic/Latino | 3 (1.6) | 45 (1.9) | 58 (2.1) | 37 (2.5) | 26 (2.6) | |
| Other | 5 (2.7) | 65 (2.8) | 57 (2.1) | 40 (2.7) | 19 (1.9) | |
| Unknown/unspecified | 27 (14.4) | 445 (19.1) | 556 (20.3) | 318 (21.5) | 185 (18.6) | |
| White | 127 (67.6) | 1584 (68.0) | 1894 (69.2) | 1000 (67.6) | 690 (69.6) | |
| Medical history, n (%) | ||||||
| Congestive heart failure | 45 (23.9) | 360 (15.5) | 409 (14.9) | 199 (13.5) | 209 (21.1) | <.001 |
| Cardiac arrhythmias | 27 (14.4) | 429 (18.4) | 517 (18.9) | 221 (14.9) | 190 (19.2) | .007 |
| Chronic pulmonary disease | 48 (25.5) | 376 (16.1) | 333 (12.2) | 219 (14.8) | 191 (19.3) | <.001 |
| Peripheral vascular disease | 28 (14.9) | 275 (11.8) | 315 (11.5) | 143 (9.7) | 106 (10.7) | .12 |
| Hypertension | 38 (20.2) | 638 (27.4) | 904 (33.0) | 500 (33.8) | 366 (36.9) | <.001 |
| Diabetes | 20 (10.6) | 439 (18.9) | 666 (24.3) | 502 (33.9) | 408 (41.1) | <.001 |
| Renal failure | 13 (6.9) | 104 (4.5) | 121 (4.4) | 45 (3.0) | 38 (3.8) | .053 |
| ICU type, n (%) | ||||||
| Cardiac | 22 (11.7) | 371 (15.9) | 475 (17.4) | 244 (16.5) | 154 (15.5) | <.001 |
| Cardiothoracic | 61 (32.4) | 1091 (46.9) | 1448 (52.9) | 809 (54.7) | 461 (46.5) | |
| Medical | 60 (31.9) | 374 (16.1) | 353 (12.9) | 185 (12.5) | 188 (19.0) | |
| Surgical | 45 (23.9) | 492 (21.1) | 461 (16.8) | 241 (16.3) | 189 (19.1) | |
| Admission values, mean (SD) | ||||||
| Systolic blood pressure, mm Hg | 120.2 (28.0) | 120.9 (24.9) | 120.6 (23.7) | 119.9 (24.3) | 120.3 (25.1) | .77 |
| Diastolic blood pressure, mm Hg | 60.5 (16.4) | 60.9 (14.3) | 61.7 (14.9) | 61.6 (14.2) | 61.7 (15.1) | .29 |
| Heart rate, beats per minute | 89.9 (20.1) | 87.1 (17.4) | 85.1 (16.7) | 85.4 (16.3) | 87.7 (16.8) | <.001 |
| Temperature, °F | 97.1 (5.1) | 96.9 (5.5) | 96.9 (5.6) | 97.0 (5.5) | 97.2 (6.3) | .75 |
| Oxygen saturation, % | 97.9 (5.6) | 98.0 (4.8) | 98.1 (5.0) | 98.0 (4.0) | 97.3 (6.6) | .001 |
| Admit SAPS, points | 16.0 (5.5) | 15.8 (5.4) | 15.2 (5.1) | 15.0 (5.2) | 14.8 (4.9) | <.001 |
| ICU values, mean (SD) | ||||||
| ICU length of stay (d) | 5.7 (7.6) | 4.8 (7.9) | 4.5 (8.3) | 4.5 (8.1) | 5.5 (7.8) | .004 |
| Urine output/first 24 h (L) | 2.1 (1.5) | 2.7 (1.9) | 2.8 (1.8) | 2.7 (1.5) | 2.5 (1.4) | <.001 |
| Daily urine output (L) | 1.9 (1.1) | 2.4 (1.3) | 2.5 (1.2) | 2.5 (1.1) | 2.4 (1.1) | <.001 |
| Peak fluid balance (L) | 3.5 (5.7) | 4.3 (6.3) | 4.5 (6.6) | 4.5 (6.8) | 4.4 (6.6) | .30 |
| Discharge fluid balance (L) | 4.1 (6.3) | 3.0 (6.0) | 2.7 (5.6) | 2.7 (5.8) | 3.2 (7.3) | .003 |
During the course of critical illness, 3946 (51.1%) patients were prescribed diuretics. In unadjusted and adjusted analyses, obesity was associated with an increased risk of ICU diuretic use (Table 2). Each 5-kg/m2 increment in admission BMI was associated with a 19% increased adjusted odds of ICU diuretic use (hazard ratio, 1.19; 95% confidence interval [CI], 1.14–1.23; P < .001).
Table 2.
Risk of diuretic use during critical illness according to BMI
| Risk of diuretic use during critical illness according to BMI
|
||||||
|---|---|---|---|---|---|---|
| BMI groups | Per 5 kg/m2 positive | |||||
| BMI < 18.5 | 18.5 ≥ BMI < 25 | 25 ≥ BMI < 30 | 30 ≥ BMI < 35 | BMI ≥ 35 | – | |
| ICU diuretic use, n (%) | 81 (43.1) | 1071 (46.0) | 1417 (51.8) | 804 (54.4) | 573 (57.8) | |
| Unadjusted | 0.89 (0.66–1.20) | 1.00 (Ref.) | 1.26 (1.13–1.41) | 1.40 (1.23–1.59) | 1.61 (1.38–1.87) | 1.13 (1.09–1.17) |
| P = .44 | P < .001 | P < .001 | P < .001 | P < .001 | ||
| Model 1a | 0.82 (0.61–1.12) | 1.00 (Ref.) | 1.32 (1.18–1.48) | 1.54 (1.34–1.76) | 1.89 (1.62–2.20) | 1.18 (1.14–1.22) |
| P = .22 | P < .001 | P < .001 | P < .001 | P < .001 | ||
| Model 2b | 0.81 (0.57–1.13) | 1.00 (Ref.) | 1.35 (1.20–1.53) | 1.56 (1.35–1.80) | 1.91 (1.61–2.26) | 1.19 (1.14–1.23) |
| P = .21 | P < .001 | P < .001 | P < .001 | P < .001 | ||
Adjusted for age, sex, and race.
Adjusted for age, sex, race, ICU type, admission SAPS, and Elixhauser comorbidities (all except for obesity).
There was no significant association between BMI and discharge fluid balance, peak fluid balance, and daily urine output. Of 6055 patients with a recorded admission medication section, 2317 (38.3%) were prescribed a diuretic before hospitalization. Inclusion of premorbid diuretic use as a covariate did not affect the association of BMI with ICU diuretic use.
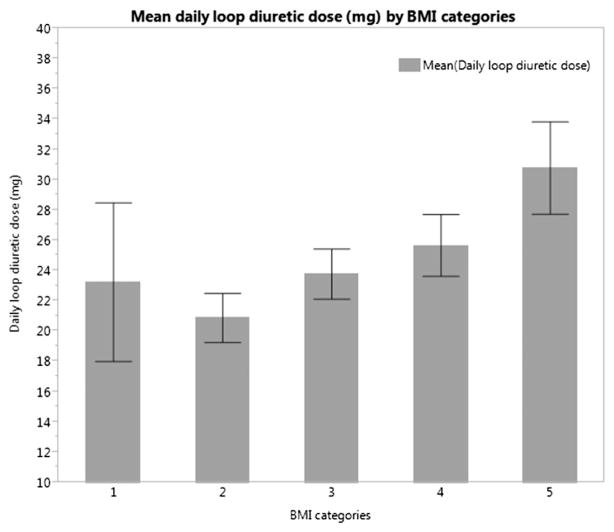
Of 3874 patients who received loop diuretics, BMI was associated with increased daily diuretic dosage (Figure). Overweight, class I obesity, and class II/III obesity were associated with a 4.18-mg (95% CI, 1.83–6.52; P < .001), 6.01-mg (95% CI, 3.28–8.74; P < .001), and 11.47-mg (95% CI, 8.38–14.57; P < .001) higher daily loop diuretic dose than those with a normal BMI. A 5-kg/m2 increase in BMI was associated with a 2.74-mg higher daily diuretic dose (95% CI, 2.00–3.47; P < .001).
Figure.
Mean daily loop diuretic dose by BMI categories (with 95% CI).
In those patients with a measured FENA on admission to the ICU, a 5-kg/m2 increase in BMI was associated with a 1.19 (1.05–1.33; P = .007) unadjusted and 1.21 (1.04–1.40; P = .009) adjusted risk of FENA less than 1%, suggestive of marked sodium avidity. The indication for why urine electrolytes were measured was not known. Finally, the association between obesity and diuretic use was not modified by an admission for sepsis (multiplicative interaction P value = .86).
4. Discussion
In this large single-center study on critically ill patients, obese patients were more likely to be administered diuretics and to receive larger doses of diuretics. Our findings further support obesity as a sodium retentive state [21,22]. We could not detect an association between obesity and peak or discharge fluid balance possibly because of the effect of diuretic use; but in a small subcohort with measured urinary electrolytes, obesity was associated with an increased risk of a FENA less than 1%.
Given the physiologic perturbations associated with obesity, including insulin resistance, altered left ventricular remodeling [23,24], oxidative stress [25–27], and heart failure [28], traditional cardiovascular risk factors and disease might explain the sodium retentiveness of obesity. However, obesity-specific factors, such as abnormalities in circulating adipokines and sympathetic nervous system activation, which are known risk factors for renal failure [13,29–31], might also contribute. In addition, the mechanical complications of obesity, including pulmonary hypertension and cor pulmonale, lead to renin activation, sodium retention, and peripheral edema.
The clinical significance of increased fluid retentiveness remains an area of ongoing research; but studies in critical illness have shown an increased risk of hypertension, arrhythmia, congestive heart failure, and mortality [6,32–36], which also extends to other patient populations, including septic shock [37], cancer [38], and lung injury [39]. To date, no study has examined the clinical consequence of fluid retention in obesity. Because increased diuretic use theoretically could lead to renal injury [14,40], whether the higher usage of diuretics in obese patients is associated with renal outcomes remains an important question.
This study has several limitations. First of all, the cross-sectional nature of our analysis limits any conclusions of causality. Secondly, despite the fact that we analyzed a large cohort and that we adjusted for many covariates, it is possible that residual confounding persists. Furthermore, because our database consisted of critically ill patients who were admitted in the ICU, we cannot generalize our findings to a noncritically ill population. Strikingly, body weights and heights were not known for more than half of the patients in our database; and diuretic use was not recorded for all patients either.
In conclusion, we found that critically ill obese patients are more likely to receive diuretics during their stay in the ICU and to receive higher dosages of loop diuretics. These findings suggest that obese patients are more likely to retain fluid and thus need careful attention to their fluid status. However, further studies are needed to validate this conclusion.
Abbreviations
- ICU
intensive care unit
- BMI
body mass index
- BIDMC
Beth Israel Deaconess Medical Center
- MIT
Massachusetts Institute of Technology
- SAPS
Simplified Acute Physiology Score
- CI
confidence interval
Footnotes
Conflict of interest: The contributing authors declare that there was no conflict of interest.
Funding: Dr Celi’s work in the Laboratory for Computational Physiology at MIT is funded by the National Institute of Biomedical Imaging and Bioengineering under grant 2R01 EB001659. Dr Danziger is supported by a Normon S. Coplon Extramural Grant from Satellite Healthcare. Dr Feng is supported by an Agency for Science, Technology, and Research (A*STAR) Graduate Scholarship. This work is supported by National Institutes of Health grant R01 EB001659.
References
- 1.Selewski DT, Cornell TT, Lombel RM, Blatt NB, Han YY, Mottes T, et al. Weight-based determination of fluid overload status and mortality in pediatric intensive care unit patients requiring continuous renal replacement therapy. Intensive Care Med. 2011;37:1166–73. doi: 10.1007/s00134-011-2231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaney E, Shaw A. Pathophysiology of fluid retention in heart failure. Contrib Nephrol. 2010;164:46–53. doi: 10.1159/000313720. [DOI] [PubMed] [Google Scholar]
- 3.Kalantar-Zadeh K, Regidor DL, Kovesdy CP, Van Wyck D, Bunnapradist S, Horwich TB, et al. Fluid retention is associated with cardiovascular mortality in patients undergoing long-term hemodialysis. Circulation. 2009;119:671–9. doi: 10.1161/CIRCULATIONAHA.108.807362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wabel P, Moissl U, Chamney P, Jirka T, Machek P, Ponce P, et al. Towards improved cardiovascular management: the necessity of combining blood pressure and fluid overload. Nephrol Dial Transplant. 2008;23:2965–71. doi: 10.1093/ndt/gfn228. [DOI] [PubMed] [Google Scholar]
- 5.Slagman MCJ, Waanders F, Hemmelder MH, Woittiez A-J, Janssen WMT, Lambers Heerspink HJ, et al. Moderate dietary sodium restriction added to angiotensin converting enzyme inhibition compared with dual blockade in lowering proteinuria and blood pressure: randomised controlled trial. BMJ. 2011;343:1–10. doi: 10.1136/bmj.d4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouchard J, Soroko SB, Chertow GM, Himmelfarb J, Ikizler TA, Paganini EP, et al. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009;76:422–7. doi: 10.1038/ki.2009.159. [DOI] [PubMed] [Google Scholar]
- 7.Vaz M, Jennings G, Turner A, Cox H, Lambert G, Esler M. Regional sympathetic nervous activity and oxygen consumption in obese normotensive human subjects. Circulation. 1997;96:3423–9. doi: 10.1161/01.cir.96.10.3423. [DOI] [PubMed] [Google Scholar]
- 8.Scherrer U, Randin D, Tappy L, Vollenweider P, Jéquier E, Nicod P. Body fat and sympathetic nerve activity in healthy subjects. Circulation. 1994;89:2634–40. doi: 10.1161/01.cir.89.6.2634. [DOI] [PubMed] [Google Scholar]
- 9.Grassi G, Seravalle G, Cattaneo BM, Bolla GB, Lanfranchi A, Colombo M, et al. Sympathetic activation in obese normotensive subjects. Hypertension. 1995;25:560–3. doi: 10.1161/01.hyp.25.4.560. [DOI] [PubMed] [Google Scholar]
- 10.Boustany CM, Bharadwaj K, Daugherty A, Brown DR, Randall DC, Cassis LA. Activation of the systemic and adipose renin-angiotensin system in rats with diet-induced obesity and hypertension. Am J Physiol Regul Integr Comp Physiol. 2004;287:R943–9. doi: 10.1152/ajpregu.00265.2004. [DOI] [PubMed] [Google Scholar]
- 11.Gorzelniak K, Engeli S, Janke J, Luft FC, Sharma AM. Hormonal regulation of the human adipose-tissue renin-angiotensin system: relationship to obesity and hypertension. J Hypertens. 2002;20:965–73. doi: 10.1097/00004872-200205000-00032. [DOI] [PubMed] [Google Scholar]
- 12.Hall JE, Louis K. Dahl Memorial Lecture. Renal and cardiovascular mechanisms of hypertension in obesity. Hypertension. 1994;23:381–94. doi: 10.1161/01.hyp.23.3.381. [DOI] [PubMed] [Google Scholar]
- 13.Rahmouni K, Correia MLG, Haynes WG, Mark AL. Obesity-associated hypertension: new insights into mechanisms. Hypertension. 2005;45:9–14. doi: 10.1161/01.HYP.0000151325.83008.b4. [DOI] [PubMed] [Google Scholar]
- 14.Mehta RL, Pascual MT, Soroko S, Chertow GM. Diuretics, mortality, and nonrecovery of renal function in acute renal failure. JAMA. 2002;288:2547–53. doi: 10.1001/jama.288.20.2547. [DOI] [PubMed] [Google Scholar]
- 15.Saeed M, Villarroel M, Reisner A, Clifford G, Lehman L, Moody G, et al. Multiparameter Intelligent Monitoring in Intensive Care II (MIMIC-II): a public-access intensive care unit database. Crit Care Med. 2011;39:952–60. doi: 10.1097/CCM.0b013e31820a92c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO. Consultation on obesity: preventing and managing the global epidemic. Geneva: World Health Organization; 1998. pp. 894pp. i–xii.pp. 1–253. [PubMed] [Google Scholar]
- 17.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Le Gall J-R, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–63. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 19.Danziger J, William JH, Scott DJ, Lee J, Lehman LW, Mark RG, et al. Proton-pump inhibitor use is associated with low serum magnesium concentrations. Kidney Int. 2013;83:692–9. doi: 10.1038/ki.2012.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sica DA, Carter B, Cushman W, Hamm L. Thiazide and loop diuretics. J Clin Hypertens. 2011;13:639–43. doi: 10.1111/j.1751-7176.2011.00512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fonarow GC, Srikanthan P, Costanzo MR, Cintron GB, Lopatin M. An obesity paradox in acute heart failure: analysis of body mass index and inhospital mortality for 108,927 patients in the Acute Decompensated Heart Failure National Registry. Am Heart J. 2007;153:74–81. doi: 10.1016/j.ahj.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Waki M, Kral JG, Mazariegos M, Wang J, Pierson RN, Heymsfield SB. Relative expansion of extracellular fluid in obese vs. nonobese women. Am J Physiol. 1991;261:E199–203. doi: 10.1152/ajpendo.1991.261.2.E199. [DOI] [PubMed] [Google Scholar]
- 23.Messerli FH, Sundgaard-Riise K, Reisin ED, Dreslinski GR, Ventura HO, Oigman W, et al. Dimorphic cardiac adaptation to obesity and arterial hypertension. Ann Intern Med. 1983;99:757–61. doi: 10.7326/0003-4819-99-6-757. [DOI] [PubMed] [Google Scholar]
- 24.Lauer MS, Anderson KM, Kannel WB, Levy D. The impact of obesity on left ventricular mass and geometry. JAMA. 1991;266:231–6. [PubMed] [Google Scholar]
- 25.Hammond IW, Devereux RB, Alderman MH, Laragh JH. Relation of blood pressure and body build to left ventricular mass in normotensive and hypertensive employed adults. J Am Coll Cardiol. 1988;12:996–1004. doi: 10.1016/0735-1097(88)90467-6. [DOI] [PubMed] [Google Scholar]
- 26.Engeli S, Sharma AM. The renin-angiotensin system and natriuretic peptides in obesity-associated hypertension. J Mol Med. 2001;79:21–9. doi: 10.1007/s001090000144. [DOI] [PubMed] [Google Scholar]
- 27.Vincent HK, Powers SK, Stewart DJ, Shanely RA, Demirel H, Naito H. Obesity is associated with increased myocardial oxidative stress. Int J Obes Relat Metab Disord. 1999;23:67–74. doi: 10.1038/sj.ijo.0800761. [DOI] [PubMed] [Google Scholar]
- 28.Kenchaiah S. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–13. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Chen X, Song Y, Caballero B, Cheskin LJ. Association between obesity and kidney disease: a systematic review and meta-analysis. Kidney Int. 2008;73:19–33. doi: 10.1038/sj.ki.5002586. [DOI] [PubMed] [Google Scholar]
- 30.De Jong PE, Verhave JC, Pinto-Sietsma SJ, Hillege HL. Obesity and target organ damage: the kidney. Int J Obes Relat Metab Disord. 2002;26(Suppl 4):S21–4. doi: 10.1038/sj.ijo.0802213. [DOI] [PubMed] [Google Scholar]
- 31.Henegar JR, Bigler SA, Henegar LK, Tyagi SC, Hall JE. Functional and structural changes in the kidney in the early stages of obesity. J Am Soc Nephrol. 2001;12:1211–7. doi: 10.1681/ASN.V1261211. [DOI] [PubMed] [Google Scholar]
- 32.Lee J, de Louw E, Niemi M, Nelson R, Mark RG, Celi LA, et al. Association between fluid balance and survival in critically ill patients. J Intern Med. 2014 doi: 10.1111/joim.12274. [epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schuller D, Mitchell JP, Calandrino FS, Schuster DP. Fluid balance during pulmonary edema. Is fluid gain a marker or a cause of poor outcome? Chest. 1991;100:1068–75. doi: 10.1378/chest.100.4.1068. [DOI] [PubMed] [Google Scholar]
- 34.Alsous F, Khamiees M, DeGirolamo A, Amoateng-Adjepong YMC. Negative fluid balance predicts survival in patients with septic shock. Chest. 2000;117:1749–54. doi: 10.1378/chest.117.6.1749. [DOI] [PubMed] [Google Scholar]
- 35.Payen D, de Pont AC, Sakr Y, Spies C, Reinhart K, Vincent JL. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care. 2008;12:R74. doi: 10.1186/cc6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Upadya A, Tilluckdharry L, Muralidharan V, Amoateng-Adjepong Y, Manthous CA. Fluid balance and weaning outcomes. Intensive Care Med. 2005;31:1643–7. doi: 10.1007/s00134-005-2801-3. [DOI] [PubMed] [Google Scholar]
- 37.Sadaka F, Juarez M, Naydenov S, O’Brien J. Fluid resuscitation in septic shock: the effect of increasing fluid balance on mortality. J Intensive Care Med. 2013;00:1–5. doi: 10.1177/0885066613478899. [DOI] [PubMed] [Google Scholar]
- 38.De Almeida JP, Palomba H, Galas FR, Fukushima JT, Duarte FA, Nagaoka D, et al. Positive fluid balance is associated with reduced survival in critically ill patients with cancer. Acta Anaesthesiol Scand. 2012;56:712–7. doi: 10.1111/j.1399-6576.2012.02717.x. [DOI] [PubMed] [Google Scholar]
- 39.Flori HR, Church G, Liu KD, Gildengorin G, Matthay MA. Positive fluid balance is associated with higher mortality and prolonged mechanical ventilation in pediatric patients with acute lung injury. Crit Care Res Pract. 2011;2011:854142. doi: 10.1155/2011/854142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Landsberg L, Aronne LJ, Beilin LJ, Burke V, Igel LI, Lloyd-Jones D, et al. Obesity-related hypertension: pathogenesis, cardiovascular risk, and treatment—a position paper of the Obesity Society and the American society of hypertension. Obesity. 2013;21:8–24. doi: 10.1002/oby.20181. [DOI] [PubMed] [Google Scholar]