Abstract
Epidermal growth factor receptor (EGFR) is a receptor tyrosine kinase. Its activation results in beneficial or detrimental consequences, depending on the particular setting. Earlier studies in the animal model of acute kidney injury showed that EGFR activation promotes renal tubular cell proliferation. Activation of EGFR by its exogenous ligands, like EGF, can enhance recovery of renal function and structure following acute kidney injury. However, recent studies indicated that EGFR activation also contributes to development and progression of renal diseases in animal models of obstructive nephropathy, diabetic nephropathy, hypertensive nephropathy, and glomerulonephritis through mechanisms involved in activation of renal interstitial fibroblasts, induction of tubular atrophy, overproduction of inflammatory factors or/and promotion of glomerular and vascular injury. This review highlights the actions and mechanisms of EGFR in a variety of acute and chronic kidney injuries.
Keywords: EGFR, signaling pathway, acute renal injury, chronic kidney diseases, clinical relevance
Introduction
The epidermal growth factor receptor (EGFR) is a family of transmembrane receptors that belongs to subclass I of the tyrosine kinase receptor superfamily. EGFR family has four members: EGFR/Human Epidermal Growth Factor Receptor-1 (HER1)/ErbB1, Neu/HER2/ErbB2, HER3/ErbB3 and HER4/ErbB4.[1] Each receptor is composed of four identical domains: an extracellular ligand-binding domain, a single membrane-spanning region, a cytoplasmic protein tyrosine kinase domain and a C-terminal tail with multiple phosphorylation sites.[2] These receptors can interact with different ligands. To date, 11 ErbB ligands have been identified. Among them, EGF, heparin-binding EGF-like growth factor (HB-EGF), transforming growth factor-α (TGF-α), amphiregulin (AR), betacellulin; epigen and epiregulin can bind to EGFR or/and ErbB4. Neuregulin (NRG)-1, NRG-2, NRG-3, and NRG-4 can bind to ErbB4 or ErbB3. Ligands for ErbB-2 have not yet been identified. EGFR is widely expressed in the mammalian kidney at sites that include proximal tubule and cortical and inner medullary collecting duct, in glomerular mesangial cells, as well as in medullary interstitial cells. ErbB2, Erb3 and ErbB4 are also exprssed in the kidney, but predominantly localized to the distal tubule and collecting duct.[1, 2] Several ErbB ligands, including EGF, HB-EGF, TGF-α, AR, are also expressed in the kidney.[1, 2]
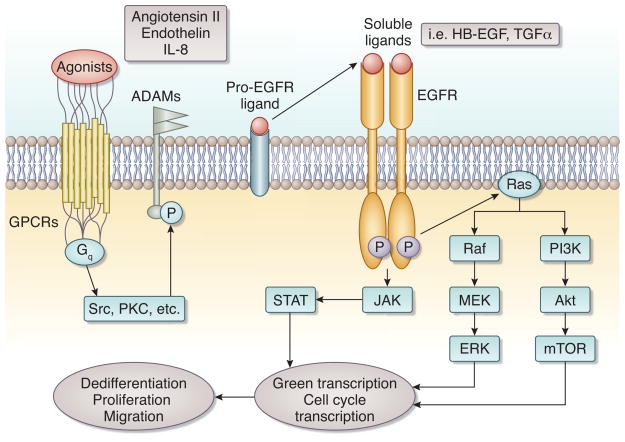
Ligand binding to EGFR induces its phosphorylation on specific tyrosine residues within the cytoplasmic tail. In addition to its cognate ligands, the EGFR can be activated by stimuli that do not directly interact with the EGFR ectodomain, including G-protein coupled receptor ligands, other receptor tyrosine kinase agonists, cytokines, and chemokines. This type of EGFR activation has been termed “transactivation”, and represents the paradigm for cross-talk between other receptors and EGFR. In this process, some intracellular kinases like PKC and Src are activated, subsequently activating proteases and disintegrin and metalloprotease (ADAM) family members.[3–5] The activated proteases and ADAMs then cleave EGFR ligands, releasing soluble forms that bind to and activate EGFR, subsequently initiating multiple intracellular signaling pathways, including the extracellular signal-regulated kinase (ERK) pathway, the Janus kinase/signal transducers and activators of transcription (JAK/STAT) pathways and the phosphoinositide-3-kinase (PI3K)/Akt pathways (Figure 1). Activation of these pathways has been implicated in cell survival, proliferation, dedifferentiation, and migration.[1, 6–8] (Figure 1) Some stimuli known to be implicated in the pathogenesis of kidney diseases such as endothelin-1 (ET-1), angiotensin II, and TGF-β1 can induce EGFR transactivation.[1, 9]
Figure 1. EGFR transactivation and downstream signaling pathways.
EGFR transactivation is induced by GPCRs, which work though intracellular kinases such as Src, PKC to phosphorylate ADAMs, which cleave EGFR ligands into soluble active moieties that activate EGFR. This leads to subsequent activation of downstream signaling pathways, including the MAPK/ERK pathway, PI3K/Akt pathway and JAK/STAT pathway. This ultimately leads to translocation of the signal from cytosol into the nucleus, triggering gene transcription and biological effects, such as cell proliferation, dedifferentiation and migration.
EGFR has been shown to be involved in the development and progression of multiple epithelial cancers, including kidney carcinoma.[10–18] In addition to its tumor biology, EGFR activation is also associated with kidney development,[2, 19] acute kidney injury and chronic kidney disease (CKD).[20–22] In this review, we focus on recent findings regarding the role and mechanism of EGFR in acute kidney injury (AKI) and CKD.
EGFR in acute kidney injury
AKI, defined as an abrupt reduction in kidney function measured as either a rise in serum creatinine or fall in urine output, can arise in a variety of clinical situations, such as ischemia/reperfusion (I/R), sepsis, trauma, and nephrotoxin exposure.[21, 23] Studies from animal models of AKI have demonstrated that the kidney possesses a remarkable capacity to recover from ischemia or other insults inducing renal tubular cell death. During the regenerative process, the remaining viable tubular cells are thought to undergo dedifferentiation, proliferation and migration, and finally to re-differentiate into mature tubular cells [21], resulting in morphological and functional recovery of renal epithelium. Although the mechanism responsible for renal regeneration following AKI is not fully understood, it has been recognized that some molecules such as vimentin and neural cell adhesion molecule, which are expressed in metanephric mesenchyme but not in mature kidneys, are re-expressed in renal tubular cells during recovery from injury.[24] This suggests that the process of renal regeneration after acute injury may recapitulate that of early renal development.
During the developmental period, renal mesenchymal cells are dedifferentiated and highly proliferative, and subjected to the regulation by numerous growth factors, such as EGFR ligands.[2] EGF was the first ligand discovered in isolated maxillary gland proteins. It induces early eye-lid opening in new-born mice through acceleration of the proliferation of epidermal basal cells.[25, 26] Organ cultures reveal that EGF and three other EGFR ligands (TGF-α, epiregulin and HB-EGF) are expressed in metanephric structures and contribute to tubulogensis and breaching [2] All these ligands exert their biological effects through EGFR. EGFR activation is also implicated in the development of kidney. This is evident by the observations that inactivation of EGFR kinase activity inhibits branching of cultured ureteric bud.[27] and EGFR knockout mice suffer from impaired epithelial development in several organs, including the kidney.[28]
The involvement of the EGFR signaling in nephrogenesis and the mitogenic potential of adult proximal tubule cells compelled researchers to explore its role in renal repair after acute injury. An increase in EGFR phosphorylation was detected in the renal proximal tubules in a variety of experimental models of AKI, including ischemia/reperfusion, aminoglycoside toxicity, and folic acid administration.[29–31] Increased expression of EGFR ligands, HB-EGF in particular, was also identified in the kidney after acute tubular injury induced by multiple insults.[29–31] In waved-2 mice, a mouse strain that has a point mutation in EGFR that results in a 90% reduction in receptor tyrosine kinase activity, renal function recovery was much slower after acute renal injury.[32] Similarly, renal function recovery were significantly delayed following ischemia and reperfusion injury in mice with a specific EGFR deletion in the renal proximal tubule or treated with erlotinib, a specific EGFR inhibitor.[22] On the contrary, activation of EGFR with exogenous EGF or HB-EGF can accelerate renal recovery from acute ischemic injury.[33, 34] These data provide strong evidence that EGFR is critically invovled in promoting kidney recovery from acute injury.
The mechanism by which EGFR accelerates renal recovery has not been completely understood yet. Using genetic tracing approach, Humphreys et al. recently demonstrated that the restoration of injured renal epithelium is primarily mediated by the proliferation of surviving tubular epithelial cells.[35] As dedifferentiation of renal tubular cells is a prerequisite for their proliferation in response to acute injury, we have examined the role of EGFR in renal tubular cell dedifferentiation in primary culture. After oxidant injury induced by H2O2, surviving renal proximal tubular cells (RPTC) acquired a dedifferentiated phenotype along with activation of EGFR. Inhibition of EGFR with selective inhibitors blocked RPTC dedifferentiation.[36] EGFR inactivation also suppressed proliferation of RPTC in response to HB-EGF and epiregulin.[37, 38] Consistent with these observations, blockade of EGFR also reduced renal tubular cell proliferation in vivo animal models of AKI.[22] Thus, EGFR activation may promote renal recovery by enhancing renal regeneration. Since recent animal studies indicated that regeneration of tubular cells occurs predominately in injured intrinsic tubular cells,[39] future studies are necessary to elucidate the mechanism of renal tubular cell dedifferentiation and the possible role of EGFR in this process after acute kidney injury.
EGFR in chronic kidney disease
EGFR and renal fibrosis
Renal fibrosis has been considered a failed regenerative process that can facilitate progression to chronic kidney disease (CKD). After acute injury, tissue repair can either completely restore the integrity of damaged tissue when injury is mild or lead to fibrosis when the injury is more severe or is superimposed on baseline kidney abnormalities.[40] Although EGFR activation has been shown to contribute to renal repair and functional recovery, studies have also indicated that its activation is critically involved in the development and progression of renal fibrosis. It is evident that mice overexpressing a dominant negative EGFR construct exhibited significantly less tubulointerstitial injury in the kidney compared with wild type littermates after subtotal renal ablation or following chronic Ang II infusion.[4, 41] A decrease in renal fibrosis was also observed in mice with deletion of EGFR in proximal renal tubular cells after angiotensin II infusion or in Waved-2 mice that have reduced EGFR kinase activity after urethral obstruction.[42, 43]. Consistent with these observations, pharmacologic blockade of EGFR with gefitinib or erlotinib also inhibits renal deterioration and fibrogensis induced by Ang II, UUO or 5/6 renal nephrectomy. [41] [42, 43]. Finally, adminstration of gefitinib was able to prevent the development of renal vascular and glomerular fibrosis in a rat model of NG-nitro-L-arginine methyl ester (L-NAME)-induced hypertension.[44]
Given its central role in renal fibrosis, the mechanism by which EGFR is activated after chronic injury and leads to renal fibrosis has been investigated. Compared with transient activation of EGFR after acute and mild injury, chronic renal injury induced prolonged phosphorylation of EGFR.[42, 43] The sustained activation of EGFR is triggered by ROS-dependent phosphorylation of Src.[42] and is required for activation of renal interstitial fibroblasts and gene expression of multiple profibrogenic cytokines, including transforming growth factor-β1 (TGF-β1).[43] Although several EGFR ligands have been reported to express in diseased kidney, TGF-α may be a major EGFR ligand in the setting of CKD since it mediates AngII-induced EGFR transactivation during AngII-induced nephropathy.[4] Its expression also increases genetic susceptibility to CKD in the sensitive FVB/N mouse strain.[45] More importantly, like EGFR depletion, TGF-α gene inactivation protected FVB/N mice from renal deterioration after nephron reduction.[46] Therefore, inhibition of EGFR signaling may be a potentiatl therapeutic approach for CKD treatment.
EGFR and diabetic nephropathy
Diabetic nephropathy is a common complication in patients with diabetes mellitus, which is characterized by hyperfiltration and glomerular hypertrophy.[47–51] Renal enlargement is associated with excessive sodium and water reabsorption due to dysregulated renal tubular cell sodium and water transport,[52] whereas glomerular hypertrophy is associated with glomerular matrix accumulation and podocyte injury.[20][47] Although the precise mechanisms underlying diabetic nephropathy are not completely clear, local production of active growth factors like EGF and subsequent EGFR activation may be critically involved in the pathogenesis of diabetic kidney disease.[47, 53] Numerous stimuli can activate the EGFR, beyond EGF itself, including high glucose and Agiotensin II [54]. Expression of EGF and HB-EGF within the kidney is increased after the induction of diabetes with STZ.[55, 56] Blockade of EGFR activity with PKI166 resulted in a significant reduction in diabetes-associated renal enlargement and glomerular hypertrophy.[47] PKI166 treatment was also effective in preserving podocytes and attenuating albuminuria in an experimental model of long-term diabetic nephropathy.[20]
Several possible mechanisms may account for the role of EGFR in diabetic nephropathy. First, EGFR activation can up-regulate serum glucocorticoid regulated kinase-1, a key regulator of the sodium-hydrogen exchanger-3 (NHE3) that is responsible for sodium reabsorption in the proximal tubule in diabetic nephropathy.[47, 53] Second, angiotensin II-induced transactivation of EGFR is required for gene expression of transporter 1 (GLUT1), an important facilitative glucose transporter. Finally, transactivation of EGFR mediates high glucose–induced TGF-β upregulation in cultured mesangial cells [57] and collagen I expression in diabetic glomeruli.[58]. Since EGFR activation is required for the pathogenesis of both early and later stage of diabetes-related kidney disease in anima models,[47, 53] EGFR signaling might be an interesting therapeutic target in the treatment of diabetic nephropathy.
EGFR and hypertensive nephropathy
Emerging evidence suggests that EGFR plays a critical role in kidney damage associated with high blood pressure. It has been shown that EGFR expression is increased in the kidneys of deoxycorticosterone acetate (DOCA)-salt-induced hypertensive rats and is prominently localized in the media of afferent and efferent arterioles and the aorta.[59, 60] DOCA-salt hypertensive rats developed kidney damage as demonstrated by increased proteinuria and enhanced renal artery responsiveness. This kidney dysfunction could be significantly prevented by EGFR inhibition with AG1478.[59] Administration of gefitinib also improves renal function and protects against the development of renal vascular and glomerular fibrosis in rats with hypertension-induced renal disease.[44]. Moreover, EGFR activation with EGF or transactivation with Ang II or endothelin leads to vasoconstriction,[61–64] and blockade of EGFR activity suppresses vasoconstriction and attenuates left ventricular hypertrophy and blood pressure in spontaneously hypertensive rats.[62, 63] Mechanistic studies suggest that EGFR-mediated renal arteriolar contraction and hypertension is in part associated with promotion of the afferent arteriolar intracellular Ca2+ influx[64] and stimulation of vascular smooth muscle cell proliferation.[60, 62, 65, 66]
EGFR and polycystic kidney disease
Polycystic kidney disease (PKD) is a common genetic disease in which mutations of cilia-localized proteins polycystin 1 and polycystin 2 (PKD1 and PKD2), lead to the formation and enlargement of multiple cysts in both kidneys. The disease encompasses two genetically distinct conditions: autosomal dominant PKD (ADPKD) and autosomal recessive PKD (ARPKD). Under normal condition, EGFR is expressed at the basolateral membranes in adult tubular epithelial cells. [67–69] A large number of primary PKD genetic defects, however, disturb the polarity of EGFR, resulting in its localization to and increased expression on the apical surface of cyst epithelium.[67, 70, 71] Using genetic mutations of EGFR with decreased tyrosine kinase activity in a murine model of ARPKD, researchers have demonstrated that a modified form of EGFR could inhibit the increase in EGFR-specific tyrosine kinase activity, which results in an amelioration of the decline in renal function and a substantial reduction in cyst formation in the collecting ducts.[72] Further studies [70] showed that inhibition of EGFR tyrosine kinase activity with EKI-785 in vivo resulted in a significant decrease in the number and size of renal collecting tubule cystic lesions, and improved renal tubular function in murine models of ARPKD. These studies suggest the importance of EGFR in modulating formation of renal cysts.
Multiple EGFR ligands, including EGF, TGF-α, amphiregulin and HB-EGF, have been reported to be expressed in proximal tubule cysts of cystic ARPKD kidneys.[73–75]. HB-EGF was also observed on the apical surface of cystic collecting tubules.[75]. This suggests that increased shedding of growth factors “upstream” into the urine may exert proliferative effects distally on abnormal collecting tubule epithelia that express apical EGFR and that HB-EGF may activate EGFR present on cystic collecting tubule epithelium by autocrine and/or paracrine mechanisms.
EGFR and glomerulonephritis
Rapidly progressive glomerulonephritis (RPGN) is a devastating disease process characterized by a rapid loss of renal function through the formation of crescent-shaped necrotizing lesions in the glomeruli with accumulation of CD4+ T cells and macrophages and proliferation of intrinsic glomerular cells.[76, 77] Recently, a link between the HB-EGF-EGFR pathway and the course of crescentic RPGN has been identified in the murine model of anti-GBM nephrotoxic serum (NTS)-induced nephritis.[76] Expression of HB-EGF in parietal cells and podocytes was markedly increased after the onset of crescentic glomerulonephritis along with sustained activation of EGFR in podocytes.[76] In cultured podocytes, HB-EGF also induced their dedifferentiation and transformation into a proliferative and migratory phenotype through activation of EGFR.[76, 77] However, HB-EGF-deficient mice displayed decreased EGFR phosphorylation in glomeruli and were significantly protected against RPGN.[76] Pharmacological inhibition of EGFR also effectively attenuated albuminuria and glomerular injury and improved renal function in this animal model, even after the onset of acute renal failure.[76] These data suggest that EGFR plays a pathophysiological role in crescentic RPGN and that targeting the HB-EGF-EGFR pathway may be beneficial in the treatment of RPGN. In addition to RPGN, HB-EGF was reported to be upregulated in the kidney in several other animal models of acute glomerular injury, such as puromycin aminonucleoside-induced focal glomerular sclerosis[78] and passive Heymann nephritis.79] This suggests that HB-EGF may also be involved in pathological changes in the glomerulus. Future studies are necessary to further explore the role of HB-EGF and EGFR in the pathogenesis of these diseases.
EGFR and allograft nephropathy
Pathological analysis of human renal allografts biopsies indicated that EGFR expression was expressed in tubular epithelial cells and along glomerular capillary walls or in mesangium.[80] A significant correlation was also observed between tubular EGFR expression, interstitial fibrosis and tubular atrophy, in chronic allograft nephropathy.[80, 81] These findings, together with the fibrosis-promoting effect of EGFR in CKD, suggest a possible role for EGFR in the pathogenesis of allograft nephropathy. Additional studies are needed to examine whether EGFR inhibition will alter the course of this pathologic process.
EGFR and peritoneal fibrosis
Peritoneal membrane fibrosis is a frequent complication of long-term peritoneal dialysis, ultimately resulting in peritoneal sclerosis and ultrafiltration failure. The mesothelial cell monolayer, a main component of the peritoneal membrane,[82] requires constant repair in response to episodes of peritonitis (acute injury) and to long-term toxicity as a result of exposure to dextrose in peritoneal dialysate (chronic injury).[83] In patients with long-term continuous ambulatory peritoneal dialysis (CAPD), this repair process gradually fails, resulting in mesothelial cell loss and progressive peritoneal fibrogenesis.[83, 84] EGFR and its ligands may be associated with the development and progression of peritoneal fibrosis during peritoneal dialysis. This is suggested by several observations: (1) HB-EGF is expressed in human peritoneal mesothelial cells and peritoneal macrophages;[83] (2) activation of EGFR by HB-EGF induces proliferation of cultured human peritoneal mesothelial cells (HPMC) and conversion into a more fibroblastoid phenotype;[83] and (3) exposure of HPMC to EGF promoted their conversion into a fibroblastoid phenotype, resulting in increased extracellular matrix protein synthesis.[85]
Conclusion and perspective
The expression of EGFR has been implicated in the initiation and progression of various cancers through promotion of tumor cell proliferation and invasion and inhibition of apoptosis.[86] Strategies to block EGFR have also been recognized as therapeutic options for some epithelial cancers.[16, 87–89] Recent studies indicated that EGFR plays an important role in acute and chronic renal diseases, but with bidirectional outcomes: whereas EGFR activation is required for renal regeneration and functional recovery after AKI, its activation also contributes to the initiation and progression of renal fibrosis in various animal models of CKD. While the detailed mechanism by which EGFR activation leads to beneficial and detrimental outcomes are not clear, EGFR-mediated renal fibrogenesis may be the result of renal maladaptive repair in response to severe injury. As EGFR transactivation provides a converging signaling pathway whereby different stimuli modulate kidney damage and pharmacological blockade of EGFR has been shown to attenuate renal fibrosis and glomerulonephritis, targeting the EGFR pathway may hold the therapeutic potential for patients with CKD.
Acknowledgments
This work was supported by grants from National Institutes of Health (DK-071997 and DK-085065 to SZ) and by a grant (PWZxk2010-02) from the Department of Public Health, Pudong New Distric, Shanghai, China.
Footnotes
Disclosure
No interest to disclosure
References
- 1.Melenhorst WB, Mulder GM, Xi Q, et al. Epidermal growth factor receptor signaling in the kidney: key roles in physiology and disease. Hypertension. 2008;52:987–993. doi: 10.1161/HYPERTENSIONAHA.108.113860. [DOI] [PubMed] [Google Scholar]
- 2.Zeng F, Singh AB, Harris RC. The role of the EGF family of ligands and receptors in renal development, physiology and pathophysiology. Exp Cell Res. 2009;315:602–610. doi: 10.1016/j.yexcr.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huovila AP, Turner AJ, Pelto-Huikko M, et al. Shedding light on ADAM metalloproteinases. Trends Biochem Sci. 2005;30:413–422. doi: 10.1016/j.tibs.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Lautrette A, Li S, Alili R, et al. Angiotensin II and EGF receptor cross-talk in chronic kidney diseases: a new therapeutic approach. Nat Med. 2005;11:867–874. doi: 10.1038/nm1275. [DOI] [PubMed] [Google Scholar]
- 5.Asakura M, Kitakaze M, Takashima S, et al. Cardiac hypertrophy is inhibited by antagonism of ADAM12 processing of HB-EGF: metalloproteinase inhibitors as a new therapy. Nat Med. 2002;8:35–40. doi: 10.1038/nm0102-35. [DOI] [PubMed] [Google Scholar]
- 6.Schlessinger J. Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell. 2002;110:669–672. doi: 10.1016/s0092-8674(02)00966-2. [DOI] [PubMed] [Google Scholar]
- 7.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 8.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Levesque LO, Anand-Srivastava MB. Epidermal growth factor receptor transactivation by endogenous vasoactive peptides contributes to hyperproliferation of vascular smooth muscle cells of SHR. Am J Physiol Heart Circ Physiol. 2010;299:H1959–1967. doi: 10.1152/ajpheart.00526.2010. [DOI] [PubMed] [Google Scholar]
- 10.Feigin ME, Muthuswamy SK. ErbB receptors and cell polarity: new pathways and paradigms for understanding cell migration and invasion. Exp Cell Res. 2009;315:707–716. doi: 10.1016/j.yexcr.2008.10.034. [DOI] [PubMed] [Google Scholar]
- 11.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 12.Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi M, Iwamatsu A, Shinohara-Kanda A, et al. Activation of ErbB3-PI3-kinase pathway is correlated with malignant phenotypes of adenocarcinomas. Oncogene. 2003;22:1294–1301. doi: 10.1038/sj.onc.1206256. [DOI] [PubMed] [Google Scholar]
- 14.Minner S, Rump D, Tennstedt P, et al. Epidermal growth factor receptor protein expression and genomic alterations in renal cell carcinoma. Cancer. 2011 doi: 10.1002/cncr.26436. [DOI] [PubMed] [Google Scholar]
- 15.Patereli A, Alexiou GA, Stefanaki K, et al. Expression of epidermal growth factor receptor and HER-2 in pediatric embryonal brain tumors. Pediatr Neurosurg. 2010;46:188–192. doi: 10.1159/000316640. [DOI] [PubMed] [Google Scholar]
- 16.Rego RL, Foster NR, Smyrk TC, et al. Prognostic effect of activated EGFR expression in human colon carcinomas: comparison with EGFR status. Br J Cancer. 2010;102:165–172. doi: 10.1038/sj.bjc.6605473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo G, Long J, Qiu L, et al. Role of epidermal growth factor receptor expression on patient survival in pancreatic cancer: a meta-analysis. Pancreatology. 2011;11:595–600. doi: 10.1159/000334465. [DOI] [PubMed] [Google Scholar]
- 18.Naik DS, Sharma S, Ray A, et al. Epidermal growth factor receptor expression in urinary bladder cancer. Indian J Urol. 2011;27:208–214. doi: 10.4103/0970-1591.82839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodyer PR, Cybulsky A, Goodyer C. Expression of the epidermal growth factor receptor in fetal kidney. Pediatr Nephrol. 1993;7:612–615. doi: 10.1007/BF00852567. [DOI] [PubMed] [Google Scholar]
- 20.Advani A, Wiggins KJ, Cox AJ, et al. Inhibition of the epidermal growth factor receptor preserves podocytes and attenuates albuminuria in experimental diabetic nephropathy. Nephrology (Carlton) 2011;16:573–581. doi: 10.1111/j.1440-1797.2011.01451.x. [DOI] [PubMed] [Google Scholar]
- 21.Wen X, Murugan R, Peng Z, et al. Pathophysiology of acute kidney injury: a new perspective. Contrib Nephrol. 2010;165:39–45. doi: 10.1159/000313743. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Chen JK, Harris RC. Deletion of the epidermal growth factor receptor in renal proximal tubule epithelial cells delays recovery from acute kidney injury. Kidney Int. 2012 doi: 10.1038/ki.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonventre JV. Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J Am Soc Nephrol. 2003;14(Suppl 1):S55–61. doi: 10.1097/01.asn.0000067652.51441.21. [DOI] [PubMed] [Google Scholar]
- 25.Cohen S. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new-born animal. J Biol Chem. 1962;237:1555–1562. [PubMed] [Google Scholar]
- 26.Carev D, Saraga M, Saraga-Babic M. Expression of intermediate filaments, EGF and TGF-alpha in early human kidney development. J Mol Histol. 2008;39:227–235. doi: 10.1007/s10735-007-9157-7. [DOI] [PubMed] [Google Scholar]
- 27.Takemura T, Hino S, Okada M, et al. Role of membrane-bound heparin-binding epidermal growth factor-like growth factor (HB-EGF) in renal epithelial cell branching. Kidney Int. 2002;61:1968–1979. doi: 10.1046/j.1523-1755.2002.00358.x. [DOI] [PubMed] [Google Scholar]
- 28.Threadgill DW, Dlugosz AA, Hansen LA, et al. Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science. 1995;269:230–234. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]
- 29.Hise MK, Salmanullah M, Liu L, et al. Control of the epidermal growth factor receptor and its ligands during renal injury. Nephron. 2001;88:71–79. doi: 10.1159/000045962. [DOI] [PubMed] [Google Scholar]
- 30.Homma T, Sakai M, Cheng HF, et al. Induction of heparin-binding epidermal growth factor-like growth factor mRNA in rat kidney after acute injury. J Clin Invest. 1995;96:1018–1025. doi: 10.1172/JCI118087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakai M, Zhang M, Homma T, et al. Production of heparin binding epidermal growth factor-like growth factor in the early phase of regeneration after acute renal injury. Isolation and localization of bioactive molecules. J Clin Invest. 1997;99:2128–2138. doi: 10.1172/JCI119386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Z, Chen JK, Wang SW, et al. Importance of functional EGF receptors in recovery from acute nephrotoxic injury. J Am Soc Nephrol. 2003;14:3147–3154. doi: 10.1097/01.asn.0000098681.56240.1a. [DOI] [PubMed] [Google Scholar]
- 33.Humes HD, Cieslinski DA, Coimbra TM, et al. Epidermal growth factor enhances renal tubule cell regeneration and repair and accelerates the recovery of renal function in postischemic acute renal failure. J Clin Invest. 1989;84:1757–1761. doi: 10.1172/JCI114359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Norman J, Tsau YK, Bacay A, et al. Epidermal growth factor accelerates functional recovery from ischaemic acute tubular necrosis in the rat: role of the epidermal growth factor receptor. Clin Sci (Lond) 1990;78:445–450. doi: 10.1042/cs0780445. [DOI] [PubMed] [Google Scholar]
- 35.Humphreys BD, Valerius MT, Kobayashi A, et al. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008;2:284–291. doi: 10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 36.Zhuang S, Yan Y, Han J, et al. p38 kinase-mediated transactivation of the epidermal growth factor receptor is required for dedifferentiation of renal epithelial cells after oxidant injury. J Biol Chem. 2005;280:21036–21042. doi: 10.1074/jbc.M413300200. [DOI] [PubMed] [Google Scholar]
- 37.Zhuang S, Dang Y, Schnellmann RG. Requirement of the epidermal growth factor receptor in renal epithelial cell proliferation and migration. Am J Physiol Renal Physiol. 2004;287:F365–372. doi: 10.1152/ajprenal.00035.2004. [DOI] [PubMed] [Google Scholar]
- 38.Zhuang S, Yan Y, Daubert RA, et al. Epiregulin promotes proliferation and migration of renal proximal tubular cells. Am J Physiol Renal Physiol. 2007;293:F219–226. doi: 10.1152/ajprenal.00082.2007. [DOI] [PubMed] [Google Scholar]
- 39.Humphreys BD, Czerniak S, DiRocco DP, et al. Repair of injured proximal tubule does not involve specialized progenitors. Proc Natl Acad Sci U S A. 108:9226–9231. doi: 10.1073/pnas.1100629108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang L, Besschetnova TY, Brooks CR, et al. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med. 2010;16:535–543. doi: 10.1038/nm.2144. 531p following 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Terzi F, Burtin M, Hekmati M, et al. Targeted expression of a dominant-negative EGF-R in the kidney reduces tubulo-interstitial lesions after renal injury. J Clin Invest. 2000;106:225–234. doi: 10.1172/JCI8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen J, Chen JK, Nagai K, et al. EGFR Signaling Promotes TGFbeta-Dependent Renal Fibrosis. J Am Soc Nephrol. 2012 doi: 10.1681/ASN.2011070645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu N, Guo JK, Pang M, et al. Genetic or Pharmacologic Blockade of EGFR Inhibits Renal Fibrosis. J Am Soc Nephrol. 2012 doi: 10.1681/ASN.2011050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Francois H, Placier S, Flamant M, et al. Prevention of renal vascular and glomerular fibrosis by epidermal growth factor receptor inhibition. Faseb J. 2004;18:926–928. doi: 10.1096/fj.03-0702fje. [DOI] [PubMed] [Google Scholar]
- 45.Laouari D, Burtin M, Phelep A, et al. TGF-alpha mediates genetic susceptibility to chronic kidney disease. J Am Soc Nephrol. 22:327–335. doi: 10.1681/ASN.2010040356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laouari D, Burtin M, Phelep A, et al. A transcriptional network underlies susceptibility to kidney disease progression. EMBO Mol Med. doi: 10.1002/emmm.201101127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wassef L, Kelly DJ, Gilbert RE. Epidermal growth factor receptor inhibition attenuates early kidney enlargement in experimental diabetes. Kidney Int. 2004;66:1805–1814. doi: 10.1111/j.1523-1755.2004.00955.x. [DOI] [PubMed] [Google Scholar]
- 48.Mogensen CE, Andersen MJ. Increased kidney size and glomerular filtration rate in early juvenile diabetes. Diabetes. 1973;22:706–712. doi: 10.2337/diab.22.9.706. [DOI] [PubMed] [Google Scholar]
- 49.Seyer-Hansen K. Renal hypertrophy in experimental diabetes mellitus. Kidney Int. 1983;23:643–646. doi: 10.1038/ki.1983.71. [DOI] [PubMed] [Google Scholar]
- 50.Kleinman KS, Fine LG. Prognostic implications of renal hypertrophy in diabetes mellitus. Diabetes Metab Rev. 1988;4:179–189. doi: 10.1002/dmr.5610040207. [DOI] [PubMed] [Google Scholar]
- 51.Inomata S. Renal hypertrophy as a prognostic index for the progression of diabetic renal disease in non-insulin-dependent diabetes mellitus. J Diabetes Complications. 1993;7:28–33. doi: 10.1016/1056-8727(93)90020-y. [DOI] [PubMed] [Google Scholar]
- 52.Panchapakesan U, Pollock C, Saad S. Renal epidermal growth factor receptor: its role in sodium and water homeostasis in diabetic nephropathy. Clin Exp Pharmacol Physiol. 2011;38:84–88. doi: 10.1111/j.1440-1681.2010.05472.x. [DOI] [PubMed] [Google Scholar]
- 53.Gilbert RE, Huang Q, Thai K, et al. Histone deacetylase inhibition attenuates diabetes-associated kidney growth: potential role for epigenetic modification of the epidermal growth factor receptor. Kidney Int. 2011;79:1312–1321. doi: 10.1038/ki.2011.39. [DOI] [PubMed] [Google Scholar]
- 54.Konishi A, Berk BC. Epidermal growth factor receptor transactivation is regulated by glucose in vascular smooth muscle cells. J Biol Chem. 2003;278:35049–35056. doi: 10.1074/jbc.M304913200. [DOI] [PubMed] [Google Scholar]
- 55.Gilbert RE, Cox A, McNally PG, et al. Increased epidermal growth factor in experimental diabetes related kidney growth in rats. Diabetologia. 1997;40:778–785. doi: 10.1007/s001250050749. [DOI] [PubMed] [Google Scholar]
- 56.Lee YJ, Shin SJ, Lin SR, et al. Increased expression of heparin binding epidermal growth-factor-like growth factor mRNA in the kidney of streptozotocin-induced diabetic rats. Biochem Biophys Res Commun. 1995;207:216–222. doi: 10.1006/bbrc.1995.1175. [DOI] [PubMed] [Google Scholar]
- 57.Wu D, Peng F, Zhang B, et al. PKC-beta1 mediates glucose-induced Akt activation and TGF-beta1 upregulation in mesangial cells. J Am Soc Nephrol. 2009;20:554–566. doi: 10.1681/ASN.2008040445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu D, Peng F, Zhang B, et al. Collagen I induction by high glucose levels is mediated by epidermal growth factor receptor and phosphoinositide 3-kinase/Akt signalling in mesangial cells. Diabetologia. 2007;50:2008–2018. doi: 10.1007/s00125-007-0721-1. [DOI] [PubMed] [Google Scholar]
- 59.Benter IF, Canatan H, Benboubetra M, et al. Global upregulation of gene expression associated with renal dysfunction in DOCA-salt-induced hypertensive rats occurs via signaling cascades involving epidermal growth factor receptor: a microarray analysis. Vascul Pharmacol. 2009;51:101–109. doi: 10.1016/j.vph.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 60.Ying WZ, Sanders PW. Enhanced expression of EGF receptor in a model of salt-sensitive hypertension. Am J Physiol Renal Physiol. 2005;289:F314–321. doi: 10.1152/ajprenal.00003.2005. [DOI] [PubMed] [Google Scholar]
- 61.Swaminathan N, Vincent M, Sassard J, et al. Elevated epidermal growth factor receptor levels in hypertensive Lyon rat kidney and aorta. Clin Exp Pharmacol Physiol. 1996;23:793–796. doi: 10.1111/j.1440-1681.1996.tb01181.x. [DOI] [PubMed] [Google Scholar]
- 62.Carmines PK, Fallet RW, Che Q, et al. Tyrosine kinase involvement in renal arteriolar constrictor responses to angiotensin II. Hypertension. 2001;37:569–573. doi: 10.1161/01.hyp.37.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Flamant M, Tharaux PL, Placier S, et al. Epidermal growth factor receptor trans-activation mediates the tonic and fibrogenic effects of endothelin in the aortic wall of transgenic mice. FASEB J. 2003;17:327–329. doi: 10.1096/fj.02-0115fje. [DOI] [PubMed] [Google Scholar]
- 64.Che Q, Carmines PK. Angiotensin II triggers EGFR tyrosine kinase-dependent Ca2+ influx in afferent arterioles. Hypertension. 2002;40:700–706. doi: 10.1161/01.hyp.0000035524.10948.93. [DOI] [PubMed] [Google Scholar]
- 65.Ushio-Fukai M, Griendling KK, Becker PL, et al. Epidermal growth factor receptor transactivation by angiotensin II requires reactive oxygen species in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2001;21:489–495. doi: 10.1161/01.atv.21.4.489. [DOI] [PubMed] [Google Scholar]
- 66.Eguchi S, Numaguchi K, Iwasaki H, et al. Calcium-dependent epidermal growth factor receptor transactivation mediates the angiotensin II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. J Biol Chem. 1998;273:8890–8896. doi: 10.1074/jbc.273.15.8890. [DOI] [PubMed] [Google Scholar]
- 67.Ryan S, Verghese S, Cianciola NL, et al. Autosomal recessive polycystic kidney disease epithelial cell model reveals multiple basolateral epidermal growth factor receptor sorting pathways. Mol Biol Cell. 2010;21:2732–2745. doi: 10.1091/mbc.E09-12-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sack E, Talor Z. High affinity binding sites for epidermal growth factor (EGF) in renal membranes. Biochem Biophys Res Commun. 1988;154:312–317. doi: 10.1016/0006-291x(88)90686-9. [DOI] [PubMed] [Google Scholar]
- 69.Goodyer PR, Kachra Z, Bell C, et al. Renal tubular cells are potential targets for epidermal growth factor. Am J Physiol. 1988;255:F1191–1196. doi: 10.1152/ajprenal.1988.255.6.F1191. [DOI] [PubMed] [Google Scholar]
- 70.Sweeney WE, Chen Y, Nakanishi K, et al. Treatment of polycystic kidney disease with a novel tyrosine kinase inhibitor. Kidney Int. 2000;57:33–40. doi: 10.1046/j.1523-1755.2000.00829.x. [DOI] [PubMed] [Google Scholar]
- 71.Du J, Wilson PD. Abnormal polarization of EGF receptors and autocrine stimulation of cyst epithelial growth in human ADPKD. Am J Physiol. 1995;269:C487–495. doi: 10.1152/ajpcell.1995.269.2.C487. [DOI] [PubMed] [Google Scholar]
- 72.Richards WG, Sweeney WE, Yoder BK, et al. Epidermal growth factor receptor activity mediates renal cyst formation in polycystic kidney disease. J Clin Invest. 1998;101:935–939. doi: 10.1172/JCI2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nakanishi K, Gattone VH, 2nd, Sweeney WE, et al. Renal dysfunction but not cystic change is ameliorated by neonatal epidermal growth factor in bpk mice. Pediatr Nephrol. 2001;16:45–50. doi: 10.1007/s004670000495. [DOI] [PubMed] [Google Scholar]
- 74.Lee DC, Chan KW, Chan SY. Expression of transforming growth factor alpha and epidermal growth factor receptor in adult polycystic kidney disease. J Urol. 1998;159:291–296. doi: 10.1016/s0022-5347(01)64084-9. [DOI] [PubMed] [Google Scholar]
- 75.MacRae Dell K, Nemo R, Sweeney WE, Jr, et al. EGF-related growth factors in the pathogenesis of murine ARPKD. Kidney Int. 2004;65:2018–2029. doi: 10.1111/j.1523-1755.2004.00623.x. [DOI] [PubMed] [Google Scholar]
- 76.Bollee G, Flamant M, Schordan S, et al. Epidermal growth factor receptor promotes glomerular injury and renal failure in rapidly progressive crescentic glomerulonephritis. Nat Med. 2011;17:1242–1250. doi: 10.1038/nm.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Harris R. EGFR signaling in podocytes at the root of glomerular disease. Nat Med. 2011;17:1188–1189. doi: 10.1038/nm.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Paizis K, Kirkland G, Khong T, et al. Heparin-binding epidermal growth factor-like growth factor is expressed in the adhesive lesions of experimental focal glomerular sclerosis. Kidney Int. 1999;55:2310–2321. doi: 10.1046/j.1523-1755.1999.00469.x. [DOI] [PubMed] [Google Scholar]
- 79.Paizis K, Kirkland G, Polihronis M, et al. Heparin-binding epidermal growth factor-like growth factor in experimental models of membranous and minimal change nephropathy. Kidney Int. 1998;53:1162–1171. doi: 10.1046/j.1523-1755.1998.00846.x. [DOI] [PubMed] [Google Scholar]
- 80.Sis B, Sarioglu S, Celik A, et al. Epidermal growth factor receptor expression in human renal allograft biopsies: an immunohistochemical study. Transpl Immunol. 2004;13:229–232. doi: 10.1016/j.trim.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 81.Pape L, Henne T, Offner G, et al. Computer-assisted quantification of fibrosis in chronic allograft nephropaty by picosirius red-staining: a new tool for predicting long-term graft function. Transplantation. 2003;76:955–958. doi: 10.1097/01.TP.0000078899.62040.E5. [DOI] [PubMed] [Google Scholar]
- 82.Michailova KN, Usunoff KG. Serosal membranes (pleura, pericardium, peritoneum). Normal structure, development and experimental pathology. Adv Anat Embryol Cell Biol. 2006;183:i–vii. 1–144. back cover. [PubMed] [Google Scholar]
- 83.Faull RJ, Stanley JM, Fraser S, et al. HB-EGF is produced in the peritoneal cavity and enhances mesothelial cell adhesion and migration. Kidney Int. 2001;59:614–624. doi: 10.1046/j.1523-1755.2001.059002614.x. [DOI] [PubMed] [Google Scholar]
- 84.Dobbie JW. Pathogenesis of peritoneal fibrosing syndromes (sclerosing peritonitis) in peritoneal dialysis. Perit Dial Int. 1992;12:14–27. [PubMed] [Google Scholar]
- 85.Leavesley DI, Stanley JM, Faull RJ. Epidermal growth factor modifies the expression and function of extracellular matrix adhesion receptors expressed by peritoneal mesothelial cells from patients on CAPD. Nephrol Dial Transplant. 1999;14:1208–1216. doi: 10.1093/ndt/14.5.1208. [DOI] [PubMed] [Google Scholar]
- 86.Arora A, Scholar EM. Role of tyrosine kinase inhibitors in cancer therapy. J Pharmacol Exp Ther. 2005;315:971–979. doi: 10.1124/jpet.105.084145. [DOI] [PubMed] [Google Scholar]
- 87.Rosell R, Robinet G, Szczesna A, et al. Randomized phase II study of cetuximab plus cisplatin/vinorelbine compared with cisplatin/vinorelbine alone as first-line therapy in EGFR-expressing advanced non-small-cell lung cancer. Ann Oncol. 2008;19:362–369. doi: 10.1093/annonc/mdm474. [DOI] [PubMed] [Google Scholar]
- 88.Swanton C, Futreal A, Eisen T. Her2-targeted therapies in non-small cell lung cancer. Clin Cancer Res. 2006;12:4377s–4383s. doi: 10.1158/1078-0432.CCR-06-0115. [DOI] [PubMed] [Google Scholar]
- 89.Cappuzzo F, Bemis L, Varella-Garcia M. HER2 mutation and response to trastuzumab therapy in non-small-cell lung cancer. N Engl J Med. 2006;354:2619–2621. doi: 10.1056/NEJMc060020. [DOI] [PubMed] [Google Scholar]