Abstract
Purpose of review
Blood flow is intimately linked with cardiovascular development, repair, and dysfunction. The current review will build on the fluid mechanical principle underlying hemodynamic shear forces, mechanotransduction, and metabolic effects.
Recent findings
Pulsatile flow produces both time- (∂τ /∂t)and spatial-varying shear stress (∂τ /∂x) to modulate vascular oxidative stress and inflammatory response with pathophysiological significance to atherosclerosis. The characteristics of hemodynamic shear forces; namely, steady laminar (∂τ /∂t= 0), pulsatile (PSS: unidirectional forward flow), and oscillatory shear stress (OSS: bidirectional with a near net 0 forward flow) modulate mechano-signal transduction to influence metabolic effects on vascular endothelial function. Atheroprotective PSS promotes anti-oxidant, anti-inflammatory, and anti-thrombotic responses, whereas atherogenic OSS induces NADPH oxidase–JNK signaling to increase mitochondrial superoxide production, protein degradation of manganese superoxide dismutase (MnSOD), and post-translational protein modifications of LDL particles in the disturbed flow-exposed regions of vasculature. In the era of tissue regeneration, shear stress has been implicated in re-activation of developmental genes; namely, Wnt and Notch signaling, for vascular development and repair.
Summary
Blood flow imparts a dynamic continuum from vascular development to repair. Augmentation of PSS confers atheroprotection and re-activation of developmental signaling pathways for regeneration.
Keywords: Hemodynamics, Shear stress, Mechanotransduction, Post-translational protein modification, Vascular repair
Introduction
Atherosclerosis is a systemic disease; however, its manifestations tend to be eccentric and focal. Detection of atherosclerotic lesions prone to rupture is of clinical importance in the management of patients for acute heart attack or stroke. In addition to genetic predisposition and epigenetic factors, the pathogenesis is modulated by a combination of biochemical and hemodynamic factors. Hemodynamic force, such as wall shear stress on the endothelial cells, modulates inflammatory and metabolic effects in the vascular system [1]. At the lateral walls of bifurcations, disturbed flow, including oscillatory flow (bidirectional and axially misaligned flow), is considered to be an inducer of oxidative stress in favor of initiating atherosclerosis, whereas in the medial walls of bifurcations, pulsatile flow (unidirectional and axially aligned flow) down-regulates inflammatory cytokines, adhesion molecules, and oxidative stress [2-4] (Fig. 1). In this review, we focus on how fluid shear stress imparts both metabolic and mechanical effects on vascular endothelial function to influence vascular remodeling with clinical implication in arterial restenosis after angioplasty [5-7]
Figure 1.
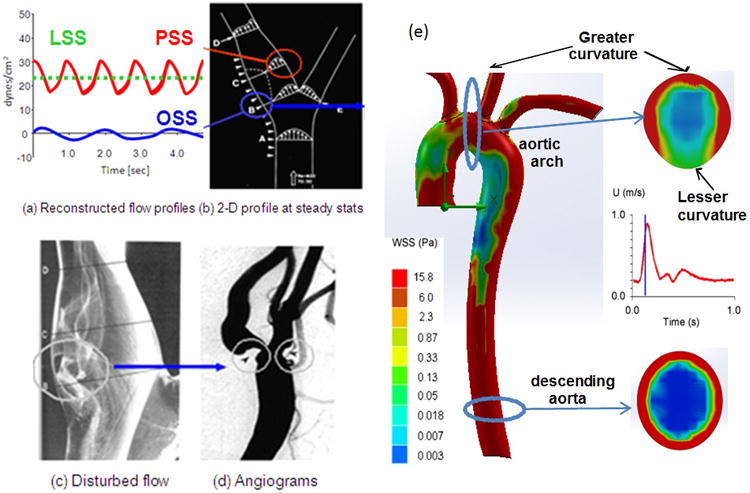
(a) Shear stress profiles at the lateral and medial walls of arterial bifurcations. (b) Pulsatile shear stress (PSS) occurs at the medial wall (red circle), whereas oscillating flow (OSS) occurs at the migrating stagnation point of the lateral wall (blue circle). (c) Flow separation and disturbed flow develops at the lateral wall. (d) Angiogram supports the predilection sites for atherosclerosis. (e) Spatial variations in wall shear stress profiles at an instantaneous moment in systole. The magnitude of wall shear stress is relatively high in the ascending aorta, greater curvature, and descending aorta. Cross-section at the aortic arch reveals an eccentric distribution of high shear stress in the greater curvature but low in the lesser curvature. Cross-section from the descending aorta reveals concentric high shear stress.
Fluid mechanical principle of hemodynamic shear forces
Fluid shear stress is generated by the frictional force by virtue of the viscosity that acts tangentially on the endoluminal surface. In the case of Couette flow, the fluid is embedded between two parallel plates separated by a displacement H. Shear force is applied to move the upper plate with velocity U while the lower plate is fixed. Shear stress (τ) is defined as the slope of tangential velocity (du/dy), and is proportional to the dynamic viscosity (μ).
At a constant pressure (P) throughout the fluid domain, the equation of fluid motion known as Navier-Stokes equation is defined as follows:
When the upper plate at y = H is moving with velocity U (Utop = U), and the lower plate at y = −H is fixed (Ubottom=0) fluid motion is linearly defined as (Figure 2a):
Figure 2.
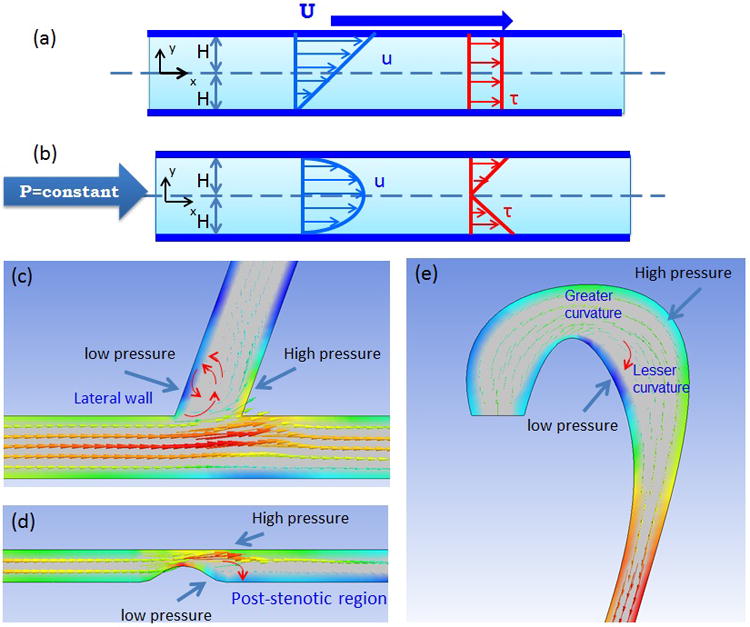
Comparison between Couette and Poiseuille flow. (a) When top plate moves with velocity U and bottom plate is fixed, fluid motion (Navier-Stoke Equation) shows a linear profile. Also, shear stress profile shows the constant profile. (b) When constant pressure is applied to fluid which is trapped between two fixed plates, fluid motion shows a parabolic flow. Shear stress profile shows a linear profile.
Anatomic variation promotes low reversal occurring from the high pressured to low pressured regions. (c) In the bifurcated region, low pressure develops at the lateral wall of bifurcation, lesser curvature, and the post-stenotic region. (d) In the wake of post-stenotic region, low pressure promotes flow separation and flow reversal. (e) In aortic arch, Greater curvature has higher pressure than the lesser curvature.
In the case of Poiseuille flow, both the upper and lower plates are fixed. The Navier-Stoke equation for 2-D blood flow at a constant pressure applied throughout the fluid domain is defined as:
The velocity profile is parabolic; that is, the velocity is maximal at the center, and zero at the wall or y = ±H for the non-slip flow (Figure 2b).
In the case of 3-D Poiseuille flow in the blood vessel, fluid shear stress at steady state (dτ/dt = 0) is directly proportional to the flow rate of blood (Q) and dynamic viscosity (μ), and inversely proportional to the cube of arterial radius (R).
Therefore, a small decrease in diameter significantly influences wall shear stress.
Fully developed Poiseuille blood flow seldom occurs in the arterial circulation in which the dynamic viscosity (μ) is not constant and the blood flow is non-Newtonian. Spatial and temporal variations in pulsatile flow prevents fully developed flow. For this reason, disturbed flow, including oscillatory flow, preferentially and geometrically occurs in the lateral wall of branching points (Figure 1). This atherogenic flow promotes vascular oxidative stress and inflammatory responses to initiate atherosclerosis [8].
Spatial variations in hemodynamic shear stress
In the arterial system, the greater curvature of aortic arch and the lateral walls of bifurcating regions are prone to develop endothelial dysfunction (Figure 1e). Oscillatory shear stress (OSS) in the aortic arch or bifurcation induces oxidative stress via nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase enzyme system production of cytosolic superoxide (O2-), and up-regulates atherogenic gene expression, including nuclear factor- kB (NF-κB)-mediated adhesion molecules and chemokines [9]. In these regions (Figure 2c-e), the time-averaged shear stress and pressure are relatively low as compared to the straight regions or the greater curvature. As fluid flows from high to lower pressure regions, blood flow tends to divert towards the lower pressured regions where the inertia force retards the fluid motion, giving rise to flow separation known as eddies. Therefore, OSS develops downstream or in the post-stenotic regions, where the time-averaged shear stress is low to promote inflammatory responses. In contrast, high shear stress develops upstream in the pre-stenotic regions, where the “shoulder” of the plaque is susceptible to fluid and mechanical mismatch or Von Mises stress to destabilize the plaque for rupture [10]. Thus, hemodynamic shear stress is low in post-stenotic but high in pre-stenotic regions [11]. The combination of OSS and low mean shear stress favors atherogenesis, whereas pulsatile and high shear stress confers protection [12]. These combined effects highlight the mechanical mechanisms underlying the rupture-prone shoulder regions, where inflammatory cells, including macrophages, release metalloproteinase (MMP). Despite the highest shear force occurring at the narrowing or the throat of stenotic lesions, OSS and low mean shear stress developed in post-stenotic regions increase the residence time for LDL and inflammatory cells, including monocytes, to transmigrate to the subendothelial layer.
In the throat of stenotic lesions, the high shear stress caused by narrowing of the plaque presents an opportunity for shear-activated nanotherapeutic thrombolytic agents to treat stenotic plaques [13]. Micro-aggregates of nanoparticles were recapitulated in the mouse model in which tissue plasminogen-coated nanoparticles disintegrate into nano-scale components in response to high fluid shear stress, leading to rapid clot dissolution in a mesenteric injury model to restore blood flow [13]. Although nanotoxicity remains a translational barrier, the integration of nanotherapeutics with hemodynamic shear force presents a promising direction to address post-stenotic lesion growth.
Despite improvement in imaging modalities such as intravascular ultrasound or magnetic resonance angiography to visualize anatomic structures, detecting areas of flow separation or low shear stress in post-stenotic regions remains challenging. Given the limitations in real-time prediction of rupture-prone regions, Ai et al. demonstrated flow reversal in a 3-D eccentric stenotic model by high frequency ultrasonic transducer (45 MHz) [14]. By interfacing microelectromechanical system (MEMS) thermal sensors with the high-frequency pulsed wave Doppler ultrasound, real-time assessment of changes in fluid shear stress upstream, downstream, and at the throat of the stenosis was validated by both computational fluid dynamics (CFD) codes and the ultrasound-acquired flow profiles. Furthermore, post-stenotic regions are prone to vascular oxidative stress and inflammatory responses, features of clinical relevance [14]. In this context, the advent of micro shear stress sensors holds promise to identify vascular regions of flow reversal with high spatial and temporal resolution [15-17]. In corollary, the application of shear-activated nanotherapeutic particles opens a new area of nanomedicine to address atherosclerotic lesions in the regions of flow separation or reversal when the blood flows though the lesser curvature of the aortic arch or the lateral wall of bifurcations. On the other hand, exercise-augmented pulsatile shear stress (PSS) up-regulates atheroprotective genes, including endothelial nitric oxide synthase (eNOS) [18-22], conferring cardioprotection.
Shear stress-mediated mechanotransduction
Shear stress imparts both metabolic and mechanical effects on vascular endothelial cells (EC) [23]. A complex flow profile develops at the arterial bifurcations; namely, flow separation and migrating stagnation points, creating low and oscillating shear stress (Figure 2c-e). In response to fluid shear stress, transmembrane proteins (including G-protein, Lectin-like oxidized LDL receptor-1 [LOX-1 receptor], Toll-like receptor, and caveolin) [24-26], junctional proteins [27], and subendothelial mechanosensors (integrin) [28] are considered to be the mechano-receptors that transmit shear forces to mechano-signal transduction (Figure. 3). In addition to mechanosensing, EC sense shear stress via deformation of the cell surface which leads to the activation of transmembrane ion channels [29, 30], realignment of endothelial cytoskeleton [31-33], and transmission of intracellular signaling pathways to modulate gene expressions [34-38].
Figure 3.
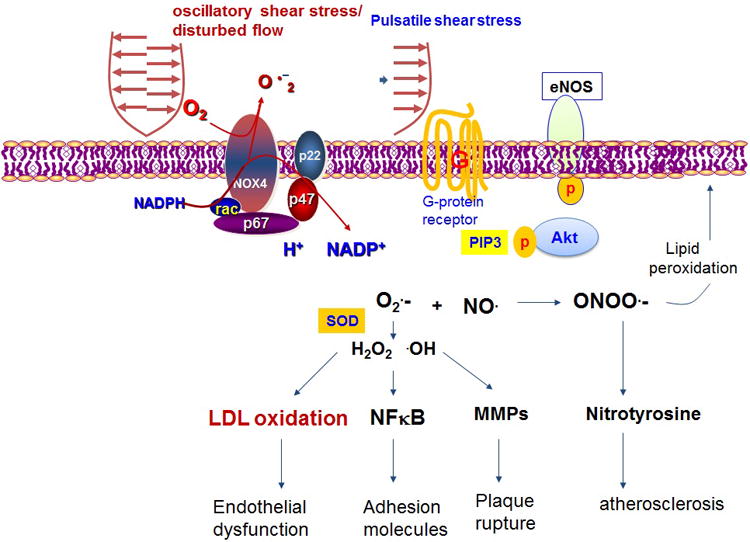
Oscillatory shear stress mediated mechanotranduction signal modulates inflammatory responses, whereas pulsatile shear stress activates G-protein and PIP3-AKT pathway, leading to phosphoyrylation of eNOS.
At the lateral walls of bifurcations, disturbed flow, including OSS is considered to be an inducer of oxidative stress. OSS-activated NADPH oxidase enzyme system promotes superoxide (O2·-) production. In the presence of superoxide dismutase (SOD), O2·- is converted to hydrogen peroxide (H2O2) and hydroxyl radicals (·OH). These two reactive oxygen species (ROS) promote LDL oxidation, NF-κB-mediated adhesion molecule and matrix metalloproteinase (MMP) expression to destabilize atherosclerotic lesions. In parallel, O2.- reacts with NO· at a rapid diffusion-limited rate to form peroxynitrite (ONOO·;-) as a substrate to lipid oxidation and nitrotyrosine formation [3, 6, 39-42]. OSS further up-regulates NADPH oxidase-dependent receptors for advanced glycation endproducts (RAGE) as an inflammatory mediator in diabetes [43]. In contrast, in the medial wall of bifurcations, pulsatile flow (PSS) down-regulates oxidative stress and inflammatory responses, but up-regulates eNOS and antioxidant expression to promote vasodilatory, anti-inflammatory, anti-oxidative and anti-thrombotic properties [2, 44*]. Furthermore, PSS or laminar flow (steady flow at dτ/dt = 0) decreases RAGE expression and attenuates RAGE signaling to inhibit NF-κB translocation to the nuclei [45].
Shear stress modulation of low density lipoprotein (LDL) post-translational modifications
The post-translational modifications of LDL particles initiate and modulate the progression of atherosclerosis. Myeloperoxidase (MPO), present in phagocytes such as macrophages, is released in response to oxidative stress and inflammatory responses. MPO produces hypochlorous acid by the reaction of H2O2 and chloride ions [46]. While this key reaction contributes significantly to the antimicrobial activity of phagocytes [47], a large body of evidence now supports oxidation of LDL by the MPO-H2O2-chloride system as a harbinger in the development of atherosclerosis [48*].
In addition to cytosolic ROS production, OSS induces mitochondrial O2.- production [49]. OSS activates NADPH oxidase-ROS-JNK signaling, leading to an increase in mitochondrial O2.- production [50-52], whereas PSS increases eNOS activities and mitochondrial membrane potential (ΔΨm) accompanied with an increase in Mn-SOD activities [53, 54]. Furthermore, oxLDL activates JNK to promote Mn-SOD ubiquitination and protein degradation [50-52]. Using a targeted proteomic approach, we have gained mechanistic insights into shear-modulated relative ratios of reactive oxygen species (ROS) and reactive nitrogen species (RNS), leading to ONOO·;- formation and specific post-translational nitration in the α and β helices of the apoB100 protein [53, 55, 56]; namely, α-1 (Tyr144), α-2 (Tyr2524), β-2 (Tyr3295), α-3 (Tyr4116), and β-2 (Tyr4211) [53]. Nitration leads to Apolipoprotein-B100 unfolding, and the modified particles are endocytosed by the scavenger receptors LOX-1, CD36 and SR-A (scavenger receptor-A), further contributing to the progression of atherosclerosis [57]. Similarly, high-performance liquid chromatography analyses of EC exposed to OSS demonstrates increased expression of the catalytic subunits of NADPH oxidase gp91phox or Nox4 with an ensuing increase in O2·- production [3]. In contrast, pulsatile shear stress (PSS) up-regulates eNOS expression accompanied with NO· production, further conferring an atheroprotective role to attenuate post-translational oxidative and nitrative modifications of LDL particles [40] [3].
Shear stress regulation of inflammatory cell recruitment
Oscillatory flow induces up-regulation of adhesion molecules and cytokines, allowing monocyte/endothelial interactions central to atherosclerosis over a dynamic range of shear stress, as demonstrated with high spatial and temporal resolution using micro-electro-mechanical systems (MEMS) sensors [39]. Indeed, EC exposed to low shear stress and flow reversal respond to inflammatory stimuli with increased monocyte binding [58], an effect mediated in part by the endothelial expression of ICAM-1 (intercellular adhesion molecule-1) [59] and release of the central atherogenic chemokine MCP-1 (monocyte chemoattractant protein-1) [60].
The transcription factor Krüppel-like factor 2 (KLF2) is an atheroprotective molecule induced by statin [61] and resveratrol [62] therapy. Recently, shear stress-induced KLF2 expression has been implicated in regulating endothelial metabolism[44**]. KLF2 is selectively induced in vascular EC exposed to the biomechanics of atheroprotected regions of the vasculature [63, 64]. Laminar shear stress reduced endothelial glycolysis by repressing the expression of phosphofructokinase-2/fructose-2,6-bisphosphatase-3 (PFKFB3) in a KLF2-dependent manner to maintain the quiescent metabolic state of EC and to inhibit angiogenesis. KLF2 further confers anti-inflammatory, anti-thrombotic, and anti-oxidative properties. In response to disturbed flow, particular biochemical stimuli, including cytokines, high glucose, and oxidative stress, KLF2 expression are reduced, resulting in an increase in glycolysis and angiogenesis [65**]. In human carotid arteries, the induction of KLF2 results in the regulation of endothelial transcriptional programs controlling inflammation, thrombosis, vascular tone, and blood vessel development [63]. KLF2, therefore, serves as a mechano-sensitive and atheroprotective transcription factor [63]. However, the underlying mechanisms whereby shear stress regulates cellular metabolism, including glycolysis and mitochondrial redox state, to maintain endothelial homeostasis remains to be explored.
Shear stress regulation of atherothrombosis
The most common complication of atherosclerosis is fibrous cap rupture leading to plaque thrombosis and clinical manifestation of acute coronary syndromes or stroke. The biomechanical properties of plaque mineralization were recapitulated in in vitro or in silico models. When cultures of calcifying vascular cells (CVC), a subpopulation of smooth muscle cells that spontaneously mineralize, are subjected to increasing magnitude in pulsatile shear stress, calcification does not increase plaque vulnerability to fluid shear stress, but may contribute a slight stabilization [66]. Destabilization of atherosclerotic plaques occur in the presence of active metabolic states; namely, oxidized lipids and activated MMP are released by macrophages [4]. Disturbed flow or extreme high shear stress also regulates the expression of tissue factor, also known as factor III, to initiate the coagulation cascade following plaque rupture and subsequent thrombotic events [67].
Shear stress modulation of vascular development and repair
In the era of stem cell and regenerative medicine, hemodynamic shear stress provides a biomechanical cure to modulate the microenvironment for vascular differentiation and repair. The transcription factor Runx1 (Runt-related transcription factor 1) is a master regulator of hematopoiesis [68]. Hemodynamic shear forces increase the expression of Runx1 in CD41+c-Kit+ hematopoietic progenitor cells, thus augmenting their colony-forming potential [68]. Additionally, shear force-mediated mechanotransduction is implicated in the differentiation of stem cells to EC [69-71], and modulates hematopoietic and multilineage engraftment potential during embryogenesis [72**]. The effects on hematopoiesis are mediated in part by a cascade downstream of wall shear stress to regulate calcium efflux and to activate the prostaglandin E2-cyclic adenosine monophosphate-protein (AMP) kinase A signaling axis [72**].
Steady laminar shear stress was further demonstrated to affect pluripotency, as well as germ specification to the mesodermal, endodermal, and ectodermal lineages, as indicated by gene expression of OCT4, T-BRACHY, AFP, and NES in mouse embryonic cells [73]. OSS induces directional reorganization of F-actin to mediate the fate choice of mesenchymal stem cells (MSCs) through the regulation of the β-catenin/Wnt signaling pathway in a time-dependent manner [74*]. OSS also activates angiopoietin-2 (Ang-2) expression critical to angiogenesis via canonical β-catenin/Wnt signaling in human aortic EC [75*]. Low and disturbed flow patterns up-regulate Notch1 expression in EC with translational implication for arteriovenous identity [76]. Steady shear stress induces VEGF-Notch signaling pathway to increase expression of the arterial endothelial marker EphrinB2, but down-regulates the venous endothelial marker EphB4 in murine embryonic stem (ES) cells [77]. Thus, the hemodynamic cue in the vascular microenvironment modulates vascular differentiation and proliferation.
Conclusion
Shear stress imparts both metabolic and mechanical effects on vascular EC, with a clinical implication in the focal and eccentric nature of atherosclerotic plaques. Physiologic shear stress up-regulates vasodilators, antioxidant enzymes, and tissue plasminogen activator (tPA) to confer atheroprotective responses, whereas oscillatory shear stress induces vasoconstriction, growth factors, and adhesion molecules to prime atherogenic responses. PSS increases endothelial mitochondrial membrane potential (ΔΨm) accompanied by a decrease in mitochondrial superoxide production (mtO2·;-), whereas OSS oxidized LDL increase mtO2·- production to promote apoptotic pathways. Recently, shear stress-reactivated developmental Wnt-Ang-2 signaling in mature vascular EC was implicated in vascular formation and repair. Shear stress-activated VEGF-Notch signaling regulates the fate of arteriovenous differentiation. In this context, the spatial (∂τ /∂x) and temporal (∂τ /∂t) variations in shear stress largely determine the focal nature of vascular oxidative stress and pro-inflammatory states. While sedentary life-style promotes flow separation and disturbed flow, exercise-augmented pulsatile shear stress remains a timeless therapeutic strategy for maintaining endothelial homeostasis.
Key Points.
The spatial (∂τ /∂x) and temporal (∂τ /∂t) variations in shear stress largely determine the focal nature of vascular oxidative stress and pro-inflammatory states.
OSS activates the NADPH oxidase enzyme system promoting superoxide (O2·-) production leading to LDL oxidation, NF-κB-mediated adhesion molecule and MMP expression and atherosclerotic lesion destabilization.
PSS increases endothelial mitochondrial membrane potential (ΔΨm), down-regulates oxidative stress by up-regulating eNOS and decreasing mitochondrial superoxide production, and also inhibits inflammatory responses such as RAGE expression and NF-κB translocation to the nucleus.
Shear stress-activated developmental Wnt-Ang-2 signaling in mature vascular EC is implicated in vascular formation and repair.
Acknowledgments
We would like to thank all members of Cardiovascular Engineering Research Laboratory for his assistance with the study
Funding: National Institutes of Health HL118650 (T.K.H.), HL083015 (T.K.H.), HL111437 (T.K.H.), T32HL007895 (R.R.S.P.), and AHA Pre-Doctoral Fellowship 15PRE21400019 (J.L.)
Financial support and sponsorship: This work was supported by the NIH, AHA, and UCLA STAR program fellowship.
Footnotes
Conflicts of interest: We have no conflicts of interest.
References and Recommended Reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arteriosclerosis, thrombosis, and vascular biology. 2005;25:29–38. doi: 10.1161/01.ATV.0000150649.39934.13. [DOI] [PubMed] [Google Scholar]
- 2.Harrison D, Griendling KK, Landmesser U, et al. Role of oxidative stress in atherosclerosis. The American journal of cardiology. 2003;91:7A–11A. doi: 10.1016/s0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- 3.Hwang J, Ing MH, Salazar A, et al. Pulsatile versus oscillatory shear stress regulates NADPH oxidase subunit expression: implication for native LDL oxidation. Circ Res. 2003;93:1225–1232. doi: 10.1161/01.RES.0000104087.29395.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li R, Mittelstein D, Lee J, et al. A dynamic model of calcific nodule destabilization in response to monocyte- and oxidized lipid-induced matrix metalloproteinases. American journal of physiology Cell physiology. 2012;302:C658–665. doi: 10.1152/ajpcell.00313.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stone PH, Coskun AU, Kinlay S, et al. Effect of endothelial shear stress on the progression of coronary artery disease, vascular remodeling, and in-stent restenosis in humans: in vivo 6-month follow-up study. Circulation. 2003;108:438–444. doi: 10.1161/01.CIR.0000080882.35274.AD. [DOI] [PubMed] [Google Scholar]
- 6.Takabe W, Jen N, Ai L, et al. Oscillatory shear stress induces mitochondrial superoxide production: implication of NADPH oxidase and c-Jun NH2-terminal kinase signaling. Antioxidants & redox signaling. 2011;15:1379–1388. doi: 10.1089/ars.2010.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng C, Tempel D, van Haperen R, et al. Shear stress-induced changes in atherosclerotic plaque composition are modulated by chemokines. The Journal of clinical investigation. 2007;117:616–626. doi: 10.1172/JCI28180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu F, Lee J, Jen N, et al. Elevated electrochemical impedance in the endoluminal regions with high shear stress: implication for assessing lipid-rich atherosclerotic lesions. Biosensors & bioelectronics. 2013;43:237–244. doi: 10.1016/j.bios.2012.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jen N, Yu F, Lee J, et al. Atrial fibrillation pacing decreases intravascular shear stress in a New Zealand white rabbit model: implications in endothelial function. Biomechanics and modeling in mechanobiology. 2013;12:735–745. doi: 10.1007/s10237-012-0437-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10**.Cheng C, Tempel D, van Haperen R, et al. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation. 2006;113:2744–2753. doi: 10.1161/CIRCULATIONAHA.105.590018. These in vitro observation shows fundamental mechanisms of superficial erosion versus fibrous cap rupture.TLR2 and neutrophils have an important role to propagate superficial erosion while monocytes/macrophages participate in plaque rupture. Also, superficial erosion induces enthothelial cell apoptosis. [DOI] [PubMed] [Google Scholar]
- 11.Sadeghi MR, Shirani E, Tafazzoli-Shadpour M, Samaee M. The effects of stenosis severity on the hemodynamic parameters-assessment of the correlation between stress phase angle and wall shear stress. Journal of biomechanics. 2011;44:2614–2626. doi: 10.1016/j.jbiomech.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 12.Thim T, Hagensen MK, Horlyck A, et al. Wall shear stress and local plaque development in stenosed carotid arteries of hypercholesterolemic minipigs. Journal of cardiovascular disease research. 2012;3:76–83. doi: 10.4103/0975-3583.95358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korin N, Kanapathipillai M, Matthews BD, et al. Shear-activated nanotherapeutics for drug targeting to obstructed blood vessels. Science. 2012;337:738–742. doi: 10.1126/science.1217815. [DOI] [PubMed] [Google Scholar]
- 14.Ai L, Zhang L, Dai W, et al. Real-time assessment of flow reversal in an eccentric arterial stenotic model. Journal of biomechanics. 2010;43:2678–2683. doi: 10.1016/j.jbiomech.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu H, Ai L, Rouhanizadeh M, et al. Flexible polymer sensors for in vivo intravascular shear stress analysis. Microelectromechanical Systems, Journal of. 2008;17:1178–1186. [Google Scholar]
- 16.Ai L, Yu H, Dai W, et al. Real-time intravascular shear stress in the rabbit abdominal aorta. Biomedical Engineering, IEEE Transactions on. 2009;56:1755–1764. doi: 10.1109/TBME.2009.2013455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu F, Ai L, Dai W, et al. MEMS thermal sensors to detect changes in heat transfer in the pre-atherosclerotic regions of fat-fed New Zealand white rabbits. Annals of biomedical engineering. 2011;39:1736–1744. doi: 10.1007/s10439-011-0283-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davies PF, Polacek DC, Handen JS, et al. A spatial approach to transcriptional profiling: mechanotransduction and the focal origin of atherosclerosis. Trends in biotechnology. 1999;17:347–351. doi: 10.1016/s0167-7799(99)01348-7. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Cardena G, Comander JI, Blackman BR, et al. Mechanosensitive endothelial gene expression profiles: scripts for the role of hemodynamics in atherogenesis? Annals of the New York Academy of Sciences. 2001;947:1–6. [PubMed] [Google Scholar]
- 20.Libby P. Coronary artery injury and the biology of atherosclerosis: inflammation, thrombosis, and stabilization. The American journal of cardiology. 2000;86:3J–8J. doi: 10.1016/s0002-9149(00)01339-4. discussion 8J-9J. [DOI] [PubMed] [Google Scholar]
- 21.Corson MA, James NL, Latta SE, et al. Phosphorylation of endothelial nitric oxide synthase in response to fluid shear stress. Circ Res. 1996;79:984–991. doi: 10.1161/01.res.79.5.984. [DOI] [PubMed] [Google Scholar]
- 22.Moncada S. Adventures in vascular biology: a tale of two mediators. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2006;361:735–759. doi: 10.1098/rstb.2005.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glagov S, Zarins C, Giddens DP, Ku DN. Hemodynamics and atherosclerosis. Insights and perspectives gained from studies of human arteries. Archives of pathology & laboratory medicine. 1988;112:1018–1031. [PubMed] [Google Scholar]
- 24.Makino A, Prossnitz ER, Bunemann M, et al. G protein-coupled receptors serve as mechanosensors for fluid shear stress in neutrophils. American journal of physiology Cell physiology. 2006;290:C1633–1639. doi: 10.1152/ajpcell.00576.2005. [DOI] [PubMed] [Google Scholar]
- 25.Murase T, Kume N, Korenaga R, et al. Fluid shear stress transcriptionally induces lectin-like oxidized LDL receptor-1 in vascular endothelial cells. Circ Res. 1998;83:328–333. doi: 10.1161/01.res.83.3.328. [DOI] [PubMed] [Google Scholar]
- 26.Wang P, Zhu F, Tong Z, Konstantopoulos K. Response of chondrocytes to shear stress: antagonistic effects of the binding partners Toll-like receptor 4 and caveolin-1. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011;25:3401–3415. doi: 10.1096/fj.11-184861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tarbell JM. Shear stress and the endothelial transport barrier. Cardiovascular research. 2010;87:320–330. doi: 10.1093/cvr/cvq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urbich C, Walter DH, Zeiher AM, Dimmeler S. Laminar shear stress upregulates integrin expression: role in endothelial cell adhesion and apoptosis. Circ Res. 2000;87:683–689. doi: 10.1161/01.res.87.8.683. [DOI] [PubMed] [Google Scholar]
- 29.Nilius B, Droogmans G. Ion channels and their functional role in vascular endothelium. Physiological reviews. 2001;81:1415–1459. doi: 10.1152/physrev.2001.81.4.1415. [DOI] [PubMed] [Google Scholar]
- 30.Brakemeier S, Kersten A, Eichler I, et al. Shear stress-induced up-regulation of the intermediate-conductance Ca(2+)-activated K(+) channel in human endothelium. Cardiovascular research. 2003;60:488–496. doi: 10.1016/j.cardiores.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 31.McCue S, Noria S, Langille BL. Shear-induced reorganization of endothelial cell cytoskeleton and adhesion complexes. Trends in cardiovascular medicine. 2004;14:143–151. doi: 10.1016/j.tcm.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Davies PF, Robotewskyj A, Griem ML. Quantitative studies of endothelial cell adhesion. Directional remodeling of focal adhesion sites in response to flow forces. The Journal of clinical investigation. 1994;93:2031–2038. doi: 10.1172/JCI117197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Helmke BP, Goldman RD, Davies PF. Rapid displacement of vimentin intermediate filaments in living endothelial cells exposed to flow. Circ Res. 2000;86:745–752. doi: 10.1161/01.res.86.7.745. [DOI] [PubMed] [Google Scholar]
- 34.Loufrani L, Dubroca C, You D, et al. Absence of dystrophin in mice reduces NO-dependent vascular function and vascular density: total recovery after a treatment with the aminoglycoside gentamicin. Arterioscler Thromb Vasc Biol. 2004;24:671–676. doi: 10.1161/01.ATV.0000118683.99628.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rizzo V, Morton C, DePaola N, et al. Recruitment of endothelial caveolae into mechanotransduction pathways by flow conditioning in vitro. American journal of physiology Heart and circulatory physiology. 2003;285:H1720–1729. doi: 10.1152/ajpheart.00344.2002. [DOI] [PubMed] [Google Scholar]
- 36.Osawa M, Masuda M, Kusano K, Fujiwara K. Evidence for a role of platelet endothelial cell adhesion molecule-1 in endothelial cell mechanosignal transduction: is it a mechanoresponsive molecule? The Journal of cell biology. 2002;158:773–785. doi: 10.1083/jcb.200205049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cowan DB, Lye SJ, Langille BL. Regulation of vascular connexin43 gene expression by mechanical loads. Circ Res. 1998;82:786–793. doi: 10.1161/01.res.82.7.786. [DOI] [PubMed] [Google Scholar]
- 38.Gudi S, Huvar I, White CR, et al. Rapid activation of Ras by fluid flow is mediated by Galpha(q) and Gbetagamma subunits of heterotrimeric G proteins in human endothelial cells. Arterioscler Thromb Vasc Biol. 2003;23:994–1000. doi: 10.1161/01.ATV.0000073314.51987.84. [DOI] [PubMed] [Google Scholar]
- 39.Hsiai TK, Cho SK, Wong PK, et al. Monocyte recruitment to endothelial cells in response to oscillatory shear stress. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2003;17:1648–1657. doi: 10.1096/fj.02-1064com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsiai TK, Hwang J, Barr ML, et al. Hemodynamics influences vascular peroxynitrite formation: Implication for low-density lipoprotein apo-B-100 nitration. Free radical biology & medicine. 2007;42:519–529. doi: 10.1016/j.freeradbiomed.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takabe W, Li R, Ai L, et al. Oxidized low-density lipoprotein-activated c-Jun NH2-terminal kinase regulates manganese superoxide dismutase ubiquitination: implication for mitochondrial redox status and apoptosis. Arterioscler Thromb Vasc Biol. 2010;30:436–441. doi: 10.1161/ATVBAHA.109.202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brennan ML, Wu W, Fu X, et al. A tale of two controversies: defining both the role of peroxidases in nitrotyrosine formation in vivo using eosinophil peroxidase and myeloperoxidase-deficient mice, and the nature of peroxidase-generated reactive nitrogen species. The Journal of biological chemistry. 2002;277:17415–17427. doi: 10.1074/jbc.M112400200. [DOI] [PubMed] [Google Scholar]
- 43.DeVerse JS, Bailey KA, Jackson KN, Passerini AG. Shear stress modulates RAGE-mediated inflammation in a model of diabetes-induced metabolic stress. American journal of physiology Heart and circulatory physiology. 2012;302:H2498–2508. doi: 10.1152/ajpheart.00869.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun X, Feinberg MW. Regulation of endothelial cell metabolism: just go with the flow. Arterioscler Thromb Vasc Biol. 2015;35:13–15. doi: 10.1161/ATVBAHA.114.304869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ha CH, Kim S, Chung J, et al. Inhibitory effect of soluble RAGE in disturbed flow-induced atherogenesis. International journal of molecular medicine. 2013;32:373–380. doi: 10.3892/ijmm.2013.1393. [DOI] [PubMed] [Google Scholar]
- 46.Klebanoff SJ. Myeloperoxidase: friend and foe. Journal of leukocyte biology. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 47.Arnhold J, Flemmig J. Human myeloperoxidase in innate and acquired immunity. Archives of biochemistry and biophysics. 2010;500:92–106. doi: 10.1016/j.abb.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 48*.Delporte C, Boudjeltia KZ, Noyon C, et al. Impact of myeloperoxidase-LDL interactions on enzyme activity and subsequent posttranslational oxidative modifications of apoB-100. Journal of lipid research. 2014;55:747–757. doi: 10.1194/jlr.M047449. This article demonstrates that myeloperoxidase activity increases up to 90% when adsorbed at the surface of LDL, and illustrates the specificity of the myeloperoxidase product hypochlorous acid to oxidize LDL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li R, Jen N, Yu F, Hsiai TK. Assessing mitochondrial redox status by flow cytometric methods: vascular response to fluid shear stress. Current protocols in cytometry / editorial board, J Paul Robinson, managing editor … [et al] 2011;Chapter 9:Unit9 37. doi: 10.1002/0471142956.cy0937s58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takabe W, Li R, Ai L, et al. Oxidized Low-Density Lipoprotein-Activated c-Jun NH2-Terminal Kinase Regulates Manganese Superoxide Dismutase Ubiquitination: Implication for Mitochondrial Redox Status and Apoptosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30:436–441. doi: 10.1161/ATVBAHA.109.202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jen N, Takabe W, Li R, et al. Oscillatory fluid shear stress-induced JNK activation via NADPH oxidase implicates mitochondrial superoxide production in endothelial cells. The FASEB Journal. 2010;24:784.713. [Google Scholar]
- 52.Takabe W, Jen N, Ai L, et al. Oscillatory Shear Stress Induces Mitochondrial Superoxide Production: Implication of NADPH Oxidase and c-Jun NH2-terminal Kinase Signaling. Antioxidants & redox signaling. 2010 doi: 10.1089/ars.2010.3645. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hsiai TK, Hwang J, Barr ML, et al. Hemodynamics influences vascular peroxynitrite formation: Implication for low-density lipoprotein apo-B-100 nitration. Free Radical Biology and Medicine. 2007;42:519–529. doi: 10.1016/j.freeradbiomed.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li R, Beebe T, Cui J, et al. Pulsatile shear stress increased mitochondrial membrane potential: Implication of Mn-SOD. Biochemical and Biophysical Research Communications. 2009;388:406–412. doi: 10.1016/j.bbrc.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ai L, Rouhanizadeh M, Wu JC, et al. Shear stress influences spatial variations in vascular Mn-SOD expression: implication for LDL nitration. American Journal of Physiology- Cell Physiology. 2008;294:C1576–C1585. doi: 10.1152/ajpcell.00518.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hamilton RT, Asatryan L, Nilsen JT, et al. LDL protein nitration: Implication for LDL protein unfolding. Archives of biochemistry and biophysics. 2008;479:1–14. doi: 10.1016/j.abb.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hamilton RT, Asatryan L, Nilsen JT, et al. LDL protein nitration: implication for LDL protein unfolding. Archives of biochemistry and biophysics. 2008;479:1–14. doi: 10.1016/j.abb.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Honda HM, Hsiai T, Wortham CM, et al. A complex flow pattern of low shear stress and flow reversal promotes monocyte binding to endothelial cells. Atherosclerosis. 2001;158:385–390. doi: 10.1016/s0021-9150(01)00462-2. [DOI] [PubMed] [Google Scholar]
- 59.Nagel T, Resnick N, Atkinson WJ, et al. Shear stress selectively upregulates intercellular adhesion molecule-1 expression in cultured human vascular endothelial cells. The Journal of clinical investigation. 1994;94:885–891. doi: 10.1172/JCI117410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hsiai TK, Cho SK, Reddy S, et al. Pulsatile flow regulates monocyte adhesion to oxidized lipid-induced endothelial cells. Arteriosclerosis, thrombosis, and vascular biology. 2001;21:1770–1776. doi: 10.1161/hq1001.097104. [DOI] [PubMed] [Google Scholar]
- 61.Parmar KM, Nambudiri V, Dai G, et al. Statins exert endothelial atheroprotective effects via the KLF2 transcription factor. The Journal of biological chemistry. 2005;280:26714–26719. doi: 10.1074/jbc.C500144200. [DOI] [PubMed] [Google Scholar]
- 62.Gracia-Sancho J, Villarreal G, Jr, Zhang Y, Garcia-Cardena G. Activation of SIRT1 by resveratrol induces KLF2 expression conferring an endothelial vasoprotective phenotype. Cardiovascular research. 2010;85:514–519. doi: 10.1093/cvr/cvp337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parmar KM, Larman HB, Dai G, et al. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. The Journal of clinical investigation. 2006;116:49–58. doi: 10.1172/JCI24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu W, Xiao H, Laguna-Fernandez A, et al. Flow-Dependent Regulation of Kruppel-Like Factor 2 Is Mediated by MicroRNA-92a. Circulation. 2011;124:633–641. doi: 10.1161/CIRCULATIONAHA.110.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65**.Doddaballapur A, Michalik KM, Manavski Y, et al. Laminar shear stress inhibits endothelial cell metabolism via KLF2-mediated repression of PFKFB3. Arterioscler Thromb Vasc Biol. 2015;35:137–145. doi: 10.1161/ATVBAHA.114.304277. This article shows laminar shear stress regulation of KLF2 expression in endothelial cells which leads to direct repression of PFKFB3 transcription, thereby reducing glycolysis and mitochondrial content. [DOI] [PubMed] [Google Scholar]
- 66.Lin TC, Tintut Y, Lyman A, et al. Mechanical response of a calcified plaque model to fluid shear force. Annals of biomedical engineering. 2006;34:1535–1541. doi: 10.1007/s10439-006-9182-9. [DOI] [PubMed] [Google Scholar]
- 67.Lin MC, Almus-Jacobs F, Chen HH, et al. Shear stress induction of the tissue factor gene. The Journal of clinical investigation. 1997;99:737–744. doi: 10.1172/JCI119219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Adamo L, Naveiras O, Wenzel PL, et al. Biomechanical forces promote embryonic haematopoiesis. Nature. 2009;459:1131–1135. doi: 10.1038/nature08073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yamamoto K, Sokabe T, Watabe T, et al. Fluid shear stress induces differentiation of Flk-1-positive embryonic stem cells into vascular endothelial cells in vitro. American journal of physiology Heart and circulatory physiology. 2005;288:H1915–1924. doi: 10.1152/ajpheart.00956.2004. [DOI] [PubMed] [Google Scholar]
- 70.Obi S, Yamamoto K, Shimizu N, et al. Fluid shear stress induces arterial differentiation of endothelial progenitor cells. Journal of applied physiology. 2009;106:203–211. doi: 10.1152/japplphysiol.00197.2008. [DOI] [PubMed] [Google Scholar]
- 71.Toh YC, Voldman J. Fluid shear stress primes mouse embryonic stem cells for differentiation in a self-renewing environment via heparan sulfate proteoglycans transduction. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011;25:1208–1217. doi: 10.1096/fj.10-168971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72**.Diaz MF, Li N, Lee HJ, et al. Biomechanical forces promote blood development through prostaglandin E2 and the cAMP-PKA signaling axis. The Journal of experimental medicine. 2015 doi: 10.1084/jem.20142235. This investigation illustrates the role of fluid shear stress-mediated regulation of the PGE2-cAMP-PKA pathway expression, leading to long-term multilineage engrafment of early hematopoietic tissues during emrbyogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wolfe RP, Leleux J, Nerem RM, Ahsan T. Effects of shear stress on germ lineage specification of embryonic stem cells. Integrative biology : quantitative biosciences from nano to macro. 2012;4:1263–1273. doi: 10.1039/c2ib20040f. [DOI] [PubMed] [Google Scholar]
- 74*.Kuo YC, Chang TH, Hsu WT, et al. Oscillatory shear stress mediates directional reorganization of actin cytoskeleton and alters differentiation propensity of mesenchymal stem cells. Stem cells. 2015;33:429–442. doi: 10.1002/stem.1860. The authors demonstrate that oscillatory shear stress is mechanosensed by intercellular junction molecules, induces directional reorganization of F-actin and ultimately mediates the fate choice of mesenchymal stem cells. [DOI] [PubMed] [Google Scholar]
- 75*.Li R, Beebe T, Jen N, et al. Shear stress-activated Wnt-angiopoietin-2 signaling recapitulates vascular repair in zebrafish embryos. Arterioscler Thromb Vasc Biol. 2014;34:2268–2275. doi: 10.1161/ATVBAHA.114.303345. This study shows that oscillatory shear stress induces Ang2 via canonical Wnt signaling in vascular endothelial cells with translational implications in vascular development and repair. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jahnsen ED, Trindade A, Zaun HC, et al. Notch1 is pan-endothelial at the onset of flow and regulated by flow. PLoS One. 2015;10:e0122622. doi: 10.1371/journal.pone.0122622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Masumura T, Yamamoto K, Shimizu N, et al. Shear stress increases expression of the arterial endothelial marker ephrinB2 in murine ES cells via the VEGF-Notch signaling pathways. Arterioscler Thromb Vasc Biol. 2009;29:2125–2131. doi: 10.1161/ATVBAHA.109.193185. [DOI] [PubMed] [Google Scholar]


